Chapter 15 Erythrocyte and leucocyte cytochemistry
Erythrocyte cytochemistry
Siderocytes and Sideroblasts
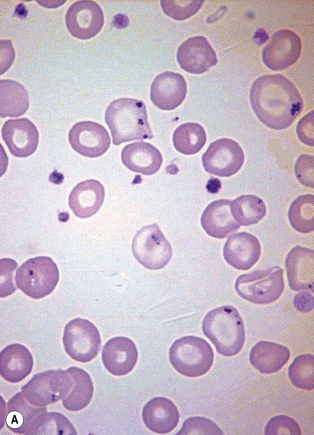
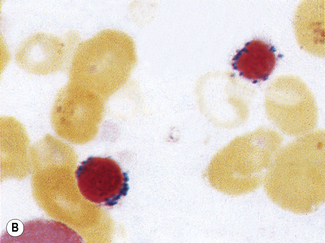
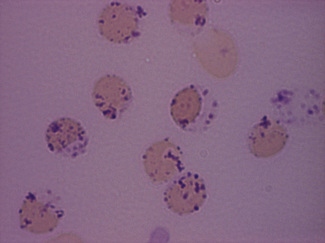
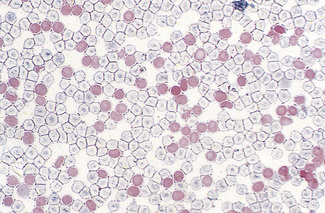
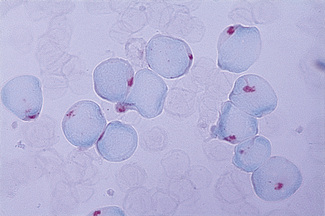
Siderocytes are red cells containing granules of non-haem iron. They were originally described by Grüneberg1 in small numbers in the blood of normal rat, mouse and human embryos and in large numbers in mice with a congenital anaemia. The granules are formed of a water-insoluble complex of ferric iron, lipid, protein and carbohydrate. This siderotic material (or haemosiderin) reacts with potassium ferrocyanide to form a blue compound, ferriferrocyanide; this reaction is the basis of a positive Prussian-blue (Perls’) reaction. The material also stains with Romanowsky dyes and then appears as basophilic granules, which have been referred to as ‘Pappenheimer bodies’ (Fig. 15.1).2 By contrast, ferritin, which is a water-soluble non-haem compound of iron with the protein apoferritin, is not detectable by Perls’ reaction. Ferritin is normally present in all cells in the body, whereas, in health, haemosiderin is mainly found in macrophages in the bone marrow, liver (Kupffer cells) and spleen. When the body is overloaded with iron, as in haemochromatosis or transfusional haemosiderosis, excess iron is also found in other tissues.
In health, siderotic granules can normally be seen, in preparations stained by Perls’ reaction, in the cytoplasm of many of the erythroblasts of human bone marrow and in marrow reticulocytes.3 However, they are not normally seen in human peripheral blood red cells. After splenectomy, siderocytes can always be found in the peripheral blood, often in large numbers. The reason for this is probably because reticulocytes, after delivery from the marrow, are normally sequestered for a time in the spleen and there they complete haem synthesis, utilizing, for this purpose, the iron stored in their cytoplasm within the siderotic granules. After splenectomy, this stage of reticulocyte maturation has to take place in the bloodstream, with the result that, even in an otherwise healthy person, a small percentage of siderocytes can then be found in the peripheral blood. The spleen is also probably able to remove large siderotic granules – as may be found in disease – from red cells by a process of pitting,4 and in its absence such granules persist in the red cells throughout their lifespan.
Method of Staining Siderotic Granules
Leave the slides in the solution for about 10 min at about 20°C. Wash well in running tap water for 20 min, rinse thoroughly in distilled water and then counterstain with 1 g/l aqueous neutral red or eosin for 10–15 s. Care must be taken to avoid contamination by iron that may have been present on the slides or in staining dishes. Prepare the glassware by soaking in 3 mol/l HCl before washing (see p. 623). For quality control, a positive bone marrow film should always be stained together with the test films.
Prussian-blue staining can be applied to films that have previously been stained by Romanowsky dyes, even after years of storage. It is advisable to let the films stand in methanol overnight to remove most of the Romanowsky stain. The film should be checked before carrying out Perls’ reaction to ensure that there is no residual blue staining that could obscure Prussian-blue staining. Sundberg and Bromann described a technique whereby films were stained first by a Romanowsky dye (Wright’s stain) and then overstained by the acid-ferrocyanide method.5 This can give beautiful pictures, but the small blue-stained iron-containing granules tend to be masked in young erythroblasts by the general basophilia of the cell cytoplasm. Hayhoe and Quaglino described a method for combined periodic acid–Schiff (PAS) and iron staining.6 This may be helpful in the investigation of abnormal erythropoiesis in which the erythroblasts give a positive PAS reaction (see p. 343). A rapid method has been described for demonstrating siderotic granules by staining with 1% bromochlorphenol blue for 1 min.7 Iron-containing granules stain dark purple.
Significance of siderocytes
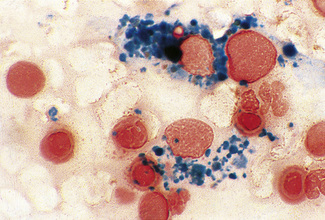
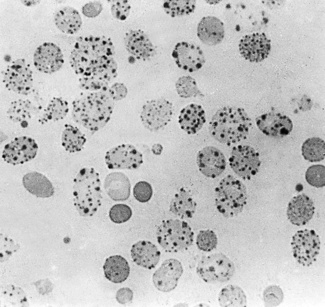
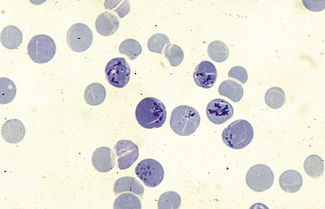
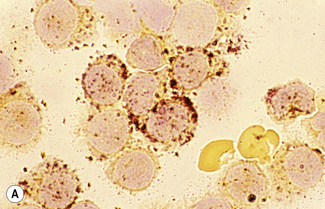
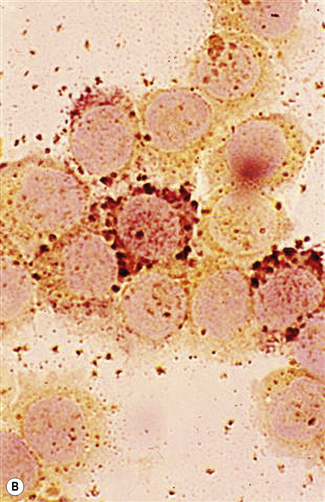
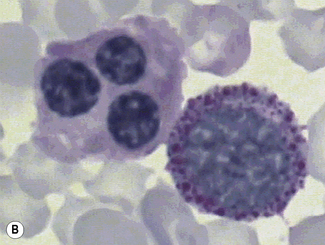
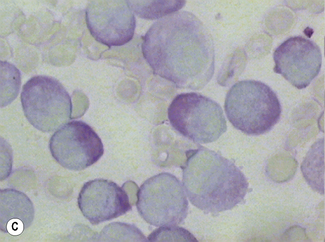
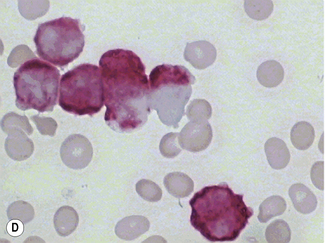
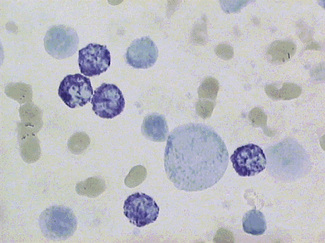
Siderocytes contain one or two (rarely many) small, unevenly distributed iron-containing granules that stain a Prussian-blue colour. There are normally a few very small scattered siderotic granules in about 40% of late erythroblasts.3 They stain faintly and may be difficult to see by light microscopy. The percentage of erythroblasts recognizable as sideroblasts is increased in haemolytic anaemias and megaloblastic anaemias and in haemochromatosis and haemosiderosis, in proportion to the degree of saturation of transferrin (i.e. to the amount of iron available). A disproportionate increase in the percentage of erythroblasts that are sideroblasts occurs when the synthesis of haemoglobin is impaired, in which case the siderotic granules are both more numerous and larger than normal (Fig. 15.2). When there is a defect in haem synthesis, the granules are deposited in mitochondria and frequently appear to be arranged in a collar around the nucleus (Fig. 15.3) giving the ‘ring sideroblasts’ characteristic of sideroblastic anaemias. In contrast, the distribution of the granules within the cell tends to be mainly normal in conditions in which globin synthesis alone is affected (e.g. in thalassaemia) or when there is iron overload.
There are several types of sideroblastic anaemia. These include the congenital (hereditary) type, pyridoxine (vitamin B6) deficiency (rarely), sideroblastic anaemia caused by B6 antagonists (e.g. drugs used in antituberculosis therapy) and secondary sideroblastic anaemia in alcoholism and lead poisoning. The presence of ring sideroblasts is a defining feature of refractory anaemia with ring sideroblasts8 and refractory cytopenia with multilineage dysplasia and ring sideroblasts, two of the World Health Organization (WHO) categories of myelodysplastic syndrome (MDS). They may also occur in other categories of MDS. Ring sideroblasts are not uncommon in other haematological neoplasms, including primary myelofibrosis and acute myeloid leukaemia (AML), particularly erythroleukaemia and the WHO categories of therapy-related AML and AML with multilineage myelodysplasia. Ring sideroblasts have been defined as erythroblasts with at least five siderotic granules surrounding at least one-third of the nucleus.9,10
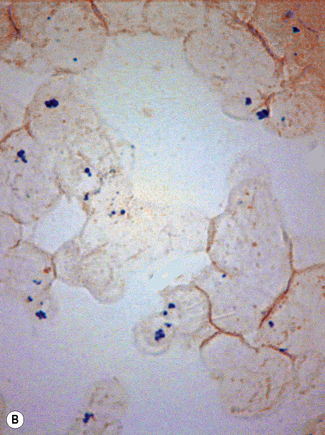
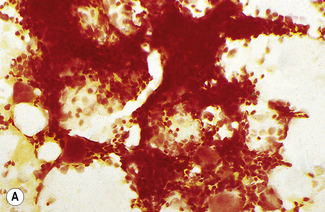
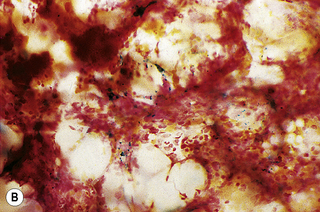
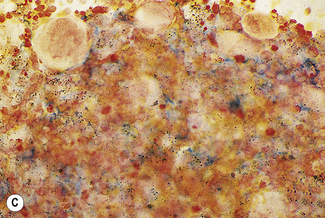
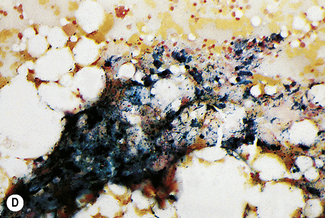
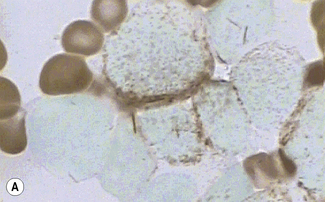
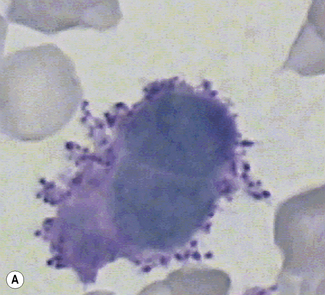
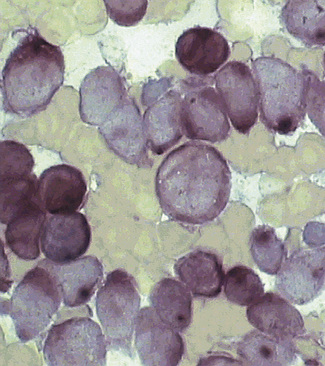
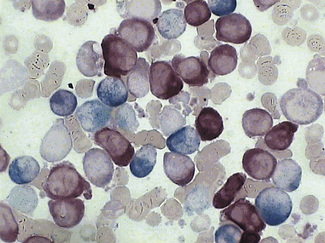
In addition to the siderotic granules within erythroblasts, haemosiderin can normally be seen in marrow films as accumulations of small granules, lying free or in macrophages in marrow fragments.11 The amount of haemosiderin will be markedly increased in patients with increased iron stores, whereas haemosiderin is absent in iron deficiency anaemia (Fig. 15.4). In practice, staining to demonstrate iron stores in marrow fragments and siderotic granules in erythroblasts is a simple and valuable diagnostic procedure and should be applied as a routine to marrow films from the initial bone marrow aspirate of each patient.
In chronic infections and in other examples of anaemia of chronic disease, the iron stores may be increased, with much siderotic material in macrophages but little or none visible in erythroblasts. Markedly excessive iron in macrophages is also a feature of thalassaemia intermedia and major and some dyserythropoietic anaemias. Conversely, absence of iron is diagnostic of iron deficiency or iron depletion (the latter term indicating the state in which storage iron is absent but anaemia is not yet evident). One study has shown that to establish the absence of stainable iron, at least seven particles must be examined, if necessary using more than one slide for this purpose.12 There is no cytochemical method of demonstrating ferritin; methods of assay are described in Chapter 9.
Haemoglobin Derivatives
Heinz Bodies in Red Cells
Heinz, in 1890, was the first to describe in detail inclusions in red cells developing as the result of the action of acetylphenylhydrazine on the blood.13 It is now known that Heinz bodies can be produced by the action on red cells of a wide range of aromatic nitro- and amino-compounds, as well as by inorganic oxidizing agents such as potassium chlorate. They also occur when one or other of the globin chains of haemoglobin is unstable. In man, the finding of Heinz bodies is a sign of either chemical poisoning, drug toxicity, glucose-6-phosphate dehydrogenase (G6PD) deficiency or the presence of an unstable haemoglobin (e.g. Hb Köln). When of chemical or drug origin, Heinz bodies are likely to be visible in red cells only if the patient has been splenectomized previously or when large doses of the chemical or drug have been taken. When they are due to an unstable haemoglobin, they are rarely visible in freshly withdrawn red cells except after splenectomy. They may nevertheless develop in vitro in the blood of patients who have not been splenectomized if the specimen is incubated for 24–48 h.14 Heinz bodies are a late sign of oxidative damage and represent an end-product of the degradation of haemoglobin. Reviews dealing with Heinz bodies include those by Jacob15 and by White.16
Demonstration of Heinz Bodies
Unstained preparations
The degradation product of an unstable haemoglobin (e.g. Hb Köln) exhibits green fluorescence when excited by blue light at 370 nm in a fluorescence microscope.17
Stained preparations
Dissolve approximately 0.5 g of methyl violet in 100 ml of 9 g/l NaCl and filter. Add 1 volume of blood (in any anticoagulant) to 4 volumes of the methyl violet solution and allow the suspension to stand for about 10 min at room temperature. Then prepare films and allow them to dry or view the suspension of cells between slide and coverglass. The Heinz bodies stain an intense purple (Fig. 15.5).
Heinz bodies also stain with other basic dyes. Brilliant green stains them well and none of the stain is taken up by the remainder of the red cell.18 Rhodanile blue (5 g/l solution in 10 g/l NaCl) stains them rapidly19 (i.e. within 2 min), at which time reticulocytes are only weakly stained. Compared with methyl violet, Heinz bodies stain less intensely with brilliant cresyl blue or New methylene blue. Nevertheless, they may be readily seen as pale blue bodies in a well-stained reticulocyte preparation, if the preparation is not counterstained.
Demonstration of Haemoglobin H Inclusions
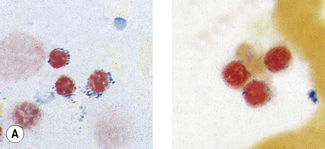
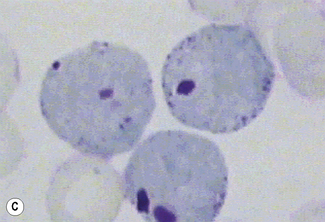
Patients with α thalassaemia, who form haemoglobin H (β4), have red cells in which multiple blue-green spherical inclusions develop on exposure to brilliant cresyl blue or New methylene blue as in reticulocyte preparations20 (Fig. 15.6). This is mainly a feature of haemoglobin H disease, but small numbers of similar cells may be seen in α thalassaemia trait, particularly, but not only, in α° thalassaemia heterozygosity.
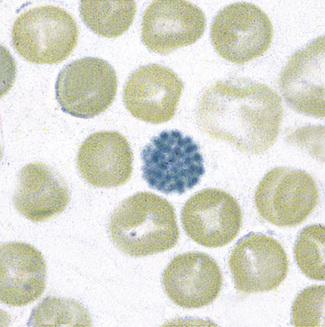
Figure 15.6 Denaturation of haemoglobin H by brilliant cresyl blue. The round bodies consist of precipitated Hb H.
Method
Mix together in a small tube, as for staining reticulocytes (see p. 33), equal volumes of fresh blood or blood collected into ethylenediaminetetra-acetic acid (EDTA) and 10 g/l brilliant cresyl blue or 20 g/l New methylene blue in iso-osmotic phosphate buffer pH 7.4. Leave the preparation at 37°C for 3 h and make films at intervals during this time. Allow the films to dry and examine them without counter-staining. Haemoglobin H precipitates as multiple pale-staining greenish-blue, almost spherical, bodies of varying size (Fig. 15.7), which can be clearly differentiated from the darker-staining reticulofilamentous material of reticulocytes (Fig. 15.8).
It should be noted that a haemoglobin H preparation is not recommended when precise diagnosis of the type of α thalassaemia trait is required (e.g. in antenatal diagnosis). DNA analysis is then indicated (see p. 147).
Carboxyhaemoglobin and Methaemoglobin
Carboxyhaemoglobin- and methaemoglobin-containing cells can be demonstrated cytochemically. These methods are described by Kleihauer and Betke.21 They have little practical value in modern practice.
Fetal Haemoglobin
An acid-elution cytochemical method that was introduced by Kleihauer et al.22 is a sensitive procedure to identify individual cells containing haemoglobin F even when few are present. Their detection in the maternal circulation has provided valuable information on the pathogenesis of haemolytic disease of the newborn.
The identification of cells containing haemoglobin F depends on the fact that they resist acid elution to a greater extent than do normal cells; thus, in the technique described in the following, they appear as isolated, darkly stained cells among a background of palely staining ghost cells. The occasional cells that stain to an intermediate degree are less easy to evaluate; some may be reticulocytes because these also resist acid elution to some extent. The following method, in which elution is carried out at pH 1.5, is recommended.23
Reagents
Method
Prepare fresh air-dried films. Immediately after drying, fix the films for 5 min in 80% ethanol in a Coplin jar. Then rinse the slides rapidly in water and stand them vertically on blotting paper for about 10 min to dry. Next, place the slides for 20 s in a Coplin jar containing the elution solution. Then wash the slides thoroughly in water and finally place them in the counterstain for 2 min. Rinse in tap water and allow them to dry in the air. Fetal cells stain red and adult ghost cells stain pale pink (Fig. 15.9). Films prepared (a) from a mixture of cord blood and adult blood and (b) from normal adult blood should be stained alongside the test films as positive and negative controls, respectively.
A number of modifications of the Kleihauer method have been proposed. In one, New methylene blue is incorporated in the buffer solution, the reaction time is prolonged and buffer is used for washing the films.24 The advantage of this technique is that reticulocytes stain blue, whereas cells containing haemoglobin F stain pink.
An immunofluorescent staining method has been developed based on the use of a specific antibody against haemoglobin F, which does not react with haemoglobin A.25 By using a double-labelling procedure with rhodamine-labelled antibody against γ globin and a fluorescein-labelled antibody against β globin, it is possible to detect the presence of haemoglobin F and haemoglobin A in the same cell.26
Haemoglobin S and Other Haemoglobin Variants
Immunodiffusion with specific antibodies has been used for the identification of haemoglobin S, haemoglobin A2 and haemoglobin F in red cells.27,28 An alternative method is by detection of cells after labelling the cells with fluorescein isothiocyanate (FITC).27 By a double-labelling method similar to that described earlier, it is possible to identify haemoglobin S as well as another haemoglobin in individual cells.
Leucocyte cytochemistry
Leucocyte cytochemistry encompasses the techniques used to identify diagnostically useful enzymes or other substances in the cytoplasm of haemopoietic cells. These techniques are particularly useful for the characterization of immature cells in AML and the identification of maturation abnormalities in the myelodysplastic syndromes and myeloproliferative neoplasms. There are many variations in the staining techniques, as discussed in the recommendations of an Expert Panel of the International Committee (now Council) for Standardization in Haematology.29,30 Detailed reference works discussing the theoretical and practical aspects of cytochemistry are available.31 The use of cytochemistry to characterize lymphoproliferative disorders has been largely superseded by immunological techniques (see Chapter 16). The results of cytochemical tests should always be interpreted in relation to Romanowsky stains and immunological techniques. Control blood or marrow slides should always be stained in parallel to ensure the quality of the staining. The principal uses of cytochemistry are as follows:
Myeloperoxidase
Myeloperoxidase (MPO) is located in the primary and secondary granules of neutrophils and their precursors, in eosinophil granules and in the azurophilic granules of monocytes. The MPO in eosinophil granules is cyanide resistant, whereas that in neutrophils and monocytes is cyanide sensitive. MPO splits H2O2 and in the presence of a chromogenic electron donor forms an insoluble reaction product. Various benzidine substitutes have been used, of which 3,3′-diaminobenzidine (DAB) is the preferred chromogen.33,34 The reaction product is stable, insoluble and non-diffusible. Staining can be enhanced by immersing the slides in copper sulphate or nitrate, but this is generally not required in normal diagnostic practice. Alternative non-benzidine-based techniques use 4-chloro-1-naphthol (4CN)35 or 3-amino-9-ethylcarbazole.36 The former gives very crisp staining but is soluble in some mounting media and immersion oil; the latter shows some diffusibility and does not stain as strongly as DAB.
Method with 3,3′-Diaminobenzidine
Method
Results and interpretation
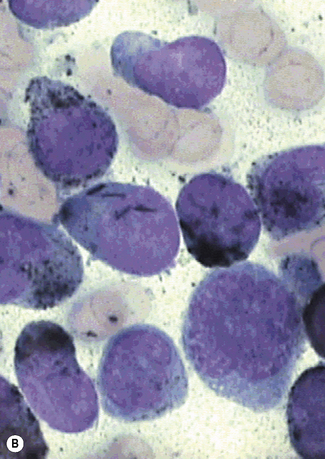
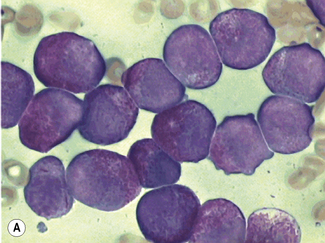
The reaction product is brown and granular (Fig. 15.10A). Red cells and erythroid precursors show diffuse brown cytoplasmic staining. The most primitive myeloblasts are negative, with granular positivity appearing progressively as they mature toward the promyelocyte stage. The positivity may be localized to the Golgi region. Promyelocytes and myelocytes are the most strongly staining cells in the granulocyte series, with positive (primary) granules packing the cytoplasm. Metamyelocytes and neutrophils have progressively fewer positive (secondary) granules. Eosinophil granules stain strongly and the large specific eosinophil granules are easily distinguished from neutrophil granules. Eosinophil granule peroxidase is distinct biochemically and immunologically from neutrophil peroxidase. Monoblasts and monocytes may be negative or positive. When positive, the granules are smaller than in neutrophils and diffusely scattered throughout the cytoplasm. MPO activity is present in basophil granules but is not demonstrable in mature basophils by the DAB reaction described earlier.
Sudan Black B
Sudan Black B (SBB) is a lipophilic dye that binds irreversibly to an undefined granule component in granulocytes, eosinophils and some monocytes. It cannot be extracted from the stained granules by organic dye solvents and gives comparable information to that of MPO staining.37 The currently used staining solution is essentially that described by Sheehan and Storey.38
Reagents
Method
Results and Interpretation
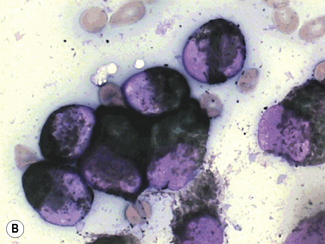
The reaction product is black and granular. The results are essentially similar to those seen with MPO staining, both in normal and leukaemic cells (Fig. 15.10B). MPO-negative neutrophils are also SBB negative. The only notable difference is in eosinophil granules, which have a clear core when stained with SBB. Rare cases (1–2%) of acute lymphoblastic leukaemia (ALL) show non-granular smudgy positivity not seen with MPO staining.39 Basophils are generally not positive but may show bright red/purple metachromatic staining of the granules.
Neutrophil Alkaline Phosphatase
Alkaline phosphatase activity is found predominantly in mature neutrophils, with some activity in metamyelocytes. Although demonstrated as a granular reaction product in the cytoplasm, enzyme activity is associated with a poorly characterized intracytoplasmic membranous component distinct from primary or secondary granules.40 Other leucocytes are generally negative, but rare cases of lymphoid malignancies show cytochemically demonstrable activity.41 Bone marrow macrophages are positive. Early methods of demonstrating alkaline phosphatase relied on the use of glycerophosphate or other phosphomonoesters as the substrate at alkaline pH, with a final black reaction product of lead sulphide.42 Azo-dye techniques are simpler, giving equally good results. These methods use substituted naphthols as the substrate and it is the liberated naphthol rather than phosphate that is used to combine with the azo-dye to give the final reaction product.43–45
Reagents
Method
Technical considerations
N,N-dimethylformamide may dissolve some types of plastic; therefore a glass tube should be used to dissolve the substrate. Blood films should be made soon after blood collection, preferably within 30 min because neutrophil alkaline phosphatase (NAP) activity decreases rapidly in EDTA-anticoagulated blood. Once spread, the blood film should be stained within 6 h. A control film with a predictably high score, e.g. from a patient with reactive neutrophilia or from a pregnant woman, should be processed together with the patient film. The technical aspects of blood film preparation and the effects of fixation on NAP activity are discussed by Kaplow.46
Results and Interpretation
The reaction product is blue and granular. The intensity of reaction product in neutrophils varies from negative to strongly positive, with coarse granules filling the cytoplasm and overlying the nucleus (Fig. 15.11). An overall score is obtained by assessing the stain intensity in 100 consecutive neutrophils, with each neutrophil scored on a scale of 1–4 as follows:
The overall possible score will range between 0 and 400 (assessed on 100 neutrophils). Reported normal ranges show some variations, owing possibly in part to variations in scoring criteria and methodology: 13–160 (mean 61);46 14–100 (mean 46);47 37–98 (mean 68);48 11–134 (mean 48).49 A normal range should therefore be established in each laboratory.
In normal individuals, it is rare to find any neutrophils with a score of 3 and a score of 4 should not be present. There is some physiological variation in NAP scores. Newborn babies, children and pregnant women have high scores and premenopausal women have, on average, scores one-third higher than those of men.40 In pathological states, the most significant diagnostic use of the NAP score is in CML. In the chronic phase of the disease, the score is almost invariably low, usually zero. Transient increases may occur with inter-current infection. In myeloid blast transformation or accelerated phase, the score rises. Low scores are also commonly found in paroxysmal nocturnal haemoglobinuria (PNH) and the very rare condition of hereditary hypophosphatasia. There are many causes of a raised NAP score, notably in the neutrophilia of infection, polycythaemia vera, leukaemoid reactions and Hodgkin lymphoma. In aplastic anaemia, the NAP score is high, but it falls if PNH supervenes. With the greater use of cytogenetic and molecular genetic techniques to confirm the diagnosis of CML, the NAP score is rarely needed.
Acid Phosphatase Reaction, including Tartrate-Resistant Acid Phosphatase Reaction
Cytochemically demonstrable acid phosphatase is ubiquitous in haemopoietic cells. The staining intensity of different cell types is somewhat variable according to the method used. Its main diagnostic use is in the diagnosis of T-cell ALL and hairy cell leukaemia.50,51 These diseases are more reliably diagnosed and characterized by immunophenotyping when this is available (see Chapter 16). The unmodified acid phosphatase stain is now largely redundant but the tartrate-resistant acid phosphatase stain is still useful for confirmation of the diagnosis of hairy cell leukaemia when immunophenotyping is not available. The pararosaniline method given in the following section, modified from Goldberg and Barka,52 is recommended for demonstrating positivity in T lymphoid cells. Use of Fast Garnet GBC as coupler may be preferred for the demonstration of tartrate-resistant acid phosphatase activity.29,51
Reagents
Method
Results and Interpretation
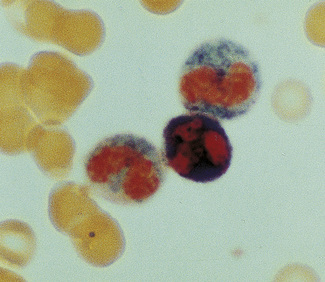
The reaction product is red with a mixture of granular and diffuse positivity (Fig. 15.12). In T cells, acid phosphatase is an early differentiation feature. Almost all acute and chronic T-lineage leukaemias show strong activity. In T-lineage ALL, the activity is usually highly localized (polar). Granulocytes are strongly positive. Monocytes, eosinophils and platelets show variable positivity. In the bone marrow, macrophages, plasma cells and megakaryocytes are strongly positive.
In hairy cell leukaemia the majority of leukaemic cells react equally positively in the presence and absence of tartaric acid (Fig. 15.13).
Periodic Acid–Schiff Reaction
Periodic acid specifically oxidizes 1–2 glycol groups to produce stable dialdehydes. These dialdehydes give a red reaction product when exposed to Schiff’s reagent (leucobasic fuchsin). Positive reactions occur with carbohydrates, principally glycogen, but also monosaccharides, polysaccharides, glycoproteins, mucoproteins, phosphorylated sugars, inositol derivatives and cerebrosides.53 Glycogen can be distinguished from other positively reacting substances by its sensitivity to diastase digestion. In haemopoietic cells, the main source of positive reactions is glycogen.
Reagents
Method
Results and Interpretation
The reaction product is red, with intensity ranging from pink to bright red (Figs 15.14, 15.15). Cytoplasmic positivity may be diffuse or granular. Granulocyte precursors show diffuse weak positivity, with neutrophils showing intense confluent granular positivity. Eosinophil granules are negative, with diffuse cytoplasmic positivity. Basophils may be negative but often show large irregular blocks of positive material not related to the granules. Monocytes and their precursors show variable diffuse positivity with superimposed fine granules, often at the periphery of the cytoplasm. Normal erythroid precursors and red cells are negative. Megakaryocytes and platelets show variable, usually intense, diffuse positivity with superimposed fine granules, coarse granules and large blocks. Granular positivity with negative background cytoplasm is found in 10–40% of peripheral lymphocytes, with no detectable differences between T and B cells.54,55 Lymphoblasts show variable PAS-positive cytoplasmic granules or blocks on a clear background; it is block positivity on a clear background that is most characteristic of lymphoblasts rather than myeloblasts. When immunophenotyping is available, the PAS reaction is redundant for the diagnosis of ALL. It can still be useful in AML and MDS to identify abnormal erythroblasts and dysplastic megakaryocytes and to demonstrate the cytoplasmic blush that helps to confirm a diagnosis of acute promyelocytic leukaemia (Fig. 15.15).
Esterases
Leucocyte esterases are a group of enzymes that hydrolyse acyl or chloroacyl esters of α-naphthol or naphthol AS. Li et al.56 identified nine esterase isoenzymes using polyacrylamide gel electrophoresis of leucocyte extracts from normal and pathological cells. The gels were stained in parallel with cell smears. The isoenzymes fell into two groups: bands 1, 2, 7, 8 and 9 corresponded to the ‘specific’ esterase of neutrophils, staining specifically with naphthol AS-D chloroacetate esterase (chloroacetate esterase, CAE), whereas bands 3, 4, 5 and 6 corresponded to ‘non-specific’ esterase (NSE), staining with α-naphthyl acetate esterase (ANAE) and α-naphthyl butyrate esterase (butyrate esterase, ANBE). Band 4 was best demonstrated by ANBE and band 5 by ANAE. The NSEs are inhibited by sodium fluoride (NaF). Naphthol AS acetate and naphthol AS-D acetate react with both specific and non-specific esterases, but only the reaction with the NSEs is inhibited by NaF. The methods using parallel slides with and without NaF are not generally used anymore because it is usually more informative to perform a combination of chloroacetate esterase and one of the ‘non-specific’ esterase stains on a single slide. The combined methods have the advantage of demonstrating pathological double staining of individual cells. All the esterase stains can be performed using a variety of coupling reagents, each of which gives a different coloured reaction product. The methods outlined as follows have been chosen for their simplicity and reliability.
Naphthol AS-D Chloroacetate Esterase
Reagents
Results and interpretation
The reaction product is bright red (Fig. 15.15D). It is confined to cells of the neutrophil series and mast cells. Cytoplasmic CAE activity appears as myeloblasts mature to promyelocytes. Positivity in myeloblasts is rare, but promyelocytes and myelocytes stain strongly, with the reaction product filling the cytoplasm. More mature cells stain strongly but less intensely. It is therefore useful as a marker of cytoplasmic maturation in myeloid leukaemias. In acute promyelocytic leukaemia, the cells show heavy cytoplasmic staining. The characteristic multiple Auer rods stain positively, often with a hollow core. It is rare to see CAE-positive Auer rods in other forms of AML except in cases with the t(8;21) translocation.57
α-Naphthyl Butyrate Esterase
Reagents
Method
Technical considerations
The reaction product is soluble in immersion oil and synthetic mounting media. If slides are to be looked at repeatedly, they should be mounted in an aqueous mounting medium (e.g. Apathy’s gum-arabic mountant or glycerin/gelatin). There may be batch-to-batch variation of the Fast Garnet GBC. Staining can be controlled by removing the control slide from the incubation medium after 20 min and examining it wet under a low-power (e.g. ×20) objective, returning it to the incubation medium while still wet. When the monocytes show as dark brown, staining is complete. Hexazotized pararosaniline is an alternative coupling reagent, which gives an insoluble brown reaction product and is suitable for mounting in synthetic mounting media.56
Results and interpretation
The reaction product is brown and granular (Fig. 15.16). The majority of monocytes (>80%) stain strongly, the remainder showing some weak staining. Negative monocytes are rare. Neutrophils, eosinophils, basophils and platelets are negative. B lymphocytes are negative and T lymphocytes are more variably stained. In the bone marrow, monocytes, monocyte precursors and macrophages stain strongly. α-Naphthyl butyrate is more specific for identifying a monocytic component in AML than α-naphthyl acetate (see later).
α-Naphthyl Acetate Esterase
Reagents
Sequential Combined Esterase Stain Using ANAE and CAE
Method
Results and interpretation
The ANAE gives a brown reaction product and the CAE gives a granular bright blue product (Fig. 15.17). Staining patterns are identical to those seen with the two stains used separately. The double-staining technique avoids the need to compare results from separate slides and reveals aberrant staining patterns. In myelomonocytic leukaemias, cells staining with both esterases may be present. In MDS and AML with dysplastic granulocytes, double staining of individual cells may be present. This may be helpful when a diagnosis of MDS is not otherwise certain, but the same abnormal pattern may be seen in non-clonal dysplastic states such as megaloblastic anaemia.
Single Incubation Double Esterase (Naphthol AS-D Chloroacetate and α-Naphthyl Butyrate)58
Results and interpretation
The CAE reaction product is bright blue (granulocytes); the ANBE product is dark green/brown (monocytes). ANBE does not stain megakaryocytes or T cells as strongly as α-naphthyl acetate. Lam et al. suggest the use of hexazotized pararosaniline as coupling reagent in a single incubation combined esterase, which gives contrasting bright red and brown reaction products.59
In AML, the stain is useful for identifying monocytic and granulocytic components.
Toluidine Blue Stain
Results and Interpretation
The granules of basophils and mast cells stain a bright red/purple and are discrete and distinct (Fig. 15.18). Nuclei stain blue and cells with abundant RNA may show a blue tint to the cytoplasm. Although toluidine blue is said to be specific for these granules, with >10 min incubation, the primary granules of promyelocytes are stained red/purple. However, these are smaller and finer than the mast cell or basophil granules and easily distinguished.
Cytochemical Reactions and Leukaemia Classification
Myelodysplastic Syndromes and Acute Myeloid Leukaemia
MDS is an acquired clonal preleukaemic bone marrow disorder characterized largely by a cellular or hypercellular marrow, peripheral cytopenias and variable morphological abnormalities of the haemopoietic cells. The classification system proposed by the French-American-British (FAB) cooperative group in 198260 was widely used for many years but is now being superseded by the WHO classification (see Chapter 23). A Perls’ reaction for haemosiderin is essential for the demonstration of ring sideroblasts. Other cytochemical evidence of dysplasia includes double staining of cells with chloroacetate and ANAE, the presence of SBB- or MPO-negative neutrophils and the presence of Auer rods (identified with SBB and MPO).
Acute Lymphoblastic Leukaemia
The modern diagnosis and classification of ALL is by cytology, followed by immunophenotyping (see Chapter 16). If immunophenotyping is not available, cytochemistry remains important. It should be noted that, on Romanowsky staining, lymphoblasts may rarely contain fine azurophilic granules. However, Auer rods are never seen and MPO and CAE are negative, whether or not fine granules are present. Occasionally granules give a weak reaction with SBB. Although not lineage specific, the pattern of any PAS positivity may be helpful.31 In ALL, 95% of cases show positive blocks or granules of bright red PAS-positive material. This may be present in very few blasts (<1%) or the majority. The critical difference from granular or block positivity in other leukaemic cells is the glass-clear background cytoplasm in lymphoblasts. Myeloblasts, monoblasts, leukaemic erythroblasts and megakaryoblasts all show some degree of diffuse cytoplasmic positivity and occasionally block positivity is seen. Acid phosphatase staining is more likely to give focal positivity in T-lineage than B-lineage acute leukaemias, but the difference is not clear enough to be of diagnostic certainty and focal positivity is sometimes also seen in the erythroblasts of erythroleukaemia. Esterase staining is generally unhelpful; some T-cell cases show focal positivity with ANAE, but this is not specific.
Chronic Lymphoproliferative Disorders
Chronic lymphoproliferative disorders are now characterized by immunophenotyping (Chapter 16). The reactions for acid hydrolases (acid phosphatase, ANAE, β-glucuronidase and β-glucosaminidase) show focal positivity in most T-cell disorders but are negative in B-cell disorders. The tartrate-resistant acid phosphatase reaction for hairy cell leukaemia is the only cytochemical stain that is sufficiently specific to still be regarded as diagnostically useful (in the absence of immunophenotyping).
1 Grüneberg H. Siderocytes: a new kind of erythrocyte. Nature (London). 1941;148:114-115.
2 Pappenheimer A.M., Thompson K.P., Parker D.D., et al. Anaemia associated with unidentified erythrocytic inclusions after splenectomy. Q J Med. 1945;14:75-100.
3 Kaplan E., Zuelzer W.W., Mouriquand C. Sideroblasts: a study of stainable nonhemoglobin iron in marrow normoblasts. Blood. 1954;9:203-213.
4 Crosby W.H. Siderocytes and the spleen. Blood. 1957;12:165-170.
5 Sundberg R.D., Bromann H. The application of the Prussian blue stain to previously stained films of blood and bone marrow. Blood. 1955;10:160-166.
6 Hayhoe F.G., Quaglino D. Refractory sideroblastic anaemia and erythraemic myelosis: possible relationship and cytochemical observations. Br J Haematol. 1960;6:381-387.
7 Kass L., Eickholt M.M. Rapid detection of ringed sideroblasts with bromchlorphenol blue. Am J Clin Pathol. 1978;70:738-740.
8 Bennett J.M. Classification of the myelodysplastic syndromes. Clin Haematol. 1986;15:909-923.
9 Mufti G.J., Bennett J.M., Goasgen J., et al. Diagnosis and classification of MDS: International Working Group on Morphology of MDS (IWGM-MDS) consensus proposals for the definition and enumeration of myeloblasts and ring sideroblasts. Haematologica. 2008;93:1712-1717.
10 Hasserjian R.P., Gatterman N., Bennett J.M., et al. Refractory anaemia with ring sideroblasts. In: Swerdlow S.H., Campo E., Harris N.L., et al, editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008:96-97.
11 Rath C.E., Finch C.A. Sternal marrow hemosiderin: a method for the determination of available iron stores in man. J Lab Clin Med. 1948;33:81-86.
12 Hughes D.A., Stuart-Smith S.E., Bain B.J. How should stainable iron in bone marrow films be assessed? J Clin Pathol. 2004;57:1038-1040.
13 Heinz R. Morphologische Veränderungen der rother Blutkörperchen durche Gifte. Virchows Archives. 1890;122:112.
14 Dacie J.V., Grimes A.J., Meisler A., et al. Hereditary Heinz-body anaemia: a report of studies on five patients with mild anaemia. Br J Haematol. 1964;10:388-402.
15 Jacob H.S. Mechanisms of Heinz body formation and attachment to red cell membrane. Semin Hematol. 1970;7:341-354.
16 White J.M. The unstable haemoglobins. Br Med Bull. 1976;32:219-222.
17 Eisinger J., Flores J., Tyson J.A., et al. Fluorescent cytoplasm and Heinz bodies of hemoglobin Köln erythrocytes: evidence for intracellular heme catabolism. Blood. 1985;65:886-893.
18 Schwab M.L., Lewis A.E. An improved stain for Heinz bodies. Am J Clin Pathol. 1969;51:673.
19 Simpson C.F., Carlisle J.W., Mallard L. Rhodanile blue: a rapid and selective stain for Heinz bodies. Stain Technol. 1970;45:221-223.
20 Gouttas A., Fessas Ph, Tsevrenis H., et al. Description d’une nouvelle variété d’anémie hémolytique congénitale. Sang. 1955;26:911-919.
21 Kleihauer E., Betke K. Elution procedure for the demonstration of methaemoglobin in red cells of human blood smears. Nature (London). 1963;199:1196-1197.
22 Kleihauer E., Braun H., Betke K. Demonstration von fetalem Hämoglobin in den Erythrocyten eines Blutausstrichs. Klin Wochenschr. 1957;35:637-638.
23 Nierhaus K., Betke K. Eine vereinfachte Modifikation der säuren Elution für die cytologische Darstellung von fetalem Hämoglobin. Klin Wochenschr. 1968;46:47.
24 Clayton E.M., Felhaus W.D., Phythyon J.M. The demonstration of fetal erythrocytes in the presence of adult red blood cells. Am J Clin Pathol. 1963;40:487-490.
25 Tomoda Y. Demonstration of foetal erythrocytes by immunofluorescent staining. Nature (London). 1964;202:910-911.
26 Thorpe S.J., Huehns E.G. A new approach for the antenatal diagnosis of β-thalassaemia: a double labelling immunofluorescence microscopy technique. Br J Haematol. 1983;53:103-112.
27 Headings V., Bhattacharya S., Shukla S., et al. Identification of specific hemoglobins within individual erythrocytes. Blood. 1975;45:263-271.
28 Papayannopoulou Th., McGuire T.C., Lim G., et al. Identification of haemoglobin S in red cells and normoblasts using fluorescent anti-Hb antibodies. Br J Haematol. 1976;34:25-31.
29 Shibata A., Bennett J.M., Castoldi G.L., et al. Recommended methods for cytological procedures in haematology. Clin Lab Haematol. 1985;7:55-74.
30 International Council for Standardization in Haematology. Procedures for the classification of acute leukaemias. Leuk Lymphoma. 1993;11:37-48.
31 Hayhoe F.G., Quaglino D. Haematological Cytochemistry, 2nd ed. Churchill Livingstone, Edinburgh, 1988.
32 Borowitz M.J., Béné M.-C., Harris N.L., et al. Acute leukaemia of ambiguous lineage. In: Swerdlow S.H., Campo E., Harris N.L., et al, editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008:150-155.
33 Graham R.C., Karnovsky M.J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966;14:291-302.
34 Novikoff A.B., Goldfischer S. Visualization of peroxisomes (microbodies and mitochondria with diaminobenzidine). J Histochem Cytochem. 1969;17:675-680.
35 Elias J.M. A rapid sensitive myeloperoxidase stain using 4-chloro-1-naphthol. Am J Clin Pathol. 1980;73:797-799.
36 Graham R.C., Lundholm U., Karnovsky M.J. Cytochemical demonstration of peroxidase activity with 3-amino-9-ethylcarbazole. J Histochem Cytochem. 1965;13:150-152.
37 Lillie R.D., Burtner H.J. Stable sudanophilia of human neutrophil leucocytes in relation to peroxidase and oxidase. J Histochem Cytochem. 1953;1:8-26.
38 Sheehan H.L., Storey G.W. An improved method of staining leucocyte granules with Sudan Black B. J Pathol Bacteriol. 1974;49:580.
39 Stass S.A., Pui C.-H., Melvin S., et al. Sudan black B positive acute lymphoblastic leukaemia. Br J Haematol. 1984;57:413-421.
40 Rosner F., Lee S.L. Endocrine relationships of leukocyte alkaline phosphatase. Blood. 1965;5:356-369.
41 Poppema S., Elema J.D., Halie M.R. Alkaline phosphatase positive lymphomas: a morphologic, immunologic and enzyme histochemical study. Cancer. 1981;47:1303-1312.
42 Gomori G. Microscopic Histochemistry: Principles and Practice. Chicago, IL: University of Chicago Press; 1952.
43 Kaplow L.S. A histochemical procedure for localizing and evaluating leucocyte alkaline phosphatase activity in smears of blood and marrow. Blood. 1955;10:1023-1029.
44 Kaplow L.S. Cytochemistry of leukocyte alkaline phosphatase: use of complex naphthol AS phosphates in azo-dye coupling technics. Am J Clin Pathol. 1963;39:439-449.
45 Rustin G.J., Wilson P.D., Peters T.J. Studies on the subcellular localization of human neutrophil alkaline phosphatase. J Cell Sci. 1979;36:401-412.
46 Kaplow L.S. Leukocyte alkaline phosphatase cytochemistry: applications and methods. Ann N Y Acad Sci. 1968;155:911.
47 Hayhoe F.G.J., Quaglino D. Cytochemical demonstration and measurement of leucocyte alkaline phosphatase in normal and pathological states by modified azo-dye coupling technique. Br J Haematol. 1958;4:375-389.
48 Rutenberg A.B., Rosales C.L., Bennett J.M. An important histochemical method for the demonstration of leukocyte alkaline phosphatase activity: clinical applications. J Lab Clin Med. 1965;65:698-705.
49 Bendix-Hansen K., Helleberg-Rasmussen I. I. Evaluation of neutrophil alkaline phosphatase: untreated myeloid leukaemia, lymphoid leukaemia and normal humans. Scand J Haematol. 1985;34:264-269.
50 Yam L.T., Li C.Y., Lam K.W. Tartrate-resistant acid phosphatase isoenzyme in the reticulum cells of leukemic reticuloendotheliosis. N Engl J Med. 1971;284:357.
51 Li C.Y., Yam L.T., Lam K.W. Acid phosphatase isoenzyme in human leucocytes in normal and pathologic conditions. J Histochem Cytochem. 1970;18:473-481.
52 Goldberg A.F., Barka T. Acid phosphatase activity in human blood cells. Nature. 1962;195:297.
53 Hotchkiss R.D. A microchemical reaction resulting in the staining of polysaccharide structures in fixed tissue preparations. Arch Biochem. 1948;16:131-141.
54 Higgy K.E., Burns G.F., Hayhoe F.G.J. Discrimination of B, T and null lymphocytes by esterase cytochemistry. Scand J Haematol. 1977;18:437-438.
55 Quaglino D., Hayhoe F.G.J. Observations on the periodic acid–Schiff reaction in lymphoproliferative diseases. J Pathol Bacteriol. 1959;78:521-532.
56 Li C.Y., Yam L.T., Lam K.W. Esterases in human leucocytes. J Histochem Cytochem. 1973;21:1.
57 Swirsky D.M., Li Y.S., Matthews J.G., et al. Translocation in acute granulocytic leukaemia: cytological, cytochemical and clinical features. Br J Haematol. 1983;56:199-213.
58 Swirsky D.M. Single incubation double esterase cytochemical reaction using a single coupling reagent. J Clin Pathol. 1984;37:1187-1190.
59 Lam K.W., Li C.Y., Yam L.T. Simultaneous demonstration of non-specific esterase and chloro-acetate esterase in human blood cells. Stain Technol. 1985;60:169-172.
60 Bennett J.M., Catovsky D., Daniel M.-T., et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189-199.