3 Evaluation of the Patient with Diastolic Dysfunction
Restrictive Cardiomyopathy
Background
| Site of Involvement | Classification |
|---|---|
| Myocardium | Noninfiltrative |
TABLE 3-2 OVERALL ECHOCARDIOGRAPHIC APPROACH FOR ASSESSING DIASTOLIC DYSFUNCTION
| Modality | View/Technique | Findings |
|---|---|---|
| 2D | Apical, parasternal, subcostal | |
| Pulsed wave Doppler | 4-chamber: Ultrasound beam aimed at an angle of 20 degrees laterally to apex, and the sample volume between the tips of the mitral valve leaflets | |
| Suprasternal short-axis | ||
| Subcostal | ||
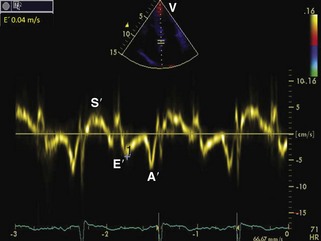
| Tissue Doppler imaging | 4-Chamber: 2-mm sample volume at the medial mitral annulus with the Doppler beam parallel to the longitudinal movement. Gain settings and wall filters should be low and velocity scale expanded. | Varying E, E/E patterns (see text for details) |
TABLE 3-3 Approaches and Findings in RCM
| Modality | General Findings | Specific Findings |
|---|---|---|
| Chest radiograph | Normal-sized heart. Dilated atria. Signs of pulmonary congestion or interstitial edema. Pleural effusion may occur. |
Mediastinal lymphadenopathy; pulmonary parenchymal disease in sarcoidosis. |
| Electrocardiogram | Nonspecific ST- and T-wave abnormalities. Conduction abnormalities. Same chamber and wall dimensions and functions as in echocardiography. |
Low voltage in precordial leads is seen in ~50% of patients with amyloidosis with cardiac involvement. Pseudo-infarct pattern (QS wave in consecutive leads) is seen in ~50% of patients with cardiac amyloidosis. Fibrosis caused by cardiac sarcoidosis can be detected with late gadolinium enhancement. Trilaminar appearance (normal myocardium, thickened fibrotic endocardium, and overlying thrombus) may be detectable in endomyocardial diseases. Global reduction in T2* cardiac tissue is commonly seen in hemochromatosis. |
| CT | Same chamber and wall dimensions and functions as in echocardiography. | |
| Biposy | In most cases nonspecific. | Apple-green birefringence, electron microscopy findings in amyloidosis. |
| Radionuclide imaging | Mainly nonspecific findings in RCM. | With increased cardiac involvement in amyloidosis, Tc-99m labeled tracers are detectable. |
| Cardiac catheterization | Nonspecific findings of diastolic dysfunction (increased diastolic pressures, right-sided square root sign, and often LVEDP ≥5 mm Hg greater than RVEDP). |
LVEDP, LV end-diastolic pressure; RVEDP, RV end-diastolic pressure; Tc-99m, 99-mtechnetium.
Echocardiographic Approach
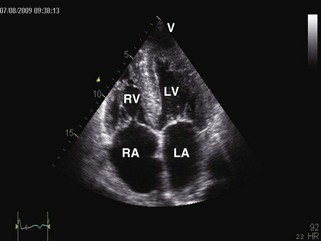
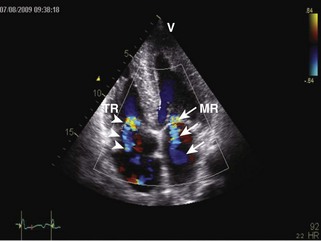
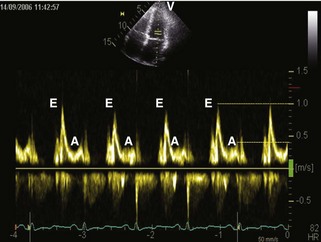
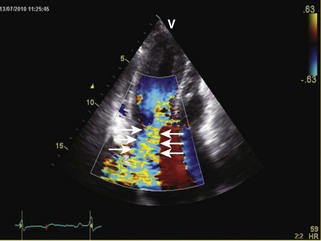
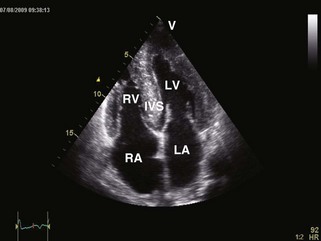
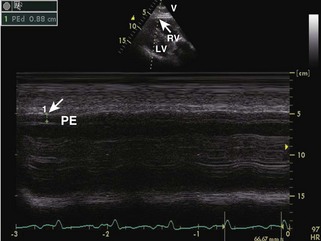
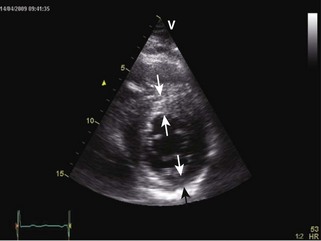
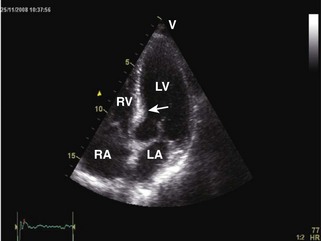
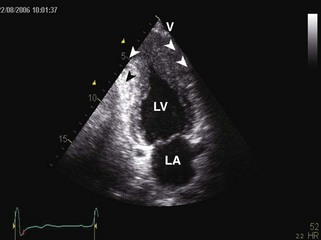
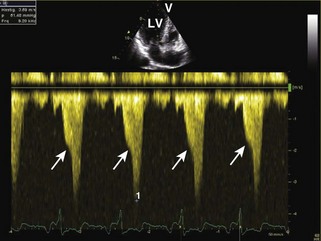
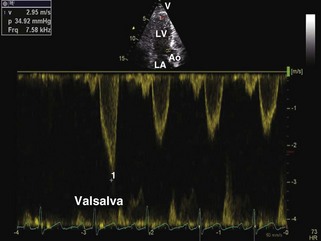
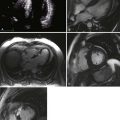
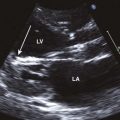
General characteristic findings in RCM include (Figures 3-2 through 3-5):
Specific Diagnoses
Anatomic Imaging (Acquisition/Analysis/Pitfalls)
Endomyocardial Fibrosis
Key Points
Hypertrophic Cardioymopathy
Key Points
Dynamic LV Outflow Tract (LVOT) Obstruction
Mitral Regurgitation
Diastolic Filling Patterns
Overview of Echocardiographic Approach
Acquisition
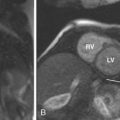
Wall Thickness
LVOT Obstruction
Analysis/Pitfalls
A number of echocardiographic parameters (2D, M-mode, TDI, and strain) have been demonstrated to have diagnostic value in HCM.6
Differential Diagnosis
Alternative Approaches
Estimating Clinical Prognosis
RCM versus Constrictive Pericarditis
Approach
Monitoring Effects of Treatment
Control of Blood Pressure (BP)
Mass
Diastolic Function
1 Nagueh SF, Lakkis NM, Middleton KJ, Spencer WH3rd, Zoghbi WA, Quiñones MA. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation. 1999;99:254-261.
2 Nishimura RA, Appleton CP, Redfield MM, Ilstrup DM, Holmes DRJr, Tajik AJ. Noninvasive Doppler echocardiographic evaluation of left ventricular filling pressures in patients with cardiomyopathies: A simultaneous Doppler echocardiographic and cardiac catheterization study. J Am Coll Cardiol. 1996;28:1226-1233.
3 Ho CY, Sweitzer NK, McDonough B, et al. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation. 2002;105:2992-2997.
4 Ho CY, Carlsen C, Thune JJ, et al. Echocardiographic strain imaging to assess early and late consequences of sarcomere mutations in hypertrophic cardiomyopathy. Circ Cardiovasc Genet. 2009;2:314-321.
5 Nagueh SF, Lakkis NM, Middleton KJ, et al. Changes in left ventricular diastolic function 6 months after nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy. Circulation. 1999;99:344-347.
6 Afonso LC, Bernal J, Bax JJ, Abraham TP. Echocardiography in hypertrophic cardiomyopathy: The role of conventional and emerging technologies. JACC Cardiovasc Imaging. 2008;1:787-800.
7 Rickers C, Wilke NM, Jerosch-Herold M, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. 2005;112:855-861.
8 O’Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867-874.
9 Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committe and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the Europena Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440-1463.
10 Borlaug BA, Melenovsky V, Redfield MM, et al. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570-1577.
11 Tapp RJ, Sharp A, Stanton AV, et al. Differential effects of antihypertensive treatment on left ventricular diastolic function: an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol. 2010;55:1875-1881.
1 Dal-Bianco JP, Sengupta PP, Mookadam F, Chandrasekaran K, Tajik AJ, Khandheria BK. Role of echocardiography in the diagnosis of constrictive pericarditis. J Am Soc Echocardiogr. 2009;22:24-33.
2 Geske JB, Sorajja P, Nishimura R, Oomen SR. Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hyertrophic cardiomyopathy: Correlation with direct left atrial pressure measurement at catheterization. Circulation. 2007;116:2662-2665.
3 Ha JW, Ommen SR, Tajik AJ, et al. Differentiation of constrictive pericarditis from restrictive cardiomyopathy using mitral annular velocity by tissue Doppler echocardiography. Am J Cardiol. 2004;94:316-319.
4 Nagueh SF, Mahmarian JJ. Noninvasive cardiac imaging in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;48:2410-2422.
5 Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107-133.