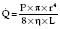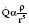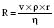Chapter 12 Equipment
Anaesthetic Equipment
Gases
Cylinders
Gas cylinders are manufactured from chromium molybdenum steel as a seamless tube.
Colours. Conform to International Standards Organization (Table 12.1).
| Body | Shoulder | |
|---|---|---|
| Oxygen | Black | White |
| Nitrous oxide | French blue | French blue |
| Air | Grey | Black/white |
| Carbon dioxide | Grey | Grey |
| Helium | Brown | Brown |
| Cyclopropane | Orange | Orange |
| Entonox | French blue | French blue/white |
| Nitric oxide | Pink | Pink |
Anaesthetic machine safety features
Checking Anaesthetic Equipment 3
Association of Anaesthetists of Great Britain and Ireland 2004
Vaporizers
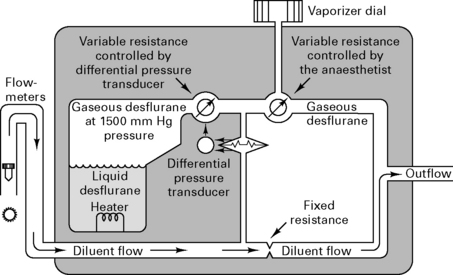
Because desflurane has such a high saturated vapour pressure (88 kPa), standard vaporizers are unsuitable for its storage and delivery. Use of a conventional vaporizer would require very high fresh gas flows to achieve 1 MAC equivalent of desflurane. The low boiling point of desflurane (24°C) also makes a conventional vaporizer unsuitable. The Tech 6 desflurane vaporizer (Fig. 12.1) uses a servo-controlled electronic system which heats the vaporizer chamber to a constant 39°C (higher than the boiling point) at a pressure of 1500 mmHg. The desflurane is delivered into the fresh gas flow (FGF) at equal pressures through a pressure-regulating valve which increases desflurane delivery as the FGF increases. Unlike conventional ventilators, use of the Tech 6 vaporizer at high altitude requires manual adjustment to increase desflurane concentrations.
Ventilators
Andrews J.J., Johnston R.V. The new Tech 6 desflurane vaporizer. Anesth Analg. 1993;76:1338-1341.
Association of Anaesthetists of Great Britain and Ireland. Checking anaesthetic equipment, vol 3. AAGBI, London, 2004.
Cartwright D.P., Freeman M.F. Vaporisers. Anaesthesia. 1999;54:519-520.
Davey A., Moyle J.T., Ward C.S., editors. Ward’s anaesthetic equipment, ed 3, London: WB Saunders, 1992.
Gardner M.C., Adams A.P. Anaesthetic vaporizers: design and function. Curr Anaesth Crit Care. 1996;7:315-321.
Howell R.S.C. Medical gases (1) Manufacture and uses. Kaufman L., editor. Anaesthesia review, vol 7. Churchill Livingstone, Edinburgh, 1989;87-104.
Howell R.S.C. Medical gases (2) Distribution. Kaufman L., editor. Anaesthesia review, vol 8. Churchill Livingstone, Edinburgh, 1990;195-210.
Breathing Circuits
Mapleson’s classification of breathing systems
| Mapleson classification | Spontaneous ventilation | IPPV |
|---|---|---|
| A | 70 mL/kg per min | 2.5 × MV |
| B | 2.5 × MV | 2.5 × MV |
| C | 2.5 × MV | 2.5 × MV |
| D | 2.5 × MV | 70 mL/kg per min |
| E – Adult | 2.5 × MV | 2.5 × MV |
| E < 20 kg | 3 (1000 + 100 mL/kg) | 1000 + 100 mL/kg (minimum flow = 3 L) |
| or | 3 (5 × frequency × kg) | 5 × frequency × kg |
| Lack (coaxial A) | 50 mL/kg per min | |
| Bain (coaxial D) | 70 mL/kg per min |
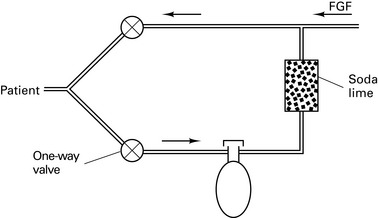
Circle systems
Re-breathing was introduced by Snow in 1850. Circle systems were pioneered by Sword in 1926.
Anaesthetic circuits
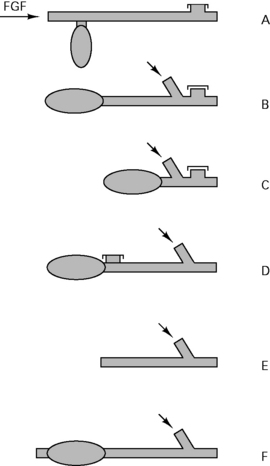
Circle layout
There are 64 different possible combinations of layout. The most efficient has been found to be that shown in Figure 12.4.
Principles of closed circuit volatile administration
Direct administration of volatiles into the circuit was pioneered by Lowe.
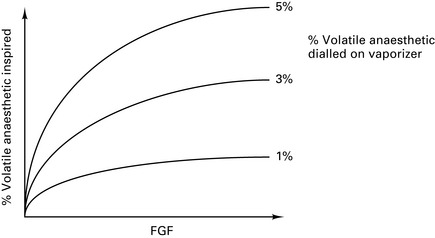
Vaporizer outside circle (VOC)
At high FGFs, the volatile concentration inspired by the patient will approach that leaving the vaporizer (Fig. 12.5).
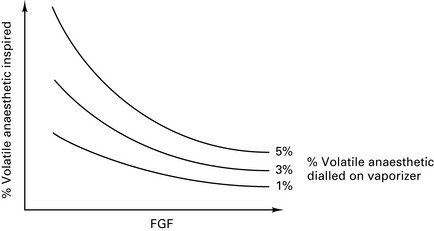
Vaporizer inside circle (VIC)
At low FGFs, the volatile concentration inspired by the patient will be much higher than that leaving the vaporizer (Fig. 12.6).
Baxter P.J., Garton K., Kharasch E.D. Mechanistic aspects of carbon monoxide formation from volatile anesthetics. Anesthesiology. 1998;89:929-941.
Committee on Safety of Medicines. Circle systems and volatile agents. Curr Prob. 1997;23:7.
Jones M.J. Breathing systems and vaporizers. In: Nimmo W.S., Rowbotham D.J., Smith G., editors. Anaesthesia. ed 2. Oxford: Blackwell Scientific; 1994:486-505.
Nunn G. Low-flow anaesthesia. Contin Educ Anaesth, Crit Care Pain. 2008;8:1-4.
Schober P., Loer S.A. Closed system anaesthesia – historical aspects and recent developments. Eur J Anaesthesiol. 2006;23:914-920.
Monitoring
Recommendations for Standards of Monitoring During Anaesthesia and Recovery
Association of Anaesthetists of Great Britain and Ireland 2007 (4E)
Inspired oxygen concentration
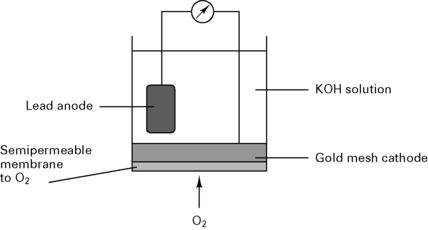
Fuel cell
In a fuel cell (Fig. 12.7), the current is proportional to the partial pressure of oxygen:
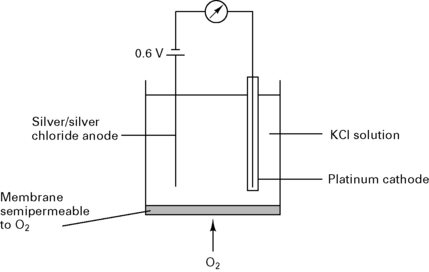
Clarke electrode
In a Clarke electrode (Fig. 12.8) the current is proportional to the partial pressure of oxygen. This type of electrode is usually used in blood gas machines.
Pulse oximeter
Mechanism
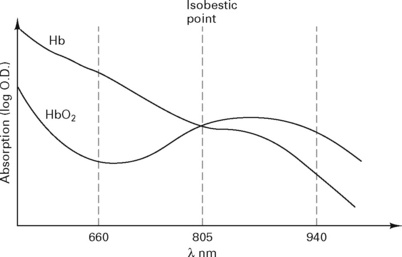
Light is transmitted through tissue at two alternating wavelengths:
Beer’s law, used to calculate the absorption (Fig. 12.9), states:
It = intensity of reflected light
Io= intensity of incident light
d = distance light is transmitted through liquid
e = extinction coefficient of solute.
The pulse oximeter measures the variation in absorption caused by the arterial pulse, cancelling out the effects of other tissues, venous blood and background light (Fig. 12.10). It is accurate to within 2%, but falls to ± 5% with saturations below 80%.
Capnography
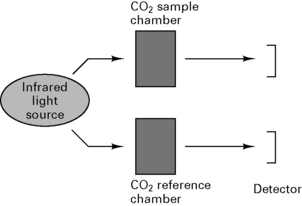
Uses spectrophotometry to measure absorption of CO2 in sample chamber (Beer’s law) and compares results with known CO2 concentration in a reference chamber (Fig. 12.11).
Arrangement of sampling chamber
Clinical uses
Volatile agent monitoring
Infrared absorption spectroscopy
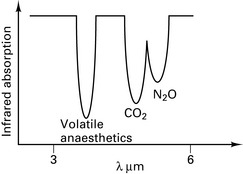
Asymmetric, polyatomic molecules absorb infrared radiation, e.g. CO2, N2O. H2 and O2 do not. Similar arrangement as the capnograph CO2 detector. Volatile agents have overlapping absorption spectra (Fig. 12.12) and therefore the gas being measured must be specified.
Non-invasive blood pressure
Association of Anaesthetists of Great Britain and Ireland. Recommendations for standards of monitoring during anaesthesia and recovery: report of a Working Party, ed 4. London: AAGBI; 2007.
Lennmarken C., Vegfors M. Advances in pulse oximetry. Curr Opin Anaesth. 1998;11:639-644.
Moyle J.T.B. Pulse oximetry. Principles and practice series. London: BMJ Publishing; 1994.
O’Flaherty D. Capnography. Principles and practice series. London: BMJ Publishing; 1994.
Runciman W.B., Ludbrook G.L. Monitoring. In: Nimmo W.S., Rowbotham D.J., Smith G., editors. Anaesthesia. ed 2. Oxford: Blackwell Scientific; 1994:704-739.
Physics
Gas laws
Henry’s law. Amount of gas dissolved ∝ partial pressure of the gas.
Fick’s law. Rate of diffusion across a membrane ∝ concentration gradient.
Graham’s law. Rate of diffusion ∝ 1/molecular weight.
Charles’ law. The volume of a gas changes in proportion to the change in temperature.
Boyle’s law. The volume of a gas is inversely proportional to pressure.
Temperature
Critical temperature. Temperature above which a gas cannot be liquefied.
Critical pressure. Pressure above which a gas at its critical temperature cannot be liquefied.
Gas flow
Hagen–Poiseuille equation. For laminar flow:
where P = pressure, r = tube radius, L = tube length, η = viscosity.
When Reynold’s number (R) exceeds 2000, flow becomes turbulent:
Venturi devices use the Bernoulli effect for suction, e.g. Venturi oxygen mask.
Humidification
Absolute humidity is the mass of water vapour present in a given volume of air.

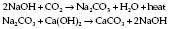
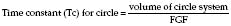
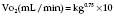
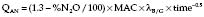
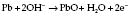
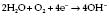
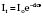

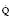 values. Alveoli emptying last have the least ventilation, the lowest V/
values. Alveoli emptying last have the least ventilation, the lowest V/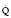 and thus the highest PETCO2.
and thus the highest PETCO2.