CHAPTER 205 Evaluation and Management of the Stridulous Child
Advances in medical technology impact the likely underlying diagnoses of modern-day stridor and its management.1 Developments in neonatal intensive care have seen improved neonatal survival and the associated challenges of the airway pathology seen in these children. Routine childhood immunization with the Haemophilus influenzae type B (HiB) vaccine has led to a precipitous decline in epiglottitis, making it a rarity. Improvements in endoscopic equipment have revolutionized the quality of optical images obtainable and captured for digital recording. It has also allowed for the development of endoscopic interventions in the compromised pediatric airway, many of which may now be used in combination to avoid tracheotomy or open airway surgery.
Definition and Physical Principles
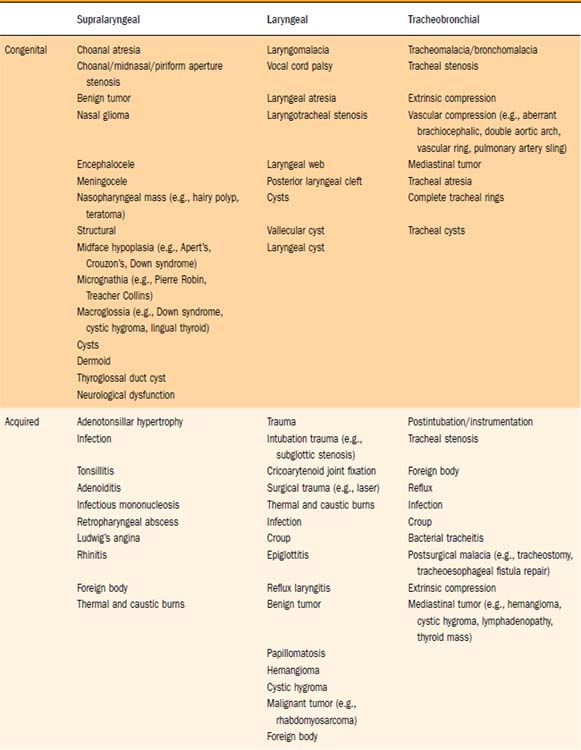
Stridor is an audible respiratory noise derived from turbulent airflow due to narrowing or obstruction of the upper airway. Stridor may be inspiratory, biphasic, or expiratory in nature. It is classically a harsh sound, which can vary in quality from a squeak to a whistling noise. Stertor describes the snoring-like noise, which typically originates from nasopharyngeal or oropharyngeal obstruction. Clinically, however, the supraglottic larynx can occasionally produce this quality of noise. Obstruction from all levels of the airway should thus be considered when approaching the differential diagnoses of airway obstruction (Table 205-1).
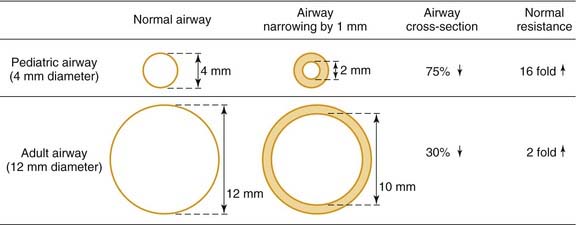
It is helpful to review the physical principles of tubular flow according to Poiseuille’s law, which states that Q = [πr4(P1-P2)]/8ηL; where Q is flow, r is radius, P is pressure, η is viscosity, and L is length of tube. From this law, resistance is inversely proportional to the radius to the fourth power. From a practical viewpoint, this explains why minor narrowing in a child’s airway is of much greater consequence than in an adult. For example, 1 mm of edema in a 4-mm diameter pediatric airway will reduce the cross-sectional area by 75% (16-fold increase in resistance), whereas in a 12-mm adult airway, the same 1 mm of edema reduces the airway area by just 30% (twofold increase in resistance) as demonstrated in Fig. 205-1.2
Assessment
Stridor may be characteristic of a particular pathology but is never diagnostic. The diagnosis can be confirmed with certainty only after endoscopy. However, the combination of a thorough history, examination, and investigation can in some conditions (e.g., mild laryngomalacia) provide sufficient diagnostic probability to avoid initial rigid endoscopy. Seventeen percent of infants with laryngomalacia have a synchronous lesion that may be contributing to their airway compromise.3 This may be missed without assessment via rigid endoscopy.
History
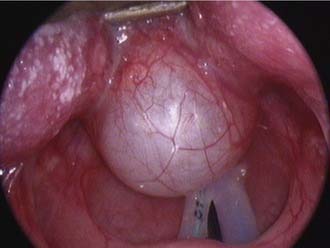
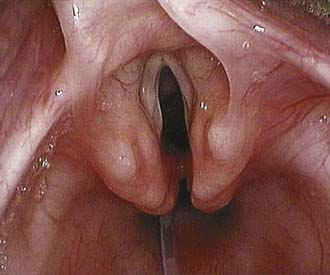
Features of the stridor, including the timing of onset, progression, variability, and presence of exacerbating or relieving factors should be carefully established. Stridor present from birth suggests an underlying anatomic cause. This generally denotes a fixed congenital narrowing such as a laryngeal web, subglottic stenosis, or tracheal narrowing.4 Dynamic conditions such as laryngomalacia typically become evident in the first few weeks of life, while congenital vocal cord palsy is also a common cause of neonatal stridor. A gradual increase in severity of stridor or airway compromise implies progressive obstruction. The obstruction may be luminal (as in a subglottic hemangioma) or extrinsic (as with a mediastinal mass or vascular anomaly). Alternatively over a longer period of time, increasing stridor may coincide with increased respiratory demands as the child becomes more active. Typically, laryngomalacia improves during rest or sleep but is worsened by crying, feeding, or physical activity. Airway obstruction associated with supine positioning may occur with supralaryngeal obstruction, such as micrognathia wherein there is associated airway obstruction by the tongue base. More rarely a pedunculated laryngeal mass, for example, a vallecular cyst, can give positional variation in the stridor as it is displaced in and out of the airway (Fig. 205-2). Improved airway obstruction with crying may occur in gross nasal obstruction such as bilateral choanal atresia.
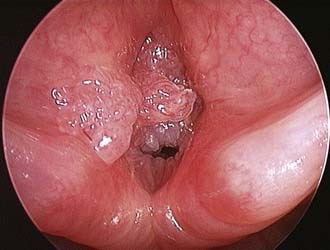
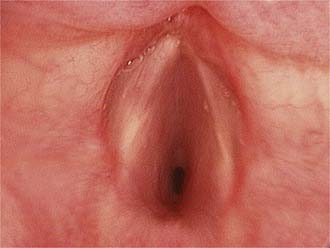
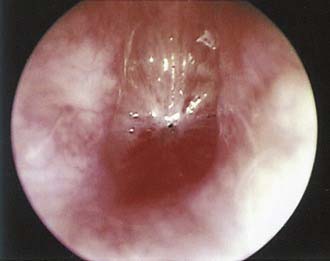
Airway obstruction produces a number of associated symptoms (Table 205-2) that may be useful in formulating a differential diagnosis. Parents have often observed signs of increased respiratory work, including tracheal tug, subcostal recession, and suprasternal or sternal indrawing. Apneas with cyanosis may occur and parents often attempt resuscitation if these are severe. These episodes are typical of severe tracheobronchomalacia and are sometimes termed dying spells. The differential diagnosis includes underlying congenital cardiac disease, hence these episodes should be investigated further. Cough is typical of tracheoesophageal fistula and tracheomalacia, and raises the possibility of aspiration. Hoarseness suggests laryngeal pathology such as laryngeal papillomatosis (Fig. 205-3), whereas supralaryngeal pathology may give a muffled voice. Voice change may also occur in vocal cord palsy, although these children can also present with airway compromise and a normal voice.5,6 Tachypnea and dyspnea are not limited to upper airway obstruction, but a clear description of exertional dyspnea in an older child may provide a useful functional assessment of severity.
Table 205-2 Features of History and Examination in the Stridulous Child
| Symptoms | Signs |
|---|---|
The baby may feed slowly due to airway obstruction and “run out of breath” or “come up for air” during feeds. Bottle-fed babies may require thickened feeds or a “slow teat” (i.e., one with small holes). Uncomplicated slow feeding, although often a source of significant maternal anxiety, is not necessarily of concern in isolation. However, if there is failure to thrive and documented poor weight gain on the centile growth chart, further investigation and possible intervention should be considered. Laryngomalacia commonly gives rise to feeding difficulties but it is important to consider other causes. Choking episodes, documented aspiration, or recurrent chest infections may occur with vocal cord palsy, tracheoesophageal fistula, or rarely a laryngeal cleft (see Fig. 205-21). Gastroesophageal reflux is also common in infants with stridor and may exacerbate airway obstruction via edema.
The obstetric and perinatal histories are often relevant, especially if the child was born prematurely and required ventilation. Parents should be specifically asked about neonatal intubation. This history must be taken carefully because the passage of nasogastric tubes for feeding or nasal/oral mucous suctioning may be mistaken for intubation. Previous intubation places the child at an increased risk of acquired subglottic stenosis (see Fig. 205-13). If the child was recently intubated or is being assessed due to failed extubation, it is worthwhile to obtain details, including size of the endotracheal tube used in comparison to the predicted age-appropriate tube, presence of a leak during intubation, use of steroids at the time of extubation, and any previous history of failed extubations.
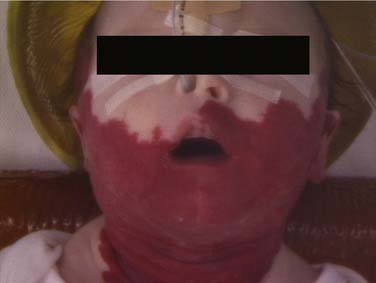
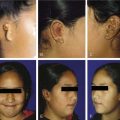
Other comorbidities may predispose to airway obstruction, for example, vocal cord palsy in Arnold-Chiari malformation or birth injury, neurologic disease giving rise to hypotonia, or iatrogenic injury following cardiac surgery. A previously diagnosed syndrome may alert the clinician to potential pathology that is recognized as a component of the syndrome, for example, laryngeal webs in velocardiofacial syndrome. Vascular malformations, congenital cardiac disease, or vascular anomalies may give extrinsic compression of the airway and subsequent tracheomalacia. Parents should be asked about the presence of any “birthmarks,” in that 50% of children with a subglottic hemangioma have a cutaneous lesion at the time of diagnosis (Fig. 205-4).7 There is a markedly increased risk for children with cutaneous hemangiomas in a “beard” distribution, with 63% having significant airway involvement (Fig. 205-5).8 The clinician should be particularly vigilant in assessing children with a history of recurrent episodes of laryngotracheitis or bronchiolitis that has been slow to settle. These children ought to be thoroughly assessed for an underlying source of airway obstruction that is being exacerbated by superimposed upper respiratory tract infection (Fig. 205-6).
Examination
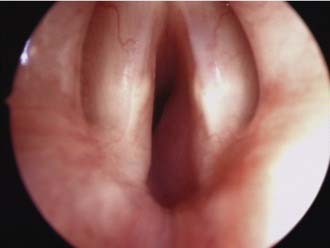
An overall assessment of the degree of respiratory distress and airway compromise should be expedited in the acute situation and a full examination completed if the child’s condition is stable (see Table 205-2). Observation of the child at rest in the parent’s arms provides an initial assessment of the degree of respiratory distress, the characteristics of any stridor, and whether the child appears systemically unwell. It also allows time to gain the child’s confidence before any further examination. A careful general examination is necessary to avoid missing subtle features of related syndromes or of other general pediatric disease. Syndromic features, if present, may suggest likely sources of airway obstruction associated with the particular syndrome, for example, micrognathia in Pierre Robin sequence, macroglossia in Beckwith-Wiedemann syndrome, or anterior glottic webs in velocardiofacial syndrome (Fig. 205-7).
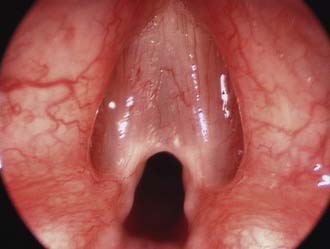
Figure 205-7. Anterior glottic web presenting with stridor in a child with velocardiofacial syndrome.
Simple inspection of the child reveals valuable information regarding the work of breathing and degree of obstruction. Potential findings of increased respiratory work include suprasternal or sternal recession, subcostal indrawing, “see-saw” or paradoxical abdominal movement during respiration, nasal flaring, tracheal tug, anxiety, irritability, or evidence of fatigue. The amount of recession is a better indicator of the severity of airway compromise than the degree of stridor. Stridor can paradoxically become quieter and less apparent as the obstruction worsens due to the diminishing airflow and may be a sign of impending respiratory arrest. Pallor and subsequent cyanosis are late events in pediatric patients and no comfort should be taken from the fact that a child still appears pink. Children may position themselves to optimize their own airway as is classically described in epiglottitis, where children prefer to sit upright with their head in a “sniffing” position. Pectus excavatum may be seen in children with chronic airway obstruction due to negative intrathoracic pressure in their highly compliant rib cage.2
Inspiratory stridor typically occurs due to obstruction at the level of the supraglottis or glottis. Obstruction arising in the bronchi or lower trachea classically produces an expiratory stridor or prolongation of the expiratory phase. Biphasic stridor can occur with obstruction anywhere in the laryngotracheobronchial tree but is classically associated with either upper tracheal or subglottic pathology where it is attributed to the fixed luminal diameter of the cricoid. The characteristic sound of stridor, even in a common condition such as laryngomalacia,9 is so variable as to be of little diagnostic use in isolation. Laryngomalacia has been traditionally described as having stridor that is of a musical quality, while stridor in vocal cord palsy has a breathy quality, viral laryngotracheitis has a barking cough and tracheomalacia has a brassy cough. It is important to recognize that these descriptions are highly subjective and their assessment is not reliably reproducible.
Radiology
Soft tissue lateral neck x-ray examination demonstrates the airway outline from the nasopharynx to the subglottis. Posterior-anterior chest x-ray study shows the lung fields and mediastinum. Persistent air trapping may be demonstrated on the side of a bronchial foreign body in inspiratory/expiratory views. A decubitus view may be useful in young children, where normally the dependent lung deflates.10 A Cincinnati (high-kilovoltage filter) view enhances the tracheal air column while de-emphasizing bony cervical spine to demonstrate the major airways. These x-ray techniques may be useful as screening investigations (Fig. 205-8).11,12 Careful consideration of the stability of the child’s airway is mandatory and radiology should not be undertaken if there is any potential for acute airway deterioration. Policy for suspected epiglottitis differs between centers; however, the result of the x-ray study may not influence management13 and may be associated with significant risk to the child. Further imaging should be performed according to the endoscopy findings.
Videofluoroscopy is an excellent way of demonstrating tracheomalacia and can be combined with a contrast swallow to exclude vascular compression,14,15 tracheoesophageal fistula, or aspiration. It may demonstrate diaphragmatic immobility on the side of a foreign body airway obstruction. Bronchograms using safe nonionic contrast media are useful for outlining the luminal surface of the lower airway, demonstrating tracheobronchial stenosis and malacia. Opening pressures of the collapsed bronchi and lower trachea can also be measured to determine the level of airway support needed. Computed tomography (CT) and magnetic resonance imaging (MRI)12,16,17 continue to lack sensitivity in assessing airway stenoses and cannot replace endoscopic assessment. They are useful in demonstrating vascular anomalies and extrinsic compression of the airway (Fig. 205-9). Virtual endoscopy uses radiologic data to create computer simulations that may be viewed as one would conventional endoscopy. Helical CT with multiplanar reconstruction provides three-dimensional images, which are used in real time to simulate endoscopy views. It is noninvasive and allows retrograde luminal airway views. Although it demonstrates some fixed airway stenoses, it is not helpful in detecting obstruction during dynamic movement as occurs in tracheomalacia or vocal cord palsy. There is also a lack of detail, with airway pathology such as glottic webs being poorly demonstrated.18
Other Investigations
Vocal cord ultrasound can be used, with experience, to demonstrate vocal cord palsy to complement the endoscopic findings.19 The role of laryngeal EMG in the management of vocal cord immobility is yet to be fully defined.20 Airway obstruction that worsens during sleep is usually a feature of pharyngeal obstruction, such as adenotonsillar obstruction or craniofacial anomaly. Laryngotracheal pathology at any level including laryngomalacia may, however, occasionally worsen during sleep, thus requiring sleep study investigation.21 Pediatric lung function tests are seldom used because of compliance and cooperation issues. Flow volume loops22 may help localize the site of obstruction and other tests such as peak flows or ventilation-perfusion scans may be used upon the advice of a pulmonologist. Gastroesophageal reflux23–26 is not discussed in detail in this chapter but can be investigated via double probe pH studies, contrast studies, milk scan, or esophagoscopy with lower esophageal biopsy. Echocardiography detects congenital heart disease but does not demonstrate all abnormal vasculature, thus it cannot be used to exclude vascular anomalies.
Endoscopy
Pediatric airway endoscopy requires a full range of specialized pediatric endoscopy equipment and an experienced team. An inadequate evaluation needs to be repeated, and referral to an experienced center should be made as required to ensure a single comprehensive and definitive evaluation. A systematic approach provides a diagnosis in most cases.27
Flexible Endoscopy in the Office or Ward
The introduction of ultrathin endoscopes28 in a range of diameters with good optics has allowed even neonates to undergo endoscopy without the need for a general anesthetic. This is usually considered a screening procedure, in that the view of the larynx may be suboptimal. Rigid endoscopy of the airway is superior in examining the airway for structural abnormalities. Flexible endoscopy is useful in assessing the dynamic airway for vocal cord movement or features of laryngomalacia. A systematic approach must be adopted, observing first the nasal cavity followed by the postnasal space, oropharynx, supraglottis, and glottis during dynamic respiration and phonation. It is difficult to obtain good views of the subglottis. This is purely a diagnostic procedure, with no opportunity for therapeutic procedures, in contrast to a rigid laryngotracheobronchoscopy. Flexible endoscopy under sedation in an endoscopy suite is widely practiced by pediatricians and pulmonologists29–31 and is used by otolaryngologists as an adjunct to rigid endoscopy.32 Rigid endoscopy is helpful if the diagnosis remains unclear despite flexible endoscopy, if there is clinical suspicion of a second airway lesion, or if subglottic/tracheal or bronchial pathology is suspected.
Laryngotracheobronchoscopy
Laryngotracheobronchoscopy33 is the gold standard in the evaluation of the stridulous child. It enables a thorough assessment to be performed while the airway is maintained and allows findings to be visually recorded for future reference. The airway may be formally sized to grade any narrowing. It also may incorporate therapeutic procedures, including foreign body removal or the endoscopic management of airway pathology. It requires an experienced team skilled in airway assessment, including the surgeon, anesthetist, and nursing assistant, who work closely to ensure an optimal and safe examination. The use of a video is essential to facilitate training and allows the anesthetist and nurse to follow the procedure and status of the airway on the monitor.
It is vital that accurate records are kept in a standardized form within a department.34 Digital prints provide a valid record of static conditions while video clips record dynamic findings. Digital images and video recordings should be saved and archived. This provides an invaluable source of information for sequential clinical comparisons, teaching, and medicolegal purposes.
Anesthetic Considerations
This procedure requires a cooperative approach to airway management with both the anesthetic team and surgeon sharing the control of the airway. The use of atropine premedication provides a dry surgical field and improves the efficacy of topical anesthesia. It is most effective via intramuscular injection. Anesthesia is often induced using slow masked inhalational anaesthesia. Halothane is a volatile agent that has been traditionally used and is nonirritating to the airway, allowing for smooth maintenance of anesthesia during instrumentation of the airway. Halothane is now difficult to obtain and other inhalational agents such as isoflurane, sevoflurane, and intravenous agents are being used. Sevoflurane35 is also nonirritating, rapid in onset, and allows rapid delivery of high concentrations. Topical lidocaine is applied to the airway to minimize airway stimulation during the procedure and helps avoid laryngospasm. Lidocaine dose needs to be carefully measured to avoid overdosage.36,37
Anesthetic practice varies between centers but most units use spontaneous respiration for pediatric patients rather than paralysis and jet ventilation simulating normal respiration. Jet ventilation prevents coughing or gagging38–40 but requires pressures above physiologic levels38,41 with an associated risk of pneumothorax in neonates and smaller children. Dynamic conditions such as malacia and cord palsy cannot be identified. Apneic endoscopy is also possible after initial hyperventilation with 100% oxygen, although progressive hypoxemia and hypercarbia limit the operating time and condition of the patient.42
A laryngeal mask43 may be useful for fiberoptic bronchoscopy particularly if the patient is difficult to intubate due to mandibular hypoplasia.
Operative Technique
A suspension laryngoscope and microscope are traditionally used to examine the larynx, with a ventilating bronchoscope to examine the tracheobronchial tree (Fig. 205-10). Rigid telescopes provide excellent images and many centers now perform the assessment using a telescope, with digital images recorded at each anatomic level. High-quality images may be captured via the use of a wide Storz photographic telescope. A 4-mm rigid telescope should provide adequate images for data records, provided there is careful focusing and appropriate camera settings to ensure optimal captured images. For routine examination, the telescope and camera can be held in the left hand with a probe used in the right while the laryngoscope is in suspension. The microscope is used if there is to be manipulation of the airway requiring both hands of the operating surgeon. Differing techniques are adopted, according to individual departmental preferences. The procedure may be combined with rigid esophagoscopy, according to departmental protocols.
Preparation and Positioning
It is essential to anticipate equipment requirements so all equipment can be checked and prepared before commencement. A range of Hopkins rod telescopes should be available, which includes all lengths and diameters that may be required, so the endoscopist is fully prepared for all eventualities. Charts that predict age-appropriate sizes of bronchoscopes should be consulted and be readily available in the operating room.44 The appropriate bronchoscope needs to be checked and assembled and at least one size smaller must be on hand. A 30-degree telescope may be used to enable assessment of the supraglottis without splinting. It may also assist assessment of an anteriorly placed larynx. A microscope should be available with the 400-mm lens to be used with standard laryngeal instruments. A 350-mm lens may be useful in small neonates by “bringing the patient closer” to allow easier manipulation of larynx.
Microlaryngoscopy Technique
A suspension straight-blade laryngoscope is gently inserted while assessing the overall appearance of the laryngopharynx, taking care to protect the teeth, lips, and oral mucosa while keeping the tongue midline to provide a well-centered view. The laryngoscope is placed in the vallecula and the epiglottis is carefully lifted forward. An overall assessment of the larynx can be made while an endotracheal tube is in situ and while providing a degree of stability, particularly in a compromised pediatric airway. Removal of the endotracheal tube allows a superior view and a probe may be used to independently palpate the arytenoids to assess the mobility of the cricoarytenoid joints and any limitation in joint movement. Absence of independent arytenoid movement on palpation is indicative of interarytenoid scarring. A posterior laryngeal cleft should be excluded by passing the probe between the arytenoids to allow comparison of the inferior limit of the interarytenoid groove with the posterior commissure. The subglottis should also be inspected from above. Topical decongestion and vasoconstriction of the airway may be helpful, particularly in the presence of low-grade inflammation or edema. Any airway narrowing should be formally sized. The largest endotracheal tube that permits a leak at less than 30 cm H2O of pressure provides a measure of the airway diameter. Subglottic stenosis may then be graded using the Cotton-Myer grading system,45 thus allowing reproducible assessment of the stenosis and aiding in treatment selection. If there is airway compromise or instability, assessment time may be limited. It is thus essential to be prepared to move ahead with bronchoscopy at any stage.
Bronchoscopy Technique
Traditionally, a ventilating bronchoscope (Fig. 205-11) has been used to assess the distal airway, which provides a means of actively ventilating the patient during the procedure, if required. An age-appropriate sized bronchoscope is used unless stenosis is suspected. A smaller-diameter rigid Hopkins rod telescope can be used as an alternative to examine the airway in a spontaneously breathing child, particularly if there is significant subglottic stenosis. The rigid telescope may reduce local airway trauma and lessen airway splinting, while allowing the distal airway to be visualized.
An anesthetic laryngoscope is placed in the vallecula while passing the bevel of the bronchoscope through the vocal cords under vision or via the monitor. The subglottis, trachea, carina, and main bronchi are systematically examined and findings recorded via digital images and/or videos. Evidence of tracheomalacia should be sought in the absence of positive airway pressure to avoid splinting of the airway and using a small bronchoscope withdrawn from the area being assessed. The ratio of cartilage to trachealis is significant in recording the type of malacia. The presence of complete tracheal rings is of importance when considering slide tracheoplasty for the treatment of long segment tracheal stenosis.46 Extrinsic compression and prominent transmitted pulsation may be evident where there is an underlying vascular anomaly, which can be confirmed with imaging.
Dynamic Airway Assessment
An excellent technique for dynamic assessment of vocal cord movement utilizes a laryngeal mask with a fiberoptic bronchoscope passed through to visualize the airway. The flexible endoscope is positioned just proximal to the laryngeal inlet to observe airway dynamics.47
Complications
Complications are unusual in rigid laryngobronchoscopy with a reported overall complication rate of 1.9%.48 Possible complications include oral injury, subglottic injury, pneumothorax, cardiac complications, loss of control of the airway, and bleeding. Blood in the airway impairs oxygenation and ventilation, rather than giving rise to difficulties due to volume of blood loss.42 Risks associated with procedural complication include cardiac anomalies, airway foreign body, tracheal stenosis, and biopsy or abscess drainage,48 and particular care should be taken in these cases.
Postoperative Care and Planning
Airway obstruction may increase following the procedure due to underlying pathology, edema, blood, secretions, or postintubation croup. Edema is usually maximal within 4 hours and settles by 24 hours. Humidified oxygen by mask is initially tried. Nebulized epinephrine (1 : 1000) may be tried but the child must be kept under observation and reviewed within an hour for recurrence due to its limited duration of action.42 The patient may be given intraoperative dexamethasone if there is a potential for edema from instrumentation of the airway. Steroids may be continued in the postoperative period depending on the endoscopy findings and underlying airway pathology. The patient must be recovered by nursing staff experienced in the care of pediatric airways and remain as an inpatient for close observation until the airway is adequately stable for discharge.
Treatment of Acute Airway Obstruction
Medical Treatment
The child who arrives in the emergency department with acute airway obstruction should undergo rapid evaluation. Assessment, history, and active resuscitation should proceed while measures are taken to stabilize the airway. The physician should determine the degree of airway obstruction by observing the child for recession and work of respiration while taking a brief history from the accompanying adult. Details will be obtained regarding the length of history, recent systemic illness, and possibility of foreign body inhalation. The nursing staff should check the oxygen saturation and set up humidified oxygen. Another team member should contact the operating room and anesthesiologist to advise that the child may require intubation. The otolaryngologist may also be contacted and asked to attend the intubation,49 depending on the unit’s protocol. This situation clearly benefits from careful planning, with most units having a protocol for how to deal with the stridulous child.
Supplemental oxygen reduces the ventilatory requirement to maintain adequate oxygen saturation in cases of airway obstruction with normal alveolar function. The potential for CO2 retention should be kept in mind. Heliox (helium-oxygen gas mixture) may be used to relieve respiratory distress, decrease the work of breathing, and improve gas exchange.50 It does this due to its low density, which results in a lower resistance to gas flow.51,52 It has no direct effect on the underlying pathology and thus must be viewed only as a “therapeutic bridge,” allowing time for definitive treatment to be commenced.50 Steroids and nebulized epinephrine may also be used to help optimize the airway in the acute situation.
Laryngotracheitis (viral croup) can be treated with intravenous, oral, or nebulized steroids. There is no objective evidence that raising the humidity of the inspired air is beneficial.53 Nebulized budesonide54 is used in the home environment for children with recurrent croup. Corticosteroids are beneficial and are routinely recommended for the treatment of croup.55 Klassen56 recommends that children with croup symptoms and increased work of breathing should be treated with either nebulized budesonide (2 mg) or oral/intramuscular dexamethasone (0.15 to 0.6 mg/kg). Oral dexamethasone is easily administered, widely available, and low in cost. L-epinephrine (5 mL of 1 : 1000) or racemic epinephrine (0.5 mL) should be considered if there is moderate or severe distress because it has been found to give clinical improvement and reduce the need for intubation.57
Other infective causes of airway obstruction respond promptly to antibiotic therapy. This includes bacterial tracheitis or epiglottitis, which is rare, although vaccine failure58,59 has been documented. Appropriate intravenous antibiotic therapy should be commenced while the airway is stabilized via short-term intubation if required.
Therapeutic Intubation
The child is “gassed down” slowly and the larynx is inspected to exclude epiglottitis. The child may then be manageable on a nasal pharyngeal airway with continuous positive airway pressure (CPAP). If intubation is required, it is important to minimize any damage to the inflamed subglottic mucosa. This is achieved by gentle intubation with a small, soft tube that is adequate for ventilation and suction of secretions. A nasotracheal tube is preferable to an oral intubation to help minimize tube movement. The secure nasotracheal tube ought to be placed and assessed by suctioning the secretions and testing for a leak. Any persisting element of airway obstruction or tenacious secretions should be further investigated by passing a ventilating bronchoscope. This helps exclude bacterial tracheitis60,61 or a foreign body. Endoscopic suctioning and clearance of debris from the airway may be performed and is particularly beneficial in bacterial tracheitis. Significant tracheobronchomalacia may also give persisting obstruction after intubation and will usually respond to CPAP.
Emergency Tracheotomy
Emergency tracheotomy is a rare occurrence in departments with experienced pediatric anesthesiologists.49,62 Otolaryngologists are often asked to attend a potentially difficult intubation but are rarely needed. If emergent tracheotomy is required, however, the surgical set and appropriate tube must be immediately available. The neonate needs to be in a straight supine position with the neck extended over a roll. A long, vertical, strictly midline incision is made through skin and down to the trachea, guided by palpation of the trachea. The main danger lies in drifting off the midline and a finger on either side of the larynx is helpful. If a pediatric tracheotomy tube is not available, a pediatric endotracheal tube can be used, ensuring it remains above the carina.
Endoscopic Removal of Foreign Bodies
Foreign body removal has been greatly facilitated by the introduction of foreign body optical extraction forceps that have a central channel for an extra-long Hopkins rod telescope. Different forceps are available for the removal of various types of foreign bodies. The foreign body and adjacent airway can be carefully examined while the removal is planned using a fine suction catheter in the bronchoscope alongside the rigid telescope. The use of topical adrenaline before any attempt at removal optimizes local conditions by vasoconstricting the mucosa and reducing bleeding.63
Approach to Postextubation Stridor
Extubation should not be attempted until the child has an optimal chance of success. Failed extubation requiring subsequent reintubation predisposes to further deterioration of the airway with additional edema, ulceration, and perichondritis. There should be no active respiratory infection, minimal pressure support, and a normal oxygen requirement at the time of extubation. Reflux should be adequately treated and steroids commenced if indicated. Extubation should proceed once sedation has worn off and the child is awake to maximize the likelihood of success. If postextubation stridor and obstruction occur, the airway can be further optimized with nebulized epinephrine,64 intravenous dexamethasone65 before and after extubation, and continuous positive airway pressure via nasal cannula. Therapeutic reintubation for laryngeal rest has been demonstrated to be beneficial,66–68 allowing any reversible component of the airway inflammation to settle.
If extubation fails, despite appropriate medical measures and laryngeal rest, endoscopic procedures are being increasingly used to optimize the airway (Table 205-3). Alternatively, a cricoid split may be performed. Single-stage laryngotracheal reconstruction can be used in patients who fail cricoid split or who have a mature stenosis. Tracheotomy is reserved for those who are not suitable for an alternative approach. These surgical options are discussed later.
Table 205-3 Management Options for the Difficult to Extubate Neonate
Expected Airway Obstruction in the Postpartum Neonate
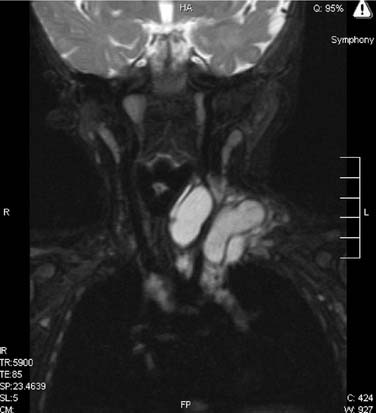
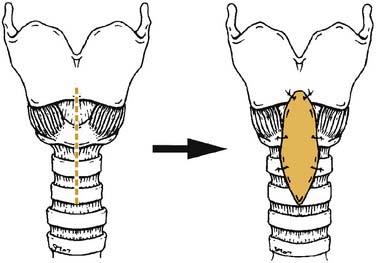
Antenatal diagnosis of airway obstruction enables a definitive plan of management to be formulated before delivery. Routine prenatal ultrasonography may diagnose large masses, including cervical teratoma,69,70 cystic hygroma,71 or rhabdomyosarcoma72 with associated airway obstruction. Further details of the anatomy can then be defined using MRI.73,74 Airway obstruction may be detected on ultrasound, even in the absence of a mass. Antenatally diagnosed congenital high airway obstruction syndrome (CHAOS) exhibits features including tracheal dilation and lung hypertrophy, as seen in laryngeal atresia.75 Polyhydramnios may also occur if an obstructing lesion is compressing the esophagus and impairing fetal swallowing.76 Other ultrasound features may include diaphragmatic eversion, ascites, and ultimately fetal hydrops.77 Holinger and others first described the successful airway management of an antenatally diagnosed cervical teratoma in 1985.70 The technique of securing the neonatal airway while the uteroplacental blood flow was maintained was subsequently described in the early 1990s.73,78 This procedure has become known as the ex utero intrapartum treatment (EXIT)79,80 or OOPS (operation on placental support)71 and should be considered when perinatal airway obstruction is anticipated. The multidisciplinary team usually includes pediatricians, obstetricians, anesthetists, and ENT surgeons. Other specialties may be involved, depending on the underlying pathology. At planned cesarean section, adequate uteroplacental flow is maintained while the airway is established. The placenta allows continued gas exchange while the airway is stabilized. Direct laryngoscopy and intubation is then performed. Rigid laryngobronchoscopy may be used to define the anatomy and possibly place a guidewire for intubation guidance if there is marked distortion or poor visualization of the airway. If intubation is not possible, the trachea is exposed and a tracheotomy performed. The surgeon should be mindful that a large neck mass will hyperextend the neck and anteriorly displace the trachea. This may mean that the tracheotomy is placed more distally than planned. Once the airway is secured the umbilical cord is clamped and the delivery is completed.76 Extracorporeal membranous oxygenation can be used particularly in the presence of severe congenital cardiac disease.81 Resection of an obstructing mass is usually delayed until the child’s condition is stabilized.81
Unexpected Airway Obstruction in the Postpartum Neonate
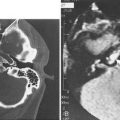
Supralaryngeal
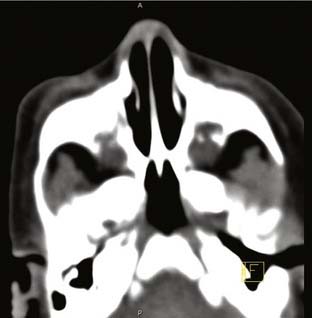
Nasal or nasopharyngeal obstruction can be highly significant in the newborn who is an obligate nasal breather. Nasal suction removes secretions and allows assessment of the patency of the nasal airway. Choanal atresia is the most common diagnosis in complete nasal obstruction and can be investigated via CT scan (Fig. 205-12). It may be difficult to pass a nasal suction catheter in a neonate with prominent turbinates or, less commonly, piriform aperture stenosis, midnasal stenosis, or choanal stenosis. Nasopharyngeal masses such as teratomas may also obstruct the airway. Nasal or nasopharyngeal airway obstruction is relieved by an oral airway. Oropharyngeal sources of obstruction (such as micrognathia with a retroposed tongue) may also be improved by positioning and oral airway placement.
Laryngeal
Laryngeal obstruction shows no improvement with an oral airway but is relieved by intubation. Airway obstruction associated with severe laryngomalacia, vocal cord palsy, laryngeal cysts, or polyps will improve with intubation. A small-shouldered tube may be temporarily positioned through a laryngeal stenosis to secure the airway. Some units now advocate using a laryngeal mask for neonatal resuscitation82 to avoid exacerbation of the stenosis by attempts at intubation. It may be possible to ventilate a neonate with laryngeal atresia and a low tracheoesophageal fistula although this is rare. In these cases, there will be none of the characteristic findings of congenital upper airway obstruction on antenatal ultrasound. On closer examination in this instance, the tube is found in the esophagus with ventilation occurring via the fistula.83
Tracheobronchial
Obstruction due to distal tracheal or bronchial pathology, including tracheobronchomalacia, may not improve with either intubation or tracheotomy. Positive pressure will aid in splinting the airway and improve malacia. It is less helpful for distal airway stenosis. Use of an extended (long) tracheotomy tube will physically support tracheomalacia but will not assist in carinal malacia or bronchomalacia. A tapering long-segment tracheal stenosis presents a difficult clinical situation, because it is often possible to insert only a very small tracheotomy tube. In addition the tracheotomy severely limits the therapeutic options for surgical reconstruction of the stenosis. The patient with a long-segment tracheal stenosis requires referral for consideration of slide tracheoplasty, which is recognized as the best treatment for this condition in the current era.46
Treatment of Chronic Airway Obstruction
Medical Management
There are several conditions presenting with chronic airway compromise that may be managed expectantly with regular clinical review and supportive therapy only (e.g., mild to moderate laryngomalacia, unilateral vocal cord palsy, grade I subglottic stenosis). The input of allied health departments such as speech and language therapy and dietetics is beneficial with regard to concurrent feeding, swallowing, and aspiration issues. Respiratory review to manage underlying lung disease and the consideration of CPAP may be appropriate in some situations. The use of a nasopharyngeal airway to bypass supralaryngeal airway obstruction is highly effective and relatively noninvasive.84 It may be used to avoid surgery in certain cases of midface hypoplasia, neuromuscular disorders, glossoptosis, and micrognathia/retrognathia. It is also a valuable postoperative adjunct following adenotonsillectomy in the child with severe obstructive sleep apnea.
Gastroesophageal reflux is thought to aggravate airway conditions and possibly contribute to apnea, recurrent upper respiratory tract infections, laryngomalacia, and subglottic stenosis, although consistent evidence to support the hypothesis is limited.85,86 Adjuvant antireflux treatment is generally well tolerated and effective in the pediatric population. Its use, although empirical at times, may improve airway symptoms, circumvent the need for surgery, and enhance surgical outcomes.
Systemic steroids are often used intermittently to treat acute deterioration in the setting of chronic airway obstruction. Long-term doses require careful monitoring for side effects but have been successfully used as an adjuvant treatment for conditions such as subglottic hemangioma in many series.87–89 Airway edema from croup responds well to steroids and some children with this condition benefit from having dexamethasone available for recurrent attacks at home.57
Tracheotomy
The indications for insertion of a pediatric tracheotomy have changed over the past decades.90 Tracheotomy is more commonly requested to bypass chronic airway obstruction from craniofacial or upper airway abnormalities, or to support prolonged ventilation than for acute infective conditions such as laryngotracheobronchitis and epiglottitis (Table 205-4). Tracheotomy may be a temporary treatment while waiting for the child to outgrow the underlying condition (e.g., subglottic hemangioma or severe laryngomalacia), or until the child is old enough to undergo other definitive procedures. Its insertion may be permanent but is generally not undertaken without consideration of care requirements and the potential morbidity associated with it. The overall complication rate is up to 44% and tracheotomy-related death as high as 3.6% in some series.91,92
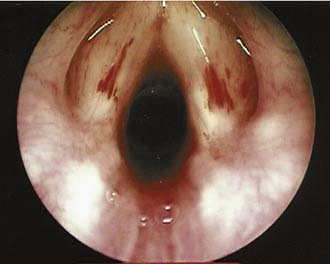
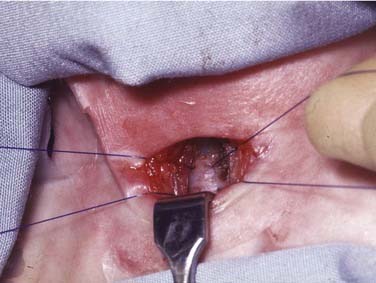
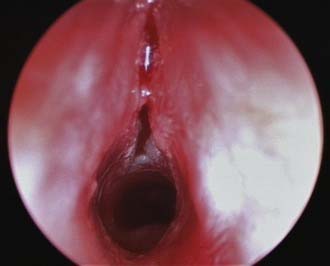
Techniques to avoid tracheotomy include the use of maximal medical treatments to reduce edema and inflammation while surgical alternatives are directed toward addressing the site of obstruction and minimizing resistance to airflow. For example, in developing subglottic stenosis, the options of laryngeal rest,67 cricoid split,93–95 endoscopic balloon dilatation with mitomycin C,96 and single-stage laryngotracheal reconstruction97,98 may avoid the need for tracheotomy. A case of early subglottic stenosis and its response to balloon dilatation is shown in Figs. 205-13 and 205-14.
Surgical Procedure
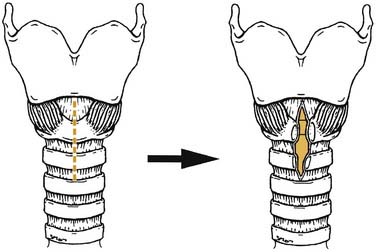
The operation is usually conducted under general anesthesia with the child intubated or a laryngeal mask in place. The position is supine with a small shoulder roll to provide slight neck extension. An adhesive tape under the chin retracts any dewlap and anchors the head (Fig. 205-15). Skin marking of the cricoid and sternal notch is useful although may be difficult to palpate in a small infant. Either a horizontal or short vertical skin incision can be used. In children, removal of a subcutaneous fat plug is helpful and the thyroid isthmus can be simply divided using bipolar diathermy. It is crucial to stay midline in a pediatric tracheotomy and one should frequently pause to check the dissection plane. A vertical incision is made through the third and fourth tracheal rings without removal of cartilage and nonabsorbable stay sutures placed on either side of the incision (Fig. 205-16). These sutures assist in replacement of a tube, which is dislodged before the tract is established. The sutures are removed with the first tube change at 1 week. If a future laryngotracheal reconstruction (LTR) or open laryngeal cleft repair is planned, the tracheal opening may be placed lower, being mindful of the innominate (brachiocephalic) vessels and lung apices. Occasionally, a high innominate vein needs to be protected from the tracheotomy tube by a small inferiorly based flap instead of the vertical incision. Vicryl maturation sutures from the tracheal opening to the skin reduce the chance of false passage creation and assist in establishment of the stomal tract. They have not been shown to increase the rate of tracheocutaeous fistula.99,100
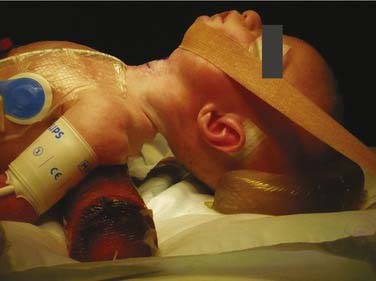
Figure 205-15. Ideal positioning for pediatric airway surgery using shoulder roll, head ring, and chin strap to stabilize the head.
Local policy will dictate tracheotomy tube type but beware inserting one that is too long, particularly in neonates, for whom special short tubes are available. Consideration of the underlying condition, patient anatomy, and frequency for tube change influences the choice of tracheotomy tube.101 An extended length tube may be required if the neck and chest wall are bulky, or length may be required internally to support tracheomalacia or a tracheal stenosis. Cuffed tracheotomy tubes are placed infrequently in the pediatric population except to protect the airway in the presence of aspiration. The use of a cuffed tube is usually unnecessary and avoided if possible in that the pediatric airway is particularly sensitive to ischemic insult and prone to ulceration, granulation, and ultimately stenosis.102 Lateral skin sutures to close the tracheotomy neck wound should not be applied tightly because this predisposes to surgical emphysema.
Tracheotomy Complications
Early complications such as accidental tube dislodgment may be a life-threatening emergency in a small child. This can be prevented by adjusting the tapes carefully to only allow one fingerbreadth between the skin and the tapes, and by providing stay sutures through the trachea on either side of the incision (see Fig. 205-16). These sutures are pulled apart in the event of tube dislodgment to open the incision, bring the trachea to the surface, and guide tube reinsertion into the tracheal lumen. Conventional adult tracheal dilators are usually too large, but a small, curved artery clamp may help. The technique of using a cut suction catheter through the lumen of an appropriate sized tracheotomy tube to guide its insertion has the advantage of confirming tracheal position by successful suctioning of secretions and the opportunity for jet ventilation to maintain oxygenation if required.103 An emergency box should always be at the child’s bedside containing two spare tracheotomy tubes (one a size smaller), suction catheters, lubricant, scissors, and spare tapes. Tube blockage is avoided by humidification, frequent suctioning, and an awareness of the problem.
Postoperatively, a chest x-ray study is imperative to check the tube length and exclude a rare pneumothorax, which can occur from puncturing the lung apex or from positive-pressure ventilation. Hemorrhage is rarely a complication in children if the thyroid isthmus has been divided with electrocautery rather than ligated after division between clamps. Subcutaneous emphysema may be prevented by having a snug fit of the tube in the trachea and avoiding tight wound closure and excessively high-pressure ventilation. Tube dislodgment and blockage remain the most important late complications and are responsible for tracheotomy-related mortality. Stomal and tracheal granulations are the result of physical factors such as movement and plasticizers in the tube, combined with infective factors from the skin and airway. The use of antibiotic ointment around the tube may improve but never remove the granulations. Longstanding suprastomal granulations within the tracheal lumen are associated with the development of suprastomal collapse, which is commonly responsible for difficult decannulation that requires further surgery. Tracheal erosion may occur because of a poor-fitting tracheotomy tube, particularly if metal. Erosion can involve the mediastinum, esophagus, or major vessel. An acquired tracheoesophageal fistula requires closure with an open operation and interposition of muscle. Small bleeds around or from the tracheotomy tube may herald the dramatic erosion of a major vessel.104 Although suprastomal and tracheal granulations can give rise to small hemorrhages, any tracheotomy bleeding should be taken seriously and investigated radiologically or endoscopically. In very longstanding tracheotomy, the tube may migrate and require surgical repositioning.
Tracheotomy Decannulation
Adults and children with a short-duration tracheotomy (less than 1 month) can be decannulated after appropriate assessment by simply having the tube removed and the stoma covered with an occlusive dressing.105 Most children, however, are more difficult to decannulate because of multiple factors including suprastomal collapse and granulation, the relatively small size of the airway, and, possibly, psychological dependence or attachment to their tracheotomy tube. A more controlled process for decannulation is used in these children. A recent formal endoscopic examination of the airway, ideally within 1 month, is required before any attempt is made to try decannulation.106 The most common causes of failed decannulation are significant suprastomal collapse and granulation, and this should be addressed at the time of surgery.
The child is admitted to hospital for a supervised trial of “ward decannulation.” The tracheotomy tube is downsized in a stepwise manner, usually one to two sizes smaller each day until the smallest tube has been tolerated. The tracheotomy tube is then blocked for 24 hours and the child is kept on the ward for careful observation at night. If the trial of capping is uneventful, the tube is completely removed and the stoma covered with an occlusive dressing. A further 48-hour period of observation is recommended in that failure may occur at any stage. In the case of failed decannulation, the smallest tube is simply reinserted in the stoma and gradually upsized. Time may be required to allow the airway to grow; however, a repeat endoscopy should be performed before any further trials of decannulation to exclude concurrent pathology such as cord fixation or palsy, suprastomal granuloma or collapse, and tracheomalacia.107
Endoscopic Treatment of the Larynx and Tracheobronchial Tree
Microinstrumentation
Sharp dissection using cupped forceps and microscissors is precise and causes minimal damage to surrounding structures, and the mucosal injury is generally quick to heal. Cystic lesions and nodules may be successfully marsupialized or resected with less trauma than with a laser. Indeed, in inexperienced hands the laser probably has greater potential for damage. Aryepiglottic trimming for laryngomalacia108 was originally described using sharp dissection. The aryepiglottic folds may simply be released or a small wedge of bulky arytenoid mucosa removed to prevent the forward prolapse of the posterior larynx and arytenoids, which is typical of the condition. Microinstrumentation with the sickle knife and sharp blades can be used to perform other procedures such as an endoscopic cricoid split (Fig. 205-17). In these cases, decompression of the cricoid ring followed by short-term stenting with an endotracheal tube may allow sufficient expansion of the subglottis to enable extubation and avert the need for a tracheostomy in early subglottic stenosis.109
Laser
The CO2 laser for the larynx, and more recently the KTP laser for the trachea and bronchi, have been considered an essential part of the equipment needed to manage the pediatric airway. Other lasers such as the Nd:YAG and thulium laser have also been used in the respiratory tract. Newer techniques and instrumentation, however, may supersede use of the laser due to the delicate nature of the pediatric airway and its sensitivity to thermal injury. A number of improvements, including super pulsing, have reduced the collateral damage that occurs around the intended area of vaporization, making the laser relatively safe and effective in experienced hands. One should always err on the side of conservatism when using the laser in the pediatric airway (Fig. 205-18). Great care must be taken not to damage normal tissues and produce a secondary stenosis. A laser-safe tube must be used to provide anesthesia in all these cases; it is withdrawn into the pharynx and the inspired oxygen concentration is reduced to allow safe lasering of an unobstructed field.
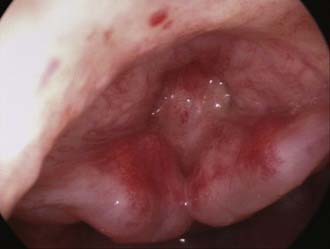
Figure 205-18. Acquired complete glottic stenosis following excessive laser treatment for subglottic hemangioma.
The thulium laser is a continuous wave laser with a wavelength of 2013 nm. It has the advantage of being deliverable down a glass fiber with precise cutting and coagulation characteristics, and limited deep tissue penetration. Its use in the pediatric airway for laryngomalacia, laryngeal cysts, vocal cord palsy, tracheal hemangioma, and papillomatosis has recently been described.110
Techniques employed with the laser include excision of a wide variety of lesions,111 vaporization of tissue, the encouragement of controlled scarring, and its use as a precise dissection tool. Postintubation granulomas can be accurately excised or vaporized, and cystic lesions marsupialized or debulked with good hemostasis and minimal postoperative edema. Subglottic hemangioma can be controlled with regular treatments, although the time to resolution is unchanged and there is a potential risk of subglottic scarring and stenosis especially with the CO2 laser.87–89 Propanolol is a recent therapy that can cause hemangiomas to regress.
Laser arytenoidectomy may be used for vocal cord palsy to remove the arytenoid and posterior vocal cord. This provides a posterior airway while preserving the function of the anterior vocal cord for phonation. Laryngeal papillomatosis responds well to vaporization with the laser, with potential reduction of viable viral particles that may seed down the trachea. Laser epiglottoplasty and supraglottoplasty for laryngomalacia is quick and bloodless,112 and when used in the vallecula, scarring is promoted with elevation of a prolapsing epiglottis. Similarly, KTP laser to suprastomal granulomas secondary to tracheostomy tube placement may improve the airway lumen and stiffen up the anterior tracheal wall, facilitating subsequent decannulation.113,114 Attempts to use the laser to divide anterior webs or subglottic stenosis, however, have generally been unsuccessful due to the tendency for recurrent and sometimes more severe stenoses.
Balloons
Endoscopic treatment of the pediatric airway using vascular-type balloons has become more popular in the last decade. They may be positioned under direct vision using the Hopkins rod or operating microscope and gradually inflated until the desired diameter and pressure are reached. The radial force generated is gentle and there is little mucosal injury. When using a spontaneous inhalational anesthetic technique, the anesthetist must be prepared for a period of total airway occlusion and apnea. One should avoid exceeding the burst pressure of the balloon or overstretching the airway, which potentially risks the development of cartilaginous injury and malacia. There are several situations in which endoscopic balloon dilatation is used for the stridulous child: in the case of early evolving stenosis following airway surgery or intubation, after division of posterior glottic scar bands, with endoscopic cricoid split, and to assist or maintain expansion of tracheal and bronchial stents.96,115–117
Microdébrider
The powered microdébrider was initially introduced to otolaryngologists as an additional tool in the treatment of nasal polyps and endoscopic sinus surgery. The development of longer blades with smaller angled cutting surfaces has seen its use migrate to the airway, including the pediatric airway. The concurrent suction attached to the handpiece allows abnormal tissue to be drawn into the blade and precise excision follows. This has been particularly suitable for cases of recurrent respiratory papillomatosis in which preservation of the submucosal tissues is crucial to avoid damage to the vocal ligament and scarring. In fact, a recent survey of members of the American Society of Pediatric Otolaryngology has noted that the microdébrider is now favored for the treatment of laryngeal papillomatosis over the CO2 laser.118 The technique is relatively fast, safe, and well tolerated. It has also been shown to cause equivalent immediate postoperative pain, greater improvement in voice quality and the overall procedure cost is lower in some centers.119–121 The blade may be passed into the subglottis and upper trachea but access may be limited distally. The microdébrider has also been used to remove granulations and even subglottic hemangioma in some centers.88,122
Stents, Keels, and Grafts
Maintenance of the pediatric airway occasionally requires techniques of anatomic augmentation or the use of foreign materials to prevent collapse and to discourage stenosis. Many of these procedures can be performed endoscopically, thus avoiding an open operation and related potential morbidity. Glottic and subglottic stents may be positioned, secured, and later removed endoscopically. Expandable metallic stents used for tracheal and bronchial pathology can be placed endoscopically under radiologic guidance. Silastic sheeting used as a keel at the anterior commissure in cases of anterior webbing may be temporarily sutured into place through the neck. The use of the Montgomery T-tube in specialized circumstances of laryngotracheal disease can be inserted endoscopically using a heavy Prolene suture to guide the proximal limb toward the larynx. Recent reports of insertion of cartilage expansion grafts into the posterior larynx following endoscopic posterior cricoid split are also promising.123
Endoscopic Sutures
The development of endoscopic instrumentation such as the Lichtenberger needle holder has expanded the options available to the endoscopic airway surgeon. Type I and II laryngotracheoesophageal clefts may be sutured closed after endoscopic trimming of the common mucosal cleft wall. Interrupted sutures are used to close the defect on the tracheal and esophageal aspects with particular attention given to repairing the distal-most point where a fistula may persist. Two-layered repairs of type III laryngeal clefts have also been described, although the technique remains challenging.124 In cases of vocal cord paralysis, endoscopic arytenoidopexy using a lateralizing suture has been performed with some success.125,126 This may be temporizing or permanent.
Endoscopic Administration of Topical Treatments
Certain topical treatments can be applied endoscopically to the pediatric airway to assist in the management of the chronically stridulous child. Direct intralesional injection of papillomas with the antiviral cidofovir after debulking of the lesions is effective in reducing recurrence in approximately 60% of severe cases.127 Its mechanism of action is via inhibition of the HPV viral DNA polymerization process; however, its use is reserved for those patients with aggressively progressing disease as the long-term side effects and potential for malignant transformation have yet to be documented.128 Corticosteroids may be injected into evolving stenoses and into subglottic hemangiomas followed by a short period of postoperative intubation. The results have been encouraging so far.87 Mitomycin C is an antineoplastic agent that has been used against solid tumors in the breast, lung, pancreas, and bladder. It was first used in the airway in 1998129 and has since been applied topically to inhibit fibroblast growth, granulation tissue formation, and cicatricial scarring in many sites including the airway. The optimal dosage has yet to be agreed upon, although most centers tend to prefer 0.2 to 0.4 mg/mL applied on cotton pledgets for up to 5 minutes.130
Principles of Open Laryngeal Surgery
Open procedures may definitively address anatomic narrowing by allowing removal of tissue (stenoses, granulomas and other pathology), decompression of the lumen (cricoid split; Fig. 205-19), or expansion and augmentation with cartilage grafting (laryngotracheal reconstruction (Fig. 205-20), surgical decannulation and stomal reconstruction). Complete resection of a severe stenosis with end-to-end anastomosis is appropriate in some settings (cricotracheal resection). Subglottic hemangiomata may be definitively excised with a single stage open resection incorporating the techniques of cartilage augmentation into the repair. Laryngotracheoesophageal clefts can be repaired in two layers using a temporalis fascia or tibial periosteum graft as a reinforcing layer (Fig. 205-21). Maximal supportive medical therapy in the perioperative period with nutritional support, steroids, antibiotics, and antireflux treatment are important adjuncts to open surgery. Endoscopic follow-up is essential to monitor outcomes, granulations, and tendency to restenosis. This may need to be frequent in the early postoperative stages. (See Chapters 202 and 206 for further coverage of laryngeal surgery.)
Axon PR, Hartley C, Rothera MP. Endoscopic balloon dilatation of subglottic stenosis. J Laryngol Otol. 1995;109:876.
Benjamin B. Prolonged intubation injuries of the larynx: endoscopic diagnosis, classification, and treatment. Ann Otol Rhinol Laryngol Suppl. 1993;160:1.
Bitar MA, Moukarbel RV, Zalzal GH. Management of congenital subglottic hemangioma: trends and success over the past 17 years. Otolaryngol Head Neck Surg. 2005;132:226.
Cherry JD. Clinical practice. Croup. N Engl J Med. 2008;358:384.
Craig MF, Bajaj Y, Hartley BE. Maturation sutures for the paediatric tracheostomy—an extra safety measure. J Laryngol Otol. 2005;119:985.
de Jong AL, Kuppersmith RB, Sulek M, et al. Vocal cord paralysis in infants and children. Otolaryngol Clin North Am. 2000;33:131.
Durden F, Sobol SE. Balloon laryngoplasty as a primary treatment for subglottic stenosis. Arch Otolaryngol Head Neck Surg. 2007;133:772.
Eze NN, Wyatt ME, Hartley BE. The role of the anterior cricoid split in facilitating extubation in infants. Int J Pediatr Otorhinolaryngol. 2005;69:843.
Gupta VK, Cheifetz IM. Heliox administration in the pediatric intensive care unit: an evidence-based review. Pediatr Crit Care Med. 2005;6:2041.
Hartley BE, Cotton RT. Paediatric airway stenosis: laryngotracheal reconstruction or cricotracheal resection? Clin Otolaryngol Allied Sci. 2000;25:342.
Inglis AFJr, Perkins JA, Manning SC, et al. Endoscopic posterior cricoid split and rib grafting in 10 children. Laryngoscope. 2003;113:2004.
Jaquet Y, Lang F, Pilloud R, et al. Partial cricotracheal resection for pediatric subglottic stenosis: long-term outcome in 57 patients. J Thorac Cardiovasc Surg. 2005;130:726.
Lichtenberger G. Reversible immediate and definitive lateralization of paralyzed vocal cords. Eur Arch Otorhinolaryngol. 1999;256:407.
MacKenzie TC, Crombleholme TM, Flake AW. The ex-utero intrapartum treatment. Curr Opin Pediatr. 2002;14:453.
Mathur NN, Peek GJ, Bailey CM, et al. Strategies for managing type IV laryngotracheoesophageal clefts at Great Ormond Street Hospital for Children. Int J Pediatr Otorhinolaryngol. 2006;70:1901.
Pasquale K, Wiatrak B, Woolley A, et al. Microdebrider versus CO2 laser removal of recurrent respiratory papillomas: a prospective analysis. Laryngoscope. 2003;113:139.
Rahbar R, Nicollas R, Roger G, et al. The biology and management of subglottic hemangioma: past, present, future. Laryngoscope. 2004;114:1880.
Rahbar R, Rouillon I, Roger G, et al. The presentation and management of laryngeal cleft: a 10-year experience. Arch Otolaryngol Head Neck Surg. 2006;132:1335.
Sharp HR, Hartley BE. KTP laser treatment of suprastomal obstruction prior to decannulation in paediatric tracheostomy. Int J Pediatr Otorhinolaryngol. 2002;66:125.
Senders CW. Use of mitomycin C in the pediatric airway. Curr Opin Otolaryngol Head Neck Surg. 2004;12:473.
Soma MA, Albert DM. Cidofovir: to use or not to use? Curr Opin Otolaryngol Head Neck Surg. 2008;16:86.
Tweedie DJ, Skilbeck CJ, Cochrane LA, et al. Choosing a paediatric tracheostomy tube: an update on current practice. J Laryngol Otol. 2008;122:161.
Vijayasekaran S, White DR, Hartley BE, et al. Open excision of subglottic hemangiomas to avoid tracheostomy. Arch Otolaryngol Head Neck Surg. 2006;132:159.
Wyatt ME, Bailey CM, Whiteside JC. Update on paediatric tracheostomy tubes. J Laryngol Otol. 1999;113:35.
Wyatt ME, Hartley BE. Laryngotracheal reconstruction in congenital laryngeal webs and atresias. Otolaryngol Head Neck Surg. 2005;132:232.
1. Bent J. Pediatric laryngotracheal obstruction: current perspectives on stridor. Laryngoscope. 2006;116:1059.
2. Hartnick CJ, Cotton RT. Stridor and airway obstruction, 4th ed. Bluestone CD, editor. Pediatric Otolaryngology, Vol 2. Philadelphia: Saunders. 2003.
3. Gonzalez C, Reilly JS, Bluestone CD. Synchronous airway lesions in infancy. Ann Otol Rhinol Laryngol. 1987;96(1 Pt 1):77-80.
4. Pedraza Pena LR, Rodriguez Santana JR, Sifontes JE. Neonatal stridor: a life-threatening condition. P R Health Sci J. 1994;13:33.
5. Cohen SR, Geller KA, Birns JW, et al. Laryngeal paralysis in children: a long-term retrospective study. Ann Otol Rhinol Laryngol. 1982;91(4 Pt 1):417.
6. de Jong AL, Kuppersmith RB, Sulek M, et al. Vocal cord paralysis in infants and children. Otolaryngol Clin North Am. 2000;33:131.
7. Cohen SR. Unusual lesions of the larynx, trachea and bronchial tree. Ann Otol Rhinol Laryngol. 1969;78:476.
8. Orlow SJ, Isakoff MS, Blei F. Increased risk of symptomatic hemangiomas of the airway in association with cutaneous hemangiomas in a “beard” distribution. J Pediatr. 1997;131:646.
9. Nussbaum E, Maggi JC. Laryngomalacia in children. Chest. 1990;98:942.
10. Capitanio MA, Kirkpatrick JA. The lateral decubitus film. An aid in determining air-trapping in children. Radiology. 1972;103:460.
11. Walner DL, Ouanounou S, Donnelly LF, et al. Utility of radiographs in the evaluation of pediatric upper airway obstruction. Ann Otol Rhinol Laryngol. 1999;108:378-383.
12. Vogl T, Wilimzig C, Hofmann U, et al. MRI in tracheal stenosis by innominate artery in children. Pediatr Radiol. 1991;21:89.
13. Dawson KP, Steinberg A, Capaldi N. The lateral radiograph of neck in laryngo-tracheo-bronchitis (croup). J Qual Clin Pract. 1994;14:39.
14. Backer CL, Ilbawi MN, Idriss FS, et al. Vascular anomalies causing tracheoesophageal compression. Review of experience in children. J Thorac Cardiovasc Surg. 1989;97:725.
15. Han MT, Hall DG, Manche A, et al. Double aortic arch causing tracheoesophageal compression. Am J Surg. 1993;165:628.
16. Hofmann U, Hofmann D, Vogl T, et al. Magnetic resonance imaging as a new diagnostic criterion in paediatric airway obstruction. Prog Pediatr Surg. 1991;27:221.
17. Vogl T, Wilimzig C, Bilaniuk LT, et al. MR imaging in pediatric airway obstruction. J Comput Assist Tomogr. 1990;14:182.
18. Burke AJ, Vining DJ, McGuirt WFJr, et al. Evaluation of airway obstruction using virtual endoscopy. Laryngoscope. 2000;110:23.
19. Vats A, Worley GA, de Bruyn R, et al. Laryngeal ultrasound to assess vocal fold paralysis in children. J Laryngol Otol. 2004;118:429.
20. Scott AR, Chong PS, Randolph GW, et al. Intraoperative laryngeal electromyography in children with vocal fold immobility: a simplified technique. Int J Pediatr Otorhinolaryngol. 2008;72:31.
21. Goldberg S, Shatz A, Picard E, et al. Endoscopic findings in children with obstructive sleep apnea: effects of age and hypotonia. Pediatr Pulmonol. 2005;40:205.
22. Contencin P, Gumpert LC, de Gaudemar I, et al. Non-endoscopic techniques for the evaluation of the pediatric airway. Int J Pediatr Otorhinolaryngol. 1997;41:347.
23. Burton DM, Pransky SM, Katz RM, et al. Pediatric airway manifestations of gastroesophageal reflux. Ann Otol Rhinol Laryngol. 1992;101:742.
24. Contencin P, Maurage C, Ployet MJ, et al. Gastroesophageal reflux and ENT disorders in childhood. Int J Pediatr Otorhinolaryngol. 1995;32(Suppl):S135.
25. Krishnamoorthy M, Mintz A, Liem T, et al. Diagnosis and treatment of respiratory symptoms of initially unsuspected gastroesophageal reflux in infants. Am Surg. 1994;60:783.
26. Nielson DW, Heldt GP, Tooley WH. Stridor and gastroesophageal reflux in infants. Pediatrics. 1990;85:1034.
27. Zalzal GH. Stridor and airway compromise. Pediatr Clin North Am. 1989;36(6):1389-1402.
28. Nussbaum E. Usefulness of miniature flexible fiberoptic bronchoscopy in children. Chest. 1994;106:1438.
29. Eber E, Zach M. [Flexible fiberoptic bronchoscopy in pediatrics—an analysis of 420 examinations]. Wien Klin Wochenschr. 1995;107:246.
30. Raine J, Warner JO. Fibreoptic bronchoscopy without general anaesthetic. Arch Dis Child. 1991;66:481.
31. Todres ID, Noviski N. Flexible fiberoptic bronchoscopy: a practical guide to examining infants and children. Mt Sinai J Med. 1995;62:36.
32. Handler SD. Direct laryngoscopy in children: rigid and flexible fiberoptic. Ear Nose Throat J. 1995;74:100.
33. Teitelbaum DH. Bronchoscopy and esophagoscopy in children. Curr Opin Pediatr. 1993;5:341.
34. Hoeve LJ, Rombout J. Pediatric laryngobronchoscopy. 1332 procedures stored in a data base. Int J Pediatr Otorhinolaryngol. 1992;24:73.
35. Johannesson GP, Floren M, Lindahl SG. Sevoflurane for ENT-surgery in children. A comparison with halothane. Acta Anaesthesiol Scand. 1995;39:546.
36. Amitai Y, Zylber-Katz E, Avital A, et al. Serum lidocaine concentrations in children during bronchoscopy with topical anesthesia. Chest. 1990;98:1370.
37. Sitbon P, Laffon M, Lesage V, et al. Lidocaine plasma concentrations in pediatric patients after providing airway topical anesthesia from a calibrated device. Anesth Analg. 1996;82(5):1003-1006.
38. Depierraz B, Ravussin P, Brossard E, et al. Percutaneous transtracheal jet ventilation for paediatric endoscopic laser treatment of laryngeal and subglottic lesions. Can J Anaesth. 1994;41:1200.
39. Evans KL, Keene MH, Bristow AS. High-frequency jet ventilation—a review of its role in laryngology. J Laryngol Otol. 1994;108:23.
40. Shikowitz MJ, Abramson AL, Liberatore L. Endolaryngeal jet ventilation: a 10-year review. Laryngoscope. 1991;101:455.
41. Ostfeld E, Ovadia L. Bilateral tension pneumothorax during pediatric bronchoscopy (high-frequency jet injection ventilation). Int J Pediatr Otorhinolaryngol. 1984;7:301.
42. Holinger L, Lusk RP, Green CG, editors. Pediatric Laryngology and Bronchoesophagology. Philadelphia: Lippincott-Raven Publishers, 1997.
43. Tunkel DE, Fisher QA. Pediatric flexible fiberoptic bronchoscopy through the laryngeal mask airway. Arch Otolaryngol Head Neck Surg. 1996;122:1364.
44. Wyatt ME, Bailey CM, Whiteside JC. Update on paediatric tracheostomy tubes. J Laryngol Otol. 1999;113:35.
45. Myer CM3rd, O’Connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol. 1994;103(Pt 1):319.
46. Elliott M, Hartley BE, Wallis C, et al. Slide tracheoplasty. Curr Opin Otolaryngol Head Neck Surg. 2008;16:75.
47. aWengen DF, Probst RR, Frei FJ. Flexible laryngoscopy in neonates and infants: insertion through a median opening in the face mask. Int J Pediatr Otorhinolaryngol. 1991;21:183.
48. Hoeve LJ, Rombout J, Meursing AE. Complications of rigid laryngo-bronchoscopy in children. Int J Pediatr Otorhinolaryngol. 1993;26:47.
49. Parsons DS, Smith RB, Mair EA, et al. Unique case presentations of acute epiglottic swelling and a protocol for acute airway compromise. Laryngoscope. 1996;106:1287.
50. Gupta VK, Cheifetz IM. Heliox administration in the pediatric intensive care unit: an evidence-based review. Pediatr Crit Care Med. 2005;6:204.
51. Wolfson MR, Bhutani VK, Shaffer TH, et al. Mechanics and energetics of breathing helium in infants with bronchopulmonary dysplasia. J Pediatr. 1984;104:752.
52. Rogers M. Textbook of Pediatric Intensive Care, 3rd ed. Baltimore: Lippincott Williams & Wilkins; 1996.
53. Moore M, Little P. Humidified air inhalation for treating croup. Cochrane Database Syst Rev. 2006;3:CD002870.
54. Fitzgerald D, Mellis C, Johnson M, et al. Nebulized budesonide is as effective as nebulized adrenaline in moderately severe croup. Pediatrics. 1996;97:722.
55. Russell K, Wiebe N, Saenz A, et al. Glucocorticoids for croup. Cochrane Database Syst Rev 2004:CD001955.
56. Klassen TP. Croup. A current perspective. Pediatr Clin North Am. 1999;46:1167.
57. Cherry JD. Clinical practice. Croup. N Engl J Med. 2008;358:384.
58. Gonzalez Valdepena H, Wald ER, Rose E, et al. Epiglottitis and Haemophilus influenzae immunization: the Pittsburgh experience—a five-year review. Pediatrics. 1995;96(Pt 1):424.
59. Herceg A. The decline of Haemophilus influenzae type b disease in Australia. Commun Dis Intell. 1997;21:173.
60. Rotta AT, Wiryawan B. Respiratory emergencies in children. Respir Care. 2003;48:248.
61. Stroud RH, Friedman NR. An update on inflammatory disorders of the pediatric airway: epiglottitis, croup, and tracheitis. Am J Otolaryngol. 2001;22:268.
62. Robb PJ. Failure of intubation in acute inflammatory airway obstruction in childhood. J Laryngol Otol. 1985;99:993.
63. Harries ML, Albert DM. Bronchoscopic foreign bodies: overcoming granulation tissue. J Otolaryngol. 1993;22:134.
64. Nutman J, Brooks LJ, Deakins KM, et al. Racemic versus l-epinephrine aerosol in the treatment of postextubation laryngeal edema: results from a prospective, randomized, double-blind study. Crit Care Med. 1994;22:1591.
65. Couser RJ, Ferrara TB, Falde B, et al. Effectiveness of dexamethasone in preventing extubation failure in preterm infants at increased risk for airway edema. J Pediatr. 1992;121:591.
66. Gould SJ, Young M. Subglottic ulceration and healing following endotracheal intubation in the neonate: a morphometric study. Ann Otol Rhinol Laryngol. 1992;101:815.
67. Graham JM. Formal reintubation for incipient neonatal subglottic stenosis. J Laryngol Otol. 1994;108:474.
68. Hoeve LJ, Eskici O, Verwoerd CD. Therapeutic reintubation for post-intubation laryngotracheal injury in preterm infants. Int J Pediatr Otorhinolaryngol. 1995;31:7.
69. Holinger LD, Birnholz JC. Management of infants with prenatal ultrasound diagnosis of airway obstruction by teratoma. Ann Otol Rhinol Laryngol. 1987;96(Pt 1):61.
70. Holinger LD, Birnholz JC, Bruce DR, et al. Management of an infant with prenatal ultrasound diagnosis of upper airway obstruction. Int J Pediatr Otorhinolaryngol. 1985;10:263.
71. Skarsgard ED, Chitkara U, Krane EJ, et al. The OOPS procedure (operation on placental support): in utero airway management of the fetus with prenatally diagnosed tracheal obstruction. J Pediatr Surg. 1996;31:826.
72. Skelton VA, Goodwin A. Perinatal management of a neonate with airway obstruction caused by rhabdomyosarcoma of the tongue. Br J Anaesth. 1999;83:951.
73. Catalano PJ, Urken ML, Alvarez M, et al. New approach to the management of airway obstruction in “high risk” neonates. Arch Otolaryngol Head Neck Surg. 1992;118:306.
74. Hubbard AM, Crombleholme TM, Adzick NS. Prenatal MRI evaluation of giant neck masses in preparation for the fetal exit procedure. Am J Perinatol. 1998;15:253.
75. DeCou JM, Jones DC, Jacobs HD, et al. Successful ex utero intrapartum treatment (EXIT) procedure for congenital high airway obstruction syndrome (CHAOS) owing to laryngeal atresia. J Pediatr Surg. 1998;33:1563.
76. MacKenzie TC, Crombleholme TM, Flake AW. The ex-utero intrapartum treatment. Curr Opin Pediatr. 2002;14:453.
77. Hedrick MH, Ferro MM, Filly RA, et al. Congenital high airway obstruction syndrome (CHAOS): a potential for perinatal intervention. J Pediatr Surg. 1994;29:271.
78. Zerella JT, Finberg FJ. Obstruction of the neonatal airway from teratomas. Surg Gynecol Obstet. 1990;170:126.
79. Bui TH, Grunewald C, Frenckner B, et al. Successful EXIT (ex utero intrapartum treatment) procedure in a fetus diagnosed prenatally with congenital high-airway obstruction syndrome due to laryngeal atresia. Eur J Pediatr Surg. 2000;10:328.
80. Larsen ME, Larsen JW, Hamersley SL, et al. Successful management of fetal cervical teratoma using the EXIT procedure. J Matern Fetal Med. 1999;8:295.
81. Kelly MF, Berenholz L, Rizzo KA, et al. Approach for oxygenation of the newborn with airway obstruction due to a cervical mass. Ann Otol Rhinol Laryngol. 1990;99(Pt 1):179.
82. Gandini D, Brimacombe JR. Neonatal resuscitation with the laryngeal mask airway in normal and low birth weight infants. Anesth Analg. 1999;89:642.
83. Cohen MS, Rothschild MA, Moscoso J, et al. Perinatal management of unanticipated congenital laryngeal atresia. Arch Otolaryngol Head Neck Surg. 1998;124:1368.
84. Tweedie DJ, Skilbeck CJ, Lloyd-Thomas AR, et al. The nasopharyngeal prong airway: an effective post-operative adjunct after adenotonsillectomy for obstructive sleep apnoea in children. Int J Pediatr Otorhinolaryngol. 2007;71:563.
85. Stavroulaki P. Diagnostic and management problems of laryngopharyngeal reflux disease in children. Int J Pediatr Otorhinolaryngol. 2006;70:579.
86. Rosbe KW, Kenna MA, Auerbach AD. Extraesophageal reflux in pediatric patients with upper respiratory symptoms. Arch Otolaryngol Head Neck Surg. 2003;129:1213.
87. Bitar MA, Moukarbel RV, Zalzal GH. Management of congenital subglottic hemangioma: trends and success over the past 17 years. Otolaryngol Head Neck Surg. 2005;132:226.
88. Pransky SM, Canto C. Management of subglottic hemangioma. Curr Opin Otolaryngol Head Neck Surg. 2004;12:509.
89. Rahbar R, Nicollas R, Roger G, et al. The biology and management of subglottic hemangioma: past, present, future. Laryngoscope. 2004;114:1880.
90. Hoeve LJ, Joosten KF. [The child with a tracheostomy, past and present: different indications, different children, different care]. Ned Tijdschr Geneeskd. 2007;151:2308.
91. Carron JD, Derkay CS, Strope GL, et al. Pediatric tracheotomies: changing indications and outcomes. Laryngoscope. 2000;110:1099.
92. Lewis CW, Carron JD, Perkins JA, et al. Tracheotomy in pediatric patients: a national perspective. Arch Otolaryngol Head Neck Surg. 2003;129:523.
93. Balakrishnan A. The cricoid split: an alternative to paediatric tracheostomy. Ann Acad Med Singapore. 1991;20:700.
94. Berkowitz RG. Experience with anterior cricoid split for infantile subglottic stenosis. J Paediatr Child Health. 1994;30:345.
95. Richardson MA, Inglis AFJr. A comparison of anterior cricoid split with and without costal cartilage graft for acquired subglottic stenosis. Int J Pediatr Otorhinolaryngol. 1991;22:187.
96. Axon PR, Hartley C, Rothera MP. Endoscopic balloon dilatation of subglottic stenosis. J Laryngol Otol. 1995;109:876.
97. McQueen CT, Shapiro NL, Leighton S, et al. Single-stage laryngotracheal reconstruction: the Great Ormond Street experience and guidelines for patient selection. Arch Otolaryngol Head Neck Surg. 1999;125:320.
98. Saunders MW, Thirlwall A, Jacob A, et al. Single-or-two-stage laryngotracheal reconstruction; comparison of outcomes. Int J Pediatr Otorhinolaryngol. 1999;50:51.
99. Craig MF, Bajaj Y, Hartley BE. Maturation sutures for the paediatric tracheostomy—an extra safety measure. J Laryngol Otol. 2005;119:985.
100. Park JY, Suskind DL, Prater D, et al. Maturation of the pediatric tracheostomy stoma: effect on complications. Ann Otol Rhinol Laryngol. 1999;108:1115.
101. Tweedie DJ, Skilbeck CJ, Cochrane LA, et al. Choosing a paediatric tracheostomy tube: an update on current practice. J Laryngol Otol. 2008;122:161.
102. Benjamin B. Prolonged intubation injuries of the larynx: endoscopic diagnosis, classification, and treatment. Ann Otol Rhinol Laryngol Suppl. 1993;160:1.
103. Lyons MJ, Cooke J, Cochrane LA, et al. Safe reliable atraumatic replacement of misplaced paediatric tracheostomy tubes. Int J Pediatr Otorhinolaryngol. 2007;71:1743.
104. Terada Y, Yamabuki K, Fukushima T, et al. [A case report of surgically treated tracheobrachiocephalic artery fistula following tracheostomy]. Kyobu Geka. 1993;46:862.
105. Prescott CA. Factors that influence successful decannulation after surgery for laryngo-tracheal stenosis in children. Int J Pediatr Otorhinolaryngol. 1994;30:183.
106. Lee TS, Wu Y. Bedside fiberoptic bronchoscopy for tracheostomy decannulation. Respir Med. 1995;89:571.
107. Friedberg J, Giberson W. Failed tracheotomy decannulation in children. J Otolaryngol. 1992;21:404.
108. Kelly SM, Gray SD. Unilateral endoscopic supraglottoplasty for severe laryngomalacia. Arch Otolaryngol Head Neck Surg. 1995;121:1351.
109. Cochrane LA, Albert DM. Endoscopic Cricoid Split in the Neonate. American Society of Pediatric Otolaryngology. San Diego, 2007.
110. Ayari-Khalfallah S, Fuchsmann C, Froehlich P. Thulium laser in airway diseases in children. Curr Opin Otolaryngol Head Neck Surg. 2008;16:55.
111. Bagwell CE. CO2 laser excision of pediatric airway lesions. J Pediatr Surg. 1990;25:1152.
112. Lee KS, Chen BN, Yang CC, et al. CO2 laser supraglottoplasty for severe laryngomalacia: a study of symptomatic improvement. Int J Pediatr Otorhinolaryngol. 2007;71:889.
113. Mandell DL, Yellon RF. Endoscopic KTP laser excision of severe tracheotomy-associated suprastomal collapse. Int J Pediatr Otorhinolaryngol. 2004;68:1423.
114. Sharp HR, Hartley BE. KTP laser treatment of suprastomal obstruction prior to decannulation in paediatric tracheostomy. Int J Pediatr Otorhinolaryngol. 2002;66:125.
115. Bagwell CE, Talbert JL, Tepas JJ3rd. Balloon dilatation of long-segment tracheal stenoses. J Pediatr Surg. 1991;26:153.
116. Durden F, Sobol SE. Balloon laryngoplasty as a primary treatment for subglottic stenosis. Arch Otolaryngol Head Neck Surg. 2007;133:772.
117. Maeda K, Yasufuku M, Yamamoto T. A new approach to the treatment of congenital tracheal stenosis: Balloon tracheoplasty and expandable metallic stenting. J Pediatr Surg. 2001;36:1646.
118. Schraff S, Derkay CS, Burke B, et al. American Society of Pediatric Otolaryngology members’ experience with recurrent respiratory papillomatosis and the use of adjuvant therapy. Arch Otolaryngol Head Neck Surg. 2004;130:1039.
119. El-Bitar MA, Zalzal GH. Powered instrumentation in the treatment of recurrent respiratory papillomatosis: an alternative to the carbon dioxide laser. Arch Otolaryngol Head Neck Surg. 2002;128:425.
120. Pasquale K, Wiatrak B, Woolley A, et al. Microdebrider versus CO2 laser removal of recurrent respiratory papillomas: a prospective analysis. Laryngoscope. 2003;113:139.
121. Patel RS, MacKenzie K. Powered laryngeal shavers and laryngeal papillomatosis: a preliminary report. Clin Otolaryngol Allied Sci. 2000;25:358.
122. Lunn W, Garland R, Ashiku S, et al. Microdebrider bronchoscopy: a new tool for the interventional bronchoscopist. Ann Thorac Surg. 2005;80:1485.
123. Inglis AFJr, Perkins JA, Manning SC, et al. Endoscopic posterior cricoid split and rib grafting in 10 children. Laryngoscope. 2003;113:2004.
124. Sandu K, Monnier P. Endoscopic laryngotracheal cleft repair without tracheotomy or intubation. Laryngoscope. 2006;116:630.
125. Lichtenberger G. Reversible immediate and definitive lateralization of paralyzed vocal cords. Eur Arch Otorhinolaryngol. 1999;256:407.
126. Mathur NN, Kumar S, Bothra R. Simple method of vocal cord lateralization in bilateral abductor cord paralysis in paediatric patients. Int J Pediatr Otorhinolaryngol. 2004;68:15.
127. Chadha NK, James AL. Antiviral agents for the treatment of recurrent respiratory papillomatosis: a systematic review of the English-language literature. Otolaryngol Head Neck Surg. 2007;136:863.
128. Soma MA, Albert DM. Cidofovir: to use or not to use? Curr Opin Otolaryngol Head Neck Surg. 2008;16:86.
129. Ward RF, April MM. Mitomycin-C in the treatment of tracheal cicatrix after tracheal reconstruction. Int J Pediatr Otorhinolaryngol. 1998;44:221.
130. Senders CW. Use of mitomycin C in the pediatric airway. Curr Opin Otolaryngol Head Neck Surg. 2004;12:473.
131. Benjamin B, Inglis A. Minor congenital laryngeal clefts: diagnosis and classification. Ann Otol Rhinol Laryngol. 1989;98:417.