25 Essentials of Endocrinology
Diabetes Mellitus*
The incidence of both type 1 and type 2 diabetes mellitus in children is increasing worldwide.1–6 The use of insulin pumps and various multicomponent insulin regimens has increased the complexity of perioperative management of children with diabetes. Anesthesiologists must carefully consider the pathophysiology of the disease, as well as each child’s specific diabetes treatment regimen, glycemic control, intended surgery, and anticipated postoperative course, when devising an appropriate perioperative management plan. Standardized algorithms for perioperative diabetes management improve care7–9 without significantly increasing costs9; several guidelines and studies of perioperative management of children with diabetes are available in the literature and examples are included.10–15
Classification and Epidemiology in Children
Type 1 and type 2 are the most common forms of diabetes.16,17 Type 1 diabetes is characterized by absolute deficiency of insulin, which usually results from immune-mediated destruction of pancreatic beta cells.17 In contrast, type 2 diabetes results from a combination of insulin resistance and a relative deficiency of insulin.16,17 Children with type 2 diabetes typically are overweight and frequently have a first- or second-degree relative with type 2 diabetes.16 However, the increasing prevalence of obesity in children18 has made distinction between type 1 and type 2 diabetes, at times, rather difficult. Children with phenotypic characteristics of type 2 diabetes may have pancreatic autoimmunity,19–21 and approximately 35% of children with diabetes who require exogenous insulin at diagnosis are overweight or obese,22,23 consistent with the prevalence of childhood overweight and obesity in the general U.S. pediatric population.24 Other forms of diabetes may be less commonly encountered (Table 25-1). Additional modifications to the perioperative treatment regimen may be necessary when diabetes is associated with genetic syndromes and/or other endocrinopathies, such as adrenal insufficiency (see later discussion).
TABLE 25-1 Classification of Less Common Forms of Diabetes Mellitus
Modified from Rhodes ET, Ferrari LR, Wolfsdorf JI. Perioperative management of pediatric surgical patients with diabetes mellitus. Anesth Analg 2005;101:986-99.
Although the worldwide incidence of type 1 diabetes is quite variable,25 the incidence is increasing in almost all populations throughout the world.4 In the United States, the SEARCH for Diabetes in Youth Study, which began in 2000, provides the most comprehensive estimates of the prevalence and incidence of type 1 and type 2 diabetes among youth less than 20 years of age in the United States.26–30 Type 1 diabetes remains the most common form of diabetes observed among youth in the United States, with the greatest prevalence (2 per 1000) among non-Hispanic Caucasians.29 The epidemic of obesity has also contributed to a progressive increase in the incidence and prevalence of type 2 diabetes in U.S. children.1 In the SEARCH study, the prevalence of type 2 diabetes in 10- to 19-year-old youth ranged from 0.18 per 1000 among non-Hispanic Caucasian youth to 1.45 per 1000 among Navajo youth, and the incidence ranged from 3.7 per 100,000 per year among non-Hispanic Caucasian youth to 27.7 per 100,000 per year among Navajo youth.29,30 Increases in the incidence and prevalence of type 2 diabetes have been noted in other parts of the world.2,3,5,6,31–34
General Management Principles
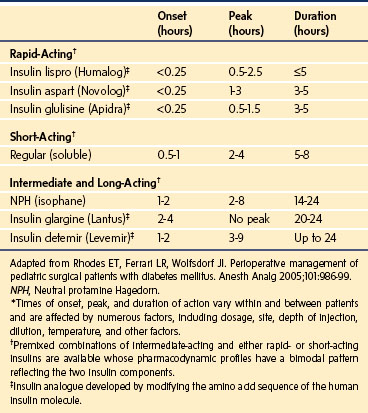
Type 1 diabetes always requires treatment with insulin. However, an increasing number of insulin preparations (Table 25-2) and delivery systems are available. The least complex insulin regimens consist of two or three insulin injections per day. They incorporate a combination of an intermediate-acting insulin (e.g., neutral protamine Hagedorn [NPH]) and/or a long-acting insulin (e.g., insulin detemir [Levemir] or insulin glargine [Lantus]) for basal coverage with a short- or rapid-acting insulin (e.g., regular, insulin aspart [NovoLog], insulin lispro [Humalog], or insulin glulisine [Apidra]) to provide prandial glycemic coverage. More intensive insulin regimens are being used with greater frequency and typically consist of insulin glargine, a long-acting insulin, which provides a relatively constant 24-hour basal concentration of circulating insulin without a pronounced peak, to simulate basal insulin secretion,35 in conjunction with rapid-acting insulin administered with food. Some studies have demonstrated superior glycemic control with such regimens as compared with regimens using NPH insulin and regular insulin,36 although the ideal insulin regimen for children with type 1 diabetes remains controversial.37 Another long-acting insulin, insulin detemir, may also be used in intensive insulin regimens and has demonstrated more predictable glucose-reducing effects than both NPH insulin38–40 and insulin glargine.38
Many children with type 1 diabetes are managed with an insulin pump,41,42 a device that administers a continuous subcutaneous infusion of insulin (typically a rapid-acting insulin, see Table 25-2) at a basal rate that is supplemented by bolus doses of insulin given with meals and snacks and to correct hyperglycemia. In appropriately selected children, pump therapy has shown superiority over injection regimens.43–46 Standard insulin preparations are U100, meaning that there are 100 units of insulin per milliliter. However, very young patients with type 1 diabetes may require diluted insulin (e.g., U10) to achieve accurate dosing.47 Parents of toddlers with type 1 diabetes should be specifically questioned about their use of diluted insulin.
Most children with type 2 diabetes are managed with insulin and/or oral metformin, the only oral agent approved for use in children with diabetes in the United States.48–50 Metformin’s primary action is to decrease hepatic glucose production and, secondarily, to increase insulin sensitivity in peripheral tissues. Occasionally, other oral agents, including sulfonylureas, which promote insulin secretion, and thiazolidinediones, which increase insulin sensitivity in muscle and adipose tissue, are used in adolescents.48,51 Nutritional therapy is always included in the management of children with type 2 diabetes. Studies, such as the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) trial,52,53 are evaluating the optimal treatment for type 2 diabetes. Monotherapy with metformin was associated with durable glycemic control in approximately half of children and adolescents with type 2 diabetes. The addition of rosiglitazone, but not an intensive lifestyle intervention, was superior to metformin alone.
New medications introduced to manage adults with diabetes may eventually become available for use in children. Incretins, including glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (GLP-1), are gastrointestinal hormones released after eating that stimulate insulin secretion and are necessary for normal glucose tolerance.54,55 GLP-1 acts through a G protein–coupled receptor to promote glucose-dependent insulin secretion, suppression of glucagon secretion, slowing of gastric emptying, and reduction in food intake.54 Exenatide (Byetta) is a GLP-1 receptor agonist widely used in adults with type 2 diabetes as an adjunctive therapy for those taking metformin and/or a sulfonylurea.56 Inhibitors of dipeptidyl peptidase-IV,57 the enzyme that degrades GLP-1, are also increasingly being used for the treatment of type 2 diabetes in adults, but have not been approved for use in children. Pramlintide acetate (Symlin) is a synthetic amylin receptor agonist that can be used as an adjunct to insulin therapy in adults with type 1 or type 2 diabetes.58–61 Amylin, a 37-amino acid polypeptide islet hormone cosecreted with insulin from beta islet cells,54 has three effects: delays gastric emptying, inhibits glucagon secretion, and modulates satiety.54
Metabolic Response to Surgery
Trauma of any kind, and surgery in particular, causes a complex neuroendocrine stress response, including suppression of insulin secretion and increased production of counter-regulatory hormones (frequently called “stress hormones” in the anesthesiology literature), particularly cortisol and catecholamines.62,63 Insulin is the primary anabolic hormone that promotes glucose uptake in muscle and adipose tissue while suppressing glucose production (glycogenolysis and gluconeogenesis) by the liver.64 The counter-regulatory hormones, which include epinephrine, glucagon, cortisol, and growth hormone, exert the opposite effects, resulting in resistance to insulin action65–67 and an increase in blood glucose concentration by: (1) stimulating glycogenolysis and gluconeogenesis in the liver, (2) increasing lipolysis and ketogenesis, and (3) inhibiting glucose uptake and utilization in muscle and fat. Glucagon, secreted by alpha cells in the pancreatic islets, suppresses insulin secretion while stimulating hepatic glycogenolysis, gluconeogenesis, and ketogenesis.64,65 Epinephrine, which acts via β2– and α2-adrenergic receptors, stimulates glucagon production, increases glycogenolysis and gluconeogenesis, stimulates lipolysis, decreases insulin secretion, and decreases glucose utilization in insulin-sensitive tissues.64 Cortisol stimulates gluconeogenesis, proteolysis, and lipolysis, and decreases glucose utilization.65,68 Growth hormone augments glucose production, decreases glucose utilization, and accelerates lipolysis.69 Pro-inflammatory cytokines may further stimulate secretion of counter-regulatory hormones and alter insulin receptor signaling.67 These changes increase catabolism, as evidenced by increased hepatic glucose production and breakdown of protein and fat. In the diabetic patient with absolute or relative insulin deficiency, the enhanced catabolism resulting from surgical trauma can lead to marked hyperglycemia and even diabetic ketoacidosis.70 These metabolic effects may be exacerbated by a prolonged fast before and during surgery.
Metabolic Response to Anesthesia
Although adequate analgesia is essential to minimize the neuroendocrine stress response to surgery, some anesthetics may also independently contribute to perioperative hyperglycemia.10,71 Inhalation anesthetics, such as isoflurane, may cause hyperglycemia by inhibiting insulin secretion72,73; the hyperglycemia results from both impaired glucose uptake and increased glucose production.71 In contrast, epidural analgesia with local anesthetics prevents this hyperglycemic effect74,75 through an inhibitory effect on endogenous glucose production.71 Similarly, intravenous (IV) anesthesia with opioids mitigates the hyperglycemic response of surgery71,76,77 through its apparent neutral effect on endogenous glucose production but decrease in glucose clearance.71 Although these differences are important to consider, the metabolic effects of anesthesia are relatively minor compared with the direct effects of surgery.10
Adverse Consequences of Hyperglycemia
Hyperglycemia can impair wound healing by hindering collagen production, which may decrease the tensile strength of the surgical wound.78 Hyperglycemia may also have adverse effects on neutrophil function, including decreased chemotaxis, phagocytosis, and bactericidal killing.79–82 Evidence from controlled experimental studies in rabbits demonstrated that these effects may be reversed, in part, by glycemia-independent effects of insulin.83 However, the overall benefits of intensive insulin therapy, including a reduction in mortality, were derived mainly from maintenance of normoglycemia, whereas glycemia-independent actions of insulin exert only minor, organ-specific effects.83
Clinical studies in humans have not consistently supported the relationship between perioperative glycemic control and short-term risk of infection or morbidity.84,85 However, several studies in diabetic adults undergoing surgery have shown an association between postoperative hyperglycemia and infectious complications.86,87 A meta-analysis showed that patients in surgical intensive care units (ICUs) appear to benefit from intensive insulin therapy, whereas patients in other ICU settings do not.88 These outcomes have contributed to confusion regarding specific glycemic targets and the means for achieving them in both critically and noncritically ill patients. A recent consensus statement89 recommended that an insulin infusion should be used to control hyperglycemia in the majority of critically ill patients in the ICU setting, with a starting threshold of no greater than 180 mg/dL. Once IV insulin therapy has been initiated, the glucose level should be maintained between 140 and 180 mg/dL. With no prospective, randomized controlled trial data for establishing specific guidelines in noncritically ill patients treated with insulin, pre-meal glucose targets should generally be less than 140 mg/dL and random blood glucose values less than 180 mg/dL, as long as these targets can be safely achieved. To avoid hypoglycemia, one should reassess the insulin regimen if blood glucose levels decline below 100 mg/dL. Modification of the insulin regimen is necessary when blood glucose values are less than 70 mg/dL, unless the event is easily explained by other factors (such as a missed meal).89
The single pediatric trial to date demonstrated shorter length of stay, attenuated inflammatory response, and decreased mortality in patients randomized to targeting of age-adjusted normoglycemia.90 However, the rate of severe hypoglycemia (less than 40 mg/dL) in that study was too frequent, at 25% of the total cohort, making the implementation of this approach ill-advised without further evidence from ongoing multicenter pediatric trials. Other retrospective studies of critically ill children managed in pediatric ICUs have also demonstrated associations of hyperglycemia with morbidity and mortality.91–94 In the 1980s and 1990s the dogma of tight glucose control replaced an era in which the mantra of caring for diabetic surgical patients was “keep them sweet,” but in more recent years glucose control has become controversial and is unresolved, with conflicting evidence; a systematic review of the literature concluded that there are insufficient data regarding the best strategy or regimen to attain target blood glucose levels in ambulatory surgical patients with diabetes mellitus.95 The recommended practice for subspecialties, such as cardiac, neurosurgical, and solid organ transplant surgical patients, requires still more study, and it must be highlighted that few of the published investigations and meta-analyses have focused specifically on the pediatric age group.
Preoperative Assessment
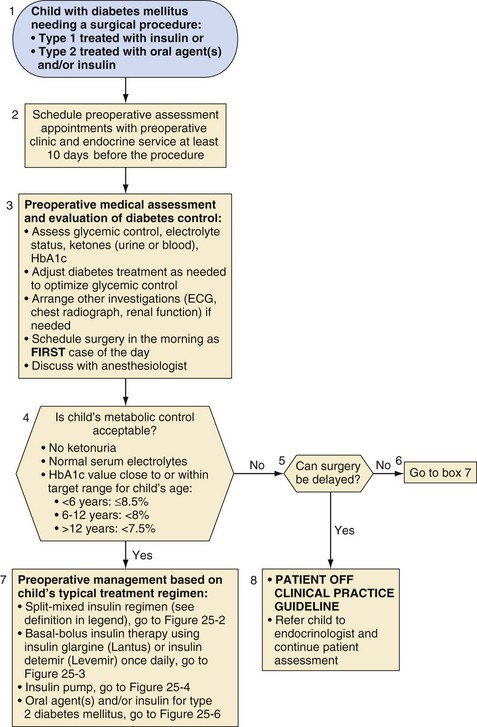
When feasible, children with diabetes should not undergo elective surgery until they are metabolically stable (Fig. 25-1, box 4); that is, there is no ketosis, serum electrolytes are normal, and the glycated hemoglobin (HbA1c) value is close to or within the ideal range for the child’s age. Although there is no consensus regarding the ideal metabolic targets for control of diabetes in children,96 the American Diabetes Association recommends an HbA1c less than or equal to 8.5% for children younger than 6 years of age; less than 8% for children 6 to 12 years of age; and less than 7.5% for 13 years of age or older, with a more stringent target of less than 7% to be considered in this last age group, for children in whom this can be achieved without excessive hypoglycemia,96 as well as for those with type 2 diabetes.16 The preoperative consultation to assess the adequacy of metabolic control should be scheduled at least 10 days before the procedure (see Fig. 25-1, box 3). If metabolic control is poor, surgery should be delayed if possible. Both the endocrinology and anesthesiology services should participate in this assessment. Whenever possible, surgery for children with diabetes should be scheduled as the first case in the morning so that prolonged fasting is avoided and diabetes treatment regimens may be most easily adjusted.
Preoperative Management
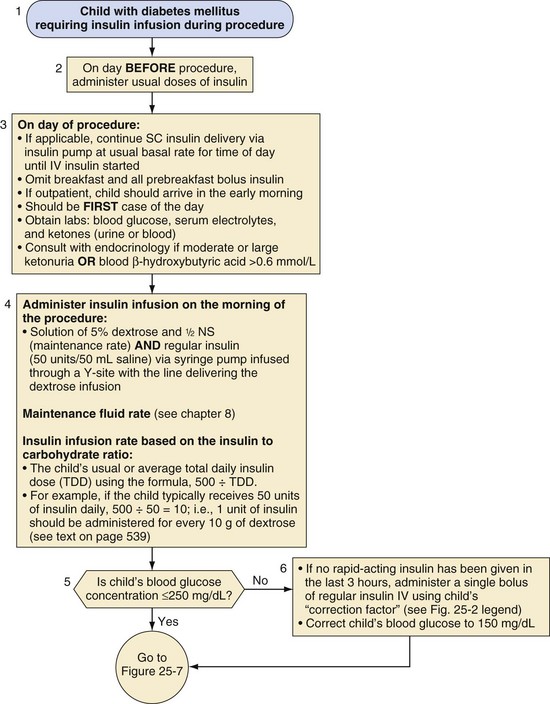
The regimen for managing diabetes before, during, and after a surgical or diagnostic procedure that requires the child to fast should aim to maintain near-normoglycemia, that is, blood glucose levels of 100 to 200 mg/dL. In this blood glucose range there will be a reduced risk of osmotic diuresis, dehydration, electrolyte imbalance, metabolic acidosis, infection, and hypoglycemia in the sedated child who may be unaware of hypoglycemia or unable to communicate with staff.97 The child need not be admitted before the day of surgery, but rather early on the same morning as the surgery or procedure. Parents should receive explicit written instructions regarding appropriate modifications of their child’s diabetes regimen before and for the day of surgery. On admission to the hospital, metabolic control should be assessed, including a preoperative determination of glucose concentration. On the morning of surgery, no rapid- or short-acting acting insulin should be administered unless the blood glucose level exceeds 250 mg/dL. However, admission to the hospital before major surgical procedures in children10 and adults has been recommended if the metabolic status needs to be optimized preoperatively.65 If the surgery must be delayed for any reason, frequent blood glucose monitoring is mandatory to prevent perioperative hypoglycemia or hyperglycemia.
If the blood glucose exceeds 250 mg/dL, a conservative dose of rapid-acting insulin (e.g., insulin lispro or insulin aspart) or short-acting insulin (regular) is administered to restore near-normoglycemia. This is achieved using the child’s usual sliding scale or a “correction factor.” The insulin “correction factor” is the decrease in the blood glucose concentration expected after administering 1 unit of rapid- or short-acting insulin. This can be calculated using the “1500 rule”: divide 1500 by the child’s usual total daily dose (TDD) of insulin. For example, if a child typically receives 30 units of insulin daily, this child’s “correction factor” would be 1500 ÷ 30 = 50. Therefore 1 unit of rapid- or short-acting insulin would be expected to decrease the child’s blood glucose concentration by approximately 50 mg/dL. Various correction factors have been described, including a “1500 rule” for short-acting insulin (regular) and an “1800 rule” for rapid-acting insulin, such as insulin lispro.91,98 For simplicity and because of insulin resistance stimulated by surgical stress, the “1500 rule” is appropriate in this setting, even with the use of a rapid-acting insulin. To then calculate an appropriate corrective dose of insulin to restore near-normoglycemia, the anesthesiologist should aim for a target blood glucose concentration of 150 mg/dL.
More detailed preoperative recommendations must be based on the individual child’s usual treatment regimen. For most children with diabetes undergoing minor outpatient surgical procedures, insulin can be provided perioperatively with subcutaneous injections. In adults, especially those with type 2 diabetes, this practice is commonly,97 although not universally, preferred.99 Others routinely recommend insulin infusions even for minor outpatient surgical procedures in children.10 Several reports suggest that better glycemic control can be achieved in the perioperative period with a continuous intravenous infusion rather than subcutaneous insulin administration.12,100,101 However, these studies were conducted before the availability of rapid-acting insulins whose rapid and reproducible effects after subcutaneous administration102 may more closely match the ability to titrate IV-administered short-acting insulin. Subcutaneous insulin lispro has been shown to be as efficacious as regular IV insulin for treatment of uncomplicated diabetic ketoacidosis in children.103 Whatever the management strategy for the diabetes, it is most important that it is coordinated between the anesthesiology and endocrinology services, and the modifications to the child’s diabetic regimen at home needs to be communicated clearly and in a timely manner to the family.
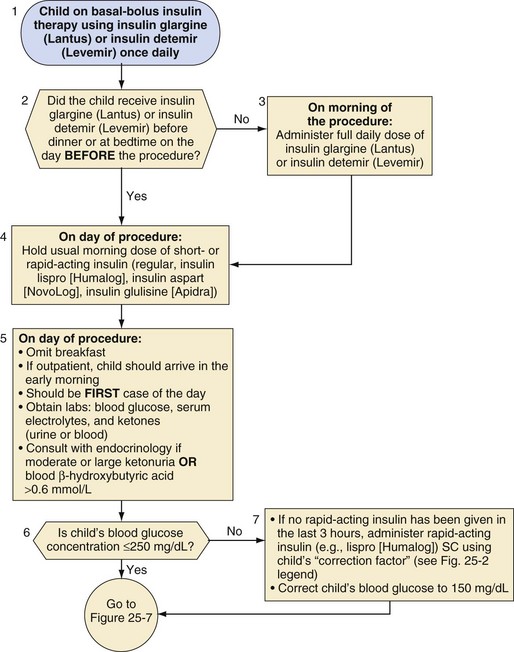
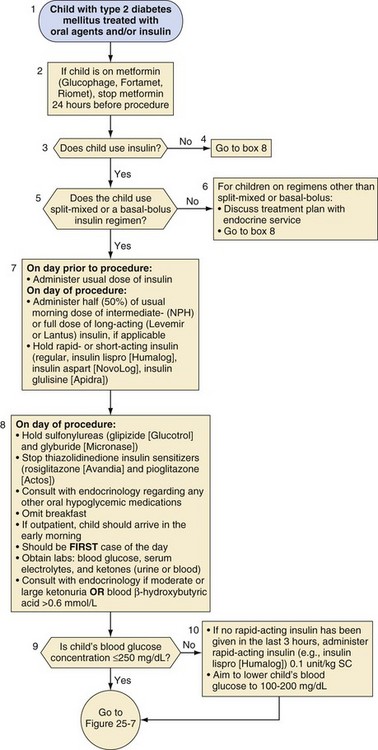
Split-mixed insulin regimens involve two to three injections per day with a combination of NPH or insulin detemir plus a rapid- or short-acting insulin. For children who use such a regimen, 50% of the usual morning dose of NPH or the full usual dose of insulin detemir should be administered on the morning of the procedure (Fig. 25-2). For the child whose basal insulin is once-daily insulin glargine or insulin detemir, no additional insulin is given on the morning of the surgery if the child received a dose in the evening of the previous day (Fig. 25-3). However, if insulin glargine or detemir is typically administered in the morning, the full dose should be administered on the morning of the procedure to prevent ketosis.
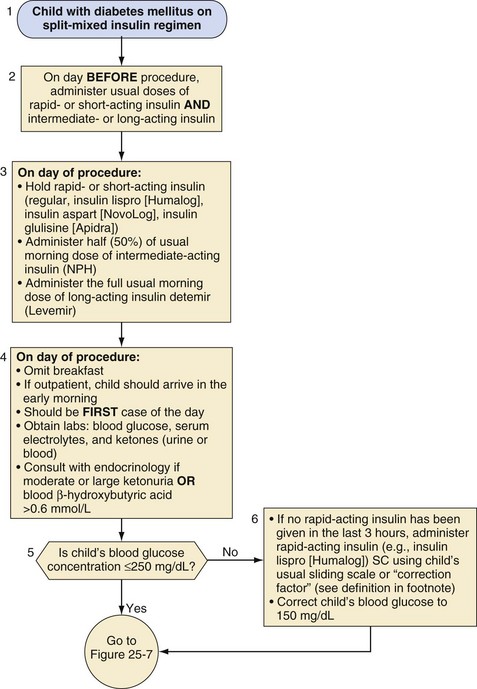
1. Divide 1500 by child’s total daily dose (TDD).
2. If daily dose varies (i.e., use of sliding scales), use the average daily dose in the past week to determine TDD.
3. Example: if TDD = 50 units, then insulin correction factor is 1 unit insulin lispro (Humalog) to reduce blood glucose by 30 mg/dL.
NPH, Neutral protamine Hagedorn; SC, subcutaneous.
(Modified from Rhodes ET, Ferrari LR, Wolfsdorf JI. Perioperative management of pediatric surgical patients with diabetes mellitus. Anesth Analg 2005;101:986-99.)
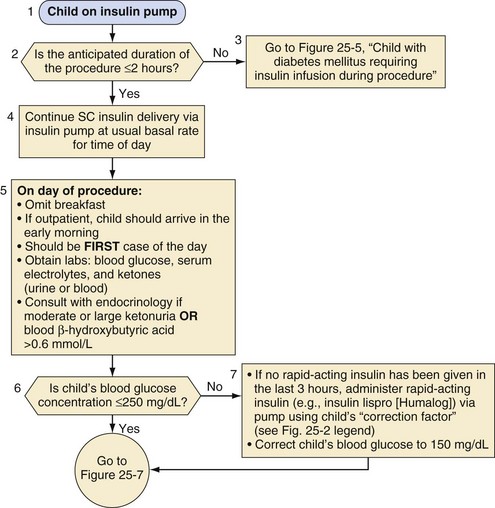
Management of the child on an insulin pump depends on the duration of the surgical procedure (Fig. 25-4). Those undergoing minor procedures expected to last less than 2 hours can continue to receive their usual basal rate via their insulin pump. However, this approach requires that the anesthesiologist is familiar and comfortable with the use of the pump in the operating room. Other protocols include transitioning patients who use an insulin pump to IV insulin infusion or subcutaneous insulin glargine.65 For procedures longer than 2 hours, children should be transitioned to an IV insulin infusion, as described in the next section.
Major Surgery and Intravenous Insulin Infusions
For children who require major surgery, especially procedures anticipated to last more than 2 hours, an IV insulin infusion is the preferred perioperative diabetes management plan (Fig. 25-5). Studies in children12 and adults7 have demonstrated that glycemic control is superior with infusions of IV insulin compared with subcutaneous injections. These children should receive their usual doses of insulin on the day before the procedure. On the morning of the procedure, an IV infusion of 5% dextrose in half-normal saline should be started at a maintenance rate, and an IV insulin infusion should also be provided to accommodate the dextrose infusion to maintain blood glucose in the target range of 100 to 200 mg/dL (see Fig. 25-5). The maintenance rate for IV fluids in a child depends on body size and can be calculated either based on body weight (4 mL/kg/hr for the first 10 kg body weight, 2 mL/kg/hr for 11 to 20 kg, and then 1 mL/kg/hr for each kg over 20 kg) or body surface area (1.5 L/m2/day). The insulin dose varies with the child’s pubertal status; prepubertal children are relatively more sensitive to insulin than pubertal adolescents.104 In prepubertal children with type 1 diabetes, after the remission (honeymoon) period, the insulin requirement is typically 0.6 to 0.8 unit/kg/day, whereas in adolescents, the requirement is 1 to 1.5 units/kg/day.105,106 Children with type 2 diabetes may require even greater doses of insulin because of their insulin resistance. With IV insulin, a suitable initial ratio of insulin to dextrose may be estimated from the child’s usual or average total daily insulin dose (TDD) using the formula 500 ÷ TDD. For example, if the child typically receives 50 units of insulin daily, then 500 ÷ 50 = 10. Therefore, in this example, 1 unit of insulin would be administered via a “piggyback infusion” for each 10 g of dextrose contained in the maintenance IV infusion to prevent hyperglycemia. Note that a maintenance IV solution of 5% dextrose contains 10 g of dextrose in every 200 mL. Only regular insulin should be used for IV infusions.
Special Considerations for Preoperative Management of Type 2 Diabetes
Children with type 2 diabetes may require insulin or one of several oral antihyperglycemic agents (Fig. 25-6). Metformin should be discontinued 24 hours before a procedure because of its long half-life and the risk of lactic acidosis in the presence of dehydration, hypoxemia, or poor tissue perfusion.107 Other oral agents, such as sulfonylureas and thiazolidinediones, may be discontinued on the morning of the procedure. Figure 25-6 outlines additional recommendations for children with type 2 diabetes who use a split-mixed or basal-bolus insulin regimen. Adjustments for other insulin regimens or oral hypoglycemic agents in type 2 diabetes should be determined in consultation with an endocrinologist or diabetologist.
Intraoperative Management
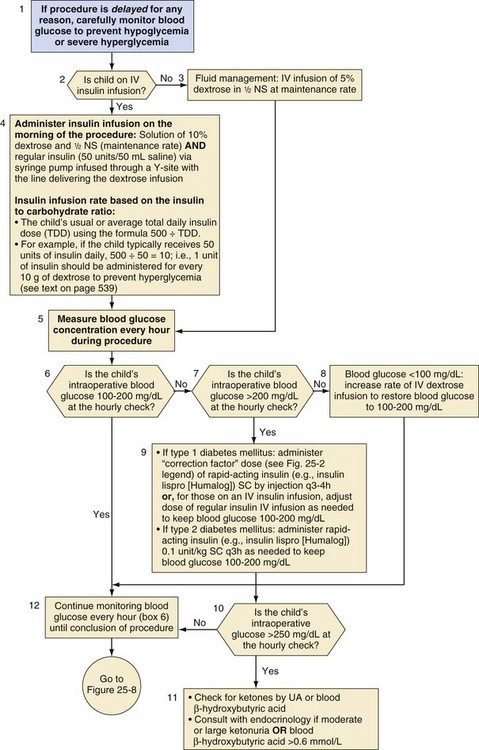
The insulin and fluid regimen during and after surgery depends on the duration of the procedure. If the procedure is likely to be brief (e.g., ≤1 hour) and one can reasonably anticipate that the child will be able to drink soon after the procedure, it may not be necessary to start a glucose-containing IV infusion. If the duration of fasting is likely to be more prolonged, an IV infusion should be started at a maintenance rate as described earlier (Fig. 25-7). Intraoperative maintenance fluid should then be replaced with a comparable glucose-containing solution. Replacement of insensible losses and intravascular volume owing to blood or other body fluid losses should be with an appropriate isotonic solution (e.g., lactated Ringer’s solution or normal saline).
Although some protocols include potassium chloride in the maintenance IV fluid solution,10,65,97 this practice should generally be avoided because of the danger of inadvertent intraoperative administration of large quantities of potassium during fluid resuscitation. Children undergoing a brief procedure with a baseline normal serum potassium concentration and well-controlled diabetes have a small risk of hypokalemia. Those undergoing more prolonged surgeries or emergent surgeries during which metabolic decompensation is more likely, require intraoperative assessment of electrolytes and appropriate adjustment of the electrolyte composition of their IV solution. In all cases, blood glucose concentrations should be measured hourly and either insulin or dextrose adjusted, as necessary, to maintain blood glucose in the target range of 100 to 200 mg/dL. If the blood glucose exceeds 250 mg/dL, urine or blood ketones should also be measured (see Fig. 25-7). Either an increase in the rate of continuous insulin infusion or subcutaneous administration of a rapid-acting insulin analog is used to correct intraoperative hyperglycemia. Intraoperative IV bolus of regular insulin is not recommended because this causes a rapid supraphysiologic increase in serum insulin concentration, which, owing to insulin’s short half-life (approximately 5 minutes), will have a short-lived effect on blood glucose levels. In contrast, subcutaneously administered rapid-acting insulin analogs have a typical and reproducible pharmacologic profile.
Postoperative Management
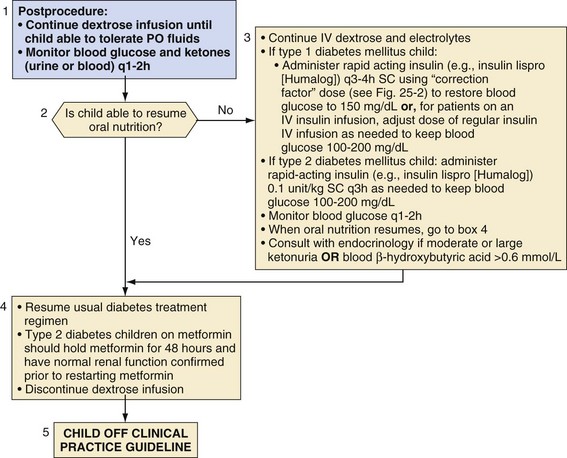
As soon as the child is able to resume drinking and eating normally, the usual diabetes regimen, including insulin and/or oral agents, may be reinstituted and the dextrose infusion discontinued, if applicable (Fig. 25-8). One exception to this approach is for children with type 2 diabetes who take metformin; metformin should be held for 48 hours and renal function must be within normal limits before its resumption. For children who are unable to eat or drink, IV dextrose and electrolyte solution should be continued until oral intake is restored. An infusion of IV short-acting insulin (regular) or intermittent subcutaneous rapid-acting insulin should be administered, not more than every 3 hours, to maintain blood glucose in the target range of 100 to 200 mg/dL. Frequent blood glucose monitoring and monitoring blood or urine ketones is essential because of the variable effects of surgical trauma, inactivity, pain, anxiety, nausea and/or vomiting with poor oral intake, medications, and postoperative infection. At the time of discharge from the hospital, children and their parents or care providers should be given appropriate guidelines regarding these issues. Those who are admitted to the hospital overnight after surgery should be managed in consultation with the endocrinology service, if possible, to coordinate appropriate scheduling and subsequent dosing of insulin.
Special Surgical Situations
Children with diabetes who need urgent surgery must have a full clinical and biochemical assessment. Frequently, the problem necessitating surgery may have led to metabolic decompensation that must first be corrected and stabilized, unless the need for surgery is immediate. These children are often dehydrated; in addition to administering insulin, rehydration and electrolyte replacement is critical to address their metabolic derangements and restore normal glomerular filtration rate and renal function. In most cases, these children require emergent surgery that should be managed with an IV infusion of insulin as described earlier (see Fig. 25-5). Children with diabetic ketoacidosis require close collaboration between the anesthesiology and endocrinology services.
Diabetes Insipidus
Diagnosis of Neurosurgical Diabetes Insipidus: The Triple-Phase Response
Of special interest is the triphasic pattern of vasopressin secretion, often, but not always, observed after neurosurgical procedures that interfere with the supraoptic-hypophyseal tract.108 After surgery, an initial phase of transient DI may be observed, lasting between 12 hours and 2 days. This may be explained by local edema that interferes with normal vasopressin secretion. If significant vasopressin-secreting cell damage has occurred, release of stored vasopressin from damaged neurons leads to a second phase that involves water retention. The syndrome of inappropriate antidiuretic hormone secretion (SIADH) may last up to 10 days. Finally, a third phase, permanent neurogenic DI, may follow if more than 90% of vasopressin cells are destroyed. Pronounced SIADH in the second phase generally portends permanent DI in the final phase of the triple response. In children with vasopressin and cortisol deficiency (e.g., in combined anterior and posterior hypopituitarism after neurosurgical treatment of craniopharyngioma), symptoms of DI may be masked because cortisol deficiency impairs renal free water clearance. Institution of glucocorticoid therapy may precipitate polyuria, leading to the diagnosis of DI (Fig. 25-9).
Perioperative Management of Minor Procedures
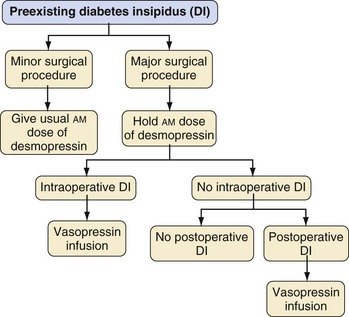
Children with preexisting DI who are scheduled for a minor procedure, i.e., a procedure without significant blood loss and not followed by a period of further fasting or fluid shifts (e.g., myringotomy, radiologic imaging, peripheral orthopedic procedures) are treated differently from those undergoing a more major procedure associated with blood loss or fluid shifts and delayed postoperative resumption of fluid intake (see Fig. 25-9).
Children undergoing minor procedures under anesthesia should receive their usual morning dose of desmopressin (DDAVP) (Table 25-3). The anesthetic technique is tailored to the procedure (e.g., IV sedation for MRI, general endotracheal anesthesia for tonsillectomy). Arterial and urinary catheters are not required, and recovery takes place in the postanesthesia care unit (PACU). Once the morning dose of desmopressin has been administered, intraoperative and postoperative fluids should be restricted to the rate of 1 L/m2/24 hours, i.e., to match insensible free water losses and obligatory urine output. Oral fluids may be offered once the child is awake. Although modern PACU policies often no longer require successful fluid intake before discharging the ambulatory patient, discharging the child with DI should be delayed until the child is able to freely take oral fluids without vomiting. Subsequent doses of desmopressin should be administered according to the child’s usual preoperative schedule.
TABLE 25-3 Medications for Management of Central Diabetes Insipidus
| Desmopressin acetate (100-, 200-µg tablets; nasal tube 10 µg/0.1 mL; nasal spray 10 µg/0.1 mL: delivers 0.1 mL/spray) | |
| Oral | Dose: 100-400 µg q12h |
| Oral doses are 10-20 times intranasal doses of desmopressin | |
| Onset of action: ~15-30 minutes | |
| Duration: 8-12 hours | |
| May develop tachyphylaxis | |
| Intranasal | Dose: ~10-20 µg/dose q12-24h |
| Onset of action: 5-15 minutes | |
| Duration: 8-12 hours | |
| Vasopressin (Pitressin) (20 units/mL; 0.5-mL, 1-mL, and 10-mL vials; conversion: 1 unit = 2.5 µg) | |
| Intravenous | Dose: 1.5 milliunits/kg/hr |
| Onset of action: minutes | |
| Half life: 5-10 minutes | |
Perioperative Management of Major Procedures
A major surgical procedure is defined as an operation associated with the potential for significant blood loss; intraoperative or postoperative hemodynamic, neurologic, or respiratory instability; entry into a body cavity (e.g., craniotomy, abdominal surgery, thoracic surgery); craniofacial or airway surgery; major orthopedic surgery (e.g., spine surgery, tumor resection or amputation, major osteotomies); and/or surgery followed by delayed resumption of unrestricted fluid intake. The child with DI should be scheduled as the first case of the day. On the day before the procedure, the child treated with desmopressin should receive the usual morning dose, but only 50% of the usual evening or bedtime dose (see Fig. 25-9). On the morning of the procedure, desmopressin is withheld. The child undergoing a major procedure should receive general anesthesia with conventional monitoring, as well as insertion of arterial and urinary catheters, and after surgery is admitted to the ICU. A central venous catheter for monitoring central venous pressure, although not indicated solely for the management of DI, is useful in the postoperative period.
Postoperative Management
Aqueous vasopressin at a dose of 1.5 milliunits/kg/hr results in a supranormal blood vasopressin concentration of approximately 10 pg/mL, twice that needed for full antidiuretic activity.109 The effect of vasopressin is maximal within 2 hours after starting an infusion.109
After hypothalamic, but not trans-sphenoidal surgery, greater initial concentrations of vasopressin are occasionally required to treat acute DI. This may be attributed to the release of a substance related to vasopressin from the damaged hypothalamo-neurohypophyseal system, which acts as an antagonist to normal vasopressin activity.110 Much greater rates of vasopressin infusions, resulting in plasma concentrations greater than 1000 pg/mL, should be avoided because they may cause cutaneous necrosis,111 rhabdomyolysis,111,112 and cardiac rhythm disturbances.112
Post–intensive Care Unit Management
Children treated with vasopressin for postneurosurgical DI should be switched from IV to oral fluid intake at the earliest opportunity. With an intact thirst mechanism and access to free water, the patient will better regulate blood osmolality. Once oral intake has been resumed without nausea and vomiting (often by the morning of the day after surgery), the vasopressin infusion should be stopped; all IV infusions should be stopped to avoid iatrogenic fluid overload, and oral fluids are permitted freely. Desmopressin is reinstituted (nasally or orally) in the child with preexisting DI or begun in the child who has new-onset DI (see Table 25-3).
Syndrome of Inappropriate Antidiuretic Hormone Secretion
SIADH is characterized by hypotonic hyponatremia, urine osmolality in excess of plasma osmolality, natriuresis in the absence of edema and volume depletion, and normal renal and adrenal function.113 The dilutional hyponatremia of SIADH develops because of persistent detectable or increased plasma arginine vasopressin (AVP; also known as antidiuretic hormone [ADH]) concentrations in the presence of continued fluid intake. Chronic hyponatremia, however, is the result of a combination of water retention and sodium excretion.114 The major causes of SIADH are neurologic diseases, neoplasia, lung diseases, and medications (Table 25-4).115 Inappropriate infusion of hypotonic fluids in the postoperative period is among the most common causes.116 The clinical manifestations are principally neuromuscular (headache, nausea, vomiting, muscle cramps, lethargy, restlessness, disorientation, and depressed reflexes). The severity of symptoms is related to both the absolute serum sodium concentration (most patients with serum sodium greater than 125 mEq/L are asymptomatic) and its rate of decrease, especially if greater than 0.5 mEq/L/hr.
TABLE 25-4 Causes of Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH)
Perioperative Management
Hypertonic (3%) saline should be used with caution in children. Rapid correction of chronic hyponatremia can lead to serious, permanent, and even fatal neurologic complications from osmotic demyelination (central pontine myelinolysis).117 Only children with neurologic symptoms (e.g., altered level of consciousness, coma, or seizures) attributable to acute hyponatremia of less than 3 days’ duration require rapid initial correction of the sodium deficit (use 3% hypertonic saline to deliver 5 mEq/kg per hour). The rate of increase of serum sodium concentration in states of chronic hyponatremia should not exceed 0.5 mEq/L/hr (or 8 to 10 mEq/L/24 hours).118,119 The saline infusion should stop when the absolute concentration of serum sodium reaches 120 to 125 mEq/L. Hypertonic saline is usually combined with furosemide to limit treatment-induced expansion of the extracellular fluid volume.119 Thereafter, treatment should consist of fluid restriction.
Thyroid Disorders
Thyroid hormones play an important role in metabolic processes, growth, and development in children.120 The thyroid gland develops from the embryonic pharyngeal floor and descends along the thyroglossal duct to its final position in the anterior neck. Thyroid hormone production is controlled by the hypothalamic-pituitary-thyroid axis. Two principal thyroid hormones are produced. Although thyroxine (T4) is the predominant circulating thyroid hormone, triiodothyronine (T3), primarily formed by peripheral conversion from T4 by a family of deiodinases, is the major physiologically active thyroid hormone. Serum T3 and T4 concentrations in turn regulate hypothalamic thyrotropin-releasing hormone (TRH) and pituitary thyroid stimulating hormone (TSH) secretion via negative feedback. Thyroid hormones are transported in the blood by carrier proteins, including thyroxine-binding globulin (TBG), prealbumin, and albumin. Protein-bound T4 and T3 are not biologically active. Only 0.03% of circulating T4 and 0.3% of T3 are unbound and active.121,122
Hypothyroidism
Classification and Epidemiology
Hypothyroidism, the most common thyroid disorder in children, can range from subclinical to overt disease. In subclinical hypothyroidism, children maintain a normal T4 level by increasing TSH release. Over time, however, thyroid function can decompensate despite increased TSH stimulation and progress to overt thyroid hormone deficiency.123–125 Iodine deficiency continues to be the foremost cause of hypothyroidism worldwide.126 However, in the United States and other regions in which the intake of iodine is adequate, autoimmune thyroid disease is the most common cause of hypothyroidism.124 Other less frequent causes of hypothyroidism include secondary and tertiary processes resulting from pituitary or hypothalamic insufficiency, respectively, which are relevant in many surgical situations, especially tumors of the central nervous system (CNS).
Biochemical Tests of Thyroid Function
In general, measurement of serum TSH and either total T4 or free T4 suffices for assessment of thyroid function. However, several conditions exist in which thyroid hormone levels appear abnormal yet the individual is clinically euthyroid. These conditions may lead to erroneous diagnosis and inappropriate treatment for hypothyroidism or hyperthyroidism. TSH is the most sensitive test for diagnosing primary thyroid disorders and generally precedes noticeable changes in total T4 and T3 levels. Thyroid function can also be assessed by measurement of unbound or free T4. When the total T4 level is reduced but free T4 and TSH values are normal, TBG deficiency is the most likely diagnosis. No treatment is required for TBG deficiency because these individuals have normal concentrations of free T4 and are euthyroid. Likewise, medical intervention is unnecessary for familial dysalbuminemic hyperthyroxinemia, which manifests with increased total T4 levels but normal free T4 and TSH values.127
Importantly, if the hypothalamic-pituitary axis is not intact, TSH becomes a useless measurement of thyroid function. Therefore, in cases of secondary or tertiary (central) thyroid disorders, diagnosis and treatment is based solely on serum T4 levels and clinical signs. During times of stress or acute illness, increased conversion of T3 to a metabolically inactive form (reverse T3) occurs. In addition, T4 and TSH values may both be reduced, reflecting a decreased metabolic state. Individuals are considered euthyroid based on non-elevated TSH values but the differential diagnosis includes central hypothyroidism, a distinction that often cannot be made by laboratory evaluation. Controversy still exists regarding possible benefits of using T4 or T3 to treat euthyroid sick syndrome, in which there are abnormal thyroid tests in the setting of a nonthyroid illness, such as a critical illness. Typically, children with euthyroid sick syndrome have a reduced T4 level with a normal or low-normal TSH level, indicating a decreased metabolic state.128–131
Clinical Manifestations
Because thyroid hormone affects all metabolically active cells, hormone deficiency leads to a wide array of systemic abnormalities. Classic signs and symptoms of hypothyroidism in children include short stature resulting from a decline in growth rate, fatigue, cold intolerance, weight gain, dry skin, hair loss, constipation, hoarse voice, and coarse facial features. Myxedema coma is a severe manifestation of hypothyroidism that can occur in profoundly hypothyroid individuals exposed to an external stress, such as infection, surgery, hypnotics, or cold temperature. Myxedema coma can result in severe life-threatening heart failure or coma132,133 and should be considered in any postoperative child with unexplained cardiovascular dysfunction, difficulty weaning from ventilator support, or delirium.97 Other medical conditions associated with hypothyroidism include anemia, hyperlipidemia, and pubertal disorders.
Neonatal Hypothyroidism
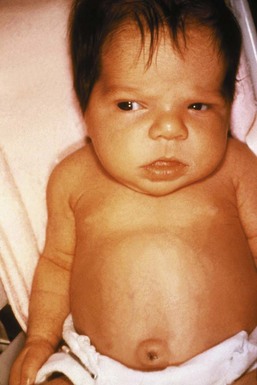
Congenital hypothyroidism remains the most frequent cause of preventable mental developmental delay. Thyroid dysgenesis or agenesis accounts for the majority of cases, whereas a smaller percentage results from thyroid dyshormonogenesis and secondary or tertiary hypothyroidism.134 Because neonates do not exhibit the classic signs or symptoms of hypothyroidism, testing for congenital hypothyroidism occurs with the neonatal screen. Newborn screening programs that employ a strategy based on TSH thresholds detect only primary thyroid deficiency and not the central hypothyroidism that accompanies hypopituitarism or CNS anomalies, such as septo-optic dysplasia. Early detection and implementation of thyroid replacement are essential to avoid permanent neurologic sequelae. The classic syndrome includes macroglossia, umbilical hernia, constipation, hypothermia, a low hoarse cry, and neonatal jaundice (Fig. 25-10). In general, affected children who receive adequate thyroid replacement starting early in the neonatal period lead normal lives.
Treatment
The goal of thyroid replacement is to normalize T4 within 1-2 weeks and TSH within 4 weeks, and consequently reverse the metabolic derangements caused by hypothyroidism. In general, daily levothyroxine (LT4) replacement leads to normalization in T4 levels within a week but equilibration of TSH occurs more slowly, over 4 to 6 weeks.135 The appropriate starting LT4 dose varies with age and disease state. For neonates, the starting dose is 10 to 15 µg/kg/day, which is much greater than the conventional replacement dose of 2 to 4 µg/kg/day in older children and adolescents.136 In healthy children with acute hypothyroidism (e.g., post thyroidectomy), full LT4 replacement can be started immediately. However, symptomatic children with chronic hypothyroidism may develop pseudotumor cerebri or slipped capital femoral epiphysis if they are started on full LT4 doses. Therefore treatment should begin at one-fourth of the expected dose, with slow titration every 4 to 6 weeks.137,138 After dose stabilization, children and adolescents should continue to have regular clinical examinations and TSH monitoring owing to increased dose requirements during puberty and pregnancy.139,140 Thyroid replacement can be given parenterally if needed. The IV dose of LT4 is approximately half of the oral dose and should be given once daily.
Preoperative Management
Because thyroid hormones play a critical role in regulating metabolism, children should be clinically and biochemically euthyroid before any type of elective surgery. Several case studies have shown decreased cardiac function, diminished breathing capacity, and increased sensitivity to anesthetic agents with moderate to severe hypothyroidism.141–143 Children with known hypothyroidism should have documented normal thyroid function tests before surgery. For those with undiagnosed hypothyroidism, a detailed history should be obtained regarding previous thyroid disorders, head and neck radiation, radioiodine therapy, thyroid surgery, and family history of thyroid disease.144 In addition, children with autoimmune disorders, type 1 diabetes mellitus, celiac disease, and children with genetic syndromes such as trisomy 21 and Turner syndrome are at increased risk for developing autoimmune thyroiditis and should be screened if symptomatic.145–147
Children with subclinical or mild hypothyroidism often must undergo urgent surgery without delay. Thyroid replacement can be started and continued with the same regimen as described in the outpatient setting. For children with moderate to severe hypothyroidism, however, surgery should be postponed, if possible, until thyroid hormone levels normalize on replacement therapy. However, if urgent surgery is required, perioperative treatment with IV LT4 with or without glucocorticoids should be given to prevent myxedema coma.97,144 An exception to this strategy applies to the child who is scheduled for cardiovascular surgery or cardiac catheterization in which thyroid replacement could precipitate or worsen unstable coronary syndromes. Several studies in adults reported no adverse outcomes in cardiac patients undergoing surgery without thyroid replacement.97,144,148 Therefore thyroid replacement could be initiated postoperatively in children with cardiovascular disease undergoing cardiac surgery or cardiac evaluation.
Hyperthyroidism
Classification and Epidemiology
Hyperthyroidism is a condition caused by excess circulating thyroid hormones resulting in an increase in metabolic activity of various peripheral tissues in the body. Almost all children with hyperthyroidism have suppressed serum TSH concentrations resulting from negative feedback by increased concentrations of T4 and T3. Hyperthyroidism can be either overt or subclinical.149 Overt hyperthyroidism is characterized by both clinical and biochemical manifestations of the disease. A child with subclinical hyperthyroidism typically is asymptomatic but with a suppressed TSH level.
Hyperthyroidism occurs less frequently than hypothyroidism in children and is nearly always caused by Graves disease. Other causes of childhood thyrotoxicosis include autoimmune thyroiditis (Hashimoto thyroiditis), infections of the thyroid gland, autonomously functioning thyroid nodules, iodine-induced hyperthyroidism, McCune-Albright syndrome, TSH-producing pituitary adenomas, and thyroid hormone ingestion.150 A rare thyroid disorder that mimics hyperthyroidism is thyroid hormone resistance. However, unlike other causes of childhood thyrotoxicosis, thyroid hormone resistance should not be treated with antithyroid medications.151,152
Graves Disease
Graves disease, an autoimmune disorder, is the most common cause of childhood hyperthyroidism. The pathophysiology of Graves disease involves thyroid-stimulating immunoglobulins that bind to the TSH receptor causing hyperstimulation of the thyroid gland. Most children with Graves disease have a diffuse goiter, and some develop autoimmune ophthalmopathy and myxedema. Because few children with Graves disease enter spontaneous remission, treatment of hyperthyroidism is required. Current treatment options include antithyroid medications (methimazole; propylthiouracil [PTU] is no longer recommended for treatment of Graves disease in children because of the risk of hepatotoxicity), surgical removal of the thyroid gland, or radioiodine.153,154 Although virtually all children enter remission during therapy with antithyroid drugs, side effects are relatively common (see later discussion) and the majority of children treated for 2 to 3 years relapse shortly after discontinuation of therapy.155,156 Radioactive ablation is usually definitive and carries less risk than surgery.157
Thyroiditis
The term thyroiditis is used to describe a heterogeneous group of disorders that result in inflammation of the thyroid gland with subsequent release of preformed thyroid hormone. Transient hyperthyroidism can result from the initial inflammatory process, but symptoms generally last up to about 8 weeks, until preformed stored thyroid hormone is depleted. Some children will develop hypothyroidism after the recovery phase because of lymphocytic infiltration of the gland and destruction of thyroid tissue. Treatment with antithyroid drugs (thioamides) that block formation of thyroid hormone is not indicated for transient hyperthyroidism, but for symptomatic children, β-blockers should be used during the thyrotoxic phase to control symptoms.158
Thyroiditis can also manifest as painful inflammation of the gland and fever owing to bacterial or viral infection. Haemophilus influenzae, group A streptococci, and Staphylococcus are the most frequent causes of acute thyroiditis, which can be associated with thyroid gland cutaneous fistulas.159 Viral infections of the thyroid gland are less severe and result in subacute thyroiditis.160 Owing to the difficulty in distinguishing bacterial from viral infections, all cases of infectious thyroiditis are treated with antibiotics.
Clinical Manifestations
Most of the symptoms that children experience are the same regardless of the cause of hyperthyroidism. Classic signs and symptoms of hyperthyroidism include goiter, tachycardia and/or palpitations, tremor, brisk reflexes, heat intolerance, dyspnea, insomnia, diarrhea, nervousness, and weight loss despite a normal or increased appetite. Many children with hyperthyroidism have inability to concentrate, resulting in poor school performance, and may be initially mistaken to have attention deficit hyperactivity disorder. Other medical conditions associated with long-standing hyperthyroidism include osteoporosis and irregular menses.161
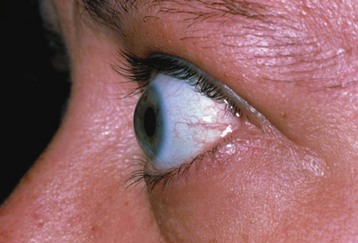
Individuals with Graves disease may develop additional autoimmune manifestations, such as ophthalmopathy and pretibial myxedema. Eye involvement in Graves disease is characterized by inflammation of the extraocular muscles, connective tissue, and orbital fat, which results in proptosis, restricted eye movement, and periorbital edema.162,163 Children with Graves ophthalmopathy may complain of eye irritation or dryness because of lid retraction (Fig. 25-11). If left untreated, corneal ulceration may develop and lead to irreversible eye damage, including blindness. Unfortunately, ophthalmopathy usually persists in spite of treatment of the hyperthyroidism.153,164
Treatment
Antithyroid medications remain the first line of treatment for many physicians, because one-third of children enter remission after several years of drug therapy.155,165 Long-term remission rates are greater in pubertal than prepubertal children. Other favorable prognostic indicators include a small thyroid gland and normal levels of TSH receptor antibodies at the time of diagnosis.166,167 Methimazole (a thioamide) is the mainstay of antithyroid therapy. Minor adverse effects occur in 25% of children treated with thioamides and include increases in liver enzymes, neutropenia, rash, and lymphadenopathy. Serious adverse effects, such as agranulocytosis and liver failure, are rare, occurring in up to 1% of individuals.168
Perioperative Management
For children who relapse after antithyroid drug therapy or who require immediate definitive therapy, radioiodine is a safe and effective alternative to thyroidectomy. Treatment consists of iodine-131 uptake in the thyroid gland, which leads to cell destruction by internal radiation. Several longitudinal studies have shown that children are not at increased risk for developing thyroid cancer if appropriate ablative doses of radioiodine are used.169,170 In cases in which radioiodine is contraindicated, surgical removal of the entire thyroid gland can also cure the hyperthyroid state. Children should be euthyroid or slightly hypothyroid before surgery to avoid precipitation of thyroid storm. In addition, because of potential serious complications from neck surgery, only surgeons with expertise in performing thyroidectomies in children should perform the surgery.171
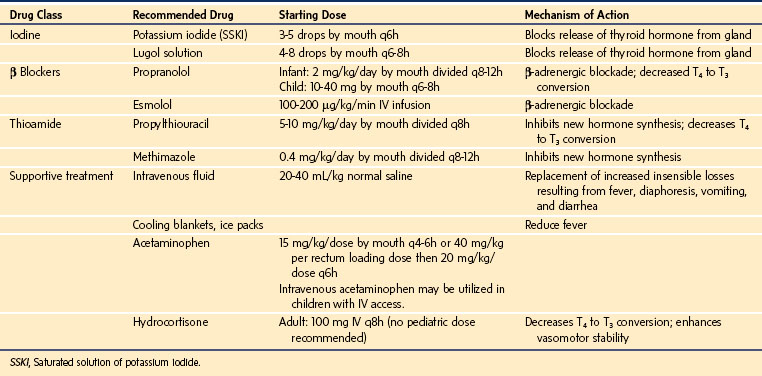
In special circumstances, perioperative rapid control of hyperthyroidism can be achieved by using large doses of oral iodine (Lugol solution or saturated potassium iodine), which inhibit thyroid hormone synthesis. The effect is short-lived (several days) because of eventual escape from the Wolff-Chaikoff effect (iodine inhibition of thyroid hormone release); therefore, the addition of a thioamide is necessary for long-term treatment. Rarely, untreated or undertreated hyperthyroidism can lead to thyroid storm (approximately 1 in 1,000,000 young children), a potentially lethal complication.172 Thyroid storm may be difficult to differentiate from an acute malignant hyperthermia (MH) reaction.173,174 However, in contrast to MH, thyroid storm has a varied onset (usually an insidious onset 6 to 18 hours postoperatively, although it may develop precipitously during surgery), it induces a less severe acidosis than MH, and the serum creatine phosphokinase remains unchanged. Because the serum creatine phosphokinase peaks at 12 to 18 hours after an MH reaction, it is a late sign to differentiate from MH. Interestingly, IV dantrolene will ameliorate some of the clinical manifestations of thyroid storm, including rigidity and hyperthermia.174,175 Thyroid storm usually responds to symptomatic treatment, including parenteral β-blockers, large doses of glucocorticoids (block conversion of T4 to T3 and prevent relative adrenal insufficiency), and propylthiouracil, the last intervention administered with a nasogastric tube.173 If left untreated, the mortality rate resulting from thyroid storm is 20% to 30%.172 Several factors may precipitate thyroid storm, including surgery, infection, and stress. Because of the dramatically increased metabolic state, acute management of hyperthyroidism and targeted symptomatic treatment is essential to avoid death.132,150,173 If the presentation proves difficult to distinguish from MH (both diseases may present with tachycardia, rigidity, and fever), it would be prudent to administer dantrolene 2.5 mg/kg IV in case the presumptive diagnosis of thyroid storm is incorrect.175 However, if the hypermetabolic signs abate after dantrolene, one cannot conclude that the reaction was MH, as dantrolene does attenuate the hypermetabolic signs of thyroid storm.174 The specific treatment for thyroid storm, however, remains a multidrug approach to (1) halt production and release of thyroid hormone, (2) prevent conversion of T4 to T3, (3) antagonize the peripheral (adrenergic) effects of thyroid hormone, and (4) control systemic disturbances with supportive therapy (Table 25-5).133,153,168,176–179
Parathyroid and Calcium Disorders
Physiology of Calcium Homeostasis
The four parathyroid glands are usually present in pairs on the posterior aspect of the superior and inferior poles of the thyroid gland, with the inferior pair occasionally ectopic elsewhere in the neck or chest. Parathyroid hormone (PTH) is released from secretory granules in response to a decrease in serum ionized calcium; its secretion is inhibited by hyperphosphatemia, profound hypomagnesemia or hypermagnesemia, or increased 1,25-dihydroxyvitamin D (calcitriol). The calcium-sensing receptor in the parathyroid mediates PTH release and also directly regulates calcium reabsorption in the distal tubule. Inactivating mutations in the calcium-sensing receptor gene result in a greater serum calcium set point in familial hypocalciuric hypercalcemia, now a relatively common and incidental diagnosis that is benign unless homozygotic, whereas activating mutations result in autosomal dominant hypocalcemia with hypercalciuria.180,181 PTH has three primary modes of increasing serum calcium: (1) calcium reabsorption in the proximal convoluted tubule, and concomitant phosphaturia in the proximal tubule; (2) upregulation of osteoclast-mediated calcium and phosphate release from bone; and (3) renal conversion of 25-hydroxyvitamin D to the active metabolite, calcitriol. In turn, calcitriol increases absorption of calcium and phosphate from the gut and has direct calcium-releasing effects on bone. Calcitonin is secreted by the thyroid C cells and has calcium-reducing properties via its own G protein–coupled receptor. It has no known physiologic role at this time.
Hypocalcemia
Neonatal Hypocalcemia
Neonatal hypocalcemia owing to prematurity and maternal diabetes is common but is typically transient. Rare causes include maternal hyperparathyroidism, excessive diuretic use or phosphate load, and transient neonatal hypoparathyroidism.182 Neonatal hypocalcemia requires exclusion of 22q11.2 deletion syndrome (which includes DiGeorge syndrome, velocardiofacial syndrome [VCF], and other clinical manifestations) by genetic testing.183–185 The hypocalcemia of VCF results from an incomplete hypoparathyroidism that tends to normalize eventually but may recur. Reduced PTH or PTH resistance (pseudohypoparathyroidism) cause hyperphosphatemia beyond the already increased normal range in neonates, whereas vitamin D deficiency or resistance is associated with low to normal serum phosphate levels. Maternal 25-hydroxyvitamin D deficiency is another common cause of early or late infantile hypocalcemia in the United States, regardless of the infant’s dietary intake.186,187 Childhood hypocalcemia with reduced PTH may be the first endocrine sign of polyglandular autoimmune disease type I, now called autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED).188
Childhood Hypocalcemia
Persistent hypocalcemia in infants and children can have many manifestations, including poor feeding, tetany, seizures, laryngospasm, paresthesias, and muscle cramping. Initial evaluation of hypocalcemia, regardless of age, includes measurement of serum calcium, phosphate, magnesium, alkaline phosphatase, creatinine, PTH, 25-hydroxyvitamin D, and 1,25-dihydroxyvitamin D, plus urine calcium, phosphate, and creatinine. Therapy for acute hypocalcemia includes parenteral calcium, followed by oral calcium supplementation and 25-hydroxyvitamin D if reduced,187 and calcitriol as a substitute for PTH, because PTH itself, an anabolic bone agent in adults,189 is contraindicated in children owing to concerns about its possible role in osteosarcoma.
Perioperative Management
Hypocalcemia is most frequently managed by IV infusion of calcium in the form of calcium chloride or calcium gluconate, with frequent measurement of serum ionized calcium levels to guide therapy (see Chapter 10). Calcium salts release free calcium ions and should be given via a central venous catheter because their hypertonicity and the increase concentration of ionized calcium causes intense local vasoconstriction that may lead to necrosis of the skin and subcutaneous tissues and possible gangrene of the affected limb if administered via a peripheral IV cannula that becomes interstitial. In the postoperative period after the child is tolerating oral intake, IV calcium supplementation may be converted to oral supplementation.
Hypercalcemia
Hyperparathyroidism (HPT) is much less common in children than in adults.190 In most cases of HPT in children, an adenoma is present that is unresponsive to the increased serum calcium level. Other uncommon causes of HPT in children include generalized parathyroid hyperplasia and, rarely, parathyroid carcinoma.191
Parathyroid hyperplasia occurs in familial forms of HPT, including multiple endocrine neoplasia type 1 (MEN 1), in which HPT is a nearly universal manifestation of the mutation of the menin gene; the hyperparathyroid abnormality occurs more commonly and earlier in presentation than the associated pancreatic, pituitary, and gastrointestinal neuroendocrine tumors associated with MEN 1.192,193 HPT is a less common manifestation of MEN 2A, in which medullary thyroid carcinoma and pheochromocytoma occur as a result of the proto-oncogene mutation.194 Secondary HPT with relatively normal calcium levels is a common pediatric phenomenon that accompanies renal failure, renal tubular acidosis, and hypophosphatemic rickets.195 When hypercalcemia is present, the differential diagnosis includes Williams syndrome, vitamin A intoxication, vitamin D excess and conversion to 1,25-dihydroxyvitamin D in granulomatous disorders, and infantile subcutaneous fat necrosis.196 Solid tumors may secrete increased concentrations of parathyroid hormone–related protein (PTHrP). Tumors, such as leukemias, lymphomas, and others, may release excess cytokines and osteoclast-activating factors, with the hypercalcemia leading to the tumor diagnosis.197,198
Signs and symptoms of hypercalcemia are usually nonspecific, such as nausea and vomiting, polyuria, and fatigue. Initial laboratory evaluation of hypercalcemia matches the list for hypocalcemia given earlier.199
Perioperative Management
Management of acute hypercalcemia begins with isotonic rehydration and administration of furosemide to induce calciuria. Treatment then proceeds to administration of calcitonin, to which tachyphylaxis may occur within 1 or 2 days, or to glucocorticoids. In the absence of a definitive therapy, such as surgical excision of the pathologic source of PTH, chronic hypercalcemia is responsive to bisphosphonates, such as pamidronate, which can be given by infusion every 3 months.200,201 Acute management after parathyroidectomy requires careful monitoring and replacement of calcium and possibly the use of calcitriol for persistent hypocalcemia. In isolated parathyroid hyperplasia, MEN, or secondary HPT, surgeons may elect to leave a portion of one gland in the forearm to avoid permanent hypoparathyroidism, which is challenging to manage.
Adrenal Endocrinopathies
Physiology
Adrenal steroidogenesis begins with cholesterol as precursor and results in three types of steroids: mineralocorticoids, glucocorticoids, and sex steroids (androgen precursors).202 Most of the enzymes involved in adrenal (or gonadal) steroidogenesis are cytochrome P450s. The adrenal cortex comprises three zones: the outer zona glomerulosa exclusively synthesizes mineralocorticoids owing to localized expression of CYP11B2, whereas the zona fasciculata and reticularis synthesize glucocorticoid and androgens owing to localized expression of CYP17.203 Cortisol, the end-product of the glucocorticoid pathway, is the primary regulator of the hypothalamic-pituitary-adrenal (HPA) axis, in which hypothalamic corticotropin-releasing hormone regulates pituitary adrenocorticotropic hormone (ACTH) secretion and downstream adrenal production of all three categories of steroid. ACTH release follows a diurnal pattern with a peak at 0400 to 0800 hours and, most importantly, markedly increases in response to trauma, acute illness, high fever, and hypoglycemia.
Mineralocorticoid production is primarily controlled by the renin-angiotensin system, in which angiotensinogen secreted by the liver is cleaved by renin to angiotensin I, which is then converted to angiotensin II.204 The mineralocorticoid pathway is also responsive to ACTH, as demonstrated by a rise in all mineralocorticoid precursors and the end-product aldosterone within minutes of exogenous ACTH stimulation. However, hypopituitarism does not lead to mineralocorticoid deficiency because an intact renin-angiotensin system independently stimulates aldosterone synthesis. Nevertheless, patients with ACTH deficiency may present with hyponatremia (glucocorticoids are required for free water excretion) and hypotension. The latter consequence of failure of the adrenal to release an adequate amount of glucocorticoid may be attributable to the role of cortisol in enhancing vascular responsiveness to catecholamines and inotropic activity.205 This essential aspect of glucocorticoid activity is the most lethal potential consequence of both primary adrenal insufficiency and hypothalamic-pituitary deficiency and requires the utmost caution on the part of pediatrician, endocrinologist, surgeon, or anesthesiologist. When any question regarding adequacy of the HPA arises, it is prudent to provide exogenous glucocorticoid, particularly perioperatively.206
Causes of Adrenal Insufficiency
Primary adrenal insufficiency is suspected from features such as weight loss, nausea and vomiting, poor appetite, and, specifically, the characteristic skin hyperpigmentation that is secondary to melanocyte-stimulating hormone receptor cross-stimulation by ACTH (which is present in extraordinarily large concentrations).207 Primary adrenal insufficiency is uncommon in countries with advanced health care systems now that infectious diseases, such as tuberculosis, are less prevalent, but adrenal hemorrhage still causes adrenal failure.208 Autoimmune adrenal insufficiency, sometimes as part of a polyendocrinopathy syndrome, is now the most common cause and includes both glucocorticoid and mineralocorticoid deficiency. Glucocorticoid deficiency with or without mineralocorticoid loss is characteristic of congenital adrenal hyperplasia.209 Rare disorders causing adrenal insufficiency include adrenoleukodystrophy,210 congenital adrenal hypoplasia, X-linked adrenal hypoplasia congenita, and ACTH receptor defects.211
Congenital or acquired lesions of the hypothalamus or pituitary may lead to ACTH deficiency. In general, these lesions cause other pituitary deficiencies, particularly growth hormone or TSH deficiency; isolated ACTH deficiency is rare. Both growth hormone and ACTH deficiency may cause hypoglycemia in infancy. CNS malformations leading to ACTH deficiency are usually detectable by MRI. A notable CNS anomaly with hypopituitarism is septo-optic dysplasia,29,212–214 which may be associated with an assortment of midline defects, such as an absent or underdeveloped septum pellucidum and optic nerve hypoplasia, but isolated pituitary agenesis or hypoplasia is more common. Acquired lesions leading to hypopituitarism include hydrocephalus, meningitis, infiltrative disorders,215 and tumors such as craniopharyngioma or histiocytosis X.216 Tumor resection, especially craniopharyngioma, cranial irradiation, and chemotherapy, can lead to multiple pituitary deficiencies, often evolving over many years.217
Testing for Adrenal Insufficiency
Primary adrenal insufficiency may be effectively assessed in two ways,218 either by simultaneous measurement of serum cortisol and plasma ACTH concentrations or by stimulating the adrenal with exogenous ACTH (cosyntropin [Cortrosyn], the biologically active fragment of ACTH containing the first 24 amino acids). A markedly increased random plasma ACTH concentration is definitive evidence of primary adrenal insufficiency, but levels can be moderately increased by the stress of phlebotomy itself. Therefore blood for plasma ACTH levels should be obtained from an indwelling catheter to avoid false-positive values.
Hypothalamic-pituitary insufficiency is more difficult to assess. The complete absence of ACTH levels, as in the obvious case of pituitary ablation or destruction, leads eventually to adrenal atrophy and complete unresponsiveness to cosyntropin stimulation, a finding that necessitates maintenance glucocorticoid replacement. However, an “adequate” response to cosyntropin implies only tonic ACTH secretion and does not guarantee the ability to generate a normal surge of ACTH during stress. As a consequence, any patient with a CNS lesion that might cause HPA deficiency should receive stress doses of glucocorticoid (see later discussion). In practical terms, a post–cosyntropin-treatment cortisol value of 10 to 20 µg/dL in an at-risk child suggests the need for stress coverage, whereas a value less than 10 µg/dL indicates long-term replacement.218,219
Perioperative Management of Adrenal Insufficiency
Glucocorticoid Dosing
Conventional stress glucocorticoid coverage is 3 to 10 times the patient’s usual replacement dose, depending on the severity of illness or trauma, although this practice is not evidence based.220 Therefore, with little risk associated with the transient use of excess glucocorticoid, for children who are on oral replacement therapy we recommend exogenous glucocorticoid replacement using three to five times the patient’s usual oral maintenance dose for intercurrent illness or fever (greater than 101° F) and five to ten times the oral maintenance dose for major surgery, during critical illness, or in major emergencies. For children who require stress glucocorticoid coverage but who do not take outpatient steroids, or who are unable to take medications by mouth, we recommend using parenteral cortisol (Solu-Cortef) at a dose of 0.5 mg/kg/day every 12 hours for illness associated with nausea, vomiting, diarrhea, or fever (greater than 101° F) and 0.5 to 1 mg/kg every 6 hours for perioperative, intensive care, or emergency department indications for up to 72 hours.221
Iatrogenic Adrenal Suppression and Tapering
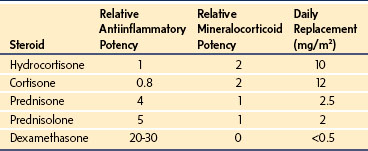
The glucocorticoid taper has two meanings. The tempo of therapeutic tapers of prednisone by gastroenterologists, rheumatologists, nephrologists, and oncologists, and of dexamethasone by surgeons, is entirely dependent on their assessment of the immune or inflammatory process. As long as the glucocorticoid dose is in excess of the replacement dose, and regardless of the dose, the HPA axis continues to be suppressed. It is only when the steroid dose is tapered down to the daily replacement dose (approximately 10 mg/m2 hydrocortisone, 2 mg/m2 prednisone, or less than 0.5 mg/m2 dexamethasone) that the endocrine taper begins. At that point, the prednisone should be switched to hydrocortisone for ease of starting a gradual taper from replacement therapy to reduced doses while function of the HPA axis is reestablished. In the perioperative period, steroid coverage should be administered if there is any concern about the integrity of the HPA axis during a steroid taper (Table 25-6).
Hypercortisolism (Cushing Syndrome)
Excess cortisol, whether exogenous or endogenous, results in muscle wasting, truncal obesity, moon facies, hypertension, hyperglycemia, osteopenia, and growth deceleration.222,223 Iatrogenic hypercortisolism is common in pediatrics,224 whereas Cushing disease or syndrome is rare.225 Cushing disease refers to an ACTH-secreting pituitary adenoma. Cushing syndrome refers to other conditions of excess glucocorticoid, including ectopic ACTH-secreting tumors226 and adrenal tumors that secrete cortisol.227 An adrenal tumor (adenoma or carcinoma) is the most likely cause of hypercortisolism in a young child. These tumors can secrete any combination of steroids and commonly cosecrete androgens, resulting in virilization.228 A unilateral glucocorticoid-secreting tumor typically causes HPA axis suppression, requiring careful tapering of hydrocortisone replacement after resection to reestablish normal cortisol production in the contralateral adrenal gland.229
In a child with signs of hypercortisolism (and not simply obesity), screening tests include an overnight small dose dexamethasone suppression test, measurement of 24-hour urinary free cortisol and creatinine, and measurement of nocturnal salivary cortisol concentrations.230 If screening tests suggest a diagnosis of hypercortisolism, additional adrenal steroids should be measured and an adrenal computed tomographic scan or magnetic resonance image obtained. In an adolescent with pure glucocorticoid effects and suspicious results of a corticotropin-releasing hormone stimulation test or longer dexamethasone suppression test231 suggestive of a pituitary ACTH-secreting adenoma (Cushing disease), pituitary magnetic resonance imaging is warranted.232 Because pituitary adenomas can be very small, bilateral inferior petrosal sinus sampling may be necessary to locate the adenoma.233
Perioperative Management of Hypercortisolism
Therapy for Cushing disease and Cushing syndrome is surgical.234,235 There are no specific anesthetic considerations except the supportive management of secondary manifestations, such as obesity and its attendant airway concerns, hypertension, and skin and bone fragility.
2000 Type 2 diabetes in children and adolescents. American Diabetes Association. [No authors listed]. Diabetes Care. 2000;23:381–389.
Andersen LJ, Andersen JL, Schutten HJ, et al. Antidiuretic effect of subnormal levels of arginine vasopressin in normal humans. Am J Physiol. 1990;259:R53–R60.
Baylis PH. The syndrome of inappropriate antidiuretic hormone secretion. Int J Biochem Cell Biol. 2003;35:1495–1499.
LaFranchi S. Congenital hypothyroidism: etiologies, diagnosis, and management. Thyroid. 1999;9:735–740.
Rhodes ET, Ferrari LR, Wolfsdorf JI. Perioperative management of pediatric surgical patients with diabetes mellitus. Anesth Analg. 2005;101:986–999.
Rivkees SA. The treatment of Graves’ disease in children. J Pediatr Endocrinol Metab. 2006;19:1095–1111.
Sarlis NJ, Gourgiotis L. Thyroid emergencies. Rev Endocr Metab Disord. 2003;4:129–136.
Seckl JR, Dunger DB, Lightman SL. Neurohypophyseal peptide function during early postoperative diabetes insipidus. Brain. 1987;110:737–746.
Silverstein J, Klingensmith G, Copeland K, et al. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care. 2005;28:186–212.
Sterns RH, Riggs JE, Schochet SS, Jr. Osmotic demyelination syndrome following correction of hyponatremia. N Engl J Med. 1986;314:1535–1542.
Wolfsdorf J, Glaser N, Sperling MA. Diabetic ketoacidosis in infants, children, and adolescents: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1150–1159.
1 Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136:664–672.
2 Kiess W, Bottner A, Raile K, et al. Type 2 diabetes mellitus in children and adolescents: a review from a European perspective. Horm Res. 2003;59:177–184.
3 Likitmaskul S, Kiattisathavee P, Chaichanwatanakul K, et al. Increasing prevalence of type 2 diabetes mellitus in Thai children and adolescents associated with increasing prevalence of obesity. J Pediatr Endocrinol Metab. 2003;16:71–77.
4 Onkamo P, Vaananen S, Karvonen M, et al. Worldwide increase in incidence of Type I diabetes—the analysis of the data on published incidence trends. Diabetologia. 1999;42:1395–1403.
5 Urakami T, Kubota S, Nitadori Y, et al. Annual incidence and clinical characteristics of type 2 diabetes in children as detected by urine glucose screening in the Tokyo metropolitan area. Diabetes Care. 2005;28:1876–1881.
6 Wiegand S, Maikowski U, Blankenstein O, et al. Type 2 diabetes and impaired glucose tolerance in European children and adolescents with obesity—a problem that is no longer restricted to minority groups. Eur J Endocrinol. 2004;151:199–206.
7 Gonzalez-Michaca L, Ahumada M, Ponce-de-Leon S. Insulin subcutaneous application vs. continuous infusion for postoperative blood glucose control in patients with non-insulin-dependent diabetes mellitus. Arch Med Res. 2002;33:48–52.
8 Markovitz LJ, Wiechmann RJ, Harris N, et al. Description and evaluation of a glycemic management protocol for patients with diabetes undergoing heart surgery. Endocr Pract. 2002;8:10–18.
9 Vora AC, Saleem TM, Polomano RC, et al. Improved perioperative glycemic control by continuous insulin infusion under supervision of an endocrinologist does not increase costs in patients with diabetes. Endocr Pract. 2004;10:112–118.
10 Chadwick V, Wilkinson KA. Diabetes mellitus and the pediatric anesthetist. Paediatr Anaesth. 2004;14:716–723.
11 Holvey SM. Surgery in the child with diabetes. Pediatr Clin North Am. 1969;16:671–679.
12 Kaufman FR, Devgan S, Roe TF, et al. Perioperative management with prolonged intravenous insulin infusion versus subcutaneous insulin in children with type I diabetes mellitus. J Diabetes Complications. 1996;10:6–11.
13 Kirschner RM. Diabetes in pediatric ambulatory surgical patients. J Post Anesth Nurs. 1993;8:322–326.
14 Rhodes ET, Ferrari LR, Wolfsdorf JI. Perioperative management of pediatric surgical patients with diabetes mellitus. Anesth Analg. 2005;101:986–999.
15 Betts P, Brink S, Silink M, et al. Management of children and adolescents with diabetes requiring surgery. Pediatr Diabetes. 2009:102169–102174.
16 American Diabetes Association. Type 2 diabetes in children and adolescents. [no authors listed]. Diabetes Care. 2000;23:381–389.
17 American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:S62–S69.
18 Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555.
19 Gilliam LK, Brooks-Worrell BM, Palmer JP, et al. Autoimmunity and clinical course in children with type 1, type 2, and type 1.5 diabetes. J Autoimmun. 2005;25:244–250.
20 Hathout EH, Thomas W, El-Shahawy M, et al. Diabetic autoimmune markers in children and adolescents with type 2 diabetes. Pediatrics. 2001;107:E102.
21 Klingensmith GJ, Pyle L, Arslanian S, et al. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype: results from the TODAY study. Diabetes Care. 2010;33:1970–1975.
22 Libman IM, Pietropaolo M, Arslanian SA, et al. Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diabetes Care. 2003;26:2871–2875.
23 Liu LL, Lawrence JM, Davis C, et al. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2010;11:4–11.
24 Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303:242–249.
25 DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med. 2006;23:857–866.
26 Lawrence JM, Mayer-Davis EJ, Reynolds K, et al. Diabetes in Hispanic American youth: prevalence, incidence, demographics, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32:S123–S132.
27 Liu LL, Yi JP, Beyer J, et al. Type 1 and Type 2 diabetes in Asian and Pacific Islander U.S. youth: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32:S133–S140.
28 Mayer-Davis EJ, Beyer J, Bell RA, et al. Diabetes in African American youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32:S112–S122.
29 Bell RA, Mayer-Davis EJ, Beyer JW, et al. Diabetes in non-Hispanic Caucasian youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32:S102–S111.
30 Dabelea D, DeGroat J, Sorrelman C, et al. Diabetes in Navajo youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32:S141–S147.
31 Chan JC, Cheung CK, Swaminathan R, et al. Obesity, albuminuria and hypertension among Hong Kong Chinese with non-insulin-dependent diabetes mellitus (NIDDM). Postgrad Med J. 1993;69:204–210.
32 Kadiki OA, Reddy MR, Marzouk AA. Incidence of insulin-dependent diabetes (IDDM) and non-insulin-dependent diabetes (NIDDM) (0-34 years at onset) in Benghazi, Libya. Diabetes Res Clin Pract. 1996;32:165–173.
33 Kitagawa T, Owada M, Urakami T, et al. Increased incidence of non-insulin dependent diabetes mellitus among Japanese schoolchildren correlates with an increased intake of animal protein and fat. Clin Pediatr (Phila). 1998;37:111–115.
34 McGrath NM, Parker GN, Dawson P. Early presentation of type 2 diabetes mellitus in young New Zealand Maori. Diabetes Res Clin Pract. 1999;43:205–209.
35 Owens DR, Griffiths S. Insulin glargine (Lantus). Int J Clin Pract. 2002;56:460–466.
36 Murphy NP, Keane SM, Ong KK, et al. Randomized cross-over trial of insulin glargine plus lispro or NPH insulin plus regular human insulin in adolescents with type 1 diabetes on intensive insulin regimens. Diabetes Care. 2003;26:799–804.
37 Holl RW, Swift PG, Mortensen HB, et al. Insulin injection regimens and metabolic control in an international survey of adolescents with type 1 diabetes over 3 years: results from the Hvidore study group. Eur J Pediatr. 2003;162:22–29.
38 Heise T, Nosek L, Ronn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614–1620.
39 Kolendorf K, Ross GP, Pavlic-Renar I, et al. Insulin detemir lowers the risk of hypoglycaemia and provides more consistent plasma glucose levels compared with NPH insulin in Type 1 diabetes. Diabet Med. 2006;23:729–735.
40 Pieber TR, Draeger E, Kristensen A, et al. Comparison of three multiple injection regimens for Type 1 diabetes: morning plus dinner or bedtime administration of insulin detemir vs. morning plus bedtime NPH insulin. Diabet Med. 2005;22:850–857.
41 Tamborlane WV. Fulfilling the promise of insulin pump therapy in childhood diabetes. Pediatr Diabetes. 2006;7:S4–10.
42 Weinzimer SA, Swan KL, Sikes KA, et al. Emerging evidence for the use of insulin pump therapy in infants, toddlers, and preschool-aged children with type 1 diabetes. Pediatr Diabetes. 2006;7:S15–S19.
43 Berhe T, Postellon D, Wilson B, et al. Feasibility and safety of insulin pump therapy in children aged 2 to 7 years with type 1 diabetes: a retrospective study. Pediatrics. 2006;117:2132–2137.
44 Nimri R, Weintrob N, Benzaquen H, et al. Insulin pump therapy in youth with type 1 diabetes: a retrospective paired study. Pediatrics. 2006;117:2126–2131.
45 Weintrob N, Benzaquen H, Galatzer A, et al. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens in children with type 1 diabetes: a randomized open crossover trial. Pediatrics. 2003;112:559–564.
46 Willi SM, Planton J, Egede L, et al. Benefits of continuous subcutaneous insulin infusion in children with type 1 diabetes. J Pediatr. 2003;143:796–801.
47 Casella SJ, Mongilio MK, Plotnick LP, et al. Accuracy and precision of low-dose insulin administration. Pediatrics. 1993;91:1155–1157.
48 Gahagan S, Silverstein J. Prevention and treatment of type 2 diabetes mellitus in children, with special emphasis on American Indian and Alaska Native children. American Academy of Pediatrics Committee on Native American Child Health. Pediatrics. 2003;112:e328.
49 Rapaport R, Silverstein JH, Garzarella L, et al. Type 1 and type 2 diabetes mellitus in childhood in the United States: practice patterns by pediatric endocrinologists. J Pediatr Endocrinol Metab. 2004;17:871–877.
50 Fleischman A, Rhodes ET. Management of obesity, insulin resistance and type 2 diabetes in children: consensus and controversy. Diabetes Metab Syndr Obes. 2009:2185–2202.
51 Gottschalk M, Danne T, Cara J, et al. Glimepiride (GLIM) versus metformin (MET) as monotherapy in pediatric subjects with T2DM: a single blind comparison study (abstract). Diabetes. 2005;54:A65.
52 TODAY Study Group. Design of a family-based lifestyle intervention for youth with type 2 diabetes: the TODAY study. Int J Obes (Lond). 2010;34:217–226.
53 Zeitler P, Hirst K, Pyle L, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. NEJM. 2012;366:2247–2256.
54 Jeha GS, Heptulla RA. Newer therapeutic options for children with diabetes mellitus: theoretical and practical considerations. Pediatr Diabetes. 2006;7:122–138.
55 Vilsboll T, Holst JJ. Incretins, insulin secretion and Type 2 diabetes mellitus. Diabetologia. 2004;47:357–366.
56 Iltz JL, Baker DE, Setter SM, et al. Exenatide: an incretin mimetic for the treatment of type 2 diabetes mellitus. Clin Ther. 2006;28:652–665.
57 Ahren B, Landin-Olsson M, Jansson PA, et al. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:2078–2084.
58 Fineman M, Weyer C, Maggs DG, et al. The human amylin analog, pramlintide, reduces postprandial hyperglucagonemia in patients with type 2 diabetes mellitus. Horm Metab Res. 2002;34:504–508.
59 Heptulla RA, Rodriguez LM, Bomgaars L, et al. The role of amylin and glucagon in the dampening of glycemic excursions in children with type 1 diabetes. Diabetes. 2005;54:1100–1107.
60 Weyer C, Gottlieb A, Kim DD, et al. Pramlintide reduces postprandial glucose excursions when added to regular insulin or insulin lispro in subjects with type 1 diabetes: a dose-timing study. Diabetes Care. 2003;26:3074–3079.
61 Whitehouse F, Kruger DF, Fineman M, et al. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care. 2002;25:724–730.
62 Allison SP, Tomlin PJ, Chamberlain MJ. Some effects of anaesthesia and surgery on carbohydrate and fat metabolism. Br J Anaesth. 1969;41:588–593.
63 Halter JB, Pflug AE. Relationship of impaired insulin secretion during surgical stress to anesthesia and catecholamine release. J Clin Endocrinol Metab. 1980;51:1093–1098.
64 Cryer PE. Glucose counterregulatory hormones. In: LeRoith D, Taylor SI, Olefsky JM, eds. Diabetes mellitus a fundamental and clinical text. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:199–206.
65 Glister BC, Vigersky RA. Perioperative management of type 1 diabetes mellitus. Endocrinol Metab Clin North Am. 2003;32:411–436.
66 Thorell A, Nygren J, Ljungqvist O. Insulin resistance: a marker of surgical stress. Curr Opin Clin Nutr Metab Care. 1999;2:69–78.
67 Van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest. 2004;114:1187–1195.
68 De Feo P, Perriello G, Torlone E, et al. Contribution of cortisol to glucose counterregulation in humans. Am J Physiol. 1989;257:E35–E42.
69 De Feo P, Perriello G, Torlone E, et al. Demonstration of a role for growth hormone in glucose counterregulation. Am J Physiol. 1989;256:E835–E843.
70 Hirsch IB, McGill JB. Role of insulin in management of surgical patients with diabetes mellitus. Diabetes Care. 1990;13:980–991.
71 Lattermann R, Schricker T, Wachter U, et al. Understanding the mechanisms by which isoflurane modifies the hyperglycemic response to surgery. Anesth Analg. 2001;93:121–127.
72 Desborough JP, Jones PM, Persaud SJ, et al. Isoflurane inhibits insulin secretion from isolated rat pancreatic islets of Langerhans. Br J Anaesth. 1993;71:873–876.
73 Vore SJ, Aycock ED, Veldhuis JD, et al. Anesthesia rapidly suppresses insulin pulse mass but enhances the orderliness of insulin secretory process. Am J Physiol Endocrinol Metab. 2001;281:E93–E99.
74 Engquist A, Brandt MR, Fernandes A, et al. The blocking effect of epidural analgesia on the adrenocortical and hyperglycemic responses to surgery. Acta Anaesthesiol Scand. 1977;21:330–335.
75 Kehlet H, Brandt MR, Hansen AP, et al. Effect of epidural analgesia on metabolic profiles during and after surgery. Br J Surg. 1979;66:543–546.
76 Giesecke AH, Spier DJ, Jenkins MT. Management of diabetes mellitus during anesthesia and surgery. Tex Med. 1964;60:840–843.
77 Schricker T, Carli F, Schreiber M, et al. Propofol/sufentanil anesthesia suppresses the metabolic and endocrine response during, not after, lower abdominal surgery. Anesth Analg. 2000;90:450–455.
78 Rosenberg CS. Wound healing in the patient with diabetes mellitus. Nurs Clin North Am. 1990;25:247–261.
79 Delamaire M, Maugendre D, Moreno M, et al. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14:29–34.
80 Gallacher SJ, Thomson G, Fraser WD, et al. Neutrophil bactericidal function in diabetes mellitus: evidence for association with blood glucose control. Diabet Med. 1995;12:916–920.
81 Marhoffer W, Stein M, Maeser E, et al. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care. 1992;15:256–260.
82 Wilson RM, Reeves WG. Neutrophil phagocytosis and killing in insulin-dependent diabetes. Clin Exp Immunol. 1986;63:478–484.
83 Ellger B, Debaveye Y, Vanhorebeek I, et al. Survival benefits of intensive insulin therapy in critical illness: impact of maintaining normoglycemia versus glycemia-independent actions of insulin. Diabetes. 2006;55:1096–1105.
84 Hjortrup A, Sorensen C, Dyremose E, et al. Influence of diabetes mellitus on operative risk. Br J Surg. 1985;72:783–785.
85 MacKenzie CR, Charlson ME. Assessment of perioperative risk in the patient with diabetes mellitus. Surg Gynecol Obstet. 1988;167:293–299.
86 Golden SH, Peart-Vigilance C, Kao WH, et al. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care. 1999;22:1408–1414.
87 Zerr KJ, Furnary AP, Grunkemeier GL, et al. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg. 1997;63:356–361.
88 Griesdale DEG, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821–827.
89 Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119–1131.
90 Vlasselaers D, Milants I, Desmet L, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373:547–556.
91 Yates AR, Dyke PC, 2nd., Taeed R, et al. Hyperglycemia is a marker for poor outcome in the postoperative pediatric cardiac patient. Pediatr Crit Care Med. 2006;7:351–355.
92 Faustino EV, Apkon M. Persistent hyperglycemia in critically ill children. J Pediatr. 2005;146:30–34.
93 Srinivasan V, Spinella PC, Drott HR, et al. Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med. 2004;5:329–336.
94 Ulate KP, Lima Falcao GC, Bielefeld MR, et al. Strict glycemic targets need not be so strict: a more permissive glycemic range for critically ill children. Pediatrics. 2008;122:e898–e904.
95 Joshi GP, Chung F, Vann MA, et al. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111:1378–1387.
96 Standards of medical care in diabetes. Diabetes Care. 2005;28:S4–36.
97 Schiff RL, Welsh GA. Perioperative evaluation and management of the patient with endocrine dysfunction. Med Clin North Am. 2003;87:175–192.
98 Wolfsdorf J, ed. Intensive diabetes management, 4th ed, Alexandria, Va.: American Diabetes Association, 2009.
99 Marks JB. Perioperative management of diabetes. Am Fam Physician. 2003;67:93–100.
100 Christiansen CL, Schurizek BA, Malling B, et al. Insulin treatment of the insulin-dependent diabetic patient undergoing minor surgery. Continuous intravenous infusion compared with subcutaneous administration. Anaesthesia. 1988;43:533–537.
101 Meyer EJ, Lorenzi M, Bohannon NV, et al. Diabetic management by insulin infusion during major surgery. Am J Surg. 1979;137:323–327.
102 ter Braak EW, Woodworth JR, Bianchi R, et al. Injection site effects on the pharmacokinetics and glucodynamics of insulin lispro and regular insulin. Diabetes Care. 1996;19:1437–1440.
103 Della Manna T, Steinmetz L, Campos PR, et al. Subcutaneous use of a fast-acting insulin analog: an alternative treatment for pediatric patients with diabetic ketoacidosis. Diabetes Care. 2005;28:1856–1861.
104 Roemmich JN, Clark PA, Lusk M, et al. Pubertal alterations in growth and body composition. VI. Pubertal insulin resistance: relation to adiposity, body fat distribution and hormone release. Int J Obes Relat Metab Disord. 2002;26:701–709.
105 Kerouz N, el-Hayek R, Langhough R, et al. Insulin doses in children using conventional therapy for insulin dependent diabetes. Diabetes Res Clin Pract. 1995;29:113–120.
106 Danne T, Mortensen HB, Hougaard P, et al. Persistent differences among centers over 3 years in glycemic control and hypoglycemia in a study of 3,805 children and adolescents with type 1 diabetes from the Hvidore Study Group. Diabetes Care. 2001;24:1342–1347.
107 Mercker SK, Maier C, Neumann G, et al. Lactic acidosis as a serious perioperative complication of antidiabetic biguanide medication with metformin. Anesthesiology. 1997;87:1003–1005.
108 Seckl JR, Dunger DB, Lightman SL. Neurohypophyseal peptide function during early postoperative diabetes insipidus. Brain. 1987;110:737–746.
109 Andersen LJ, Andersen JL, Schutten HJ, et al. Antidiuretic effect of subnormal levels of arginine vasopressin in normal humans. Am J Physiol. 1990;259:R53–R60.
110 Seckl JR, Dunger DB, Bevan JS, et al. Vasopressin antagonist in early postoperative diabetes insipidus. Lancet. 1990;335:1353–1356.
111 Moreno-Sanchez D, Casis B, Martin A, et al. Rhabdomyolysis and cutaneous necrosis following intravenous vasopressin infusion. Gastroenterology. 1991;101:529–532.
112 Mauro VF, Bingle JF, Ginn SM, et al. Torsade de pointes in a patient receiving intravenous vasopressin. Crit Care Med. 1988;16:200–201.
113 Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967;42:790–806.
114 Rose B, Post T. Clinical physiology of acid-base and electrolyte disorders, 5th ed. New York: McGraw-Hill; 2001.
115 Baylis PH. The syndrome of inappropriate antidiuretic hormone secretion. Int J Biochem Cell Biol. 2003;35:1495–1499.
116 Burrows FA, Shutack JG, Crone RK. Inappropriate secretion of antidiuretic hormone in a postsurgical pediatric population. Crit Care Med. 1983;11:527–531.
117 Sterns RH, Riggs JE, Schochet SS, Jr. Osmotic demyelination syndrome following correction of hyponatremia. N Engl J Med. 1986;314:1535–1542.
118 Ellis SJ. Severe hyponatraemia: complications and treatment. QJM. 1995;88:905–909.
119 Adrogue HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581–1589.
120 Weiss RE, Murata Y, Cua K, et al. Thyroid hormone action on liver, heart, and energy expenditure in thyroid hormone receptor beta-deficient mice. Endocrinology. 1998;139:4945–4952.
121 Fisher DA, Nelson JC, Carlton EI, et al. Maturation of human hypothalamic-pituitary-thyroid function and control. Thyroid. 2000;10:229–234.
122 Fisher DA, Klein AH. Thyroid development and disorders of thyroid function in the newborn. N Engl J Med. 1981;304:702–712.
123 Jaruratanasirikul S, Leethanaporn K, Khuntigij P, et al. The clinical course of Hashimoto’s thyroiditis in children and adolescents: 6 years longitudinal follow-up. J Pediatr Endocrinol Metab. 2001;14:177–184.
124 Lafranchi S. Thyroiditis and acquired hypothyroidism. Pediatr Ann. 1992;21:29. 32-9
125 Gordin A, Lamberg BA. Spontaneous hypothyroidism in symptomless autoimmune thyroiditis. A long-term follow-up study. Clin Endocrinol (Oxf). 1981;15:537–543.
126 Delange F. The disorders induced by iodine deficiency. Thyroid. 1994;4:107–128.
127 Fisher DA. Physiological variations in thyroid hormones: physiological and pathophysiological considerations. Clin Chem. 1996;42:135–139.
128 Klemperer JD, Klein I, Gomez M, et al. Thyroid hormone treatment after coronary-artery bypass surgery. N Engl J Med. 1995;333:1522–1527.
129 Stathatos N, Wartofsky L. The euthyroid sick syndrome: is there a physiologic rationale for thyroid hormone treatment? J Endocrinol Invest. 2003;26:1174–1179.
130 Haas NA, Camphausen CK, Kececioglu D. Clinical review: thyroid hormone replacement in children after cardiac surgery–is it worth a try? Crit Care. 2006;10:213.
131 De Groot LJ. Non-thyroidal illness syndrome is a manifestation of hypothalamic-pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit Care Clin. 2006;22:57–86.
132 Sarlis NJ, Gourgiotis L. Thyroid emergencies. Rev Endocr Metab Disord. 2003;4:129–136.
133 Wall CR. Myxedema coma: diagnosis and treatment. Am Fam Physician. 2000;62:2485–2490.
134 LaFranchi S. Congenital hypothyroidism: etiologies, diagnosis, and management. Thyroid. 1999;9:735–740.
135 Czernichow P, Wolf B, Fermanian J, et al. Twenty-four hour variations of thyroid hormones and thyrotrophin concentrations in hypothyroid infants treated with L-thyroxine. Clin Endocrinol (Oxf). 1984;21:393–397.
136 Selva KA, Mandel SH, Rien L, et al. Initial treatment dose of L-thyroxine in congenital hypothyroidism. J Pediatr. 2002;141:786–792.
137 Van Dop C, Conte FA, Koch TK, et al. Pseudotumor cerebri associated with initiation of levothyroxine therapy for juvenile hypothyroidism. N Engl J Med. 1983;308:1076–1080.
138 Hirano T, Stamelos S, Harris V, et al. Association of primary hypothyroidism and slipped capital femoral epiphysis. J Pediatr. 1978;93:262–264.
139 Mandel SJ, Larsen PR, Seely EW, et al. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med. 1990;323:91–96.
140 Clarke N, Kabadi UM. Optimizing treatment of hypothyroidism. Treat Endocrinol. 2004;3:217–221.
141 Weinberg AD, Brennan MD, Gorman CA, et al. Outcome of anesthesia and surgery in hypothyroid patients. Arch Intern Med. 1983;143:893–897.
142 Ladenson PW, Levin AA, Ridgway EC, et al. Complications of surgery in hypothyroid patients. Am J Med. 1984;77:261–266.
143 Cone AM. Anaesthesia in a patient with untreated myxoedema coma. Anaesth Intensive Care. 1994;22:295–298.
144 Stathatos N, Wartofsky L. Perioperative management of patients with hypothyroidism. Endocrinol Metab Clin North Am. 2003;32:503–518.
145 Roldan MB, Alonso M, Barrio R. Thyroid autoimmunity in children and adolescents with Type 1 diabetes mellitus. Diabetes Nutr Metab. 1999;12:27–31.
146 Hardy O, Worley G, Lee MM, et al. Hypothyroidism in Down syndrome: screening guidelines and testing methodology. Am J Med Genet A. 2004;124:436–437.
147 El-Mansoury M, Bryman I, Berntorp K, et al. Hypothyroidism is common in Turner syndrome: results of a five-year follow-up. J Clin Endocrinol Metab. 2005;90:2131–2135.
148 Drucker DJ, Burrow GN. Cardiovascular surgery in the hypothyroid patient. Arch Intern Med. 1985;145:1585–1587.
149 Woeber KA. Observations concerning the natural history of subclinical hyperthyroidism. Thyroid. 2005;15:687–691.
150 Zimmerman D, Lteif AN. Thyrotoxicosis in children. Endocrinol Metab Clin North Am. 1998;27:109–126.
151 Weiss RE, Refetoff S. Treatment of resistance to thyroid hormone – primum non nocere. J Clin Endocrinol Metab. 1999;84:401–404.
152 Weiss RE, Refetoff S. Resistance to thyroid hormone. Rev Endocr Metab Disord. 2000;1:97–108.
153 Rivkees SA. The treatment of Graves’ disease in children. J Pediatr Endocrinol Metab. 2006;19:1095–1111.
154 Wartofsky L, Glinoer D, Solomon B, et al. Differences and similarities in the treatment of diffuse goiter in Europe and the United States. Exp Clin Endocrinol. 1991;97:243–251.
155 Lippe BM, Landaw EM, Kaplan SA. Hyperthyroidism in children treated with long term medical therapy: twenty-five percent remission every two years. J Clin Endocrinol Metab. 1987;64:1241–1245.
156 Torring O, Tallstedt L, Wallin G, et al. Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine—a prospective, randomized study. Thyroid Study Group. J Clin Endocrinol Metab. 1996;81:2986–2993.
157 Wartofsky L. Radioiodine therapy for Graves’ disease: case selection and restrictions recommended to patients in North America. Thyroid. 1997;7:213–216.
158 Nabhan ZM, Kreher NC, Eugster EA. Hashitoxicosis in children: clinical features and natural history. J Pediatr. 2005;146:533–536.
159 Chang P, Tsai WY, Lee PI, et al. Clinical characteristics and management of acute suppurative thyroiditis in children. J Formos Med Assoc. 2002;101:468–471.
160 Ogawa E, Katsushima Y, Fujiwara I, et al. Subacute thyroiditis in children: patient report and review of the literature. J Pediatr Endocrinol Metab. 2003;16:897–900.
161 Zimmerman D, Gan-Gaisano M. Hyperthyroidism in children and adolescents. Pediatr Clin North Am. 1990;37:1273–1295.
162 Volpe R. The pathogenesis of Graves’ disease. Endocr Pract. 1995;1:103–115.
163 Bartley GB, Gorman CA. Diagnostic criteria for Graves’ ophthalmopathy. Am J Ophthalmol. 1995;119:792–795.
164 Lee HB, Rodgers IR, Woog JJ. Evaluation and management of Graves’ orbitopathy. Otolaryngol Clin North Am. 2006;39:923–942.
165 Laurberg P. Remission of Graves’ disease during anti-thyroid drug therapy. Time to reconsider the mechanism? Eur J Endocrinol. 2006;155:783–786.
166 Barrio R, Lopez-Capape M, Martinez-Badas I, et al. Graves’ disease in children and adolescents: response to long-term treatment. Acta Paediatr. 2005;94:1583–1589.
167 Vitti P, Rago T, Chiovato L, et al. Clinical features of patients with Graves’ disease undergoing remission after antithyroid drug treatment. Thyroid. 1997;7:369–375.
168 Cooper DS. Antithyroid drugs. N Engl J. Med 2005;352:905–917.
169 Behar R, Arganini M, Wu TC, et al. Graves’ disease and thyroid cancer. Surgery. 1986;100:1121–1127.
170 Rivkees SA, Sklar C, Freemark M. Clinical review 99: The management of Graves’ disease in children, with special emphasis on radioiodine treatment. J Clin Endocrinol Metab. 1998;83:3767–3776.
171 Argov S, Duek D. The vanishing surgical treatment of Graves’ disease: review of current literature and experience with 50 patients. Curr Surg. 1982;39:158–162.
172 Tietgens ST, Leinung MC. Thyroid storm. Med Clin North Am. 1995;79:169–184.
173 Morrison MP, Schroeder A. Intraoperative identification and management of thyroid storm in children. Otolaryngol Head Neck Surg. 2007;136:132–133.
174 Bennett MH, Wainwright AP. Acute thyroid crisis on induction of anaesthesia. Anaesthesia. 1989;44:28–30.
175 Nishiyama K, Kitahara A, Natsume H, et al. Malignant hyperthermia in a patient with Graves’ disease during subtotal thyroidectomy. Endocr J. 2001;48:227–232.
176 Birrell G, Cheetham T. Juvenile thyrotoxicosis; can we do better? Arch Dis Child. 2004;89:745–750.
177 Nayak B, Burman K. Thyrotoxicosis and thyroid storm. Endocrinol Metab Clin North Am. 2006;35:663–686.
178 Knighton JD, Crosse MM. Anaesthetic management of childhood thyrotoxicosis and the use of esmolol. Anaesthesia. 1997;52:67–70.
179 Langley RW, Burch HB. Perioperative management of the thyrotoxic patient. Endocrinol Metab Clin North Am. 2003;32:519–534.
180 Pidasheva S, D’Souza-Li L, Canaff L, et al. CASRdb: calcium-sensing receptor locus-specific database for mutations causing familial (benign) hypocalciuric hypercalcemia, neonatal severe hyperparathyroidism, and autosomal dominant hypocalcemia. Hum Mutat. 2004;24:107–111.
181 Raue F, Haag C, Schulze E, et al. The role of the extracellular calcium-sensing receptor in health and disease. Exp Clin Endocrinol Diabetes. 2006;114:397–405.
182 Hsu SC, Levine MA. Perinatal calcium metabolism: physiology and pathophysiology. Semin Neonatol. 2004;9:23–36.
183 Greenhalgh KL, Aligianis IA, Bromilow G, et al. 22q11 deletion: a multisystem disorder requiring multidisciplinary input. Arch Dis Child. 2003;88:523–524.
184 Oskarsdottir S, Persson C, Eriksson BO, et al. Presenting phenotype in 100 children with the 22q11 deletion syndrome. Eur J Pediatr. 2005;164:146–153.
185 Shprintzen RJ, Higgins AM, Antshel K, et al. Velo-cardio-facial syndrome. Curr Opin Pediatr. 2005;17:725–730.
186 Ashraf A, Mick G, Atchison J, et al. Prevalence of hypovitaminosis D in early infantile hypocalcemia. J Pediatr Endocrinol Metab. 2006;19:1025–1031.
187 Ladhani S, Srinivasan L, Buchanan C, et al. Presentation of vitamin D deficiency. Arch Dis Child. 2004;89:781–784.
188 Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab. 2006;91:2843–2850.
189 Quattrocchi E, Kourlas H. Teriparatide: a review. Clin Ther. 2004;26:841–854.
190 Kollars J, Zarroug AE, van Heerden J, et al. Primary hyperparathyroidism in pediatric patients. Pediatrics. 2005;115:974–980.
191 Iacobone M, Lumachi F, Favia G. Up-to-date on parathyroid carcinoma: analysis of an experience of 19 cases. J Surg Oncol. 2004;88:223–228.
192 Learoyd DL, Gosnell J, Elston MS, et al. Experience of prophylactic thyroidectomy in multiple endocrine neoplasia type 2A kindreds with RET codon 804 mutations. Clin Endocrinol (Oxf). 2005;63:636–641.
193 Marx SJ, Simonds WF, Agarwal SK, et al. Hyperparathyroidism in hereditary syndromes: special expressions and special managements. J Bone Miner Res. 2002;17:N37–N43.
194 Kahraman T, de Groot JW, Rouwe C, et al. Acceptable age for prophylactic surgery in children with multiple endocrine neoplasia type 2a. Eur J Surg Oncol. 2003;29:331–335.
195 Waller SC, Ridout D, Cantor T, et al. Parathyroid hormone and growth in children with chronic renal failure. Kidney Int. 2005;67:2338–2345.
196 Amenta S, Sofocleous C, Kolialexi A, et al. Clinical manifestations and molecular investigation of 50 patients with Williams syndrome in the Greek population. Pediatr Res. 2005;57:789–795.
197 Jacobs TP, Bilezikian JP. Clinical review: rare causes of hypercalcemia. J Clin Endocrinol Metab. 2005;90:6316–6322.
198 Kerdudo C, Aerts I, Fattet S, et al. Hypercalcemia and childhood cancer: a 7-year experience. J Pediatr Hematol Oncol. 2005;27:23–27.
199 Rodriguez Soriano J. Neonatal hypercalcemia. J Nephrol. 2003;16:606–608.
200 Alos N, Eugene D, Fillion M, et al. Pamidronate: treatment for severe hypercalcemia in neonatal subcutaneous fat necrosis. Horm Res. 2006;65:289–294.
201 Shaw NJ, Bishop NJ. Bisphosphonate treatment of bone disease. Arch Dis Child. 2005;90:494–499.
202 Arlt W, Stewart PM. Adrenal corticosteroid biosynthesis, metabolism, and action. Endocrinol Metab Clin North Am. 2005;34:293–313.
203 Hammer GD, Parker KL, Schimmer BP. Minireview: transcriptional regulation of adrenocortical development. Endocrinology. 2005;146:1018–1024.
204 White PC. Abnormalities of aldosterone synthesis and action in children. Curr Opin Pediatr. 1997;9:424–430.
205 Ulick S. Cortisol as mineralocorticoid. J Clin Endocrinol Metab. 1996;81:1307–1308.
206 Langer M, Modi BP, Agus M. Adrenal insufficiency in the critically ill neonate and child. Curr Opin Pediatr. 2006;18:448–453.
207 Wise-Faberowski L, Soriano SG, Ferrari L, et al. Perioperative management of diabetes insipidus in children [corrected]. J Neurosurg Anesthesiol. 2004;16:14–19.
208 Nieman LK, Chanco Turner ML. Addison’s disease. Clin Dermatol. 2006;24:276–280.
209 Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365:2125–2136.
210 Moser H, Dubey P, Fatemi A. Progress in X-linked adrenoleukodystrophy. Curr Opin Neurol. 2004;17:263–269.
211 New MI. Inborn errors of adrenal steroidogenesis. Mol Cell Endocrinol. 2003;211:75–83.
212 Garcia ML, Ty EB, Taban M, et al. Systemic and ocular findings in 100 patients with optic nerve hypoplasia. J Child Neurol. 2006;21:949–956.
213 Haddad NG, Eugster EA. Hypopituitarism and neurodevelopmental abnormalities in relation to central nervous system structural defects in children with optic nerve hypoplasia. J Pediatr Endocrinol Metab. 2005;18:853–858.
214 Polizzi A, Pavone P, Iannetti P, et al. Septo-optic dysplasia complex: a heterogeneous malformation syndrome. Pediatr Neurol. 2006;34:66–71.
215 Urban RJ. Hypopituitarism after acute brain injury. Growth Horm IGF Res. 2006;16:S25–S29.
216 Hopper N, Albanese A, Ghirardello S, et al. The pre-operative endocrine assessment of craniopharyngiomas. J Pediatr Endocrinol Metab. 2006;19:325–327.
217 Rose SR, Danish RK, Kearney NS, et al. ACTH deficiency in childhood cancer survivors. Pediatr Blood Cancer. 2005;45:808–813.
218 Nieman LK. Dynamic evaluation of adrenal hypofunction. J Endocrinol Invest. 2003;26:74–82.
219 Yaegashi M, Boujoukos AJ. The low-dose ACTH test in the ICU: not ready for prime time. Crit Care. 2006;10:313.
220 Aneja R, Carcillo JA. What is the rationale for use of hydrocortisone therapy for infection related adrenal insufficiency and septic shock? Arch Dis Child. 2007;92:165–169.
221 Jacobi J. Corticosteroid replacement in critically ill patients. Crit Care Clin. 2006;22:245–253.
222 Mancini T, Doga M, Mazziotti G, et al. Cushing’s syndrome and bone. Pituitary. 2004;7:249–252.
223 Stratakis CA. Cortisol and growth hormone: clinical implications of a complex, dynamic relationship. Pediatr Endocrinol Rev. 2006;3:333–338.
224 Frank GR, Speiser PW, Griffin KJ, et al. Safety of medications and hormones used in pediatric endocrinology: adrenal. Pediatr Endocrinol Rev. 2004;2:134–145.
225 Storr HL, Mitchell H, Swords FM, et al. Clinical features, diagnosis, treatment and molecular studies in paediatric Cushing’s syndrome due to primary nodular adrenocortical hyperplasia. Clin Endocrinol (Oxf). 2004;61:553–559.
226 Ilias I, Torpy DJ, Pacak K, et al. Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90:4955–4962.
227 Lacroix A, Bourdeau I. Bilateral adrenal Cushing’s syndrome: macronodular adrenal hyperplasia and primary pigmented nodular adrenocortical disease. Endocrinol Metab Clin North Am. 2005;34:441–458.
228 Mayer SK, Oligny LL, Deal C, et al. Childhood adrenocortical tumors: case series and reevaluation of prognosis: a 24-year experience. J Pediatr Surg. 1997;32:911–915.
229 Shen WT, Lee J, Kebebew E, et al. Selective use of steroid replacement after adrenalectomy: lessons from 331 consecutive cases. Arch Surg. 2006;141:771–774. discussion 4-6
230 Trilck M, Flitsch J, Ludecke DK, et al. Salivary cortisol measurement – a reliable method for the diagnosis of Cushing’s syndrome. Exp Clin Endocrinol Diabetes. 2005;113:225–230.
231 Dias R, Storr HL, Perry LA, et al. The discriminatory value of the low-dose dexamethasone suppression test in the investigation of paediatric Cushing’s syndrome. Horm Res. 2006;65:159–162.
232 Batista D, Courkoutsakis NA, Oldfield EH, et al. Detection of adrenocorticotropin-secreting pituitary adenomas by magnetic resonance imaging in children and adolescents with Cushing disease. J Clin Endocrinol Metab. 2005;90:5134–5140.
233 Batista D, Gennari M, Riar J, et al. An assessment of petrosal sinus sampling for localization of pituitary microadenomas in children with Cushing disease. J Clin Endocrinol Metab. 2006;91:221–224.
234 Chanson P, Salenave S. Diagnosis and treatment of pituitary adenomas. Minerva Endocrinol. 2004;29:241–275.
235 Chen JC, Amar AP, Choi S, et al. Transsphenoidal microsurgical treatment of Cushing disease: postoperative assessment of surgical efficacy by application of an overnight low-dose dexamethasone suppression test. J Neurosurg. 2003;98:967–973.