CHAPTER 2 Evaluation of the Symptomatic Patient: Diagnostic Breast Imaging
Screening mammography is used to detect breast cancer in asymptomatic women. In order to be cost-effective and efficient, screening mammography is performed by a technologist and batch-read at a later time by the radiologist. Most screening mammography findings for which patients are called back prove to be benign. The average callback rate for screening mammography is about 8% to 10%.1 Of these callbacks, only about 15% will prove to be suspicious after diagnostic workup and require biopsy. Of the biopsies, about one third (30% to 35%) will yield a diagnosis of cancer. Screening mammography is intended for detection, not analysis, of potential abnormalities. Diagnostic evaluations involve the use of additional mammographic views, such as spot compression and magnification, and other breast evaluation techniques, such as ultrasound, physical exam, and ductography, to analyze potential abnormalities identified on screening mammography and to evaluate symptomatic patients. A diagnostic study is directed and supervised by a radiologist, and patients are given their results at the conclusion of the examination. Questions to be addressed through workup of a screening mammography finding are:
Magnification views are utilized to evaluate morphologic features, such as the margins of masses and the shapes of microcalcifications. The Breast Imaging Reporting and Data System (BI-RADS) lexicon is a classification scheme used to help standardize the description and disposition of breast lesions seen on mammography, ultrasound, and MRI.2 BI-RADS categories 1 and 2 are used to describe a negative study and a study in which there are benign findings, respectively. Category 3 is used to describe probably benign findings, with a less than 2% chance of malignancy, which can be followed in 6 months.3 Patients with new or enlarging solid masses or increasing clustered microcalcifications that are not classically benign require biopsy. Category 4 is used to describe suspicious findings (greater than 2% chance of malignancy) that require biopsy. BI-RADS category 5 lesions are highly suspicious findings, having a 95% or higher likelihood of malignancy. Category 6 is used in patients with a known breast cancer who are undergoing neoadjuvant chemotherapy or additional imaging studies (Table 1).
Another primary role of diagnostic workups is to evaluate symptomatic patients. Commonly evaluated complaints include palpable lumps, an area of thickening, pain, and nipple discharge. The use of ultrasound in combination with mammography is extremely important in the symptomatic patient because some breast cancers may not be detected mammographically. Real-time evaluation by the radiologist is often necessary to detect subtle cancers. A report by the Physicians Insurers Association of America (PIAA) noted that a large percentage of suits involve women presenting with clinical symptoms and that a common reason for litigation was the claim that the physical symptoms “failed to impress” the physician.4 The negative predictive value of a negative mammography and ultrasound is estimated to be 95% to 99%.5–8 However, despite this high negative predictive value, a biopsy may still be warranted in the setting of a suspicious clinical finding.
MAGNETIC RESONANCE IMAGING (MRI) OF THE BREAST
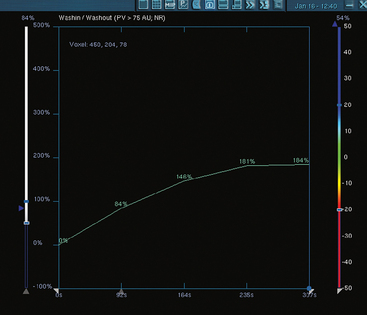
Dynamic contrast-enhanced (DCE) MRI of the breast has been shown to be extremely sensitive in the detection of invasive breast cancer and is not limited by breast tissue density. Current indications include high-risk screening, evaluation for an unknown primary carcinoma, preoperative evaluation in patients with known breast cancer, evaluating response to neoadjuvant therapy, and suspected recurrence. However, the utility of DCE MRI has been limited by its variable sensitivity for ductal carcinoma in situ (DCIS). In general, the role of DCE MRI as a problem-solving tool in the evaluation of suspicious imaging or clinical findings is unclear. A negative MRI should not be used to avoid biopsy of suspicious findings on mammography or ultrasound or of a suspicious clinical finding.
NEEDLE BIOPSY PROCEDURES
Lesions categorized as suspicious following diagnostic workup require biopsy. Image-guided percutaneous needle biopsy techniques are firmly established as a valid replacement for surgical excisional biopsy, both in the evaluation of suspicious imaging findings and in diagnosing breast cancer.9–19 Multiple studies have demonstrated the accuracy of percutaneous large-core breast biopsy for nonpalpable lesions to be comparable to that of needle localization and open surgical biopsy. Percutaneous needle biopsy is less expensive and less invasive than surgical biopsy and does not deform or significantly scar the breast. Its use has decreased the number of unnecessary surgical biopsies for benign lesions and reduces the number of surgeries required to treat patients with cancer. Instead of two surgical procedures to diagnose and treat breast cancer (excisional biopsy for diagnosis, followed later by a separate sentinel node biopsy or axillary dissection), a single surgical procedure can often be performed. The average number of surgeries performed in women with cancers diagnosed preoperatively using percutaneous needle biopsy is significantly lower than in women whose cancer was surgically diagnosed.20–24 In addition, a preoperative tissue diagnosis allows patients to have a more informed and thorough discussion of cancer treatment options before surgery.
Ultrasound is the guidance method of choice for percutaneous interventional procedures of nonpalpable breast lesions. In addition, ultrasound guidance may be useful for selected palpable masses that are small, mobile, or vaguely palpable, in which palpation-guided biopsy may prove difficult.25,26 Ultrasound as a guidance modality for cyst aspiration, needle localization, and biopsy procedures is preferred for several reasons. These include patient tolerance, accuracy, speed, real-time visualization, accessibility to all areas of the breast and axilla, relatively low cost, and lack of ionizing radiation. Most ultrasound-guided biopsy procedures are performed for masses. Even when seen on ultrasound, microcalcifications are usually best biopsied stereotactically, unless an associated mass is identified on ultrasound.
Cyst Aspiration
Benign (simple) cysts can be accurately characterized using high-resolution ultrasound imaging and do not require aspiration or biopsy.27,28 Incidental complicated cysts (circumscribed round or oval masses with posterior enhancement, homogeneous low-level internal echoes, or mobile debris) can be safely followed.29–32 Aspiration is reasonable for isolated complicated cysts that are enlarging or newly palpable. In addition, simple cysts may be aspirated in patients desiring symptomatic relief. For incidental nonpalpable lesions that cannot be reliably classified as simple or complicated cysts, and would require biopsy if solid, ultrasound-guided cyst aspiration is indicated for differentiation.
Routinely sending aspirated cyst fluid to cytology can lead to unnecessary surgical biopsies because of false-positive fluid analysis. Unless the fluid is unusually bloody, it can be discarded.33–35 Bloody cyst fluid, although most likely benign, has been associated with intracystic papillomas. In cases in which bloody fluid is obtained upon aspiration and the cyst does not completely resolve, core needle biopsy of the residual cyst may yield a more definitive diagnosis than fluid analysis, possibly averting unnecessary surgical biopsy.
Fine-Needle Aspiration Biopsy
High-quality, fine-needle aspiration breast biopsies (FNABs) require both a skilled cytopathologist and on-site evaluation to establish adequacy of sampling. Although FNAB can provide quicker results and reduce patient anxiety because of faster processing time, accuracy should not be compromised for the sake of speed. Several studies, including the multicenter randomized trial performed by the Radiation Oncology Diagnosis Group V (RDOGV), have shown large-core needle biopsy to be superior to FNAB.36–38 In addition, unlike core needle biopsy, FNAB cannot reliably distinguish in situ from invasive carcinoma. Patients whose cancer has been diagnosed as invasive preoperatively can undergo a lumpectomy and lymph node dissection in a single surgical procedure, as opposed to two separate surgeries when diagnosed using FNAB. Given its superior accuracy, diagnostic utility, and safety, with few significant complications, core needle biopsy is preferred over FNAB.
14-Gauge Core Needle Biopsy
Image-guided percutaneous needle biopsy is a less invasive and less expensive alternative to surgical excisional biopsy in the diagnosis of breast cancer. Fourteen-gauge needles have been found to be more accurate than smaller-diameter core biopsy needles.39 To ensure accurate sampling, it is important to obtain an adequate number of samples and to be certain that the biopsy needle traverses the mass.
Vacuum-Assisted Core Biopsy
Vacuum-assisted core biopsy devices allow the acquisition of larger amounts of tissue per core biopsy specimen. Compared to 14-gauge spring-loaded biopsies, which on average yield 18 mg of tissue per core specimen, the 11-gauge vacuum-assisted devices can obtain core samples averaging 95 mg.40 Complication rates for 11-gauge vacuum-assisted biopsy have not been shown to be significantly different than those for 14-gauge automated core needle biopsy.41,42
Larger tissue volume devices have facilitated the sampling of microcalcifications during stereotactic biopsy. In addition, the frequency of histologic underestimation, imaging-histology discordance, and rebiopsy is lower for 11-gauge vacuum-assisted stereotactic biopsy than for 14-gauge core needle biopsy.43–48 However, because these types of sampling errors occur in only a small percentage of patients who undergo 14-gauge ultrasound-guided biopsy, improvements in these rates may benefit relatively few patients. Philpotts and colleagues42 reported no significant differences in the outcomes of sonographically guided core biopsies performed with the automated gun compared with those performed with a vacuum-assisted device, in terms of missed cancers, underestimation, or the need (immediate or delayed) for a second biopsy.
If enough samples are taken, vacuum core biopsy devices can remove a large portion of a lesion. However, they are only approved for diagnostic purposes and not for excision or removal. Although there is little data available, removal of most of a lesion through vacuum core biopsy has been suggested for possible relief of painful, symptomatic fibroadenomas. Removing all imaging evidence of a lesion does not equate to complete excision of the pathologic abnormality. Liberman and colleagues49 reported that for cancers in which the imaging findings had been removed by vacuum-assisted biopsy, 79% had residual cancer at excision. The use of large-volume vacuum core biopsy, instead of open surgical excision, to evaluate high-risk lesions found at 14-gauge biopsy (papillomas, radial scars, atypia, or suspected phyllodes tumors) has not been extensively studied. Further investigation is needed to assess the potential benefits of completely removing the imaging findings of lesions versus sampling them.
Imaging-Histology Correlation and Follow-up
The false-negative rate for ultrasound-guided core-needle biopsy procedures is between 0% and 1.26%.50 It is important to remember that needle biopsy of breast lesions is a sampling procedure and that undersampling can occur. Low falsenegative rates and high accuracy are only attained by combining precise targeting and sufficient sampling with systematic radiologic and pathologic correlation.
In order for needle biopsy procedures to attain a level of accuracy similar to that of needle localization and surgical excisional biopsy, pathology results must be correlated with the imaging findings.18,51–53 A discordant finding, one in which the histopathologic findings do not provide a sufficient explanation for suspicious imaging findings, warrants a repeat biopsy. This is usually in the form of a surgical biopsy, owing to the possibility of a second discordant result on repeat needle biopsy. Imaging-histologic discordance rates for 14-gauge ultrasound-guided needle biopsy have been reported as high as 7.7%. Crystal and associates18 found that 12 of 323 cancers were missed at initial ultrasound-guided 14-gauge needle biopsy. Of these missed cancers, 7 were found immediately at rebiopsy prompted by discordant results, 2 at immediate rebiopsy because of indeterminate pathology findings, and 3 at later follow-up. Sauer and coworkers,51 in their study of 962 lesions that underwent 14-gauge core biopsy under three-dimensional ultrasound guidance, reported a 3% discordance rate, of which 27.6% proved to be malignant on rebiopsy. Additionally, Liberman and colleagues52 reported a 3.3% discordance rate out of 580 lesions biopsied under ultrasound guidance. At rebiopsy, 10.5% of these discordant lesions proved to be carcinoma. Therefore, careful imaginghistologic correlation will allow the detection of a significant number of false-negative results immediately after needle biopsy, thereby avoiding delays in diagnosis.
HIGH-RISK LESIONS FOUND AT CORE NEEDLE BIOPSY
Lobular Neoplasia and Atypical Ductal Hyperplasia
Underestimation of disease occurs when a lesion diagnosed as benign or atypical by core needle biopsy is upgraded to cancer upon surgical excision. Although the upgrade rate of atypical ductal hyperplasia (ADH) at needle biopsy to DCIS at surgical excision has decreased because of larger tissue volumes obtained with 11-gauge vacuum-assisted technique, upgrade rates as high as 21% have been reported.54,55 Even when 100% of the mammographic lesion was removed at stereotactic biopsy, 8% of cases with ADH were still upgraded from ADH to DCIS at excision.54 Therefore, a diagnosis of ADH at core needle biopsy warrants surgical excision.
The diagnosis of lobular neoplasia (lobular carcinoma in situ [LCIS] and atypical lobular hyperplasia) confers an increased risk for developing infiltrating carcinoma (ductal or lobular) in either breast. However, recommendations for surgical excision following a diagnosis of lobular neoplasia on core needle biopsy have not been standardized. Recent studies have suggested that, similar to ADH, surgical excision may be warranted when a diagnosis of lobular neoplasia is obtained at core needle biopsy.56–58 However, as more data is accumulated, the decision to excise may be further refined based on the variety of LCIS and the relationship of the pathology to the radiographic findings that prompted biopsy.
Radial Scars and Papillary Lesions
Radial scars, also termed complex sclerosing lesions when larger than 1 cm, are not infrequently associated with DCIS, tubular carcinoma, ADH, and LCIS.59–61 Therefore, surgical excision is recommended when a diagnosis of radial scar is obtained at core needle biopsy. Although further data are needed, recent studies have suggested that in selected subsets of patients, incidental radial scars found at core needle biopsy can potentially be safely followed rather than excised.62–65
A diagnosis of benign papilloma found at core needle biopsy of a radiographically suspicious mass should be considered discordant and calls for surgical excision. In addition, atypical papillary lesions should be excised.66,67 For benign papillary lesions that are incidental or concordant with the imaging findings, recommendations for imaging follow-up versus excision have not been standardized. A recent study and literature review reports upgrade rates for benign papillomas diagnosed by core biopsy of 5% and 8%.68 However, upgrade rates of benign papillomas diagnosed by large-gauge vacuum core biopsy may be lower.
Cellular Fibroadenomas and Phyllodes Tumors
Phyllodes tumors are relatively uncommon, accounting for less than 1% of all breast neoplasms.69 Although they are most often benign, they can be locally aggressive. An actively growing cellular fibroadenoma may be difficult for the pathologist to distinguish from a phyllodes tumor when sampled by core biopsy. Therefore, surgical excision should be considered when a diagnosis of cellular fibroadenoma is obtained at core biopsy of a mass that is rapidly growing, clinically discordant, or has unusual imaging features.70
1 Rosenberg RD, Yankaskas BC, Abraham LA, et al. Performance benchmarks for screening mammography. Radiology. 2006;241:55-66.
2 American College of Radiology (ACR). Breast Imaging Reporting and Data System Atlas (BI-RADS® Atlas). Reston, Va: American College of Radiology, 2003.
3 Sickles EA. Periodic mammographic follow-up of probably benign lesions: results in 3,184 consecutive cases. Radiology. 1991;179(2):463-468.
4 Report from the Physician Insurers Association of America (PIAA), 2005.
5 Dennis MA, Parker SR, Klaus AJ, et al. Breast biopsy avoidance: the value of normal mammograms and normal sonograms in the setting of a palpable lump. Radiology. 2001;219:186-191.
6 Soo MS, Rosen EL, Baker JA, et al. Negative predictive value of sonography with mammography in patients with palpable breast lesions. AJR Am J Roentgenol. 2001;177:1167-1170.
7 Moy L, Slanetz PJ, Moore R, et al. Specificity of mammography and US in the evaluation of a palpable abnormality: retrospective review. Radiology. 2002;225:176-181.
8 Houssami N, Irwig L, Simpson JM, et al. Sydney breast imaging accuracy study: comparative sensitivity and specificity of mammography and sonography in young women with symptoms. AJR Am J Roentgenol. 2003;180:935-940.
9 Liberman L, Kaplan JB. Percutaneous core biopsy of nonpalpable breast lesions: utility and impact on cost of diagnosis. Breast Dis. 2001;13:49-57.
10 Mainiero MB, Gareen IF, Bird CE, et al. Preferential use of sonographically guided biopsy to minimize patient discomfort and procedure time in a percutaneous image-guided breast biopsy program. J Ultrasound Med. 2002;21:1221-1226.
11 Rubin E, Mennemeyer ST, Desmond RA, et al. Reducing the cost of diagnosis of breast carcinoma: impact of ultrasound and imaging-guided biopsies on a clinical breast practice. Cancer. 2001;91(2):324-332.
12 Parker SH, Jobe WE, Dennis MA, et al. US-guided automated large-core breast biopsy. Radiology. 1993:187.
13 Parker SH, Burbank F, Jackman RJ, et al. Percutaneous large-core breast biopsy: a multi-institutional study. Radiology. 1994:193.
14 Crowe JPJr, Rim A, Patrick RJ, et al. Does core needle breast biopsy accurately reflect breast pathology? Surgery. 2003;134:526-528.
15 Meloni GB, Dessole S, Becchere MP, et al. Ultrasound-guided Mammotome vacuum biopsy for the diagnosis of impalpable breast lesions. Ultrasound Obstet Gynecol. 2001:18.
16 Simon JR, Kalbhen CL, Cooper RA, Flisak ME. Accuracy and complication rates of US-guided vacuum-assisted core breast biopsy: initial results. Radiology. 2000:215.
17 Buchberger W, Niehoff A, Obrist P, et al. Sonographically guided core needle biopsy of the breast: technique, accuracy and indications. Radiology. 2002:42.
18 Crystal P, Koretz M, Shcharynsky S, et al. Accuracy of sonographically guided 14-gauge core-needle biopsy: results of 715 consecutive breast biopsies with at least two-year follow-up of benign lesions. J Clin Ultrasound. 2005:33.
19 Smith DN, Rosenfield Darling ML, Meyer JE, et al. The utility of ultrasonographically guided large-core needle biopsy: results from 500 consecutive breast biopsies. J Ultrasound Med. 2001:20.
20 Smith DN, Christian R, Meyer JE. Large-core needle biopsy of nonpalpable breast cancers. The impact on subsequent surgical excisions. Arch Surg. 1997;132:260.
21 Kaufman CS, Delbecq R, Jacobson L. Excising the reexcision: stereotactic core-needle biopsy decreases need for reexcision of breast cancer. World J Surg. 1998;22:1028.
22 Liberman L, LaTrenta LR, Dershaw DD, et al. Impact of core biopsy on the surgical management of impalpable breast cancer. AJR Am J Roentgenol. 1997:168.
23 Lind DS, Minter R, Steinbach B, et al. Stereotactic core biopsy reduces the reexcision rate and the cost of mammographically detected cancer. J Surg Res. 1998;78(1):23-26.
24 Yim JH, Barton P, Weber B, et al. Mammographically detected breast cancer. Benefits of stereotactic core versus wire localization biopsy. Ann Surg. 1996;223:697-700.
25 Liberman L, Ernberg LA, Heerdt A, et al. Palpable breast masses: is there a role for percutaneous imaging-guided core biopsy? AJR Am J Roentgenol. 2000:175.
26 Lorenzen J, Welger J, Lisboa BW, et al. Percutaneous core-needle biopsy of palpable breast tumors. Do we need ultrasound guidance? Rofo. 2002:174.
27 Hilton SV, Leopold GR, Olson LK, Willson SA. Real-time breast sonography: application in 300 consecutive patients. AJR Am J Roentgenol. 1986:147.
28 Vargas HI, Vargas MP, Gonzalez KD, et al. Outcomes of sonography-based management of breast cysts. Am J Surg. 2004:188.
29 Berg WA, Campassi CI, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology. 2003:227.
30 Buchberger W, DeKoekkoek-Doll P, Springer P, et al. Incidental findings on sonography of the breast: clinical significance and diagnostic workup. AJR Am J Roentgenol. 1999:173.
31 Mendelson EB, Berg WA, Merritt CR. Toward a standardized breast ultrasound lexicon, BI-RADS: ultrasound. Semin Roentgenol. 2001:36.
32 Venta LA, Kim JP, Pelloski CE, Morrow M. Management of complex breast cysts. AJR Am J Roentgenol. 1999:173.
33 Ciatto S, Cariaggi P, Bulgaresi P. The value of routine cytologic examination of breast cyst fluids. Acta Cytol. 1987:31.
34 Hindle WH, Arias RD, Florentine B, Whang J. Lack of utility in clinical practice of cytologic examination of nonbloody cyst fluid from palpable breast cysts. Am J Obstet Gynecol. 2000:182.
35 Smith DN, Kaelin CM, Korbin CD, et al. Impalpable breast cysts: utility of cytologic examination of fluid obtained with radiologically guided aspiration. Radiology. 1997:204.
36 Pisano ED, Fajardo LL, Caudry DJ, et al. Fine-needle aspiration biopsy of nonpalpable breast lesions in a multicenter clinical trial: results from the radiologic diagnostic oncology group V. Radiology. 2001:219.
37 Symmans WF, Cangiarella JF, Gottlieb S, et al. What is the role of cytopathologists in stereotaxic needle biopsy diagnosis of nonpalpable mammographic abnormalities? Diagn Cytopathol. 2001:24.
38 Clarke D, Sudhakaran N, Gateley CA. Replace fine needle aspiration cytology with automated core biopsy in the triple assessment of breast cancer. Ann R Coll Surg Engl. 2001:83.
39 Nath ME, Robinson TM, Tobon H, et al. Automated large-core needle biopsy of surgically removed breast lesions: comparison of samples obtained with 14-, 16-, and 18-gauge needles. Radiology. 1995:197.
40 Berg WA, Krebs TL, Campassi C, et al. Evaluation of 14- and 11-gauge directional, vacuum-assisted biopsy probes and 14-gauge biopsy guns in a breast parenchymal model. Radiology. 1997;205(1):203-208.
41 Kettritz U, Rotter K, Schreer I, et al. Stereotactic vacuum-assisted breast biopsy in 2874 patients: a multicenter study. Cancer. 2004:100.
42 Philpotts LE, Hooley RJ, Lee CH. Comparison of automated versus vacuum-assisted biopsy methods for sonographically guided core biopsy of the breast. AJR Am J Roentgenol. 2003:180.
43 Kettritz U, Rotter K, Schreer I, et al. Stereotactic vacuum-assisted breast biopsy in 2874 patients: a multicenter study. Cancer. 2004:100.
44 Darling ML, Smith DN, Lester SC, et al. Atypical ductal hyperplasia and ductal carcinoma in situ as revealed by large-core needle breast biopsy: results of surgical excision. AJR Am J Roentgenol. 2000:175.
45 Jackman RJ, Burbank F, Parker SH, et al. Atypical ductal hyperplasia diagnosed at stereotactic breast biopsy: improved reliability with 14-gauge, directional, vacuum-assisted biopsy. Radiology. 1997:204.
46 Jackman RJ, Nowels KW, Rodriguez-Soto J, et al. Stereotactic, automated, large-core needle biopsy of nonpalpable breast lesions: false-negative and histologic underestimation rates after long-term follow-up. Radiology. 1999:210.
47 Philpotts LE, Lee CH, Horvath LJ, et al. Underestimation of breast cancer with 11-gauge vacuum suction biopsy. AJR Am J Roentgenol. 2000:175.
48 Philpotts LE, Shaheen NA, Carter D, et al. Comparison of rebiopsy rates after stereotactic core needle biopsy of the breast with 11-gauge vacuum suction probe versus 14-gauge needle and automatic gun. AJR Am J Roentgenol. 1999:172.
49 Liberman L, Kaplan JB, Morris EA, et al. To excise or to sample the mammographic target: what is the goal of stereotactic 11-gauge vacuum-assisted breast biopsy? AJR Am J Roentgenol. 2002:179.
50 Memarsadeghi M, Pfarl G, Riedl C, et al. Value of 14-gauge ultrasound-guided large-core needle biopsy of breast lesions: own results in comparison with the literature. Rofo. 2003:175.
51 Sauer G, Deissler H, Strunz K, et al. Ultrasound-guided large-core needle biopsies of breast lesions: analysis of 962 cases to determine the number of samples for reliable tumour classification. Br J Cancer. 2005:92.
52 Liberman L, Drotman M, Morris EA, et al. Imaging-histologic discordance at percutaneous breast biopsy. Cancer. 2000:89.
53 Lee CH, Philpotts LE, Horvath LJ, Tocino I. Follow-up of breast lesions diagnosed as benign with stereotactic core-needle biopsy: frequency of mammographic change and false-negative rate. Radiology. 1999:212.
54 Jackman RJ, Birdwell RL, Ikeda DM. Atypical ductal hyperplasia: can some lesions be defined as probably benign after stereotactic 11-gauge vacuum-assisted biopsy, eliminating the recommendation for surgical excision? Radiology. 2002;224(2):548-554.
55 Winchester DJ, Bernstein JR, Jeske JM, et al. Upstaging of atypical ductal hyperplasia after vacuum-assisted 11-gauge stereotactic core needle biopsy. Arch Surg. 2003;138:622-623.
56 Lechner MC, Jackman RJ, Brem RF, et al. Lobular carcinoma in situ and atypical lobular hyperplasia at percutaneous biopsy with surgical correlation: a multi-institutional study [abstract]. Radiology. 1999;213(P):106.
57 Arpino G, Allred DC, Mohsin SK, et al. Lobular neoplasia on core-needle biopsy: clinical significance. Cancer. 2004:101.
58 Foster MC, Helvie MA, Gregory NE, et al. Lobular carcinoma in situ or atypical lobular hyperplasia at core-needle biopsy: is excisional biopsy necessary? Radiology. 2004:231.
59 Sloane JP, Mayers MM. Carcinoma and atypical hyperplasia in radial scars and complex sclerosing lesions: importance of lesion size and patient age. Histopathology. 1993;23:225-231.
60 Frouge C, Tristant H, Guinebretiere J-M, et al. Mammographic lesions suggestive of radial scars: microscopic findings in 40 cases. Radiology. 1995;195:623-625.
61 Hassell P, Klein-Parker H, Worth A, Poon P. Radial sclerosing lesions of the breast: mammographic and pathologic correlation. Can Assoc Radiol J. 1999;50:370-375.
62 Philpotts LE, Shaheen NA, Jain KS, et al. Uncommon high-risk lesions of the breast diagnosed at stereotactic core-needle biopsy: clinical importance. Radiology. 2000;216:831-837.
63 Kirwan SE, Denton ERE, Nash RM, et al. Multiple 14G stereotactic core biopsies in the diagnosis of mammographically detected stellate lesions of the breast. Clin Radiol. 2000;55(10):763-766.
64 Brenner RJ, Jackman RJ, Parker SH, et al. Percutaneous core needle biopsy of radial scars of the breast: when is excision necessary? AJR Am J Roentgenol. 2002:179.
65 Cawson JN, Malara F, Kavanagh A, et al. Fourteen-gauge needle core biopsy of mammographically evident radial scars. Is excision necessary? Cancer. 2003;97:345-351.
66 Leung J, Margolin FR, Lester SC, et al. Benign papillary breast lesions diagnosed at large-core needle biopsy: correlation with surgical pathology and clinical outcome [abstract]. AJR Am J Roentgenol. 2002;178:59-60.
67 Agoff SN, Lawton TJ. Papillary lesions of the breast with and without atypical ductal hyperplasia: can we accurately predict benign behavior from core needle biopsy? Am J Clin Pathol. 2004:122.
68 Mercado CL, Hamele-Bena D, Oken SM, et al. Papillary lesions of the breast at percutaneous core-needle biopsy. Radiology. 2006;238(3):801-808.
69 Liberman L, Bonaccio E, Hamele-Bena D, et al. Benign and malignant phyllodes tumors: mammographic and sonographic findings. Radiology. 1996:198.
70 Meyer JE, Smith DN, Lester SC, et al. Large-needle core biopsy: nonmalignant breast abnormalities evaluated with surgical excision or repeat core biopsy. Radiology. 1998:206.
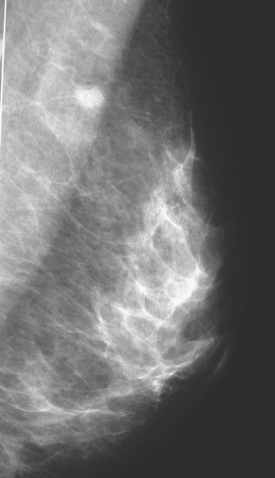
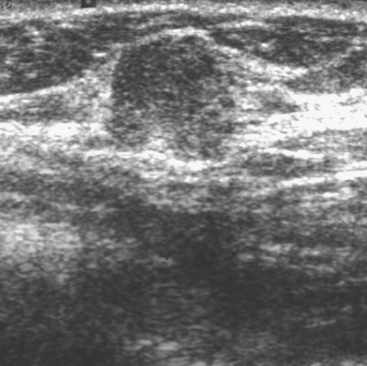
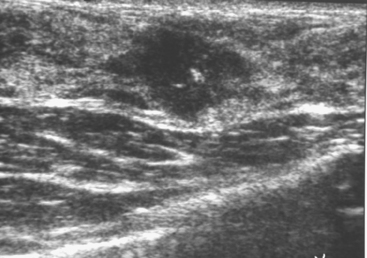
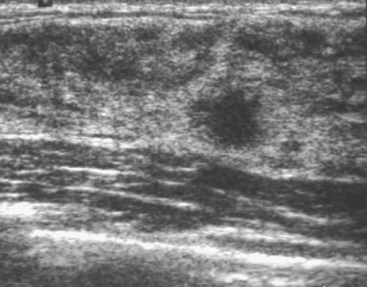
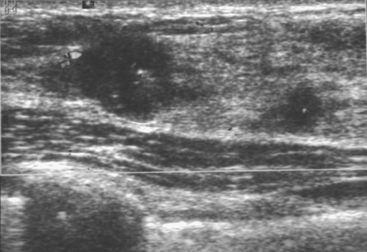
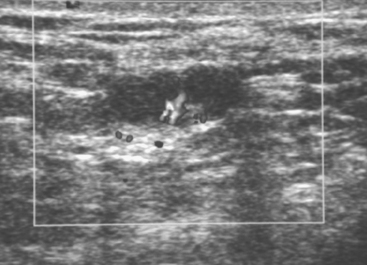
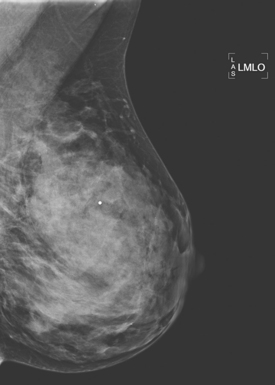
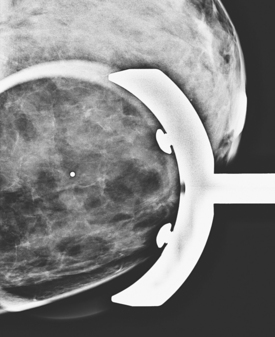
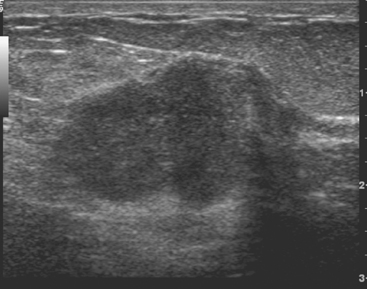
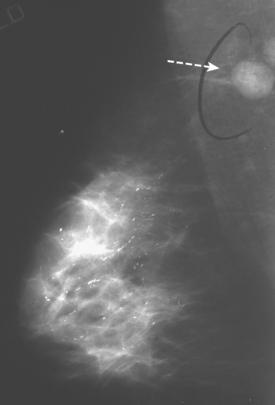
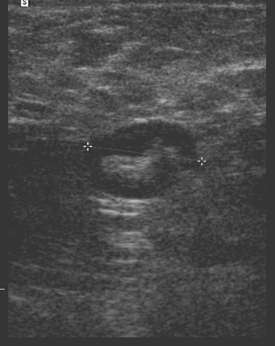
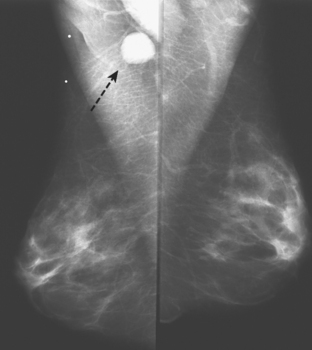
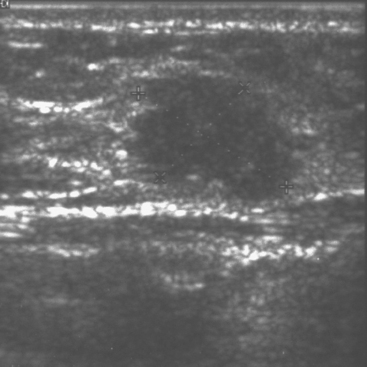
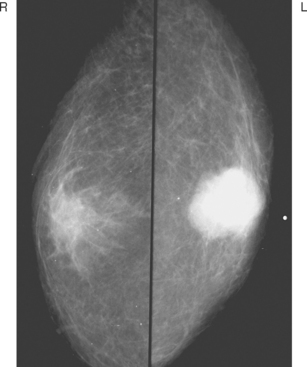
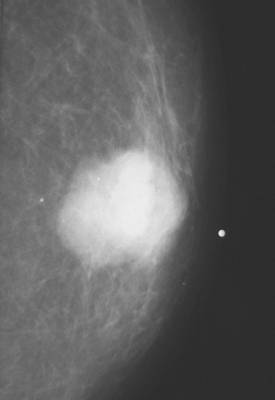
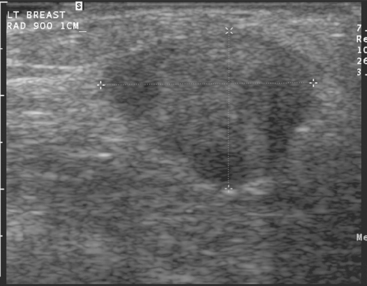
CASE 1 Palpable axillary IDC, presenting as a growing mammographic mass simulating a lymph node
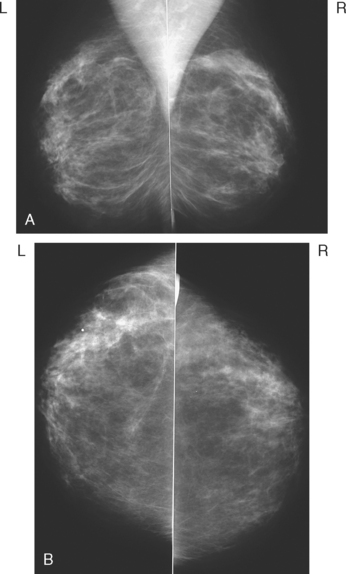
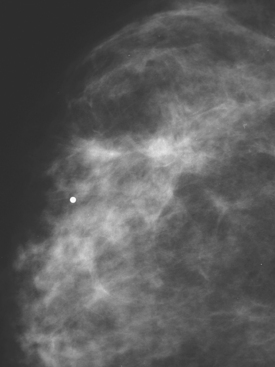
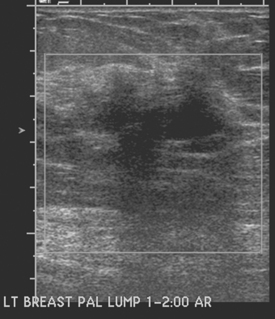
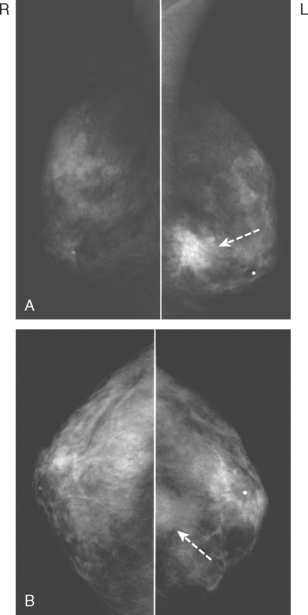
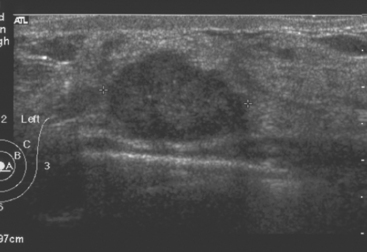
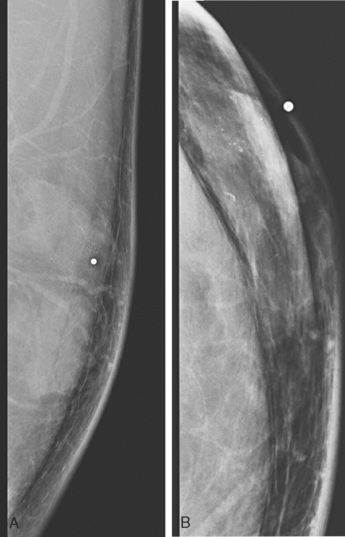
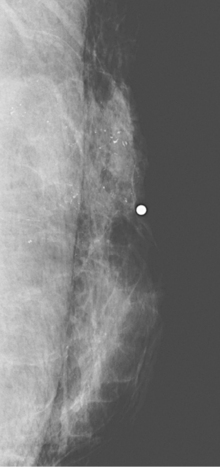
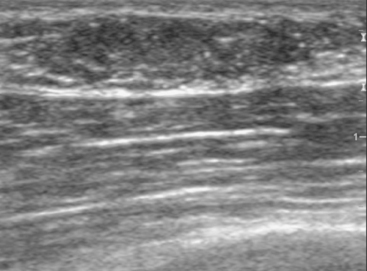
An 82-year-old woman identified a palpable right axillary tail mass. Mammography showed interval growth of a small mass (Figure 1), which had been evaluated 9 months before and was previously thought to be a lymph node (Figure 2). Ultrasound showed a corresponding 1-cm solid mass with lobular margins, with vascularity and no well-defined capsule (Figure 3). A surgical excisional biopsy revealed a 1.3-cm infiltrating ductal carcinoma (IDC) with ductal carcinoma in situ, most of which was within the invasive component. Carcinoma was transected at the anterior margin. The patient elected to undergo simple mastectomy to clear the margins, rather than undergo re-excision and radiation. No residual tumor was identified in the mastectomy specimen. An axillary sentinel lymph node was negative. The final stage was stage I, T1N0, estrogen receptor positive, HER2/neu negative.
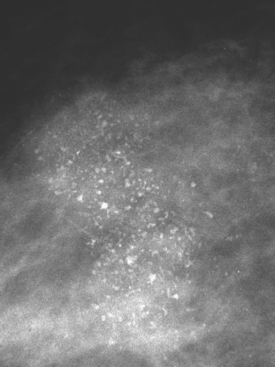
CASE 2 Palpable lump presenting as malignant microcalcifications on mammography
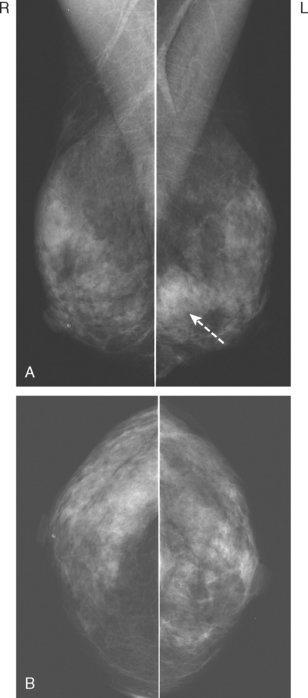
A 43-year-old woman presented with a palpable, painful left upper outer quadrant breast mass. Mammography showed a 3-cm region of suspicious pleomorphic microcalcifications in the corresponding location (Figures 1 and 2). Ultrasound showed no evidence of an invasive component. Stereotactic biopsy confirmed ductal carcinoma in situ (DCIS) with foci of invasion. Lumpectomy and axillary lymph node dissection were performed after needle localization with a single needle. The pathology demonstrated a stage II left breast cancer, T2N2, a 3.5-cm moderately differentiated infiltrating ductal carcinoma with high-grade DCIS, which was estrogen receptor and progesterone receptor positive, HER-2/neu negative, and had extensive lymphovascular invasion and multifocal margin positivity. The sentinel lymph node was positive for tumor, and subsequent axillary dissection confirmed 4 of 14 lymph nodes to be involved. A subsequent quadrantectomy was performed, with pathology showing residual intralymphatic tumor, and a lateral superficial margin was close (<0.5 mm). Chemotherapy on a study protocol and radiation were given, as well as subsequent tamoxifen therapy.
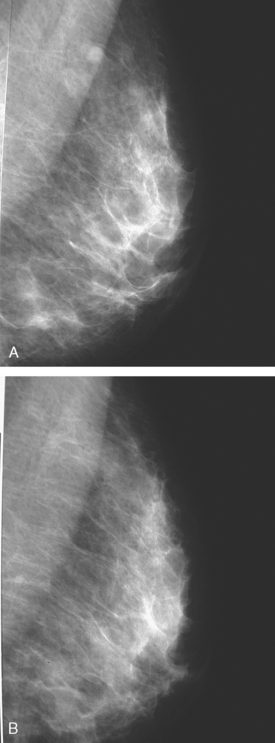
CASE 3 Palpable ILC presenting as architectural distortion
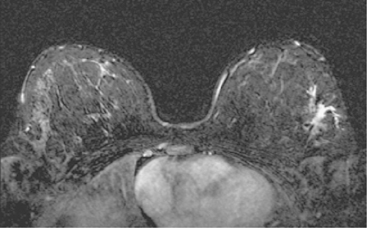
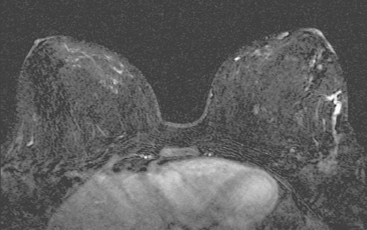
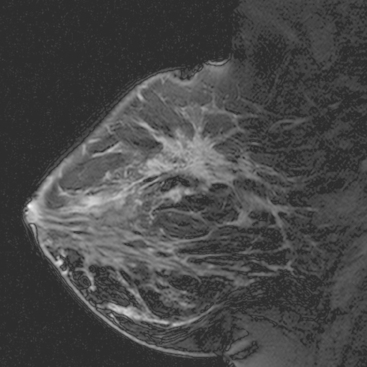
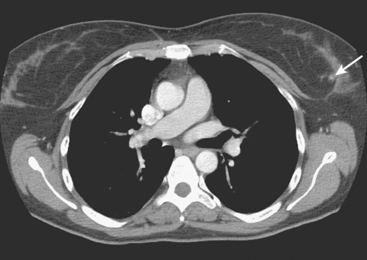
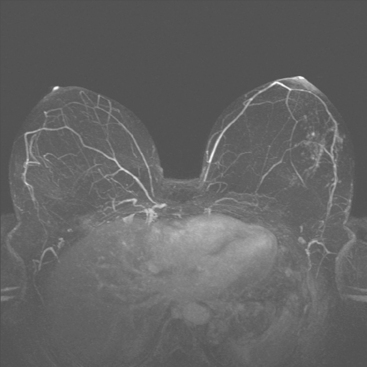
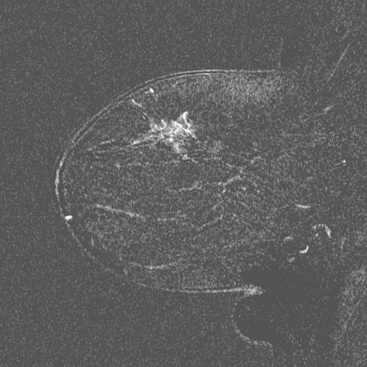
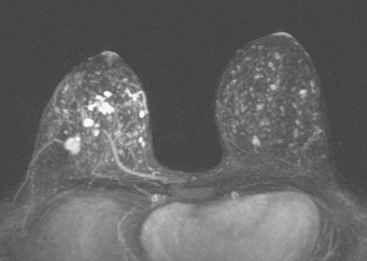
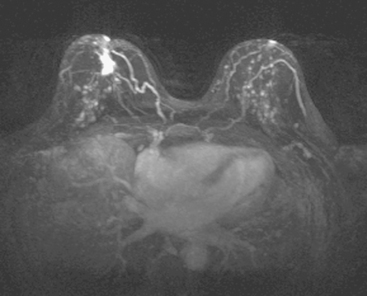
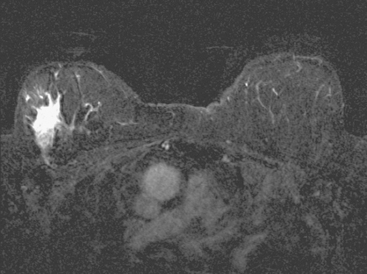
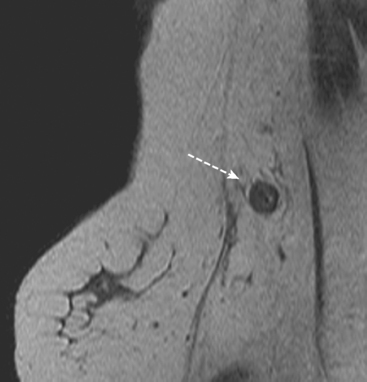
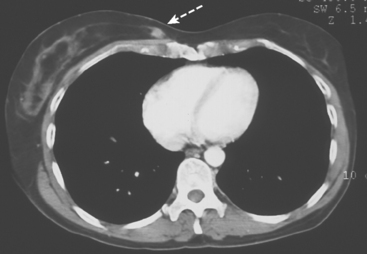
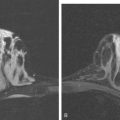
A 47-year-old woman presented for evaluation of a growing palpable lump in the left breast, noted initially 3 to 4 months before. Architectural distortion was noted on mammography at the indicated site (Figures 1 and 2). Ultrasound showed a shadowing, ill-defined mass, with maximal dimension estimated of 4.6 cm (Figure 3). Palpation-guided biopsy by a breast surgeon did not confirm the suspected malignancy, and so an image-guided biopsy was performed stereotactically of the architectural distortion. Because of the ill-defined mammographic margins of the mass, a clip was placed to mark the site of the biopsy. Histology was invasive lobular carcinoma (ILC). MRI was recommended to better delineate the extent of the neoplasm and to evaluate the opposite breast. MRI showed the mass to occupy essentially the entire upper outer quadrant and to consist of irregular, strandy enhancement with architectural distortion (Figures 4, 5, 6, and 7). Strandy extension toward both the nipple and chest wall was noted.
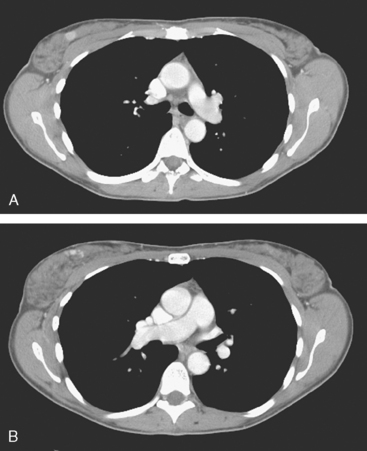
Clinical evaluations of the patient during the course of the workup had noted the large size of the palpable mass (estimated at 3 × 4 cm when initially seen by the breast surgeon, and ranging up to 8 × 7 cm when seen by the medical oncologist, after two biopsies), and the consensus was that she was not a candidate for breast conservation. However, the patient was disinclined to undergo mastectomy and elected to undergo neoadjuvant chemotherapy. Her staging evaluation included enhanced body CT scans and positron emission tomography (PET)/CT (Figures 8 and 9). The breast mass showed only modest increased metabolic activity, and no other abnormalities were identified.
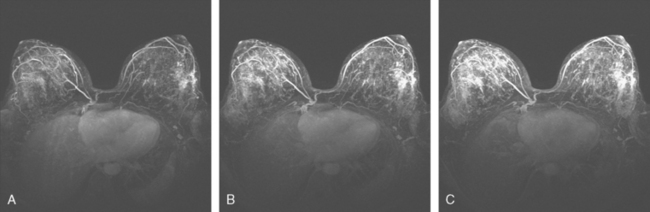
During the course of the eight rounds of neoadjuvant chemotherapy, the response of the ILC mass was periodically assessed with repeat breast MRI (Figures 10, 11, 12, 13, and 14).
TEACHING POINTS
This case provides multiple interesting takeoff points for discussion. Many typical features of ILC are depicted. The mass presented as a palpable lump, which was large clinically at presentation. Its major mammographic manifestation was architectural distortion, and it was better seen on the CC projections, which generally achieve better compression. On ultrasound, it was well seen as ill-defined hypoechogenicity, with irregular margins and shadowing. However, the margins and its size were difficult to define precisely on both mammography and ultrasound. Although ultrasound guidance for performance of image-guided biopsy is preferred by many when a lesion is seen both mammographically and sonographically, there was some concern in this case about using ultrasound for biopsy guidance because of poor margin definition. Stereotactic guidance was utilized and confirmed the diagnosis of ILC. Because of the poor mammographic definition of the mass margins, a clip was placed to mark the biopsy site. With large, well-seen lesions, clip placement after biopsy is not universal. However, with increasing neoadjuvant use of preoperative chemotherapy and the attendant possibility that a mass may disappear with treatment, the universal use of clip placement after biopsy may need to be considered. Often, it is not known at the time of a diagnostic image-guided biopsy whether a patient will undergo neoadjuvant chemotherapy, but with larger lesions and a patient highly motivated for breast conservation, this possibility should be considered and a clip placed while there is access and a visible lesion. In this case, the mass did not resolve completely and ultimately this patient elected mastectomy.
MRI was useful in this case to most precisely estimate the size of the ILC. The mass was noted clinically by all to be large, but estimates of its size increased over the course of staging evaluations. By the time the medical oncologist saw the patient, she had undergone two percutaneous biopsies, and so it was not clear whether the mass growth was due to biopsy-related bleeding. The MRI showed that the mass was sizable, even larger than the most generous clinical estimates, and provided the best delineation of measurable disease for follow-up during the course of neoadjuvant chemotherapy. MRI also is useful in such cases for evaluation of the opposite breast, given the relatively high propensity of ILC for bilaterality.
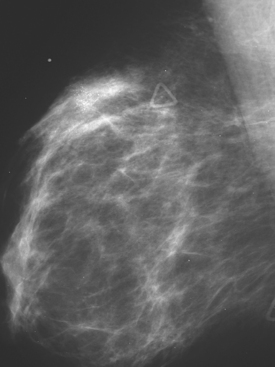
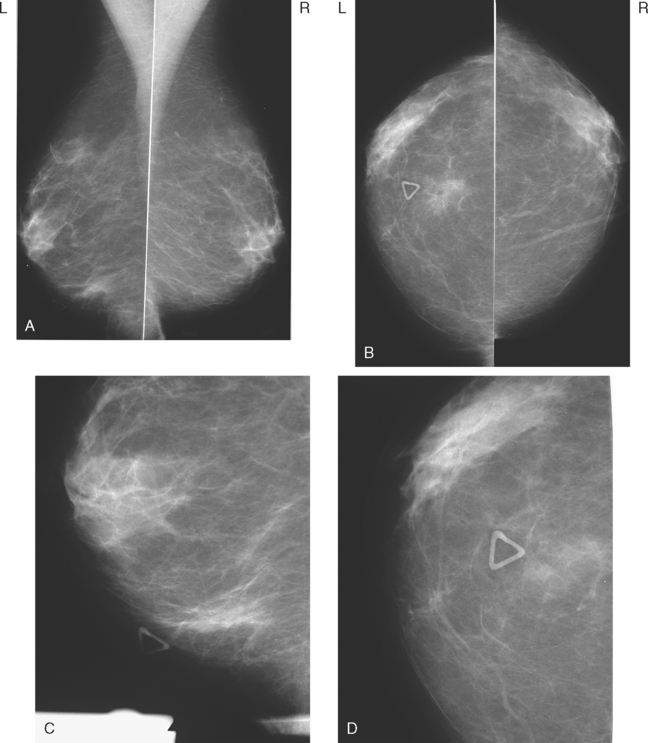
CASE 4 Palpable lump presenting as mammographic architectural distortion with microcalcifications
A 47-year-old woman with very dense breasts presented for evaluation of a newly self-noted right 12-o’clock palpable lump. Her routine screening mammogram performed 5 months before was negative (Figure 1). A diagnostic mammogram and ultrasound were performed to evaluate the palpable mass. On magnification spot compression of the mass, architectural distortion, and faint microcalcifications were appreciated, which appeared to be new (Figure 2). Ultrasound of the palpable mass showed a suspicious solid corresponding mass with angular margins and associated calcifications (Figure 3). Nearby (about 2 cm away) was a second, smaller, equally suspicious mass at 11 o’clock (Figures 4 and 5). In the axilla, a lymph node with an abnormally thickened and nodular cortical mantle was identified (Figure 6). These abnormalities were sampled with ultrasound guidance. The axillary fine-needle aspiration obtained groups of malignant cells, compatible with metastatic carcinoma of breast primary origin. The palpable mass at 12 o’clock was proved to be an infiltrating ductal carcinoma. The smaller, 11-o’clock mass was technically difficult to sample because of extreme breast density and proximity to the chest wall, and sampling yielded benign fibrotic breast tissue. Sampling error at this level was suspected prospectively.
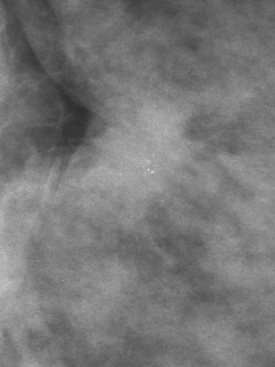
FIGURE 2 Magnification spot compression (CC projection) of the palpable right 12-o’clock breast lump shows retraction of the posterior glandular margin, with subtle architectural distortion and evidence of a mass as a double density, with associated microcalcifications. This was not seen on the screening mammogram performed 5 months earlier (see Figure 1).
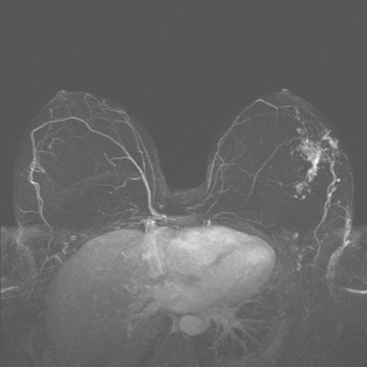
Because of the marked breast density, and proven node-positive disease, breast MRI was recommended preoperatively to supplement this patient’s local staging. This suggested that the right breast cancer was extensive (Figure 7), with suspicious enhancement in the right upper inner and right lower inner quadrants, in addition to enhancing masses at the 11- and 12-o’clock positions. The palpable known cancer at 12 o’clock was accompanied by enhancing small suspected satellite lesions. The patient’s staging was completed with performance of chest, abdomen, and pelvis enhanced CT, and positron emission tomography (PET)/CT (Figure 8), which did not suggest distant metastatic disease. Based on the MRI findings, the patient did not seem a suitable breast conservation candidate, and mastectomy and axillary dissection were performed.
The mastectomy pathology demonstrated the 12-o’clock mass to be a 1.5-cm infiltrating ductal carcinoma, and multiple foci of DCIS (ranging up to 1 cm in size) were identified, both on the periphery of the invasive cancer and in the upper inner quadrant. In addition, LCIS was found in the upper inner and lower inner quadrants. One of 12 lymph nodes showed metastatic carcinoma, with a 0.8-cm tumor deposit and focal extracapsular extension. The tumor was estrogen receptor and progesterone receptor positive and HER-2/neu negative.
TEACHING POINTS
This patient has extremely dense breast tissue. Ultrasound proved a valuable supplement to the mammographic evaluation, but even in combination, these two modalities underestimated the extent of disease in this patient’s right breast. Breast density is not a limiting factor for breast MRI, making it a valuable adjunct in the evaluation of such patients. Hormone-related enhancement can be limiting, making it advisable to schedule examinations when possible in the second week of the menstrual cycle (ideal is days 7 to 10). This rule is most readily enforceable when doing more elective studies, such as follow-up studies, or surveillance of high-risk patients. With new diagnoses of breast cancer in premenopausal patients, expediency in the workup will generally have to take precedence over optimal timing. This may make interpretation more challenging because there will often be many benign foci of enhancement to “read around.” Use of a bilateral axial technique provides a convenient built-in control in the form of the other breast, which will be screened simultaneously. In breasts of similar mammographic density, a striking difference in the number of foci and intensity of enhancement can be significant. Such findings are seen here in the right breast, where there are multiple small, intensely enhancing masses, too large to be considered fibrocystic foci of enhancement, and accordingly suspicious.
CASE 5 Palpable lump presenting as growing amorphous mammographic asymmetry
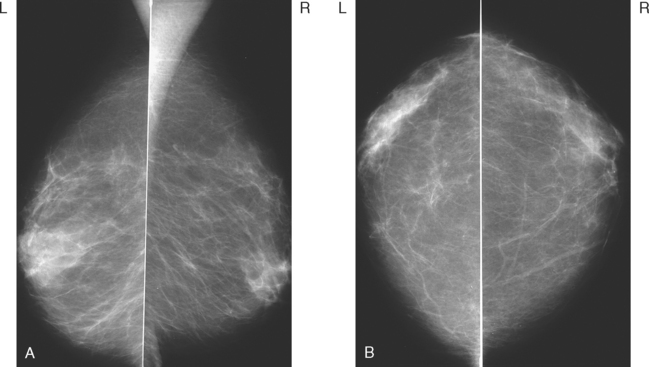
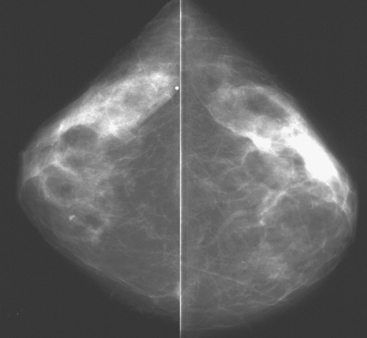
A 61-year-old woman was noted to have a palpable left breast mass on clinical examination by her physician. It was described as a 1-cm, irregular, firm, and mobile mass. Mammography showed amorphous parenchyma-like density at the corresponding level (Figure 1). Subsequent comparison showed that the asymmetry had been developing over the preceding years (Figures 2 and 3). The patient was seen for surgical consultation, and a palpation-guided fine-needle aspiration was performed. Atypical cells were obtained. An excisional biopsy confirmed a stage I, T1N0, 1.3-cm, welldifferentiated invasive ductal carcinoma, with positive margins. Re-excision and sentinel node procedure followed, with pathology showing no residual tumor and negative sentinel nodes. External-beam radiation therapy with a boost to the lumpectomy bed was also given.
TEACHING POINTS
The mammograms showed the breasts to be largely fatty, with a small amount of retroareolar density. In the inferior left breast, at the site of the lump indicated by the patient, there was amorphous, tissue-like density, with no architectural distortion or other concerning features. However, comparison showed that the asymmetry had been developing over the preceding years.
CASE 6 Palpable lump presenting as developing mammographic density
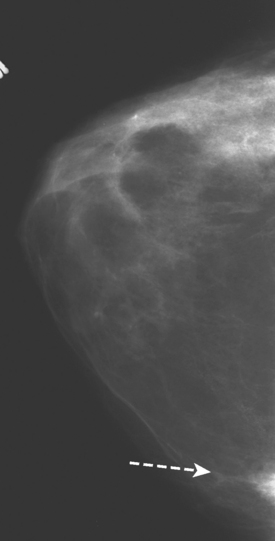
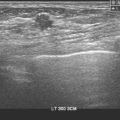
A 48-year-old woman presented with a palpable mass in the lower inner left breast. Routine mammographic views demonstrated a large dense mass at the site of the palpable finding (Figures 1 and 2). Ultrasound confirmed the presence of an irregular solid mass (Figure 3). Ultrasound-guided core needle biopsy yielded a diagnosis of low-grade invasive ductal carcinoma.
CASE 7 Large, palpable, mammographically occult invasive carcinoma
A 49-year-old woman presented with a new palpable mass in the medial left breast. Mammographic evaluation showed no corresponding abnormality (Figures 1, 2, and 3). Ultrasound demonstrated a large hypoechoic solid mass, corresponding to the palpable finding, as well as a small adjacent satellite lesion (Figures 4 and 5). Subsequent ultrasound-guided core needle biopsy yielded a diagnosis of high-grade invasive ductal carcinoma. The large cancer is easily seen on a preoperative MRI examination (Figure 6).
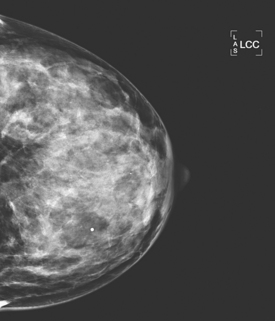
FIGURE 2 Left CC mammographic view. No mass is seen at the level of the palpable lump on either view.
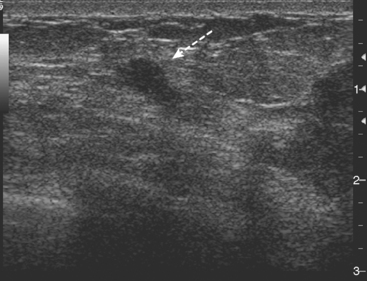
FIGURE 5 Ultrasound also showed a 5-mm satellite lesion (arrow) adjacent to the index lesion (to the right).
TEACHING POINTS
This case illustrates that even large cancers may be occult on mammography, depending on location and the density of the patient’s breast tissue. Both diagnostic mammography and targeted ultrasound should be used in the imaging evaluation of a palpable mass in a woman with dense breasts. Using this combined approach, mammography will permit detection of almost all cancers that display visible calcifications, as well as many noncalcified cancers. Noncalcified cancers that are obscured by dense fibroglandular tissue at mammography can usually be identified on ultrasound. In women with dense breasts and a palpable mass, the likelihood of malignancy when both mammography and ultrasound are negative is about 1% to 5%. This case also demonstrates the importance of evaluating the tissue surrounding a suspicious finding for adjacent satellite lesions. By identifying any satellite lesions, the extent of the disease can be better defined. If conservative therapy is performed, needle localization to include these satellite lesions will improve the probability of obtaining clear margins at surgery.
Dennis MA, Parker SR, Klaus AJ, et al. Breast biopsy avoidance: the value of normal mammograms and normal sonograms in the setting of a palpable lump. Radiology. 2001;219:186-191.
Durfee SM, Selland DG, Smith DN, et al. Sonographic evaluation of clinically palpable breast cancers invisible on mammography. Breast J. 2000;6:247-251.
Houssami N, Irwig L, Simpson JM, et al. Sydney breast imaging accuracy study: comparative sensitivity and specificity of mammography and sonography in young women with symptoms. AJR Am J Roentgenol. 2003;180:935-940.
Moy L, Slanetz PJ, Moore R, et al. Specificity of mammography and US in the evaluation of a palpable abnormality: retrospective review. Radiology. 2002;225:176-181.
Soo MS, Rosen EL, Baker JA, et al. Negative predictive value of sonography with mammography in patients with palpable breast lesions. AJR Am J Roentgenol. 2001;177:1167-1170.
Weinstein SP, Conant EF, Orel SO, et al. Retrospective review of palpable breast lesions after negative mammography and sonography. J Womens Imaging. 2000;2:15-18.
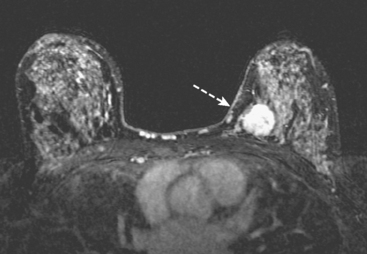
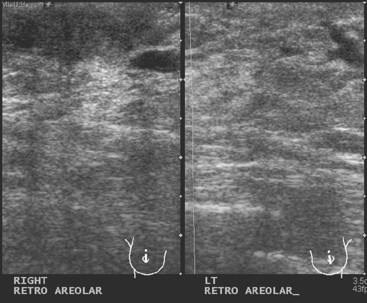
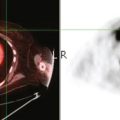
CASE 8 Breast cancer involving the nipple-areolar complex, not identified on conventional imaging, demonstrated by MRI
A 68-year-old woman noted several months of change in the right nipple-areolar complex, with tenderness. Mammographic and sonographic evaluations were considered negative, although asymmetry was noted on ultrasound of the retroareolar area compared with the opposite side (Figures 1 and 2). It was thought to be within normal limits, and a referral for breast surgical evaluation was made. Retraction and palpable hard nodularity were noted on physical examination by the breast surgeon, who performed a palpation-guided core needle biopsy. This confirmed infiltrating ductal carcinoma (IDC).
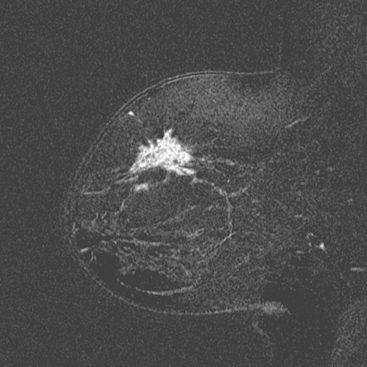
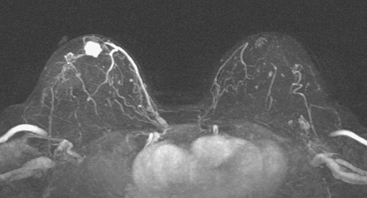
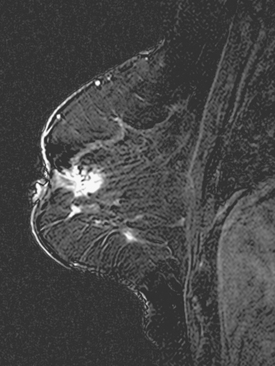
A breast MRI readily demonstrated an intensely enhancing, irregularly shaped subareolar mass at the level in question (Figure 3).
The patient was treated surgically with a mastectomy, which demonstrated a stage IIB, T2N1, estrogen receptor–positive, HER-2/neu negative IDC. Chemotherapy was interrupted after four cycles because of reactivation of chronic hepatitis B, and the patient was placed on anastrozole (Arimidex). Chest wall radiation was also performed.
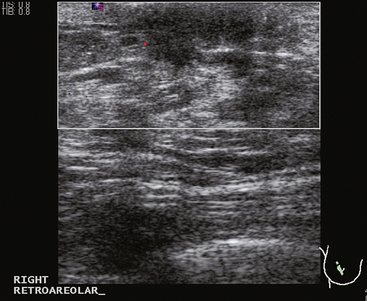
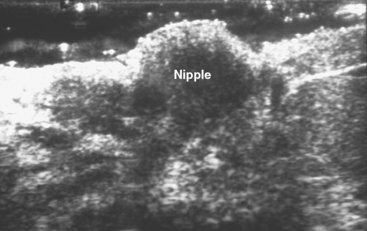
CASE 9 Mammographically occult retroareolar breast cancer presenting as nipple retraction
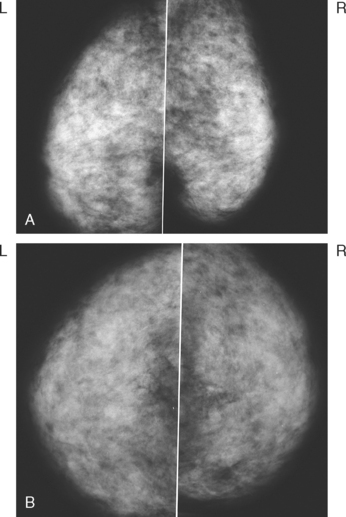
A 72-year-old woman presented for evaluation of right nipple change, noted for several years, but pronounced in the last year. A mammogram performed 2 months earlier was negative (Figure 1). Retraction of the nipple-areolar complex was confirmed on physical examination, with an area of irregular firmness on palpation at 1 o’clock. A palpation-guided core needle biopsy yielded only benign tissue and did not confirm the clinically suspected breast cancer. Sonographic examination was undertaken next and was negative (Figure 2). Breast MRI was obtained and demonstrated an irregular, intensely enhancing retroareolar mass, measuring up to 2.5 cm (Figures 3, 4, and 5). Repeat breast sonography (see Figure 2), with the MRI information, identified a 9-mm, irregularly shaped, hypoechoic retroareolar breast mass, which was successfully sampled with ultrasound guidance, confirming infiltrating ductal carcinoma (IDC).
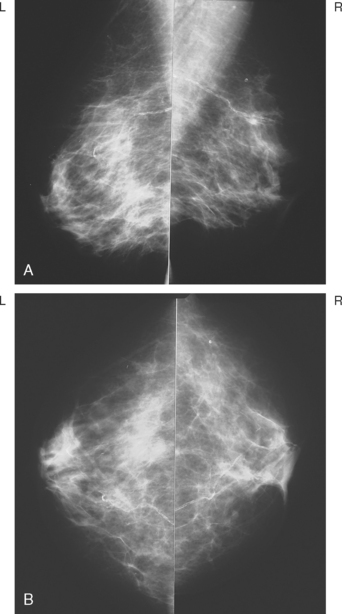
FIGURE 1 MLO (A) and CC (B) mammograms show moderate parenchymal density and no definite abnormality.
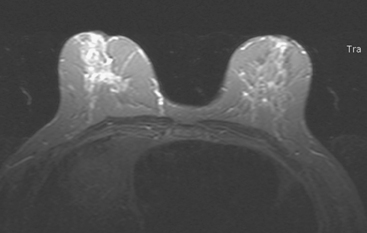
FIGURE 4 Axial short tau inversion recovery (STIR) image at the same level as Figure 3, for correlation.
The patient was treated with a mastectomy and sentinel lymph node sampling. The specimen showed a 2.5-cm IDC, with high-grade ductal carcinoma in situ involving 25% of the tumor, estrogen receptor positive, HER-2/neu negative, with clear margins and no angiolymphatic involvement. Three sentinel lymph nodes were all negative. Final stage was IIA, T2N0M0. The patient was placed on tamoxifen.
TEACHING POINTS
Similar to Case 8 in this chapter, the nipple-areolar complex proved in this case to be a breast imaging blind spot. At times, breast cancers presenting as nipple changes or retraction can be more clinically compelling than the imaging findings. The convergence of ductular structures at the nipple-areolar complex makes this region difficult to evaluate mammographically, and it can be challenging to overcome the dense shadowing that can normally emanate from the nipple-areolar complex to adequately assess this region sonographically. In such a case, with high clinical suspicion, an inconclusive biopsy and falsely reassuring imaging, the unambiguous breast MRI provides confirmation that there is a breast cancer underlying the clinical changes and serves as a roadmap for repeat ultrasound evaluation and biopsy with ultrasound guidance.
CASE 10 Importance of clear communication and accurate history; inaccurate history of biopsy “scar” leads to near-miss of a spiculated cancer
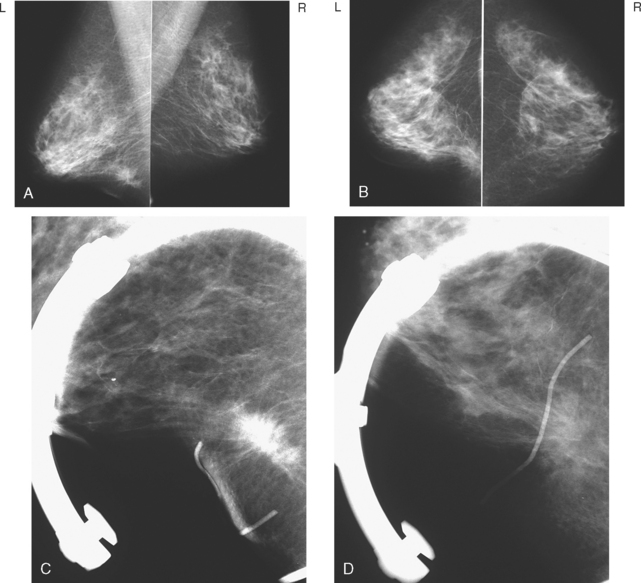
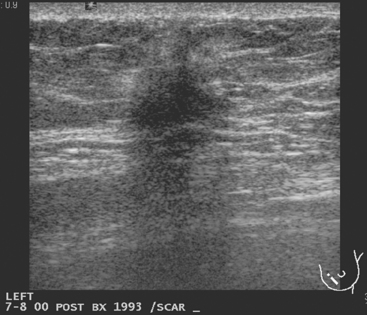
A 67-year-old woman was referred for breast imaging evaluation of a left breast palpable abnormality noted by her physician. However, the location of the area of concern was not indicated by the physician, and the patient did not seem to be aware of the clinical question. She did indicate to the technologist that she had had a previous benign biopsy. The technologist diagrammed a linear surgical scar in the left lower inner quadrant, and to physical inspection, there was a linear skin indentation at this level. Mammograms obtained with a scar marker in place showed a dense “scar” at the marked level, with architectural distortion and spiculation (Figure 1). The mammogram interpreter thought the scar was more prominent than on prior examinations and recommended ultrasound for further evaluation. The ultrasound technologist reported that she could see shadowing from the scar, but no other abnormalities (Figure 2). On physician real-time evaluation, the patient’s history was reviewed, and it became apparent that the patient’s prior biopsy had been stereotactic, and that she had never had breast surgery before! With this information, the presumed scar assumed increased significance as a spiculated, highly suspicious, probable breast cancer. With the room lights on, the linear scar diagrammed by both the mammographic and ultrasound technologists was revealed to be a linear pucker in the patient’s skin due to retraction by the cancer.
TEACHING POINTS
This case could have had a disastrous outcome because of the incomplete and misleading history. From the referral, which indicated a left breast abnormality, but not the location, to the patient’s incomplete knowledge of her physician’s concerns, this case was plagued by poor communication. The patient did indicate to the technologist her history of a prior benign breast biopsy. The technologist may have mistaken the linear skin retraction for a scar site, or been misled by the patient’s description of her prior biopsy into diagramming a surgical scar site on her history form and marking it on her films. In any case, misinformation was conveyed to the interpreting mammographer. If the cancer was changing more slowly, this might have been passed off as the surgical scar purported by the history. The ultrasound technologist was also misled by the history from the mammogram. Fortunately for the ultrasound interpreter, verifying the history by phrasing the questions differently uncovered the confusion. Although in the same quadrant, her prior biopsy had been stereotactic (reports were subsequently made available from another facility, verifying this), and she had never had breast surgery. At that point, the true significance of what had been regarded as scar became abundantly apparent.
CASE 11 Axillary nodal presentation of ILC, primary occult on conventional imaging, found by MRI
A 59-year-old woman presented to her physician with a new palpable right axillary mass. Palpation-directed fine-needle aspiration of the axillary mass revealed metastatic adenocarcinoma. Physical exam of the right breast was unremarkable except for pain noted by the patient in the lateral aspect of the right breast. Mammographic evaluation showed the enlarged right axillary node but no primary breast carcinoma (Figure 1). Ultrasound was also performed of the right breast but failed to show a breast mass (Figures 2 and 3). MRI was obtained to search for a primary breast carcinoma. The MRI demonstrated a large area of abnormal enhancement in the lateral right breast that proved on biopsy to be invasive lobular carcinoma (ILC) (Figures 4 and 5).
TEACHING POINTS
This case illustrates the use of MRI in the evaluation of patients presenting with an axillary metastasis of unknown origin. If conventional imaging by mammography and ultrasonography fails to detect a primary breast cancer, MRI can be useful. Orel and colleagues observed that MRI identified the primary breast carcinoma in 86% of 22 women studied with axillary nodal presentation of breast cancer. A review of six studies suggests that MRI may have a sensitivity as high as 94% in the detection of occult breast malignancy in patients with axillary metastasis.
Blue Cross Blue Shield (BCBSA), Technology Evaluation Center (TEC). Breast MRI for detection or diagnosis of primary or recurrent breast cancer. TEC Assessment Program. Chicago, IL: BCBSA; April 2004;19. (1)
Orel SG, Weinstein SP, Schnall MD, et al. Breast MR imaging in patients with axillary node metastases and unknown primary malignancy. Radiology. 1999;212:543-549.
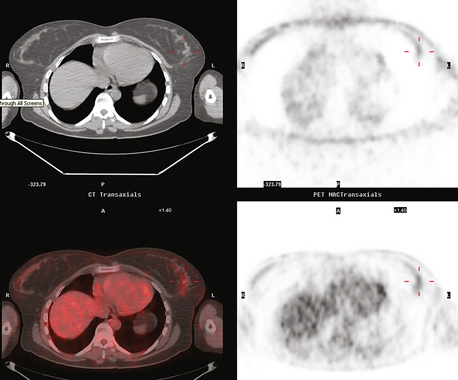
CASE 12 Axillary nodal presentation with negative mammogram, primary found on PET*
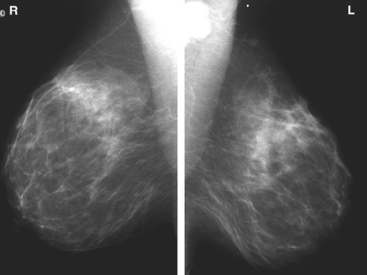
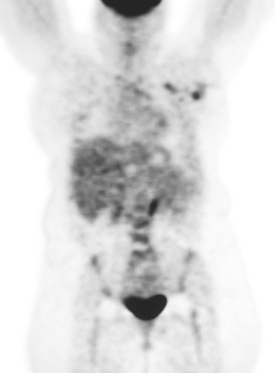
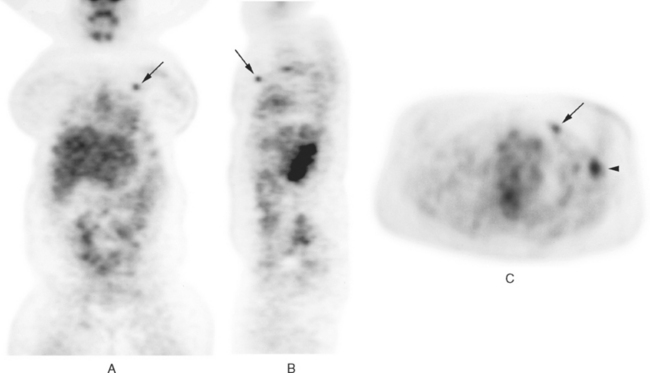
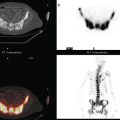
A 39-year-old woman presented with left breast pain and axillary adenopathy. Mammography noted the enlarged lymph nodes in the axilla but did not identify a source within the breast (Figure 1). The left axilla was sampled, and metastatic carcinoma compatible with a breast primary was confirmed. She was referred for positron emission tomography (PET) to search for an unknown primary lesion.
PET imaging showed three hypermetabolic foci in the left axilla, corresponding to the known residual palpable lymph nodes (Figure 2). In addition, a separate, small, hypermetabolic focus was identified in the upper inner quadrant of the left breast (Figure 3). The mammogram was re-reviewed with this information, and a neodensity was noted in the medial breast compared with the prior year’s study (Figure 4). Further mammographic and ultrasonographic workup confirmed the abnormality (Figures 5, 6, and 7). Planned mastectomy was deferred in favor of core needle biopsy of the suspected primary lesion. Poorly differentiated invasive ductal carcinoma was confirmed. The patient’s management was changed from mastectomy to lumpectomy. At pathology, a 1.5-cm poorly differentiated invasive ductal carcinoma was identified, and 3 of 12 lymph nodes from axillary dissection were positive for malignancy. The largest lymph node measured 2.1 cm. Further therapy was undertaken with adjuvant chemotherapy and radiation.
TEACHING POINTS
PET imaging was the initial study to locate this patient’s breast carcinoma primary lesion. Overlooked initially on mammography, the addition of the PET information enabled the mammographic workup to be redirected and the lesion successfully localized and sampled. The PET information also changed this patient’s management. Until the lesion was confirmed on mammographic and ultrasonographic workup, it was considered unable to be localized, and the patient was slated for mastectomy without consideration of breast conservation therapy. If the lesion had not been confirmed on the additional breast imaging workup, enhanced breast MRI could have been considered as an appropriate next step in the imaging pursuit of this highly persuasive PET scan abnormality.
CASE 13 Axillary nodal presentation with initially negative mammogram, medial primary found on CT
A 52-year-old woman presented to her physician with a palpable right axillary lymph node. Fine-needle aspiration of the lymph node revealed an adenocarcinoma. To search for a primary breast carcinoma as the source of the right axillary metastasis, a mammogram was performed. The mammogram showed the abnormal right axillary adenopathy, but no evidence of a primary breast carcinoma (Figures 1 and 2). The patient underwent subsequent staging CT scan of the thorax. A small enhancing lesion was seen on the CT scan in the far medial right breast (Figure 3). Additional mammographic views were performed, including a medial exaggerated CC view (Figure 4). At the far posteromedial breast, a small irregular mass was identified, corresponding to the lesion on CT. Targeted ultrasound demonstrated a solid mass (Figure 5). Subsequent core needle biopsy confirmed an intermediate-grade invasive ductal carcinoma. The patient was treated with lumpectomy, axillary node dissection, and chemotherapy.
CASE 14 Male breast cancer and gynecomastia
A 70-year-old man presented with a palpable mass in the subareolar left breast. Standard mammographic views showed a dense mass in the subareolar region, corresponding to the palpable lump (Figures 1 and 2). In the contralateral breast, gynecomastia was noted. Ultrasound confirmed the palpable finding in the left breast to be a solid, lobular mass (Figure 3). Subsequent core needle biopsy confirmed an invasive ductal carcinoma.
TEACHING POINTS
Male breast cancer is uncommon. It accounts for less than 1% of all breast cancer. Family history is a known risk factor for male breast cancer. Men with a first-degree relative with breast cancer have up to a 4 times increased risk for breast cancer. Most male breast cancers are hormone sensitive, and estrogen is implicated as an etiologic agent in some cases. Known risk factors include BRCA2 gene mutation, Klinefelter’s syndrome (extra X chromosome, associated with testicular insufficiency and gynecomastia), and radiation exposure at a young age. Other risk factors include age, estrogen therapy for prostate cancer or gender reassignment, diseases associated with hyperestrogenism (e.g., liver disease), and conditions resulting in androgen deficiency (testicular dysfunction). Although a correlation between gynecomastia and male breast cancer has been suspected, with up to 40% of male breast cancer patients having gynecomastia, histologic progression from gynecomastia to cancer has never been demonstrated.
CASE 15 Male breast cancer with microcalcifications
A 42-year-old man presented with a palpable mass in the upper outer left breast. Mammographic views showed an area of asymmetric density with associated microcalcifications in the upper outer left breast in the area of the palpable lump (Figures 1 and 2). Ultrasound evaluation identified a solid mass corresponding to the palpable mass (Figure 3). Subsequent core needle biopsy revealed an invasive ductal carcinoma and associated ductal carcinoma in situ.
* Case from: Kipper MS, Tartar M. Case 3. In Clinical Atlas of PET. Philadelphia: Saunders, 2004.