Esophagectomy
Introduction
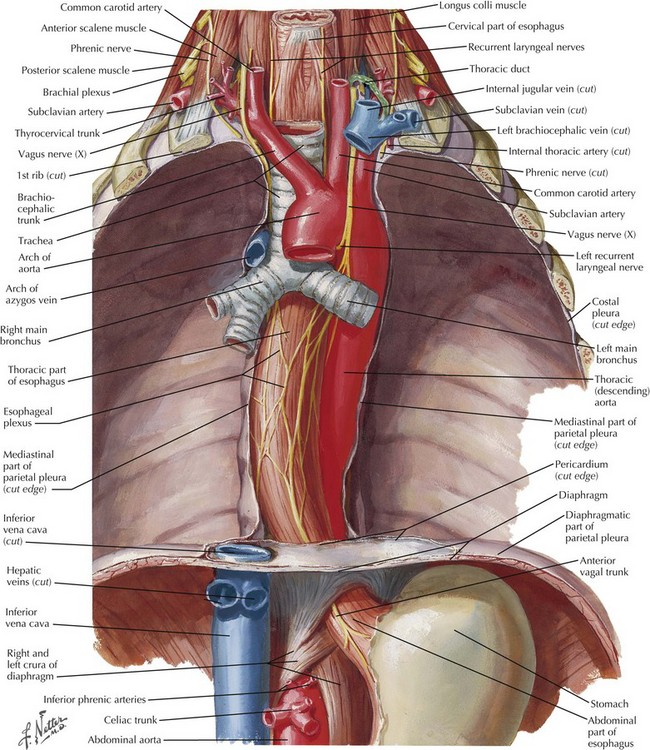
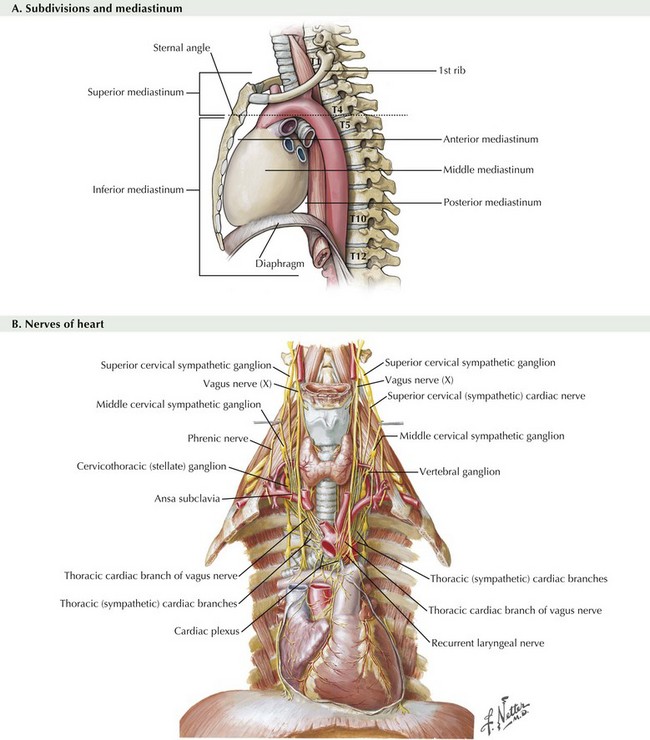
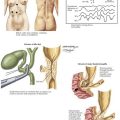
Franz Torek performed the first successful esophagectomy in 1913, after this high-risk surgery had been described 30 years with no patient survivors. Improvements in technique and patient care have reduced the mortality and morbidity, but esophagectomy remains one of the most dangerous surgeries performed, with a mortality rate of 4% to 10% and a complication rate of 20% to 60%. This dramatic risk profile results from (1) difficult anatomic position of the esophagus (Fig. 5-1), (2) involvement of three body cavities in the resection, (3) tenuous blood supply of all reconstruction options, and (4) nutrition impact on the surgical patient.
Indications
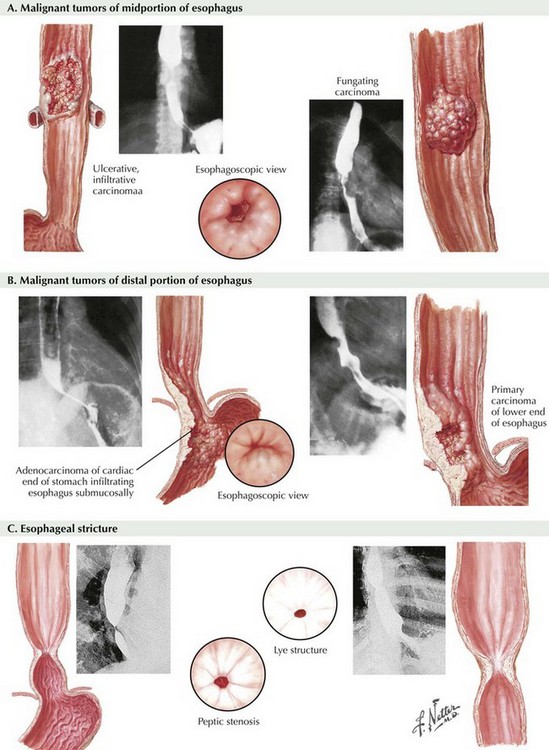
Because of the associated morbidity, most esophagectomies are performed on cancer patients (Fig. 5-2, A and B). Benign indications arise from absolute loss of function caused by end-stage motility disorders or from refractory strictures (Fig. 5-2, C). Worldwide, the primary esophageal cancer is squamous cell type, the result of dietary insults or smoking. In North America, however, this is no longer true: 80% or more of esophageal cancers are now adenocarcinomas related to gastroesophageal reflux and Barrett syndrome (metaplasia).
Transhiatal Esophagectomy
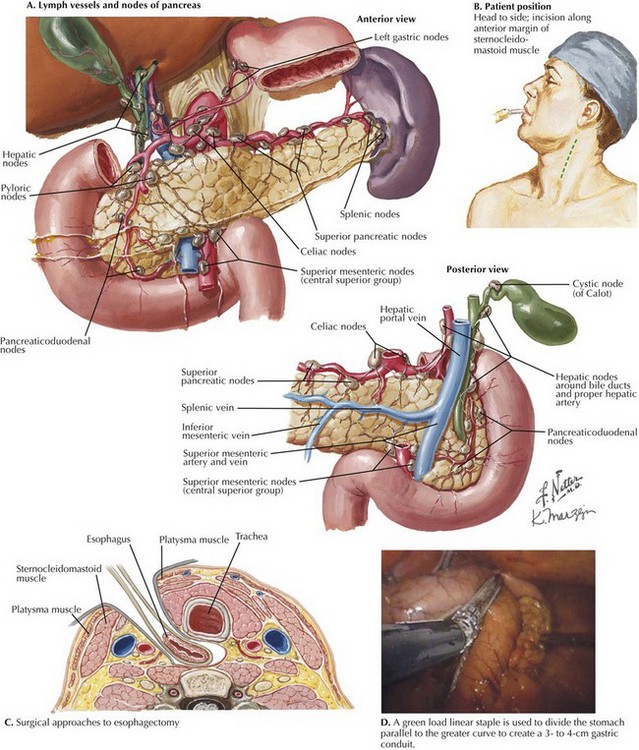
The transhiatal esophagectomy is performed with the patient supine and the left side of the neck prepped and exposed. Either an upper midline incision or, increasingly, a five-port laparoscopic access is performed. Exploration is performed to rule out disseminated tumor, which would obviate resection in most cases. The greater curvature of the stomach is retracted anteriorly, and the gastrocolic omentum is divided from the spleen to the hepatic flexure, wide of the gastroepiploic blood supply, which is critical for the viability of the gastric graft (Fig. 5-3, A). Once the greater curvature is fully mobilized, the retrogastric adhesions to the peritoneum are divided. The lesser curvature of the stomach is then mobilized, a Kocher maneuver is performed to mobilize the duodenum, and the gastrohepatic ligament is divided near its attachment to the liver (Fig. 5-3, B). This exposes the D1 node area, which is routinely dissected.
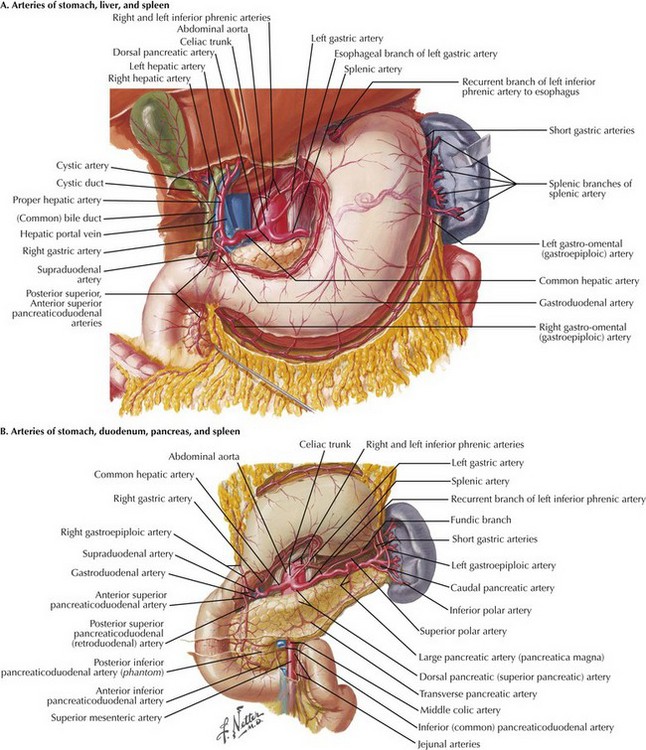
FIGURE 5–3 Anatomy of the lesser curve, second portion of the duodenum, and gastrohepatic ligament (stomach is rotated cephalad).
Node dissection margins include the porta hepatis (hepatic portal) structures, the hepatic artery, and the vena cava (Fig. 5-4, A). Dense nodal tissue around the base of the left gastric artery is dissected, and the coronary vein and left gastric artery are divided with an energy sealing device, stapler, or suture ligation. Node tissue is left in continuity with the specimen. The hiatus is typically opened by excising a rim of the hiatal musculature and entering the mediastinum in the plane of the mediastinal pleura, pericardium, and preaortic and prespinal planes. This maneuver is more feasible with a laparoscopic approach and allows good lymphatic clearance of the lower mediastinum.
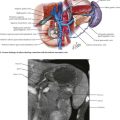
Open transhiatal surgery is performed by the surgeon, with the smallest hand on the team cupping the esophagus in the extended fingers and bluntly stripping the esophagus up to the proximal mediastinum. Care must be taken at the upper end of the blind dissection to avoid injury to the recurrent laryngeal nerve or membranous portion of the trachea (see Fig. 5-1).
Laparoscopic mediastinal dissection is performed by advancing the angled laparoscope into the mediastinum with the surgeon’s instruments. At the same time, a 5- to 7-cm incision is made in the left side of the neck from the sternal notch along the anterior margin of the sternocleidomastoid muscle (Fig. 5-4, B). The platysma muscle is divided and the sternocleidomastoid is retracted laterally (Fig. 5-4, C). After dividing the omohyoid muscle, the esophagus is located and surrounded by a soft drain. The upper mediastinal dissection is performed with lubricated gauze on a long, curved vascular clamp to meet with the dissection from below.
The gastric conduit is prepared by grasping the longest portion of the gastric cardia and stretching it into the left upper quadrant. Starting at the “crow’s foot,” a green load linear staple is used to divide the stomach parallel to the greater curvature to create a 3- to 4-cm gastric conduit (Fig. 5-4, D). Once transected, the specimen is placed in a plastic specimen bag and withdrawn through the neck. A chest tube is inserted through the neck and into the abdomen, and the gastric conduit is sutured to it and pulled up into the neck. A hand-sewn or stapled anastomosis is created to restore continuity.
Ivor Lewis and McKEOWN Approaches
Ivor Lewis Esophagectomy
The gastrocolonic omentum is divided, staying 1 to 2 cm away from the critical gastroepiploic vessels of the greater curvature of the stomach (see Fig. 5-3, A). This dissection is extended medially past the second portion of the duodenum and laterally to the inferior pole of the spleen. This allows the stomach to be reflected anteriorly, exposing the lesser sac to allow the surgeon to check for metastatic disease, and exposes the right gastric artery to allow dissection of station 8 lymph nodes, if indicated (see Fig. 5-4, A). The greater curvature of the stomach is further mobilized by dividing the short gastric vessels, once again wide of the gastric margin. This dissection is taken to the left crus. For the lesser curvature, the gastrohepatic omentum is divided from the 2nd part of the duodenum up to the right crus. The base of the left gastric artery is identified either from the right side of the stomach or from underneath the stomach. It is skeletonized and divided to provide access to the preaortic plane, which will be extended into the mediastinum.
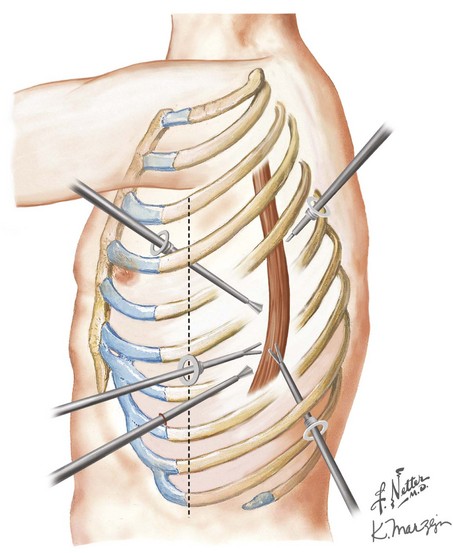
The patient is then repositioned in a left lateral decubitus position to allow access to the right side of the chest (5th intercostal posterolateral thoracotomy or four-port thoracoscopy). The right mediastinal pleura is widely excised in continuity with the esophagus. The esophagus is isolated away from the tumor and surrounded by a plastic drain for retraction. A wide resection of the esophagus is performed, incorporating surrounding node-bearing tissues with the specimen (Fig. 5-5). The esophagus is divided above the azygos vein, high in the mediastinum, with an endoscopic linear cutting stapler. The specimen is pulled up into the chest, along with the attached gastric conduit. A staple or hand-sewn anastomosis is performed, according to surgeon preference.
Modified McKeown (Three-Hole) Esophagectomy
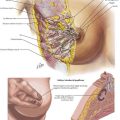
The three-hole procedure begins with the patient in the left lateral decubitus position with the arm carefully positioned overhead. A 5th or 6th intercostal posterolateral thoracotomy is done or a four-port thoracoscopic access is created. Wide resection of the right mediastinal pleura leaves the pleura with the esophagus, particularly over the area of the tumor. The azygos vein is typically divided with ties or a vascular stapler (Fig. 5-6, A). The esophagus is surrounded with a drain for retraction, and with the use of cautery or ultrasonic coagulating shears, the esophagus with all of its surrounding lymph node–bearing tissues is progressively mobilized.
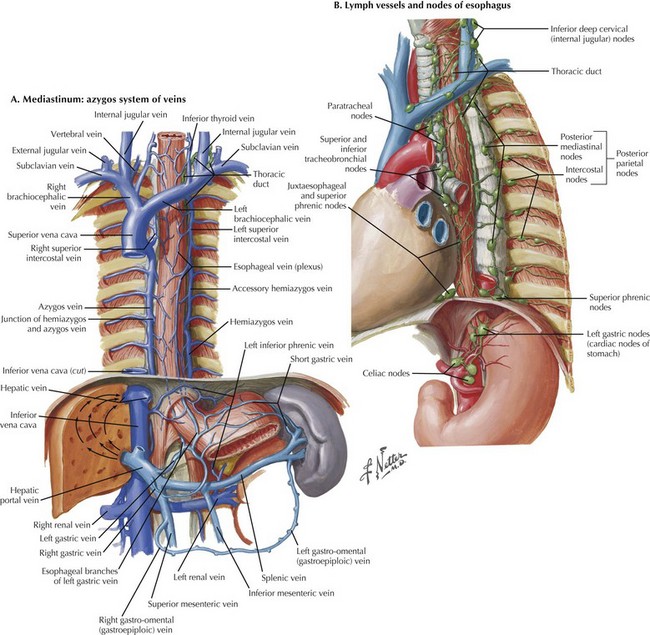
FIGURE 5–6 Modified McKeown esophagectomy: azygos system of veins and esophageal lymph nodes/vessels.
Controversy surrounds the required extent of the dissection. Some argue that both the azygos vein and thoracic duct need to be resected with the periesophageal tissue, whereas others believe that only the periesophageal nodes require resection (Fig. 5-6, B). The proximal dissection should be taken as far as possible into the thoracic outlet and the distal dissection down to the crura.
The upper esophageal dissection is performed by staying on the esophagus and using mostly blunt dissection. Care must be taken to avoid the recurrent laryngeal nerve in this area as well (Fig. 5-7, B). Small tumors can be brought out through the neck incision, pulling the interposition up at the same time. Larger cancers should be pulled into the abdomen, placed in an impermeable specimen bag, and withdrawn through a small abdominal incision. The anastomosis is performed with staples (circular or double linear firing) or is hand-sewn. Drains are placed and the incisions are closed.
Bogoevski, D, Bockhorn, M, Koenig, A, et al. How radical should surgery be for early esophageal cancer? World J Surg. 2011;35(6):1311–1320.
Briez, N, Piessen, G, Bonnetain, F, et al. Open versus laparoscopically assisted oesophagectomy for cancer: a multicentre randomised controlled phase III trial: the MIRO trial. BMC Cancer. 2011;11:310.
Defoe, SG, Pennathur, A, Flickinger, JC, et al. Retrospective review of patients with locally advanced esophageal cancer treated at the University of Pittsburgh. Am J Clin Oncol. 2011;34(6):587–592.
Jaroszewski, DE, Williams, DG, Fleischer, DE, et al. An early experience using the technique of transoral OrVil EEA stapler for minimally invasive transthoracic esophagectomy. Ann Thorac Surg. 2011;92(5):1862–1869.
Ng, T, Vezeridis, MP. Advances in the surgical treatment of esophageal cancer. J Surg Oncol. 2010;101(8):725–729.
Palmes, D, Brüwer, M, Bader, FG, et al. Diagnostic evaluation, surgical technique, and perioperative management after esophagectomy: consensus statement of the German Advanced Surgical Treatment Study Group. Langenbecks Arch Surg. 2011;396(6):857–866.
Wajed, SA, Veeramootoo, D, Shore, AC. Surgical optimisation of the gastric conduit for minimally invasive oesophagectomy. Surg Endosc. 2012;26(1):271–276.