Chapter 14 Esophageal Cancer
Introduction
Esophageal cancer is a devastating diagnosis with a high morbidity and mortality. The overall 5-year survival for all stages combined remains dismally low at approximately 17%.1 Although it is a relatively uncommon malignancy, accounting for less than 1% of all malignancies, it has been steadily increasing in incidence since the 1980s, with an estimated 16,470 new cases and an estimated 14,530 deaths in the United States in 2009.2 Up to 38% of patients with esophageal cancer present late with locally advanced or metastatic disease and are not suitable for curative resection.3 However, there have been significant improvements in both staging and therapeutic options in recent years, leading to a greater understanding of the biology of the disease. Optimal management depends on effective multidisciplinary care that combines multimodality staging and combined modality treatment incorporating chemotherapy, radiation therapy, and surgery. For the radiologist, appropriate imaging requires a full understanding of the behavior and staging of esophageal cancer in addition to a familiarity with the treatment options available. For the surgeon and clinician, recognition of the limitations and pitfalls of each imaging modality is important in order to appropriately utilize the information obtained from imaging.
Epidemiology and Risk Factors
Esophageal cancer is a worldwide disease with an incidence that varies according to geographic location. The most endemic areas include Asia, southern and eastern Africa, northern France, and Great Britain.4 In the United States, esophageal cancer accounts for less than 1% of all cancers, but it has a high mortality and is responsible for 3% of all cancer deaths.2 The risk of esophageal cancer rises with age, with a median age at diagnosis of approximately 60 years. Males have a more than threefold risk compared with females.2
The two most common histologic types of esophageal cancer are squamous cell carcinoma (SCC) and adenocarcinoma (AC). Historically, SCC has been the more common of the two types; however, since the early 1980s, there has been a steady decline in the incidence of SCC and an even greater increase in the incidence of AC, particularly in white males.4
Risk factors for the development of SCC of the esophagus include alcohol intake, smoking, and lower socioeconomic status.5,6 Risk factors for AC of the esophagus include Barrett’s esophagus (epithelial metaplasia related to chronic gastroesophageal reflux), smoking, and increased body mass index; alcohol does not appear to be a significant risk factor for AC. Protective factors include high intakes of fresh fruit and vegetables and antioxidants and use of aspirin and other nonsteroidal anti-inflammatory drugs.7–9
An explanation for the rising incidence of AC of the esophagus and its striking male predominance (7:1) is unclear. Epidemiologic data do not support direct correlations with the increased incidence of gastroesophageal reflux, the more widespread use of lower esophageal sphincter–relaxing medications, the greater incidence of obesity, or the decreased incidence of Helicobacter pylori infection.10 Tobacco use among males has decreased in recent years, which may partly account for the decreasing incidence in SCC of the esophagus but does not explain the increasing incidence of AC. Screening those at increased risk of esophageal carcinoma is possible through the identification of certain risk factors (based on age, body mass index, and reflux symptomatology) but would consume considerable healthcare resources and is currently not justifiable on a population basis. However, in view of the increasing incidence of this disease and its high morbidity and mortality, the issue of surveillance may be revisited in the future.
Anatomy and Pathology
SCC and AC are both epithelial in origin but otherwise differ greatly in anatomic proclivity, risk factors, pathologic behavior and prognosis, and for staging and clinical purposes, they can be considered as separate diseases. SCC is a disease of nicotine and alcohol abuse, risk factors that affect the entire esophagus and partly explain why SCC arises in the mid- and upper thoracic esophagus above the level of the carina in up to 65% of cases.11 AC, conversely, is strongly linked to gastroesophageal reflux disease and Barrett’s esophagus and is almost always located below the level of the carina.12 SCC of the esophagus is also associated with a significantly worse prognosis than is AC.13
Because the behavior and surgical options for esophageal cancer vary according to its location, the esophagus is divided into four distinct anatomic regions14: the cervical esophagus extends from the cricopharyngeus muscle to the suprasternal notch; the upper thoracic esophagus extends from the suprasternal notch to the lower border of the azygos vein; the midthoracic esophagus extends from the lower border of the azygos vein to the inferior pulmonary veins; and the lower thoracic esophagus extends from the inferior pulmonary veins to the stomach and includes a variable portion of the intra-abdominal esophagus and the gastroesophageal junction.15 Gastroesophageal junction tumors have been further subdivided into types I, II, and III according to the relative extent of involvement of the esophagus or gastric fundus.16 According to the American Joint Committee on Cancer (AJCC) staging classification, Siewert type III tumors (those with an epicenter within 5 cm of the gastroesophageal junction but with proximal extension into the gastroesophageal junction or lower thoracic esophagus) are considered as primary esophageal tumors, and more distal tumors are staged as gastric carcinomas.
Histologically, the esophageal wall comprises the mucosa, the submucosa, the muscularis propria, and the adventitia. Local tumor invasion is determined according to the involvement of each of these histologic layers (Figure 14-1). There is no serosa surrounding the esophagus, facilitating local tumor invasion into pleura, pericardium, diaphragm, or peritoneum.
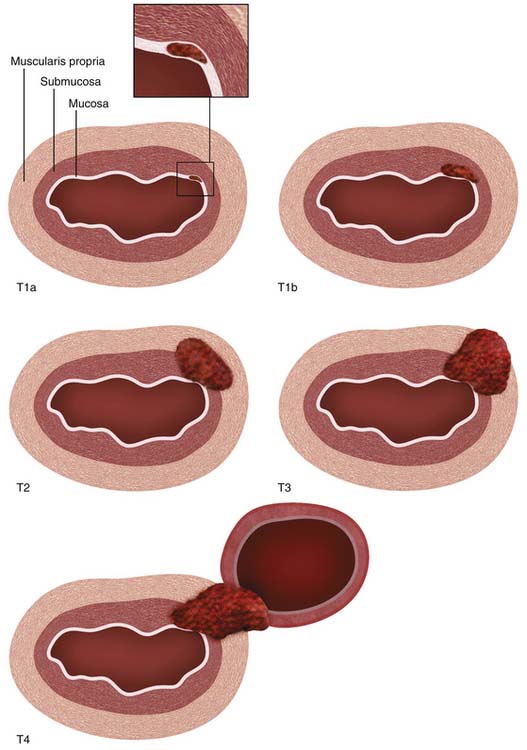
Figure 14-1 Schematic illustration of T descriptor of esophageal cancer. See Table 14-1 for comprehensive description.
A rich lymphatic network drains the esophagus with channels running both radially and longitudinally, facilitating early and distant dissemination of lymphatic metastases. Most of the lymphatics are concentrated in the submucosa, but they are also present in the lamina propria of the mucosa and connect to periesophageal lymph node stations and with the thoracic duct.17 The longitudinal lymphatic plexus in the submucosa facilitates orthogonal drainage both cranially and caudally, with the result that lymph node metastases may be located distant to the primary tumor without involvement of adjacent lymph nodes (“skip” metastases).
Locoregional lymph nodes are defined as those lymph nodes that are located close to the esophagus and that would commonly be resected at the time of surgery (Figure 14-2). These include lower cervical and supraclavicular lymph nodes; intrathoracic lymph nodes in the periesophageal region, mediastinum, and hilar regions; diaphragmatic lymph nodes adjacent to the crura; left gastric and paracardial lymph nodes; and common hepatic, splenic, and celiac lymph nodes.15
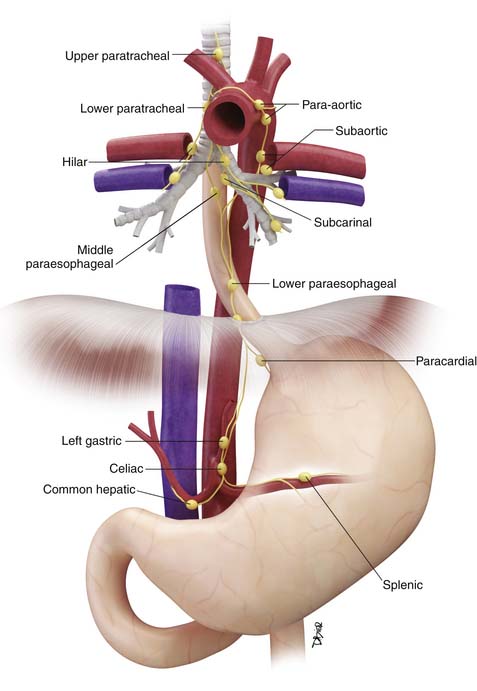
Figure 14-2 Schematic illustration of locoregional lymph nodes in esophageal cancer. See text for further discussion.
Key Points Anatomy and pathology
• SCC of the esophagus is more commonly located above the level of the carina and associated with a significantly worse prognosis than AC.
• Esophagus does not have a serosa, thereby facilitating adjacent organ invasion by tumor.
• Extensive submucosal lymphatic plexus facilitates both orthogonal and radial spread of metastatic disease in the lymphatics.
Clinical Presentation
Most esophageal carinomas, particularly AC, arise in the distal esophagus or gastroesophageal junction.18 Symptoms are often initially silent or nonspecific and are most often attributed to underlying chronic gastroesophageal reflux, manifesting as chronic or recurrent dyspepsia. More specific symptoms and signs, when they occur, typically present late, most often with weight loss, dysphagia, or odynophagia, by which time the primary tumor is locally advanced.
Tumors in the more proximal cervical esophagus may also present with dysphagia or signs of local tumor extension such as dysphonia or sympathetic nerve dysfunction.
By the time of presentation, distant metastases may already be present in up to 20% to 30% of patients and are often occult on conventional imaging.3,19 Advanced weight loss is a poor prognostic sign and nutritional replacement is an important facet of treatment.
Staging Evaluation
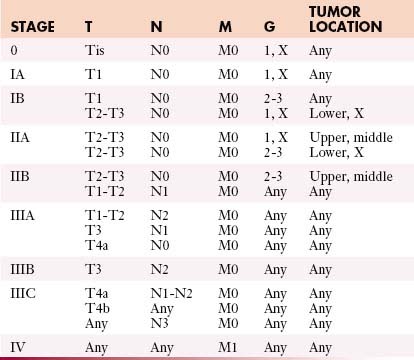
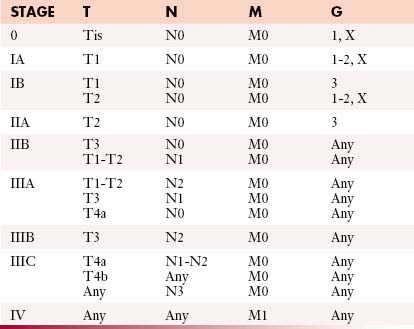
The most commonly used international staging system for esophageal cancer is the tumor-node-metastasis-grade (TNMG) classification published by the AJCC, currently in its seventh edition.15 According to this system, tumor descriptors are assigned based on the extent of local tumor invasion (T), nodal involvement (N), presence of distant metastases (M), and histologic grade (G) (Table 14-1). Stages are formulated according to combinations of descriptors that are associated with similar prognoses and are intended as a guide to management and prognosis (Tables 14-2 and 14-3).
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1a | Tumor invades lamina propria or muscularis mucosae |
| T1b | Tumor invades submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades adventitia |
| T4a | Resectable tumor invading pleura, pericardium, or diaphragm |
| T4b | Unresectable tumor invading other adjacent structures such as aorta, vertebral body, or trachea |
| Regional Lymph Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in one or two regional lymph nodes |
| N2 | Metastasis in three to six regional lymph nodes |
| N3 | Metastasis in seven or more regional lymph nodes |
| Distant Metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| Histologic Grade (G) | |
| GX | Grade cannot be assessed—stage grouping as G1 |
| G1 | Well differentiated |
| G2 | Moderately differentiated |
| G3 | Poorly differentiated |
| G4 | Undifferentiated—stage grouping as G3 squamous |
The seventh edition of the AJCC staging classification incorporates several important revisions compared with the sixth edition. Most importantly, different staging classifications are used depending on whether the primary tumor is SCC (see Table 14-2) or AC (see Table 14-3), based on worldwide evidence that histopathologic type has an influence on prognosis (SCC in general having a worse prognosis than AC).13 However, the TNMG descriptors remain the same for both histopathologic types.
The T descriptor ranges from Tis (carcinoma in situ) to T4 (tumor invades into adjacent structures) (see Figure 14-1). New since the sixth edition of the AJCC staging system, T1 is now subdivided into T1a and T1b, depending on whether tumors are confined to the mucosa (T1a) or whether there is invasion of the submucosa (T1b). Also new since the previous edition, T4 is now subdivided into T4a (potentially resectable invasion of the pleura, pericardium, or diaphragm) and T4b (unresectable invasion into other adjacent structures such as the aorta and vertebral bodies). According to the National Comprehensive Cancer Network (NCCN) clinical practice guidelines,20 T1a or T1b tumors without nodal metastases are amenable to surgical monotherapy (endoscopic resection or esophagectomy) but more advanced tumors will require combined modality treatment involving chemotherapy, radiotherapy, and surgery. T4b tumors are unresectable.
The N descriptor has been simplified since the sixth edition with removal of the M1a descriptor (nonregional lymph node involvement). Celiac and supraclavicular lymph node metastases are now considered as locoregional lymph node metastases (rather than as M1a disease as in the sixth edition) (see Figure 14-2). The number of involved lymph nodes has been shown to have an important influence on survival and this is reflected in the subdivision of the N descriptor into N1 (1 or 2 lymph nodes involved), N2 (3-6 lymph nodes involved), and N3 (>6 lymph nodes involved). This subdivision also implies that an adequate lymph node dissection must be performed to ensure adequate pathologic staging and also to optimize survival. A recommended number of lymph nodes that should be removed has not been universally determined, but most would agree that at least 10 lymph nodes should be removed for early-stage carcinomas, and more (20-30 lymph nodes) for advanced tumors.21–26
Nonregional lymph node metastases and distant visceral metastases are both designated an M descriptor (previously subdivided into M1a and M1b) and are associated with a uniformly poor prognosis, with a 5-year survival of only 3%.1
Key Points Staging
• Different staging systems for SCC and AC.
• T1 descriptor subdivided into T1a (tumor confined to mucosa) and T1b (extension of tumor into submucosa).
• T4 descriptor subdivided into T4a (limited invasion of pleura, pericardium, or diaphragm) and T4b (invasion into other adjacent organs).
• N descriptor subdivided into N1 (1-2 lymph node metastases), N2 (3-6 lymph node metastases) and N3 (>6 lymph node metastases).
• M1 descriptor includes both nonregional lymph node metastases and distant visceral metastases.
Patterns of Tumor Spread
Local Extension
Most esophageal carcinomas are epithelial in origin (i.e., SCC and AC) and normally progress in a radial fashion from involvement of the mucosa, to breach of the muscularis mucosae with extension into the submucosa, to invasion of the muscularis propria and involvement of adjacent organs. The absence of a serosa means that, once tumor has invaded through the muscularis propria and adventitia, local organ involvement readily occurs. Once tumor has invaded into the submucosa, it can also spread longitudinally by several centimeters before involving the muscularis propria. Endoscopic ultrasound is the most accurate modality for assessing the depth of tumor invasion; however, even by this means, it is difficult to confidently identify tumors that are limited to the mucosa or to diagnose local organ invasion.27
Lymphatic
Lymph node metastases spread radially to adjacent periesophageal lymph nodes and longitudinally via the submucosal lymphatic plexus and via the thoracic duct to more distant lymph node stations. Longitudinal lymphatic spread is in a roughly predictable fashion: tumors located in the cervical and upper thoracic esophagus drain preferentially in a cranial direction to cervical lymph nodes; tumors in the distal esophagus and gastroesophageal junction drain caudally to intra-abdominal lymph nodes; and tumors in the midthoracic esophagus may drain in either direction.28 However, lymphatic spread is not limited to these trends; lymph node metastases along the recurrent laryngeal nerves in the neck can still occur with distal esophageal tumors.28,29 Furthermore, because of the rich submucosal lymphatic plexus, “skip” metastases to distant lymph node stations may occur while bypassing locoregional lymph nodes; such skip metastases are found in 10% to 20% of resected tumors, and in surgery performed with a curative intent, these are an important argument for extended lymph node dissection.27,29
Although lymphatic spread of esophageal cancer occurs early, the probability of lymph node metastases increases with greater local tumor invasion. Lymph node metastases occur in up to 33% of patients with early intramucosal tumors, 67% of intramural tumors, and 89% of tumors that have invaded through the esophageal muscularis propria.27,30
Hematogenous
Hematogenous metastases to distant organ sites are common and are estimated to be present in up to 18% to 30% of patients at the time of presentation.3,31 The risk of hematogenous spread of tumor increases with more advanced local tumor invasion and nodal involvement, but can also occur early with small primary tumors and no apparent nodal metastases. The most common sites of distant visceral metastases include the liver, bones, lungs, and adrenal glands, but they may also occur in more unusual sites (brain, skeletal muscle, subcutaneous fat, and thyroid gland) and may be occult by CT imaging.32 The detection of distant metastases constitutes incurable disease and is associated with a very poor prognosis.
Peritoneal
Locally advanced tumors of the intra-abdominal esophagus or gastroesophageal junction can spill tumor cells directly into the peritoneal cavity and seed distant intra-abdominal or intrapelvic sites. Peritoneal metastatic disease constitutes a contraindication to surgery but is difficult to diagnose prospectively by imaging owing to the typically small volume of disease and its occult nature on cross-sectional images. In general, the diagnosis is made either at the time of surgery or preoperatively by laparoscopic exploration.33 The peritoneum represents an important source of recurrent disease after definitive therapy.
Key Points Tumor spread
• Lymphatic and hematogenous metastases occur early and their probability increases with locally advanced tumor.
• “Skip” nodal metastases occur in 10% to 20% of resected tumors.
• Hematogenous metastases are present in up to 30% of patients at the time of initial presentation.
• Hematogenous and peritoneal metastases may be occult on CT imaging.
Imaging
EGD is critical for initial diagnosis and EUS is an extremely useful tool for nodal evaluation in specialized centers, but a full discussion of these endoscopic techniques is beyond the scope of this chapter. Barium studies are important for evaluating the length and severity of stricture, which can be used to assess the need for stent placement and for guiding radiation therapy planning, but do not provide useful staging information. Standard radiologic baseline staging is based on contrast-enhanced CT and, for patients with potentially resectable tumors, PET/CT (Table 14-4).
PET/CT imaging with 2[18F] fluoro-2-deoxy-D-glucose (18F-FDG) has greatly changed the way in which esophageal cancer is managed and should now be considered an indispensable imaging modality for baseline staging and therapeutic response evaluation. It provides both functional and anatomic information of great use for guiding clinical decision-making; however, its optimal utilization requires an understanding of its limitations and the interpretative pitfalls that may be encountered. Because conventional contrast-enhanced CT (Table 14-5) provides better-quality images of the soft tissues and particularly of the lungs, both CT and PET/CT are complementary modalities for the evaluation of esophageal cancer.
| PARAMETER | |
|---|---|
| Volume of interest | Midcervical neck to symphysis pubis |
| kVp | 100-140 |
| Oral contrast medium | 500-1000 mL dilute contrast medium, 45 min before imaging |
| Intravenous contrast medium | 300-350 mg/dL nonionic iodinated contrast |
| Injection technique | 100-120 mL at 3-5 mL/sec |
| Scan phase (time from end of injection to start of scan [in sec]) | Thorax: phase of peak pulmonary arterial enhancement (20-25 sec) Abdomen/pelvis: portal venous phase (50-60 sec) |
| Image re-formats | 2-3-mm-thin axial slices in soft tissue (center, 50 HU; width, 350 HU) and lung (center, 600 HU; width, 1600 HU) windows with appropriate vendor-specified soft tissue and lung/bone reconstruction algorithms |
| Optional additional multiplanar re-formats | Coronal and sagittal oblique |
Primary Tumor
Computed Tomography
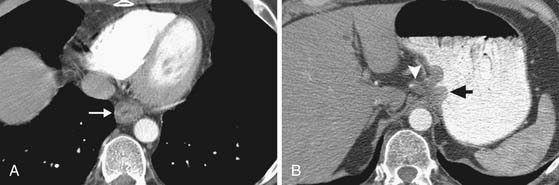
On axial CT scans, the primary tumor may be visualized as a focal or diffuse area of esophageal wall thickening (Figure 14-3). An esophageal wall thickness greater than 5 mm has been described as abnormal34; however, in practice, it is difficult to accurately measure the esophageal wall thickness because it is frequently nondistended and often cannot be accurately differentiated from adjacent structures such as the thoracic duct and small periesophageal lymph nodes. Compared to EGD/EUS, CT has a relatively poor sensitivity for depiction of the primary mass (~67%) and cannot resolve invasion of the different histologic layers of the esophageal wall.35
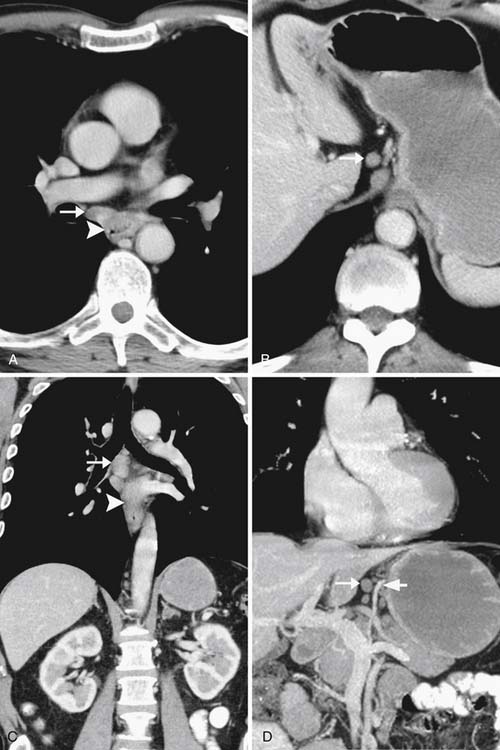
Invasion into adjacent structures (T4 disease) may be suggested on CT by contiguity between the esophagus and the adjacent organs and loss of the normal periesophageal fat planes. Aortic invasion is suggested by encasement greater than 90 degrees and diaphragmatic invasion by loss of the retrocrural fat planes (Figure 14-4). Tracheal invasion may be suspected where a midesophageal tumor bulges into the posterior membranous portion of the tracheal wall (which normally has a straight or convex shape). In practice, however, the fat planes are often absent without invasive disease, and periesophageal fat stranding may also occur secondary to fibrosis or inflammation incited by the primary tumor. In the absence of evidence of frank invasion into surrounding structures, a T4 status is often impossible to determine with certainty by any modality and is a diagnosis most often made at the time of attempted resection.
Positron-Emission Tomography/Computed Tomography
Most, but not all, esophageal malignancies are FDG-avid. Although there is some evidence to suggest that the metabolic activity of the primary tumor (as measured by the maximum standard uptake value [SUVmax] or metabolic tumor volume [MTV]) is of prognostic value, there are conflicting reports as to the reliability of this correlation.36–39 Although an understanding of the relationship between FDG uptake and tumor biology is still evolving, the baseline metabolic activity of esophageal cancer has not yet been incorporated into routine clinical decision-making or staging.
In patients with esophageal cancer, FDG uptake in the esophagus is most often secondary to activity in the primary tumor itself but can also occur secondary to multiple additional factors that may confound the apparent longitudinal extent of the tumor. Esophagitis or mucosal ulceration secondary to acid reflux is a common cause of false-positive FDG uptake, appearing as linear or focal areas of high activity in the distal esophagus. After endoscopic esophageal stent placement, there is often intense surrounding FDG uptake that cannot be differentiated from the primary tumor itself (Figure 14-5). Another frequent cause of false-positive FDG uptake in the esophagus is inflammation after endoscopic biopsy of the mucosa. For all of these reasons, evaluation of the apparent metabolic activity of the primary esophageal tumor should be correlated with the recent clinical history and findings at endoscopy.
Nodal Disease
Computed Tomography
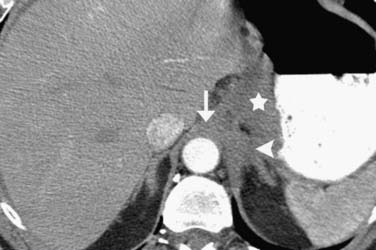
CT has a relatively poor diagnostic accuracy for the diagnosis of nodal metastases, depending entirely on size criteria that are neither sensitive nor specific. As a general rule, lymph nodes that are greater than 1 cm in short axis are considered suspicious for malignancy; however, smaller lymph nodes may still harbor metastatic disease, and large lymph nodes may simply be reactive. The lymph node status is best determined by EGD/EUS and fine-needle aspiration (FNA). The reported diagnostic accuracy of CT varies considerably from study to study according to the gold standard used; in one study of 75 patients in which tissue confirmation was used as the gold standard, CT had a sensitivity and specificity of 84% and 67%, respectively.40
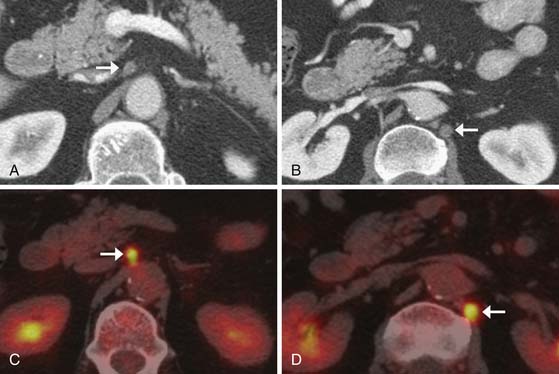
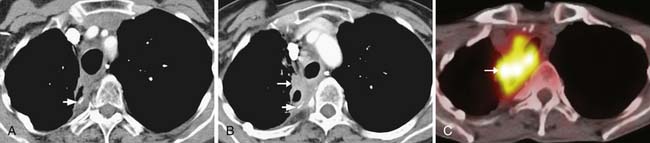
Thin-slice (2-3 mm) axial and multiplanar re-formats facilitate more accurate anatomic localization of lymph nodes, particularly in the upper abdomen where it is easy to confuse left gastric and celiac lymph nodes and where prominent lobulations of pancreatic tissue may be mistaken for peripancreatic lymph nodes. These re-formats can increase reader confidence and provide the surgeon with a better preoperative assessment (Figure 14-6).
Positron-Emission Tomography/Computed Tomography
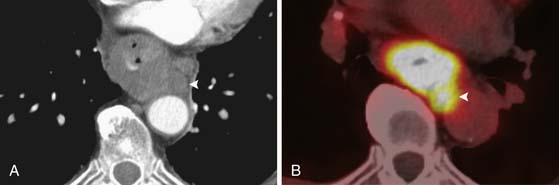
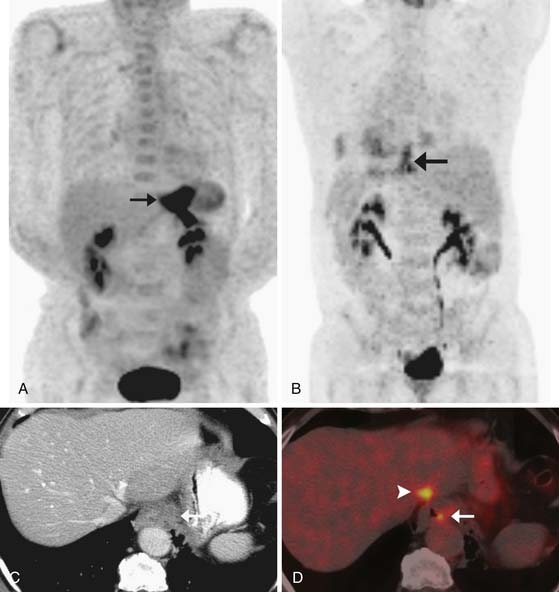
Compared with CT, PET/CT has a similar sensitivity (51-82%) and a slightly greater specificity (60-84%) for the detection of nodal metastases in patients with esophageal cancer.40,41 Evaluation of the presence of nodal metastases by PET/CT is limited by its inability to detect micrometastases and by its difficulty in resolving FDG-avid lymph nodes that are located close to the primary tumor owing to the “bloom” effect from activity in the primary mass (Figures 14-7 and 14-8). PET/CT does have greater specificity than CT in identifying nodal metastases, which is useful for directing EUS-guided FNA of specific lymph nodes and for alerting the surgeon to possible nodal metastases in atypical locations. A combination of EGD/EUS and PET/CT is the optimal method for prospectively evaluating the N status of esophageal cancer. As discussed previously, the main objective of PET/CT is the identification of celiac or other lymph nodes that lie outside the typical locoregional nodal field, the presence of which will have a great influence on surgical decision-making (see Figure 14-8).
Metastases
Computed Tomography
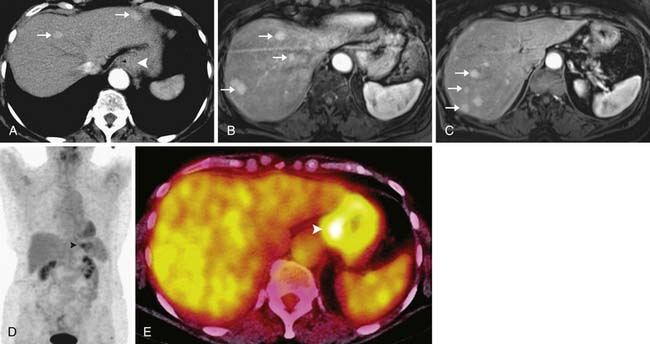
Metastases from esophageal cancer most commonly occur in the liver, lungs, and bones.31 Most of these metastases are readily detectable by CT with a sensitivity of approximately 81%,40 particularly when multiphasic imaging of the liver is included as part of the baseline CT scanning protocol. CT is also the ideal imaging modality for detection of pulmonary metastases and has greater sensitivity in this regard than PET/CT, in which images of the lungs are not acquired in full inspiration and in which pulmonary metastases may be too small for resolution by PET scanning.
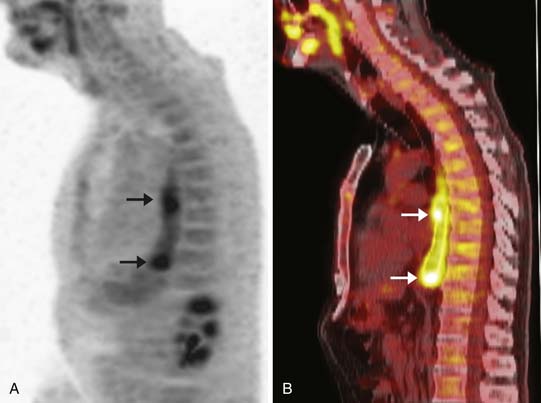
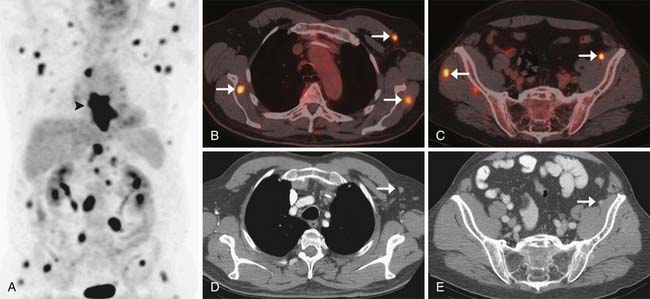
However, CT has a relatively poor specificity (82%)40 and cannot readily differentiate between indeterminate pulmonary nodules and metastatic disease. Furthermore, 7% to 20% of esophageal cancer metastases are occult or are difficult to prospectively diagnose by CT alone (Figure 14-9).19,32 A combination of CT and PET/CT is the optimal method for detection of metastatic disease from esophageal cancer.
Positron-Emission Tomography/Computed Tomography
PET/CT is the imaging modality with the highest sensitivity and specificity for the detection of distant metastases (estimated at 81% and 91% in one study of 75 patients with tissue confirmation); in up to 20% of patients, it has been reported to diagnose distant metastases that were not detectable by other means (see Figure 14-9).19 PET/CT improves baseline staging over CT and EUS and can obviate futile surgery.3 The increased sensitivity and specificity of PET/CT over other imaging modalities for the detection of distant metastases makes it an indispensable tool in the evaluation of patients with newly diagnosed esophageal cancer.
Common causes of false-positive PET findings include FDG uptake by foci of infection or inflammation, brown fat, physiologic bowel activity, skeletal muscle activity (particularly in the intercostal muscles and diaphragmatic crura due to breathing), and fractures. However, false-negative findings can also occur because not all AC is FDG-avid, particularly if necrotic or mucinous (Figure 14-10). For these reasons, PET/CT should always be interpreted in correlation with the findings from other investigations. A full discussion of these interpretative pitfalls is beyond the scope of this discussion but has been well covered in other publications.42–44 Because the detection of metastatic disease by PET/CT will have such a profound influence on subsequent clinical decision-making, consideration should always be given to biopsy of the abnormal lesions to confirm the presence of malignancy.
Treatment
Esophagectomy remains the only curative option for patients with esophageal cancer without metastatic disease. However, only 30% to 40% of patients with esophageal cancer have resectable disease at the time of presentation, and 3- to 5-year survival is low (6-37%) for patients treated with surgery alone, mainly owing to disease recurrence at distant sites.45–50 This has stimulated much interest in the use of perioperative chemotherapy and radiotherapy to improve the probability of a complete resection and to treat systemic metastases. For patients who are medically fit and for whom esophagectomy is planned, current NCCN clinical practice guidelines recommend combined modality treatment for any cancer with invasion beyond the submucosa or with nodal metastases.20 Neoadjuvant concurrent chemoradiation therapy represents the standard of care for combined modality treatment in most institutions, although there is evidence to support the use of neoadjuvant chemotherapy alone or postoperative adjuvant chemoradiation therapy.50–54
Neoadjuvant Chemoradiation Therapy
Concurrent neoadjuvant chemoradiation therapy combining platinum-based chemotherapy and 5-fluorouracil (5-FU) with radiation therapy to the primary tumor is the most commonly used regimen in most U.S. institutions. Meta-analyses have reported a significant survival benefit at 3 years compared with surgery alone (odds ratio 0.45-0.66), mainly owing to a higher chance of complete R0 resection after combined modality treatment and a reduced likelihood of local tumor recurrence51,52; however, there is no reduction in the incidence of recurrent distant metastases, which remain the most important cause of treatment failure.
The selection of patients for combination therapy relies on accurate TNM staging at baseline evaluation, combining the findings of EGD/EUS, CT, and PET/CT. Specifically, the detection of transmural tumor invasion on EUS examination and the identification of nodal metastases on CT or PET/CT will be indications for combined modality treatment.
Radiation Therapy
The goal of radiation therapy in the management of esophageal cancer, as part of either preoperative or definitive treatment with concurrent systemic therapy, is to improve locoregional disease control by delivering sufficient tumoricidal radiation dose while minimizing adjacent normal tissue radiation exposure. To achieve this end, it is critical for the radiation oncologist to be able to identify the areas with gross tumor involvement as well as those areas at risk for microscopic disease extension.55 To define the gross tumor volume (GTV), the radiation oncologist must rely on all available imaging studies, including barium swallow, CT scan of chest and abdomen with oral and intravenous contrast, EUS, and PET/CT scanning.56–58 The determination of microscopic disease extent, or clinical target volume (CTV), is more complex, given the intricate network of submucosal lymphatic drainage of the esophagus via which microscopic dissemination occurs. Based on pathologic analysis of prospectively collected surgical esophageal carcinoma specimens without neoadjuvant treatment, a 3-cm proximal and distal margin on the gross tumor in the esophagus is deemed ample, because microscopic disease was identified within 3 cm of the gross tumor in 94% of cases, except in gastroesophageal junction tumors, which extended to 5 cm distally.56,59 Similar analysis of the radial extent of esophageal cancer microscopic spread is not available; however, a 1-cm radial margin is accepted in current cooperative Radiation Therapy Oncology Group protocols. The CTV must also include the high-risk lymph node basins. The risk of nodal involvement is related to both primary tumor stage and location.56 For all patients, the periesophageal lymph nodes are included in the CTV, whereas coverage of the supraclavicular or celiac nodes is variable, depending on the site and size of the primary tumor. To account for daily setup uncertainty and internal target motion due to respiration, peristalsis, and cardiac contraction, an additional margin of 13 to 18 mm craniocaudally and 4 to 8 mm radially (planning target volume [PTV]) is needed to ensure that adequate radiation dose is delivered to the CTV.59
Most modern radiation treatment planning uses CT scanning of the chest and abdomen, preferably with oral and intravenous contrast, for delineation of the GTV, CTV, and PTV, as described previously. The advantage of CT-based planning is that not only is the target better visualized but also the adjacent critical normal tissue structures can be contoured and a more conformal treatment plan can be developed using three-dimensional (3D) reconstruction of the target and avoidance structures. From the 3D information, it is possible to estimate the radiation dose to be delivered to any given percent volume of the target or avoidance structure. In addition, the CT scans provide tissue density information, which allows for correction of the tissue heterogeneity and, thus, estimation of radiation absorption of the tissues delivered by a particular radiation plan. The most commonly accepted form of radiation treatment delivery for esophageal cancer in use today is based on 3D conformal treatment planning. Another approach that modulates the dose intensity of the radiation beam to minimize dose to the adjacent critical normal tissues while providing full tumor target coverage, aptly called intensity-modulated radiation therapy (IMRT), is still considered experimental in the treatment of esophageal cancer, given the uncertainty of target motion and potential for unanticipated over- and/or underdosing of both target and avoidance structures.59 The conventional dose of radiation for the treatment of esophageal cancer in both the neoadjuvant and the definitive setting with concurrent chemotherapy is 50 to 50.4 Gy delivered once daily 5 days per week in 1.8- to 2.0-Gy fractions using megavoltage external beam radiotherapy. Prospective studies investigating dose-intensification using external beam radiotherapy or brachytherapy boost techniques have not shown improved disease control (in the range of 40-55%) or overall survival (~30% alive at 3 yr).59–61 The use of dose fraction size of 2 Gy or less is supported by the observed increased postoperative mortality rate in patients treated with greater than 2 Gy per fraction versus the lower-fraction dose on meta-analysis of trimodality therapy versus surgery alone for esophageal cancer patients.52
Whereas esophageal cancer is a treatable disease, it remains rarely curable, as exemplified by its continued ranking among the top 10 causes of cancer deaths in U.S. males.2 The locoregional control rate, and consequently patient survival, remains poor, even with the best current practice. With continuing advances in imaging, treatment planning, and treatment delivery, radiation therapy, as part of a multidisciplinary approach to the management of patients with esophageal cancer, offers promise of greater success in outcomes in this patient population. The importance of improved target identification and localization using a wide array of radiologic techniques is paramount to achieving this success.
Surgery
Esophageal resection may be performed using a transhiatal approach, a combined transthoracic and abdominal approach, a three-field approach (incorporating cervical, thoracic, and abdominal incisions), or minimally invasive techniques. Opinions differ regarding optimal resection techniques, but there have been only a limited number of comparative trials with none showing clear superiority of one surgical approach over another.62 The choice of which technique to use often depends on individual factors such as surgeon preference, patient performance status, cancer stage, or tumor location. Despite the numerous options, the one critical principle of esophageal cancer surgery is to achieve a complete resection.
The inability to demonstrate a significant survival advantage between the various approaches has much to do with the fact that most patients recur systemically, thus rendering any local control issue irrelevant.63 What has been suggested is that the tumor location and number of nodes involved may be pertinent to the efficacy of the approach.64 There is general agreement, however, that an operation that fails locally is difficult to salvage and a complete resection of all tumor and involved nodes (R0 resection) is superior to an incomplete resection. Retrospective review of a high volume of patients has shown that the ability to achieve an R0 resection is an independent predictor of long-term survival.65
Proximally located (e.g., upper and mid esophageal) tumors often require a total esophagectomy with a cervical anastomosis because it is difficult to achieve negative margins with subtotal resections (e.g., Ivor Lewis esophagectomy). A three-field (McKeown) approach utilizes a right thoracotomy or thoracoscopy combined with laparotomy and left cervicotomy for esophageal resection and (typically) a two-field, abdominal and thoracic lymphadenectomy. Some surgeons have advocated the THE approach for tumors of the middle esophagus but we have been concerned by associated high locoregional recurrence rates.46 Because THE renders performance of an adequate thoracic and mediastinal lymphadenectomy difficult, we have not recommended this approach unless the tumor is at the earliest stage (Tis or T1a) at which there is minimal risk of lymph node involvement.
Although it is generally agreed that surgical resection is the primary form of therapy for local and locoregional disease, there is controversy over the value and extent of lymphadenectomy. Some believe that certain patients with affected lymph nodes can be successfully cured with an aggressive surgical approach that focuses on wide peritumoral soft tissue excision and extended lymphadenectomy using a transthoracic approach with en bloc lymphadenectomy.66 The downside to this approach is an increase in perioperative morbidity associated with the more extensive resection. Unfortunately, we are currently unable to predict which patients with locally advanced disease would benefit from immediate resection and extensive lymphadenectomy versus neoadjuvant chemoradiotherapy followed by resection. Furthermore, it is unclear whether more extensive dissection actually leads to improved survival or whether these superior results are a function of more accurate staging (stage-migration effect).
Therapeutic Response Evaluation
Surgical series have consistently shown that patients in whom there has been a good pathologic response to neoadjuvant treatment have an improved survival after complete R0 esophagectomy compared with those who do not demonstrate a pathologic response, of the order of 69% 3-year survival for responders and 27% for nonresponders.46,48,67–70 Surgical series show that 10% to 28% of patients show pathologic response. It has, therefore, been suggested that esophagectomy may not be appropriate for all patients and that, in patients who do not respond to neoadjuvant therapy, additional nonsurgical treatment may be indicated rather than proceeding to esophagectomy. The difficulty in identifying such patients and the limited available treatment options, however, remain as obstacles to the introduction of such a selection process into standard treatment protocols.
Conventional practice is to evaluate treatment response by repeat EGD/EUS and CT scanning after neoadjuvant therapy and, in patients without new metastatic disease, to proceed directly to surgery. However, EGD/EUS is not technically feasible owing to luminal stenosis in up to 6% of patients and is susceptible to overstaging residual disease.71–73 The sensitivity of EUS-directed FNA is also limited by the often extensive necrosis and fibrosis that occur after chemoradiation therapy. Similarly, CT cannot accurately evaluate the esophageal wall and is unable to differentiate esophageal wall thickening due to viable tumor from that due to treatment-related inflammation and fibrosis.
Because PET/CT shows functional rather than pure morphologic information, there has been much interest in its use for the evaluation of therapeutic response to neoadjuvant therapy and for the detection of residual viable malignancy. Multiple studies have shown that therapeutic response, as measured either by a percentage decrease in SUVmax or by a single posttreatment SUVmax (Figure 14-11), correlates with histologic response in resected specimens and may be used to select pathologic responders who are most likely to benefit from esophagectomy.39,72,74–80 Conversely, persistent FDG uptake in the tumor above a certain threshold has been shown to correlate with persistent viable macroscopic malignancy and a poor clinical outcome.39,74 However, this is not a consistent finding and definitions of a “metabolic” response vary considerably from institution to institution, most often being derived post hoc from analysis of receiver-operating curves.81,82 Furthermore, the not-insignificant overlap between perceived responders and nonresponders has made it difficult to incorporate posttreatment PET findings into clinical decision-making.
Interpretative difficulties that may be encountered when using PET/CT for the assessment of therapeutic response include FDG uptake secondary to biopsy or chemoradiotherapy-induced esophagitis or ulceration that may be confused with malignancy.72,81,83,84 Thus, most investigators recommend that PET/CT be performed no earlier than 3 weeks after the completion of chemoradiation therapy if it is to be used for local tumor response assessment. In addition, PET/CT cannot detect residual microscopic disease, and for this reason, esophagectomy (or definitive chemoradiation therapy) should still be offered to patients who have had an apparent complete metabolic response to neoadjuvant therapy.
Whole body PET/CT performed after neoadjuvant therapy is also useful for detecting new interval metastases that were not present at baseline evaluation. New metastases may occur in 8% to 17% of patients, most commonly with more locally advanced tumors at baseline (stages III-IVa).72,75,76,81 Of note, such metastases may occur even when there has been an apparent complete local metabolic response to therapy, and they may be occasionally occult on CT scanning or lie outside the usual range of restaging scans.81 Although the incidence of such new metastases is relatively rare, repeat PET/CT after neoadjuvant therapy and before surgery should be considered in patients who have locally advanced tumors at baseline and in those who may not be ideally fit for surgery.
In the context of therapeutic response assessment earlier during the course of neoadjuvant therapy (e.g., after 1-2 cycles of induction chemotherapy rather than at the completion of neoadjuvant therapy), there is convincing early evidence to suggest that PET/CT can predict the response of the primary tumor: in patients with FDG-avid tumors at baseline who undergo repeat PET/CT after one or two cycles of chemotherapy, a failure of metabolic activity (as measured by SUVmax) to decrease by 30% to 35% is predictive of a poor pathologic response and a worse 3-year survival after esophagectomy.76,85,86
The most important clinical trial to date that prospectively evaluated the utility of PET/CT for early therapeutic response assessment is the MUNICON (metabolic response evaluation for individualisation of neoadjuvant chemotherapy in oesophageal and oesophagogastric adenocarcinoma) phase II trial.86 In this study, 119 patients with potentially resectable distal esophageal tumors underwent two cycles of platinum and fluorouracil-based neoadjuvant chemotherapy followed by PET/CT scanning for response evaluation. Patients who showed a 35% decrease in SUVmax in the primary tumor were considered to represent responders and proceeded to 12 more cycles of additional chemotherapy followed by esophagectomy, whereas patients who did not show a decrease in FDG uptake did not receive any further neoadjuvant therapy, but rather proceeded directly to surgery. Of the 54 patients who showed a metabolic response after 2 weeks, 50 proceeded to esophagectomy. At a median follow-up of 2.3 years, median overall survival and event-free survival were significantly higher in metabolic responders than in nonresponders. Of clinical significance, all of the patients without an apparent metabolic response at 2 weeks had no histologic response on examination of the esophagectomy specimens, and their clinical outcome was worse. Also of note, of the 50 patients with an apparent metabolic response and who underwent esophagectomy, only 29 patients had evidence of a major or subtotal histologic response; the other 21 patients did not have a histologic response and their overall and event-free survival were not significantly different from those patients without a metabolic response.
Key Points Treatment and therapeutic response assessment
• In surgically fit patients, neoadjuvant chemoradiation therapy (followed by esophagectomy or definitive chemoradiation therapy) represents a standard of care for any tumor that has invaded beyond the submucosa or that is associated with nodal metastases.
• A survival benefit from esophagectomy is seen only in patients who have a pathologic response to neoadjuvant therapy.
• Preliminary evidence indicates that PET/CT can identify responders to neoadjuvant therapy when performed after two cycles of induction chemotherapy.
Surveillance
Following definitive treatment by esophagectomy or combined modality treatment, tumor recurrence most often occurs in the form of distant metastases rather than local recurrence and is associated with a poor prognosis.51,87 Local tumor recurrence may present months or years later and may manifest clinically as recurrent dysphagia or unexplained weight loss. On CT, local tumor recurrence should be suspected if there is new soft tissue thickening adjacent to the surgical anastomosis or new regional lymphadenopathy (Figure 14-12). Distant metastatic disease may occur in the peritoneum, nonregional lymph nodes or distant visceral sites (lungs, brain, bones, or muscle). PET/CT can provide additional information in up to 27% of patients compared with conventional imaging in this regard.88
Local practices regarding routine surveillance vary, but NCCN guidelines recommend a history and physical examination every 3 to 6 months for 3 years, with imaging reserved for instances of suspected tumor recurrence. More formal imaging surveillance may allow earlier institution of systemic or palliative therapy, but data to support improved clinical outcomes in this regard are lacking. Clinical thresholds for repeat imaging (CT or PET/CT) are likely to be governed by local resources, patient-specific factors, and remaining treatment options.88
Complications of Therapy
Surgical perioperative mortality has greatly reduced in recent decades from approximately 20% in the 1960s to rates of 5% to 7% in the late 1990s, mainly due to improved surgical technique and perioperative care.14,89,90 Early postoperative surgical complications relatively specific to esophagectomy include anastomotic leaks (occurring in ≤ 18% of patients), recurrent laryngeal nerve palsy, graft necrosis, and chylothorax.90
Anastomotic leaks occurring early in the postoperative period (2-3 days postoperatively) are usually due to technical complications whereas leaks occurring later (3-7 days) are most likely ischemic in etiology.14 Small asymptomatic leaks may be detected on routine postoperative fluoroscopy studies and are usually self-limited. Symptomatic leaks may also be treated conservatively but require drainage of extraluminal fluid collections, either by image guidance or by repeat surgery. Such patients are normally very unwell and unfit for upright fluoroscopic studies and are more appropriately evaluated by CT. In such instances, imaging should include an initial noncontrast study, followed by a contrast-enhanced scan immediately after the oral administration of dilute non-ionic contrast material by mouth or nasogastric tube. CT can identify the site of leak by the detection of adjacent extraluminal radiodense oral contrast medium and can also depict abnormal fluid collections that may require drainage. Small foci of extraluminal contrast extravasation should be differentiated from surgical clips (hence, the value of an initial noncontrast scan).
In cases of leaks that may be ischemic in etiology, endoscopic confirmation of the viability of the anastomosis and gastric remnant is important because immediate surgery will be necessary for resection of necrotic tissue and esophageal diversion.14
Later surgical complications include anastomotic strictures (seen in up to one third of patients), delayed gastric emptying, and gastroesophageal reflux.14
Key Points Complications of therapy
• Chemoradiation therapy can be complicated by tumor swelling and fistula formation; maintenance of nutritional needs is of critical importance in reducing treatment-related morbidity.
• Anastomotic leaks are the most common cause of postoperative morbidity after esophagectomy; CT can localize symptomatic leaks and aid in percutaneous draining of fluid collections.
New Therapies
New management strategies for esophageal cancer are likely to focus on optimizing patient selection for esophagectomy. At the moment, PET/CT appears to be the most promising tool for this purpose, with preliminary phase II data indicating that it can be used early in the course of induction chemotherapy to identify nonresponders who may not be suitable candidates for surgery.86 Unfortunately, limited therapeutic options are available to patients who have not responded to neoadjuvant therapy, with little evidence to suggest that more prolonged or intensive chemoradiation therapy is of any additional benefit. It may be that such patients will require a combination of definitive chemoradiation therapy and salvage esophagectomy, with surgery contributing a survival advantage that must be balanced against the significant morbidity and mortality of the treatment regimen.91,92
Systemic disease remains the most important cause of disease recurrence, even in patients treated with combined local and systemic therapy. Traditional chemotherapy has been based on combinations of platinum, 5-FU–based agents, taxanes, and irinotecan.93 Newer targeted therapies such as the epidermal growth factor receptor (EGFR) inhibitors (e.g., tyrosine kinase inhibitors such as erlotinib and Her-2/neu inhibitors such as trastuzumab) are based on preclinical evidence that blockade of EGFR may enhance radiation response. There is limited early phase I/II evidence to support that such agents, when used in combination with conventional chemotherapy agents, are well-tolerated and may have a beneficial effect.94–96 Vascular endothelial growth factor (VEGF) is overexpressed in esophageal cancer, and this has been shown to correlate with the depth of tumor invasion and the presence of nodal and distant metastases. Early phase II evidence has shown that the inhibition of angiogenesis by VEGF inhibitors (e.g., bevacizumab) has a favorable effect on response rate, time to disease progression, and overall survival.97 Complications of VEGF inhibition are related to its angiogenic effect and include mucosal perforation, hemorrhage, and thromboembolic events. Although targeted therapies are showing initial clinical promise in improving both local and systemic disease control, further study is required before they are adopted into the standard treatment armamentarium.
Conclusions
Despite the morbid nature of esophageal cancer and its grim mortality statistics, there have been significant recent improvements in imaging, surgical techniques, and chemoradiation therapy. Good clinical outcomes can still be achieved through optimization of all facets of management, which requires close interdisciplinary collaboration. From the imaging perspective, it is important for the radiologist to be familiar with the current staging system and the anatomy and patterns of spread of malignancy and to have an understanding of the influence of imaging on clinical decision-making. PET/CT is an increasingly important imaging modality for baseline staging and response assessment and a potentially useful tool for stratifying management according to prognosis. Specifically, the concept of early therapeutic response assessment by PET/CT to optimize individual patient management is likely to attract greater attention in future clinical trials.
1. . American Cancer Society. Esophageal cancer. Available at http://www.cancer.org/downloads/PRO/EsophagealCancer.pdf
2. Jemal A., Siegel R., Ward E., et al. Cancer statistics, 2009. CA Cancer J Clin. 2009(59):225-249.
3. van Westreenen H.L., Heeren P.A., van Dullemen H.M., et al. Positron emission tomography with F-18-fluorodeoxyglucose in a combined staging strategy of esophageal cancer prevents unnecessary surgical explorations. J Gastrointest Surg. 2005;9:54-61.
4. Holmes R.S., Vaughan T.L. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2-9.
5. Blot W.J., McLaughlin J.K. The changing epidemiology of esophageal cancer. Semin Oncol. 1999;26:2-8.
6. Brown L.M., Devesa S.S. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin North Am. 2002;11:235-256.
7. Shaheen N., Ransohoff D.F. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: clinical applications. JAMA. 2002;287:1982-1986.
8. Tran G.D., Sun X.D., Abnet C.C., et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456-463.
9. Farrow D.C., Vaughan T.L., Hansten P.D., et al. Use of aspirin and other nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:97-102.
10. Lagergren J. Adenocarcinoma of oesophagus: what exactly is the size of the problem and who is at risk? Gut. 2005;54:i1-i5.
11. Siewert J.R., Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Semin Radiat Oncol. 2006;17:38-44.
12. Siewert J.R., Feith M., Stein H.J. Biologic and clinical variations of adenocarcinoma at the esophgago-gastric junction: Relevance of a topographic-anatomic subclassification. J Surg Oncol. 2005;90:139-146.
13. Siewert J.R., Stein H.J., Feith M., et al. Histological tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg. 2001;234:360-367.
14. Lerut T., Coosemans W., Decker G., et al. Surgical techniques. J Surg Oncol. 2005;92:218-229.
15. Esophagus and esophagogastric junction. AJCC Cancer Staging Handbook, 7th ed., New York: Springer; 2010:103-115.
16. Siewert J.R., Stein H.J. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457-1459.
17. Kuge K., Murakami G., Mizobuchi S., et al. Submucosal territory of the direct lymphatic drainage system to the thoracic duct in the human esophagus. J Thorac Cardiovasc Surg. 2003;125:1343-1349.
18. Rice T.W., Rusch V.W., Apperson-Hansen C., et al. Worldwide esophageal cancer collaboration. Dis Esophagus. 2009;22:1-8.
19. Weber W.A., Ott K. Imaging of esophageal and gastric cancer. Semin Oncol. 2004;31:530-541.
20. National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
21. Hofstetter W., Correa A.M., Bekele N., et al. Proposed modification of nodal status in AJCC esophageal cancer staging system. Ann Thorac Surg. 2007;84:365-373. discussion 374–375
22. Altorki N.K., Zhou X.K., Stiles B., et al. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg. 2008;248:221-226.
23. Peyre C.G., Hagen J.A., DeMeester S.R., et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549-556.
24. Peyre C.G., Hagen J.A., DeMeester S.R., et al. Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg. 2008;248:979-985.
25. Rizk N., Venkatraman E., Park B., et al. The prognostic importance of the number of involved lymph nodes in esophageal cancer: implications for revisions of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg. 2006;132:1374-1381.
26. Greenstein A.J., Litle V.R., Swanson S.J., et al. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer. 2008;112:1239-1246.
27. DeMeester T.R. Esophageal carcinoma: current controversies. Semin Surg Oncol. 1997;13:217-233.
28. Tsurumaru M., Kajiyama Y., Udagawa H., Akiyama H. Outcomes of extended lymph node dissection for squamous cell carcinoma of the thoracic esophagus. Ann Thorac Cardiovasc Surg. 2001;7:325-329.
29. Lee P.C., Port J.L., Paul S., et al. Predictors of long-term survival after resection of esophageal carcinoma with nonregional nodal metastases. Ann Thorac Surg. 2009;88:186-192. discussion 192–193
30. Clark G.W., Peters J.H., Ireland A.P., et al. Nodal metastasis and sites of recurrence after en bloc esophagectomy for adenocarcinoma. Ann Thorac Surg. 1994;58:646-653. discussion 653–654
31. Quint L.E., Hepburn L.M., Francis I.R., et al. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer. 1995;76:1120-1125.
32. Bruzzi J.F., Truong M.T., Macapinlac H., et al. Integrated CT-PET imaging of esophageal cancer: unexpected and unusual distribution of distant organ metastases. Curr Probl Diagn Radiol. 2007;36:21-29.
33. Yau K.K., Siu W.T., Cheung H.Y., et al. Immediate preoperative laparoscopic staging for squamous cell carcinoma of the esophagus. Surg Endosc. 2006;20:307-310.
34. Desai R.K., Tagliabue J.R., Wegryn S.A., Einstein D.M. CT evaluation of wall thickening in the alimentary tract. Radiographics. 1991;11:771-783. discussion 784
35. Rasanen J.V., Sihvo E.I., Knuuti M.J., et al. Prospective analysis of accuracy of positron emission tomography, computed tomography, and endoscopic ultrasonography in staging of adenocarcinoma of the esophagus and the esophagogastric junction. Ann Surg Oncol. 2003;10:954-960.
36. Kato H., Nakajima M., Sohda M., et al. The clinical application of (18)F-fluorodeoxyglucose positron emission tomography to predict survival in patients with operable esophageal cancer. Cancer. 2009;115:3196-3203.
37. Hyun S.H., Choi J.Y., Shim Y.M., et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010;17:115-122.
38. Levine E.A., Farmer M.R., Clark P., et al. Predictive value of 18-fluoro-deoxy-glucose-positron emission tomography (18F-FDG-PET) in the identification of responders to chemoradiation therapy for the treatment of locally advanced esophageal cancer. Ann Surg. 2006;243:472-478.
39. Swisher S.G., Maish M., Erasmus J.J., et al. Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg. 2004;78:1152-1160. discussion 1160
40. Lowe V.J., Booya F., Fletcher J.G., et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol. 2005;7:422-430.
41. van Westreenen H.L., Westerterp M., Bossuyt P.M., et al. Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol. 2004;22:3805-3812.
42. Gorospe L., Raman S., Echeveste J., et al. Whole-body PET/CT: spectrum of physiological variants, artifacts and interpretative pitfalls in cancer patients. Nucl Med Commun. 2005;26:671-687.
43. Truong M.T., Pan T., Erasmus J.J. Pitfalls in integrated CT-PET of the thorax: implications in oncologic imaging. J Thorac Imaging. 2006;21:111-122.
44. Truong M.T., Erasmus J.J., Munden R.F., et al. Focal FDG uptake in mediastinal brown fat mimicking malignancy: a potential pitfall resolved on PET/CT. AJR Am J Roentgenol. 2004;183:1127-1132.
45. Walsh T.N., Noonan N., Hollywood D., et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462-467.
46. Urba S.G., Orringer M.B., Turrisi A., et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305-313.
47. Nygaard K., Hagen S., Hansen H.S., et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg. 1992;16:1104-1109. discussion 1110
48. Le Prise E., Etienne P.L., Meunier B., et al. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer. 1994;73:1779-1784.
49. Kelsen D.P., Ginsberg R., Pajak T.F., et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979-1984.
50. Cunningham D., Allum W.H., Stenning S.P., et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20.
51. Urschel J.D., Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185:538-543.
52. Fiorica F., Di Bona D., Schepis F., et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004;53:925-930.
53. Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727-1733.
54. Macdonald J.S., Smalley S.R., Benedetti J., et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730.
55. Jones D. ICRU Report 50: Prescribing, recording and reporting photon beam therapy. Med Phys. 1994;21:833-834.
56. Gao X., Qiao X., Wu F., et al. Pathological analysis for clinical target volume margin for radiotherapy in patients with esophageal and gastroesophageal junction carcinoma. Int J Radiat Oncol Biol Phys. 2007;67:389-396.
57. Konski A., Doss M., Milestone B., et al. The integration of 18-fluoro-deoxy-glucose positron emission tomography and endoscopic ultrasound in the treatment-planning process for esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1123-1128.
58. Vesprini D., Ung Y., Kamra J., et al. The addition of 18-fluorodeoxyglucose positron emission tomography (FDG-PET) to CT based radiotherapy planning of carcinoma of the esophagus decreases both the intra- and interobserver variability of GTV delineation. Int J Radiat Oncol Biol Phys. 2006;66:S299-300.
59. Hazard L., Yang G.P., McAleer M., et al. Principles and techniques for radiation therapy for esophageal and gastroesophageal junction cancers. J Natl Compr Canc Netw. 2008;6:870-878.
60. Minsky B.D., Neuberg D., Kelsen D.P., et al. Final report of Intergroup Trial 0122 (ECOG PE-289, TROG 90-12): phase II trial of neoadjuvant chemotherapy plus concurrent chemotherapy and high-dose radiation for squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 1999;43:517-523.
61. Gaspar L., Winter K., Kocha W., et al. A phase I/II study of external beam radiation, brachytherapy, and concurrent chemotherapy for patients with localized carcinoma of the esophagus (Radiation Therapy Oncology Group Study 9207): final report. Cancer. 2000;88:988-995.
62. Omloo J.M., Lagarde S.M., Hulscher J.B., et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg. 2007;246:992-1000. discussion 1000–1001
63. Orringer M.B., Marshall B., Chang A.C., et al. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg. 2007;246:363-372. discussion 372–374
64. Nigro J.J., Hagen J.A., DeMeester T.R., et al. Prevalence and location of nodal metastases in distal esophageal adenocarcinoma confined to the wall: implications for therapy. J Thorac Cardiovasc Surg. 1999;117:16-23. discussion 23–25
65. Hofstetter W., Swisher S.G., Correa A.M., et al. Treatment outcomes of resected esophageal cancer. Ann Surg. 2002;236:376-384. discussion 384–385
66. Hagen J.A., DeMeester S.R., Peters J.H., et al. Curative resection for esophageal adenocarcinoma: analysis of 100 en bloc esophagectomies. Ann Surg. 2001;234:520-530. discussion 530–531
67. Burmeister B.H., Smithers B.M., Gebski V., et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659-668.
68. Swisher S.G., Ajani J.A., Komaki R., et al. Long-term outcome of phase II trial evaluating chemotherapy, chemoradiotherapy, and surgery for locoregionally advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 2003;57:120-127.
69. Berger A.C., Farma J., Scott W.J., et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330-4337.
70. Urba S.G., Orringer M.B., Ianettonni M., et al. Concurrent cisplatin, paclitaxel, and radiotherapy as preoperative treatment for patients with locoregional esophageal carcinoma. Cancer. 2003;98:2177-2183.
71. Westerterp M., van Westreenen H.L., Reitsma J.B., et al. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy—systematic review. Radiology. 2005;236:841-851.
72. Cerfolio R.J., Bryant A.S., Ohja B., et al. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg. 2005;129:1232-1241.
73. Zuccaro G.Jr., Rice T.W., Goldblum J., et al. Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol. 1999;94:906-912.
74. Swisher S.G., Erasmus J., Maish M., et al. 2-Fluoro-2-deoxy-d-glucose positron emission tomography imaging is predictive of pathologic response and survival after preoperative chemoradiation in patients with esophageal carcinoma. Cancer. 2004;101:1776-1785.
75. Flamen P., Van Cutsem E., Lerut A., et al. Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol. 2002;13:361-368.
76. Weber W.A., Ott K., Becker K., et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19:3058-3065.
77. Kato H., Kuwano H., Nakajima M., et al. Usefulness of positron emission tomography for assessing the response of neoadjuvant chemoradiotherapy in patients with esophageal cancer. Am J Surg. 2002;184:279-283.
78. Brucher B.L., Weber W., Bauer M., et al. Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Surg. 2001;233:300-309.
79. Downey R.J., Akhurst T., Ilson D., et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol. 2003;21:428-432.
80. Wieder H.A., Beer A.J., Lordick F., et al. Comparison of changes in tumor metabolic activity and tumor size during chemotherapy of adenocarcinomas of the esophagogastric junction. J Nucl Med. 2005;46:2029-2034.
81. Bruzzi J.F., Swisher S.G., Truong M.T., et al. Detection of interval distant metastases: clinical utility of integrated CT-PET imaging in patients with esophageal carcinoma after neoadjuvant therapy. Cancer. 2007;109:125-134.
82. Vallbohmer D., Holscher A.H., Dietlein M., et al. [18F]-Fluorodeoxyglucose-positron emission tomography for the assessment of histopathologic response and prognosis after completion of neoadjuvant chemoradiation in esophageal cancer. Ann Surg. 2009;250:888-894.
83. Brink I., Hentschel M., Bley T.A., et al. Effects of neoadjuvant radio-chemotherapy on 18F-FDG-PET in esophageal carcinoma. Eur J Surg Oncol. 2004;30:544-550.
84. Erasmus J., Munden R., Truong M.T., et al. Pre-operative chemoradiation-induced ulceration in patients with esophageal cancer: a confounding factor in tumor response assessment in integrated CT-PET imaging. J Thorac Oncol. 2006;1:478-486.
85. Wieder H.A., Brucher B.L., Zimmermann F., et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22:900-908.
86. Lordick F., Ott K., Krause B.J., et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8:797-805.
87. Korst R.J., Rusch V.W., Venkatraman E., et al. Proposed revision of the staging classification for esophageal cancer. J Thorac Cardiovasc Surg. 1998;115:660-669. discussion 669–670
88. Flamen P., Lerut A., Van Cutsem E., et al. The utility of positron emission tomography for the diagnosis and staging of recurrent esophageal cancer. J Thorac Cardiovasc Surg. 2000;120:1085-1092.
89. Akiyama H., Tsurumaru M., Udagawa H., Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg. 1994;220:364-372. discussion 372–373
90. Gockel I., Exner C., Junginger T. Morbidity and mortality after esophagectomy for esophageal carcinoma: a risk analysis. World J Surg Oncol. 2005;3:37.
91. Stahl M., Wilke H., Stuschke M., et al. Clinical response to induction chemotherapy predicts local control and long-term survival in multimodal treatment of patients with locally advanced esophageal cancer. J Cancer Res Clin Oncol. 2005;131:67-72.
92. Swisher S.G., Wynn P., Putnam J.B., et al. Salvage esophagectomy for recurrent tumors after definitive chemotherapy and radiotherapy. J Thorac Cardiovasc Surg. 2002;123:175-183.
93. Ajani J.A., Walsh G., Komaki R., et al. Preoperative induction of CPT-11 and cisplatin chemotherapy followed by chemoradiotherapy in patients with locoregional carcinoma of the esophagus or gastroesophageal junction. Cancer. 2004;100:2347-2354.
94. Dragovich T., McCoy S., Fenoglio-Preiser C.M., et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 2006;24:4922-4927.
95. Safran H., Dipetrillo T., Akerman P., et al. Phase I/II study of trastuzumab, paclitaxel, cisplatin and radiation for locally advanced, HER2 overexpressing, esophageal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2007;67:405-409.
96. Pande A.U., Iyer R.V., Rani A., et al. Epidermal growth factor receptor-directed therapy in esophageal cancer. Oncology. 2007;73:281-289.
97. Shah M.A., Ramanathan R.K., Ilson D.H., et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201-5206.