Epithelial Neoplasms of the Appendix
Joseph Misdraji
Mucinous Epithelial Tumors
Nomenclature
The classification, nomenclature, and histologic criteria of mucinous epithelial tumors in the appendix have been the source of considerable controversy. These tumors may be confined to the mucosa, in which case they are often classified as “adenomas,” but they may also penetrate deeply into, or through, the appendiceal wall and disseminate to the peritoneal cavity, resulting in a clinical syndrome known as pseudomyxoma peritonei (PP). The exact nature (benign versus malignant, invasive versus noninvasive) of these tumors when they disseminate to the peritoneum is controversial. Based purely on morphologic evaluation of tissue, some authorities interpret these lesions as ruptured adenomas with dissemination of adenomatous epithelium.1–6 Arguments in favor of this theory refer to the presence of low-grade cytologic features in the appendix and in the peritoneum, the lack of destructive invasion in the appendix, and the appearance of tumor in the peritoneum, which shows tumor coating surfaces of organs without definite evidence of stromal invasion. Others, including members of the American Joint Committee on Cancer (AJCC) and the World Health Organization (WHO), consider peritoneal dissemination to represent a cardinal feature of malignancy and classify these appendiceal tumors as adenocarcinomas.7–11 Arguments in favor of this theory include the facts that other mucinous tumors in humans can also be deceptively bland; direct organ invasion does occur in the ovary and spleen in some circumstances; and, most importantly, classification of these lesions as a ruptured benign adenoma does not account for the progressive biologic behavior of the tumor once it has spread to the peritoneum. These authorities suggest that “rupture” actually represents “pushing” or “broad front” invasion of a true carcinoma.
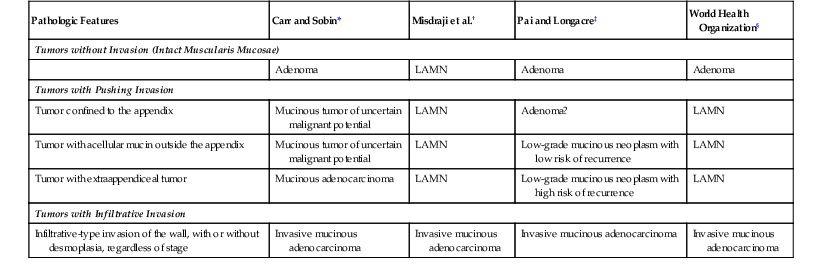
Because the criteria for invasion in appendiceal tumors are not well defined, and in order to account for the possibility of pushing invasion, various terms have been proposed for this group of epithelial tumors, particularly those that dissect into the wall and disseminate to the peritoneum. Some authors have proposed the term “mucinous tumor of uncertain malignant potential” for tumors that appear to push deeply into the appendix wall but do not show dissemination beyond the appendix.12 Others have proposed the term “low-grade appendiceal mucinous neoplasm”13 for the entire spectrum of these tumors, including those that show peritoneal dissemination. Pai and colleagues14 classified tumors that are associated with extraappendiceal mucin as “low-grade mucinous neoplasm with low risk of recurrence” if the mucin located outside the appendix was acellular or “low-grade mucinous neoplasm with high risk of recurrence” if it contained neoplastic epithelium. A comparison of the classification systems used for these tumors is presented in Table 28.1. In the most recent WHO classification of appendiceal tumors, the term low-grade appendiceal mucinous neoplasm (LAMN) is used for low-grade mucinous tumors that reveal pushing invasion, and mucinous adenocarcinoma is used for high-grade appendiceal tumors and ones with conventional features of tissue invasion (Box 28.1).10
Table 28.1
Classifications of Low-Grade Appendiceal Mucinous Neoplasms of the Appendix
| Pathologic Features | Carr and Sobin* | Misdraji et al.† | Pai and Longacre‡ | World Health Organization§ |
| Tumors without Invasion (Intact Muscularis Mucosae) | ||||
| Adenoma | LAMN | Adenoma | Adenoma | |
| Tumors with Pushing Invasion | ||||
| Tumor confined to the appendix | Mucinous tumor of uncertain malignant potential | LAMN | Adenoma? | LAMN |
| Tumor with acellular mucin outside the appendix | Mucinous tumor of uncertain malignant potential | LAMN | Low-grade mucinous neoplasm with low risk of recurrence | LAMN |
| Tumor with extraappendiceal tumor | Mucinous adenocarcinoma | LAMN | Low-grade mucinous neoplasm with high risk of recurrence | LAMN |
| Tumors with Infiltrative Invasion | ||||
| Infiltrative-type invasion of the wall, with or without desmoplasia, regardless of stage | Invasive mucinous adenocarcinoma | Invasive mucinous adenocarcinoma | Invasive mucinous adenocarcinoma | Invasive mucinous adenocarcinoma |
* Data from Carr NJ, McCarthy WF, Sobin LH. Epithelial noncarcinoid tumors and tumor-like lesions of the appendix: a clinicopathologic study of 184 patients with a multivariate analysis of prognostic factors. Cancer. 1995;75:757-768.
† Data from Misdraji J, Yantiss RK, Graeme-Cook FM, et al. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27:1089-1103.
‡ Data from Pai RK, Beck AH, Norton JA, Longacre TA. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol. 2009;33:1425-1439.
§ Data from Carr NJ, Sobin LH. Adenocarcinoma of the appendix. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, eds. WHO Classification of Tumours of the Digestive System. Lyon: International Agency for Research on Cancer (IARC); 2010:122-125.
LAMN, Low-grade appendiceal mucinous neoplasm.
Adenoma
Clinical Features
Adenomas typically occur in patients in their fifth decade of life, but the age range is wide, with cases reported in patients between 18 and 87 years of age.3,5,8,15 There is a female predominance.3,5,8,15 Abdominal pain that mimics acute appendicitis is the most common clinical presentation.5,8 Less often, an appendiceal adenoma manifests as an abdominal mass or as intussusception of the appendix.5,8,16–24 Approximately half of the cases are discovered in asymptomatic patients, particularly in women who have undergone an appendectomy during gynecologic surgery. Occasionally, a patient with adenoma in the appendix has concomitant colonic adenocarcinoma,5,8,15 but whether this represents more than a chance association is uncertain.
Pathogenesis
Appendiceal adenomas show frequent KRAS mutations25–27 and infrequent loss of chromosome 5q.25,28 However, one study of four adenomas found no loss of heterozygosity of the adenomatous polyposis coli (APC) or deleted in colorectal carcinoma (DCC) genes,27 and abnormal nuclear accumulation of β-catenin, a sign of APC loss of function, has not been found in adenomas.29,30 Adenomas are microsatellite stable and lack BRAF mutations.27,29
Pathology
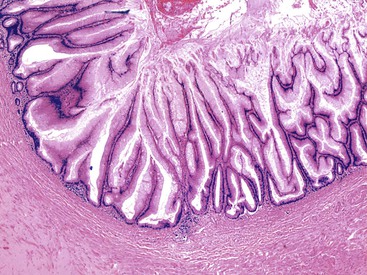
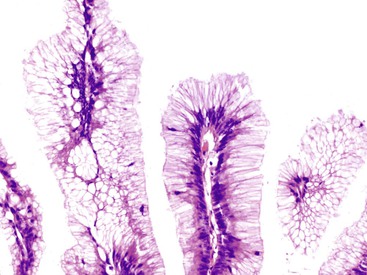
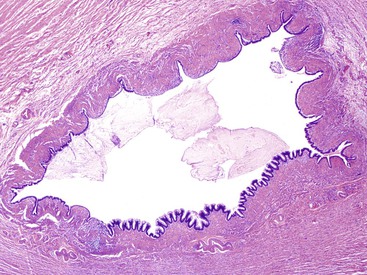
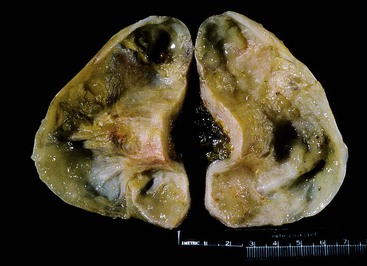
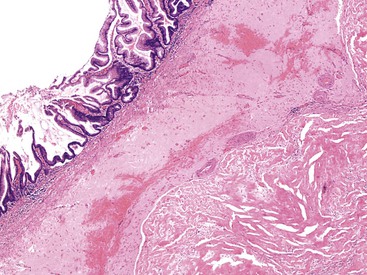
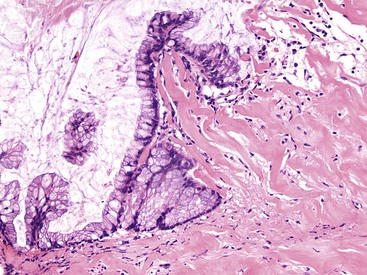
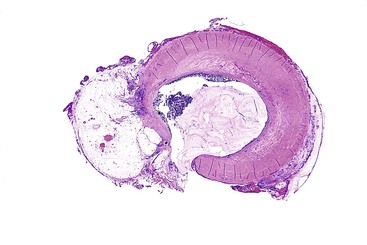
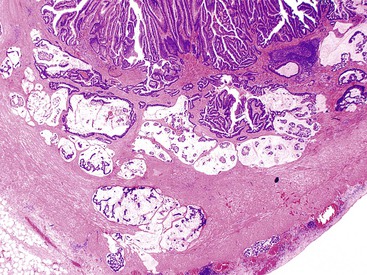
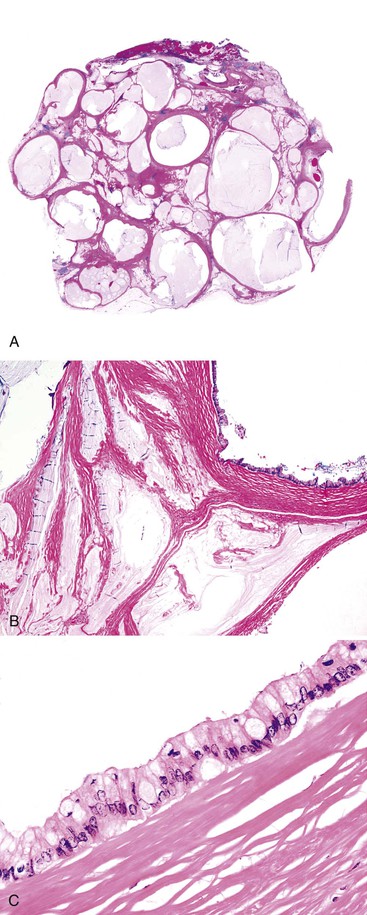
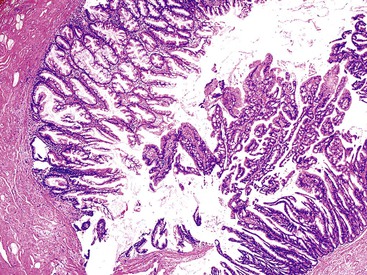
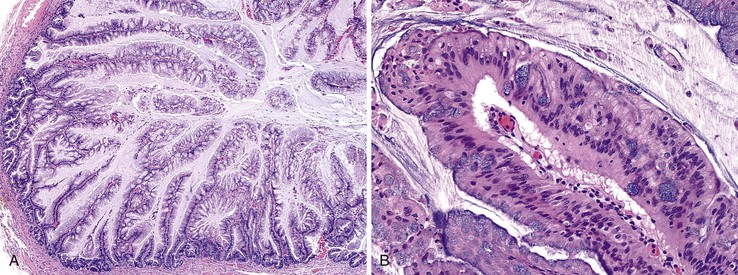
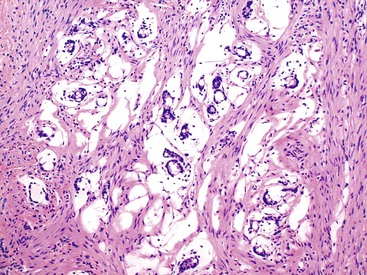
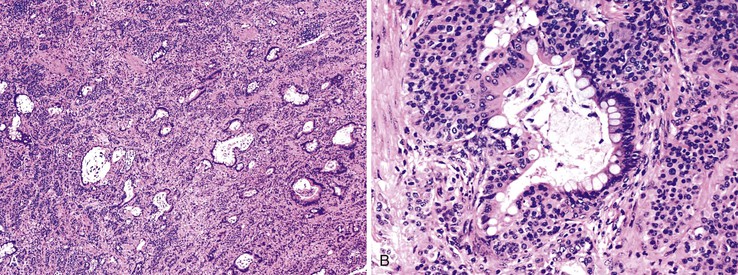
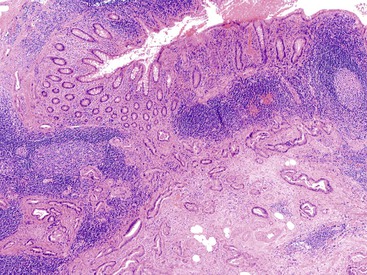
Grossly, an appendix with an adenoma may be unremarkable, or it may be dilated with tenacious mucin. The serosa is typically smooth unless there are adhesions, but gross mucin is absent.8 The wall may be thin and fibrous, and after evacuation of the mucus, the inner surface appears granular or corrugated.8 Microscopically, these tumors are by definition confined to the mucosa, show intact muscularis mucosae, and contain no mucin in the wall or outside the appendix. The tumor consists of a proliferation of mucinous epithelial cells, often with atrophy of the lymphoid tissue (Fig. 28.1). The tumor is usually composed of slender villi with scant lamina propria; the villi are lined by mucinous epithelial cells, often with abundant intracytoplasmic mucin (Fig. 28.2).1,12,13 The glands may have straight luminal edges, or they may have luminal serration that raises the suspicion of a serrated epithelial neoplasm (see later discussion). In some cases, mucin accumulation leads to cystic dilatation of the appendix. In these cases, one may see a flat, undulating, or short villous pattern of the neoplastic epithelium (Fig. 28.3). These findings in the appendix are often termed cystadenoma.
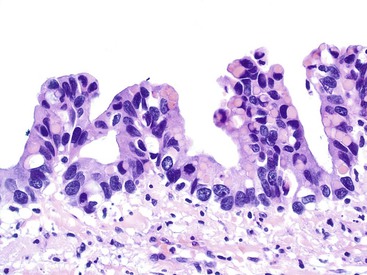
The tumors are graded as either low or high. Most tumors are low grade. They show nuclear enlargement, elongation, hyperchromasia, pseudostratification, rare mitoses, and apoptotic nuclear debris,8,13,31 but the nuclei are limited to the basal portion of the cell. High-grade dysplasia reveals large pleomorphic nuclei, prominent nucleoli, increased mitotic figures, and full-thickness nuclear pseudostratification (Fig. 28.4).
Localized tubular adenomas of the type that occur in the colon are rare in the appendix.32,33 However, they may occur in patients with familial adenomatous polyposis.7,12 The histologic appearance of these polyps is identical to those that occur in the colon, and these may be low or high grade as well.
Differential Diagnosis
Retention Cyst
In cystic tumors, attenuation and denudation of the lining may make distinction between an appendiceal adenoma and a retention cyst difficult. In this case, multiple sections, and deep cuts of each section, may be necessary to detect (or exclude) the presence of neoplastic epithelium. Cysts larger than 2 cm are likely neoplastic. Examination of the lining may reveal residual villi focally that indicate a neoplasm. Convincing low-grade dysplasia with nuclear pseudostratification, nuclear enlargement, apoptotic debris, and infrequent mitoses also warrant a diagnosis of cystadenoma.
Low-Grade Appendiceal Mucinous Neoplasm
Distinction between a mucinous adenoma and a LAMN rests on evaluation of the muscularis mucosae, which is intact in a mucinous adenoma and is breached by epithelium in a LAMN (see later discussion).
Serrated Polyp of the Appendix
Mucinous adenomas can have serrated glandular lumina that can mimic serrated polyps of the appendix (discussed later).
Natural History
Appendiceal adenomas are benign lesions, and they are cured by appendectomy.
Low-Grade Appendiceal Mucinous Neoplasm
Clinical Features
LAMNs usually manifest in the sixth decade of life, although the age range is broad (20 to 89 years).13,35 As with adenomas, there is a predilection for females.14 Presentation with an abdominal mass or with an ovarian metastasis is relatively common.8,13,35 In fact, diagnosis of the ovarian tumor may predate discovery of the appendiceal tumor. Other presentations include abdominal pain or distention.13,14 Rarely, a ruptured LAMN manifests with mucin in a hernia sac.36–38 A retrocecal LAMN can manifest as a retroperitoneal mass or as pseudomyxoma extraperitonei.39–44 Approximately 15% to 20% of LAMNs are found incidentally in patients undergoing surgery for unrelated conditions.13,14
Pathogenesis
LAMNs show frequent KRAS mutations.25–27,45 Loss of chromosome 5q has been described,25,28 but one study of 31 LAMNs found no loss of heterozygosity of the APC or DCC genes.27 LAMNs have not been shown to demonstrate microsatellite instability or features of the mutator pathway, such as BRAF mutations.27,29 A recent study found GNAS mutations in 50% of 32 LAMNs.45 Activating GNAS mutations cause constitutive activation of adenylate cyclase and elevated cyclic adenosine monophosphate (cAMP) levels. In cell lines, this was associated with increased expression of the mucins MUC2 and MUC5AC,45 raising the intriguing possibility that GNAS mutations play a role in the prominent mucin production that is a hallmark of LAMNs and PP.
Bibi and colleagues showed that N-cadherin expression is increased in appendiceal mucinous tumors and PP, whereas E-cadherin expression is reduced in tumors relative to normal mucosa.46 They also showed vimentin expression in these tumors. They suggested that a shift in cadherin phenotype and vimentin expression may reflect an epithelial-mesenchymal transition state in which epithelial cells switch to mesenchymal phenotype, a process that presumably promotes metastasis.
Pathology
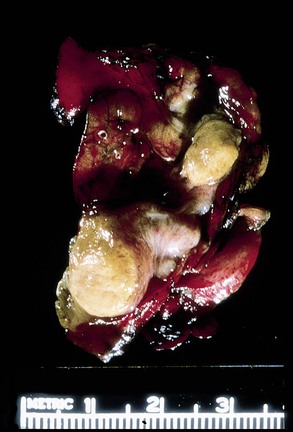
Appendices with LAMNs may appear grossly unremarkable, or they may be cystically dilated and filled with tenacious mucin.8,13,35,47 The wall may be thin or fibrotic, hyalinized, and calcified (Fig. 28.5).8,13 When calcifications are extensive, they can lead to the development of a thin-walled appendix with a hard, gritty texture, often referred to as “porcelain appendix.” Rupture with mucin present in the wall or on the serosal surface may be evident grossly.8,13,35 Infrequently, the appendix may be completely encased in mucin or fused to a right ovarian tumor.13,35,47 The appendix lining may be smooth, granular, or corrugated with intervening smooth areas, similar to adenomas.8 Focal outpouchings of the lumen filled with mucin have been described.35
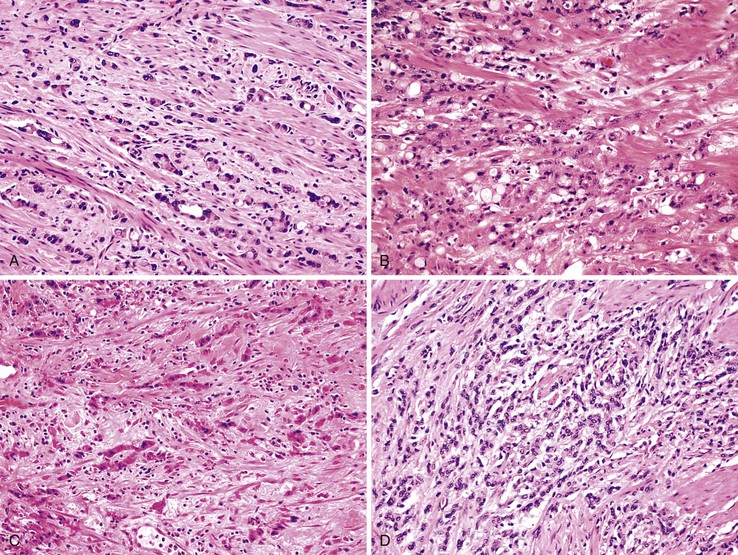
On microscopic examination, these tumors show a villous or flat, neoplastic, mucinous epithelium, often associated with atrophy of lymphoid tissue, similar to adenomas.1,12,13 In villous tumors, the villi are typically filiform and contain scant lamina propria. The epithelium contains abundant cytoplasmic mucin that may compress nuclei. Cells with small, compressed nuclei may appear deceptively benign or even non-neoplastic.1 The villi are usually straight, but occasionally there is variable degree of luminal serration. These cases may resemble hyperplastic polyps or a serrated neoplasm (see later discussion). Mucin accumulation may be extensive; cystic dilatation may develop in these cases, usually in association with attenuation of the lining epithelium. The epithelium may be flat, forming a single layer of epithelium, or undulating. Denudation of the epithelium is common in these tumors,1 particularly in those with attenuated epithelium, and the distinction between a LAMN and a retention cyst can be difficult (see later discussion). In some cases, the entire appendiceal mucosa is denuded of epithelium. In these cases, extensive sampling may be needed to identify rare foci of neoplastic epithelium.7,8 The tumors are composed of columnar cells that are rich in mucin and display low-grade nuclear atypia consisting of nuclear elongation, hyperchromasia, pseudostratification, inconspicuous nucleoli, occasional mitoses, and apoptotic nuclear debris.8,13,31 High-grade tumors are classified as mucinous adenocarcinoma (see later discussion).
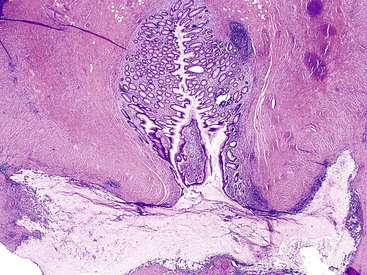
The appendiceal wall in LAMNs may show alterations of the muscularis mucosae and even extensive effacement of normal appendiceal landmarks in some cases. Fibrosis and hyalinization of the wall are common. In some cases, the hyalinized tissue in the wall is nodular (Fig. 28.6), and this may represent evidence of prior episodes of mucin leakage and subsequent tissue repair.13 Dystrophic calcification is common, particularly in areas with mucin.1,47
One distinctive feature of LAMN is the pattern of invasion of the underlying appendiceal wall. Neoplastic epithelium, mucin, or a combination of both may dissect into or through the wall of the appendix, lying very close to the serosa.35 Fibrosis of the appendiceal wall may result in growth of neoplastic epithelium along fibrotic or hyalinized stroma rather than atop normal lamina propria and muscularis mucosae (Fig. 28.7). Tumor dissecting into the wall may resemble involvement of preexisting diverticula, but, in contrast to true diverticular involvement, tumor involving the wall lacks lamina propria (Fig. 28.8). LAMNs typically do not invade in a conventional manner, with production of desmoplastic stroma combined with an infiltrative and irregular glandular proliferation. Tumors that invade and perforate the appendix may appear similar to a ruptured adenoma, but in the new WHO classification, as discussed earlier, any tumor with mucin or epithelium within or outside the wall of the appendix should be classified as LAMN, not an adenoma (Fig. 28.9).
Appendiceal mucinous neoplasms and peritoneal metastasis usually express keratin 20, and approximately 25% to 40% coexpress keratin 7.46,48 CDX2 and MUC2 are expressed in almost 100% of appendiceal mucinous tumors and PP.46,48–51 In contrast, MUC1 is typically negative in adenomas (although it may be positive in adenocarcinomas).48 MUC5AC and MUC6 are variably expressed in these tumors and therefore are not of diagnostic value.48
Differential Diagnosis
Retention Cyst
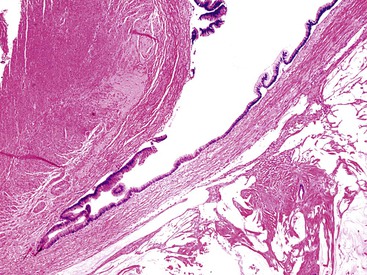
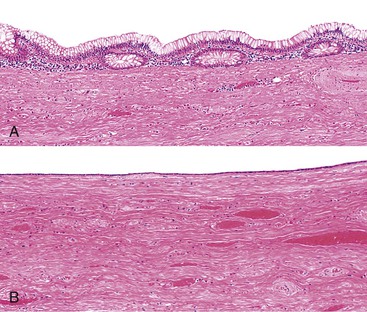
Retention cysts are caused by obstruction of the appendiceal lumen,5 although the cause of the obstruction may not be readily apparent. Retention cysts are relatively uncommon lesions. They only rarely attain a size larger than 2 cm in greatest diameter.7 Appendices larger than 2 cm in diameter are usually neoplastic (either adenomas or LAMNs). Retention cysts may be fibrotic and chronically inflamed and may show extensive epithelial denudation similar to adenomas and LAMNs. The mucosa may be atrophic but retain some semblance of crypt architecture with lamina propria, or it may be composed of a single layer of attenuated cuboidal epithelium (Fig. 28.10).3,12 In these cases, the appendix should be submitted in its entirety for microscopic evaluation to detect or exclude a neoplasm. Any atypicality, such as nuclear enlargement, elongation, hyperchromaticity, or mitoses, particularly if atypical, should raise suspicion for a neoplasm. In particular, villous epithelial proliferations are not consistent with a retention cyst and indicate a neoplasm. Retention cysts may also rupture and result in the presence of mucin in the peritoneal cavity.12 However, unlike PP resulting from a neoplasm, the mucin in these cases is usually confined to the localized area of rupture and does not continue to accumulate after appendectomy. Microscopic examination of the mucin may demonstrate varying degrees of tissue reaction and organization, showing granulation tissue in various degrees of development and mesothelial hyperplasia. Epithelial cells are not present in peritoneal mucin in cases of ruptured retention cyst. Therefore, its presence should exclude this diagnosis, unless contamination of mucin with epithelium occurred as a technical complication of specimen prosection.
Ruptured Appendiceal Diverticulum
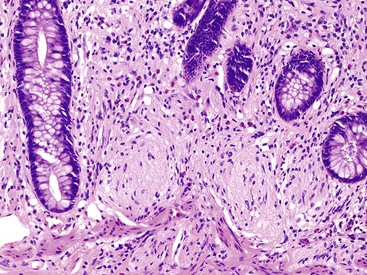
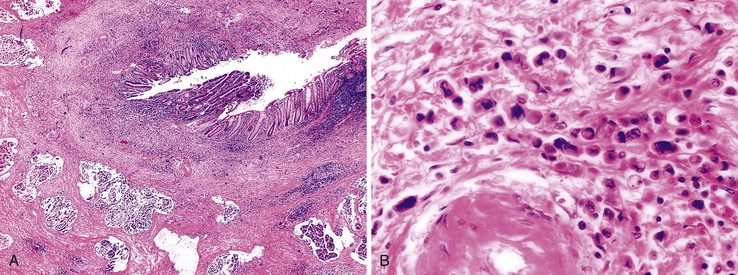
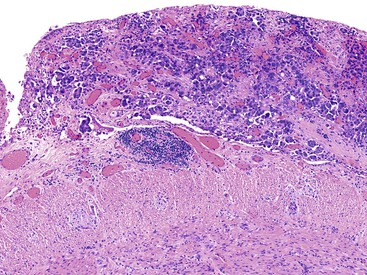
Appendiceal diverticula may, on occasion, rupture and cause release of mucin into the wall and onto the serosa, which may raise concern for a mucinous neoplasm (Fig. 28.11).52 In addition, hyperplastic and reactive changes of the epithelium within the diverticula may be confused with a neoplastic process. An everted diverticulum, or fragments of diverticular lining on the serosa, may be confused with localized PP. There are some features that pathologists can use to differentiate ruptured diverticulum from a mucinous neoplasm (Box 28.2). In patients with appendiceal diverticular disease, the appendix often shows several diverticula, and in these cases, the finding of intact diverticula assists the pathologist in establishing a correct diagnosis of benign diverticular disease. Hyperplastic and reactive changes show architectural alterations that are more pronounced in the superficial rather than the basal portions of the mucosa. Gland serration, crypt disarray, and hyperplastic changes with abundant mucin-producing cells are usually located in the upper half of the mucosa. Non-neoplastic crypts are normally separated by lamina propria and show little or no crowding. In contrast, typical LAMNs show back-to-back crypts with scant lamina propria and elongated, slender villi that protrude into the lumen of the appendix. The nuclei in some neoplasms are larger and show elongation, hyperchromaticity, and mitoses. One other helpful hint is that appendices with diverticula often show transformation of the lamina propria into neural tissue, termed “mucosal neuroma” (Fig. 28.12). This may result from the effects of obstruction.
Natural History
The prognosis of LAMN is highly dependent on the presence or absence of neoplastic epithelium outside the appendix.8,13,14 For instance, LAMNs that are confined to the appendix, without extraappendiceal mucin, are almost always cured by appendectomy, similar to adenomas. LAMNs associated with acellular mucin in the peritoneal cavity confined to the right lower quadrant carry a low risk of recurrence or progression to PP. In a multiinstitutional study by Yantiss and associates,53 the authors collected 65 patients with ruptured appendiceal mucinous tumors, all of whom had at least 6 months of follow-up. Of the 50 patients with acellular mucin in the right lower quadrant, only 2 (4%) had local recurrence after 56 and 92 months of follow-up. In contrast, 15 tumors were associated with clusters of low-grade neoplastic epithelium within the extraappendiceal mucin. Five of these cases (33%) recurred as diffuse PP within 24 to 87 months, and 1 patient died of disease at 60 months. Pai and colleagues14 studied 116 patients with mucinous tumors and came to similar conclusions. They had follow-up for 14 patients with acellular mucin in the right lower quadrant; 1 (7%) of these patients had a recurrence during the follow-up period. Three (75%) of 4 patients with extraappendiceal mucin with neoplastic epithelium limited to the right lower quarter had a recurrence. From these studies, it can be concluded that appendiceal mucinous tumors with extraappendiceal acellular mucin in the right lower quadrant only rarely recur but that tumors with extraappendiceal mucin with neoplastic epithelium recur more often. Therefore, evaluation of the appendix specimen for extraappendiceal mucin and neoplastic epithelium should be performed, in total, for all cases.
In contrast, LAMNs with diffuse (nonlocalized) peritoneal seeding of neoplastic epithelium and mucin have a rather progressive clinical course that frequently results in death of the patient unless managed aggressively (see Pseudomyxoma Peritonei).
Treatment
Simple appendectomy is considered sufficient treatment for LAMNs that are confined to the appendix if the tumor is removed in total with a negative proximal margin. Given the possibility of peritoneal recurrence in patients with ruptured LAMNs, those with acellular mucin on the periappendiceal serosa should be followed closely to ensure that localized or diffuse PP does not develop. Radiographic imaging of the abdomen and pelvis is often the preferred method of surveillance. Tumors that have spread to the right lower quadrant have a higher risk of recurrence, but the appropriate management for these patients is controversial. Right hemicolectomy offers no additional benefit over appendectomy alone for patients with LAMN, even those with extraappendiceal mucin and neoplastic epithelium.54,55 Tumors that have disseminated widely throughout the peritoneal cavity are treated either by debulking or, more aggressively, with multiple peritonectomies and heated intraperitoneal chemotherapy (see Pseudomyxoma Peritonei).
Adenocarcinoma
Clinical Features
Adenocarcinomas of the appendix are uncommon; Collins found an incidence of 0.082% among 50,000 appendectomy specimens.56 There is an increased incidence among men in some series4,57–64 but not in others.65–68 Patients are usually in their fifth to seventh decade of life at presentation57,59–61,66,68 and usually are seen with symptoms of acute appendicitis. Less common modes of presentation include a palpable mass, obstruction, gastrointestinal (GI) bleeding, and symptoms related to metastases.3,4,59–62,64–67,69–71
Pathogenesis
Appendiceal adenocarcinomas show frequent allelic loss of chromosome 18q but less frequent mutations of SMAD4 (DPC4), suggesting that tumor suppressor genes located on 18q may play a role in their pathogenesis.72 Studies have found frequent mutations of KRAS.27,29,45,73 A recent study found absent GNAS mutations in three mucinous adenocarcinomas.45 Studies have been mixed with regard to TP53. Some have reported absent TP53 expression,27,73 whereas others have reported TP53 expression in most adenocarcinomas29,48 or TP53 mutations in approximately 75% by gene sequencing.48 Mutations in CTNNB1 (β-catenin) were not found in a series of 28 appendiceal adenocarcinomas,72 and no loss of heterozygosity for APC or DCC was detected in another series of 6 adenocarcinomas.27 Although mucinous carcinomas in the right colon are frequently associated with defective DNA mismatch repair and microsatellite instability,74–76 most appendiceal adenocarcinomas studied to date have been microsatellite stable.27,73 However, in one study, four adenocarcinomas arising in the background of serrated lesions29 showed loss of the mismatch repair gene MLH1 and reduced expression of O-6-methylguanine-DNA methyltransferase (MGMT), with high microsatellite instability (MSI-H) in one tumor. A second tumor showed reduced MGMT expression. One tumor showed two different KRAS mutations and a BRAF mutation, indicating genetic instability in this tumor. In another study of nine appendiceal adenocarcinomas, a single case showed absent expression of MSH2 and MSH6, and none showed absent MLH1 expression.77
Pathology
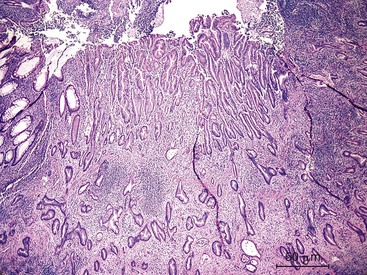
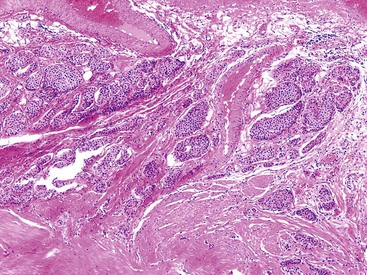
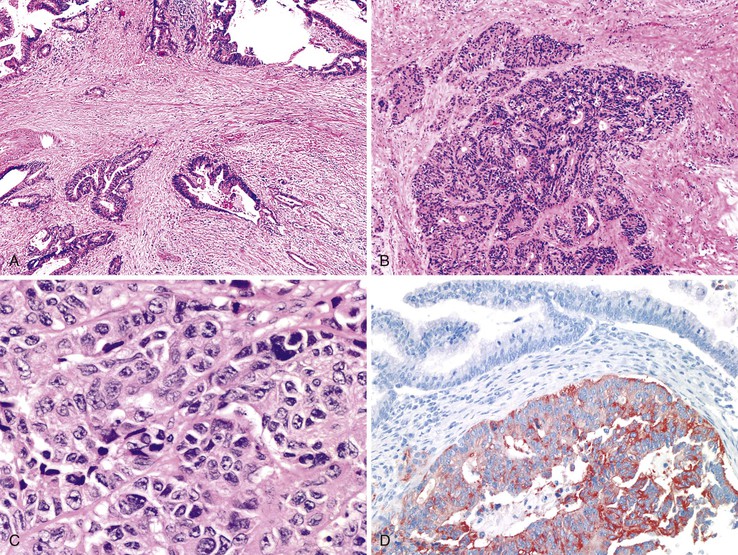
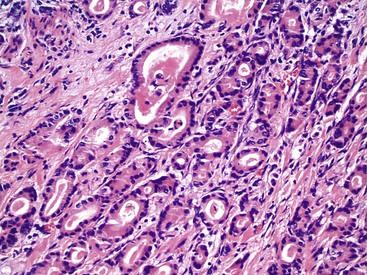
Grossly, appendiceal carcinomas can be polypoid, ulcerating, or infiltrative.62,63,71 Obstruction of the lumen may result in cystic dilation of the appendix. The proximal third of the appendix is involved more often than the distal portions.58,66,69
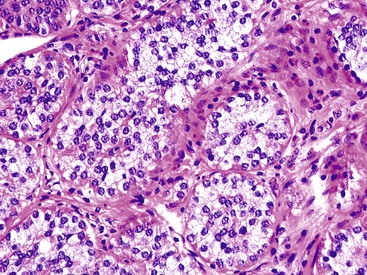
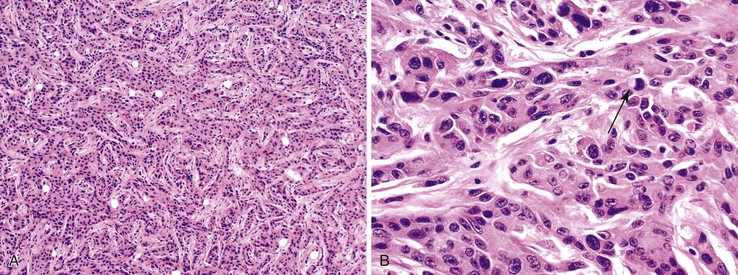
The classification of carcinomas is indicated in Box 28.1. They are classified as adenocarcinoma not otherwise specified, mucinous adenocarcinoma, LAMN (see prior discussion), signet ring cell adenocarcinoma, or undifferentiated carcinoma.10 Mucinous adenocarcinoma accounts for approximately 40% of all appendiceal adenocarcinomas.60 The histology of invasive mucinous adenocarcinoma of the appendix is similar to that of mucinous adenocarcinoma occurring elsewhere in the colon. These tumors show irregular pools of mucin of various sizes and shapes infiltrating the wall of the appendix and associated with cytologically malignant glandular epithelium, the latter usually arranged as strips, clusters, or complex glandular structures (Fig. 28.13). Mucinous cystadenocarcinomas may rupture and metastasize to the peritoneum or ovaries67,70 or, less commonly, they may metastasize via a hematogenous route.3,54,61,73 Peritoneal metastasis may reveal large volumes of extracellular mucin.31 Histologically, peritoneal metastasis resembles that occurring in LAMNs, except that the mucin is more cellular and the neoplastic epithelium shows a greater degree of architectural complexity and higher-grade cytologic atypia (Fig. 28.14). In this circumstance, the peritoneal metastasis is termed peritoneal mucinous adenocarcinoma.6 Other organs may be affected, such as small or large bowel, liver, or ovary.6,79
Nonmucinous appendiceal carcinomas show a range of morphologic features of the invasive component. For instance, the tumors may resemble colonic adenocarcinomas by revealing malignant glands lined by neoplastic columnar epithelium with dirty necrosis. Some tumors are composed of well-differentiated tubular glands lined by cuboidal shaped cells without dirty necrosis, and with only a mild amount of extracellular mucin, reminiscent of pancreaticobiliary adenocarcinomas. Adenocarcinomas arising in the background of serrated polyps may have serrated gland morphology, with or without extracellular mucin (see Serrated Tumors of the Appendix).29
Signet ring cell adenocarcinomas are rare in the appendix. These tumors are associated with a poor prognosis because of rapid dissemination within the peritoneal cavity.80,81 The histology is similar to that of signet ring cell adenocarcinomas at other sites in the GI tract (Fig. 28.15). They are composed of single cells, clusters, and/or sheets of signet ring cells, some with extracellular mucin. Some tumors show areas reminiscent of goblet cell carcinoid tumor, suggesting that the adenocarcinoma arose from the latter (see Goblet Cell Carcinoid).7
Differential Diagnosis
Low-Grade Appendiceal Mucinous Neoplasm versus Mucinous Adenocarcinoma
The distinction between LAMN and mucinous adenocarcinoma is based on evaluation of the characteristics of the “invasive” component and on the cytologic features of the neoplastic epithelium. LAMNs show prominent fibrosis of the wall with “pushing” invasion. Mucinous adenocarcinomas usually show conventional patterns of invasion, exhibiting irregular pools of mucin harboring high-grade epithelium in complex architectural configurations or irregularly shaped glands with a desmoplastic stromal response. LAMNs, by definition, are low-grade tumors, whereas mucinous adenocarcinomas may be low or high grade.
Natural History
The reported 5-year survival rate for patients with appendiceal adenocarcinoma ranges from 18.7%57 to 55%.65 Patients with mucinous adenocarcinomas have a better prognosis than those with non-mucinous adenocarcinomas.65,66,70 Several studies have shown that histologic grade4,57,69 and Duke stage correlate with prognosis.4,65,66,69,70,82,83 Patients with peritoneal carcinomatosis have a poor prognosis.6,60,68,70
Treatment
Invasive adenocarcinoma of the appendix warrants treatment by right hemicolectomy, with lymph node dissection, to potentially achieve a cure.65,67,70,82 Most studies have shown that right hemicolectomy offers improved 5-year survival compared with appendectomy alone.31,59,65,67,82,83 Some authorities advocate oophorectomy in women, both for staging purposes and to remove a common site of metastasis.65,70 Treatment of patients with peritoneal spread depends on the resectability of the tumor. Patients whose tumors are resectable should be treated aggressively with peritonectomy and postoperative intraperitoneal chemotherapy.54,84,85
Pseudomyxoma Peritonei
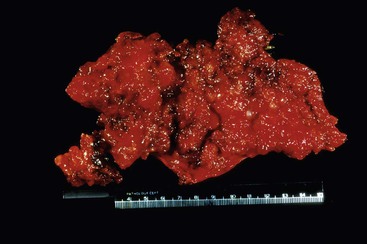
The term pseudomyxoma peritonei refers to the accumulation of mucin within the peritoneal cavity. Historically, the term was used to describe mucin in the peritoneal cavity regardless of the presence or absence of neoplastic epithelial cells,1,47,86,87 but more recently, it has been reserved for cases in which neoplastic epithelial cells are present within the peritoneal mucin.8,88,89 This condition most often occurs as a result of peritoneal spread of a mucinous neoplasm from the appendix (Fig. 28.16) but has been described with mucinous tumors from other sites, including colon, ovary, gallbladder, pancreas, and urachus.
Clinical Features
Spread of mucinous neoplastic epithelium to the peritoneal cavity occurs most often in association with LAMN and mucinous adenocarcinomas. PP develops in approximately 20% of patients with a mucinous tumor of the appendix.90 PP typically manifests in the sixth to seventh decades of life, and in some series, women predominate over men.88,90,91 Along with the peritoneal cavity, the ovaries are commonly involved with tumor as well.35,88,90 Peritoneal mucinous deposits tend to accumulate in particular areas, such as the greater omentum, the undersurface of the right hemidiaphragm, the pelvis, the right retrohepatic space, the left abdominal gutter, and the ligament of Treitz.92 This so-called “redistribution phenomenon” occurs because tumor accumulates at anatomic locations where ascitic fluid is resorbed from the abdomen and in those areas most subjected to pooling in the abdomen.
Pathology, Nomenclature, and Outcome
The morphologic characteristics, particularly the degree of atypia of the neoplastic epithelium (grade), correlate with the grade and degree of “invasiveness” of the primary appendiceal tumor.6 Peritoneal tumors in patients with LAMN demonstrate abundant mucin; hyalinized, fibrotic stroma; and a variable quantity of strips of low-grade (or even non–neoplastic-appearing) mucinous epithelium (Fig. 28.17). This is the appearance of classic PP. Peritoneal mucinous tumors with high-grade cytologic atypia of the neoplastic epithelium and more complex architecture are usually associated with mucinous adenocarcinomas of the appendix and are more appropriately termed peritoneal mucinous adenocarcinoma (see Fig. 28.14).13,79 Progression from low grade to high grade has been described in patients after failure of cytoreduction and chemotherapy treatment.93,94
Ronnett and co-workers separated peritoneal mucinous tumors into two categories based on cytologic and architectural features.79 They proposed the term disseminated peritoneal adenomucinosis (DPAM) for “noninvasive” mucinous implants derived from an appendiceal “adenoma” (i.e., LAMN) that contains scant strips of nonstratified mucinous epithelium with minimal to moderate atypia, at most focal tufting, and no significant mitotic activity. In contrast, peritoneal tumors characterized by more abundant epithelium arranged as glands, nests, or individual cells and with marked cytologic atypia were classified as peritoneal mucinous carcinomatosis (PMCA). These peritoneal tumors are derived from an appendiceal or intestinal mucinous adenocarcinoma and are characterized by the presence of parenchymal organ invasion and lymph node metastases in the primary site.6 Five- and 10-year survival rates were 75% and 68% for patients with DPAM and 14% and 3% for patients with PMCA, respectively. These authors also described an intermediate category for tumors that are mostly DPAM but also contain focal areas of well-differentiated adenocarcinoma.79 Initially, these patients appeared to have an intermediate prognosis, but additional follow-up showed that their tumors behaved similarly to PMCA.95
In contrast, a study of 101 patients with PP by Bradley and co-workers found that patients with DPAM and intermediate-grade peritoneal mucinous tumors had a similar outcome and had a better prognosis than those with high-grade peritoneal tumors.96 Because they found no difference in outcome between patients with DPAM and those with intermediate-grade histology (or well-differentiated adenocarcinoma), they argued that DPAM is equivalent to well-differentiated mucinous adenocarcinoma. As a result, they proposed a two-level classification: (1) mucinous carcinoma peritonei, low grade, and (2) mucinous carcinoma peritonei, high grade. They found that the histology of the primary appendiceal tumor was not predictive of behavior, because “ruptured adenomas” behaved similarly to well-differentiated invasive adenocarcinomas.
Although these studies support grading of peritoneal mucinous tumors, the appropriate nomenclature for peritoneal mucinous neoplasia remains controversial. The WHO discourages use of the term DPAM because the concept of a ruptured adenoma with dissemination of adenomatous epithelium cannot be reconciled with the low-grade malignant behavior of PP.10
Treatment
The treatment of PP has not been standardized. Surgical tumor debulking was the mainstay of therapy for decades, but most patients had disease recurrence that required repeated surgical debulking procedures until, eventually, adhesions precluded additional surgery and the patient succumbed to the tumor.97,98 Survival rates for patients who underwent debulking have been reported to be 86% at 2 years, 53% to 67% at 5 years, and 32% at10 years.97,99
Sugarbaker and colleagues54,84,85,100 pioneered an aggressive treatment approach that uses peritonectomies to achieve complete cytoreduction and hyperthermic intraoperative peritoneal chemotherapy (HIPEC), supplemented by additional cycles of early postoperative intraperitoneal chemotherapy (EPIC). The cytoreduction procedures include peritonectomies as well as resection of affected organs. Women require hysterectomy and bilateral salpingo-oophorectomy.101 Using this aggressive approach, they achieved 5- and 10-year survival rates of 71.9% and 54.5%, respectively.54 Other groups have reported similar survival rates by using cytoreduction surgery and HIPEC/EPIC. In particular, long-term (10-year) survival has been reported to be better in some studies.102–104
Several factors have been associated with success in the management of PP. One of the most important is the need to achieve complete cytoreduction, which is accomplished in 40-91% of cases.101,104 In one study, patients who had complete cytoreduction had a 5 and 10 year survival rate of 87% and 74%, respectively, whereas those in which complete cytoreduction could not be achieved, and thus underwent debulking only, had 34% and 23% 5 and 10 year survival rates.104 In this regard, the experience of the surgeon may play a role, with specialized centers achieving better survival statistics.102,103 Extensive involvement of the small bowel or mesentery predicts a high likelihood of incomplete cytoreduction. Tumor grade is a factor that affects outcome.102,103 Patients with low-grade peritoneal disease (DPAM) show improved survival compared with those with peritoneal carcinomatosis, with 5-year survival rates of 86% and 50%, respectively.100
Some authors remain skeptical of such aggressive treatment, given the high associated morbidity and mortality of these procedures.98,101 Similar survival rates have been achieved by using less aggressive debulking surgery, particularly for patients with low-grade histology and complete cytoreduction.98,99,105
Serrated Tumors of the Appendix
Serrated polyps of the colorectum—hyperplastic polyps, sessile serrated adenomas/polyps (SSA/Ps), traditional serrated adenomas (TSAs)—are now recognized as precursors in the serrated pathway of colorectal tumorigenesis, and this is believed to occur in the appendix as well for a small proportion of tumors.106 In the appendix, serrated polyps are classified in a similar manner (Box 28.3).
Clinical Features
Serrated polyps in the appendix are most often detected as an incidental finding or in patients with presenting symptoms of acute appendicitis.7,8,15,107 They often affect older adults, and they are found in men and women equally.29 They may also be seen in appendices with invasive cancer and probably represent a precursor lesion in some of those tumors (Fig. 28.18).29,108 Studies have been inconsistent regarding an association between serrated neoplasia of the appendix and concomitant colorectal neoplasms.29,109
Pathogenesis
Mucosal hyperplasia is occasionally seen in appendices that have been inflamed, obstructed, or ulcerated, presumably as a reaction to prior inflammation. Hyperplastic, serrated proliferations in this setting may not be neoplastic. However, there is evidence that a small proportion of appendiceal cancers develop through the serrated pathway of carcinogenesis, indicating that many serrated lesions are neoplastic. For instance, in a large study of serrated polyps of the appendix, Yantiss and colleagues29 found reduced MLH1 and MGMT expression across the entire spectrum of serrated lesions, although loss of MLH1 immunoreactivity was not accompanied by MSI-H. In their study, most serrated polyps in the appendix had either BRAF (29%) or KRAS (34%) mutations. Among the nondysplastic serrated polyps, the prevalence of BRAF mutation was less than in colorectal serrated lesions. BRAF mutations were less common in dysplastic serrated polyps and in invasive carcinomas arising in serrated polyps than in nondysplastic polyps. This finding suggests that the biologic potential of BRAF in the appendix is limited. Also of interest is the fact that most BRAF mutations were present in only a minor portion of the DNA (usually ≤25%). In contrast, most polyps with KRAS mutations showed alterations in more than 25% of extracted DNA, and most of these were dysplastic polyps, indicating that KRAS mutations may be more important in the biology of appendiceal serrated neoplasms. Finally, in four cancers that developed within serrated polyps, a clear relationship between the molecular changes in the serrated lesion and those in the carcinoma was not established. None of the serrated polyps adjacent to carcinomas harbored BRAF mutations. In addition, most carcinomas also lacked BRAF mutations, except for one case in which a BRAF mutation was identified in a small portion of DNA in the carcinoma but not in the associated serrated polyp.
Abnormal nuclear localization of β-catenin is a reflection of loss of APC function, part of the canonical adenoma–carcinoma sequence in the colorectum. To date, studies have failed to demonstrate abnormal nuclear localization of β-catenin in serrated lesions in the appendix.29,30
Pathology
As in the colon, distinction among the various serrated lesions in the appendix is difficult given the morphologic overlap.
Hyperplastic Polyp/Diffuse Mucosal Hyperplasia
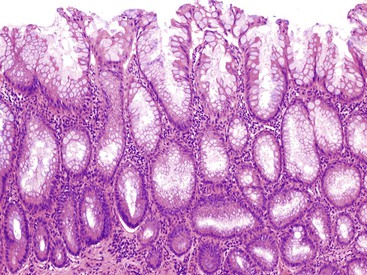
Mucosal hyperplasia in the appendix may be localized and polypoid (hyperplastic polyp) or diffuse and nonpolypoid. In the appendix, hyperplastic polyps are unusual, whereas nonpolypoid mucosal hyperplasia is relatively more common.7 In some cases, mucosal hyperplasia can involve large portions of the appendix or the entire circumference of the lumen. Grossly, an appendix with mucosal hyperplasia is usually unremarkable, but the lumen may be slightly dilated with mucinous material, and the mucosa may appear grossly thickened, with minute papillary areas.8 Histologically, mucosal hyperplasia shows elongation of crypts with delicate papillary structures and glandular infolding, producing a serrated luminal contour.8,107 The glands are lined by mucinous cells that differentiate progressively toward the surface, with columnar cells having apical mucin vacuoles alternating with goblet cells.8,29,107,108 The crypts taper down to a preserved proliferative zone with regular cells containing small, round nuclei.108 Mitoses are largely restricted to the basal area.8,107 A prominent basement membrane may be seen, and the muscularis mucosae is intact.7,8 Essentially, these tumors appear similar histologically to hyperplastic polyps that occur in the colon (see Chapter 22) (Fig. 28.19).8
Sessile Serrated Adenoma/Polyp
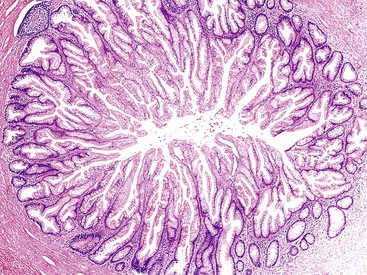
In comparison to hyperplastic polyps, sessile serrated adenoma/polyps (SSA/Ps) show architectural and cytologic atypia and abnormal crypt proliferation. The latter is characterized by displacement of the proliferative zone into the more superficial portions of the crypts. SSA/Ps demonstrate architectural distortion characterized by basal crypts that are branched or grow in a horizontal fashion.29 This results in boot-shaped or inverted T-shaped crypts. Other features include prominent serration and dilatation of the crypts, particularly at the base of the mucosa; fewer neuroendocrine cells; dystrophic goblet cells; increased mitotic figures; and mild nuclear atypia (Fig. 28.20).29 SSA/Ps may develop cytologic dysplasia; these polyps are often designated as “mixed polyps” when they show areas that resemble hyperplasia or SSA/P and other areas with cytologic dysplasia.29 Dysplasia may be of the conventional (adenomatous, intestinal) type or serrated. In either case, dysplastic epithelium shows enlarged ovoid nuclei, increased nucleus-to-cytoplasm (N : C) ratio, hyperchromaticity, nuclear debris, infrequent mitoses, and pseudostratification.29 High-grade dysplasia shows micropapillary epithelial infolding or cribriform architecture with increased nuclear irregularity, nucleoli, hyperchromasia, loss of nuclear polarity, and increased mitoses (Fig. 28.21).29
Traditional Serrated Adenoma
Traditional serrated adenoma (TSA) was originally described in 1990 by Longacre and Fenoglio-Preiser.110 These tumors show a villous growth pattern with complex serration, epithelial cells with abundant eosinophilic cytoplasm and elongated nuclei, and little or no proliferative activity (Fig. 28.22). Conventional dysplasia may also develop in TSAs. One report of 10 serrated adenomas in the appendix (defined by the presence of serrated fronds in more than 50% of dysplastic structures) showed invasive carcinoma in 4, suggesting that TSAs in the appendix may be more aggressive than those that occur in the colon.108 However, in a large series by Yantiss and co-workers,29 a strong association between TSAs and adenocarcinoma was not seen.
Differential Diagnosis
Appendiceal Adenoma and LAMN
On occasion, it is difficult to distinguish serrated lesions of the appendix from adenomas or LAMNs that show serrated crypt architecture. In contrast to adenomas and LAMNs, which often show slender villi with scant lamina propria, most serrated lesions show crypts separated by lamina propria and lack villous architecture (although TSAs can be villous). Complex serration favors a serrated lesion. Tumors that are associated with PP are LAMNs, because the available evidence indicates that serrated lesions do not spread to the peritoneum as PP.
Neuroendocrine Tumors
Well-Differentiated Neuroendocrine Tumor (Carcinoid Tumor)
Clinical Features
Well-differentiated neuroendocrine tumors (NETs) are found in approximately 0.32% to 0.6% of surgically removed appendices.90–92 They occur with greatest frequency in the fourth to fifth decade of life,111–115 although they can occur at any age, including childhood.116–121 Females are affected more often than males, even after accounting for the increased rate of appendectomy among women.111–115,119,122 Most NETs are found incidentally,112,123 although some manifest clinically with appendicitis or recurrent abdominal pain,113,122 particularly in children.116–119,121 In some cases, these tumors, particularly those that arise at the base of the appendix, contribute to the development of appendicitis by occluding the lumen.113,123 Clinical presentation with the carcinoid syndrome (flushing, diarrhea, bronchoconstriction, right-sided endocardial fibrosis) is exceptional in patients with primary NETs of the appendix.116,124
Pathogenesis
Evidence suggests that appendiceal NETs arise from subepithelial neuroendocrine cells.125,126 Argentaffin cells within the lamina propria were initially described by Masson in 1928.127 These cells are associated with S100-positive, unmyelinated nerve fibers in the periglandular plexus.128 The number of subepithelial neuroendocrine cells peaks in the third or fourth decade of life and also increases in frequency in the tip of the appendix.115,129,130 Increased numbers of argentaffin cells in the crypts of appendices that harbor NETs has been reported.131 However, other studies have shown that endocrine cells are not increased in number in the crypts adjacent to NETs.125 Gastrin-releasing peptide and its receptor are coexpressed by some NETs, suggesting a paracrine or autocrine pathway of tumorigenesis,132 but this theory is controversial.
Pathology
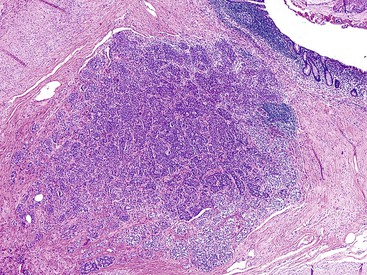
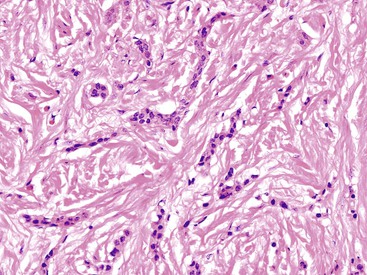
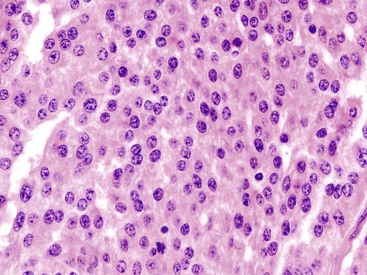
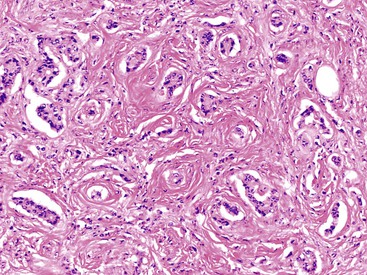
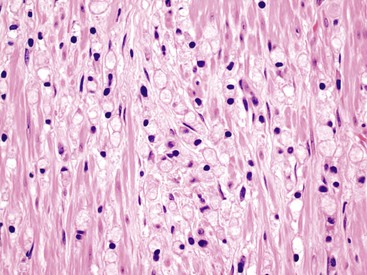
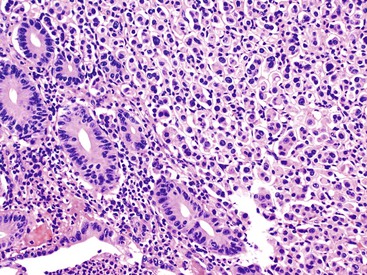
Appendiceal NETs are typically yellow-tan in color with firm consistency (Fig. 28.23). They are most commonly located at the appendiceal tip (70% of cases), followed by the body and then the base.112–114,123 Approximately 75% are smaller than 1 cm in diameter, and they may be hard to detect at gross examination. Approximately 5% are larger than 2 cm.112–114,133 Microscopically, most are enterochromaffin (EC) cell tumors that demonstrate an insular pattern of growth with peripheral palisading of tumor cells (Fig. 28.24).113 L cell tumors are less common. They demonstrate more prominent trabecular architecture (Fig. 28.25). In both types, tumor cells are typically uniform in size and shape and contain a modest amount of eosinophilic, sometimes finely granular, cytoplasm. Tumor cell nuclei show the classic “salt and pepper” chromatin pattern of NET in the GI tract (Fig. 28.26).113 The intervening stroma may be variably fibrotic and may contain a reactive neural proliferation. In some cases, the stroma is densely fibrotic with entrapment of tumor cells (scirrhous pattern).113,114 The overlying mucosa is usually unremarkable, although in some cases, the tumor appears to arise from the deep compartment of the mucosa. NETs extend deeply into the wall and even to the peritoneal surface in 33% to 66% of cases.113,133 Lymphatic invasion is common,113,133 but it can be difficult to differentiate retraction artifact from lymphatic invasion.122,134,135 Perineural invasion is also common; it is reported in approximately 35% of tumors.114 Infiltration of the mesoappendix occurs in 7.5% to 27% of cases (Fig. 28.27),114,122,133 and this feature is related to tumor size. Syracuse and associates found invasion of the mesoappendix in 26% of grossly evident tumors but in only 4% of tumors discovered on microscopic examination.123
Rare NETs demonstrate clear or foamy cytoplasm because of lipid accumulation. This change is usually focal in the tumor, but it may be diffuse. These tumors are termed “lipid-rich”, “clear cell,” or “balloon cell” NETs and are considered to be a morphologic variant, with an immunohistochemical profile and clinical behavior similar to those of classic EC NETs (Fig. 28.28).136,137 Some lipid droplets coalesce to form larger droplets,137 and tumors with cells containing prominent lipid droplets should be distinguished from goblet cell carcinoid tumors. The latter show large mucin vacuoles that appear to push aside the nucleus. Rare NETs show spindle cell morphology.138
Grading of appendiceal NETs is based on mitotic counts or the Ki67 proliferation index, similar to NETs in the rest of the GI tract. Tumors with fewer than two mitoses per 10 high-power fields (HPFs) or a Ki67 index of 2% or less are graded as G1. Tumors with more than two mitoses per 10 HPFs or a Ki67 index of 3% to 20% are graded as G2. Tumors with mitotic counts higher than 20 per 10 HPFs or a Ki67 index greater than 20% are classified as neuroendocrine carcinomas (see later discussion).
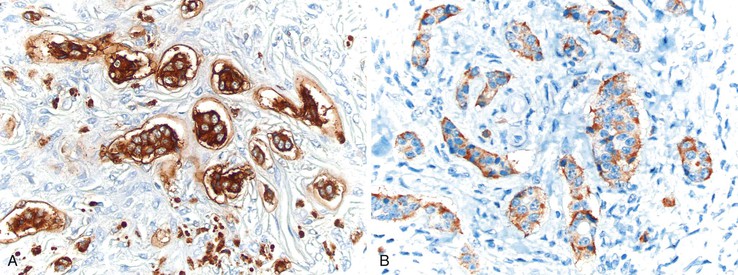
Immunohistochemically, EC tumors stain positively with conventional neuroendocrine markers, including chromogranin, synaptophysin, and neuron-specific enolase (NSE). They also express serotonin and substance P.139,140 S100-positive cells may be identified surrounding tumor islands, analogous to sustentacular cells.125,128,141 EC tumors are negative for carcinoembryonic antigen (CEA).141 TP53 is typically negative in appendiceal NET.142 L cell carcinoids stain for enteroglucagon, pancreatic polypeptide (PP/PYY), and CEA.139,140,143 These tumors stain less commonly for chromogranin A but consistently with chromogranin B and synaptophysin.141,143,144
Differential Diagnosis
Goblet Cell Carcinoid
Clear cell NETs and NETs with a prominent glandular pattern can mimic goblet cell carcinoid tumor. Goblet cell carcinoid tumors are typically composed of smaller nests of cells with goblet-like or signet ring–like features interspersed with eosinophilic granular cells, similar in appearance to Paneth cells. The vacuoles in goblet cell carcinoid tumors are single in number and large in size compared to clear cell NETs, which contain foamy, microvesicular, or rarified cytoplasm. Also, goblet cell carcinoid tumors usually infiltrate the appendix in a circumferential manner, unlike typical NETs, which tend to form a single mass lesion.
Metastatic Carcinoma
Occasionally, an appendiceal NET (particularly the L cell variant) can be mistaken for a metastatic carcinoma, such as metastatic breast carcinoma. Attention to the nuclear chromatin pattern, bland nuclear features, and low mitotic activity in NETs helps avoid this diagnostic pitfall.
Natural History
Patients with appendiceal NETs usually have an excellent prognosis. Lymph node metastases are uncommon, and liver metastases are rare.112–114,145 The reported frequency of metastasis ranges from 1.4% to 8.8%,114,146 but is highly dependent on tumor size: 0% for tumors smaller than 1 cm, 3% to 6.7% for tumors of 1 to 2 cm, and 21% to 30% for tumors larger than 2 cm in maximum dimension.140,147 Patients with local disease have a 5-year survival rate of 92% to 100%, but this rate decreases for patients with regional metastasis (81%) or distant metastasis (31%).111,112,148 Several studies have examined prognostic features of appendiceal NETs. The only consistent finding has been that tumors smaller than 2 cm metastasize infrequently and that those smaller than 1 cm are essentially always benign.113,114,149 Other factors that have been evaluated but dismissed as reliable prognostic factors include depth of invasion, location of the tumor along the length of the appendix, perineural invasion, lymphatic invasion, and serosal involvement.112,114,133,149 Studies that have examined the significance of mesoappendix involvement have yielded conflicting results. Some authors report an increased likelihood of metastatic disease independent of tumor size,123,150 whereas others have shown that mesoappendiceal invasion has no effect on prognosis for tumors smaller than 2 cm in diameter.114,134 Still, most authorities believe that the presence of mesoappendiceal involvement and its extent (limited versus extensive) should be documented in the pathology report.151
Treatment
Simple appendectomy is considered curative for small NETs (<1 cm), provided that the margin of resection is negative for tumor. According to some authors, the presence of tumor at the margin of resection should prompt consideration of ileocolectomy.122,123 Because tumors larger than 2 cm in diameter carry an increased risk of metastatic disease, right hemicolectomy is recommended, particularly if there is involvement of the mesoappendix or vascular invasion.149 However, fewer than 20% of hemicolectomy specimens show residual tumor or lymph node metastases.112,133,145
The management of tumors that measure between 1 and 2 cm has not been standardized. Other factors should be considered in the decision process, such as mesoappendix and serosal involvement, angiolymphatic invasion, gross evidence of regional lymph node metastases at the time of appendectomy, patient age, and comorbidities.112,145,147 Given that these tumors have a low risk of metastatic disease and follow an indolent clinical course even when metastatic, conservative management is advised for older patients.112,147,149 Appendiceal NETs in children without metastasis at presentation are often clinically benign.116 Several authors recommend right hemicolectomy in patients of this age group only for tumors that demonstrate regional lymph node metastases, incomplete resection, or in other unusual circumstances.118–120 However, one study found that tumor size greater than 1.5 cm in children and adolescents was predictive of lymph node involvement, with a sensitivity of 77.8% and a specificity of 66.7%, and recommended right hemicolectomy in such cases.152
Tubular Carcinoid
Tubular carcinoid tumors are typically small neoplasms composed of discrete tubules lined by bland, cuboidal-shaped epithelial cells with a small amount of luminal mucin (Fig. 28.29).153,154 Rarely, they may contain a few goblet cells or Paneth cells.153 Mitotic figures are absent or very rare.153 These tumors are typically located in the tip of the appendix, often involve the submucosa and muscularis propria, and are almost always an incidental finding at appendectomy. The tubules appear compressed within a fibrotic stroma, which may render them difficult to recognize on routinely stained slides. Keratin immunohistochemistry may be helpful to delineate the true size of the tumor. Unlike classic NETs, tubular carcinoids stain with CEA and glucagon (Fig. 28.30) but not with serotonin.141 Synaptophysin is positive, but chromogranin immunohistochemistry may be variable and weak.141,153,154 Tubular carcinoids are considered by some to represent L cell carcinoids with a prominent acinar pattern.144
Differential Diagnosis
Goblet Cell Carcinoid
Tubular carcinoid tumors can have a small nested appearance similar to goblet cell carcinoid tumors. However, in goblet cell carcinoids, most cells are of the goblet cell type, whereas goblet cells are absent or infrequent in tubular carcinoids. Tubular carcinoid tumors are not typically associated with abundant extracellular mucin and are typically much smaller than goblet cell carcinoid tumors. They do not grow circumferentially around the appendiceal lumen.
Metastatic Adenocarcinoma
The small tubular pattern of tubular carcinoid tumors can be mistaken for metastatic breast or prostate cancer. Positive staining for CEA can add to the confusion with adenocarcinoma. Tubular carcinoids do not show nuclear features of malignancy, and they express neuroendocrine markers.
Natural History
Tubular carcinoid tumors are invariably benign.
Treatment
Appendectomy is curative for tubular carcinoid.
Neuroendocrine Carcinoma
Neuroendocrine carcinomas of the appendix are similar to those that occur elsewhere in the GI tract. They are poorly differentiated tumors that frequently demonstrate neuroendocrine differentiation when evaluated immunohistochemically and carry a poor prognosis. They are classified as small cell or large cell type. Large cell tumors have more abundant cytoplasm, vesicular nuclei, and prominent nucleoli.155 These tumors are rare in the appendix.155
Mixed Glandular and Endocrine Neoplasms
Nomenclature
Tumors with both glandular and endocrine differentiation are known as amphicrine tumors. In the appendix, these tumors are designated as goblet cell carcinoid. Synonyms include mucinous carcinoid tumor,156 adenocarcinoid,157 crypt cell carcinoma,158 and microglandular carcinoma.4 Related tumors with aggressive histologic patterns have been designated as either mixed carcinoid-adenocarcinoma or adenocarcinoma ex goblet cell carcinoid.153,159 They are classified by the WHO as mixed adenoneuroendocrine carcinoma (MANEC) (see later discussion).
Goblet Cell Tumors
Goblet cell tumors are a distinctive type of tumor composed of clusters of goblet-like mucinous cells, endocrine cells, and a variable number of Paneth cells.
Pathogenesis
Isaacson demonstrated that many goblet cell tumors show staining for immunoglobulin A, secretory component, and lysozyme, all of which are features of intestinal crypt cells, and proposed the term “crypt cell carcinoma.”158 Others have also noted similarity between the tumor nests and normal crypts. These observations raised theories regarding the derivation of these tumors and whether they represent proliferation of pluripotential crypt cells or are simply neoplasms that retain the capacity to differentiate into crypt-like units.153,157,158
Ultrastructural and molecular studies have shown that the pathogenesis of goblet cell tumors shares features with both conventional NETs and adenocarcinomas. Electron microscopic studies have provided evidence of both mucin production and neuroendocrine differentiation (electron-dense granules) in the same cells.136,170–173 van Eeden and colleagues174 showed absent staining for TP53 and nuclear β-catenin expression, normal DPC4 expression, and rare KRAS mutations in 16 goblet cell tumors, similar to conventional carcinoid tumors. However, they also found expression of CEA and cytokeratin 7 (CK7), similar to adenocarcinomas and unlike conventional carcinoid tumors. Goblet cell tumors were also marked with an antibody against the transcription factor Atoh1/Math1, which is involved in the differentiation of intestinal stem cells toward progenitor cells with a secretory phenotype.
Some molecular studies have demonstrated a greater degree of similarity between goblet cell tumors and conventional carcinoid tumors. Stancu and colleagues160 found allelic loss of chromosomes 11q, 16q, and 18q as frequently in goblet cell tumors as in ileal carcinoids. They also found absent KRAS, CTNNB1 (β-catenin), and DPC4 mutations and no TP53 overexpression by immunohistochemistry. These findings are similar to the molecular features of conventional carcinoid tumors. Ramnani and associates175 were also unable to detect mutations of KRAS in 22 goblet cell tumors. They found mutations of TP53 in 25% of goblet cell tumors and in 44% of classic carcinoid tumors of the appendix.
Pathology
Goblet Cell Carcinoid
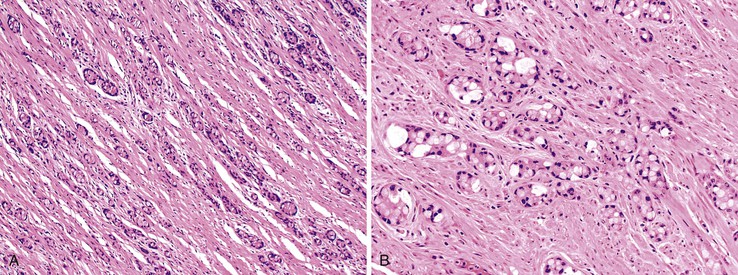
Goblet cell carcinoid tumors usually appear as an area of circumferential thickening of the appendix and may be recognized only on microscopic examination of the specimen. Tumor size averages 2 cm.160 Microscopically, goblet cell carcinoids show circumferential infiltration of the appendix by discrete nests of mucinous cells that resemble goblet cells or, in cases in which the mucin compresses the nucleus to the edge of the cell, signet ring cells (Fig. 28.31).156 The mucin within goblet-like cells stains positive with periodic acid–Schiff (PAS) or Alcian blue stain.156 Admixed with goblet-like or signet ring–like cells are cells with eosinophilic cytoplasm and, occasionally, Paneth cells (Fig. 28.32).153,157,158,176,177
Most tumor cell clusters lack central lumina.156,177 Cell clusters that contain central lumina resemble intestinal crypts.153,157 Nuclear pleomorphism is typically mild, and mitoses are infrequent. Perineural and intralymphatic invasion may be present.156 Extracellular mucin pools harboring clusters of tumor cells are not an uncommon finding, but, unlike pure mucinous adenocarcinomas, the epithelium within pools of mucin usually remains clustered, without the formation of large cribriform-shaped units. Some cell clusters reveal preserved central lumina resembling “floating” crypts (Fig. 28.33).153,177
The tumor typically does not elicit a stromal reaction. Subtle atypical changes may be seen in the overlying mucosa, consisting of goblet cell disarray and slight nuclear enlargement.31 Tumor cells may infiltrate the lamina propria diffusely without the appearance of a clear point of origin, or they may seem to arise from the crypts.177 Immunohistochemically, goblet cell carcinoid tumors are keratin and CEA positive.153,171,176 They also express Cam 5.2, CK7, CK20, CDX2, and E-cadherin.174 Silver stains show the presence of argentaffin and argyrophil cells; these can be numerous, but some cases show few or only rare positive cells.4,153,156,158,176–178 Similar results are obtained by immunohistochemistry for chromogranin and synaptophysin.159,174,179 In some cases, small numbers of cells may be positive for glucagon, particularly in areas that resemble a tubular carcinoid tumor.153
Mixed Adenoneuroendocrine Carcinoma (MANEC)
In 1990, Burke and colleagues defined carcinomatous growth patterns in goblet cell carcinoids as fused or cribriform glands, single-file structures, diffusely infiltrating signet ring cells, or sheets of tumor cells.153 They also classified tumors as having carcinomatous growth patterns if they demonstrated compressed goblet cell nests with small glands and signet ring cells with little or no intervening stroma, or extracellular mucin pools harboring glandular epithelium that demonstrated gland fusion and lacked lumina (Box 28.4). Tumors lacking significant carcinomatous growth patterns (<25%) were designated goblet cell carcinoid. Tumors with greater than 50% carcinomatous growth pattern were classified as mixed carcinoid-adenocarcinoma. In Burke’s series, no tumor had between 25% and 50% carcinomatous growth patterns.
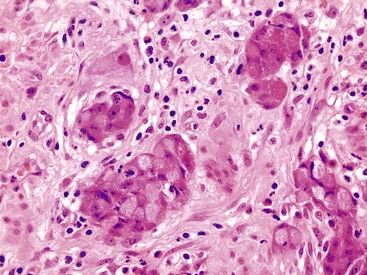
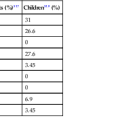
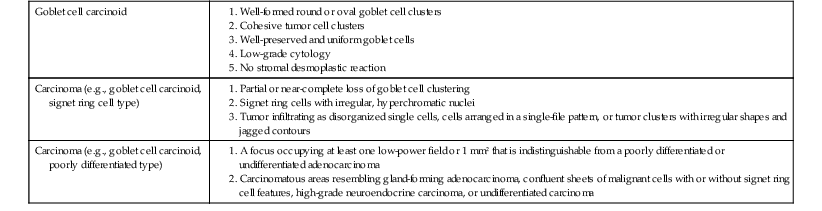
In 2008, Tang and co-workers159 proposed a classification of goblet cell carcinoid tumors (Table 28.2) that distinguishes pure goblet cell carcinoid tumor from carcinomas that arise in association with them (carcinoma ex goblet cell carcinoid, signet ring type or poorly differentiated type). By definition, these two carcinomas should contain, at minimum, focal areas in the tumor that are recognizable histologically as goblet cell carcinoid tumors. As described earlier, pure goblet cell carcinoids are characterized by the presence of cohesive, well-formed, round or oval goblet cell clusters with well preserved and uniform goblet cells and low-grade cytologic features (low N : C ratio, no or only mild pleomorphism, low mitotic rate). In contrast, adenocarcinoma ex goblet cell carcinoid, signet ring cell type demonstrates partial or near-complete loss of goblet cell clustering as a major criterion. Tumors consist of signet ring cells with hyperchromatic and irregular nuclei that infiltrate in the form of disorganized single cells, cells arranged in a single-file pattern, or tumor clusters with irregular shapes and jagged contours (Fig. 28.34). Adenocarcinoma ex goblet cell carcinoid, poorly differentiated carcinoma subtype, is defined as a goblet cell carcinoid tumor with at least one focus (occupying at least 1 low-power field or 1 mm2) that is indistinguishable from a conventional poorly differentiated or undifferentiated adenocarcinoma (Fig. 28.35). These carcinomatous areas resemble conventional gland-forming adenocarcinomas of the GI tract, or consist of sheets of malignant cells with or without signet ring cell features, or show morphologic features of a high-grade neuroendocrine carcinoma or undifferentiated carcinoma. In their study, tumors in this group showed positive staining for TP53 and MUC1 but absent MUC2 expression, suggesting progression of the goblet cell tumor.
Table 28.2
Classification of Goblet Cell Tumors of the Appendix
| Goblet cell carcinoid |
From Tang LH, Shia J, Soslow RA, et al. Pathologic classification and clinical behavior of the spectrum of goblet cell carcinoid tumors of the appendix. Am J Surg Pathol. 2008;32:1429-1443.
Differential Diagnosis
Signet Ring Cell Adenocarcinoma versus Pure Goblet Cell Carcinoid Tumor
Goblet cell carcinoid tumors may be confused with signet ring cell carcinoma. Signet ring cell carcinomas are more disorganized in their growth pattern and more atypical cytologically (increased mitoses, necrosis, pleomorphism) than pure goblet cell carcinoid tumors. If a portion of the tumor shows pure goblet cell carcinoid tumor morphology, then the tumor should be classified as an adenocarcinoma ex goblet cell carcinoid (see earlier discussion). Because pure goblet cell carcinoid tumors can reveal focal areas in which the tumor cells infiltrate singly (Fig. 28.36), this feature is insufficient for a diagnosis of primary signet ring cell carcinoma of the appendix.
Conventional Adenocarcinoma versus Pure Goblet Cell Carcinoid Tumor
Occasionally, the glandular profiles in pure goblet cell carcinoid tumors can be focally dilated and irregular, or they can form strips or incomplete glands, in which case distinction from a primary mucinous adenocarcinoma or another variant of adenocarcinoma may be difficult. Furthermore, immunohistochemistry for neuroendocrine markers can be misleading, because some pure goblet cell carcinoid tumors stain weakly, focally, or not at all, and some adenocarcinomas contain areas of neuroendocrine cell differentiation. Glandular differentiation with columnar cells, strips of glandular epithelium, and cribriform glands favors a diagnosis of adenocarcinoma. A precursor lesion in the lumen of the appendix (adenoma) suggests an adenocarcinoma because goblet cell tumors do not usually have a recognizable precursor lesion, although the two may coexist as a collision tumor.180 As mentioned earlier, the presence of a discrete area of the tumor that shows goblet cell carcinoid features is sufficient for a diagnosis of carcinoma ex goblet cell carcinoid.
Metastatic Adenocarcinoma versus Pure Goblet Cell Carcinoid Tumor
Some metastatic adenocarcinomas to the appendix can assume a nested appearance, mimicking goblet cell carcinoid tumor. For instance, metastatic breast carcinoma, prostate cancer, pancreatic acinar cell carcinoma, and other tumors can assume this morphologic appearance. However, metastatic tumors usually show a higher degree of cytologic atypia and more frequent mitoses.
Natural History
In general, goblet cell carcinoid tumors have a prognosis intermediate between that of conventional carcinoid tumors and that of mucinous adenocarcinomas.81,157 Various investigators have attempted to define more precisely the histologic features that predict malignant behavior. Features that have been associated with a worse prognosis include tumor extension beyond the appendix at presentation, atypical histologic features, and more than 2 mitoses per 10 HPFs.157,178 The Ki67 index has not proved useful for predicting prognosis.181 Perineural invasion and lymphatic invasion are common but do not correlate with prognosis.31,157
Outcome correlates well with the pathologic classifications of Burke153 and Tang.159 In Burke’s series,153 patients with goblet cell carcinoid had a uniformly benign course, whereas 8 of 10 patients with mixed carcinoid-adenocarcinoma died of the disease (average follow-up, 16 months). These latter tumors were highly likely to have spread outside the appendix at presentation and to metastasize to the cecum, ileum, serosal surfaces, and lymph nodes. In Tang’s series,159 the 3-year survival rates for pure goblet cell carcinoid tumor; adenocarcinoma ex goblet cell carcinoid, signet ring cell type; and adenocarcinoma ex goblet cell carcinoid, poorly differentiated type were 100%, 85%, and 17%, respectively, and the 5-year rates were 100%, 36%, and 0%. The prognostic significance of the histologic category persisted even when only stage IV tumors were analyzed.
Treatment
The treatment of goblet cell tumors is not standardized, partly because of the wide spectrum of cases for which this term has been applied historically. Whether appendectomy alone is sufficient for tumors that are confined to the appendix and do not show atypical histologic features is subject to debate. Some authors recommend right hemicolectomy for all patients, but others do not.136,161 A positive proximal appendiceal margin should prompt serious consideration for right hemicolectomy. Tumors that show extension beyond the appendix are best treated with right hemicolectomy and possibly chemotherapy, particularly if there is evidence of progression to adenocarcinoma. Oophorectomy should be considered for women with tumors that demonstrate poorly differentiated histology or high stage.159
Other Mixed Glandular and Endocrine Tumors
Rare tumors in the appendix demonstrate a combination of glandular and endocrine elements but are difficult to classify according to the current classifications; they represent either collision tumors or composite tumors. Others may be examples of disproportionate glandular or endocrine differentiation in an amphicrine tumor. Examples include composite classic carcinoid tumor and adenocarcinoma,182,183 (Fig. 28.37) goblet cell carcinoid with mucinous cystadenoma,180 combined classic carcinoid and goblet cell carcinoid,184,185 and mixed adenocarcinoma and high-grade neuroendocrine carcinoma (Fig. 28.38).138,139
Secondary Tumors (Other Than Lymphoma)
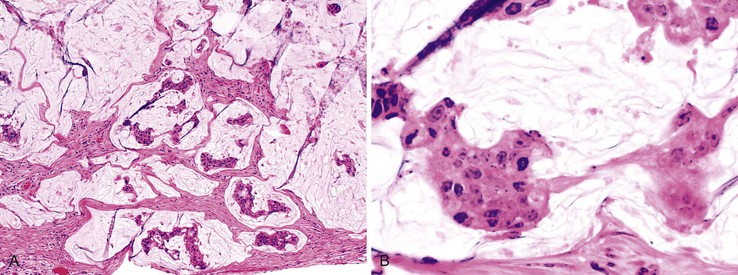
In females, the most common secondary tumors are metastatic ovarian carcinoma, particularly serous adenocarcinoma, and peritoneal serous carcinoma (Fig. 28.39). The usual scenario is involvement of the appendix with advanced-stage ovarian carcinoma.186–189 As many as 80% of women with advanced ovarian cancer have appendiceal metastases.186 However, patients with early disease may, rarely, be found to have appendiceal serosal involvement on microscopic examination. Therefore, some authors advocate appendectomy as part of the primary staging procedure for all patients with ovarian carcinoma.163,190
Rarely, gastric carcinoma, breast carcinoma (Fig. 28.40),191,192 bronchogenic adenocarcinoma and pulmonary small cell carcinoma,193–197 endometrial carcinoma,198 cervical carcinoma,199 nasopharyngeal carcinoma,200 cholangiocarcinoma (Fig. 28.41),201 mediastinal choriocarcinoma,202 transitional cell carcinoma,203 and prostatic adenocarcinoma may metastasize to the appendix.203,204 Metastatic tumors may obstruct the appendiceal lumen, resulting in the development of acute appendicitis. In some cases, this is the initial manifestation of the malignant neoplasm. Occasionally, it is difficult to determine the location of the primary tumor, particularly in cases with bulky disease. In those instances, determining the location of the greatest bulk of disease can be helpful. For example, Krukenberg tumors with extensive involvement of the appendix but also of the stomach are more likely to be primary in the stomach, because pure signet ring cell carcinomas are statistically more common in that organ than in the appendix. Metastatic tumors can also mimic primary appendiceal tumors, particularly mucinous adenocarcinoma, goblet cell carcinoid, and NET (Fig. 28.42).204