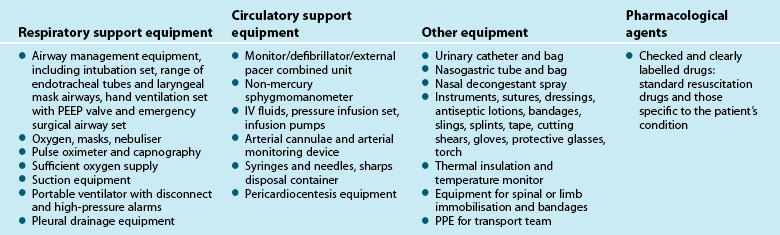
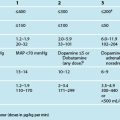
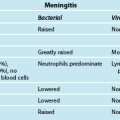
6 Essential Nursing Care of the Critically Ill Patient
After reading this chapter, you should be able to:
• identify risks posed to critically ill patients relating to inadequate physical care and hygiene
• describe best practice in the provision of physical care and hygiene
• understand the key elements of safe transfer of critically ill patients within the hospital setting
• understand the principles of infection-control risk identification and management for critically ill patients
Introduction
Comfort is a paramount concern in intensive care. The two key areas of care – reducing risk and providing quality care – are closely related and served by a series of principles (see Table 6.1). Good risk management is an important component of quality care; if patients are assessed thoroughly and on a continuing basis then problems may be detected and treated early, preventing the development of unnecessary complications. These principles underpin this chapter. Additionally, it is important always to treat the patient as a person. Although this chapter focuses on the physical dimension of nursing care, patients’ psychosocial care should not be ignored (see Chapters 7 and 8). Further, while this chapter describes essential nursing care, care bundles, which encompass a number of these activities, are described in Chapter 3.
Personal Hygiene
It is important to provide the critically ill patient with effective personal hygiene as poor hygiene may increase the risk of bacterial colonisation and subsequent infection,1 or lead to surgical infection.2 Daily bed-baths are usually provided for most critically ill patients, although their effectiveness at reducing bacterial colonisation is questionable.1 Personal hygiene is also closely related to an individual’s esteem and sense of wellbeing. It may also influence family members’ perception of the quality of care the patient is receiving and the confidence they have in the staff’s ability to care for their loved one.
Assessment of Personal Hygiene
Assessment of critical care patients’ personal hygiene should be undertaken on two levels: first, determining what patients are able to do for themselves and what they want and second, the nurse’s professional assessment of what is required. As with all aspects of care, the patient has the right to refuse personal hygiene measures. Many critical care patients are unable to participate in decision making, and in these cases it falls to the nurse at the bedside to determine what level of care is necessary.Washing patients provides opportunities for the nurse to assess the patient’s skin and tissue. Often this enables the nurse to: pick up vital clues about the patient’s health status; identify tissue damage that requires treatment; and identify dressings or wounds that require attention. There are a number of areas to consider when assessing the skin (see Table 6.2). Excessive moisture on the patient’s skin from sweat can be problematic, particularly in skinfolds. Perspiration is a normal insensible loss, and is invisible. Body sweat is usually related to temperature and is observed on all skin surfaces, especially the forehead, axillae and groins. Emotional sweating is stress-related and is observed on the palms of the hands, soles of the feet, forehead and axillae.
| Factor | Observations |
|---|---|
| Colour of the skin |
Basic Hygiene
The length of time taken to wash a patient and the environmental temperature are factors that affect cooling. Water on exposed skin causes rapid heat loss through conduction, convection and radiation, and for many years tepid sponging was used in critical care as a method of cooling pyrexic patients.3 Vasoconstriction increases the patient’s perception of cold and the possibility of shivering,4 which can affect the patient’s cardiovascular stability. When shivering occurs, vulnerable patients, with low energy reserves, can rapidly use energy to keep warm. The higher oxygen consumption associated with shivering may be particularly significant in elderly patients.4
A range of cleansing solutions is available for washing. Although soap is effective in facilitating the removal of bacteria, it can cause dryness of the skin. Aqueous cream, which can be used as a soap substitute, or emulsifying ointments are preferable, as they have moisturising properties, although the latter is greasier.5 Topical emollients (moisturisers) either trap water or draw water into the dermis, and help to protect damaged skin by creating a waterproof barrier.5 Baby care products are often used, although these may be the least effective due to their low oil content.5 Specific topical treatments may be required for patients with skin diseases such as dermatitis. Disposable cloths should be used for washing, as linen flannels have been shown to harbour bacteria. Complete disposable wash kits are available with potential advantages of being effective for patient’s skin cleaning without requiring rinsing and therefore drying the skin, and being disposable may reduce potential for infection and certainly reduces linen costs.1
Personal hygiene involves washing the patient’s hair as necessary, shaving the patient, management of cerumen in ears and care of finger and toe nails. While normal shampoo can be used, hair caps and washing products are available that are easier to use for bed ridden patients. Male facial hair should be managed as per the patient’s normal routine, such as maintaining a beard or shaving. Ears should be gently inspected for debris or injury. If assessed as appropriate, wax softening drops may be needed for 3–5 days if cerumen is present and causing the patient difficulties with their hearing.6 Maintaining clean nails is another aspect of personal hygiene. Care should be taken if nails require trimming, especially if the patient has brittle nails or is diabetic.
Skin Tears
Dependent patients who require total care are at greatest risk of skin tears. Injuries result from routine activities such as dressing, bathing, positioning and transferring.7 The elderly, those with fragile skin (particularly those with a history of previous skin tears), those who require the use of devices to assist lifting, those who are cognitively or sensorily impaired, and those who have skin problems such as oedema, purpura or ecchymosis are at greatest risk. Most skin tears occur on the arms and the back of the hands. The Payne-Martin classification system8 uses three categories to describe skin tears: skin tears without tissue loss; skin tears with partial tissue loss; and skin tears with complete tissue loss.
Skin tears can be prevented by careful handling of patients to reduce skin friction and shear during repositioning and transfers. Padded bed rails, pillows and blankets can be used to protect and support arms and legs. Paper-type or non-adherent dressings should be used on frail skin, and should be removed gently and slowly. Wraps or nets can be used instead of surgical tape to secure dressings and drains in place. Application of a moisturising lotion to dry skin helps to keep it adequately hydrated. Treatment of skin tears7 is outlined in Table 6.3. The focus of nursing care should be on careful cleansing and protection of the skin tear to prevent further damage and documentation of interventions and healing progress.
| Factor | Interventions |
|---|---|
| Cleansing |
• Provide appropriate topical wound care, such as a moist wound dressing.
• Remove any product with an adhesive backing with utmost care to avoid further trauma
• Secure non-adherent dressing with a gauze or tubular non-adhesive wrap
• Change dressings according to the manufacturer’s recommendations
Eye Care
The eyes are one of the most sensitive parts of the human body. If their eyes are not properly cared for, critical care patients may spend many hours in unnecessary discomfort. Simple bedside procedures like turning on lights at night or assessing pupil reactions can be uncomfortable. There are a number of physiological processes that protect the eye. For example, the eye is protected from dryness by frequent lubrication facilitated by blinking. Antimicrobial substances in tears help prevent infection, and the tear ducts provide drainage. When the eye is unable to close properly, tear film evaporates more quickly.8 If any of these defence mechanisms are compromised the eyes are at greater risk.
There is considerable risk to patients’ eyes while they are in the ICU.9 The blink response may be slowed or absent in some patients, such as individuals receiving sedatives and muscle relaxants, or those with Guillain–Barré syndrome.10 A number of complications can result, such as keratopathy, corneal ulceration and viral or bacterial conjunctivitis.9 Corneal abrasions may occur within 48 hours of ICU admission11,12 and in up to 40–60% of critically ill patients.8,12 When the eyes are exposed they are at greater risk of injury and infection, and conjunctival oedema can lead to subconjunctival haemorrhage.13 For the intensive care patient, who often has multiple intravenous lines, nasogastric tubes, ventilation tubes and their various connections, there is potential to unintentionally damage one of the eyes with one of these devices during position changes.
Eye Assessment
Eye assessment should be undertaken at least every 12 hours, even for the conscious patients who are able to blink spontaneously and usually require minimal eye care. The risk of corneal abrasion or iatrogenic trauma is greatest when patients are unable to close their eyes spontaneously,14 so these patients are at greatest risk of injury. The second at-risk group is those patients receiving positive pressure ventilation, who may develop conjunctival oedema (chemosis), sometimes referred to as ‘ventilator eye’.9 Third, patients who are exposed to high flows of air/oxygen, such as that with continuous positive airway pressure (CPAP) systems, may be vulnerable to its drying effects. Finally, all patients are at risk of eye inflammation and infection. Serious infections with bacteria such as pseudomonas can progress rapidly, resulting in blindness if not treated promptly.
Initial assessment should focus on whether the patient belongs to an at-risk group. Most critically ill patients are at some risk, but particularly those who are unable to close their eyes adequately. If the cornea is exposed, the patient is considered to be in a high-risk group.14 Based on the groups identified above, initial assessment should help determine how often eye assessment and eye care is required.
The general principles of eye assessment are shown in Table 6.4, which should include a full examination of the eye’s external structure, colour and response. A number of assessment tools have been developed for this purpose.9 Thorough eye assessment should assess appearance (which may provide indications of disease or trauma) and physical and neurological functions. If there is concern about any aspect of a patient’s eyes, a referral for assessment should be made to an ophthalmologist.
| External structure | Colour | Reaction |
|---|---|---|
Essential Eye Care
The goals of eye care are to provide comfort and protect the eyes from injury and infection. Eye care and the administration of artificial tears should be provided as required, if the patient complains of sore or dry eyes, or if there is visible evidence of encrustation. If a patient is receiving high-flow oxygen therapy via a mask, they may benefit from regular 4-hourly administration of artificial tears to lubricate the eyes,9 although this may be unnecessary while they are sleeping.
Dawson offers an eye care protocol for critically ill patients, which clarifies the type of eye care required according to the patient’s ability to maintain eye closure.14 The protocol requires an assessment to be made once per shift. Initially, eye closure is assessed to determine whether it is complete or whether the conjunctiva and/or the cornea are exposed. Suggested treatment is 1–4-hourly eyedrops, with further assessment to exclude keratitis or conjunctivitis. Unconscious or paralysed patients are likely to require more eye care than conscious patients. Basic eye care consists of cleaning the sclera and surrounding tissue and moistening the eyes by administering artificial tears.
For at-risk patients, the general consensus is that eye care should be performed using a sterile technique, cleansing the eye from the inside to the outside usually with saline and gauze; however, eye care regimens have not been rigorously researched.9 Cotton wool is not recommended because of the presence of particulates that may cause corneal abrasions. Eyedrops should be administered gently, inserting the drop in the uppermost part of the opened eye and as close to the eye as possible without touching it. Sometimes eyedrops can sting, so it is advisable to warn the patient of this possibility. Regular scheduled eye care with an ocular lubricant plus eye closure with tape or wrap is used to reduce the potential for corneal abrasions or subsequent corneal ulceration or infection in patients who are either paralysed or heavily sedated.15–17
Conjunctival Oedema (Chemosis)
Conjunctival oedema (chemosis) is a common problem associated with positive pressure ventilation, high positive end-expiratory pressure (PEEP) above 5 cmH2O18 and prone positioning.9 While the oedema itself usually resolves without treatment when ventilation is discontinued, it may be advisable to seek an ophthalmic opinion if there is concern. The literature is inconclusive concerning the best method of treatment for conjunctival oedema, but evidence supports the use of artificial tear ointment and maintaining eye closure as effective measures to reduce corneal abrasions.9
Severe oedema often results in the patient’s inability to maintain eye closure. Under such circumstances, the majority opinion is that eye closure may be maintained by applying a wide piece of adhesive tape horizontally to the upper part of the eyelid.9 This usually anchors the lid in the closed position, while allowing the eyelid to be opened for pupil assessment and access for eye care. It is not necessary to change the tape at each pupil assessment using this method. However, the use of tape may be inappropriate for patients whose skin is very friable. Furthermore, if the eyelid becomes sore and inflamed, taping should be discontinued and an alternative method employed to close the eyes, e.g. gel eye pads.19 When it is not possible to close the eyes, artificial tear ointment has been shown to reduce the incidence of corneal abrasion.15
If it is difficult to maintain eye closure by taping the upper part of the eyelid, the entire eye can also be covered with polyethylene film, which has been shown to reduce the incidence of corneal abrasion.18 This should be changed 4-hourly with eye care and assessment. Commercially available eye-closing tape products are also available along with gel eye dressings which may be used instead of polyethylene film.20,21 Current evidence indicates that polyethylene film is the superior and most cost-effective product for maintaining the ocular surface.9,21
Oral Hygiene
Poor oral hygiene is unpleasant, causing halitosis and discomfort. Although mouth care is one of the most basic nursing activities,22 in some cases lack of oral hygiene can lead to serious complications or increase their risk, such as ventilator-associated pneumonia in the ventilated patient. Attendance to oral hygiene including the removal of dental plaque which harbours pathogens is an imptant component of nursing care.23–26 Using a well-developed oral protocol can improve the oral health of ICU patients.27 However, the practice of mouth care is not always evidence-based,28 although evidence supports having a standardise oral care protocol to improve oral hygiene.25 Factors associated with poor quality of oral care include lack of education, insufficient time, non-prioritising of oral care, and the perception that it is unpleasant.29
Oral Assessment
Mouth care should be reviewed regularly based on a thorough assessment of the oral cavity.22 Several oral assessment tools have been designed specifically for intubated patients.30–32 Essentially, a healthy mouth is characterised by several factors,33 as identified in Box 6.1, and all of these areas should be assessed as a basis for good oral care.
Box 6.1
Characteristics of a healthy mouth
Essential Oral Care
Oral care aims to ensure a healthy oral mucosa, prevent halitosis, maintain a clean and moist oral cavity, prevent pressure sores from devices such as ETTs, prevent trauma caused by grinding of teeth or biting of the tongue, and reduce bacterial activity that leads to local and systemic infection.22
Oral care for an un-intubated conscious patient with a healthy mouth generally involves daily observation of the mucosa and twice-daily toothbrushing with a non-irritant fluoride toothpaste.22 In general, for unconscious patients oral care should be attended to 2-hourly, although the evidence is inconclusive and frequency ranges from 2- to 12-hourly.28 If the mouth is unhealthy, it may be necessary to provide oral hygiene as often as every hour.
The basic method for oral care is to use a soft toothbrush and toothpaste (even for intubated patients), as this will assist with gum care as well as cleaning teeth.25 Toothpaste loosens debris34 and fluoride helps to prevent dental caries.35 However, if it is not rinsed away properly, toothpaste dries the oral mucosa. The practice of using mouth swabs only for oral hygiene is ineffective,36 and toothbrushes perform substantially better than foam swabs in removing plaque.25,36,37 Mouth rinses have not conclusively shown benefit,26 however they may be comfortable for the patient to use. Toothbrushing every 8 hours was recommended in a recent study as being an adjunct to other ventilator associated pneumonia prevention practices38 while use of chlorhexidine toothbrushing was found to be of benefit in another study.39
Although it is an effective saliva stimulant, practices such as the use of lemon and glycerine are outdated, as glycerine causes reflex exhaustion of the saliva process, resulting in a dryer mouth.22,25 Lemon juice is to be avoided, as it can decalcify enamel.37 Commercial mouthwashes moisten and soften the mucosa and help to loosen debris, which can be washed away.26 They must be used with caution in patients with oral problems, due to their potential to cause irritation and hypersensitivity.22
There are many oral hygiene products and solutions available to suit the needs of all patients.22 Commercial mouthwashes should be used as a comfort measure to supplement toothbrushing.26 A range of other products are available to treat oral problems, for example benzydamine hydrochloride (anti-inflammatory), aqueous lignocaine (anaesthetic) and nystatin (antifungal). For patients intubated for more than 24 hours, rates of nosocomial pneumonia may be reduced by using twice-daily chlorhexidine gluconate mouthwashes,25,37,39,40 which also prevent plaque accumulation.25 This has the disadvantage of an unpleasant taste and can discolour teeth.32 For patients with crusty build-up on their teeth,25 a single application of warm dilute solution of sodium bicarbonate powder with a toothbrush is effective in removing debris and causes mucus to become less sticky, although its use has not be definitively tested. However, it can cause superficial burns and its use should be followed immediately by a thorough water rinse of the mouth to return the oral pH to normal. Hydrogen peroxide has an antiplaque effect,22 but if incorrectly diluted it can cause pain and burns to the oral mucosa41 and a predisposition to candida colonisation.22 It is not pleasant tasting and sometimes rejected by patients although it is the substance that impregnates some of the foam sticks available for oral care.37 As a preventive measure, to reduce the incidence of fungal colonisation, natural yoghurt may be used. Normal oral hygiene is followed by coating the mouth and tongue with yoghurt.
Patient Positioning and Mobilisation
Positioning patients correctly is important for their comfort and the reduction of complications associated with pressure areas42 and joint immobility. Lying in bed for long periods can be a painful experience.43 Several researchers44–48 describe neuromyopathy from critical illness and disuse atrophy from prolonged immobility contributing to intensive care acquired weakness. This weakness may contribute to prolonged ventilation, intensive care length of stay as well as delayed return to physical normality.44–53 Cardiovascular stability, respiratory function and cerebral or spinal function are all factors that influence the positioning of patients in critical care areas. Modern beds and pressure-relieving devices have helped considerably to enhance the care of critically ill patients.
The primary goals of essential nursing care for patient positioning are:
• to position the patient comfortably
• to enhance therapeutic benefits
• to ensure the limbs are supported appropriately and to maintain flexible joints
• to facilitate patient activity to minimise muscle atrophy
• to implement early mobilisation as the patient’s condition allows.
There is growing evidence that early mobilisation is an important aim for critically ill patients51–55 and an essential goal of nursing care is to support the patient in maintaining or attaining a normal level of physical function for mobility. As with many other aspects of care for the critically ill, this is best achieved through multidisciplinary team members working together. Here, physiotherapists and occupational therapists have a lead role in assessing patients and planning programs of care and activity to facilitate attaining the goals of normal physical function, while nurses contribute by ensuring the programs of care are delivered when other personnel are not available.
Assessment of Body Positioning
Body positioning assessment is based on the goals of nursing care. First, a risk assessment is made and those patients at highest risk of complications related to their position are those who are unable to move for long periods, for whatever reason.56 For example, unstable patients whose status is compromised when they are moved, patients who are in critical care for a long time, elderly and frail or malnourished patients, and patients who are unable to move themselves (e.g. due to sedation, trauma, surgery or obesity) are all at risk. Batson et al. identified several significant risk factors: patients receiving adrenaline and/or noradrenaline infusions; patients with restricted movement; and diabetic and unstable patients.57 However, even previously fit patients who experience a critical illness can develop severe limitations in their mobility. The common short- and long-term complications of immobility are pressure ulcers, venous thromboembolism and pulmonary dysfunction, each of which carries a significant co-morbidity.56
Positioning and Mobilising Patients
Positioning the patient to achieve maximum comfort, therapeutic benefit and pressure area relief and employing active and passive exercises to maintain muscle and joint integrity and progress to regaining mobility are important nursing activities. Provided there are no specific contraindications, the immobile patient should be positioned with the head raised by 30 degrees or more, as research has demonstrated that it improves mortality58 and helps reduce ventilator-associated pneumonia.59 When combined with thromboembolic prophylaxis, gastric ulcer prophylaxis and daily sedation assessment, ventilator-associated pneumonia may be reduced by around 45%.59 Good body positioning and alignment helps prevent muscle contracture, pressure ulcers and unnecessary pain or discomfort for the patient.60,61
Mobilisation for the critically ill patient can be described as a graduated increase in range of activity from positioning, passive movement, sitting upright in bed, sitting in a chair to actually ambulating.49–5153 Stiller62 describes a range of safety factors that need to be considered prior to mobilising the critically ill patient, which fall into two groups; those specific to the patient and their physical and physiological condition, and those extrinsic to the patient such as the environment, staffing and patient devices attached. Creating an individualised mobility plan which can be adapted according to patient assessment and general health progress, will optimise early movement and mobilisation.53,54,62,63
Regular musculoskeletal assessment should be made, focusing on the patient’s major muscles and joints and the degree of mobility. Table 6.5 offers a simple guide to assessment, which should include a visual and physical assessment of all limbs and joints. Provided there are no contraindications, function should be stimulated by regular passive then active movements of all limbs and joints to maintain both flexibility and comfort (see below).
| Muscles and joints | Mobility |
|---|---|
Active and Passive Exercises
It takes only seven days of bed rest to reduce muscle mass by up to 30%,64 and physical activity is essential to healthy functioning and beneficial for the cardiovascular system.54 Active exercises are those that can be performed by the patient with no, or minimal, assistance. Passive exercises are performed when patients are either too weak or incapable of active exercise. Exercises can be employed to help the recovering patient develop power and regain function, to assist in venous return and maintain the normal sensation of movement.64 They should be performed at least daily. Passive exercises put the main joints through their range of movement, which helps reduce joint stiffness and maintain muscle integrity, preventing contractures. Shoulders, hands, hips and ankles are particularly at risk of stiffness and muscle contracture.64 It is important, however, to ensure that joints and muscles are not overstretched, as this is painful for patients and can cause permanent injury. Splints may be used when the patient is resting, to maintain joints in a neutral position.64 The physiotherapist’s advice should be sought regarding the correct range of movement and the frequency of passive exercises. This is particularly important for burn-injured patients. Concern has been expressed about the effects of limb movements on head-injured patients; however, Koch et al.65 detected no significant cardiovascular or neurological changes during passive exercises in neurosurgical patients,65 and Brimioulle et al. found no detrimental effects on cerebral perfusion or intracranial pressure (ICP), whether the ICP was raised or not.66
Changing Body Position
Mobility is defined as the ability to change and control body position.67 The complications of immobilisation in critically ill patients are well documented, and include decubitus ulcer, venous thromboembolism and pulmonary dysfunction such as atelectasis, retained secretions, pneumonia, dysoxia and aspiration.56 The routine standard for immobilised patients in ICU is 2-hourly body repositioning, although this does not always happen,56 and the optimal interval for turning critically ill patients is unknown.68 In addition to providing pressure relief, it is recommended that the patient’s position be changed often to ensure comfort, relaxation and rest, to inflate both lungs, improve oxygenation69 and help mobilise airway secretions, to orient the patient to the surroundings and for a change of view, and to improve circulation to limbs through movement.50 The frequency of body repositioning should be determined according to the patient’s pressure ulcer risk (preferably using one of the assessment tools described below), clinical stability and comfort.
Good body alignment helps prevent pressure points, contractures and unnecessary pain or discomfort for the patient.60 The nurse caring for the immobile critically ill patient is most often responsible for determining patient positioning.70 Here, careful consideration should be given to factors (outlined in Table 6.6) such as haemodynamic and cardiopulmonary responses of the patient,71 the timing and method of positioning patients, and whether there are any restrictions on movement. It is important to fully consider the individual needs of patients: they may have a history of back or neck problems, and the selective use of soft or firm pillows and mattresses may be relevant. Pillows can optimise the patient’s position so that the shoulders and chest are squared, and may reduce the work of breathing for patients with chronic airways disease.42 Some pressure-relieving mattresses have an adjustable pressure control, which can be changed according to pressure relief assessment and patient comfort.42 When patients are positioned lying on one side, consideration should be given to their feeling of security; for example, ensuring that they are well supported by pillows and the bed rails are raised. Provided cerebral perfusion pressure is maintained above 50 mmHg, even severely head-injured patients can be moved safely,66 however it is important to maintain the neck in alignment to promote venous drainage (see Chapter 17), and for those with spinal injuries, log-rolling may be required (see Chapter 17).
| Factors | Comments |
|---|---|
| Haemodynamic and cardiopulmonary responses |
Pressure Area Care
The prevalence of pressure ulcers in an ICU ranges from 5% to 18%72 and the risk of developing a pressure sore is cumulative: 5% risk after 5 days; 30% risk after 10 days; and 50% risk after 20 days in the ICU.72 Pressure area risk for critically ill patients can be attributed to their immobility, lack of sensory protective mechanisms, suboptimal tissue perfusion and environmental factors that cause pressure and friction.42 The commonest locations for pressure ulcers are the sacrum, the heels and the head.72 Significant risk factors include the age of the patient, the number of days since admission, malnutrition,42,49 and delays in the use of pressure-relieving mattresses.72,73
Pressure risk assessment tools can help nurses identify at-risk patients.42 However, it is unusual for a patient in critical care to be assessed as low-risk. There are several pressure area risk assessment tools available such as Braden score67 and the revised Jackson/Cubbin pressure risk calculator74 (Table 6.7) that was designed specifically for use in ICU and provides an awareness of the many factors that need to be considered and monitored prior to and during procedures for pressure prevention. Skin assessment for pressure should be scheduled at least daily and include a review of pressure relieving devices for effectiveness or requirement for change. Skin assessment should include testing for blanching response and checking for areas of oedema, induration, redness or localised heat.42
TABLE 6.7 Components of the revised Jackson/Cubbin pressure area risk calculator74
| Risk assessment categories | Scoring |
|---|---|
Pressure ulcer prevention practices include alternating the use of pressure-relief mattresses, low-pressure mattresses and air-flow mattresses.42,73 For bariatric patients (usually those heavier than 150 kg), specialist beds and mattresses are required.
Intensive care patients are at risk of pressure ulcers and injury from a number of devices in everyday use, such as endotracheal tubes and blood pressure cuffs (see Table 6.8). Close attention to detail with frequent observation of the patient, the patient’s position, and the presence and location of equipment is required to prevent skin damage. It is important to remove aids such as compression stockings and cervical collars to assess the skin. Vulnerable patients, such as those with poor tissue perfusion, anaemia, oedema, diaphoresis and poor sensory perception42 can develop pressure ulcers relatively quickly, and pressure ulcers caused by equipment are entirely avoidable.
TABLE 6.8 Risk of pressure sores from commonly used equipment
| Risk factor | Comments |
|---|---|
| Endotracheal tubes (ETTs) | The ETT should be repositioned from one corner of the mouth to the other on a daily basis to prevent pressure on the same area of oral mucosa and lips. Care should also be taken when positioning and tying ETT tapes: friction burns may be caused if they are not secure; pressure sores may be caused if they are too tight (particularly above the ears and in the nape of the neck). Moist tapes exacerbate problems and harbour bacteria. |
| Oxygen saturation probes | Repositioning of oxygen saturation probes 1–2 hourly prevents pressure on potentially poorly perfused skin. If using ear probes, these must be positioned on the lobe of the ear and not on the cartilage, as this area is very vulnerable to pressure and heat injury. |
| Blood pressure cuffs | Non-invasive blood pressure cuffs should be regularly reattached and repositioned. If left in position without reattachment for long periods of time they can cause friction and pressure damage to skin. Care should be taken to ensure that tubing is not caught under the patient, especially after repositioning. |
| Urinary catheters, central lines and wound drainage | The patient should be checked often to ensure that invasive lines are not trapped under the patient. In addition to causing skin injury, they may function ineffectively. |
| Bed rails | Limbs should not press against bed rails; pillows should be used if the patient’s position or size makes this likely. |
| Oxygen masks | Use correct-size mask and hydrocolloid protective dressing on the bridge of the nose to assist with prevention of pressure from non-invasive or continuous positive airway pressure masks, especially when these are in constant or frequent use. |
| Splints, traction and cervical collars | Devices such as leg/foot splints, traction and cervical collars can all cause direct pressure when in constant use and friction injury if they are not fitted properly. ICU patients often have rapid body mass loss (especially muscle) following admission, so daily assessment is required. |
All pressure points and any pressure ulcers should be monitored closely. The key areas of monitoring are identified in Table 6.9, and it is important to use standardised methods to objectively assess pressure ulcers and their response to therapy. If a patient develops one pressure ulcer, there is a good chance he/she could develop another. Nursing intervention includes the placing of patients in positions that avoid pressure on the affected area(s), employing measures such as good fluid management to improve tissue perfusion, reducing the risk of infection and promoting tissue granulation with the use of appropriate dressings.
TABLE 6.9 Monitoring pressure ulcers
| Factor | Actions |
|---|---|
| Size | |
| Stage/grading | |
| Documentation | |
| Treatment | |
| Observing other sites |
• Dependent areas of the body are susceptible: sacrum, heels, back of the head, hips, shoulders, elbows, knees. • Areas of the body where equipment is causing pressure are susceptible: nose, ears, corners of the mouth, fingertips. • Areas of the body where tissue perfusion is poor are susceptible: extremities. |
The International NPUAP–EPUAP Pressure Ulcer Classification System42 grades pressures ulcers as follows:
• Stage I: Non-blanchable redness of intact skin
• Stage II: Partial thickness skin loss or blister
• Stage III: Full thickness skin loss (fat visible)
• Stage IV: Full thickness tissue loss (muscle/bone visible)
• protecting tissue from further damage with pressure re-distribution techniques
• preventing infection either localised or systemic by closely observing the ulcer for signs of infection such as friable, oedematous, pale or dusky tissue
• aiding wound healing such as use of negative pressure wound therapy for deep ulcers or foam and alginate dressings to control heavy exudate.42
Rotational Therapy
Continuous Lateral Rotation Therapy (CLRT) or Kinetic bed therapy is an intervention in which the patient is rotated continually, on a specialised bed, through a set number of degrees; it helps to relieve pressure areas and can significantly improve oxygenation.75–77 Continual lateral rotational therapy may reduce the prevalence of ventilator-associated pneumonia in patients requiring long-term ventilation.76 Appropriate evaluation of the benefits and suitability of the patient for CLRT should be undertaken by the team and the therapy implemented according to local protocols.75 In implementing this therapy, the goal is to achieve continuous rotation through the maximum angle that the patient tolerates for 18 hours per day.75,78
Venous Thromboembolism (VTE) Prophylaxis
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are separate conditions collectively referred to as venous thromboembolism (VTE).79,80 DVT is a blood clot in a major vein of the lower body, i.e. leg, thigh, pelvis, which causes disruption to venous blood flow and is often first noticed by pain and swelling of the leg. The blood clot forms due to poor venous flow, endothelial injury to the vein or increased blood clotting which may be caused by trauma, venous stasis or coagulation disorders.81 Pulmonary emboli occur when a part of a thrombosis moves through the circulation and lodge in the pulmonary circulation. VTE is a major risk factor for hospitalised patients80–83 in general and critically ill patients in particular, due to blood vessel damage, coagulation disorders and limited mobility leading to venous stasis.79 Further, around 50% of patients with DVT will also suffer a pulmonary embolism, which can be fatal causing around 10% of hospital deaths in Australia.80,82 Patients with VTE may also develop post-thrombotic syndrome where tissue injury occurs leading to pain, paraesthesia, pruritis, oedema, venous dilatation and venous ulcers.79,81
It is important to consider the individual patient (age, BMI) and their history (previous VTE, coagulation disorders) along with their current condition whether it be surgical or medical and features of their treatment (immobilisation) when determining risks for VTE.80,81,84–86 Both the risk assessment and the patient’s current condition will determine the most appropriate VTE prophylaxis strategy.80,81 Prophylaxis consists of a combination of pharmacological and mechanical interventions that may be used together or separately according to the degree of risk for VTE and/or contra-indications to particular therapies. The use of combined therapies is supported by recent reviews and guidelines.80,84,86 It is important to be guided by current best evidence in choosing the most appropriate prophylaxis regimen for your patient. The NHMRC Clinical practice guideline for the prevention of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to Australian hospitals80 provides a comprehensive guide to risks and management relating to VTE for critical care in Australia.
Low molecular weight heparin or unfractionated heparin is the most common pharmacological therapy prescribed in Australia, while other medications will be prescribed for patients according to individual factors.80,87 Special consideration of an appropriate regimen for pharmacological prophylaxis will need to be given to patients with renal and hepatic impairment.87 Heparin-induced thrombocytopenia (HIT) may develop in some patients88 so as with all heparin therapy, close monitoring of the patient’s platelet count and assessing for signs of bleeding such as bruising or haematuria will form part of the nurse’s role in managing VTE prophylaxis.
In principle, it is advised that graduated compression stockings are used for all general, cardiac, thoracic and vascular surgical patients until full mobility is achieved irrespective of pharmacological prophylaxis.80,86 Mechanical prophylaxis is provided through a range of graduated compression stockings and various pneumatic venous pump or sequential compression devices.80,81,84,86,89,90 It is important to make sure that the relevant devices are fitted correctly and monitored closely. Comparisons between a number of pneumatic pumps have been studied88–90 with all displaying relative effectiveness. The availability of battery-operated sequential compression devices can assist with the continuous application of the therapy during patient transports away from their bedside, such as to the imaging department for radiological procedures.90
Along with pharmacological and mechanical venous thromboembolism prophylaxis, maintaining patients’ hydration and implementing early mobilisation are key components of care in preventing VTE.79,80,84 Rauen et al.79 describe the most common reasons cited for lack of proper VTE prophylaxis as being lack of knowledge among healthcare providers and under-estimation of risk of VTE along with over-estimation of the potential risk of bleeding from prophylaxis. Given the risks of VTE for critically ill patients, it is clearly important that nurses contribute to lowering risks for their patients by knowing the range of risk factors for their patients, along with the appropriate pharmacological prophylaxis that may be prescribed, how to appropriately implement and manage the mechanical prophylaxis devices and most importantly facilitate the early mobilisation of the patient.
Bowel Management
Although bowel care is an essential aspect of nursing care in the critical care setting, there is little research evidence in this area. Good bowel care promotes patient comfort and reduces the risks of further problems such as nausea and vomiting. The prevention of constipation, which can occur when patients are immobile or have reduced gut motility or a poor dietary intake, is important as it may contribute to the exacerbation of other conditions, such as myocardial infarction, congestive cardiac failure, stroke and head injury.91,92 Enteral feeding is often cited in the literature as a cause of diarrhoea,93 but poor gastric fluid intake causes constipation, and improved gut motility decreases the risk of aspirations. The prevention of constipation is particularly important for patients with high cervical spinal injuries, as if left untreated it may cause potentially fatal autonomic dysreflexia.94
Bowel Assessment
Initial bowel assessment should be undertaken to determine the patient’s usual bowel habits, as less than 10% of the population have a daily bowel action, and for 1% of the population less than three times a week is normal.92 ‘Normal’ bowel function should be regarded as at least twice a week.95 In general, older patients are more susceptible to constipation.
Gut function should be assessed at the start of each nursing shift101 (see Box 6.2). Several authors91,92,96 have developed bowel care protocols for intensive care patients. The results of the McKenna et al. study93 suggest that use of a protocol improves bowel care. Rectal examination should be performed within 24 hours of ICU91 admission and it should also be undertaken if the patient has not had their bowels open for three consecutive days.92 If the bowels have not been opened during this period, action should be taken.96 For some patients in whom defecation is problematic, it may be appropriate to objectively assess the quality of faecal stools using a tool such as the Bristol stool form scale, which uses a 7-point grading system to assess stool consistency (see Table 6.10).97,98
| Grade | Description |
|---|---|
| 0 | No bowel movement |
| 1 | Separate hard lumps; like nuts; hard to pass |
| 2 | Sausage-shaped but lumpy |
| 3 | Like a sausage but with cracks on the surface |
| 4 | Like a sausage or snake but smooth and soft |
| 5 | Soft blobs with clearcut edges; easily passed |
| 6 | Fluffy pieces with ragged edges; a mushy stool |
| 7 | Watery; no solid pieces; entirely liquid |
Essential Bowel Care
Nursing care is based on managing privacy and embarrassment, increasing exercise where possible, ensuring adequate fibre and fluid in the diet, reducing unnecessary use of drugs that cause constipation, and appropriate use of laxative agents.93 Where bowel care is concerned, it is always appropriate to first explain to patients what is to be done, and to gain their consent if they are conscious. Constant reassurance is important so that patients feel safe and secure in the knowledge that their privacy will be maintained to the greatest degree possible. This is sometimes difficult when more than one nurse is required to position a patient for bowel assessment, defecation or cleansing. However, it is always important to explain to patients why more than one person is necessary and to reassure them that they will be exposed for the minimum period necessary.
Diet and Fluids
Diet and fluids are two important considerations in maintaining normal bowel function. Ensuring the appropriate administration of fluid and an adequate dietary fibre intake96 helps to prevent constipation. Enteral feeding increases faecal bulk91 and provides gastric fluid, which helps to maintain gut motility. Chapter 19 contains an in-depth discussion on the principles of enteral feeding.
Drugs
The use of sedatives is often an ascribed cause of constipation in critically ill patients. This is not due to their direct effect, but due to the subsequent immobility of patients when sedatives are used. Opiates, which are often used to control pain, slow propulsive gut contraction. The main drugs that cause constipation in critical care settings are analgesics, anaesthetic agents, anticonvulsants, diuretics and calcium channel blockers.91 While it is difficult to avoid giving these drugs, their judicious use in tandem with other preventive measures will help avoid constipation.
Constipation
Although there is no consensus,91 constipation may be defined in general as decreased frequency of defecation or bowel movements, with a hard, dry stool.99 Non-pharmacological methods to reduce constipation include exercise or moving, increasing fluid intake, and adding dietary fibre.99 These means should be implemented routinely before the need to use laxatives arises. There are many types of laxatives available, which can be given to prevent or treat constipation. Bulk-forming agents work by increasing faecal size; stimulants, such as senna, increase peristalsis; and osmotic agents draw fluid into the gut. Stimulant laxatives should not be given with faecal impaction, which should be treated using enemas.91 In general, existing protocols advise that treatment of constipation should commence with senna administration. If senna is ineffective after 2–3 days, lactulose should be commenced.91,92,96
Diarrhoea
Diarrhoea can be a major problem for intensive care patients, and in severe cases may lead to electrolyte imbalances, dehydration, malnutrition (see also Chapter 19) and skin breakdown. Furthermore, it can be very distressing for the patient, who may also suffer from distension, nausea and cramp-like pain. Investigations should be implemented to determine the cause of the diarrhoea and the patient should be managed with appropriate precautions to prevent cross contamination if the cause is infectious. If laxatives are being given they should be stopped, and a stool specimen should be obtained for microbiological examination. Antimotility drugs may be used, except with bloody diarrhoea or proven infection with E. Coli.96,100 Appropriate re-hydration should be implemented.100 If patients are being fed enterally there may be a reduction in episodes of diarrhoea if fibre-enriched feed is used.101 Fecal containment devices should be used in severe cases of diarrhoea in conjunction with all other measures to support the patient’s comfort.102 The patient should be assessed for suitability for using the incontinence system as per the manufacturer’s guidelines. An appropriate bowel therapy regimen and monitoring of these systems should be implemented to optimise functioning.
Urinary Catheter Care
Urinary catheters are inserted into most critically ill patients, and are the commonest cause of infection in the ICU.103 In principle, urinary catheters should be inserted only when deemed clinically necessary, and should be removed as soon as they are no longer required clinically. However, most critically ill patients require accurate monitoring of their urinary output and fluid balance, and a catheter is required for this reason.104 There are a number of possible alternatives to urinary catheterisation, such as intermittent catheterisation, suprapubic catheterisation, use of a male/female urinal or penile sheath and/or incontinence pads,105 although often these are not suitable for critically ill patients. Because the practice of urinary catheterisation is so common, catheter care can sometimes be relegated to a low priority. The consequences of inadequate catheter care can be distressing and detrimental to the patient, resulting in inflammation, infection and injury.
Assessment: Urinary Catheterisation
Following assessment indicating that a urinary catheter is required, its size and type should be determined. In addition to their primary purpose of urine drainage, urinary catheters may be used to monitor temperature and assess intra-abdominal pressure which may affect catheter choice. Catheters are made from several different types of material, which have varying properties, and the choice of catheter often depends on an estimation of how long it will be required. Catheters are classified as either short- or long-term. Short-term catheters should be changed after 14–28 days, according to the manufacturer’s guidelines, whereas long-term catheters may be left in place for up to 12 weeks.106 The minimum length of a male catheter is 380 mm, and for a female it is 220 mm.106
The general rule is to use the smallest size necessary that will drain the contents of the bladder,107 although narrow-bore tubes flex easily, which can be problematic in male catheterisation where the urethra rounds the prostate gland. Larger-diameter catheters may be required to drain haematuria and clots.107 All procedures involving the catheter and drainage system should be documented in the clinical notes, including size and type of catheter, balloon size and the date of insertion.
Essential Nursing Care: Urinary Catheterisation
Catheter Maintenance
The continued need for a urinary catheter should be assessed on a daily basis. Daily reminders by nursing staff to doctors results in shorter duration of catheter insertion, with a lower associated infection rate.103 The introduction of criteria that enable registered nurses to remove catheters without a doctor’s order may result in a significant reduction in catheter-related infections. Penile meatal care with soap and water104,106 should be performed at appropriate intervals for patient comfort and to keep the meatus free of encrustation and contamination. Cleansing with antiseptic solution is not recommended and can lead to multi-resistant organism infection.
Urinary catheters should be changed according to clinical need and with regard to the manufacturer’s guidelines, and the closed drainage system should be broken only for limited, clearly defined clinical reasons. Bladder washout or irrigation should be performed only for a specific clinical reason, for example catheter blockage or high risk of blood clot formation, and should not be considered as routine practice. A variety of solutions may be used for washouts107 (see Table 6.11) although research in this area is limited.108 The volume of bladder washout solution should be kept minimal, as it is a potentially irritant chemical that can cause tissue damage: 50 mL is as effective as 100 mL, and two sequential 50 mL washouts are more effective at removing encrustation than one 100 mL washout.109
| Solution | Indication |
|---|---|
| Sodium chloride 9% | For the removal of small pieces of debris. Effect is purely mechanical. May be used as required. |
| Citric acid 3.23% (Solution G) | Used to dissolve encrustations. Aids reacidification of urine. May be used up to twice daily. |
| Citric acid 6% (Solution R) | Used to unblock an encrusted catheter. Can be used before removal to reduce trauma from encrustation. May be used up to twice daily. |
| Chlorhexidine 0.02% | Used to reduce bacterial growth in the bladder, though research does not support its use. May lead to the development of resistant organisms. |
Bariatric Considerations
Obesity is known to be a major health issue around the world. While many bariatric patients will present to hospital with various health issues, obesity has its own physiological impact to be considered also, such as impaired chest expansion and respiration from a large abdomen or insulin resistance related to altered glucose metabolism.110,111 Close glucose monitoring regimens should be implemented and appropriately calculated dosages for medications be prescribed. Adapted techniques to enhance patient assessment may be required, such as auscultating over the left lateral chest wall to hear heart sounds while the patient is positioned towards their left side or using a thigh or regular blood pressure cuff on the patient’s forearm.110
Studies have found that persons who are obese contend with a negative bias within a social context112 but this same negative bias from health professionals including nurses may then interfere with their ability to obtain quality healthcare.112–114 According to Susan Bejciy-Spring, the key to providing quality, patient-centred, sensitive care to the bariatric patient is R-E-S-P-E-C-T: Rapport, Environment/Equipment, Safety, Privacy, Encouragement, Caring/Compassion and Tact.114 Simple things such as an appropriately sized gown and suitable bed linen which provide the patient with adequate covering are often not well-organised for this patient group, unless the nurse takes the time to arrange specific supplies if they are not routinely available.
Sedation in the bariatric patient needs to be carefully managed to avoid the resultant risk of respiratory failure and need for ventilation. Reducing narcotic usage through use of combinations of other analgesia along with sedatives will also reduce risk for respiratory failure.115 Bispectral index monitoring can be used to assist in the titration of sedations during procedures where levels of sedation that eliminates awareness and recall is necessary.115
Overweight patients can be challenging in any setting, and it is important to consider the health and safety of the staff involved in lifting and moving patients. Equally important is maintaining the patients’ dignity and feelings of safety and minimising their self-consciousness during repositioning, irrespective of the method required. Lifts and hoists and other equipment that are designed for heavier people should be used.116,117 A well-thought-out strategy by an inter-disciplinary group can work through the local issues within a hospital or unit and produce a Bariatric Kit, containing a range of equipment appropriate to the needs of the bariatric patients in various settings including the ICU.117
A major concern in the ICU is the positioning of the morbidly obese patient with respect to airway management and oxygenation. Boyce et al found no differences in the difficulty of airway management when patients were in the 30-degree reverse Trendelenburg (head up, feet down), supine-horizontal, or 30-degree back-up position.118 However, when patients were positioned in the reverse Trendelenburg position, their oxygen saturation dropped the least and took the shortest time to recover. Consult with the patient about techniques that work for them at home when re-positioning and mobilising. As with all patients, bariatric patients are vulnerable to fears and anxieties resulting from their illness, however additional concerns for their physical safety may be experienced, such as during re-positioning, if the activity is not arranged competently and with sensitivity.
VTE prophylaxis in bariatric patients is vital especially for those patients having bariatric surgery. Routine prophylaxis is recommended with weight adjusted dosing of medications.81,111 Combining pharmacological and mechanical prophylaxis is recommended for this high risk group. The application of leggings or sleeves for sequential compression devices or pneumatic venous pumps can often be easier than applying graduated compression stockings in any patient when they are supine in bed. Care must be taken with measuring the limb to obtain the correct size legging or stocking. Careful monitoring of the limb for signs of skin deterioration from moisture, or pressure from an ill-fitting legging, sleeve or stocking must be undertaken diligently in the bariatric patient.81 The insertion of a removable inferior vena cava (IVC) filter as a component of pulmonary embolism prophylaxis for patients undergoing bariatric surgery may occur in some institutions.111
The post-operative management of the bariatric patient will include nutrition to support tissue repair. The use of postpyloric enteral nutrition may be of benefit in reducing the risk of aspiration in the bariatric patient, as these patients often experience post-operative vomiting and nausea.115
Infection Control in the Critical Care Unit: General Principles
Effective infection control is vital in the critical care setting to prevent further health risks to critically ill patients already compromised by their disease or trauma (Box 6.3). Critically ill patients often require multiple invasive devices and therapies to manage their illness and these increase the potential risk for infection to the patient. While using therapeutic medical devices is often vital to the management of the patient, they are not without risk. Ventilator associated pneumonia (VAP), catheter associated urinary tract infections (CAUTIs) and central line associated bacteraemia (CLAB) are all aligned with invasive device use and form a significant source of healthcare acquired infections (HAIs) within critical care.119 Critical care staff themselves need to protect against contracting infections while providing care for their patients.
Box 6.3
Infection-control guidelines for the prevention of transmission of infectious diseases in the healthcare setting119
• Healthcare-associated infections are those acquired in care establishments (‘nosocomial’ infections) and infections that occur as a result of healthcare interventions (‘iatrogenic’ infections). The infection may manifest after people leave the healthcare establishment.
• A healthcare establishment is any facility that delivers healthcare services.
• Healthcare workers (HCWs) are all people delivering healthcare services, including students, trainees and mortuary attendants, who have contact with patients or with blood and body substances.
• Standard precautions are standard operating procedures that apply to the care and treatment of all patients, regardless of their perceived infection risk. They are work practices required to achieve a basic level of infection control and are recommended for the treatment and care of all patients (see Table 6.13).
• Transmission-based precautions are required when standard precautions may not be sufficient to prevent the transmission of infectious agents (e.g. in tuberculosis, measles, Creutzfeldt–Jakob disease). These precautions are tailored to the specific infectious agent concerned and may include measures to prevent airborne, droplet or contact transmission, and healthcare-associated transmission agents.
• Transmission-based precautions are recommended for patients known or suspected to be infected or colonised with disease agents that cause infections in healthcare settings and that may not be contained by standard precautions alone.
Copyright Commonwealth of Australia
When patients are admitted to critical care it is impossible to identify whether or not they are newly colonised with bacteria, or are carrying an infection, without further investigation. Standard Precautions are applied in the management of all patients regardless of the reason for their admission. Standard Precautions include hand hygiene, respiratory hygiene and cough etiquette, the use of appropriate personal protective equipment, safe handling of sharps, waste and used linen, appropriate cleaning and environmental controls, appropriate re-processing of re-usable equipment and the use of aseptic non-touch techniques during procedures.119
With the advent of Influenza H1N1 outbreaks, there has been an emphasis on respiratory hygiene and cough etiquette, which effectively means covering the mouth with a tissue when coughing or sneezing and then immediately disposing of the tissue into waste bins, followed by effective hand hygiene.119 Further Transmission-based Precautions (previously referred to as Additional Precautions) are implemented as required in response to suspicion (while awaiting confirmation from tests) or diagnosis of a condition in which Standard Precautions may not be sufficient to control the transmission of microorganisms.119 Transmission-based Precautions appropriately applied to specific microorganisms disrupt their method of transmission to other patients, visitors and healthcare workers. Transmission-based Precautions include continuation of Standard Precautions, the use of personal protective equipment specific to the risk of transmission, individual patient equipment where possible and specific cleaning protocols for shared equipment, placement of patients in single rooms (or cohorted if appropriate) and specific air filtration or circulation and environmental cleaning protocols.119
There are three types of Transmission-based Precautions recommended for Australian healthcare119 to counteract the various infectious agents: Contact Precautions, Droplet Precautions and Airborne Precautions119 (see Table 6.12) These types of precautions are applied with refinement to the use of personal protective equipment, room requirements and recommendations for visitors specific to the mode of transmission of the organism. Critical care nurses should be knowledgeable of both local and national guidelines and protocols for Infection Control in order to provide safe care to all their patients. Breaks to the consistent application of Standard Precautions and, when implemented, Transmission-based Precautions put patients at risk, especially those who are critically ill.
TABLE 6.12 Transmission-based precautions and infectious conditions
| Transmission-based precautions | Examples of infectious conditions |
|---|---|
| Contact |
Adapted from NHMRC Guidelines. NB: Standard Precautions apply for all patients at all times
While good hand hygiene is the single most effective tool in infection control,120,121 the key components of effective infection control are surveillance, prevention and control, which are described in more detail below.
Surveillance
Around 25% of ICU patients are infected prior to admission,122 so routine screening should be undertaken to detect the presence of bacteria. Ideally, all critically ill patients will be screened for MRSA and VRE on admission.123 Regular surveillance to identify rates of nosocomial infection, with feedback to critical care staff, helps to improve compliance with infection control guidelines.119,124 In the 1980s, a landmark study established that hospital-acquired infection may be reduced by around a third if surveillance and prevention programs are implemented.125
Prevention
The Australian government Department of Health and Ageing provides guidelines for infection control within the healthcare setting (see Box 6.3).119 All health services should apply these guidelines and operate within clearly defined infection-control procedures, which are based on Standard Precautions. Although formerly referred to as Universal Precautions and Additional Precautions, the recent guidelines on infection control from the NHMRC uses Standard Precautions and Transmissions-based Precautions respectively to clearly describe these levels of precautions.119 Critical care nurses should refer to their specific hospital infection-control policies regarding details of procedures that must be followed.
Control
Once an organism has been identified, the goal is to limit its spread. Although patients may be colonised with bacteria, they may not be infected. Colonisation refers to the presence of microorganisms in any amount, whereas infection means that pathological tissue injury or disease has occurred due to the invasion and multiplication of the microorganism.126 Typically, surveillance measures identify many patients who are colonised with MRSA or VRE, and although they themselves are not infected it is important to stop the spread of bacteria to patients more vulnerable and thus more susceptible to opportunistic infection, by implementing Transmission-based Precautions.127 In a study of multiresistant gram-negative bacterial infections in ICUs, several effective measures were demonstrated, which are summarised in Table 6.13.128 Due to the vulnerable nature of critically ill patients, specific issues are described in more detail including: hand hygiene, personal protective equipment (PPE), multi-resistant organisms (MROs), Healthcare associated infections (HAIs), ventilator associated pneumonia (VAP) and central line associated bacteraemia (CLAB).
TABLE 6.13 Preventive measures to reduce the spread of gramnegative infection128
Hand Hygiene
At the core of Standard Precautions is effective hand hygiene. Good hand hygiene is a simple yet effective technique that reduces the spread of bacteria. It is the most effective and least expensive method of preventing healthcare associated or nosocomial infection.120 However, hand hygiene compliance is poor,119,120 but it can be improved significantly if regular education programs, feedback and reminders are employed119–121 such as the 5 moments for hand hygiene (see Box 6.4) created by the World Health Organization (WHO) in 2009120 which has been adopted for local implementation, such as Hand Hygiene Australia.121 Evidence has led to the current recommendation of using an alcohol based hand rub for hand hygiene unless the hands are soiled.120,121,129 The use of alcohol hand rubs is associated with higher rates of hand hygiene compliance and effectiveness although effectiveness is dependent on technique.120,121,129,130
Personal Protective Equipment (PPE)
PPE may include any and all of the following: plastic aprons, gowns (single use or sterile), gloves (single use or sterile), masks ranging from surgical to particulate filter N95 mask or P2 respirators and eye protection such as goggles or face shields that also protect mucous membranes of the mouth and nose.119 Specific sequences have been outlined for putting on and taking off PPE, that minimise the risk of contamination.119
Epidemic outbreaks of SARS occurred in Canada, China, Hong Kong, Singapore and Vietnam,131 and it has been reported in over 25 countries since the WHO issued its global alert in March 2003.132 SARS was transmitted between patients, healthcare workers and hospital visitors, and large within-hospital outbreaks were associated with aerosol-generating procedures such as bronchoscopy, endotracheal intubation and the use of aerosol therapy,132 which are commonplace in critical care areas. In Hong Kong, more than 20% of cases were healthcare workers.133 Because of the high level of morbidity and mortality associated with SARS,134 the risk to healthcare staff is considerable and during the Hong Kong SARS outbreak, healthcare workers wore full head covers with a visor.135
Previous research has demonstrated relatively low rates of compliance with standard precautions, ranging from 16–44%.136 The SARS outbreaks emphasised the need for effective infection-control procedures, especially for airborne pathogens such as the SARS coronavirus (SARS-CoV). With airborne pathogens such as Pulmonary TB or SARS-CoV, Airborne Precautions137 using N95 masks (face mask with 95% or greater filter efficiency), gowns and gloves are implemented to reduce the spread of the organism, plus the use of negative air pressure rooms and strict control of family visiting.137 Additional measures may include the use of high-efficiency bacterial filters to filter patients’ expired air, closed suction systems and ventilator scavenging systems.135
The more recent Influenza H1N1 pandemic alerted everyone to the need for vigilance in infection control. The use of Droplet Precautions are the main feature of infection control for Influenza, along with early testing.138 The Influenza outbreak also drew attention to the need for vaccinations. All healthcare workers and especially those in critical care should be knowledgeable of the vaccinations that may be available to them through their employers and those that are recommended by local jurisdictions.
Multi-Resistant Organisms
MRO is a collective term for a number of infections from multi-resistant organisms. While the early diagnosis of an MRO and immediate implementation of organism-specific Transmission-based Precautions is key to management, it is true that Methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-E) have reached epidemic proportions.110 Multiple strains of MRSA have been identified, and in many studies ICUs have the highest incidence.127 In the past decade vancomycin-resistant Enterococcus (VRE) has become a serious health issue in Australia. As with MRSA, VRE transmission is associated with contact. Other resistant organisms found in critical care areas include coagulase-negative Staphylococcus, Pseudomonas aeruginosa, Acinetobacter spp, and Stenotrophomonas maltophilia.126
MRSA is endemic in hospitals throughout the world, and critical care units have a central role in its intra- and interhospital spread.127 Patients who are colonised nasally with MRSA have a significant risk of wound infection, and the risk of MRSA infection is higher in patients who have previously been colonised with MRSA and in those who have been admitted to hospital on a previous occasion. It has been found that the longer the patient remains in ICU the higher the risk of MRSA infection.127
There are a number of methods for reducing the spread of MRSA (see Table 6.13), although not all methods may be effective,133 and if the organism is not identified, its spread will continue unseen. Another key component of management of MROs is surveillance, such as the routine screening for MRSA and VRE of all patients on admission to critical care areas and on a regular basis thereafter. Once diagnosed, it is common practice to isolate MRSA patients to reduce cross-infection; however, there is recent evidence that questions its necessity.139
Healthcare Associated Infections
Nosocomial, or hospital- or healthcare-acquired, infection (HAI) is a major problem in critical care that may affect up to 20% of patients, with a mortality of around 30%.122 Critically ill patients are 5–10 times more likely to become infected than hospital ward patients.126 Multiple-drug-resistant bacteria are a worldwide problem; their acquisition by patients can lead to infection with the same bacteria,123 and multiple antibiotic therapy encourages the proliferation of resistant organisms.126 The introduction of antibiotic stewardship assists in focusing on the optimal use of antibiotics.119
Medical devices or therapies may expose the patients to potential risk of acquiring a HAI. This risk may occur during the insertion procedure or subsequent maintenance care of the medical device, unless appropriate techniques are used. The use of an aseptic technique during insertion of a device is a feature of infection control, asepsis being the elimination of pathogens. Aseptic non-touch technique (ANTT) is a format for guiding practice in the application.140,141 Standard ANTT involves standard hand hygiene, a general aseptic field and non-sterile or sterile gloves and is used for minor procedures which are simple and of short duration, that is, less than 20 minutes. Examples of procedures would include simple wound dressings and intravenous cannulation or urinary catheterisation by proficient practitioners. Surgical ANTT is used for complex or lengthy procedures such as insertion of a central venous catheter and involves the use of full barrier precautions (sterile gown and gloves, face mask), extensive drapes and critical aseptic field.119 Box 6.5 provides some basic points to guide management of the use of medical devices in critical care.
Box 6.5
Invasive device management
• Does the patient need the invasive device for effective management of their condition?
• Is the chosen device is the most suitable for the individual patient, e.g. size and type of device?
• Are the healthcare professional/s trained to safely insert and manage the device?
• Use the appropriate aseptic procedure for device insertion.
• Follow management protocols to minimise the risk of infection while the device is in situ.
• Monitor the patient for signs and symptoms of infection.
• Review the need for the device in the management of the patient daily and remove as early as possible.
Adapted from NHMRC Guidelines119
The commonest healthcare associated infections, in order of incidence, are surgical sites, urinary tract, lower respiratory tract and bloodstream.142 For the critically ill patient intravascular cannulas including central venous catheters, urinary catheters, enteral or nasogastric tubes and artificial airways and ventilation are some of the healthcare devices associated with risk. See the section on urinary catheters for information regarding catheter-associated urinary tract infections.
Ventilator-Associated Pneumonia
Ventilator-associated pneumonia (VAP) is common in intensive care and usually occurs within 48 hours of initiating ventilation.143 There are several measures that should be taken to reduce VAP.144 A number of strategies that are effective in helping to prevent infection143 are identified in Table 6.14, of which the simplest and most effective is raising the head of the bed. Effective analgesia and minimising sedation while avoidance of muscle-relaxant medications along with early mobilisation are some of the other strategies which may contribute to the reduction of VAP. Provided a heat and moisture exchanger (HME) is used, it is not necessary to routinely change ventilator circuits.145 The US Centers for Disease Control recommend changing the ventilator circuit only when it is visibly soiled or malfunctioning, and should not be changed more often than every 48 hours unless it is soiled or malfunctions.146 The use of a closed suction system for endotracheal suction does not decrease the incidence of nosocomial infection,147 but it does afford a protective barrier to the nurse performing the procedure.
| Measure | Interventions |
|---|---|
| Infection control measures |
PPE = personal protective equipment; ETT = endotrachael tube
Selective digestive decontamination has been studied extensively. In theory, the use of antimicrobial agents to reduce gut flora in intubated intensive care patients reduces the risk of pneumonia due to microaspiration (see Chapter 19). While most studies have demonstrated a reduction in the incidence of VAP, there has been an inconsistent reduction in ICU mortality, and there remains concern about the promotion of antimicrobial resistance with its prolonged use.148 Related information on respiratory failure and ventilation can be found in Chapters 14 and 15.
Central Line Associated Bacteraemia
Bloodstream infection is a serious complication often caused by intravascular catheters, particularly those that terminate close to the heart.149 The use of central lines is common in critical care areas. Catheter-related sepsis is defined by the International Sepsis Forum as at least one peripheral positive blood culture plus at least one of the following: a positive catheter tip culture, a positive hub or exit-site culture, or a positive paired central and peripheral blood culture where the central culture is positive ≥2 hours earlier than the peripheral culture or has five times the growth.150 Central line associated bactaeremia (CLAB) is one of the most important and severe infections that can occur in ICU,151 and as many as 90% of bloodstream infections may be attributable to intravascular catheters.152 Renal failure may significantly increase the risk of infection.153 Berenholtz et al. demonstrated that implementing quality improvement measures to ensure adherence to evidence-based infection control guidelines results in a significant reduction of catheter-related bloodstream infection.154
The use of antibiotic-impregnated catheters has been shown to reduce bacteraemia,155 and although it is common practice in many critical care units to routinely change intravenous administration sets, with antiseptic-coated catheters they can be used safely for up to seven days.156 Currently available evidence supports the use of maximal barriers (head cap, face mask, sterile body gown, sterile gloves and full-size body drape) during routine insertion of central venous catheters along with antiseptic solutions to prepare the skin, and catheter insertion by appropriately trained personnel.119 Chapter 3 contains information on central line care bundles and checklists. Although chlorhexidine solutions are recommended their effectiveness depends upon the strength of the solution. In Australia decontamination of the insertion site is with 0.5% chlorhexidine gluconate in 70% isopropyl alcohol.119 The use of antimicrobial ointments to prevent local colonisation is recommended for long-term tunnelled catheters used for haemodialysis.119
Nurses are responsible for the maintenance of central venous catheters once inserted, including care of the insertion site dressing and infusion line management. The types of dressing commonly used are transparent semi-permeable and more recently chlorhexidine gluconate gel dressings.119,157 Transparent dressings are advantageous because they allow direct observation of the entry site of the catheters. Dressings should be replaced whenever their seal is broken or every seven days.119 Catheter hubs are another site of colonisation for microorganisms, such as Staphylococcus epidermidis and effective hand hygiene combined with non-touch aseptic techniques when accessing the catheter hub should be implemented. Intravenous administration sets containing blood products or lipids or parenteral nutrition infusions are changed when the infusion completes or daily, while others can be left for intervals of up to 4 days or changed according to local protocols. Infusions such as propofol or nitroglycerine may have additional manufacturer guidelines regarding administration set changes.119
Transport of Critically Ill Patients: General Principles
The transport of a critically ill patient may occur for several reasons, such as from an accident site categorised as pre-hospital transport, or to move a patient to another facility for treatment which is known as interhospital transport, or within a hospital from one department to another, this being intrahospital transport.158,159 This section will focus on intrahospital transport, while interhospital transport is described in Chapter 22. A large proportion of intrahospital transports occur from the emergency department160 to the critical care unit. Patients within the ICU may require transport to imaging departments for scans or operating theatres for procedures.
Guidelines for the transport of critically ill patients are available in many countries including Australia and New Zealand158,159,161 with the principles applying equally to intrahospital as other transport.162 Specific guidelines may need to be observed for certain groups of patients, for example those with head injury. A careful assessment of risk versus benefit should be undertaken before making a decision to transport a patient.161,163 To reduce the risk of adverse events during transport, various diagnostic tests or surgical procedures should be evaluated in terms of their potential to be undertaken in the critical care unit.161,164
Assessment
As adverse effects may occur in 40–70% of critically ill patients transported within hospitals,164,165 the primary focus of assessment should be on patient safety and the prevention of adverse events. A transport ‘event’ can be any event that has an adverse impact and can be patient-, staff- or equipment-related.166 The patient may be adversely affected during transport, ranging from anxiety or pain to respiratory or cardiovascular compromise. Staff may have difficulty with managing the equipment or patient’s needs during transport and equipment related problems during transport of critically ill patients are a major consideration.164–167 Risk–benefit assessment is helpful to identify patients with a high risk of complications.163,166 For example, the potential risk of moving a severely head-injured patient with unstable intracranial pressure may outweigh the potential benefit of a CT scan. Meticulous planning for all aspects of the transport, based on a thorough assessment of the patient’s anticipated needs is the key to safe intrahospital transport.158,163,166 A comprehensive outline of information addressing key components of intrahospital transport of critically ill patients should be available to personnel at every hospital.158,159,161
Safe transport requires accurate assessment and stabilisation of the patient before transport.158 Key elements162 are identified in Box 6.6. All equipment should be checked for functionality prior to transport and while it is vital to ensure that sufficient equipment is taken to maintain the patient, unnecessary equipment complicates the logistics of managing the transport smoothly. Specifically constructed transport beds, or attachments such as equipment tables, designed to support equipment safely during transfer are useful.158,163,166 The period of transport should ideally be as short as possible, although safety should not be sacrificed for speed. Pre-planning the route of transport and good dialogue between department staff can help to maximise the efficiency of transport and reduce unnecessary delays.161,166
Essential Nursing Care during Transport
The level of experience and specialty of personnel involved in the transport of critically ill patients are factors influencing safe transport.158,161,165,166 Staff should be trained in the various aspects of patient transport,158,164,166,167 including competent management and troubleshooting of all equipment involved. There is some evidence to suggest that a designated transport team improves quality of care. Team members should be aware of their specific roles and ensure excellent communication throughout the transport procedure.
Equipment used during patient transport must be robust, lightweight and battery-powered,163 and must adhere to relevant national manufacturing and safety standards. Equipment-related complications occur in around a third of transports.164,166 All equipment must be adequately restrained during transport, and must be available continuously to the operator.158 Oxygen requirements should be calculated in advance (or it should be established that piped oxygen is available at the destination department) to ensure an adequate supply, both for the journey and for the duration of the investigation/procedure. Standard equipment for interhospital transport is identified in Table 6.15;158 and while some items may be unnecessary for all intrahospital transport, Table 6.15 provides a useful checklist so that all necessary equipment is taken. Additional specialist equipment may be required for certain patients, such as spare tracheostomy tubes in case of accidental extubation.
The need for monitoring relates to both the patient and equipment, and is identified in Table 6.16.158 Some monitoring should be continuous, such as cardiac, oxygen saturation, capnography if the patient is intubated, and arterial, pulmonary artery and intracranial monitoring if the respective devices are in situ. Intermittent monitoring of central venous pressure CVP, non-invasive blood pressure and respiratory rate should be undertaken as indicated by the patient’s condition.158,166
| Clinical patient monitoring | Equipment monitoring |
|---|---|
A complete record should be kept of all details of the patient’s condition, personnel involved, clinical events, observations and therapy given during transport. The transporting team should hand over directly to the receiving team providing continuing care for the patient,163,167 or should remain during the intervention/procedure to manage the patient’s care.
Summary
Case study
Day 1
Initial presentation
Learning activities
1. Review the patient hygiene products available in your unit. Do you have a range of products suitable for your patient population?
2. Can you identify, assess and plan definitive management specific to skin tears, pressure ulcers and venous ulcers?
3. A patient with a closed head injury has conjunctival oedema and still needs frequent neurological assessment, including assessment of pupil reactions. Outline the process to follow to ensure both eye assessment and eye protection.
4. Describe the key components of good oral hygiene.
5. Observe the positioning in bed of patients in your unit. Evaluate the position for (a) patient comfort, (b) patient security, (c) device and equipment safety, and (d) therapeutic benefit of the position.
6. What prompts decisions for patients to sit out of bed or mobilise in your unit? Do you have positioning, turning or mobilisation protocol in your unit?
7. State the evaluation tools used for pressure area risk assessment and the strategies implemented in your unit for pressure sore prevention.
8. Describe the risk evaluation and protocols for VTE prophylaxis in your unit.
9. What is the patient bowel management protocol for your unit, and is it effective? Why/why not?
10. What are the protocols for surveillance, detection and management of influenza and nosocomial infections in your unit?
11. Outline the practices used to prevent ventilator-associated pneumonia and catheter-related sepsis in your unit.
12. Review the key features of the beds and mattresses in use in your unit. Do you have scope to match specific patient requirements for beds or pressure relief mattresses?
13. Describe the preparation, equipment and monitoring of a ventilated patient with multiple infusions for transfer from the ICU to the imaging department.
Australian Wound Management Association. http://www.awma.com.au.
Australian Department of Health and Ageing. http://www.health.gov.au.
Cochrane Collaboration. http://www.cochrane.org.
College of Intensive Care Medicine of Australia and New Zealand. http://www.cicm.org.au.
Communicable Diseases Network Australia (CDNA). http://www.nphp.gov.au/workprog/cdna.
European Pressure Ulcer Advisory Panel. http://www.epuap.org.
Hand Hygiene Australia. http://www.hha.org.au.
Joint Faculty of Intensive Care Medicine. http://www.jficm.anzca.edu.au.
National Health and Medical Research Council. http://www.nhmrc.gov.au.
National Institute of Clinical Studies NICS. http://www.nhmrc.gov.au/nics/index.htm.
Therapeutics Goods Australia. http://www.tga.gov.au/index.htm.
US Centers for Disease Control and Prevention. http://www.cdc.gov.
College of Intensive Care Medicine of Australia & New Zealand. Minimum standards for transport of critically ill patients IC-10. http://www.cicm.org.au/cmsfiles, 2010. [Cited December 2010]. Available from
Khoury J, Jones M, Grim A, Dunne WM, Jr., Fraser V. Eradication of methicillin-resistant Staphylococcus aureus from a neonatal intensive care unit by active surveillance and aggressive infection control measures. Infect Control Hosp Epidemiol. 2005;26(7):616–621.
Levy MM, Baylor MS, Bernard GR, Fowler R, Franks TJ, et al. Clinical issues and research in respiratory failure from severe acute respiratory syndrome. Am J Resp Crit Care Med. 2005;171(5):518–526.
Wright M, Hebden JN, Harris AD, Shanholtz CB, Standiford HC, et al. Concise communications: aggressive control measures for resistant Acinetobacter baumannii and the impact on acquisition of methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus in a medical intensive care unit. Inf Control Hospl Epidemiol. 2004;25(2):167–168.
1 Larson EL, Ciliberti T, Chantler C, Abraham J, Lazaro EM, et al. Comparison of traditional and disposable bed baths in critically ill patients. Am J Crit Care. 2004;13(3):235–241.
2 Nguyen D, MacLeod WB, Phung DC, Cong QT, Nguyen VH, et al. Incidence and predictors of surgical-site infections in Vietnam. Infect Control Hosp Epidemiol. 2001;22(8):485–492.
3 Shackell S. Cooling hyperthermic and hyperpyrexic patients in intensive care. Nurs Crit Care. 1996;1(6):278–282.
4 Holtzclaw BJ. Shivering in acutely ill vulnerable populations. AACN Clin Issues. 2004;15(2):267–279.
5 Burr S, Penzer R. Promoting skin health. Nurs Stand. 2005;19(36):57–65.
6 NHS Quality Improvement Scotland. Ear Care Best Practice Statement. 2006 [cited 2010 November]; Available from http://www.nhshealthquality.org/nhsqi/files/EARCARE_BPS_MAY06.pdf
7 Baranoski S. Skin tears: guard against this enemy of frail skin. Nurs Manag. 2001;32(8):25–32. (Harrow)
8 Hernandez EV, Mannis MJ. Superficial keratopathy in intensive care unit patients. Am J Ophthalmol. 1997;124(2):212–216.
9 Joyce N. Eye Care for the Intensive Care Patient. Adelaide: Joanna Briggs institute for Evidence Based Nursing and Midwifery; 2002.
10 Newswanger DL, Warren CR. Guillain–Barré syndrome. Am Fam Physician. 2004;69(10):2405–2410.
11 Cortese D, Capp L, McKinley S. Moisture chamber versus lubrication for the prevention of corneal epithelial breakdown. Am J Crit Care. 1995;4(6):425–428.
12 Imanaka H, Taenaka N, Nakamura J, Aoyama K, Hosotani H. Ocular surface disorders in the critically ill. Anesth Analg. 1997;85(2):343–346.
13 Farrell M, Wray F. Eye care for ventilated patients. Intens Crit Care. 1993;9(2):137–141.
14 Dawson D, Dawson D. Development of a new eye care guideline for critically ill patients. Intens Crit Care. 2005;21(2):119–122.
15 Lenart SB, Garrity JA. Eye care for patients receiving neuromuscular blocking agents or propofol during mechanical ventilation. Am J Crit Care. 2000;9(3):188–191.
16 Sorce LR, Hamilton SM, Gauvraeu K, Mets MB, Hunter DG, et al. Preventing corneal abrasions in critically ill children receiving neuromuscular blockade: a randomized, controlled trial. Paediatr Crit Care Med. 2009;10(2):171–175.
17 Bates J, Dwyer R, O’Toole L, Kevin L, O’Hegarty N, Logan P. Corneal protection in critically ill patients: a randomized controlled trial of three methods. Clin Intensiv Care. 2004;15(1):23–26.
18 Koroloff N, Boots R, Lipman J, Thomas P, Rickard C, Coyer F. A randomised controlled study of the efficacy of hypromellose and Lacri-Lube combination versus polyethylene/cling wrap to prevent corneal epithelial breakdown in the semiconscious intensive care patient. Intens Care Med. 2004;30(6):112–116.
19 Mercieca F, Suresh P, Morton A, Tullo AB. Ocular surface disease in intensive care patients. Eye. 1999;13(Pt2):231–236.
20 Ezra DG, Chan MP, Solebo L, Malik AP, Crane E, et al. Randomised trial comparing ocular lubricants and polyarylamide hydrogel dressings in the prevention of exposure keratopathy in the critically ill. Intens Care Med. 2009;35(3):455–461.
21 So HM, Lee CC, Leung AK, Lim JM, Chan CS, Yan WW. Comparing the effectiveness of polyethylene covers (Gladwrap) with lanolin (Duratears) eye ointment to prevent corneal abrasions in critically ill patients: a randomized controlled study. Int J Nurs Stud. 2008;45(11):1565–1571.
22 Miller M, Kearney N. Oral care of patients with cancer: a review of the literature. Cancer Nurs. 2001;24(4):241–254.
23 Kunis KA, Puntillo KA. Ventilator-associated pneumonia in the ICU: its pathophysiology, risk factors and prevention. Am J Nurs. 103(8), 2003. 64AA–64GG
24 Mori H, Hirasawa H, Oda S, Shiga H, Matsuda K, Nakamura M. Oral care reduces incidence of ventilator-associated pneumonia in ICU populations. Intens Care Med. 2006;32(2):230–236.
25 O’Reilly M. Oral care of the critically ill: a review of the literature and guidelines for practice. Aust Crit Care. 2003;16(3):101–110.
26 Berry AM, Davidson PM. Beyond comfort: oral hygiene as a critical nursing activity in the intensive care unit. Intens Crit Care Nurse. 2006;22(6):318–328.
27 Fitch JA, Munro CL, Glass CA, Pellegrini JM. Oral care in the adult intensive care unit. Am J Crit Care. 1999;8(5):314–318.
28 Binkley C, Furr LA, Carrico R, McCurren C. Survey of oral care practices in US intensive care units. Am J Infect Control. 2004;32(3):161–169.
29 Furr LA, Binkley C, McCurren C, Carrico R. Factors affecting quality of oral care in intensive care units. J Adv Nurs. 2004;48(5):454–462.
30 Barnason S, Graham J, Wild MC, Jensen LB, Rasmussen D, et al. Comparison of two endotracheal tube securement techniques on unplanned extubation, oral mucosa, and facial skin integrity. J Acute Crit Care. 1998;27(6):409–417.
31 Treloar DM, Stechmiller JK. Use of a clinical assessment tool for orally intubated patients. Am J Crit Care. 1995;4(5):355–360.
32 Jenkins D. Oral care in the ICU: an important nursing role. Nurs Stand. 1989;4(7):24–28.
33 Evans G. A rationale for oral care. Nurs Stand. 2001;15(43):33–36.
34 Holmes S. The oral complications of specific anticancer therapy. Int J Nurs Stud. 1991;28(4):343–360.
35 Levine R. The scientific basis of dental health education, 3rd edn. London: Health Education Authority; 1993.
36 Pearson LS, Hutton JL. A controlled trial to compare the ability of foam swabs and toothbrushes to remove dental plaque. J Adv Nurs. 2002;39(5):480–489.
37 Berry AM, Davidson PM, Masters J, Rolls K. Systematic literature review of oral hygiene practices for intensive care patients receiving mechanical ventilation. Am J Crit Care. 2007;16(6):552–562.
38 Fields LB. Oral care intervention to reduce the incidence of ventilator-associated pneumonia in the neurologic intensive care unit. J Neurosci Nursing. 2008;40(5):291–298.
39 Munro CJ, Grap MJ, Jones DJ, McClish DK, Sessler CN. Chlorhexidine, toothbrushing, and preventing ventilator-associated penumonia in critically ill adults. Am J Crit Care. 2009;18(5):428–437.
40 Houston S, Hougland P, Anderson JJ, LaRocco M, Kennedy V, Gentry LO. Effectiveness of 0.12% chlorhexidine gluconate oral rinse in reducing prevalence of nosocomial pneumonia in patients undergoing heart surgery. Am J Crit Care. 2002;11(6):567–570.
41 Tombes MB, Gallucci B. The effects of hydrogen peroxide rinses on the normal oral mucosa. Nurs Res. 1993;42(6):332–337.
42 National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel. Pressure ulcer prevention and treatment clinical practice guideline. Washington DC: National Ulcer Advisory Panel; 2009.
43 Todres L, Fulbrook P, Albarran J. On the receiving end: A hermeneutic-phenomenological analysis of a patient’s struggle to cope while going through intensive care. Nurs Crit Care. 2000;5(4):277–287.
44 Fan E, Zanni JM, Dennison CR, Lepre SJ. Critical illness neuromyopathy and muscle weakness in patients in the intensive care unit. AACN Advanced Crit Care. 2009;20(3):243–253.
45 de Jonghe B, Lacherade JC, Sharshar T, Outin H. Intensive care unit-acquired weakness: risk factors and prevention. Crit Care Med. 2009;37(10):S309–S315.
46 de Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Duand-Zaleski I, et al. Paresis acquired in the Intensive care unit. J Med Assoc. 2002;288(22):2859–2867.
47 Griffiths RD, Hall JB. Intensive care unit-acquired weakness. Crit Care Med. 2010;38(3):779–787.
48 Needham DM, Chandolu S, Zanni J. Interruption of sedation for early rehabilitation improves outcomes in ventilated, critically ill adults. Aust J Physio. 2009;55(3):210.
49 Needham DM. Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. J Med Assoc. 2008;300(14):1685–1690.
50 Morris PE. Moving our critically ill patients: mobility barriers and benefits. Crit Care Clin. 2007;23:1–20.
51 Morris PE, Goad A, Thompson C, Taylor K, Harry B, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243.
52 Needham DM, Korupolu R, Zanni JM, Pradhan P, Colantuoni E, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536–542.
53 Vollman KM. Introduction to progressive mobility. Crit Care Nurse. 2010;30(2):S3–S5.
54 Schweickert WD, Pohlman MC, Pohlman SA, Nigos C, Pawlik AJ, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882.
55 Truong AD, Fan E, Brower RG, Needham DM. Bench-to-bedside review: mobilizing patients in the intensive care unit – from pathophysiology to clinical trials. Crit Care. 2009;13(4):167.
56 Krishnagopalan S, Johnson EW, Low LL, Kaufman LJ. Body positioning of intensive care patients: clinical practice versus standards. Crit Care Med. 2002;30(11):2588–2592.
57 Batson S, Adam S, Hall G, Quirke S. The development of a pressure area scoring system for critically ill patients: a pilot study. Intens Crit Care. 1993;9(3):146–151.
58 Berenholtz SM, Dorman T, Ngo K, Pronovost PJ. Qualitative review of intensive care unit quality indicators. J Crit Care. 2002;17(1):1–12.
59 Resar R, Pronovost P, Haraden C, Simmonds T, Rainey T, Nolan T. Using a bundle approach to improve ventilator care processes and reduce ventilator-associated pneumonia. Joint Commiss J Qual & Patient Safety. 2005;31(5):243–248.
60 Clavet H, Hebert PC, Fergusson D, Doucette S, Trudel G. Joint contracture following prolonged stay in the intensive care unit. Can Med Assoc J. 2008;178(6):691–697.
61 Jankowski IM. Tips for protecting critically ill patients from pressure ulcers. Crit Care Nurse. 2010;30(2):S7–S9.
62 Stiller K. Safety issues that should be considered when mobilizing critically ill patients. Crit Care Clin. 2007;23(1):35–53.
63 Timmermann RA. A mobility protocol for critically ill adults. Dimens Crit Care Nurs. 2007;26(5):175–179.
64 Adam S, Forrest S. ABC of intensive care: other supportive care. BMJ. 1999;319(7203):175–178.
65 Koch SM, Fogarty S, Signorino C, Parmley L, Mehlhorn U. Effect of passive range of motion on intracranial pressure in neurosurgical patients. J Crit Care. 1996;11(4):176–179.
66 Brimioulle S, Moraine JJ, Norrenberg D, Kahn RJ. Effects of positioning and exercise on intracranial pressure in a neurosurgical intensive care unit. Phys Ther. 1997;77(12):1682–1689.
67 Powers GC, Zentner T, Nelson F, Bergstrom N. Validation of the mobility subscale of the Braden Scale for predicting pressure sore risk. Nurs Res. 2004;53(5):340–346.
68 Jastremski C. Back to basics: can body positioning really make a difference in the intensive care unit? Crit Care Med. 2002;30(11):2607–2608.
69 Wheeler H. Positioning: one good turn after another? Nurs Crit Care. 1997;2(3):129–131.
70 Winkelman C, Peereboom K. Staff-perceived barriers and facilitators. Crit Care Nurse. 2010;30(2):S13–S16.
71 Jones A, Dean E. Body position change and its effect on hemodynamic and metabolic status. J Acute Crit Care. 2004;33(5):281–290.
72 Boyle M, Green M. Pressure sores in intensive care: defining their incidence and associated factors and assessing the utility of two pressure sore risk assessment tools. Aust Crit Care. 2001;14(1):24–30.
73 Weststrate J, Heule F. Prevalence of pressure ulcers, risk factors and use of pressure-relieving mattresses in ICU patients. Connect. 2001;1(3):77.
74 Jackson C. The revised Jackson/Cubbin Pressure Area Risk Calculator. Intens Crit Care. 1999;15(3):169–175.
75 Swaderner-Culpepper L. Continuous lateral rotation therapy. Crit Care Nurse. 2010;30(2):S5–S7.
76 Goddard R. Use of rotational therapy in the treatment of early acute respiratory distress syndrome (ARDS): a retrospective case report. Connect. 2004;3(3):82–85.
77 Kirschenbaum L, Azzi E, Sfeir T, Tietjen P, Astiz M. Effect of continuous lateral rotational therapy on the prevalence of ventilator-associated pneumonia in patients requiring long-term ventilatory care. Crit Care Med. 2002;30(9):1983–1986.
78 Goldhill DR, Imhoff M, McLean B, Waldman C. Rotational bed therapy to prevent and treat respiratory complications: a review and meta-analysis. Am J Crit Care. 2007;16(1):50–62.
79 Rauen CA, Flynn-Makic MB, Bridges E. Evidence-based practice habits: transforming research into bedside practice. Crit Care Nurse. 2009;29(2):46–59.
80 NHMRC. Clinical practice guideline for the prevention of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to Australian hospitals. Melbourne: National Health and Medical Research Council; 2009.
81 Kakkos SK, Caprini JA, Geroulakos G, Nicolaides AN, Stansby GP, Reddy DJ. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism in high-risk patients. Cochrane Database of Systematic Reviews. 2008:4.
82 Access Economics. The burden of venous thromboembolism in Australia. http//www.accesseconomics.com.au/publicationsreports/showreport.php, 2008. Report for the Australian and New Zealand working party on the management and prevention of venous thromboembolism. [Cited November 2010]. Available from
83 Chong BH, Braithwaite J, Harris MF, Fletcher JP. Venous thromboembolism – A major health and financial burden: how can we do better to prevent this disease. Med J Aust. 2008;189(3):134–135.
84 Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133:S381–S453.
85 Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371(9610):387–394.
86 Sachdeva A, Dalton M, Amaragiri SV, Lees T. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database of Systematic Reviews. 2010:7.
87 Douketis J, Cook D, Meade M, et al. Prophylaxis against deep vein thrombosis in critically ill patients with severe renal insufficiency with the low-molecular-weight heparin dalteparin: an assessment of safety and pharmacodynamics: the DIRECT study. Arch Intern Med. 2008;168:1805–1812.
88 Griffen M, Kakkos SK, Geroulakos G, Nicolaides AN. Comparison of three intermittent pneumatic compression systems in patients with varicose veins: a hemodynamic study. Int Angiol. 2007;26(2):158–164.
89 Lachiewicz PF, Kelley SS, Haden LR. Two mechanical devices for prophylaxis of thromoboembolism after total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg. 2004;86(8):1137–1141.
90 Kakkos SK, Griffin M, Geroulakos G, Nicolaides AN. The efficacy of a new portable sequential compression device (SCD Express) in preventing venous statis. J Vasc Surg. 2005;42(2):296–303.
91 Thorpe D, Harrison L. Bowel management: development of guidelines. Connect. 2002;2(2):61–65.
92 Hill S, Anderson J, Baker K, Bonson B, Gager M, Lake E. Management of constipation in the critically ill patient. Nurs Crit Care. 1998;3(3):134–137.
93 McKenna S, Wallis M, Brannelly A, Cawood J. The nursing management of diarrhoea and constipation before and after the implementation of a bowel management protocol. Aust Crit Care. 2001;14(1):10–16.
94 van Welzen M, Carey T. Autonomic dysreflexia: guidelines for practice. Connect. 2002;2(1):13.
95 Powell M, Rigby D. Management of bowel dysfunction: evacuation difficulties. Nurs Stand. 2000;14(47):47–54.
96 Dorman BP, Hill C, McGrath M, Mansour A, Dobson D, et al. Bowel management in the intensive care unit. Intens Crit Care Nurs. 2004;20(6):320–329.
97 International Foundation for Functional Gastrointestinal Disorders. Stool Form Guideline. http://www.aboutconstipation.org/bristol.html, 21 April 2005. [Cited October 2005]; Available from
98 Riegler G, Esposito I. Bristol scale stool form. A still valid help in medical practice and clinical research. Techniques in Coloproctology. 2001;5(3):163–164.
99 Robinson CB, Fritch M, Hullett L, Petersen MA, Sikkema S, et al. Development of a protocol to prevent opioid-induced constipation in patients with cancer: a research utilization project. Clin J Oncol Nurs. 2000;4(2):79–84.
100 Guerrant RL, van Gilder T, Steiner TS, Thielman NM, Slutsker L, et al. IDSA: practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32:331–350.
101 Rushdi TA, Pichard C, Khater YH. Control of diarrhea by fiber-enriched diet in ICU patients on enteral nutrition: A prospective randomized controlled trial. Clin Nutr. 2004;23(6):1344–1352.
102 Padmanabhan A, Stern M, Wishin J, Mangino M, Richey K, DeSane M. Clinical evaluation of a flexible fecal incontinence management system. Am J Crit Care. 2007;16(4):384–393.
103 Huang W-C, Wann S-R, Lin S-L, Kunin C, Kung M-H, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974–978.
104 Marklew A. Urinary catheter care in the intensive care unit. Nurs Crit Care. 2004;9(1):21–27.
105 Simpson L. Indwelling urethral catheters. Nurs Stand. 2001;15(46):47–54. 56
106 Pomfret I. Catheter care in the community. Nurs Stand. 2000;14(27):46–52.
107 NHS Quality Improvement Scotland. Urinary catheterisation and catheter care best practice statement. http://www.nhshealthquality.org/nhsqi/files/PUREMAN_BPW_MARCH09.pdf, June 2004. [cited November 2010]; Available from
108 Mayes J, Bliss J, Griffiths P, Mayes J, Bliss J, Griffiths P. Preventing blockage of long-term indwelling catheters in adults: are citric acid solutions effective? Brit J Comm Nurs. 2003;8(4):172–175.
109 Getliffe K. The dissolution of urinary catheter encrustation. Brit J Urology International. 2000;86(4):60–64.
110 Hurst S, Blanco K, Boyle D, Douglass L, Wikas A. Bariatric implications care nursing. Dimens Crit Care Nurs. 2004;23(2):76–83.
111 Pieracci FM, Barie PS, Pomp A. Critical care of the bariatric patient. Crit Care Med. 2006;34(6):1796–1804.
112 Puhl R, Brownell KD. Confronting and coping with weight stigma: an investigation of overweight and obese adults. Obesity. 2006;14:1802–1815.
113 Brown I. Nurses’ attitudes towards adult patients who are obese: literature review. J Adv Nurs. 2006;53:221–232.
114 Bejciy-Spring SM. Respect: a model for the sensitive treatment of the bariatric patient. Bariatric Nurs Surg Patient Care. 2008;3(1):47–56.
115 King DR, Velmahos GC. Difficulties in managing the surgical patient who is morbidly obese. Crit Care Med. 2010;38(9):S478–S482.
116 Hignett S, Griffiths P. Risk factors for moving and handling bariatric patients. Nurs Stand. 2009;24(11):40–48.
117 Nowicki T, Burns C, Fulbrook P, Jones J. Changing the mindset: an inter-disciplinary approach to management of the bariatric patient. Collegian. 2009;16:171–175.
118 Boyce JR, Ness T, Castroman P, Gleysteen JJ. A preliminary study of the optimal anesthesia positioning for the morbidly obese patient. Obes Surg. 2003;13(1):4–9.
119 NHMRC. Australian Guidelines for the Prevention and Control of Infection in Healthcare. http://www.nhmrc.gov.au, 2010. [cited November 2010]; Available from
120 WHO. Guidelines on Hand Hygiene in Healthcare 2009. [cited November 2010]; Available from http://www.who.int/patientsafety/information_centre/documents/en/index.html
121 Grayson L, Russo P, Ryan K. Hand hygiene Australia manual. Australian Commission for Safety and Quality in healthcare 2009 [cited November 2010]; Available from http://www.hha.org.au/
122 Orsi GB, Raponi M, Franchi C, Rocco M, Mancini C, Venditti M. Surveillance and infection control in an intensive care unit. Infect Control Hosp Epidemiol. 2005;26(3):321–325.
123 Troche G, Joly LM, Guibert M, Zazzo JF. Detection and treatment of antibiotic-resistant bacterial carriage in a surgical intensive care unit: a 6-year prospective survey. Infect Control Hosp Epidemiol. 2005;26(2):161–165.
124 Lemmen SW, Zolldann D, Gastmeier P, Lutticken R. Implementing and evaluating a rotating surveillance system and infection control guidelines in 4 intensive care units. Am J Infect Control. 2001;29(2):89–93.
125 Haley RW, Culver DH, White JW, Morgan WM, Emori TG, et al. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol. 1985;121(2):182–205.
126 Lim S-M, Webb SAR. Nosocomial bacterial infections in inensive care units. I: organisms and mechanisms of antibiotic resistance. Anaesthesiology. 2005;60(9):887–902.
127 Hardy KJ, Hawkey PM, Gao F, Oppenheim BA. Methicillin resistant staphylococcus aureus in the critically ill. Br J Anaesth. 2004;92(1):121–130.
128 Martins ST, Moreira M, Furtado GH, Marino CG, Machado FR, et al. Application of control measures for infections caused by multi-resistant gram-negative bacteria in intensive care unit patients. Mem Inst Oswaldo Cruz. 2004;99(3):331–334.
129 Johnson PDR, Martin R, Burrell LJ, Grabsch EA, Kirsa SW, et al. Efficacy of an alcohol/chlorhexidine hand hygiene program in a hospital with high rates of nosocomial methicillin-resistant staphylococcus aureus (MRSA) infection. Med J Aust. 2005;183(10):509–514.
130 Widmer AF, Dangel M. Alcohol-based handrub: evaluation of technique and microbiological efficacy with international infection control professionals. Infect Control Hosp Epidemiol. 2004;25(3):207–209.
131 Gamage B, Moore D, Copes R, Yassi A, Bryce E. Protecting health care workers from SARS and other respiratory pathogens: a review of the infection control literature. Am J Infect Control. 2005;33(2):114–121.
132 Lee NE, Siriarayapon P, Tappero J, Chen K, Shuey D. SARS Mobile Response Team Investigators. Infection control practices for SARS in Lao People’s Democratic Republic, Taiwan and Thailand: experience from mobile SARS containment teams. Am J Infect Control. 2004;32(7):377–383.
133 Lau PY, Chan CWH. SARS (severe acute respiratory syndrome): reflective practice of a nurse manager. J Clin Nurs. 2005;14(1):28–34.
134 Ho W, Hong Kong Hospital Authority Working Group on SARS Central Committee of Infection Control. Guideline on management of severe acute respiratory syndrome (SARS). Lancet. 2003;361(9366):1313–1315.
135 Chan D. Clinical management of SARS patients in ICU. Connect. 2003;2(3):76–79.
136 Moore D, Gamage B, Bryce E, Copes R, Yassi A, other members of The B. C. Interdisciplinary Respiratory Protection Study Group. Protecting health care workers from SARS and other respiratory pathogens: organizational and individual factors that affect adherence to infection control guidelines. Am J Infect Control. 2005;33(2):88–96.
137 WHO. Hospital Infection Control Guidance for Severe Acute respiratory Syndrome (SARS). http://www.who.int/csr/sars/infectioncontrol/en, 2003. [cited September 2005]; Available from
138 Webb SA, Seppelt IM, ANZIC Influenza Investigators. Pandemic (H1N1) 2009 influenza (“swine flu”) in Australia and New Zealand intensive care. Crit Care Resus. 2009;11(3):170–172.
139 Cepeda JA, Whitehouse T, Cooper B, Hails J, Jones K, et al. Isolation of patients in single rooms or cohorts to reduce spread of MRSA in intensive-care units: prospective two-centre study. Lancet. 2005;365(9456):295–304.
140 Pratt RJ, Pellowea CM, Wilson JA, et al. National evidence-based guidelines for preventing healthcare associated infections in NHS hospitals in England. J Hosp Infect. 2007;65:S1–64.
141 Rowley S, Clare S. Improving standards of aseptic practice through an ANTT trust-wide implementation process: a matter of prioritisation and care. British J Infect Prevention. 2009;10(1):S18–S23.
142 Bayea SC. Nosocomial infections; hand-washing compliance; comparing hand hygiene protocols; sensor-operated faucets. Assoc Perioperative Reg Nurses J. 2003;77(3):671–672.
143 Coffin S, Klompas M, Classen D, Arias K, Podgorny K, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(1):S31–S40.
144 Sole ML. Overcoming the barriers: a concerted effort to prevent ventilator-associated pneumonia. Aust Crit Care. 2005;18(3):92–94.
145 Lorente L, Lecuona M, Galvan R, Ramos MJ, Mora ML, Sierra A. Periodically changing ventilator circuits is not necessary to prevent ventilator-associated pneumonia when a heat and moisture exchanger is used. Infect Control Hosp Epidemiol. 2004;25(12):1077–1082.
146 Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing health-care-associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. Morbidity & Mortality Weekly Report Recommendations & Reports. 2004;53(3):1–36.
147 Zeitoun SS, De Barros ALB, Diccini S. A prospective, randomized study of ventilator-associated pneumonia in patients using a closed vs. open suction system. J Clin Nurs. 2003;12(4):484–489.
148 Safdar N, Crnich CJ, Maki DG. The pathogenesis of ventilator-associated pneumonia: Its relevance to developing effective strategies for prevention. Respir Care. 2005;50(6):725–739.
149 Braun BI, Kritchevsky SB, Wong ES, Solomon SL, Steele L, et al. Preventing central venous catheter-associated primary bloodstream infections: characteristics of practices among hospitals participating in the evaluation of processes and indicators in infection control (EPIC) study. Infect Control Hosp Epidemiol. 2003;24(12):926–935.
150 Calandra T, Cohen J. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33(7):1538–1548.
151 Zuschneid I, Schwab F, Geffers C, Rüden H, Gastmeier P. Reducing central venous catheter-associated primary bloodstream infections in intensive care units is possible: data from the German nosocomial infection surveillance system. Infect Control Hosp Epidemiol. 2003;24(7):501–505.
152 Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter: a randomized, controlled trial. Ann Intern Med. 1997;127(4):257.
153 Hosoglu S, Akalin S, Kidir V, Suner A, Kayabas H, Geyik MF. Prospective surveillance study for risk factors of central venous catheter-related bloodstream infections. Am J Infect Control. 2004;32(3):131–134.
154 Berenholtz S, Pronovost P, Lipsett P, Hobson D, Earsing K, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014–2020.
155 Hanna HA, Raad II, Hackett B, Wallace SK, Price KJ, et al. Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest. 2003;124(3):1030–1038.
156 Rickard C, Lipman J, Courtney M, Siversen R, Daley P. Routine changine of intravenous administration sets does not reduce colonization or infection in central venous catheters. Infect Control Hosp Epidemiol. 2004;25(8):650–655.
157 Moureau NL, Deschneau M, Pyrek J. Evaluation of the clinical performance of a chlorhexidine gluconate antimicrobial transparent dressing. J Infect Prevention. 2009;10:S13–S17.
158 College of Intensive Care Medicine Australia and New Zealand. Minimum standards for transport of critically ill patients. http://www.cicm.org.au/, 2010. [cited December 2010]; Available from
159 Australian and New Zealand College of Anaesthetists. Minimum standards for intrahospital transport of critically ill patients. http://www.anzca.edu.au/resources/professional-documents/ps39.html, 2003. [Cited November 2010]. Available from
160 Gray A, Gill S, Airey M, Williams R. Descriptive epidemiology of adult critical care transfers from the emergency department. Emergency Med J. 2003;20(3):242–246.
161 Warren J, Fromm RE, Orr RA, Rotello LC, Horst M. Medicine. ACoCC. Guidelines for the inter- and intrahospital transport of critically ill patients. Crit Care Med. 2004;32(1):256–262.
162 Gray A, Bush S, Whiteley S. Secondary transport of the critically ill and injured adult. Emergency Med J. 2004;21(3):281–285.
163 Wallace PGM, Ridley SA. ABC of intensive care: transport of critically ill patients. BMJ. 1999;319(7206):368–371.
164 Waydhas C. Intrahospital transport of critically ill patients. Crit Care. 1999;3(5):83–89. (London)
165 Lovell MA, Mudaliar MY, Klineberg PL. Intrahospital transport of critically ill patients: complications and difficulties. Anaesth Intens Care. 2001;29(4):400–405.
166 Day D. Keeping patients safe during intrahospital transport. Crit Care Nurse. 2010;30(4):18–32.
167 Chang YN, Lin LH, Chen WH, Liao HY, Hu PH, et al. Quality control work group focusing on practical guidelines for improving safety of critically ill patient transportation in the emergency department. J Emerg Nurs. 2010;36(2):140–145.