CHAPTER 114 Epidemiology and Examination
INTRODUCTION
The most current paradigm of interventional spine care admits a multiplicity of potential spine-related sources of axial and appendicular pain. There are 183 anatomic potential sites of spinal pain, including the 23 intervertebral discs, the 50 paired facet joints and most cephalad intervertebral articulations, the 60 paired nerve roots, the 48 paired costovertebral articulations, and the paired sacroiliac joints. The remainder of a myriad of possible symptom generators includes the various ligamentous, tendinous, and supporting structures; the muscles; the various neural elements; and various other interstitial elements. The sacroiliac joints proper represent a solitary pair within this virtual infinitude of potential areas of interest for the interventional spine clinician; moreover, the very fact of their clinical relevance remains controversial. Yet the potential significance of these two structures has been verified by the amount of attention they have received from interested clinicians since they were first ascribed potential clinical importance over a century ago in 1905.1
EPIDEMIOLOGY
Anatomy
The anatomic properties of the sacroiliac joints have been well studied, both at the gross and histologic scales. Grossly, the sacroiliac joint is an articulation between the ilium and the sacrum – usually S1–S4 but including the fifth lumbar vertebra up to 5% of the time.2,3 The joint’s shape is commonly described as auricular, or L-shaped, with two arms of usually unequal length: the longer arm is dorsocaudally directed, and the shorter arm is dorsocranially oriented.4 The embryologic development of the sacroiliac joint begins in the tenth week and obtains a definitive form by the four month.5 Flat until at least the time of puberty, the articular surfaces eventually become roughened, with numerous and highly variable grooves and protuberances on each opposing face.6 In the adult, there is commonly a longitudinal sacral groove at S2 which admits an iliac ridge, although this arrangement may be reversed.4
Gross supporting structures exist anteriorly and posteriorly. Stabilization against anterior motion of the sacral promontory may be aided by the anterior sacroiliac ligament7,8 and the sacrotuberous and sacrospinous ligaments.9,10 Resistance against downward translation of the sacrum may be aided by a posterior structure, the posterior sacroiliac ligament.9 The interosseous ligament, another posterior structure, is thought to be a primary joint stabilizer.10 Anterior and posterior fibrous joint capsule fibers mesh with these structures.
In keeping with the somewhat controversial nature of the sacroiliac joint, the histologic characteristics of this structure render strict definitions difficult. The cartilaginous architecture of the sacral and iliac aspects of this joint differ, the sacral face being hyaline with an overall thickness of 1–3 mm, the iliac face composed of columns of fibrocartilage oriented perpendicular to the joint surface and interposed with islands of hyaline cartilage, with a thickness of less than 1 mm.4 The overall histologic architecture of the joint has been postulated to change throughout life, beginning as a diarthrodial joint and progressively losing mobility.6 Further variation is provided by gender: a cadaveric study of 47 specimens by Vleeming found that articular interdigitation was greater in males than in females.11 Thus, the sacroiliac joints have been described as diarthrotic, synarthrotic, and amphiarthrotic.3
Physiology
Of physiologic concern is the innervation of the sacroiliac joint, although definitive understanding of this has been elusive. The anterior aspect of the joint is likely innervated by the posterior rami of the L2 through S2 roots, while the posterior portion is likely innervated by the posterior rami of L4 through S3. Innervation is thought to be highly variable, even between two joints in a given individual.12–14 Anterior joint innervation may be further subserved by the obturator nerve, superior gluteal nerve, or the lumbosacral trunk.15,16
Physiologic characterization of the sacroiliac joint, as with all joints, falls under two broad categories: kinetic and kinematic evaluation. The primary kinetic and kinematic considerations of the sacroiliac joints, by definition, involve sacroiliac force transmission and subsequent sacral motion relative to the ilia. In the standing position, superincumbent body weight is associated with an inferiorly directed translatory force acting upon the sacrum.17 An equally important induction of an anteriorly directed rotary force upon the sacral prominence relative to the ilia has been understood since the nineteenth century;18 the axis of this rotary force has been theorized to coincide with the horizontal line connecting the paired iliac tuberosities,2 although other axes of rotation have been postulated, and more than one may exist.19,20 In the standing position these two forces – one linear and the other rotatory and thus properly termed a torque – should by Newton’s first law induce a respective inferior translation and anterior rotation (or flexion, termed nutation) of the sacrum relative to the ilia, unless sacroiliac motion were completely restrained. Likewise, changing positions from standing is accompanied by changes in translatory forces and torques acting on the sacrum, and should therefore induce differing sacral translations and rotations relative to the ilia.
The physical parameter that couples the above forces acting on the sacroiliac joint with resultant sacroiliac motion is none other than joint mobility. As sacroiliac mobility has not been well quantified, the effect of sacroiliac kinetics on sacroiliac kinematics has not been well established. The presence of sacroiliac mobility in the pregnant female was suspected by Hippocrates and later by other researchers.2,19 In pregnant women, sacroiliac joint laxity has been quantified using Doppler ultrasound; side-to-side asymmetries in such laxity have been postulated to be associated with pregnancy-related pelvic pain and predictive of postpartum pelvic pain.21 In the nonpregnant patient, however, reported joint range of motion in both the normal and pathologic states varies widely. An early analysis was performed by Mennell, who noted that with a change in position from prone to sitting, the posterior superior iliac spines were displaced apart from each other by 0.5 inch.22 Attempting to verify such sacroiliac motion in normal subjects, Colachis et al. used Kirschner wires to mark bilateral iliac landmarks, and interwire distances were noted in nine different subject positions. Maximal iliac motion was found with trunk flexion from the standing position. The interiliac relationship previously described by Mennell was reversed, however, with greater posterior superior iliac spine approximation found with sitting versus the prone position.19 Subsequent research, in order to maximize sensitivity of detecting sacroiliac motion, has evaluated the extremes of motion about the sacroiliac joint, such as the reciprocal straddle position and other extremes of hip motion. Smidt et al.23 found interinnominate rotation to average 9° around an oblique sagittal axis and 5° around the transverse axis. Similar analyses by Barakatt et al.24 recorded interinnominate motions of up to 36°. Increased detail has been allowed with the use of radiologic studies. In a follow-up cadaveric study utilizing computed tomography, Smidt25 found sagittal sacroiliac motion of up to 17° with hips placed at end-ranges of motion. In contrast to such large values, Sturesson et al., in a roentgen stereophotogrammetric study of 25 individuals diagnosed with sacroiliac disorders, simulated typical motions about the joints and found mean sacral translation of 0.5 mm and usual sacral rotation of less than 3.6°.26 Such minimal amounts of rotation – less than 1.6° – were confirmed by this author in a second study involving six women, five of whom experienced postpregnancy posterior pelvic pain.27 This order of magnitude of rotation echoed similar radiostereometric results obtained by Egund et al.20 The minimal amount of sacroiliac joint mobility described in these latter studies not only contrasts the results of previously noted investigations but also tends to cast doubt on the clinician’s ability to detect any related change in joint alignment by physical examination alone. Yet the clinical detectability of sacroiliac joint misalignment has been proposed.28,29
Joint pain
Pain emanating from the sacroiliac joint may stem from multiple primary and relatively well-defined causes: a list drawing from familiar pathologic categories, such as trauma, infection, tumor, and systemic illness. In the particular case of the sacroiliac joint, these broad categories have been reported specifically as traumatic pelvic ring fracture, intrapartum diastasis, pyarthrosis, metastatic adenocarcinoma, and spondyloarthropathy.12,30,31 Even well-understood pathologies within the sacroiliac joint present with cryptic clinical findings: Bohay reported that in sacroiliac joint pyarthroses the most common symptom and sign was fever. Other common symptoms were also non-specific, including ipsilateral hip, leg, or buttock pain or low back pain. Physical examination focused on the joint is thought to be helpful in timely diagnosis of a suppurative process, and serial Patrick’s and Gaenslen’s tests and sacroiliac joint compression (described below) are recommended.31
A separate cause of sacroiliac joint pain, intrinsic to and emanating from the joint itself, has been termed sacroiliac joint dysfunction,12,32 and is postulated to stem from an anatomic derangement of the joint. Attempts have been made to characterize this entity,28,29,33–35 although its existence has not been universally accepted in the allopathic medical literature.
Borrowing from the model set forth by Dwyer and Aprill,36,37 Fortin et al. attempted to derive sacroiliac joint pain referral maps from asymptomatic volunteers. Ten subjects with no history of prior back pain underwent unilateral contrast injection into the sacroiliac joint. This was followed by analyses of the distribution of subsequently produced pain and hypoesthesia. Delineation of area of hypoesthesia was repeated after infusion of Xylocaine into the joint. Hypoesthesia after instillation of an average of 1.6 mL of contrast was noted to comprise one of three patterns: medial buttock inferior to the posterosuperior iliac spine, this area plus the superior greater trochanter, and this last area plus the superior lateral thigh. Areas of provoked pain were found to coincide with these areas of hypoesthesia. Given that the approach used was believed to minimize needle penetration of adjacent potential pain generators, including ligaments and muscle, it was inferred that these referral patterns were derived solely from the sacroiliac joint itself.38
In a follow-up study, Fortin et al. tested the clinical significance of one of the previously derived pain referral zones. Of 54 consecutive patients referred for treatment of lumbar discogenic or facet pain, 16 were tentatively diagnosed with sacroiliac joint-mediated pain based on pain diagrams that indicated maximal discomfort within a region inferior to the posterosuperior iliac spine. All of these patients subsequently demonstrated concordant pain and sensory changes after provocative instillation of contrast into the sacroiliac joint, confirming the diagnosis. Ten of these patients underwent diagnostic evaluation of the lowest two intervertebral discs and lowest two facet joints ipsilateral to their initial pain, and in all cases these structures were deemed not to be pain generators.39
However, subsequent investigation of 100 subjects with and without sacroiliac joint pain, by Schwarzer et al.,40 called into question the clinical validity of sacroiliac pain reproduction by intra-articular contrast injection. Failure to reproduce pain was noted to have some negative predictive value, while pain reproduction was noted to have little positive predictive value. The gold standard here was pain reduction with subsequent anesthetic injection into the joint. Comparison of historical features of sacroiliac joint versus nonsacroiliac joint-mediated pain yielded only a single statistically significant distinguishing factor: the presence of groin pain with sacroiliac joint-mediated pain. No potentially exacerbating or mitigating factors, such as sitting, standing, walking, flexion, or extension, were found to correlate with the presence or absence of sacroiliac joint pain.
Following Schwarzer’s use of anesthetic instillation into the joint as the diagnostic gold standard for sacroiliac pain, Dreyfuss et al.12 used this injection technique to compare historical features and physical examination findings in patients with and without sacroiliac joint pain. In a group of 85 patients who had been referred for sacroiliac injection, composite preinjection pain diagrams revealed a solitary factor that distinguished sacroiliac joint mediated pain from nonsacroiliac joint pain: the presence of pain above the L5 dermatome in patients without sacroiliac joint pain. Schwarzer’s analysis of potentially exacerbating or mitigating factors was modified slightly and extended greatly to include sitting, standing, walking, lying down, coughing/sneezing, defecation, use of heeled footwear, and job activities. Symptom relief with standing was found to be possibly specific for sacroiliac joint pain, although the statistical significance of this finding was not certain. None of the other factors was found to correlate with the presence or absence of sacroiliac joint pain. A similar lack of predictive value was found on analysis of previous response to a variety of therapeutic modalities, including certain classes of oral medication, physical therapy, manipulation, and certain modalities.
In a more focused analysis of pain referral zones, Slipman et al.41 studied 50 patients with sacroiliac pain whose diagnosis was tentatively made by means of physical examination maneuvers and confirmed by fluoroscopically guided sacroiliac joint block. Pretreatment interviews were utilized to localize the patients’ pain: buttock pain was found to be the most prevalent area of referred pain, occurring in 94% of patients, followed by lower lumbar (72%), thigh (48%), and lower leg pain (28%). Other painful areas included the foot and ankle as well as the groin, upper lumbar region, and abdomen. The greatest prevalence of buttock pain, followed by a descending prevalence of progressively distal lower extremity pain referral, is reminiscent of the previous results of Fortin et al.38,39 Slipman et al. further noted a statistically significant inverse relationship between patient age and the presence of referred pain distal to the knee.
The literature thus far, although rather scant, has already yielded some clinical insight into the nature of sacroiliac joint-mediated pain. Firstly, Schwarzer was able to confirm the very presence of such pain by alleviating this symptom with injection of anesthetic into that joint in patients whose symptoms were not relieved by similar injections into neighboring structures.40 The works of Schwarzer, Fortin, and Slipman served to establish and later validate the use of fluoroscopically guided anesthetic injection as the diagnostic gold standard for sacroiliac joint-mediated pain. Although by no means a gold standard, the pain referral zones presented first by Fortin et al. and later by Slipman et al. are at least foundations on which further clinical analyses may be conducted. Assessing possible reasons for the limited clinical usefulness of pain referral zones in the diagnosis of sacroiliac joint pain, Slipman has noted the complexity and variability of this joint’s innervation, possible sclerotomal referral patterns, and the proximity of other potential secondary pain generators activated by primary sacroiliac joint pathology.
The sacroiliac joint’s complex and incompletely understood innervation, described above, may lead to referred pain in the L2–S3 distribution. Sclerotomal referral indicates pain generated in a structure originating from a given embryonic somite being referred to another structure originating from that same somite. As the osseous structures of the spinal column originate from the ventromedial portion of their respective somites, and the muscles of the trunk and extremity originate from the corresponding posterolateral portions, sclerotomal, or somatic, referral may include a broad swath of the low back, buttock, and lower extremity. A further confounding factor is the proximity of other potential pain generators that may be directly irritated by a putative derangement in the anatomy or mechanics of the sacroiliac joint. An early study by Yeoman, involving sciatica, hints at such a pathologic mechanism,42 which has been further reported more recently.43
PHYSICAL EXAMINATION
It is perhaps the increased potential specificity of the physical examination that has led to the greater amount of published research being directed towards this subject rather than towards historical/epidemiologic data. Such research typically falls under one of two categories: physical findings in sacroiliac joint dysfunction, and provocative maneuvers in sacroiliac joint dysfunction. Along a different axis, studies evaluating physical findings or provocative maneuvers in sacroiliac joint dysfunction may also be divided another way: those that aim to determine the predictive value of specific clinical tests and those that aim to determine intertester reliability of such tests.
Physical findings
The current peer-reviewed literature contains a relative paucity of inquiry into physical findings associated with sacroiliac joint dysfunction. Excluding by definition provocative maneuvers, such findings are limited to findings obtained by pure patient observation, either through visualization or palpation. Visible signs discussed in the extant literature focus mainly on leg length discrepancy theorized to be secondary to asymmetric innominate tilt, as well as asymmetric hip range of motion. Palpable signs are theorized to detect asymmetry of the bony structures about the sacroiliac joint or abnormal sacral–innominate motion with certain patient movements. Table 114.1 summarizes some physical findings that have been reported to be associated with sacroiliac joint dysfunction.
Table 114.1 Visible and palpable physical findings in sacroiliac joint syndrome
| Visible findings | ||
|---|---|---|
| Test name | Procedure | Positive when… |
| External rotation sign – lying* | Patient is asked to lie supine as comfortably as possible. | Most comfortable position attained includes obvious external rotation of the ipsilateral hip. Passive internal rotation diminishes patient comfort. |
| External rotation sign – standing* | Patient is asked to stand at rest as comfortably as possible. | Most comfortable position attained includes external rotation and flexion of the ipsilateral hip – often supported by a plantarflexed foot. |
| Prone knee flexion test | Patient lies prone with hips and knees extended, knees are passively flexed to 90°. | One leg is initially shorter, indicating ipsilateral sacroiliac joint dysfunction. Apparent leg lengthening with knee flexion indicates ipsilateral posterior innominate rotation; shortening indicates anterior rotation. |
| Supine long sitting test | Patient lies supine with legs fully extended; examiner compares relative locations of both medial malleoli. Patient sits up, flexing hips but leaving knees extended. | Relative position of medial malleoli change with sitting up. Apparent leg length increase is due ipsilateral posterior innominate rotation or contralateral anterior innominate rotation. Apparent leg length decrease is due to opposite rotations. |
| Palpable findings | ||
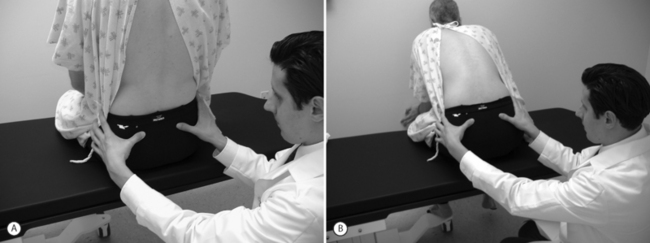
| Gillet test | Patient stands at rest; examiner places one thumb under the PSIS and the other thumb on the adjacent S2 tubercle. The patient maximally flexes the ipsilateral hip and knee. | The PSIS under the examiner’s thumb does not migrate inferiorly relative to the adjacent S2 tubercle. |
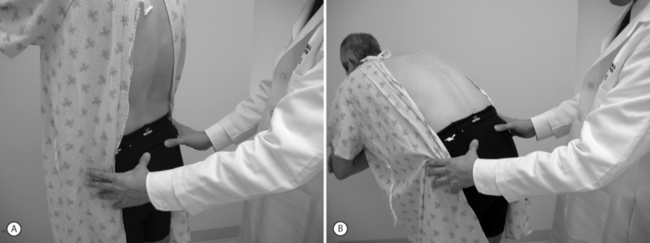
| Sitting flexion test | Patient sits at rest; examiner palpates bilateral posterior superior iliac spines. Patient bends trunk forward. | There is asymmetry in the motion of the posterosuperior iliac spines, with increased PSIS motion on the side of joint restriction. |
| Spring test | Patient supine; examiner repeatedly applies downward pressure to the superior sacrum. | Palpably decreased resistance to motion is noted. |
| Standing flexion test | Patient stands at rest; examiner palpates bilateral posterior superior iliac spines. Patient bends trunk forward. | There is asymmetry in the motion of the posterosuperior iliac spines, with increased PSIS motion on the side of joint restriction. |
| Standing/sitting iliac crest palpation | Patient stands/sits at rest; examiner palpates bilateral iliac crests. | The iliac crests are of asymmetric heights. |
| Standing/sitting posterior superior iliac spine palpation | Patient stands/sits at rest; examiner palpates bilateral posterior superior iliac spines. | The posterior superior iliac spines are of asymmetric heights. |
* Denotes findings noted by the authors.
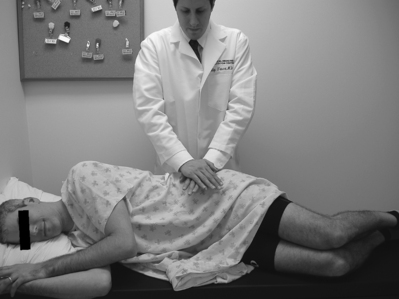
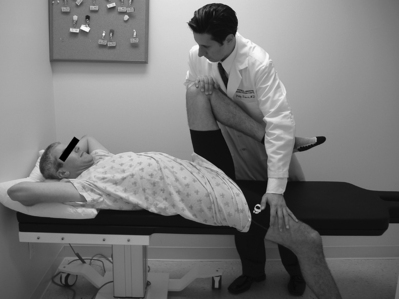
Study of visible and palpable signs has been performed by Potter and Rothstein,44 in which eight physical therapists – medical professionals whose training specifically addresses the palpation of bony structures – examined 17 patients for whom sacroiliac joint dysfunction was in the differential diagnosis. The methodologies of all 13 tests are well known and had been previously published.45 Eleven of the 13 studied were based on palpation of body landmarks of the sacrum and innominate bones plus or minus evaluation of subsequent changes in these landmarks’ relationship to each other with positional changes. These evaluations included the standing flexion test (Fig. 114.1), the sitting flexion test (Fig. 114.2), and the Gillet test (Fig. 114.3), described in Table 114.1. Two of the 13 tests were based on symptom provocation (Table 114.2): the supine iliac gapping test attempts to reproduce pain essentially through the examiner pushing the anterior ilia apart while the patient lies supine (Fig. 114.4), and the side-lying iliac compression test would reproduce pain through the examiner pushing the upward-facing ilium medially in the side-lying patient (Fig. 114.5).
Table 114.2 Provocative tests for sacroiliac joint syndrome
| Test name | Procedure | Positive with pain in… |
|---|---|---|
| Gaenslen’s test | Patient supine, one leg with hip and knee maximally flexed, the opposite innominate bone and leg is off of side of examination table with hip extended. | Joint on side off of table. |
| Patrick’s test (FABER test) | Patient prone; examiner passively simultaneously flexes, abducts, and externally rotates hip. | Ipsilateral joint. |
| Posterior shear test | Patient supine, one leg flexed at hip and knee. Examiner exerts downwards pressure on femur of flexed leg. | Joint on side of flexed hip and knee. |
| Sacral sulcus pressure | Patient prone; examiner exerts pressure just medial to posterior superior iliac spine. | Ipsilateral joint, namely area of palpation. |
| Sacral thrust | Patient prone; examiner exerts pressure on sacrum. | Ipsilateral joint. |
| Side-lying iliac compression test | Patient in lateral decubitus position; examiner applies downward pressure to iliac crest. | Topmost joint. |
| Supine iliac gapping test | Patient supine; examiner pushes anterior superior iliac spines laterally. | Ipsilateral joint. |
It was theorized that anatomic derangement of either or both sacroiliac joints would be accompanied by palpable static or dynamic changes in the relative positioning of the sacrum and the innominate bones, given the ranges of joint motion described previously. The reproducibility of such measurable changes in position was tested by each patient being evaluated in a blinded fashion by two therapists from a pool of eight, who performed all 13 tests on each patient. It was revealed that none of the testing maneuvers that were based on palpating abnormalities in sacroiliac joint positioning achieved intertester reliability of greater than 50%; all of these tests were thus deemed clinically unreliable by the authors. The two provocative maneuvers, supine iliac gapping and side-lying iliac compression tests, in contrast, achieved 94% and 76% intertester agreement, respectively. Of import, however, is the fact that in this study the intertester reliability was evaluated for each test applied in isolation.
Recognizing that no single physical examination finding or maneuver may be diagnostic of sacroiliac joint dysfunction, Cibulka et al.28 utilized the standing flexion test, the prone knee flexion test, the supine long sitting test, and palpation of the posterior superior iliac spine as a single test battery. Twenty-six patients with non-specific low back pain were evaluated by two examiners, and positive results with three of the four maneuvers was deemed sufficient for diagnosis of sacroiliac joint dysfunction. Intertester agreement analysis revealed a Cohen’s kappa value of 0.88, considered to represent excellent clinical agreement.46 A pool of only two examiners, who may have been very familiar with each other’s technique, effectively diminishes the clinical utility of this study as it applies to the examination maneuvers themselves, potentially as performed by one or more of a pool of many examiners.
More recent analysis of the intertester reliability of four testing maneuvers was performed by Riddle and Freburger47 in which paired examiners were chosen from a pool of 34. Since the ‘subjects’ in this study are not only the patients but also the examiners with their yield of examination data, this larger number of examiners is theorized to better approximate true clinical circumstances by taking into account real-world variations in examination technique. Sixty-five patients with low back pain were examined via the same four tests used by Cibulka.28 It was found that percentage single-test agreement between the paired examiners was between 44% and 63% depending on which test was used. Agreement that three of four tests were positive was between 60% and 69%. This study’s usefulness is bolstered by the larger number of included examiners; however, the quality of the patient population perhaps subtracts from the study’s utility, as a possibly low prevalence of sacroiliac joint dysfunction in this population is noted by the authors.
Turning attention from intertester reliability and towards test specificity, Dreyfuss et al. evaluated the positivity of sacroiliac joint physical findings in 101 asymptomatic subjects. In this blinded study, in which examiners did not know whether or not subjects were symptomatic, the standing flexion test, seated flexion test, and Gillet test were scrutinized. Overall, 20% of asymptomatic subjects had at least one test maneuver that was positive, with single-test false-positive rates of 13% for the standing flexion test, 8% for the seated flexion test, and 16% for the Gillet test. Overall, female subjects demonstrated more such false-positive results than males.32
Other research has focused on hip range of motion as related to sacroiliac joint dysfunction, instead of specific testing maneuvers. Early work by LaBan noted unilateral limitation in hip abduction and external rotation in the presence of sacroiliac joint inflammation.48 Basing itself on the above-mentioned studies, later work by Cibulka et al.49 noted that in subjects with low back pain not attributable to sacroiliac joint dysfunction passive hip range of motion is generally greater for external rotation than for internal rotation. This disparity was found to be increased in a hip joint ipsilateral to a posteriorly rotated innominate bone. In this research, one examiner performed the four sacroiliac joint examination maneuvers used previously by Cibulka28 and designated three of four positive results to be diagnostic of sacroiliac joint dysfunction. Intratester reliability was measured by repeat assessment with these four tests on a different day – with a kappa value of 0.86. A second examiner obtained goniometric measurements of passive hip external and internal rotation and came to the above-mentioned conclusion. If asymmetry in muscle tone is postulated as the intermediary between sacroiliac joint pathology and asymmetry in hip range of motion, it is debatable whether the asymmetry in muscle tone is the cause or the effect of the sacroiliac joint pathology.
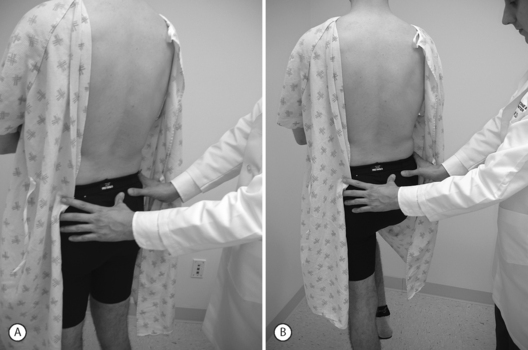
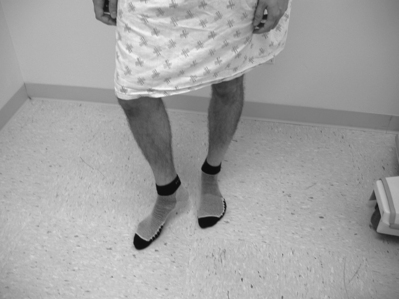
It has been the authors’ further experience that patients with sacroiliac joint syndrome, on standing, tend to place the majority of their body weight on the contralateral lower limb. More specifically, a stereotypical posture is adopted whereby the ipsilateral limb is externally rotated and the hip flexed, this flexion being maintained and assisted by a plantarflexed foot contacting the floor solely distal to the metatarsal heads (Fig. 114.6). Such a posture is assessed when the patient first stands at rest during the initial phase of our physical examination. If the patient does not stand still on his or her own, perhaps due to discomfort alleviated by constantly shifting weight, he or she is asked to stand still in a way that provides maximal comfort. If the above posture is assumed, it is taken as a relatively specific sign of sacroiliac joint syndrome. A relatively less-specific sign can present if the patient does not assume the above posture when asked to stand comfortably: his or her leg is positioned by the examiner as described above, and the patient is then asked if this provides more or less comfort than had been present when initially standing at rest. If the patient states that standing with the ipsilateral hip flexed and externally rotated does indeed provide some relief, we consider it a sign of relief from sacroiliac joint-mediated pain. To be certain, we often adjust the leg into other positions, such as internally rotated, and query the patient as to any pain relief, which in this latter case should not occur.
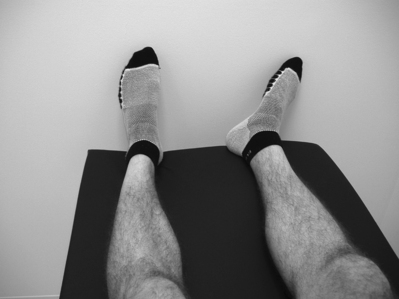
A fourth sign that the authors have noted occurs when the patient lies on the examination table at rest in the supine position with both knees fully extended: the ipsilateral leg is externally rotated when the contralateral hip is in neutral rotation This rotation is more than a few degrees – it is obvious even from a distance (Fig. 114.7). On questioning, the patient sometimes will admit to the fact that such external rotation is the usual positioning when lying supine. If the limb is rotated to the neutral position, the patient will admit that this is less comfortable, and may even axially rotate the pelvis contralaterally such that the contralateral leg externally rotates. Conversely and perhaps less specifically, a patient for whom sacroiliac joint syndrome is in the differential diagnosis, if lying supine with both lower limbs neutrally rotated, will often admit to more comfort if the examiner externally rotates the ipsilateral lower limb.
Provocative maneuvers
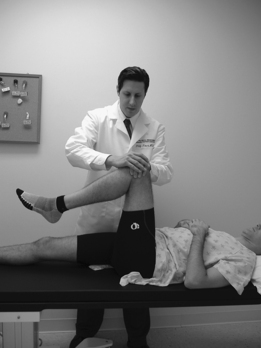
Lacking a definitively more accurate method of determining the presence of sacroiliac joint pathology per se, earlier evaluations of provocative maneuvers utilized as a gold standard the more readily diagnosable presence of ankylosing spondylitis. Russell et al.50 prospectively studied just over 100 subjects with low back pain, almost half of whom carried the diagnosis of ankylosing spondylitis based on definite radiologic abnormalities. Provocative maneuvers, in the form of ‘stress tests’ and ‘direct pressure’ tests, were performed. The former category includes Gaenslen’s test, in which the supine patient’s contralateral hip and knee are flexed and the ipsilateral innominate bone and lower limb hang off the examination table (Fig. 114.8); the side-lying iliac compression test described above; and another test where downward pressure is applied to both ilia in the supine patient. The ‘direct pressure’ tests include direct and forceful palpation over the sacroiliac joints, the sacrum, or the lower lumbar interspinous areas. Poor correlation was noted between the presence of ankylosing spondylitis and positive examination maneuver results.
Along these lines, Blower and Griffin found in a study of 66 low back pain patients that two provocative tests, namely downward bilateral iliac pressure in the supine patient and downward pressure over the inferior sacrum in the prone patient, were each specific for ankylosing spondylitis. More impressive to these authors was the fact that these maneuvers correlated better with the presence of HLA B-27 than with radiologic evidence of ankylosing spondylitis, implying to them that these tests could detect ‘presumptive ankylosing spondylitis’ which does not meet the standard criteria for definitive diagnosis due to insufficient radiologic evidence.51
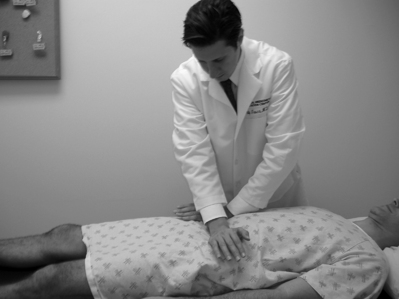
Assessment of intertester reliability of seven provocative tests was performed by Laslett et al. on 51 patients with low back pain. Tests included the previously described supine iliac gapping test, the side-lying iliac compression test, and Gaenslen’s test. Three other tests were assessed as well (see Table 114.2). One was the posterior shear test, in which pressure is transmitted from the examiner through the femur through the flexed hip and into the innominate bone in the supine patient (Fig. 114.9). The sacral thrust test entails direct downward pressure to the sacrum in the prone patient (Fig. 114.10). The cranial shear test involves cranial pressure directed on the sacrum from below the coccyx while the ipsilateral lower limb is pulled in a caudal direction, thus stabilizing the ilium. All examinations were performed by one fixed examiner and another drawn from a pool of five. Paired examinations for all tests demonstrated intertester agreement of at least 78%. Kappa values were lowest for sacral thrust at 0.52 and highest for posterior shear at 0.88.
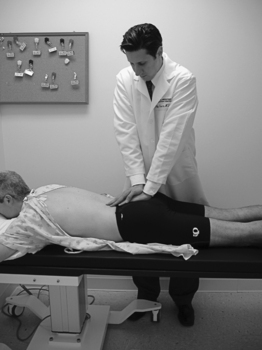
Fig. 114.10 The sacral thrust test entails direct downward pressure to the sacrum in the prone patient.
Maximizing the presumed ‘gold standard’ status of the diagnostic sacroiliac joint block, Maigne et al. used a double diagnostic block paradigm to more confidently diagnose low back pain as being of sacroiliac origin: 54 patients with symptoms suspicious for sacroiliac joint pathology underwent a fluoroscopically guided ‘screening block’ with instillation into the joint of 2 cc of 2% lidocaine.52 The 19 patients who responded to this injection returned a week later for instillation of 0.5% bupivacaine, a longer-lasting agent that allowed assessment of pain reduction during activities of daily living. Greater than 75% pain relief for at least 2 hours constituted a positive response, present in 10 patients. The following tests were studied: supine iliac gapping, side-lying iliac compression, Gaenslen’s, sacral pressure, and Patrick’s test. Two other tests included pain provocation with resisted external hip rotation in the prone patient and pressure applied to the pubic symphysis. Unfortunately, this group’s emphasis on the assumed value of the diagnostic block was counteracted by their minimal evaluation of the testing maneuvers of interest – response to the double block protocol was compared only to responses to individual tests, a proposition essentially invalidated by Potter and Rothstein.44 Furthermore, the number of examiners is not given; it is possible that there was only one examiner. Given that the tests themselves are the subject of study, a large number of patients should be accompanied by a large number of examiners to prevent idiosyncratic features of one clinician’s examination from skewing the entire data set.
A more sophisticated analysis was performed in the previously cited study by Dreyfuss et al.12 Here, 85 patients with low back pain were evaluated in terms of historical features of their symptoms as well as seven provocative maneuvers. These included the Gillet test, the posterior shear test, Gaenslen’s maneuver, sacral thrust, and palpation of the sacral sulcus. Another test utilized was Patrick’s test, also known as the ‘FABER’ test, in which the ipsilateral hip is passively flexed, abducted, and externally rotated (‘FABER’ is an acronym derived from these three motions). The seventh test, the spring test, involves palpation of motion play at the superior sacrum while the sacrum is pushed in a posteroanterior direction with the patient lying prone. Test results were studied individually and in groups; sacroiliac joint dysfunction was diagnosed based on a stringent 90% symptom reduction with instillation of 1.5 cc lidocaine and 0.5 cc Celestone Soluspan. Intertester reliability was measured between two examiners, a physician and a chiropractor. This was highest, at 87%, for sacral sulcus tenderness, but with a modest associated kappa value of 0.41. The highest kappa value was achieved by the posterior shear test: 0.64, with an associated intertester agreement of 82%. Sacral sulcus tenderness showed the highest individual sensitivity: 0.93 for the physician, 0.84 for the chiropractor. No other test had a sensitivity greater than 0.71 in either clinician’s hands. Single-test specificity was even less impressive, never exceeding 0.64 and reaching as low as 0.10 for sacral sulcus tenderness as performed by the physician. Further grouping of tests which were positive for either or both examiners failed to find any particular set of tests that attained clinically useful sensitivity, specificity, or likelihood ratios. The validity of all of the tests was thus called into question, even if intertester reliability was otherwise proven to be good.
Slipman et al.41 utilized fluoroscopically guided sacroiliac joint blocks to determine the presence of sacroiliac joint pathology in 50 consecutive patients. Provocative tests studied included Gaenslen’s, Patrick’s, and Yeoman’s tests, pressure application to the sacral sulcus, bilateral pressure to the ilia in the supine patient, side-lying compression, and standing hyperextension. This group concluded that in patients with complaints consistent with sacroiliac joint pathology, including pain over the sacral sulcus, a positive Patrick’s test, sacral sulcus pressure test, and at least one more of the other aforementioned provocative tests provided a 60% likelihood of greater that 80% pain relief following a diagnostic sacroiliac joint block with Celestone Soluspan and lidocaine. A positive Patrick’s test has been noted by Slipman to include not only ipsilateral pain reproduction but also diminished ipsilateral hip external rotation following flexion and abduction, possibly due to bony block at the sacroiliac joint. These results are in accord with the previous literature utilizing diagnostic blocks to make the definitive diagnosis of sacroiliac joint-mediated pain.
CONCLUSION
The peer-reviewed literature of the last two decades bears witness to the conceptual evolution regarding the physical examination of the patient with possible sacroiliac joint-mediated pain. Parameters that were once confidently associated with sacroiliac joint pathology were found to have poor intertester reliability and were discarded. More reproducible tests were sought, and following critical analysis it was found that sets of these tests, not solitary maneuvers, were required to achieve reliability. With the advent of the widespread use of the diagnostic sacroiliac injection, the question of external validity was brought to the fore. The validity of single tests was again replaced by the validity of test batteries. Finally, the certainty, bordering on hubris, that characterized the initial, misguided ‘common sense approach’50 to the physical examination in putative sacroiliac joint syndrome was slowly replaced by a mood and clinical tenor far more useful to the spine clinician than certainty: humility. And it is because of this humility, not in spite of it, that it is probable that the clinical assessment of sacroiliac joint syndrome will likely continue to make slow but steady progress.
1 Goldwaith JH, Osgood RB. A consideration of the pelvic articulations from an anatomical pathological and clinical standpoint. Boston Med Surg J. 1905;152:593-601.
2 Brunner C, Kissling R, Jacob HAC. The effects of morphology and histopathologic findings on the mobility of the sacroiliac joint. Spine. 1991;16:1111-1117.
3 Solonen KA. The sacroiliac joint in the light of anatomical, roentgenological, and clinical studies. Acta Orthop Scand. 1957;27:1-115.
4 Schunke GB. The anatomy and development of the sacro-iliac joint in man. Anat Rec. 1938;72:313-331.
5 Pilatte R, Vignes H. Articulation sacro-iliague (anatomique et physiologie). Le Progrès Medical [Paris]. 1919;34:479-483.
6 Sashin D. A critical analysis of the anatomy and the pathologic changes of the sacro-iliac joints. J Bone Joint Surg. 1930;28:891-910.
7 Kirkaldy-Willis WH, Hill RJ. A more precise diagnosis for low back pain. Spine. 1979;4:102-108.
8 Alderink GJ. The sacroiliac joint: review of anatomy, mechanics, and function. J Orthop Sports Phys Ther. 1991;13:71-84.
9 Kapandji IA. The physiology of the joints, vol. 3 . Churchill Livingstone, New York, 1974;54-71.
10 Williams PL. Warwick R, editor. Grays Anatomy, 36th edn.. WB Saunders, Philadelphia, 1980;473-477.
11 Vleeming A, Stoeckart R, Volers ACW, et al. Relation between form and function in the sacroiliac joint, part I: clinical anatomic aspects. Spine. 1990;15:130-136.
12 Dreyfuss P, Michaelsen M, Pauza K, et al. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine. 1996;21:2594-2602.
13 Bogduk N. The sacroiliac joint. In: Bogduk N, editor. Clinical anatomy of the lumbar spine and sacrum. 3rd edn. New York: Churchill Livingstone; 1997:177-186.
14 Bernard TNJr, Cassidy JD. The sacroiliac joint syndrome: pathophysiology, diagnosis, and management. In: Frymoyer JW, editor. The adult spine – principles and practice. 2nd edn. New York: Raven Press; 1997:2343-2366.
15 Russell A, Maksymowych W, LeClerq S. Clinical examination of the sacroiliac joint: a prospective study. Arthritis Rheum. 1981;12:1575-1577.
16 Slipman CW, Sterenfeld EB, Pauza K, et al. Sacroiliac joint syndrome: The diagnostic value of single photon emission computed tomography. ISIS Newsletter. 1994;2:2-20.
17 Hollinshead WH, Jenkins DB. The bony pervis, femur, and hip joint. In: Hollinshead WH, editor. Functional anatomy of the limbs and back. 5th edn. Philadelphia: WB Saunders; 1981:231-240.
18 Slocumb L, Terry RJ. Influence of the sacrotuberous and sacrospinous ligaments in limiting movements at the sacroiliac joint. JAMA. 1926;87:307-309.
19 Colachis SC, Worden RE, Bechtal CO, et al. Movement of the sacroiliac joint in the adult male: a preliminary report. Arch Phys Med Rehabil. 1963;44:490-498.
20 Egund N, Olsson TH, Schmid H, et al. Movements in the sacroiliac joints demonstrated with roentgen sterophotogrammetry. Acta Radiol Diagn. 1978;19:833-846.
21 Damen L, Buyruk HM, Güler-Uysal F, et al. The prognostic value of asymmetric laxity of the sacroiliac joints in pregnancy-related pelvic pain. Spine. 2002;27:2820-2824.
22 Mennell JB. The science and art of joint manipulation, vol. 2 . Blakiston, New York, 1952;24-31.
23 Smidt GL, McQuade K, Wei S-H, et al. Sacroiliac kenimatics for reciprocal straddle positions. Spine. 1995;20:1047-1054.
24 Barakatt E, Smidt GL, Dawson JD, et al. Interinnominate motion and symmetry: comparison between gymnasts and nongymnasts. J Orthop Sports Phys Ther. 1996;23:309-319.
25 Smidt GL, Wei S-H, McQuade K, et al. Sacroiliac motion for extreme hip positions. Spine. 1997;22:2073-2082.
26 Sturesson B, Selvik G, Udén A. Movements of the sacroiliac joints – a roentgen sterophotogrammetric analysis. Spine. 1989;14:162-165.
27 Sturesson B, Uden A, Vleeming A. A radiostereometric analysis of the movements of the sacroiliac joints in the reciprocal straddle position. Spine. 2000;25:214-217.
28 Cibulka MT, Delitto A, Koldehoff RM. Changes in innominate tilt after manipulation of the sacroiliac joint in patients with low back pain: an experimental study. Phys Ther. 1988;68:1359-1363.
29 Cibulka MT, Delitto A. A comparison of two different methods to treat hip pain in runners. J Orthop Sports Phys Ther. 1993;17:172-176.
30 Humphrey SM, Inman RD. Metastatic adenocarcinoma mimicking unilateral sacroiliitis. J Rheumatol. 1995;2:970-972.
31 Bohay DR, Gray JM. Sacroiliac joint pyarthrosis. Orthop Review. 1993;22(7):817-823.
32 Dreyfuss P, Dreyer S, Griffin J, et al. Positive sacroiliac screening tests in asymptomatic adults. Spine. 1994;19:1138-1143.
33 Beal MC. The sacroiliac problem: review of anatomy, mechanics, and diagnosis. J Am Osteopath Assoc. 1982;81:667-678.
34 Greenman PE. Sacroiliac dysfunction in the failed low back pain syndrome. In: Vleeming A, Mooney V, Snijders C, et al., eds. Proceedings from the First Interdisciplinary World Congress on Low Back Pain and its Relation to the Sacroiliac Joint. San Diego, CA. 1992; 329–343.
35 Cibulka MT, Koldehoff R. Clinical usefulness of a cluster of sacroiliac joint tests in patients with and without low back pain. J Orthop Sports Phys Ther. 1999;29:83-92.
36 Dwyer A, Aprill C, Bogduk N. Cervical zygapophyseal joint pain patterns I: a study in normal volunteers. Spine. 1990;15:453-457.
37 Aprill C, Dwyer A, Bogduk N. Cervical zygapophyseal joint pain patterns II: A clinical evaluation. Spine. 1990;15:458-461.
38 Fortin JD, Dwyer AP, West S, et al. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique, part I: asymptomatic volunteers. Spine. 1994;19:1475-1482.
39 Fortin JD, Aprill CN, Ponthieux RT, et al. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique, part II: Clinical evaluation. Spine. 1994;19:1483-1489.
40 Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine. 1995;20:31-37.
41 Slipman CW, Jackson HB, Lipetz JS, et al. Sacroiliac joint pain referral zones. Arch Phys Med Rehab. 2000;81:334-337.
42 Yeoman W. The relation of arthritis of the sacroiliac joint to sciatica, with an analysis of 100 cases. Lancet. 1928;2:1119-1122.
43 Hiltz DL. The sacroiliac joint as a source of sciatica. Phys Ther. 1976;15:1373.
44 Potter NA, Rothstein JM. Intertester reliability for selected clinical tests of the sacroiliac joint. Phys Ther. 1985;65:1671-1675.
45 Erhard R, Bowling R. The recognition and management of the pelvic component of low back and sciatic pain. Bulletin of the Orthopaedic Section. American Phys Ther Assoc. 1977;2:4-15.
46 Feinstein AR. Clinical epidemiology: the architecture of clinical research. Philadelphia: WB Saunders, 1985;86.
47 Riddle DL, Freburger JK. Evaluation of the presence of sacroiliac joint region dysfunction using a combination of tests: a multicenter intertester reliability study. Phys Ther. 2002;82:772-781.
48 LaBan MM, Meerschaert JR, Taylor RS, et al. Symphyseal and sacroiliac joint pain associated with pubic symphysis instability. Arch Phys Med Rehabil. 1978;59:470-472.
49 Cibulka MT, Sinacore DR, Cromer GS, et al. Unilateral hip rotation range of motion asymmetry in patients with sacroiliac joint regional pain. Spine. 1998;23:1009-1015.
50 Russell AS, Maksymowych W, LeClercq S. Clinical examination of the sacroiliac joints: a prospective study. Arthritis Rheum. 1981;24:1575-1577.
51 Blower PW, Griffin AJ. Clinical sacroiliac joint tests in ankylosing spondylitis and other causes of low back pain – 2 studies. Ann Rheum Dis. 1984;43:192-195.
52 Maigne J-Y, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine. 1996;21:1889-1892.