Chapter 10 Epilepsy
Electroencephalogram (EEG)
Normal and Abnormal
The routine EEG records cerebral electrical activity detected by “surface” or “scalp” electrodes (Fig. 10-1). Four frequency bands of cerebral activity, represented by Greek letters, emanate from the brain (Table 10-1).
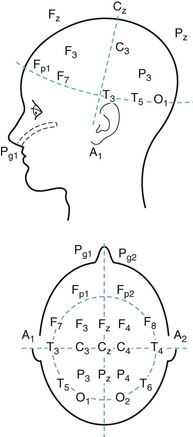
FIGURE 10-1 In the standard array of scalp electrodes, most are named for the underlying cerebral region (e.g., frontal, temporal, central, parietal, and occipital). Odd-numbered ones are on the left, and even-numbered ones on the right. The Pg electrodes attach to nasopharyngeal leads and the A electrodes, the ears (aural leads).
| Activity | Hz (cycles/second) | Usual Location |
|---|---|---|
| Alpha | 8–13 | Posterior |
| Beta | > 13 | Anterior |
| Theta | 4–7 | Generalized* |
| Delta | < 4 | Generalized* |
The normal background rhythm in an awake adult consists of waves of activity in the alpha range of 8–13 cycles per second (Hertz [Hz]) detectable mostly over the occipital region (Fig. 10-2). Neurologists refer to this pattern as the posterior dominant rhythm. It is prominent when individuals are relaxed with their eyes closed, but disappears if they open their eyes, concentrate, or are apprehensive. When people undergoing an EEG merely fix their gaze on a clock or add two single-digit numbers, faster rhythms replace alpha activity. Preoccupations, concerns, or anxiety eliminate alpha activity. Because alpha activity reflects an anxiety-free state, it represents an important parameter in “alpha training,” biofeedback, and other behavior modification techniques.
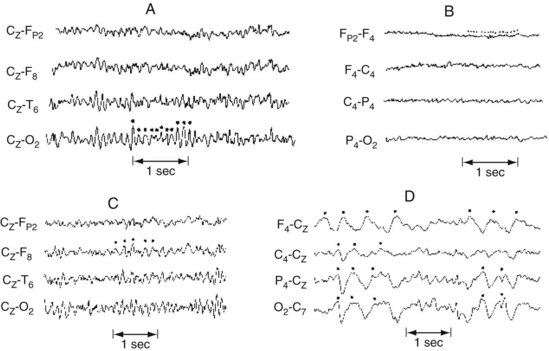
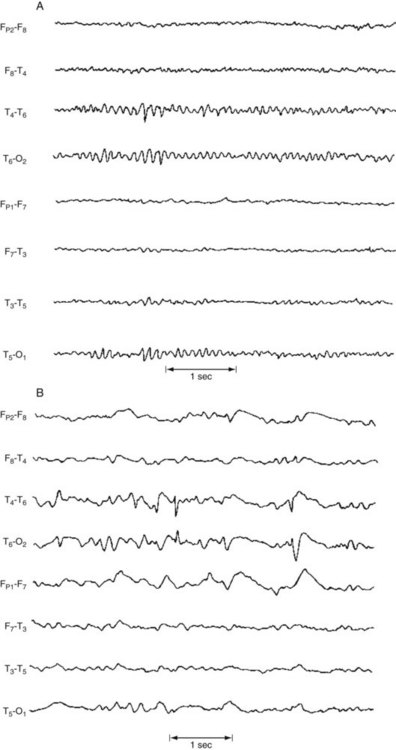
FIGURE 10-2 A, Alpha rhythm consists of regular 8–13-Hz activity overlying the occipital lobe. B, Beta rhythm consists of low-voltage, irregular >13-Hz activity overlying the frontal lobe. C, Theta rhythm consists of 4–7-Hz activity overlying the right frontal lobe. D, Delta activity consists of high-voltage <4-Hz activity present over the entire hemisphere.
Theta (4–7 Hz) and delta (< 4 Hz) activities occur normally in children and everyone during deep sleep, but are usually absent in healthy alert adults. When present over the entire brain, theta or delta activity in wakefulness often indicates a neurodegenerative illness, such as Alzheimer disease, or a metabolic derangement. Continuous focal slow activity with phase reversal in bipolar montages (Fig. 10-3) sometimes originates in an underlying cerebral lesion; however, the absence of theta or delta activity certainly does not exclude one.
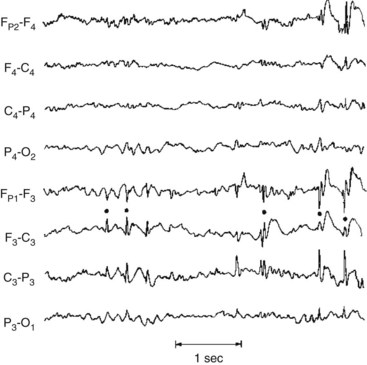
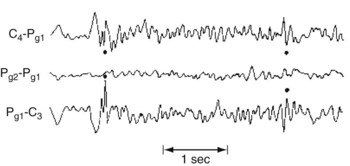
FIGURE 10-3 This bipolar montage shows four channels from the right side (upper four) and four from the left (lower four). Each side progresses from the frontal to the occipital region. On at least five occasions (marked by dots), sharp waves and spikes, in phase reversal, appear to point toward each other. These sharp waves and spikes originate from the common electrode, F3, situated over the left frontal lobe. Such isolated, phase-reversed sharp waves are associated with seizures, but, without additional clinical or EEG evidence, they are insufficient for a diagnosis of seizures.
Seizures
In some epilepsy patients, specially placed electrodes reveal abnormalities undetectable by ordinary scalp electrodes. For example, anterior temporal scalp, nasopharyngeal, or sphenoidal electrodes can detect discharges from the temporal lobe’s inferior-medial (mesial or medial) surface (Fig. 10-4).
FIGURE 10-4 Nasopharyngeal electrodes, which are inserted through the nostrils, reach the posterior pharynx. There, separated by the thin sphenoid bone, they are adjacent to the temporal lobe’s medial surface, which is the focus (origin) of about 80% of complex partial seizures. (Figures in Chapter 20 show the relatively large distance between the temporal lobe’s medial surface and the scalp, and the closeness of the temporal lobe to the sphenoid bone.) Sphenoidal electrodes are inserted through the skin to reach the lateral surface of the sphenoid wing. Electrodes in this location are near the temporal lobe’s inferior surface. (Although nasopharyngeal and sphenoidal electrodes are valuable, specially placed scalp electrodes, new arrays, electronic filters, and critical reading of the EEG may be just as accurate and less invasive.) To pinpoint a seizure focus in anticipation of its surgical removal, neurosurgeons place a grid of electrodes in the subdural space.
With children, an evaluation could actually begin with parents making videos of suspected seizures or other episodic disturbances, including temper tantrums, breath-holding spells, night terrors, other parasomnias (see Chapter 17), dopamine-responsive dystonia, and other intermittent abnormal movements (see Chapter 18).
Toxic-Metabolic Encephalopathy
During the initial phase of toxic-metabolic encephalopathy (delirium), when patients have only subtle behavioral or cognitive disturbances, theta and delta activity replaces alpha activity. The organization of the EEG deteriorates as the patient’s sensorium disintegrates. The EEG in toxic-metabolic encephalopathy almost always shows generalized slowing and disorganization. Additional EEG changes point to specific diagnoses. Hepatic and uremic encephalopathies characteristically produce triphasic waves (Fig. 10-5). In fact, with hepatic failure, triphasic waves often appear before bilirubin levels rise. While metabolic derangements are the most common cause of triphasic waves, this EEG finding may also be seen with toxic levels of several medications, including lithium. Benzodiazepine use produces beta activity. Herpes simplex encephalitis produces spikes and periodic lateralizing epileptiform discharges over the temporal lobes.
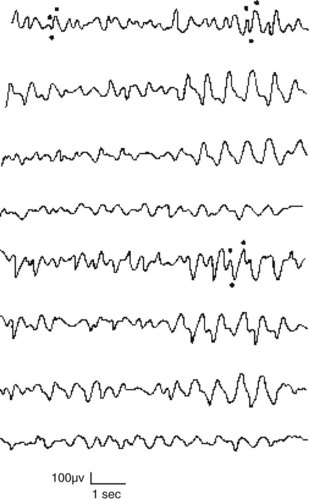
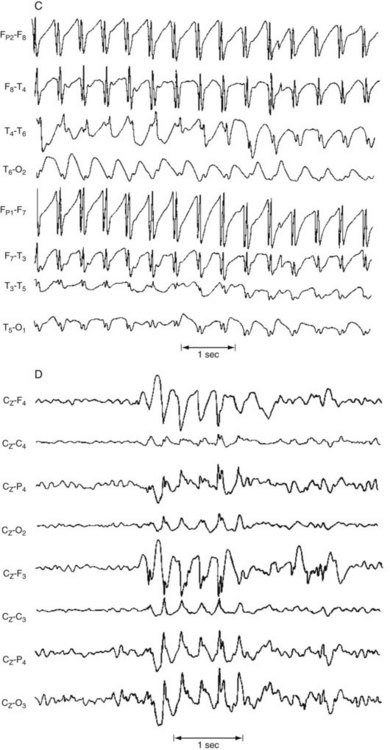
FIGURE 10-5 This EEG obtained from a patient with hepatic encephalopathy reveals characteristic triphasic waves overlying the frontal lobes (the first and fifth channels in this montage). In addition to the presence of triphasic waves, the EEG lacks the normal, organized alpha activity over the occipital lobes (the fourth and eighth channels).
Dementia
Vascular dementia also induces EEG abnormalities. However, these changes cannot reliably differentiate vascular dementia from Alzheimer disease dementia (see Chapters 7 and 11).
In contrast, the EEG is almost definitive in diagnosing subacute sclerosing panencephalitis and common, sporadic Creutzfeldt–Jakob disease (see Chapter 7). In these conditions – characterized clinically by dementia and myoclonus – the EEG shows periodic sharp-wave complexes (Fig. 10-6). (Variant Creutzfeldt–Jakob disease [“mad cow disease”] fails to produce these EEG changes [see Chapter 7].)
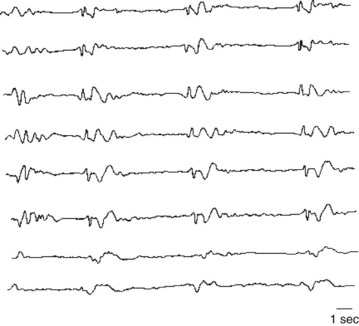
FIGURE 10-6 Periodic sharp-wave complexes classically appear in all channels as fairly regular, 1–3-second bursts of electrical activity followed by minimal activity. Neurologists often describe this EEG pattern as “burst suppression.” Myoclonic jerks represent the clinical counterpart of the periodic complexes. Together myoclonic jerks and periodic complexes are cardinal features of two illnesses characterized by dementia: subacute sclerosing panencephalitis and Creutzfeldt–Jakob disease (see Chapter 7).
The EEG can also help distinguish between pseudodementia and dementia – to the extent that they constitute separate entities (see Chapter 7). In pseudodementia the EEG ideally would remain normal, but in dementia from almost any cause, it would show slowing. For the many patients with a mixture of depression and mild dementia, the EEG cannot measure each condition’s relative contribution to cognitive impairment.
Structural Lesions
The EEG does not reliably detect or exclude structural lesions, even those that frequently cause seizures, such as brain tumors and cysticercosis. In fact, the EEG is normal in many of these conditions and, when abnormal, does not distinguish among them. Computed tomography (CT) and especially magnetic resonance imaging (MRI) are standard and, despite their expense, cost-effective for detecting structural lesions (see Chapter 20). Although CT may suffice, MRI better detects small structural lesions. More important, MRI is especially effective in detecting mesial temporal sclerosis, which underlies many complex partial seizures (see later).
Altered States of Awareness
The EEG shows distinctive changes during normal progressively deeper stages of sleep and during dreaming. Coupled with monitors of ocular movement and muscle activity in the polysomnogram, the EEG is critical in diagnosing sleep disturbances (see Chapter 17), which can include sleep-related behavioral disturbances and involuntary movement disorders, as well as seizures.
The EEG is also useful in diagnosing the locked-in syndrome, a condition in which patients cannot speak or move their trunk or limbs. Although patients in the locked-in syndrome appear comatose or demented, they remain fully alert and in possession of their cognitive capacity (see Chapter 11). The locked-in syndrome most commonly results from either an infarction in the base of the lower brainstem or extensive cranial and peripheral nerve damage. With their cerebral hemispheres and upper brainstem intact, patients retain normal cerebral activity and normal EEG activity.
Seizure Varieties
The two major seizure categories are partial (or focal)-onset seizures and primary (generalized) seizures. Most partial seizures are subclassified either as partial seizures with elementary symptoms or partial seizures with complex symptoms, and most generalized seizures are subclassified as either absence seizures (absences) or tonic-clonic seizures (Box 10-1).
Elementary Partial Seizures
Partial seizures with elementary motor symptoms, formerly called focal motor seizures, typically consist of rhythmic jerking (clonic movement) of a body region that may be limited to one finger or extend to an entire side of the body (Fig. 10-7). These seizures may evolve into partial status epilepticus or undergo secondary generalization. Sometimes, in a “Jacksonian march,” a seizure discharge spreads along the motor cortex and creates movements, which are usually clonic, beginning in one finger and extending to the entire arm and then the face.
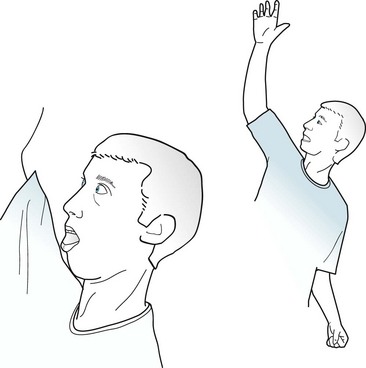
FIGURE 10-7 In this patient having a partial seizure with motor symptoms, his head, neck, and eyes deviate toward the right, his right arm extends, and his left arm flexes. A focal epileptic discharge in the contralateral left frontal lobe is producing this typical “adversive posture.”
Patients with auditory symptoms, which are attributable to temporal lobe lesions, frequently report hearing repetitive noises, musical notes, or single meaningless words. Visual symptoms, which are attributable to occipital lesions, usually consist of bright lights. Sometimes, though, these seizures may produce lines, spots, or splotches of color that move slowly across the visual field or, like a view through a kaleidoscope, rotate around the center of vision. Physicians must distinguish elaborate visual seizure phenomena from visual hallucinations due to other causes (see Box 12-1).
EEG and Etiology
During elementary partial seizures, EEGs show spikes, sharp or slow waves, or spike-wave complexes overlying the seizure focus. For example, during seizures with motor symptoms, EEG abnormalities are usually detectable in channels overlying the frontal lobe (Fig. 10-8), and, during the interictal period, EEGs may still show occasional spikes in the same channels.
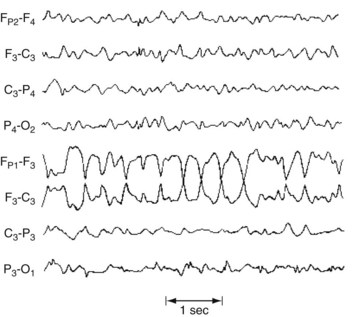
FIGURE 10-8 During a partial seizure with motor symptoms, this EEG contains a paroxysm of 4-Hz sharp-wave activity with phase reversals referable to the F3 electrode. Because the F3 electrode overlies the left frontal region, the seizure probably consists of right face or arm motor activity and, in some of the cases, a deviation of the head and eyes to the right.
In most cases, neurologists cannot determine the cause of partial seizures. Of those cases where neurologists can establish the cause, the patient’s age at the onset of the seizures is one of the most important factors. For example, when young children develop partial seizures, typical causes are congenital cerebral malformations, such as cortical dysgenesis, and neurocutaneous disorders (see Chapter 13). In young adults, common causes of elementary partial seizures are head trauma, arteriovenous malformations (AVMs), and previously asymptomatic congenital injuries. However, posttraumatic seizures are not associated with trivial head injuries, but with serious trauma, such as trauma causing more than 30 minutes of unconsciousness, depressed (not just linear) skull fractures, intracranial hematomas, and penetrating wounds.
Complex Partial Seizures
Ictal Symptoms
During most of a complex partial seizure, patients usually display a blank stare and are inattentive and uncommunicative. They always – by definition – have impaired consciousness. In most cases, they also have partial or complete memory loss, amnesia, presumably because seizure discharges besiege the limbic system in the temporal lobe. The amnesia is so striking that it may appear to be a patient’s only symptom. (Thus, physicians should strongly consider complex partial seizures among the neurologic causes of the acute amnestic syndrome [see Box 7-1].)
Physical manifestations of complex partial seizures usually consist of only simple, repetitive, purposeless movements (automatisms) of the face and hands. Present in about 80% of complex partial seizures originating in the temporal lobe, common automatisms include repetitive swallowing, mouthing, kissing, lip smacking, and lip licking – oral automatisms – or fumbling with clothing, scratching, rubbing the abdomen, or fidgeting – manual automatisms (Fig. 10-9). Other physical manifestations are simple actions, such as standing, walking, pacing, or even driving; however, sometimes these actions are simply ingrained tasks that continue despite the seizure. In addition, more than 25% of patients utter brief phrases or unintelligible sounds.
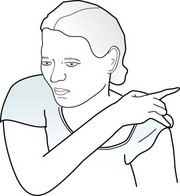
FIGURE 10-9 During complex partial seizures, patients typically appear dazed. They perform only rudimentary, purposeless actions, such as pulling on their clothing, and pay little or no attention to their surroundings or examiners. Their hands and fingers move in clumsy and misdirected patterns. Repetitive, simple oral, limb, or body movements, automatisms, such as lip smacking, occur in approximately 80% of cases with a temporal lobe focus and the majority of those with absence seizures.
Sex, Violence, and Aggression
During seizures, patients sometimes fumble with buttons, tug at their clothing, or make rudimentary masturbatory movements. They may even seem to undress partially. However, these patients are not deliberately exposing themselves or attempting to engage in sex. Except for very rare instances, seizures are unaccompanied by erotic or interactive sexual behavior. In fact, most seizure-like symptoms that develop during sexual activity, such as lightheadedness, are simply manifestations of anxiety. (On the other hand, severe headaches that develop during sexual intercourse are ominous [see coital headache and subarachnoid hemorrhage, Chapter 9].)
Immediate Postictal Symptoms
Immediately after a complex partial seizure, which has an average duration of 2–3 minutes, patients characteristically experience confusion, clouding of the sensorium, disorientation, flat affect, and sleepiness. However, seizures occasionally lead not to somnolence, inactivity, and withdrawal, but to agitation, i.e., postictal agitation. If seizures involve the brain’s language region and cause transient aphasia (see Chapter 8), postictal symptoms may be more pronounced. Similarly, if the seizure focus includes the cortical areas involved with motor function, patients may have a Todd’s hemiparesis. For 15–40 minutes after a seizure, many patients have measurable physiologic changes: Approximately 40% of them have an elevated serum prolactin concentration and focal EEG depression.
Frontal Lobe Seizures
Frontal lobe seizures, even compared to complex partial seizures of temporal lobe origin, are most apt to cause bizarre behavior and the few instances of aggressive violence. With their behavioral manifestations and EEG changes usually undetectable, frontal lobe seizures mimic PNES. In addition, when frontal lobe seizures arise exclusively during sleep, they mimic sleep disorders (see Chapter 17).
Testing During and Between Complex Partial Seizures
EEG
During a complex partial seizure, the EEG most often shows paroxysms of spikes, slow waves, or other abnormalities in channels overlying the temporal or frontotemporal region. Even though a seizure focus may be unilateral, bilateral EEG abnormalities appear because of additional foci, interhemispheric projections, or “reflections.” Nasopharyngeal and other specially placed leads may capture temporal lobe discharges that routine scalp electrodes fail to detect (Fig. 10-10).
Other Tests
Because elementary partial and complex partial seizures usually originate from structural lesions, neurologists routinely order MRIs unless patients have a contraindication (see Chapter 20). With even greater resolution than CT and freedom from artifacts produced by the bones surrounding the middle fossa, MRI reveals mesial temporal lobe sclerosis, tuberous sclerosis nodules, small strokes, and cryptic AVMs, as well as overt lesions (see Fig. 20-26). (MRI with thin cuts through the temporal lobes is often required to detect mesial temporal lobe sclerosis.)
Comorbid Conditions and Their Treatment
Cognitive Impairment
Of individuals with either a congenital intellectual disability (which the preliminary version of the DSM-5 calls Intellectual Developmental Disorder, but neurologists persist in calling “mental retardation”) or cerebral palsy, 10–20% have comorbid epilepsy (see Chapter 13). In them, epilepsy usually appears before age 5 years and its incidence increases in proportion to their physical and intellectual impairments. Of individuals institutionalized because of these disorders, 40% have epilepsy. Also, children with autism and, more so, Rett syndrome are susceptible to seizures (see before).
Destructive Behavior
Suicide
A landmark 2008 meta-analysis of clinical trials involving AEDs suggested an iatrogenic component. It found that, among epilepsy patients, AEDs represented almost a twofold risk factor for “suicidality” (suicide acts or ideation). Suicidality was greater in patients taking AEDs for epilepsy than for other indications, including mood stabilization, and among individuals taking multiple AEDs rather than a single AED. Subsequent studies found that only certain AEDs, such as those associated with causing depressive symptoms, placed epilepsy patients at risk. In sharp contrast to the 2008 study, Arana et al. (see References), in an equally credible one, determined that AEDs posed no risk of suicidality in patients with epilepsy.
Crime and Interictal Violence
Personality Traits
Classic studies, such as those by Bear and Fedio (see References), described “temporal lobe epilepsy” patients as distinctively circumstantial in thinking, hyposexual, humorless, “sticky” in interpersonal relations, and overly concerned with general philosophic and religious questions. These patients showed excessive and compulsive writing (hypergraphia). Supporting studies suggested that the presence of these abnormal traits depended on whether the seizure focus was in the right or left temporal lobe. Right-sided foci supposedly predisposed patients to anger, sadness, and elation, but left-sided ones to ruminative and intellectual tendencies.
Treatment
Antiepileptic Drugs
Neurologists routinely prescribe AEDs as the primary treatment for epilepsy (Table 10-2). They generally prefer AED monotherapy to polytherapy (polypharmacy) because it minimizes side effects, noncompliance, and cost. However, when epilepsy remains refractory to monotherapy, neurologists usually add a second AED. Also, neurologists, occasionally thinking that small doses of two AEDs may be better than large doses of one AED, prescribe two AEDs. Nevertheless, only a minority of patients benefit from the addition of a second AED, and less than 5% benefit from the addition of a third one.
| AED | Usual Daily Dose (mg) | Therapeutic Serum Concentration (µ/ml)* |
|---|---|---|
| Carbamazepine (Tegretol)† | 600–1200 | 5–12 |
| Divalproex (Depakote) | 1500–2000 | 50–100 |
| Ethosuximide (Zarontin) | 2000 | 40–100 |
| Gabapentin (Neurontin) | 900–1800 | |
| Lamotrigine (Lamictal) | 100–500 | |
| Levetiracetam (Keppra) | 1500–3000 | |
| Phenytoin (Dilantin) | 300–400 | 10–20 |
| Topiramate (Topamax) | 400 |
N.B.: These AEDs have mostly replaced phenobarbital and its closely related AED, primidone (Mysoline), which both cause sedation, cognitive impairment, and depression. Also, barbiturates, particularly when used in children and adults with brain damage, may produce a “paradoxical reaction” of excitement and hyperactivity rather than sedation. On the other hand, most children with epilepsy and comorbid hyperactivity may safely use stimulants.
*Recommended concentrations vary and should be altered depending on the clinical situation. Often a “subtherapeutic level” is sufficient, and increasing the dose will create side effects without improving seizure control.
†To reach a steady state, five “half-lives” are required (e.g., carbamazepine 4–6 days; phenytoin 5–10 days; and valproate 3–6 days).
AEDs and Pregnancy
Malformations associated with AEDs are probably induced during the first trimester when the central nervous system (CNS) forms. The most serious – meningomyelocele and other neural tube defects (see Chapter 13) – have been closely, but not exclusively, associated with both carbamazepine (0.5%) and valproate (1%). In addition, AEDs increase the rate of cleft lip, cleft palate, and ventricular septal defect.
Vagus Nerve Stimulation
Vagus nerve stimulation (VNS), a technique for reducing refractory seizures, consists of an implanted pacemaker-like device that stimulates the cervical portion of the vagus nerve (cranial nerve X). VNS sends impulses upward along the vagus nerve’s afferent fibers to synapse in the medulla’s nucleus solitary nucleus, which projects to upper brainstem, hypothalamus, limbic system, and cortex (see Chapters 4 and 16). The vagus nerve seems the most appropriate conduit to the brain because it is readily accessible in its cervical portion, contains almost entirely afferent fibers but few pain-conveying ones, and carries, on the left side, few efferent cardiac fibers.
Surgery
Another surgical requirement for patients with refractory seizures is a single frontal or temporal lobe lesion clearly identifiable on clinical, EEG, and radiographic testing. In addition, if the lesion is located in their dominant hemisphere, patients often must also undergo a Wada test, functional MRI, or similar testing to determine if surgery might entail removal of a portion of the cerebral cortex that would lead to aphasia, amnesia, or other neuropsychologic problems (see Chapter 8). If both temporal lobes were injured from birth or during surgery, patients undergoing even a unilateral temporal lobectomy may suffer permanent amnesia or the Klüver–Bucy syndrome (see Chapters 12 and 16).
A different and more invasive procedure may rarely be indicated in individuals with intractable bilateral frontal seizures or infants with atonic seizures (“drop attacks”) who have no readily resectable seizure focus. In a commissurotomy or corpus callosotomy, a neurosurgeon longitudinally severs the anterior two-thirds or entire corpus callosum, interrupting the spread of discharges between cerebral hemispheres. Because it “splits” apart the cerebral hemispheres, this procedure may cause the split-brain syndrome (see Fig. 8-9).
Generalized Seizures
Absences
Absences usually begin between ages 4 and 10 years and disappear in early adulthood. The seizures, which may occur many times daily, consist of 2–10-second lapses in attention, often accompanied by automatisms, subtle clonic limb movements, or blinking (Fig. 10-11). The blinking sometimes occurs rhythmically at 3 Hz, which is the frequency of the associated EEG abnormality. During the seizure, children maintain muscle tone and bladder control; however, they cannot carry on mental and physical activities. If they suffer from frequent seizures, children may appear inattentive, dull, or even mentally retarded. After a seizure, as though it had never occurred, children have no retrograde amnesia, confusion, agitation, or sleepiness.
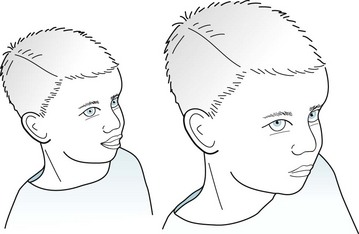
FIGURE 10-11 Seen in the midst of an absence, this 8-year-old boy has staring that has suddenly and unexpectedly interrupted his playing with a friend. He has become glassy-eyed and mute, his eyes have rolled upward, and he blinks at 3 Hz. Although he has lost consciousness, he maintains bodily tone and does not become incontinent. At the end of his seizure, which will last for only 2–10 seconds, he will resume playing and be unaware of the pause.
EEG, Etiology, and Treatment
During an absence, the EEG shows synchronous 3-Hz spike-and-wave complexes in all channels (Fig. 10-12). Even in the interictal period, an EEG reveals occasional asymptomatic bursts of 3-Hz spike-and-wave complexes lasting 1–1.5 seconds. This discharge reflects an underlying abnormality in the reciprocal circuits between the thalamus and the cerebral cortex.
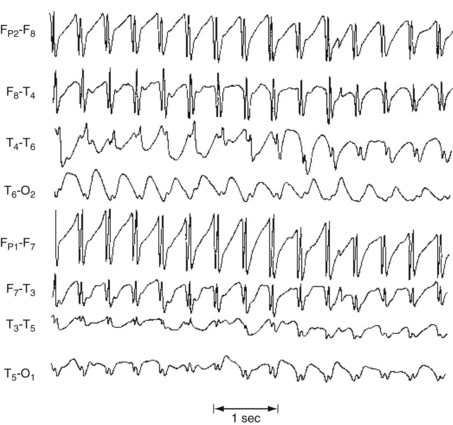
FIGURE 10-12 Absences are often so brief and liable to be confused with either inattention or complex partial seizures that neurologists often attempt to precipitate them for diagnostic purposes in an EEG laboratory. One common practice consists of asking a child suspected of having absences to count numbers slowly while hyperventilating. An absence would be evident when the counting pauses and the EEG shows regular, symmetric, and synchronous 3-Hz spike-and-wave complexes arising from and returning to a normal EEG background – as in this reproduction.
Although absences bear a superficial resemblance to complex partial seizures, physicians should distinguish the two conditions by their different manifestations, EEG abnormalities, prognoses, and treatments (Table 10-3).
TABLE 10-3 Comparison of Partial Complex and Absence Seizures
| Feature | Partial Complex | Absence |
|---|---|---|
| Aura | Often | Never |
| Consciousness | Impaired | Lost at onset |
| Movements | Usually simple, repetitive, but may include complex activity | Blinking and facial and finger automatisms |
| Postictal behavior | Amnesia, confusion, and tendency to sleep | No abnormality, except amnesia for ictus |
| Frequency | 1–2 per week | Several daily |
| Duration | 2–3 minutes | 1–10 seconds |
| Precipitants | Hyperventilation, photic stimulation | |
| Electroencephalogram | Spikes and polyspike and waves, usually over one or both temporal regions | Generalized 3-Hz spike-and-wave complexes |
| Antiepileptic drugs | Carbamazepine, phenytoin | Ethosuximide, valproate |
Tonic-Clonic Seizures
Even though they are both varieties of generalized seizures and share, at their onset, the characteristic loss of consciousness and generalized EEG abnormalities, tonic-clonic and absence seizures have completely different clinical manifestations, treatment, and prognosis. Tonic-clonic seizures begin at any age after infancy, persist through adult life, and cause massive motor activity and profound postictal residua. Although patients may have a prodrome of malaise or a depressed mood, tonic-clonic seizures usually arise as an unheralded explosion. In the initial tonic phase, patients lose consciousness, roll their eyes upward, and, as if to form a back-bending arch, extend their neck, trunk, and limbs. Immediately afterwards, in a dramatic clonic phase, patients violently and symmetrically jerk their limbs, neck, and trunk (Fig. 10-13).
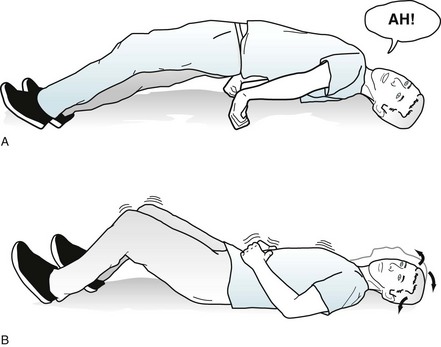
FIGURE 10-13 A, This man in the tonic phase of a tonic-clonic seizure arches his torso and extends his arms and legs. He assumes this position because of the relatively greater strength of the extensor muscles compared to the flexor muscles. Simultaneous diaphragm, chest wall, and laryngeal muscle contractions force air through his tightened larynx to produce the shrill “epileptic cry.” During this phase, he may also bite his tongue and lose control of his urine. B, In the clonic phase, his head, neck, and legs contract symmetrically and forcefully for about 10–20 seconds. Saliva, aerated and often blood-tinged from tongue lacerations, froths from his mouth. His pupils dilate and he sweats profusely. Finally, his muscular contractions lose strength. The seizure usually ends with stertorous breathing. In the immediate postictal period, he remains unresponsive. Before regaining consciousness, he often passes through a state of confusion and agitation, loosely termed “postictal psychosis.”
If electronic filters could eliminate the superimposed muscle electric artifact during the tonic phase, the EEG would show repetitive, increasingly higher-amplitude spikes occurring with increasing frequency in all channels. Then, in the clonic phase, slow waves would interrupt the spikes, which usually decrease in frequency (Fig. 10-14).
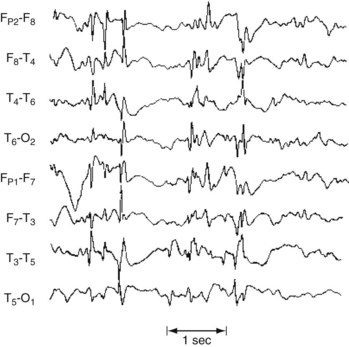
FIGURE 10-14 During a tonic-clonic seizure, the EEG ideally shows paroxysms of spikes, polyspikes, and occasional slow waves in all channels; however, muscle artifact can obscure this pattern. Even during interictal periods, the EEG contains multiple bursts of generalized spikes in the background. In contrast to occasional temporal lobe spikes, this pattern confirms a diagnosis of epilepsy in patients with seizures.
Etiology
Most seizures arising in sleep emerge during nonrapid eye movement (NREM) sleep (see Chapter 17). Rapid eye movement (REM) sleep, in contrast, remains relatively seizure-free. Some epileptic patients have seizures predominantly or exclusively during sleep, and others experience them only on awakening. To avoid committing a diagnostic error, physicians should evaluate patients with exclusively sleep-related episodes for a sleep disorder masquerading as epilepsy (see later and Chapter 17).
Nonepileptic Conditions
Related Issues
Cerebrovascular Disturbances
TIAs resemble partial seizures because both may cause momentarily impaired consciousness and physical deficits (see Chapter 11). In general, however, TIAs have slower onset and rarely cause loss of consciousness.
Of the various cerebrovascular disturbances, transient global amnesia (TGA) most closely resembles complex partial seizures (see Chapter 11). During a TGA episode, a frequent cause of transient amnesia (see Box 7-1), patients cannot remember new information, such as the date, location, and examining physicians; however, they retain basic memories, such as their name, address, and telephone number. This discrepancy separates TGA from psychogenic amnesia, in which basic as well as new information is lost.
Migraines, which also partly result from vascular disturbance, may induce episodes of confusion and personality change followed by a tendency to sleep (see Chapter 9). Migraines particularly mimic seizures when they lead to transient hemiparesis and abnormal EEGs. In fact, migraine patients have a greater than usual incidence of seizures, making migraines a risk factor for epilepsy. The diagnosis of migraines relies almost entirely on the patient’s history.
Sleep Disorders
Bizarre behavior during the night might represent a sleep disorder (see Chapter 17) rather than a nocturnal seizure. Some sleep disorders so closely mimic seizures that only polysomnography or continuous EEG-video monitoring can distinguish them. For example, children might have a night terror or another parasomnia, and older adults are liable to develop REM behavior disorder. In patients who experience repeated bouts of unresponsiveness, the narcolepsy-cataplexy syndrome, which includes several seizure-like symptoms, such as momentary loss of body tone (cataplexy) and dream-like hallucinations, represents a diagnostic alternative to seizure disorder. Unlike seizures, the narcolepsy-cataplexy syndrome has no aura, motor activity, incontinence, or subsequent symptoms. Moreover, during attacks of narcolepsy, an EEG or polysomnography shows REM activity.
Metabolic Aberrations
Hyperventilation commonly induces giddiness, confusion, and other psychologic symptoms that can be confused with seizures (see Chapter 3). When prolonged and deep, hyperventilation may precipitate seizures, but probably only in epileptic individuals.
AEDs, Surgery, and Other Treatments
Andersohn F, Schade R, Willich SN, et al. Use of antiepileptic drugs in epilepsy and the risk of self-harm or suicidal behavior. Neurology. 2010;75:335–340.
Arana A, Wentworth CE, Ayuso-Mateos JL, et al. Suicide-related events in patients treated with antiepileptic drugs. N Engl J Med. 2010;363:542–551.
Bourgeois BF. Determining the effects of antiepileptic drugs on cognitive function in pediatric patients with epilepsy. J Child Neurol. 2004;19(Suppl 1):S15–S24.
Chong DJ, Bazil CW. Update on anticonvulsant drugs. Curr Neurol Neurosci Rep. 2010;10:308–318.
French JA, Pedley TA. Initial management of epilepsy. N Engl J Med. 2008;359:166–176.
French JA, Kanner AM, Bautista J, et al. Efficacy and tolerability of the new antiepileptic drugs I: Treatment of new onset epilepsy. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2004;62:1252–1260.
French JA, Kanner AM, Bautista J, et al. Efficacy and tolerability of the new antiepileptic drugs II: Treatment of refractory epilepsy. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2004;62:1261–1273.
Jentink J, Loane MA, Dolk H, et al. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med. 2010;362:2185–2193.
Pack AM, Morrell MJ, Randall A, et al. Bone health in young women with epilepsy after one year of antiepileptic drug monotherapy. Neurology. 2008;70:1586–1593.
Patorno E, Bohn RL, Wahl PM, et al. Anticonvulsant medications and the risk of suicide, attempted suicide, or violent death. JAMA. 2010;303:1401–1409.
Patsalos PN, Perucca E. Clinically important drug interactions in epilepsy: Interactions between antiepileptic drugs and other drugs. Lancet Neurol. 2003;2:473–481.
Penovich P, Gaily E. What can we say to women of reproductive age with epilepsy? Neurology. 2005;64:938–939.
Perucca P, Carter J, Vahle V, et al. Adverse antiepileptic drug effects: Towards a clinically and neurobiologically relevant taxonomy. Neurology. 2009;72:1223–1229.
Salinsky MC, Storzbach D, Spencer DC, et al. Effects of topiramate and gabapentin on cognitive abilities in healthy volunteers. Neurology. 2005;64:792–798.
Schmitz B. Psychiatric syndromes related to antiepileptic drugs. Epilepsia. 2005;40:s65–s70.
Vinten J, Adab N, Kini U, et al. Neuropsychological effects of exposure to anticonvulsant medication in utero. Neurology. 2005;64:949–954.
Devinsky O, Barr WB, Vickrey BG, et al. Changes in depression and anxiety after resective surgery for epilepsy. Neurology. 2005;65:1744–1749.
Hemb M, Velasco TR, Parnes MS, et al. Improved outcomes in pediatric epilepsy surgery: The UCLA experience, 1986–2008. Neurology. 2010;74:1768–1775.
Langfitt JT, Westerveld M, Hamberger MJ, et al. Worsening of quality of life after epilepsy surgery: Effect of seizures and memory decline. Neurology. 2007;68:1988–1994.
Roswall LA, Engman E, Samuelsson H, et al. Cognitive outcome 10 years after temporal lobe epilepsy surgery. Neurology. 2010;74:1977–1985.
Chavel SM, Westerveld M, Spencer S. Long-term outcome of vagus nerve stimulation for refractory partial epilepsy. Epilepsy Behav. 2003;4:302–309.
Privitera MD, Welty TE, Ficker DM. Vagus nerve stimulation for partial seizures. Cochrane Database Syst Rev. 1, 2002. CD002896
Uthman B, Reichl AM, Dean JC, et al. Effectiveness of vagus nerve stimulation in epilepsy patients: A 12-year observation. Neurology. 2004;63:1124–1126.
Interictal Comorbidities and Their Treatment
Bacon D, Fisher RS, Morris JC, et al. American Academy of Neurology position statement on physician reporting of medical conditions that may affect driving competence. Neurology. 2007;68:1174–1177.
Bear DM, Fedio P. Quantitative analysis of interictal behavior in temporal lobe epilepsy. Arch Neurol. 1977;34:454–467.
Blumer D, Montouris G, Davies K, et al. Suicide in epilepsy: Psychopathology, pathogenesis, and prevention. Epilepsy Behav. 2002;3:232–241.
Devinsky O. Postictal psychosis: Common, dangerous, and treatable. Epilepsy Currents. 2008;8:31–34.
Ettinger AB, Kanner AM, eds. Psychiatric Issues in Epilepsy, 2nd ed, Philadelphia: Lippincott Williams & Wilkins, 2006.
Ettinger AB, Reed ML, Goldberg JF, et al. Prevalence of bipolar symptoms in epilepsy vs other chronic health disorders. Neurology. 2005;65:535–540.
Gross A, Devinsky O, Westbrook LE, et al. Psychotropic medication use in patients with epilepsy: Effect on seizure frequency. J Neuropsychiatry Clin Neurosci. 2000;12:4.
Hermann BP, Seidenberg M, Dow, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006;60:80–87.
Kanner AM. Depression and epilepsy: A review of multiple facets of their close relation. Neurol Clin. 2009;27:865–880.
Krauss GL, Ampaw L, Krumholz A. Individual state driving restrictions for people with epilepsy in the US. Neurology. 2001;57:1780–1785.
Lee KC Finley PR, Alldredge BK. Risk of seizures associated with psychotropic medications: Emphasis on new drugs and new findings. Expert Opin Drug Saf. 2003;2:233–247.
Nathaniel-James DA, Brown RG, Maier M, et al. Cognitive abnormalities in schizophrenia and schizophrenia-like psychosis of epilepsy. J Neuropsychiatry Clin Neurosci. 2004;16:472–479.
Schachter SC. Visions: Artists Living With Epilepsy. Amsterdam: Elsevier; 2003.
Pillmann F, Rohde A, Ullrich A, et al. Violence, criminal behavior, and the EEG. J Neuropsychiatry Clin Neurosci. 1999;11:454–457.
Tellez-Zenteno JF, Patten SB, Jette N, et al. Psychiatric comorbidity in epilepsy: A population-based analysis. Epilepsia. 2007;48:2336–2344.
Tracy JI, Dechant V, Sperling MR, et al. The association of mood with quality of life ratings in epilepsy. Neurology. 2007;68:1101–1107.
Whitman S, Coleman TE, Patmon C, et al. Epilepsy in prison: Elevated prevalence and no relationship to violence. Neurology. 1984;34:775–782.
Psychogenic Nonepileptic Seizures
Benbadis S, Agrawal V, Tatum WO. How many patients with psychogenic nonepileptic seizures also have epilepsy? Neurology. 2001;57:915–917.
Goldstein LH, Chalder T, Chigwedere C, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures. Neurology. 2010;74:1986–1994.
Marchetti RL, Kurcgant D, Neto JG, et al. Psychiatric diagnoses of patients with psychogenic nonepileptic seizures. Seizure. 2008;16:247–253.
Martin R, Burneo JG, Prasad A, et al. Frequency of epilepsy in patients with psychogenic seizures monitored by video-EEG. Neurology. 2003;61:1791–1792.
McKenzie P, Oto M, Russell A, et al. Early outcomes and predictors in 260 patients with psychogenic nonepileptic attacks. Neurology. 2010;74:64–69.
Prasad A, Mendez M, Kuzniecky RI. Teddy bears: An observational finding in patients with non-epileptic events. Neurology. 2003;61:714–715.
Reuber M, Pukrop R, Bauer J, et al. Prognostic factors in psychogenic nonepileptic seizures. Ann Neurol. 2003;53:305–311.
Szaflarski JP, Hughes C, Szaflarski M, et al. Quality of life in psychogenic nonepileptic seizures. Epilepsia. 2003;44:236–242.
Boro A, Haut S. Medical comorbidities in the treatment of epilepsy. Epilepsy Behav. 2003;4:S2–S12.
Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349:1257–1266.
Crompton DE, Berkovic SF. The borderland of epilepsy: Clinical and molecular features of phenomena that mimic epileptic seizures. Lancet Neurol. 2009;370:370–381.
Hara H. Autism and epilepsy: A retrospective follow-up study. Brain Dev. 2007;29:486–490.
Haut SR, Hall CB, Masur J, et al. Seizure occurrence: Precipitants and prediction. Neurology. 2007;69:1905–1910.
Mula M, Jauch R, Cavanna A, et al. Clinical and psychological definition of the interictal dysphoric disorder of epilepsy. Epilepsia. 2008;49:650–656.
Shahar E, Kramer U, Mahajnah M, et al. Pediatric-onset gelastic seizures: Clinical data and outcome. Pediatr Neurol. 2007;37:29–34.
Yerby MS, Kaplan P, Tran T. Risks and management of pregnancy in women with epilepsy. Cleve Clin J Med. 2004;71(Suppl 2):S25–S37.
Centorrino F, Price BH, Tuttle M, et al. EEG abnormalities during treatment with typical and atypical antipsychotics. Am J Psychiatry. 2002;159:109–115.
Coburn KL, Lauterbach EC, Boutros NN, et al. The value of quantitative electroencephalography in clinical psychiatry: A report by the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2006;18:460–500.
Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: Magnetoencephalography (MEG). Neurology. 1992;42:1–4.
Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment of digital EEG, quantitative EEG, and EEG brain mapping. Neurology. 1997;49:277–292.
Chapter 10 Questions and Answers
1–4. Match each electroencephalogram (EEG) with the appropriate interpretation (see pages 226–227):
9-d; 10-c; 11-a; 12-b; 13-f (rapid eye movement [REM] sleep); 14-i; 15-g; 16-e and j.
21–24. Identify the following statements as true or false.
21. Psychotropic-induced EEG changes may invalidate the EEG in diagnosing neurologic illness.
22. Benzodiazepines and barbiturates induce beta EEG activity.
23. Lithium at toxic levels, clozapine, and tricyclic antidepressants may cause EEG spikes and sharp waves.
24. In terms of psychotropic-induced EEG changes, clozapine, olanzapine, and trifluoperazine produce the most, and quetiapine, loxapine, and haloperidol the least.
29. Emergency room physicians admitted to the neurology service a 28-year-old man who had several seizures. Previous evaluations attributed his seizures to a congenital cerebral injury. The patient had been enrolled in a methadone program for narcotic addiction as well as an epilepsy clinic. After an intravenous benzodiazepine controlled the seizures, physicians changed his AED to phenytoin and renewed his methadone. Several days later he developed agitation, anxiety to the point of incoherence, diaphoresis, and tachycardia. Which should the consulting psychiatrist prescribe?
32. Which statement regarding depression in epilepsy patients is true?
33. Which statement regarding the schizophreniform psychosis that develops in epilepsy is false?
c. Levetiracetam is mostly excreted unchanged by the kidneys. The others require hepatic metabolism.
48. A 23-year-old medical student was experimenting with smoking marijuana. Its effect, completely different than he anticipated, was anxiety and fear. The student was brought to the emergency room with hallucinations, agitation, fever, and nystagmus. Increasing mental and physical agitation soon developed, culminating in a seizure. Of the following, which is the most likely culprit?
67. Nonconvulsive status epilepticus
68. Seizure-like activity under conscious control
69. Activity not under conscious control that mimics seizures
72. Which two statements regarding the relationship of interictal violence to epilepsy are true?
75. A commercial airliner from South America to New York crashed after exhausting its fuel supply. A young man who had been one of the passengers was brought to the hospital with blunt abdominal trauma. Shortly after arriving in the emergency room, he became agitated, incoherent, diaphoretic, and hypertensive. Although physicians found no sign of head injury, he developed status epilepticus and unstable vital signs. During one seizure, several ruptured condoms passed through his rectum. Other condoms, still intact and containing a white powder, subsequently passed out of his rectum. What is the most likely cause of his seizures and unstable vital signs?
88. Which of the following statements is untrue concerning suicide in epilepsy patients?
95. A 52-year-old woman suffered from complex partial seizures that began when she was a teenager. Multiple AED regimens had produced mental dulling and other side effects, and still allowed one or two seizures each week. One year before a psychiatric consultation she underwent partial left temporal lobectomy for seizure control. Subsequently, she remained seizure-free even without any AEDs. However, she developed postoperative anxiety, fearfulness, dysphoria, and “moodiness.” CCTV-EEG monitoring during this time showed no epileptic activity. Which would be the best strategy to restore her preoperative mental status?
98. A 25-year-old woman who has epilepsy that is well controlled with carbamazepine developed pharyngitis. Because she is allergic to penicillin, her physician prescribed erythromycin. Although she initially felt that the pharyngitis subsided, she rapidly developed ataxia, nystagmus, and diplopia. In retrospect, what is the cause of her new symptoms?
100. Two weeks before his wife brought him to the psychiatric emergency room, a 40-year-old well-regarded high-school teacher, who has had epilepsy since childhood, had a several-day cluster of eight complex partial seizures that sometimes underwent secondary generalization. Vigorous treatment in another emergency room controlled those seizures. Although too groggy and confused to return to work, he was free of seizures, oriented, and pleasant. However, he then began to push away his food, pace around the house, and complain about the neighbors’ noise intruding into his school plans. He became verbally, although not physically, aggressive with his brother. His threats of killing a neighbor finally drove his wife to bring him to the psychiatry emergency room. He has no family history of psychiatric illness. There is no evidence of his using drugs of abuse or being noncompliant with his AEDs. Which is the most likely explanation of this patient’s behavior?
101. The parents of a 5-year-old boy have noticed that for the previous 6 months their son has developed brief, completely unprovoked episodes of laughter. During the episodes, which have a duration of about 30 seconds, they cannot communicate with him or interrupt his spontaneous outburst. Also, he does not seem to be particularly happy before or during the laughter. Once the parents began to study it, they noticed that rather than laughing normally with gusto, their son seemed to be saying, “Hee, hee, hee.” A pediatric neurologist found that the boy was developmentally and physically normal. CCTV-EEG monitoring showed generalized spike-and-wave discharges during the events that it captured. MRI revealed a hypothalamic hamartoma. Which is the most accurate diagnosis?
102. Friends bring a 45-year-old man to the emergency room after they witnessed a generalized tonic-clonic seizure. He reports daily alcohol intake of 1 pint of vodka and six beers. However, he has not had anything to drink in 3 days because he ran out of money. He had a similar event 2 years ago following abstinence. His physical examination shows mild generalized tremulousness and tachycardia, but no other abnormalities. His head CT shows mild cortical atrophy for age, but is otherwise normal. Which AED should his physicians start?
104. For many years, a 34-year-old homeless woman, with epilepsy since age 10 years, has had persistent hallucinations, paranoia, and social isolation. Her seizures are usually complex partial, but many undergo secondary generalization, and occur frequently, in part because she is noncompliant with her AED regimen. She has low normal intelligence and a mild right hemiparesis. Which is the most accurate diagnosis of her condition?
106. The police bring a teenage girl, dressed in “punk clothing,” to the psychiatry emergency room because she has been found sitting alone on a park bench, confused, and belligerent. On examination, she is inattentive and unable or unwilling to reveal her personal or medical history. She does not follow requests, participate in an examination, or speak, except to repeat a single, unintelligible phrase. She has loud, repetitive lip smacking. She is afebrile and normotensive, but she has tachycardia. An extensive medical and neurologic evaluation, including an MRI, discloses no other abnormalities. Repeated tests for drugs of abuse, pregnancy, and alcohol are negative. Finally, her mother arrives and explains that the patient has been epileptic but noncompliant with her AED regimen. Which procedure(s) should the prudent physician initiate?