CHAPTER 106 Epidural Adhesiolysis
INTRODUCTION
Chances are each of us will experience low back pain at some point in our lives. The usual course is rapid improvement, but 5–10% of patients develop persistent symptoms.1 In 1997, the total impact of low back pain on industry in the United States was estimated at US$171 billion.2 The medical treatment of low back pain in 1990 amounted to US$13 billion.3 Treatment varies from conservative therapy with medication and physical therapy to minimally and highly invasive pain management interventions. Surgery is sometimes required in those patients who have progressive neurological deficits or those who have failed other therapies. Surgery is successful in the majority of patients, but an unlucky few continue to have pain and neurological symptoms. A quandary arises as to whether a repeat surgery should be attempted or an alternative intervention should be sought. This is the exact quandary that the epidural adhesiolysis procedure was designed to address. It was developed to break down scar formation, deliver site-specific corticosteroids and local anesthetic drugs directly to the target, and reduce edema with hypertonic saline. Epidural adhesiolysis has afforded patients reduction in pain and neurological symptoms without the expense and sometimes long recovery period associated with repeat surgery, and often prevents the need for surgical intervention.
PATHOPHYSIOLOGY OF EPIDURAL FIBROSIS (SCAR TISSUE) AS A CAUSE OF LOW BACK PAIN WITH RADICULOPATHY
The etiology of low back pain with radiculopathy is not well understood. Kuslich and colleagues addressed this issue when they performed 193 lumbar spine operations on patients given local anesthesia. Their study revealed that sciatica could only be produced by stimulation of a swollen, stretched, restricted (i.e. scarred) or compressed nerve root.4 Back pain could be produced by stimulation of several lumbar tissues, but the most common tissue of origin was the outer layer of the anulus fibrosus and posterior longitudinal ligament. Stimulation for pain generation of the facet joint capsule rarely generated low back pain, and facet synovium and cartilage surfaces of the facet or muscles were never tender.5
The contribution of fibrosis (scar tissue) to the etiology of low back pain has been debated.6–8 There are many possible etiologies of epidural fibrosis, including surgical trauma, an annular tear, infection, hematoma, or intrathecal contrast material.9 These etiologies are well documented in the literature. LaRocca and Macnab10 demonstrated the invasion of fibrous connective tissue into postoperative hematoma as a cause of epidural fibrosis, while Cooper and colleagues11 reported periradicular fibrosis and vascular abnormalities occurring with herniated intervertebral discs. McCarron et al.12 investigated the irritative effect of nucleus pulposus upon the dural sac, adjacent nerve roots, and nerve root sleeves independent of the influence of direct compression upon these structures. Evidence of an inflammatory reaction was identified by gross inspection and microscopic analysis of spinal cord sections after homogenized autogenous nucleus pulposus was injected into the lumbar epidural space of four dogs. In the control group consisting of four dogs injected with normal saline, the spinal cord sections were grossly normal. Parke and Watanabe showed significant evidence of adhesions in cadavers with lumbar disc herniation.13
It is widely accepted that postoperative scar renders the nerve susceptible to injury via compressive phenomena.8 It is natural for connective tissue or any kind of tissue to form fibrous layers (scar tissue) as a part of the process that transpires after disruption of the intact milieu.14 Scar tissue is generally found in three components of the epidural space. Dorsal epidural scar tissue is formed by resorption of surgical hematoma and may be involved in pain generation.15 In the ventral epidural space, dense scar tissue is formed by ventral defects in the disc, which may persist despite surgical treatment and continue to produce low back pain/radiculopathy past the surgical healing phase.16 The lateral epidural space includes epiradicular structures outside the root canals, known as the lateral recess or ‘sleeves’ containing the exiting nerve root and dorsal root ganglia, which are susceptible to lateral disc defects, facet overgrowth, and neuroforaminal stenosis.17
Although scar tissue itself is not tender, an entrapped nerve root is. Kuslich et al.4 surmised that the presence of scar tissue compounded pain associated with the nerve root by fixing it in one position and thus increasing the susceptibility of the nerve root to tension or compression. They also concluded that no other tissues in the spine are capable of producing leg pain. In a study of the relationship between peridural scar evaluated by magnetic resonance imaging (MRI) and radicular pain after lumbar discectomy, Ross et al. demonstrated that subjects with extensive peridural scarring were 3.2 times more likely to experience recurrent radicular pain.18
RADIOLOGIC DIAGNOSIS OF EPIDURAL FIBROSIS
Magnetic resonance imaging and computed tomography (CT) scanning are diagnostic tools with 50% and 70% sensitivity and specificity, respectively.14 CT-myelography may also be helpful. None of these imaging techniques can identify epidural fibrosis with 100% reliability. In contrast, epidurography is a technique used with considerable success and it is believed that epidural fibrosis is best diagnosed by performing an epidurogram.19–22 It can detect filling defects in good correlation with a patient’s symptoms in a real-time manner.22 A combination of more than one of these techniques will undoubtedly increase the ability to identify epidural fibrosis.
INDICATIONS FOR EPIDURAL ADHESIOLYSIS
Although originally designed to treat radiculopathy secondary to epidural fibrosis following surgery, the use of epidural adhesiolysis has been expanded to treat a multitude of pain etiologies. These include:23
Contraindications to epidural adhesiolysis include the following absolute contraindications:
Anticoagulant medication
Medications that prolong bleeding parameters should be withheld prior to performing epidural adhesiolysis. The length of time varies depending on the medication taken. A consultation with the patient’s primary physician should be obtained prior to stopping any of these medications. Nonsteroidal antiinflammatory drugs (NSAIDs) and aspirin should be withheld 4 days and 7–10 days prior to the procedure, respectively. Although there is much debate regarding these medications and neuraxial procedures, the authors tend to be on the conservative side. Clopidogrel (Plavix®) should be stopped 7 days prior to the adhesiolysis while ticlopidine (Ticlid®) is held 10–14 days prior.24 The warfarin (Coumadin®) withholding period is patient variable, but 5 days is usually adequate.24 Subcutaneous heparin administration should be stopped at a minimum of 12 hours prior to the procedure, while low molecular weight heparin requires a minimum of 24 hours.24 Over-the-counter medications that prolong bleeding parameters should also be withheld. These include vitamin E, gingko biloba, garlic, ginseng, and St. John’s Wort. In addition, a prothrombin time, a partial thromboplastin time, and a platelet function assay or a bleeding time is obtained to check for coagulation abnormalities. Any elevated value warrants further investigation and postponement of the procedure until those studies are complete.
TECHNIQUE
Caudal approach
An epidurogram is performed using 10 mL of a non-ionic, water-soluble contrast agent. Confirm a negative aspiration for blood or cerebrospinal fluid (CSF) prior to any injection of contrast or medication. Omnipaque® and Isovue® are the agents most frequently used and are suitable for myelography.25,26 Do not use ionic, non-water-soluble contrast agents such as Hypaque® or Renagraffin® or ionic, water-soluble agents such as Conray®.27,28 These agents are not indicated for myelography. Accidental subarachnoid injections can lead to serious untoward events such as seizure and possible death. Slowly inject the contrast agent and observe for filling defects. A normal epidurogram will have a Christmas-tree pattern with the central channel being the trunk and the outline of the nerve roots making up the branches. An abnormal epidurogram will have areas where the contrast does not fill. These are the areas of presumed scarring and typically correspond to the patient’s back and radicular complaints. If vascular uptake is observed, the needle needs to be redirected.
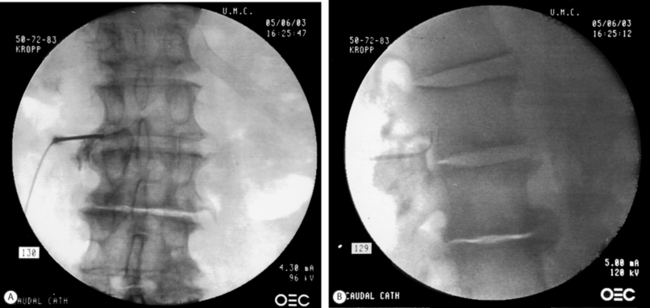
After turning the distal opening of the needle ventrolaterally, insert a TunL Kath™ or TunL-XL™ (stiffer) catheter (Epimed International®) with a bend on the distal tip through the needle. The bend should be 2.5 cm from the tip of the catheter and at a 30° angle. The bend will enable the catheter to be steered to the target level. Under continuous AP fluoroscopic guidance, advance the tip of the catheter towards the ventrolateral epidural space of the desired level. The catheter can be steered by gently twisting the catheter in a clockwise or counterclockwise direction. Avoid ‘propellering’ the tip, i.e. twisting the tip in circles, as this makes it more difficult to direct the catheter. Do not advance the catheter up the middle of the sacrum as this makes guiding the catheter to the ventrolateral epidural space more difficult. Ideal location of the tip of the catheter in the AP projection is in the foramen just below the midportion of the pedicle shadow (Fig. 106.1A). Check a lateral projection to confirm that the catheter tip is in the ventral epidural space (Fig. 106.1B).
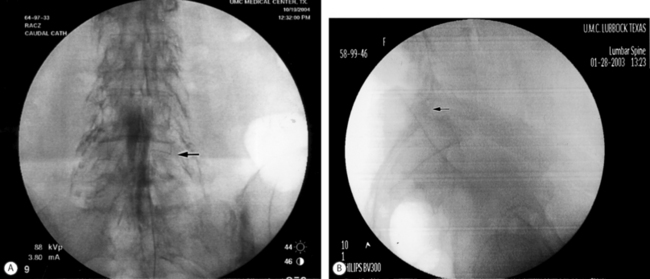
Fig. 106.1 (A) Catheter tip at the left L4–5 foramen. (B) Catheter in the ventral epidural space at L5–S1.
A 20 minute period should elapse between the last injection of the LA/S solution and the start of the hypertonic (10%) saline infusion. This is necessary to ensure that a subdural injection of the local anesthetic/steroid solution has not occurred. A subdural block can mimic a subarachnoid block but takes longer to establish. If the patient develops a subarachnoid or subdural block at any point during the procedure, the catheter should be removed and the remainder of the adhesiolysis canceled. The patient needs to be observed to document the resolution of the motor and sensory block and to document the absence of bladder/bowel dysfunction. Ten milliliters of hypertonic saline is then infused through the catheter over 30 minutes. If the patient complains of discomfort, the infusion is stopped and an additional 2–3 mL of 0.2% ropivacaine is injected and the infusion is restarted. Alternatively, 50–100 mg of fentanyl can be injected epidurally in lieu of the local anesthetic. After completion of the hypertonic saline infusion, the catheter is slowly flushed with 2 mL of preservative-free normal saline and the catheter is capped.
Transforaminal catheters
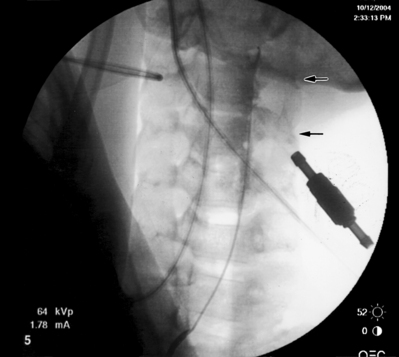
After the target level is identified with an AP fluoroscopic image, the superior endplate of the vertebra that comprises the caudal portion of the foramina is ‘squared,’ i.e. the anterior and posterior shadows of the vertebral endplate are superimposed. The angle is typically 15–20° in a caudocephalad direction. The fluoroscope is then obliqued approximately 15° to the side of the radiculopathy and adjusted until the spinous process is rotated to the opposite side. This fluoroscope positioning allows the best visualization of the superior articular process (SAP) that forms the inferoposterior portion of the targeted foramen. The image of the SAP should be superimposed upon the shadow of the disc space on the oblique view. The tip of the SAP is the target for needle placement. Raise a skin wheal slightly lateral to the shadow of the tip of the SAP. Pierce the skin with an 18-gauge needle and then insert a 16-gauge RX Coude™ needle and advance using gun-barrel technique towards the tip of the SAP. Continue to advance the needle medially towards the SAP until the tip contacts bone. Rotate the tip of the needle 180° laterally and advance about 5 mm. As the needle is advanced slowly, a clear ‘pop’ is felt as the needle penetrates the intertransverse ligament. Rotate the needle back medially 180°. Obtain a lateral fluoroscopic image. The tip of the needle should be just past the SAP in the posterior foramen. In the AP plane the tip of the needle should be just medial of the lateral border of the pedicle shadow. Under continuous AP fluoroscopy, insert the catheter slowly into the foramen and advance until the tip is past the medial border of the pedicle shadow (Fig. 106.2A). Confirm the catheter is in the anterior epidural space with a lateral image (Fig. 106.2B). Anatomically, the catheter is in the foramen above or below the exiting nerve root. If the catheter cannot be advanced, it usually means the needle is either too posterior or too lateral to the foramen. It can also indicate the foramen is too stenotic to allow passage of the catheter. The needle can be advanced a few millimeters anteriorly in relation to the foramen, and that will also move it slightly more medially into the foramen. If the catheter still will not pass, the initial insertion of the needle will need to be more lateral. Therefore, the fluoroscope angle will be about 20° instead of 15°. The curve of the needle usually facilitates easy catheter placement.
Inject 1–2 mL of contrast to confirm epidural spread. When a caudal and a transforaminal catheter are placed, the 1500 units of hyaluronidase are divided evenly between the two catheters (5 mL of the hyaluronidase/saline solution into each). The LA/S solution is also divided evenly, but a volume of 15 mL (1 mL steroid and 14 mL 0.2% ropivacaine) is used instead of 10 mL. Remove the needle under fluoroscopic guidance to make sure the catheter does not move from the original position in the epidural space. Secure and cover the catheter as described above. The hypertonic saline solution is infused at a volume of 7.5 mL per catheter over 30 minutes. It behooves the practitioner to check the position of the transforaminal catheter under fluoroscopy prior to performing the second and third infusions. The catheter may advance across the epidural space into the contralateral foramen or paraspinous muscles or more commonly back out of the epidural space into the ipsilateral paraspinous muscles. This results in deposition of the medication in the paravertebral tissue rather than in the epidural space. As with the caudal approach, remove the transforaminal catheter after the third infusion.
Cervical lysis of adhesions
Using loss of resistance technique, advance the needle into the epidural space with the tip of the RX-Coude™ pointed caudally. Once the tip is in the epidural space, rotate the tip cephalad and inject 1–2 mL of contrast to confirm entry. Inject an additional 4 mL to complete the epidurogram. As with the caudal epidurogram, look for filling defects. It is extremely important to visualize spread of the contrast in the cephalad and caudal directions. Loculation of contrast in a small area must be avoided as this can significantly increase the pressure in the epidural space and can compromise the already tenuous arterial blood supply to the spinal cord. Place a bend on the catheter as previously described for the caudal approach and insert it through the needle. The opening of the needle should be directed towards the target side. Slowly advance the catheter to the lateral gutter and direct it cephalad. Redirect the catheter as needed and once the target level has been reached, turn the tip of the catheter towards the foramen. Inject 0.5–1 mL of contrast to visualize the target nerve root. Make sure there is run-off of contrast out of the foramen (Fig. 106.3). Slowly instill 1500 units of hyaluronidase (Wydase™) dissolved in 5 mL of preservative-free normal saline (PFNS) (6 mL total). Follow this with 1–2 mL of additional contrast and observe for ‘opening-up’ of the ‘scarred-in’ nerve root. Give a 2 mL test dose of a 6 mL solution of LA/S. Our combination is 5 mL of 0.2% ropivacaine and 4 mg dexamethasone. If after 5 minutes there is no evidence of intrathecal or intravascular spread, inject the remaining 4 mL. Remove the needle, and secure and dress the catheter as previously described. Once 20 minutes have passed since the last dose of the LA/S solution and if there is no evidence of a subarachnoid or subdural block, start an infusion of 5 mL of hypertonic saline over 30 minutes. At the end of the infusion, flush the catheter with 1–2 mL of PFNS and cap the catheter.
Epidural mapping
In patients with multilevel radiculopathy and complex pain, it can be difficult to determine from where the majority of the pain is emanating. We have been using a technique, which we have termed ‘mapping,’ to locate the most painful nerve root with stimulation and then carry out the adhesiolysis at that level. There are several references in the literature regarding the use of stimulation to confirm epidural placement of a catheter and for nerve root localization.29 The TunL Kath™ and the TunL-XL™ catheter can be used as stimulating catheters to identify the nerve root(s).
OUTCOMES
Racz and Holubec first reported on epidural adhesiolysis in 1989.30 There were slight variations in the protocol compared to today’s protocol, namely the dose of local anesthetic and the fact that hyaluronidase was not used. Catheter placement was lesion-specific, i.e. the tip of the catheter was placed in the foramen corresponding to the vertebral level and side of the suspected adhesions. This retrospective analysis conducted 6–12 months postprocedure reported initial pain relief in 72.2% of patients (n=72) at time of discharge. Relief was sustained in 37.5% and 30.5% of patients at 1 and 3 months, respectively. Forty-three percent decreased their frequency and dosage of medication use and 16.7% discontinued their medications altogether. In total, 30.6% of patients returned to work or returned to daily functions.
At a presentation at the 7th World Congress on pain, Arthur and colleagues reported on epidural adhesiolysis in 100 patients of whom 50 received hyaluronidase as part of the procedure.31 In the hyaluronidase group, 81.6% of the participants had initial pain relief, with 12.3% having persistent relief; 68% of the no hyaluronidase group had relief of pain, with 14% having persistent relief.
In 1994, Stolker et al. added hyaluronidase to the procedure, but omitted the hypertonic saline. In a study of 28 patients, they reported greater than 50% pain reduction in 64% of patients at one year.32 They stressed the importance of patient selection and felt that the effectiveness of adhesiolysis was based on the effect of the hyaluronidase on the adhesions and the action of the local anesthetic and steroids on the sinuvertebral nerve.
Devulder and colleagues published a study of 34 patients with failed back surgery syndrome in whom epidural fibrosis was suspected or proved with MRI.33 An epidural catheter was inserted via the sacral hiatus to a distance of 10 cm into the caudal canal. Injections of contrast dye, local anesthetic, corticosteroid, and hypertonic saline (10%) were carried out daily for 3 days. No hyaluronidase was used. Filling defects were noted in 30 of 34 patients, but significant pain relief was only noted in seven patients at 1 month, two patients at 3 months, and none at 12 months. They concluded that epidurography may confirm epidural filling defects for contrast dye in patients with filling defects, but a better contrast dye spread, assuming scar lysis, does not guarantee sustained pain relief. This study was criticized for lack of lesion-specific catheter placement resulting in non-specific drug delivery.34
Heavner and colleagues performed a prospective randomized trial of lesion-specific epidural adhesiolysis on 59 patients with chronic intractable low back pain.35 The patients were assigned to one of four epidural adhesiolysis treatment groups: (1) hypertonic (10%) saline plus hyaluronidase, (2) hypertonic saline, (3) isotonic (0.9%) saline, or (4) isotonic saline plus hyaluronidase. All treatment groups received corticosteroid and local anesthetic. Overall across all four treatment groups, 83% of patients had significant pain relief at 1 month compared to 49% at 3 months, 43% at 6 months, and 49% at 12 months.
Manchikanti et al. performed a retrospective, randomized evaluation of a modified Racz adhesiolysis protocol in 232 patients with low back pain.36 The study involved lesion-specific catheter placement, but the usual 3-day procedure was reduced to a 2-day (group I) and 1-day (group II) procedure. Group I had 103 patients and group II had 129 patients. Other changes included changing the local anesthetic from bupivacaine to lidocaine, substituting methylprednisolone acetate or betamethasone acetate and phosphate for triamcinolone diacetate, and reduction of the volume of injectate. Of the patients in groups I and II, 62% and 58% had >50% pain relief at 1 month, respectively, with these percentages decreasing to 22% and 11% at 3 months, 8% and 7% at 6 months, and 2% and 3% at 1 year. Of significant interest is that the percentage of patients receiving >50% pain relief after four procedures increased to 79% and 90% at 1 month, 50% and 36% at 3 months, 29% and 19% at 6 months, and 7% and 8% at 1 year for groups I and II, respectively. Short-term relief of pain was demonstrated, but long-term relief was not.
In a randomized, prospective study, Manchikanti and colleagues evaluated a 1-day epidural adhesiolysis procedure against a control group of patients who received conservative therapy.37 Results showed that cumulative relief, defined as relief greater than 50% with one to three injections, in the treatment group was 97% at 3 months, 93% at 6 months, and 47% at 1 year. The study also showed that overall health status improved significantly in the adhesiolysis group.
Recently, Manchikanti et al. published their results of a randomized, double-blind, controlled study on the effectiveness of 1-day lumbar adhesiolysis and hypertonic saline neurolysis in treatment of chronic low back pain.38 Seventy-five patients whose pain was unresponsive to conservative modalities were randomized into one of three treatment groups. Group I (control group) underwent catheterization without adhesiolysis, followed by injection of local anesthetic, normal saline, and steroid. Group II consisted of catheterization and adhesiolysis, followed by injection of local anesthetic, normal saline, and steroid. Group III consisted of adhesiolysis, followed by injection of local anesthetic, hypertonic saline, and steroid. Patients were allowed to have additional injections based on the response, either after unblinding or without unblinding after 3 months. Patients without unblinding were offered either the assigned treatment or another treatment based on their response. If the patients in group I or II received adhesiolysis and injection of hypertonic saline, they were considered withdrawn, and no subsequent data were collected. Outcomes were assessed at 3, 6, and 12 months using visual analog scale pain scores, Oswestry Disability Index, opioid intake, range of motion measurement, and P-3®. Significant pain relief was defined as average relief of 50% or greater. Seventy-two percent of patients in group III, 60% of patients in group II, and 0% in group I showed significant pain relief at 12 months. The average number of treatments for 1 year were 2.76 in group II and 2.16 in group III. Duration of significant relief with the first procedure was 2.8 ± 1.49 months and 3.8±3.37 months in groups II and III, respectively. Significant pain relief (=50%) was also associated with improvement in Oswestry Disability Index, range of motion, and psychological status.
EPIDURAL ENDOSCOPIC ADHESIOLYSIS
Spinal endoscopy dates back to 1931 when Burman published the first experience with what was to become known as myeloscopy.39 Several more studies were published over the ensuing decades, and a historical review of said studies can be found in an article by Saberski and Brull.40 In 1991, Heavner and colleagues reported endoscopic evaluation of the epidural and subarachnoid spaces in rabbits, dogs, and human cadavers with aid of a flexible endoscope.41 Numerous articles followed over the next decade describing various aspects of spinal endoscopy, including clinical basis, protocol, safety, and cost-effectiveness.42–45
Epidural endoscopic adhesiolysis is a minimally invasive technique for adhesiolysis and accurate placement of injectate intended for delivery in the epidural space.45–47 It is based on the premise that the epidural space can be accessed safely by using flexible fiberoptic catheters entering via the sacral hiatus.45 It facilitates three-dimensional visualization of the contents of the epidural space and provides the physician with the ability to steer the catheter toward structures of interest. This procedure allows examination of specific nerve root pathology and treatment by injection of medication onto the nerve root, along with the ability to expand the epidural space with normal saline. Indications and patient preoperative preparation are identical to those for epidural adhesiolysis.
The epidural placement of an endoscope is most frequently performed via the sacral hiatus. This is based on anatomy, equipment, and experience.47 A midline entry of the epidural needle through the sacral hiatus is used instead of the usual paramedian approach. An epidurogram with water-soluble, non-ionic contrast is performed to visually assess the nerve roots. Insertion of the endoscope through the sacral hiatus will vary slightly depending on the type of endoscope used, but the Seldinger technique is the method of insertion used most frequently. Once inside the caudal epidural space, the endoscope is advanced using direct video and fluoroscopic guidance. There is a definite learning curve with epidural endoscopy and the operating physician should proceed with caution. In conjunction with gentle irrigation using normal saline, the myeloscope and catheter are manipulated and rotated in many directions to identify structures, namely nerve roots, at various levels. An endoscope with a working channel can facilitate placement of the epidural catheter. After the target level is reached, the epidural catheter is placed through the working channel and once it exits the distal end the endoscope is removed, leaving the catheter at the targeted nerve root (Fig. 106.4). Adhesiolysis is accomplished by the distention of the epidural space with the saline irrigation and by mechanical means using the endoscope and hyaluronidase. The LA/S and hypertonic saline protocol as previously described is then carried out.
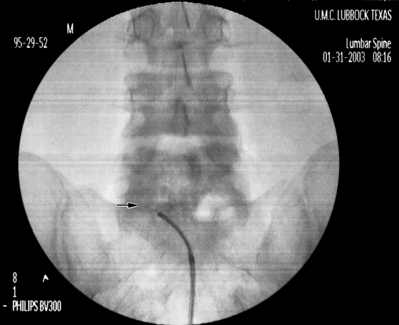
Fig. 106.4 Endoscopic epidural adhesiolysis. Note the catheter exiting the working channel of the endoscope.
There is a paucity of literature with regards to epidural adhesiolysis with myeloscopy. Richardson et al. published a prospective case series in 34 patients with severe chronic low back pain.48 All had epidural scar tissue with 14 patients having dense adhesions. Follow-up over a 12-month period showed statistically significant reductions in pain score and disability. In two separate retrospective studies, Manchikanti and colleagues evaluated the effectiveness of endoscopic adhesiolysis in 85 patients with chronic low back pain with or without history of laminectomy and 60 patients with postlumbar laminectomy syndrome.45,49 In the first study, all 85 patients received significant pain relief (≥50%) in the first month, but this decreased to 77%, 52%, and 21% at 3, 6, and 12 months, respectively. All of the patients in the second study had significant analgesia in the first month which dwindled to 80%, 52%, and 22% at the 3-, 6-, and 12-month follow-up visits, respectively. As with adhesiolysis without endoscopy, short-term relief was afforded.
PHYSICAL THERAPY
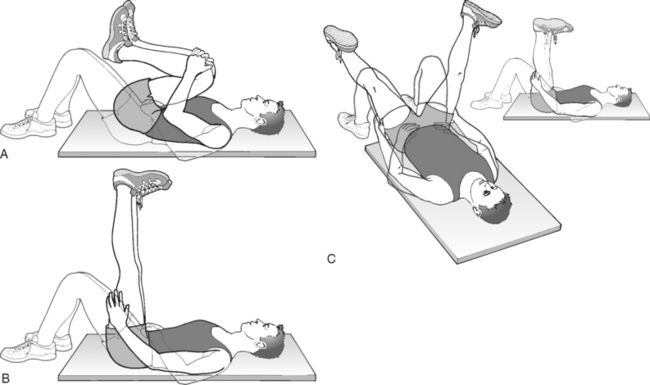
We routinely have our adhesiolysis patients engage in neural flossing exercises once the procedure is completed. These exercises involve performing repetitive, slow, rhythmic nonpainful distal initiation of dural movements (Fig. 106.5).50 The goal is to restore the normal movement of the involved nerve root in the foramen and to prevent adhesions from reforming. The patient is encouraged to do one set of ten repetitions of each of the three exercises twice a day. The patient is taught these exercises by a physical therapist prior to discharge from our facility.
CONCLUSION
Epidural adhesiolysis has evolved over the years as an important treatment option for patients with intractable cervical, thoracic, and low back and leg pain. Studies show that patients are able to enjoy significant pain relief and restoration of function over several months. Manchikanti’s studies show that the amount and duration of relief can be achieved by repeat procedures. Endoscopy offers direct visualization of the affected nerve roots in addition to mechanical adhesiolysis, and may become more mainstream as the technique is refined. More prospective, randomized, controlled studies need to be performed to further solidify epidural adhesiolysis’s position in the treatment algorithm of patients with intractable pain refractory to previous treatments.
1 Lawrence R, Helmick C, Arnett F, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778-799.
2 Straus B. Chronic pain of spinal origin: the costs of intervention. Spine. 2002;27(22):2614-2619.
3 National Center for Health Statistics. National Hospital Discharge Survey. Report No. PB92-500818. Washington DC: US Department of Health and Human Services, Centers for Disease Control, 1990.
4 Kuslich S, Ulstrom C, Michael C. The tissue origin of low back pain and sciatica. Orthop Clin North Am. 1991;22:181-187.
5 Racz G, Noe C, Heavner J. Selective spinal injections for lower back pain. Curr Rev Pain. 1999;3:333-341.
6 Anderson S. A rationale for the treatment algorithm of failed back surgery syndrome. Curr Rev Pain. 2000;4:396-406.
7 Pawl R. Arachnoiditis and epidural fibrosis: the relationship to chronic pain. Curr Rev Pain. 1998;2:93-99.
8 Cervellini P, Curri D, et al. Computed tomography of epidural fibrosis after discectomy. A comparison between symptomatic and asymptomatic patients. Neurosurgery. 1988;6:710-713.
9 Manchikanti L, Staats P, Singh V. Evidence-based practice guidelines for interventional techniques in the management of chronic spinal pain. Pain Phys. 2003;6:3-81.
10 LaRocca H, Macnab I. The laminectomy membrane: studies in its evolution, characteristics, effects and prophylaxis in dogs. J Bone Joint Surgery. 1974;5613:545-550.
11 Cooper R, Freemont A, et al. Herniated intervertebral disc-associated periradicular fibrosis and vascular abnormalities occur without inflammatory cell infiltration. Spine. 1995;20:591-598.
12 McCarron R, Wimpee M, Hudkins P, et al. The inflammatory effects of nucleus pulposus: a possible element in the pathogenesis of low back pain. Spine. 1987;12:760-764.
13 Parke W, Watanabe R. Adhesions of the ventral lumbar dura. An adjunct source of discogenic pain? Spine. 1990;15:300-303.
14 Viesca C, Racz G, Day M. Special techniques in pain management: lysis of adhesions. Anesthesiol Clin N Am. 2003;21:745-766.
15 Songer M, Ghosh L, Spencer D. Effects of sodium hyaluronate on peridural fibrosis after lumbar laminectomy and discectomy. Spine. 1990;15:550-554.
16 Key J, Ford L. Experimental intervertebral disc lesions. J Bone Joint Surg [Am]. 1948;30:621-630.
17 Olmarker K, Rydevik B. Pathophysiology of sciatica. Orthop Clin N Am. 1991;22:223-233.
18 Ross J, Robertson J, Frederickson R, et al. Association between peridural scar and recurrent radicular pain after lumbar discectomy: magnetic resonance evaluation. Neurosurgery. 1996;38:855-863.
19 Hatten HJr. Lumbar epidurography with metrizamide. Radiology. 1980;137:129-136.
20 Stewart H, Quinnell R, Dann N. Epidurography in the management of sciatica. Br J Rheumatol. 1987;26(6):424-429.
21 Devulder J, Bogaert L, Castille F, et al. Relevance of epidurography and epidural adhesiolysis in chronic failed back surgery patients. Clin J Pain. 1995;11:147-150.
22 Manchikanti L, Bakhit C, Pampati V. Role of epidurography in caudal neuroplasty. Pain Digest. 1998;8:277-281.
23 Day M, Racz G. Technique of caudal neuroplasty. Pain Digest. 1999;9(4):255-257.
24 Horlocker T, Wedel D, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med. 2003;28:172-197.
25 Omnipaque product insert. Princeton, NJ, Nycomed, Inc.
26 Isovue product insert. Princeton, NJ, Bracco Diagnostics, Inc.
27 Hypaque product insert. Princeton, NJ, Amersham Health, Inc.
28 Conray product insert. Phillipsburg, NJ, Mallinckrodt, Inc.
29 Larkin T, Carragee E, Cohen S. A novel technique for delivery of epidural steroids and diagnosing the level of nerve root pathology. J Spinal Disord Tech. 2003;16(2):186-192.
30 Racz G, Holubec J. Lysis of adhesions in the epidural space. In: Raj P, editor. Techniques of neurolysis. Boston: Kluwer Academic; 1989:57-72.
31 Arthur J, Racz G, et al. Epidural space: identification of filling defects and lysis of adhesions in the treatment of chronic painful conditions. Abstracts of the 7th World Congress on Pain. Paris: IASP Publications, 1993.
32 Stolker R, Vervest A, Gerbrand J. The management of chronic spinal pain by blockades: a review. Pain. 1994;58:1-19.
33 Devulder J, Bogaert L, Castille F, et al. Relevance of epidurography and epidural adhesiolysis in chronic failed back surgery patients. Clin J Pain. 1995;11:147-150.
34 Racz G, Heavner J. In response to article by Drs. Devulder et al. Clin J Pain. 1995;11:151-154.
35 Heavner J, Racz G, Raj P. Percutaneous epidural neuroplasty: prospective evaluation of 0.9% saline versus 10% saline with or without hyaluronidase. Reg Anesth Pain Med. 1999;24:202-207.
36 Manchikanti L, Pakanati R, Bakhit C, et al. Role of adhesiolysis and hypertonic saline neurolysis in management of low back pain: evaluation of modification of the Racz protocol. Pain Digest. 1999;9:91-96.
37 Manchikanti L, Pampati V, Fellow B, et al. Role of one day epidural adhesiolysis in management of chronic low back pain: a randomized clinical trial. Pain Phys. 2001;4:153-166.
38 Manchikanti L, Rivera J, Pampati V, et al. One day lumbar adhesiolysis and hypertonic saline neurolysis in treatment of chronic low back pain: a randomized, double-blind trial. Pain Phys. 2004;7:177-186.
39 Burman M. Myeloscopy or the direct visualization of the spinal canal and its contents. J Bone Joint Surg. 1931;13:695-696.
40 Saberski L, Brull S. Spinal and epidural endoscopy: a historical review. Yale J Biol Med. 1995;68:7-15.
41 Heavner J, Cholkhavatia S, Kizelshteyn G. Percutaneous evaluation of the epidural and subarachnoid space with the flexible endoscope. Reg Anesth. 1991;151:85.
42 Saberski L, Kitahata L. Direct visualization of the lumbosacral epidural space through the sacral hiatus. Anesth Analg. 1995;80:839-840.
43 Saberski L, Kitahata L. Review of the clinical basis and protocol for epidural endoscopy. Connecticut Med. 1996;60:70-73.
44 Saberski L. A retrospective analysis of spinal canal endoscopy and laminectomy outcomes data. Pain Phys. 2000;3:193-196.
45 Manchikanti L, Pakanati R, Pampati V, et al. The value and safety of epidural endoscopic adhesiolysis. Am J Anesthesiol. 2000;27:275-279.
46 Addison R. Spinal endoscopy. Cur Rev Pain. 1999;3:116-120.
47 Manchikanti L, Singh V. Epidural lysis of adhesions and myeloscopy. Curr Pain Headache Reports. 2002;6:427-435.
48 Richardson J, McGurgan P, Cheema S, et al. Spinal endoscopy in chronic low back pain with radiculopathy. A prospective case series. Anaesthesia. 2001;56:454-460.
49 Manchikanti L, Vidyasagar P, Bakhit C, et al. Non-endoscopic and endoscopic adhesiolysis in post lumbar laminectomy syndrome: a one-year outcome study and cost effectiveness analysis. Pain Phys. 1999;2:52-58.
50 Sizer P, Phelps V, Dedrick G, et al. Differential diagnosis and management of spinal nerve-root related pain. Pain Practice. 2002;2:98-121.