CHAPTER 27 Eosinophilic Disorders of the Gastrointestinal Tract
Eosinophilic inflammation of the gastrointestinal (GI) tract occurs in primary eosinophilic GI disease (EGID), as well as secondary to other diseases. The classification of primary EGID is traditionally according to the sites of inflammation in the GI tract (Table 27-1).1,2 The best characterized of these EGIDs, eosinophilic esophagitis (EE) and eosinophilic gastroenteritis (EG), affect all ages and exhibit prominent eosinophilic tissue infiltration. Other noteworthy diagnoses within the spectrum of EGIDs, such as food protein–induced enterocolitis (FPIEC) and eosinophilic proctitis (EP), are uniquely pediatric diagnoses, and the pathology may be characterized by a mixed inflammatory infiltrate including dense tissue eosinophilia. The cause of these disorders may not be yet well understood, but they all have a strong association with allergies, and many respond to nutritional management (see Chapter 9).3,4 The collaborative efforts of gastroenterologists, allergists, and immunologists have made significant advances in understanding the immunopathogenesis of EGID in recent years. The publication of guidelines for the diagnosis and management of EE also became possible through such efforts.5
Table 27-1 Proposed Classification and Differentiation of Primary and Secondary Eosinophilic Gastrointestinal Diseases
| PRIMARY EGID | SECONDARY EGID AND/OR DIFFERENTIAL DIAGNOSIS |
|---|---|
EE, eosinophilic esophagitis; EG, eosinophilic gastroenteritis; EGID, eosinophilic gastrointestinal disease; GERD, gastroesophageal reflux disease; HES, hypereosinophilic syndrome; IBD, inflammatory bowel disease.
Eosinophilic inflammation also occurs secondarily in the GI tract in inflammatory bowel disease (IBD), autoimmune diseases, reactions to medications,6,7 infections, hypereosinophilia syndrome (HES), tumors, and after solid organ transplantation.8,9 These disorders should be considered in the differential diagnosis of the primary eosinophilic diseases and are briefly reviewed in this chapter.
EOSINOPHIL: ROLE IN HEALTH AND DISEASE
The eosinophil, a bilobed nucleated granulocyte, differentiates from myeloid progenitor cells into its mature form containing brilliant birefringent cationic granules with a high affinity for the acidic dye eosin. It matures mainly under the influence of the hematopoiesis-specific transcription factors GATA-1, GATA-2, and PU.I, and c/EBP (enhancer-binding protein family).10 Cytokines, interleukin-3 (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF) strongly influence further development. IL-5, in particular, plays a role in the eosinophil’s differentiation and release from the bone marrow into the peripheral circulation, where it constitutes 2% to 4% of the granulocyte pool and has a circulating half-life of only 8 to 12 hours. The eosinophil then moves into resident tissues, mainly the GI tract, thymus, hematopoietic organs, and mammary glands. In the GI tract, eosinophils survive for about 1 week and finally undergo apoptosis.11
EOSINOPHILS AND THE GASTROINTESTINAL TRACT
As noted, eosinophils spend most of their lifespan in tissues, rather than circulating. The GI tract is the main nonhematopoietic organ in which eosinophils reside in the healthy state. In the GI tract, eosinophils are not homogeneously distributed. Highest concentrations are found in the cecum, ascending colon, and appendix. The esophageal epithelium is unique in being devoid of eosinophils under noninflammatory conditions.11,12 Eosinophils are normally present in the lamina propria of the gut, but the number of eosinophils regarded as pathologic for various sites along the GI tract is debated.
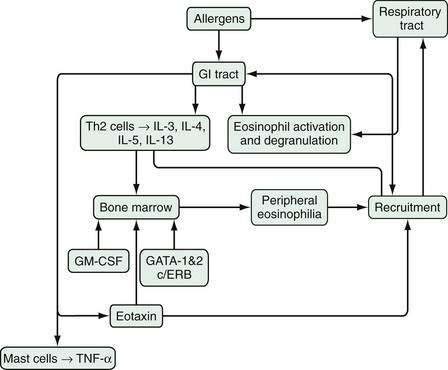
An array of stimulatory and proinflammatory factors mediate eosinophilic inflammation (Fig. 27-1). In the case of eosinophilic GI inflammation, an antigen exposure stimulates eosinophil synthesis, rolling, adhesion, diapedesis, and trafficking to the site of insult. The recruitment of eosinophils into GI segments is regulated by differential pathways involving a family of cell adhesion receptors called integrins. Investigations have shown that eosinophil movement into the small intestine and large intestine are controlled by α4β7-integrin and β2-integrin pathways, respectively.10,13,14 Eosinophils function as antigen-presenting cells and also affect the inflammatory process through specific eosinophil-derived granule proteins (EDGPs). These EDGPs include eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), eosinophil peroxidase (EPO), and major basic protein (MBP). These cationic proteins are cytotoxic to the human intestinal epithelium, possess antiviral and ribonuclease activity, and trigger degranulation of mast cells and release of cytokines (IL-1, IL-3, IL-4, IL-5, IL-13, GM-CSF, tumor necrosis factor-α [TNF-α], transforming growth factors), chemokines such as eotaxin-1 and RANTES (regulated on activation, normal T cell expressed and secreted), lipid mediators (leukotrienes, platelet-activating factor), and neuromediators (substance P, vasoactive intestinal polypeptide, nerve growth factor).15
Investigators have used various experimental models to explore the mechanisms whereby eosinophils mediate GI disease. An important puzzle includes the localization of the instigation of eosinophilic responses within the GI tract. The route of allergen exposure may determine the localization of the response. For example, oral or intragastric allergen exposure does not initiate EE but, in anesthetized mice, exposure to repeated challenges of aeroallergens induces marked EE in addition to lung eosinophilia. Interestingly, however, such aeroallergen challenge does not provoke eosinophilic inflammation in the stomach or small intestine of the mice.14,16 In human EE, therefore, sensitization likely occurs via the respiratory tract, with subsequent exposure to oral allergens leading to a hypersensitivity response and esophageal eosinophil infiltration.
Experimental studies have suggested the mechanism of this link between the lung and esophagus via T helper 2 (Th2) allergic responses in the lung and esophagus.14,16,17 Th2 cells (see Chapter 2 for more details) produce an array of cytokines, of which IL-5 is the most specific for eosinophils, inducing eosinophil growth, differentiation, activation, and survival, and enhancing responsiveness to chemoattractants such as eotaxin-1, eotaxin-2, and eotaxin-3 (eosinophil selective chemokines structurally distinguished from others on the basis of conserved cysteines). Further studies using the murine model of EE have demonstrated an important role for IL-5, IL-13, and eotaxin in this disorder.18–20 In IL-5–deficient mice, the allergen-induced EE response is ablated and, in the absence of eotaxin, it is attenuated. Furthermore, the absence of IL-5 reduces the esophageal eosinophilia induced by oral allergens after sensitization to aeroallergens, but the absence of IL-5 does not reduce intestinal eosinophilia, strongly suggesting a differential recruitment of eosinophils in EE and EG.
In humans the esophageal infiltrate in EE also includes increased numbers of T cells and mast cells and increased IL-5, TNF-α, and eotaxin.21,22 Recent evidence that IL-13 delivery into the lung induces EE further implicates Th2 cells and cytokines in the immunopathogenesis of EE.17,23 Furthermore, esophageal biopsies in EE are notable for sharing some remodeling features with airway disease in asthma, such as increased profibrotic cytokines, signaling molecules, increased vascularity, and vascular activation.24,25
In a placebo-controlled experiment using another murine model, mice challenged with oral, encapsulated ovalbumin developed peripheral blood eosinophilia and antigen-specific immunoglobulin E (IgE) and IgG1 antibodies. Their eosinophil-predominant cellular infiltrate was largely localized in the lamina propria throughout the small intestine but was also present in the esophagus, stomach, and Peyer’s patches. The mice developed gastromegaly, dysmotility, and cachexia, thought to be correlates of human EGID.26 In a mouse model of the homozygous lyp gene mutation (lyp protects against lymphocyte apoptosis), increased levels of Th2 cytokines and IgE are observed in association with clinical features of bloating, intestinal distention, wasting, splenomegaly, and increased intestinal eosinophilia.27
Some patients with EE have also been characterized as having a unique genomic transcript comprised of an increased expression of the gene encoding eosinophil-specific chemoattractant eotaxin-3 compared with healthy patients.18,28
CLINICAL ENTITIES
EOSINOPHILIC ESOPHAGITIS
In the esophagus, attention to eosinophilic infiltration has focused on the epithelium, rather than on the lamina propria. This squamous epithelium normally is devoid of eosinophils, but various disorders cause eosinophils to infiltrate the esophageal epithelium. In general, such esophageal eosinophilic infiltration is considered to be secondary to an extraesophageal cause (e.g., parasitic infections, autoimmune diseases, vasculitis, HES, medications) or an esophageal cause (e.g., gastroesophageal reflux disease [GERD])29 or to be primary EE. Primary EE may be divided into allergic or idiopathic cases, depending on whether identifiable allergens play a role (see Table 27-1). Occasional patients presenting with apparent EE have marked eosinophilic inflammation of other segments of the GI tract, and designation as EE secondary to EG or as a form of primary EE is a matter of semantics.
Primary EE, rarely diagnosed until the mid-1990s, currently represents an important esophageal disorder, particularly in children, but increasingly in adults. The emergence of this disease has paralleled the increasing incidence of allergies and asthma. Whether the increasing number of cases of EE is to the result of increased recognition of EE or a truly increased incidence of EE is still debatable.30–32 Although some of the previous lack of recognition in adults may have resulted from failure to biopsy intact-appearing esophageal mucosa, the actual prevalence of EE in adults has increased, as evident in some recent reviews.33–39 Similarly, although the routine biopsying of even normal-appearing mucosa by pediatric gastroenterologists may account for some of the predominance of EE in school-age children, it is likely that this age group currently does experience more EE, with another peak in young adulthood.40,41 Like allergic disorders, EE’s prevalence also varies markedly in different locales and perhaps in different seasons.42 The last 10 years or so have seen a dramatic increase in the diagnosis of EE around the world; this may be the result of both improved recognition and an actual increase in new cases akin to other allergic disorders. The incidence of pediatric EE in Australia increased from 0.05 to 0.89/10,000 during 1995 to 2004, according to a retrospective review.43 In the U.S. Northeast, a twofold increase in incidence was observed in a four-year period at Children’s Hospital of Philadelphia.44 In the U.S. Midwest, a fourfold increase in prevalence was reported.45 The prevalence of EE was reported as 15/100,000 inhabitants in one region of Switzerland, perhaps an underestimation because of limited expertise in its diagnosis.46 Markedly more prevalent in males, the disorder occurs in females as well. Duration of symptoms before diagnosis may vary from just a few days in those presenting with sudden episodes of food impaction to many years in those with GERD-like symptoms.4,47 Like allergies in general, EE clusters in families and an autosomal dominant pattern of inheritance have been proposed on the basis of the 10% rate of familial clustering.48 A personal history of atopy in association with EE in children and adults occurs in 50% to 80% cases, with food allergy accounting for an allergic diathesis in up to 90% children; 39% report a family history of allergies.49–52 In one series, symptoms of chronic respiratory disease were seen in 62% of patients.40 In support of the theory that aeroallergens promote the disease are cases of EE with symptomatic and biopsy-proven exacerbations during pollen season and resolution during winter months.53,54
The first report of EE in 1978 and subsequent reports have characterized the phenotype of EE.35,40,44,55,56 Symptoms of EE are similar to those of GERD, but respond poorly to antireflux medical and surgical therapy.57 Whereas younger children with EE frequently present with GERD-like symptoms, feeding problems, and abdominal pain,58,59 adolescents and adults present with obstructive presentations such as dysphagia or food impactions, with or without strictures.36,47 The degree to which these presentations represent actual structural obstruction versus dysmotility is unclear and appears to vary among patients.60 Esophageal biopsy of patients without any GI symptoms, such as some patients presenting for evaluation of respiratory symptoms, has disclosed unsuspected EE.37,61
The brief history of recognition of EE has prevented clear definition of its natural history, but EE appears to be a chronic disease with a waxing and waning course, as suggested by a noteworthy relapse rate of 80% in an eight-year follow-up of children with EE and similarly high rate of recurrent symptoms and chronic therapy in adults.62,63 It is thought that the esophageal wall is fragile and weakened in patients with chronic EE, thus predisposing to endoscopy-related and spontaneous perforation.36,64,65 Another study of the long-term (mean, 7.2 years) follow-up of EE in adults has reported persistence of eosinophilic inflammatory infiltrate (albeit significantly reduced compared with baseline) and dysphagia and a high rate of subepithelial fibrosis and sclerosis, perhaps the mechanistic link to esophageal strictures in EE.41 Although not supported by any published data, EE patients are also considered to have a low quality of life. This information raises questions regarding the pros and cons of treatment for asymptomatic patients with EE. The current literature does not clearly identify any malignant potential of the disease.66,67
EOSINOPHILIC GASTROENTERITIS
Eosinophilic gastroenteritis is a heterogeneous disorder affecting children and adults characterized by the presence of an intense eosinophilic infiltrate on histopathology of one or multiple segments, from the esophagus to the rectum.68 These eosinophilic infiltrates not only may involve various sites down the length of the GI tract, but also may occupy various sites through the depth of the wall. These inconsistencies from case to case promote unpredictability in presenting symptoms, which range from pain to dysmotility, bleeding, obstruction, or ascites.69–72 Since the initial report of EG seven decades ago, reports of EG have emerged from different parts of the world, including North America, Europe, Australia, and Asia.72–77 These reports provide important information regarding epidemiology, disease characteristics, and management.
The diagnosis of EG is rare, with an approximate incidence of 1/100,000, but it is also possible that physicians make the diagnosis of EG infrequently because of the inaccessibility of much of the length of the small bowel and of the deeper layers of the luminal wall. Therefore, the literature on EG has been somewhat anecdotal. Retrospective review of an 18-year period at a hospital in China identified 15 patients with EG, including 2 children.74 Histologic evaluation established the diagnosis in 13, and radiologic findings, combined with eosinophilic ascites, suggested it in the remaining 2 patients.
In one of the largest series, Talley and colleagues compared laboratory and clinical data on 40 adults diagnosed with EG during a 30-year period with data on 10 other patients with similar GI symptoms but no tissue eosinophilia.73 EG is most commonly diagnosed between the second and sixth decades of life, and is rarely diagnosed in infants.1,74,78 Unlike EE, which favors males, EG does not appear to manifest a significant gender disparity. It is associated with asthma and allergies in 40% to 50% of cases.79,80 Peripheral eosinophilia may be seen in up to 80% of cases, but is not a prerequisite for diagnosis.81
In published reports, the stomach (26% to 81%) and small intestine (28% to 100%) are the predominantly affected areas, but the esophagus, large intestine, and rectum may be affected as well.1,80 The depth of infiltration varies and leads to the broad spectrum of clinical manifestations in patients with EG. The classification of EG on the basis of depth of eosinophilic infiltration proposed by Klein and associates is currently the one most cited in publications.82
Mucosal Eosinophilic Gastroenteritis
Those with mucosal inflammation usually present with common, albeit nonspecific, complaints of abdominal pain, nausea, vomiting, diarrhea, fecal occult blood loss, anemia, or protein-losing enteropathy. Because of the nonspecific nature, these clinical presentations may be confused with irritable bowel syndrome, dyspepsia, peptic ulcer, pancreatitis, acute appendicitis, or IBD.83 Eosinophilic enteritis presenting in an adult as intussusception, and treated effectively with the nonsurgical option of prednisone, has been observed.76 Frequently, atopy and high IgE levels coexist.73,74 An example of eosinophilic gastritis is shown later (see Fig. 51-8B).
Muscular Eosinophilic Gastroenteritis
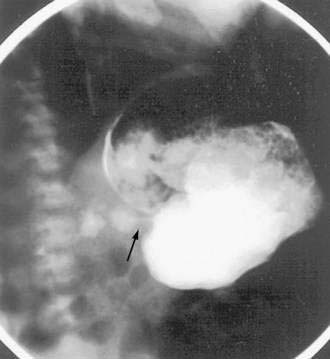
Signs and symptoms of gastric outlet and intestinal obstruction are common in those with muscular EG.84,85 The presentation of gastric outlet obstruction mimicking hypertrophic pyloric stenosis has been reported in infancy and adulthood.86,87 A hypoallergenic diet has been shown to alleviate the condition in infants (Fig. 27-2). Enteric strictures are rare, but can occur in children and adults with EG.88 As a sort of amalgam between the mucosal and muscular forms, one patient was reported to have eosinophilic inflammation of myenteric plexus and the lamina propria on colonic biopsies, producing functional intestinal obstruction.89
Serosal Eosinophilic Gastroenteritis
Involvement of the serosal layer occurs in 10% of cases of EG and typically presents as ascites. The serosal form of EG, compared with other types, is reported to be associated with significant bloating, a higher level of peripheral eosinophilia, and a better response to glucocorticoid therapy.73,90,91
The natural history of EG remains somewhat vague, although recent pediatric data has supported a protracted course, and hence the need for long-term treatment strategies, including dietary restrictions and repeated use of glucocorticoids.78
FOOD PROTEIN–INDUCED ENTEROCOLITIS
Enterocolitis signifies an inflammatory process involving the small and large intestines. FPIEC represents a symptom complex of severe vomiting and diarrhea that usually presents in infancy as a reaction to ingested proteins. The onset of symptoms is in the first few weeks of life. The trigger is most often ingestion of cow’s milk protein-based formula, but approximately half of infants also react to soy. Other food proteins, including rice, oats, and chicken, have also been implicated in individual cases.92–94 The responsible dietary antigens in the maternal diet are thought to sensitize via breast milk. The profuse, often bloody and mucoid, diarrhea is associated with weight loss and malnutrition in an ill-appearing patient. An association with methemoglobinemia noted in several cases was attributed to increased heme oxidation caused by an elevation of nitrite levels in the intestine in severe intestinal inflammation.95,96
Typically, FPIEC is caused by non-IgE mediated delayed food protein hypersensitivity, so allergy testing with skin prick tests (SPTs) and RASTs are negative; patch tests have not been adequately studied in this diagnosis.97,98 Patients lack evidence for other causes of eosinophilia, such as infections, inflammatory bowel disease, and ischemia. The diagnosis rests on clinical criteria and resolution after elimination of the causal milk and soy proteins from the diet. Most infants do well when their milk is changed to an extremely hydrolyzed formula that digests the intact proteins into small polypeptides of sizes that do not engender the hypersensitivity response. Up to 90% of such infants can tolerate milk by 3 years of age. Any milk challenge should be performed under medical supervision because of the risk of serious reactions leading to shock. Criteria for a failed challenge include vomiting, diarrhea, gross or occult fecal blood, fecal leukocytes, fecal eosinophils, and elevated white blood cell count.
EOSINOPHILIC PROCTITIS
EP uniquely affects children younger than 2 years. These children present with bloody stools, either alone or in association with diarrhea. The condition has been reported in infants receiving cow’s milk and soy protein–based formulas, as well as in exclusively breast-fed infants. A few infants suffer from eczema, but otherwise these children lack any systemic symptoms. In contrast to children with enterocolitis, these babies generally appear well. In a review, all 95 exclusively breast-fed infants evaluated for proctitis over 20 years had blood-tinged stools, and one third of them were observed to have painful defecation and eczema.99 The diagnosis may be confused with colic or GERD in infants who also present with irritability or vomiting. In a prospective study, 18% of 40 breast-fed infants presenting with rectal bleeding were diagnosed to have cow’s milk allergy on the basis of milk elimination and provocation; association with food-specific IgE and positive SPTs was uncommonly noted.100
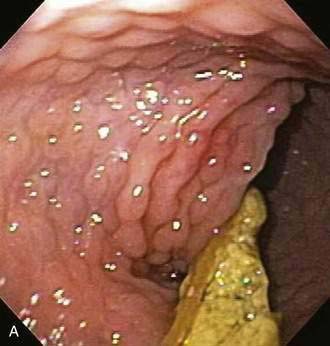
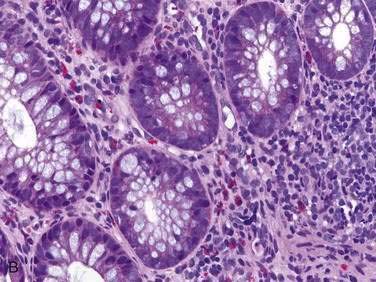
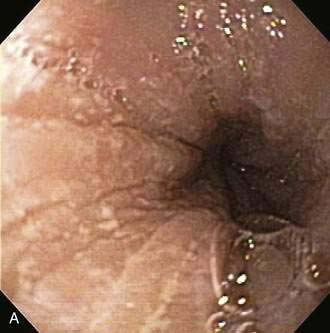
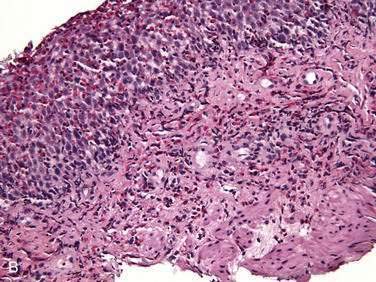
Endoscopic examination reveals focal rectal mucosal erythema, erosions, and lymphoid nodular hyperplasia (Fig. 27-3A). Histopathologic examination shows prominent eosinophil infiltration in the mucosa and lamina propria, at least 6 eosinophils/high-power field, and/or eosinophils invading crypts or the muscularis mucosae (see Fig. 27-3B).101 There is an excellent response to elimination diets using hydrolyzed or elemental formulas; by 1 year of age most infants can tolerate a rechallenge with the offending food proteins.
EVALUATION
LABORATORY EVALUATION
Peripheral eosinophilia in the context of GI symptoms is a useful clue to EGID, but the absence of eosinophilia does not exclude these diagnoses.102 It is important to note that circulating eosinophils represent a balance between bone marrow production and tissue infiltration. Moreover, the frequently observed fluctuations in peripheral eosinophil concentrations may be caused by the effects of the circadian rhythm.103 To exclude important secondary causes for GI eosinophilia, evaluation should generally include stool or duodenal aspirate for ova and parasites. In those with ascites, paracentesis may provide the only clue to the diagnosis in the form of ascitic fluid eosinophilia. Hopefully, our increasing knowledge will soon allow simple, reliable, and relatively noninvasive testing involving markers of active eosinophil inflammation (e.g., fecal ECP) for monitoring disease course and response to treatment.104
ALLERGY EVALUATION
Immunologic evidence of underlying allergy is usually lacking in most EGIDs, except those mediated by IgE antibody and typically presenting with immediate reactions or accompanied by eczema or asthma (see Chapter 9).
In addition to the lack of sensitivity of allergy testing in diagnosing EGIDs, there is a lack of specificity, with a high rate of false-positive results. The most commonly available tests, SPTs and RASTs, are used to detect IgE antibody specific to inhaled and ingested allergens. SPTs are sensitive, so negative SPTs are useful in confirming the absence of IgE-mediated reactions, if good-quality food extracts are used. In those older than 1 year, SPTs are associated with a high negative predictive value but a positive predictive value of 50% or lower. Atopy patch testing, used for non-IgE cell–mediated immunologic reactions, is gaining popularity in Europe, but has not found a routine place in most U.S. centers.105 The use of patch tests in combination with SPT in EE has been shown to identify food allergies with greater accuracy, thus leading to greater success in dietary therapy than SPT alone.52,106,107 RAST or enzyme-linked immunosorbent assays detect circulating IgE antibody against specific food antigens. The quantitative CAP fluorescent enzyme immunoassay (FEIA) has been found accurate for predicting symptomatic food hypersensitivity when compared with the gold standard of double-blind, placebo-controlled food challenges.108 Non-IgE–mediated allergies, often implicated in the pathogenesis of EGID, pose a particularly difficult challenge; the diagnosis rests on the results of elimination diets, selected oral food challenges, and biopsies. Important to note is that double-blind, placebo-controlled food challenges in the research setting and open challenges in clinics have limited usefulness for EGIDs, because delayed hypersensitivity reactions may not be apparent for a few days. An alternative approach, therefore, uses diagnostic trials of therapy with dietary restrictions such as elimination diets, the six-food elimination diet, or oligoantigenic or elemental (amino acid–based) diets.44,109
RADIOLOGIC EVALUATION
Barium esophagography is an important study in the evaluation of dysphagia and may demonstrate EE strictures, which, in contrast to the distal location of GERD strictures, are usually located in the proximal and midesophagus. High-resolution endoscopic ultrasound demonstrates increased esophageal wall thickness with expansion of mucosa, submucosa, and muscularis propria in children with EE, compared with healthy controls.110 It provides insight into the full-thickness inflammation that can lead, in turn, to esophageal dysmotility and obstructive presentations. Radiographic changes in patients with EG of the stomach may include an irregular and lacy antral surface on an upper GI series. Furthermore, a string sign may be demonstrated in gastric outlet obstruction because of antral EG (see Fig. 27-2).87 Eosinophilic infiltration of the small bowel manifests as thickening of the circular folds and wall.111 Computed tomography (CT) may show nodular, irregular, and thickened intestinal folds in the affected segments in EG.77 Deep infiltration may result in rigid bowel loops, simulating lymphoma.112 Abdominal ultrasonography is useful for detecting ascites.
ENDOSCOPY AND PATHOLOGY
Although the macroscopic appearance of the esophagus in EE may be normal, most cases are associated with one or more characteristic findings. These include furrowing, vertical lines, rings, granularity, crepe paper appearance, and whitish exudates (Fig. 27-4A).40,113–115 Esophageal biopsies sampled from areas of whitish exudates or specks contain a significantly higher eosinophilic density compared with areas without these findings.116 A small-caliber noncompliant esophagus, also termed a defiant esophagus, furrowing, and mucosal shearing on dilation are noted in some young adults with EE.33,117 Multiple esophageal biopsies should be procured along the length of the esophagus to ensure satisfactory sampling. An eosinophil density higher than 15 eosinophils/high-power field (eos/hpf) strongly suggests the diagnosis of EE, but must not be relied on as the sole diagnostic criterion. The diagnosis of EE is supported by the proper clinical context, in which a patient presents with signs of esophageal dysfunction and a poor response to a trial of proton pump inhibitor therapy.118 In contrast, the esophageal eosinophil density in patients with GERD is generally less than 7 eos/hpf.5,44
A mean eosinophil density more than or equal to 7 eos/hpf provides a sensitivity of 61%, specificity of 96%, and a predictive value for failure to respond to antireflux therapy of 86%. A mean eosinophil density less than 7 eos/hpf provides an 85% predictive value for successful antireflux therapy.119 Differentiation of EE from GERD based on eosinophil density may be even clearer in the proximal esophagus than in the distal esophagus and might be compelling in light of the proposed triggers (dietary from above in EE and acid from below in GERD), but this is not a universal finding. Other typical histologic features include a preferential juxtaluminal location of eosinophils, degranulating eosinophils, eosinophil abscesses, elongated papillae, and prominent basal layer hyperplasia (see Fig. 27-4B).5,118
Eosinophilic gastroenteritis may present with a spectrum of gastric macroscopic abnormalities, which may include erythema, focal erosions, ulcerations, and pseudopolyps.120 The gross endoscopic abnormalities in EG are most striking in the mucosal form, and include thickening of folds, erythema, and friability.75,121 The precise histologic criteria required to diagnose EG are ambiguous (see Fig. 51-8B). Normally, mucosal eosinophils may be found in low numbers in the stomach and reach higher densities (up to 30 eos/hpf) in the appendix, terminal ileum, cecum, and proximal colon.122 Furthermore, the diagnosis may be elusive, either because of patchy disease distribution or the mucosa being spared altogether, as in muscular and serosal types of EG. Degranulated eosinophils are noted in the intestinal mucosa accompanying histologic damage in EG. Although invasive, laparoscopy or open surgical exploration is most helpful for establishing the diagnosis of muscular and serosal disease. The gross findings described in serosal EG are ascites, whitish nodules, and thickening of the parietal and visceral peritoneum.91
Histologic descriptions from some case series of FPIEC feature prominent eosinophilia in colonic or small bowel biopsies and also include nonspecific findings of crypt abscesses and a diffuse inflammatory cell infiltrate in colonic biopsies, as well as variable villous injury, acute inflammation, and prominent eosinophilia in small bowel biopsies.95
Endoscopic findings in EP demonstrate disease in the rectum and sigmoid. There may be focal areas of erythema, friability, ulcerations, and lymphoid nodular hyperplasia. Histopathology consistently reveals intense eosinophilic infiltration of the mucosa, with the eosinophil concentration varying between 6 and 20 eos/hpf, and it often features degranulated eosinophils (see Fig. 27-3A and B). Characteristics of chronicity and granulomas are absent, facilitating the exclusion of IBD.100,101
DIFFERENTIAL DIAGNOSIS
INFECTIONS
Parasitic Infestations
Invasive helminthic infections frequently result in tissue and peripheral eosinophilia, which reflects an immunologic response to tissue migration; however, when migration ceases, the eosinophilia often resolves (see Chapter 110). Tissue eosinophilia is associated with hookworms (Ancylostoma caninum), pinworms (Enterobius vermicularis), Eustoma rotundatum, Giardia lamblia, Anisakis, Trichinella spiralis, Ascaris, Trichuris, and Schistosoma.123–126 Eosinophilic ascites in the absence of gastroenteritis has occurred with Toxocara canis and Strongyloides stercoralis.127–129 Fasciola hepatica can cause eosinophilia, right upper quadrant pain, fever, and hepatomegaly.130,131 Although peripheral eosinophilia is usually absent in Giardia infestations, the diagnosis is aided by stool studies (ova and cysts, Giardia antigen) and careful histologic examination of a duodenal aspirate, which has the highest diagnostic yield (90%). The larvae of Anisakis may be identified at endoscopy in the stomach in an area of mucosal edema and, in some cases, ulceration (see Chapter 51).132,133 This parasitic infestation may be underdiagnosed; a Spanish study has suggested that up to 80% of patients thought to have idiopathic EG have evidence of exposure to Anisakis, contrasted with 10% of control subjects.134
MEDICATIONS
A drug allergy may result in eosinophilic involvement of the gut.138,139 For example, a hypersensitivity reaction to carbamazepine, producing EE and resolution with drug withdrawal, has been reported. Other medications reported to induce intestinal eosinophilia include tacrolimus,9 gemfibrozil,140 enalapril,141 and interferon-α.142
CONNECTIVE TISSUE DISEASE and VASCULITIS
Several connective tissue and vasculitic disorders are reported to be associated with GI eosinophilia (see Chapter 35).6 Gastrointestinal symptoms in patients with systemic lupus erythematous have been described as being caused by eosinophilic enteritis. The signs and symptoms of the underlying disease (e.g., lupus) allow for the classification of such secondary EGIDs despite the pathology, which is similar to that seen in the primary EGIDs reviewed earlier.143 In another example of a vasculitic disease, Churg-Strauss syndrome, an eosinophilic infiltrate involves the small arteries and veins; granulomas can be found in the lungs, heart, kidneys, and subcutaneous tissues and may also occur in the stomach, small bowel, and colon.144 The diagnosis of vasculitis can be substantiated by biopsy of involved organs (skin, muscle).
INTESTINAL POLYPS
Juvenile Polyps
Juvenile polyps, also known as retention or hyperplastic polyps, are typically benign colonic tumors diagnosed most commonly in children aged 2 to 10 years who present with painless hematochezia (see Chapter 122). The polyps are usually pedunculated, solitary, and located in the rectosigmoid colon. The surface of the polyp appears lobulated because of multiple mucin retention cysts. Adenomatous transformation of a juvenile polyp is rare. Occasionally, a heavy eosinophil infiltrate is present in the stroma of a juvenile polyp, as well as in mucosal biopsies of grossly normal colon in the same patient. Our understanding of the role of eosinophils in juvenile polyps is poor.
Inflammatory Fibroid Polyps
These benign localized lesions should not be confused with EG. They typically are found in the stomach, followed by the small and large intestine, but they rarely may be in the esophagus.145 They originate in the submucosa and typically appear as polyps or nodules; peripheral eosinophilia is absent, and a history of allergy is unusual.146 Many other terms have been used to refer to these lesions, including fibroma, inflammatory pseudotumor, submucosal granuloma, and localized EG.6 Histologically, the stroma in these lesions is characterized by a concentric arrangement of proliferating spindle cells, which may be fibroblasts or endothelial cells, although their exact nature remains controversial, surrounding arborizing capillaries, with a variable eosinophil infiltration. These lesions are relatively rare, with a slight male preponderance; although they may appear at any age, they are most common in the sixth and seventh decades. Most patients present with obstructive symptoms that depend on the site of the lesion; gastric outlet obstruction and small bowel intussusception are common manifestations. Surgical excision is curative in symptomatic patients, and recurrence has not been reported. Therapy with glucocorticoids is not indicated. One retrospective study of the spectrum of colonic neoplasms revealed the most prominent stromal eosinophilia in adenomas; only 5% of hyperplastic polyps had any eosinophil infiltration, and invasive adenocarcinomas had a striking absence of any eosinophilia.147
HYPEREOSINOPHILIA SYNDROME
Occasionally, hypereosinophilia syndrome (HES), a multisystem disorder, can involve the gut and be confused with EG. The diagnostic criteria established in 1975 and still in use today are the following: blood eosinophilia exceeding 1500 cells/µL for more than six consecutive months, absence of an underlying cause of hypereosinophilia despite extensive evaluation, and presence of organ damage or dysfunction related to hypereosinophilia.148 By definition, eosinophilic infiltration of multiple organs outside the abdomen excludes the diagnosis of primary EGID. The heart, skin, and central nervous system are the major targets, with more than 50% of patients presenting with complications in one or more of these sites. Recent studies have indicated that the condition can be classified as myeloproliferative or lymphocytic, providing evidence for the existence of discrete hematologic disorders underlying these variants.148,149 Anemia and thrombocytopenia are often present.
Congestive heart failure with endocardial fibrosis (and valvular incompetence), venous and arterial thromboembolism, neuropsychiatric disturbances, mononeuritis multiplex, and fever are common clinical features. The prognosis is poor in patients with prominent organ involvement, with a 25% three-year mortality rate without treatment. The prognosis tends to be better in patients who have angioedema. Imatinib mesylate (Gleevec) is the treatment of choice for those with the myeloproliferative variant and the FIP1L1-PDGFRAα (F/P) fusion gene. Recognition of imatinib-resistant mutations has led to newer treatment options, including the development of tyrosine kinase inhibitors and anti–IL-5 monoclonal antibodies.150–152 Other treatment strategies include glucocorticoids (about one third respond), hydroxyurea, cyclosporine A, and interferon-α. In the presence of malignant transformation, chemotherapy, bone marrow transplantation, or stem cell transplantation may be considered.
INFLAMMATORY BOWEL DISEASE
In contrast to the primary eosinophilic diseases, IBD is a secondary eosinophilic disorder and is treated in greater detail in Chapters 111 and 112. Aspects specifically related to eosinophil infiltration, however, are useful to consider here. Eosinophils may be elevated in the peripheral blood and inflamed tissue in patients with Crohn’s disease and ulcerative colitis. Eosinophils are not pathognomonic of IBD, but represent a major component of the inflammatory infiltrate in active IBD.153
The increased concentrations of eosinophil granular proteins in intestinal fluid and mucosa in IBD suggest a pathogenic role for activated eosinophils. It is suggested that ulcerative colitis is a predominantly Th2-associated disease accompanied by overproduction of IL-5154 and increased serum eotaxin levels.155 Patients with ulcerative colitis seem to experience a higher prevalence of allergies than controls (52% vs. 18%). However, whether infants with allergic intestinal diseases are at risk for developing IBD in later life is controversial.156
Crohn’s disease is characterized by transmural inflammation believed to be mediated by Th1-type cytokines. Immunohistochemical analysis shows expression of TNF-α in several cell lines, including eosinophils. There is an increased serum eotaxin level in patients with Crohn’s disease,157 and fecal excretion of ECP is elevated during disease flare-ups.158
CELIAC DISEASE
Celiac disease, regarded as an immune-mediated hypersensitivity to gluten-containing grains, is characterized by variable small intestinal villous atrophy with crypt hyperplasia, intraepithelial lymphocytes, and increased cellularity of the lamina propria (see Chapter 104). Activated eosinophils are one type of infiltrating inflammatory cells in the lamina propria in celiac disease, suggesting a role for eosinophils in this disorder.159 A concurrent diagnosis of EE and celiac disease has been recognized in recent case reports, but do not provide sufficient understanding to distinguish between a pathogenic association and mere coexistence.160,161 Glucocorticoids, in addition to a gluten-free diet, were required for treatment of the EGID, perhaps pointing to the coexistence of two discrete disorders.
TRANSPLANTATION
Eosinophilic GI inflammation after solid organ transplantation is being increasingly reported (see Chapter 34).8,9,162 The precise roles of immunosuppression, therapy for rejection, and viral infections are yet to be determined. Proposed mechanisms include an imbalance of Th1 and Th2 lymphocytes as a result of immunosuppressive therapy promoting tissue eosinophilia and de novo food allergies after transplantation. In a recent study in 54 pediatric recipients of 57 liver transplants, 28% of patients developed peripheral eosinophilia.162 Of 23 patients who had an endoscopic evaluation, 6 also developed EG. Those with eosinophilia were significantly younger, had more rejection episodes, were more commonly managed with tacrolimus-based immunosuppression, and experienced more frequent episodes of detectable Ebstein-Barr viremia. Patients with EG were more frequently retransplanted.
TREATMENT
Thus far, the management of EE and EG has been largely guided by several case reports, case series, and expert opinion, which provide support for various treatments, including special diets, glucocorticoids, and investigational agents such as anti-IL5 antibody (Table 27-2). The use of mast cell inhibitors, antihistamines, and leukotriene antagonists has dwindled, simply because of the lack of supporting evidence. When this chapter was written, most of the medical treatments under discussion were not approved by the U.S. Food and Drug Administration, but their status could change as a result of ongoing research trials, and new agents are being proposed. Obstructive manifestations of EGIDs require the adjunctive therapeutic use of periodic esophageal dilations for esophageal strictures and of surgery for intestinal obstruction.
Table 27-2 Recommended Therapeutic Options for Eosinophilic Gastrointestinal Disorders
| DISORDER | RECOMMENDED THERAPEUTIC OPTIONS |
|---|---|
| Eosinophilic esophagitis |
DIET
The strong association of EGID with food allergies (see Chapter 9) prompted the use of restrictive or elemental diets.163 The degree of allergen restriction ranges from the provision of protein nitrogen exclusively as amino acids, through the use of protein hydrolysate formulas, consisting of free amino acids and peptides of varying chain lengths, manufactured via enzymatic hydrolysis of casein or whey proteins, to the simple elimination of one or several whole- food proteins via careful reading of labels. Other modifications include oligoantigenic diets, allowing choice of those foods considered unlikely to be food allergens (e.g., broccoli, apple, corn, sweet potato, olive oil, salt, sugar, lamb) and the six-food elimination diet (eliminating only wheat, milk, soy, egg, peanut, tree nuts, fish, and shellfish) to exclude the most common food allergens.
Both retrospective and prospective open-label, uncontrolled studies have indicated that elemental diets can lead to clinical and histologic improvement in children and adolescents with EE.44,51,56 The six-food elimination diet and an elemental diet positively affect clinical symptoms, but esophageal histology appears to be more responsive to an elemental diet.50,109
In EG, case reports suggest efficacy of exclusively elemental diets,78,87 but they have not yet been examined in any prospective fashion.
Most infants with EP respond to extensively hydrolyzed formulas or, in the case of breast-fed infants, maternal dietary restriction of both milk and soy. The rationale for eliminating milk and soy is the coexistence of milk and soy protein intolerance in 30% to 50% of cases. A considerable proportion of infants who are unresponsive to these basic dietary changes will show complete resolution of their symptoms when an elemental diet is instituted. Infants with FPIEC and patients with more severe cases of EG are more apt to benefit from initiation of treatment with amino acid–based diets, or even with an initial period of stabilization using intravenous fluids.93,95
THERAPEUTIC AGENTS
Glucocorticoids
Glucocorticoids, by virtue of their potent anti-inflammatory actions, are an effective and powerful treatment for EGIDs. Mechanisms of action include inhibition of eosinophil growth factors, IL-3, IL-5, and GM-CSF. Several uncontrolled studies have provided evidence for beneficial anti-inflammatory effects during short-term and long-term treatment.40,44,73 Long-term use of glucocorticoids is undesirable, however, because of serious side effects, which include fluid and electrolyte disturbances, glucose intolerance, cushingoid state, growth suppression, bone demineralization, pituitary and adrenocortical hyporesponsiveness, posterior subcapsular cataracts, and various infections.
Glucocorticoids are useful as first-line therapy to induce remission, similar to their use in IBD at a dose equivalent to prednisone, 1 to 2 mg/kg/day. This is particularly the case in patients with nonallergic EGID or those refractory to dietary therapy. In a study examining the effects of oral glucocorticoids in children with EE, all 20 children showed clinical and histologic improvement at four weeks and half of them remained well at one-year follow-up.164 An important development in the treatment of EE with regard to efficacy and tolerance is the successful use of topical fluticasone, a relatively safe alternative to systemic glucocorticoids.165,166 A randomized, double-blind, placebo controlled study has shown swallowed fluticasone to be more effective in inducing histologic remission (eos/hpf ≤ 1) in EE; its effects were more impressive in younger patients, in nonallergic EE, and in the proximal esophagus. Suggested doses for fluticasone are 440 to 880 µg/day in children and 880 to 1760 µg/day in adolescents and adults, administered in two to four divided doses. Side effects associated with fluticasone include esophageal candidiasis, herpes esophagitis, and epistaxis.167 A head-to-head comparison of prednisone and fluticasone in a randomized fashion resulted in similar clinical and histologic response rates, although with far greater side effects with the former. Both treatment groups suffered relapse of symptoms by six months of follow-up.168 Topical viscous budesonide is also an effective treatment option for EE, particularly in children with swallowing disorders or in those who find the metered-dose inhaler difficult to use.169 Recommended doses are 1 mg daily for children younger than 10 years and 2 mg/day for those 10 years and older. Another form of topical glucocorticoid delivery with potential application in EGID is non–enteric-coated budesonide administered at a dosage of 9 mg daily in patients with EG affecting the ileum and right colon.170,171
Mast Cell Inhibitors
Oral disodium cromoglycate and ketotifen have shown limited success as treatment options for patients with EGID.172 There are only sporadic case reports of the use of these agents, and most reports used them in combination with other treatment modalities.173,174 Sodium cromoglycate may be an effective agent in EG at a dosage of 200 mg orally four times daily. Ketotifen (also an antihistamine), administered in dosages of 2 to 4 mg/day for 1 to 4 months, has been effective in improving symptoms and peripheral and intestinal eosinophilia in patients with EG.
Antihistamines
Support for use of conventional antihistamines (H1 receptor antagonists) in EGID comes from the murine model of EE induced by aeroallergens and reports of GI tissue eosinophilia in association with seasonal allergies.16,54,175 A study of children with dyspepsia described clinical benefit of antihistamines in 50% of all patients with duodenal eosinophilia. Because of insufficient evidence for the efficacy of other antihistamines in EGID and the fact that a significant proportion of cases are idiopathic, antihistamines probably cannot be recommended as a mainstay of therapy.
Leukotriene Receptor Antagonists
Montelukast selectively and competitively antagonizes the leukotriene receptor Cys-LT1 expressed on bronchial smooth muscle cells and eosinophils. Montelukast thereby blocks the actions of LTD4, a potent and specific eosinophil chemoattractant. Experience with the use of montelukast in EGID is limited to a few reports in EG and EE.176–179 Although some reports disclose persistent tissue eosinophilia, they indicate that montelukast in initial dosages up to 100 mg daily (and maintenance dosages of 20 to 40 mg daily for several months) induces an improvement in peripheral eosinophilia and symptoms.178 Montelukast is approved by the U.S. Food and Drug Administration for use in children 1 year and older with asthma and allergic rhinitis. It has the potential for being a relatively safe and effective steroid-sparing therapy for EE.
Anti–Interleukin-5 Therapy (Mepolizumab)
Clinical trials have described reduced peripheral eosinophil counts and clinical benefit with mepolizumab in certain eosinophilic disorders, such as HES and EE. An open-label trial in four patients with HES suggested the efficacy and safety of mepolizumab, a humanized monoclonal antibody against IL-5.152 Three intravenous doses of anti-IL-5 at four-week intervals lowered peripheral eosinophilia during the 12 weeks of therapy and improved clinical and quality of life measurements. In the patient with HES and EE who began the trial tolerating only a liquid diet, a 10-fold reduction in tissue eosinophilia and significant improvement in vomiting and dysphagia occurred. These data clearly need confirmation in larger randomized, controlled studies.151,180–182
Anti–IgE Therapy (Omalizumab)
The humanized anti-IgE monoclonal antibody omalizumab, known to be effective therapy against allergic rhinitis and asthma, has also been described to have positive effects in EG by improving peripheral and tissue eosinophilia, serum IgE, and symptom scores.182
Other Novel and Emerging Treatments
As understanding of the role of eosinophils in EGID continues to evolve, investigation of the effects of novel agents targeting eosinophils proceeds. These agents include anti-CCR3 antibodies against eosinophil-selective adhesion molecules, a monoclonal eotaxin antibody (CAT-213), and therapeutic agents to enhance eosinophil apoptosis.183
ESOPHAGEAL DILATION AND SURGERY
Dilation of esophageal strictures may be considered as initial therapy for symptomatic relief in patients with EE presenting with dysphagia and food impactions.184 Esophageal mucosal rents and extensive linear abrasions observed in some patients on withdrawal of the endoscope may be exaggerated by bougienage. Therefore, dilation should be undertaken cautiously after pretreatment with topical or oral steroids to reduce the risk of complications.5 In one study, 7 of 13 patients with EE experienced transient relief lasting less than three months, requiring repeated dilations with limited success.185 Most patients reported chest pain after dilation, but none had esophageal perforation despite extensive mechanical trauma. Patients with EG presenting with GI obstruction or perforation are usually treated by surgery. Resection of the obstructing segment is successful in relieving obstruction, but symptoms may persist or recur, warranting close follow-up and adjunctive medical management.186,187
Canani RB, Ruotolo S, Auricchio L, et al. Diagnostic accuracy of the atopy patch test in children with food allergy-related gastrointestinal symptoms. Allergy. 2007;62:738-43. (Ref 105.)
Chehade M, Sicherer SH, Magid MS, et al. Multiple exudative ulcers and pseudopolyps in allergic eosinophilic gastroenteritis that responded to dietary therapy. J Pediatr Gastroenterol Nutr. 2007;45:354-357. (Ref 75.)
Collins MH. Histopathologic features of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:59-71. (Ref 118.)
Foroughi S, Foster B, Kim N, et al. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J Allergy Clin Immunol. 2007;120:594-601. (Ref 182.)
Fox VL. Eosinophilic esophagitis: Endoscopic findings. Gastrointest Endosc Clin N Am. 2008;18:45-57. (Ref 115.)
Fulkerson PC, Rothenberg ME. Origin, regulation and physiological function of intestinal oeosinophils. Best Pract Res Clin Gastroenterol. 2008;22:411-23. (Ref 10.)
Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342-63. (Ref 5.)
Hogan SP, Mishra A, Brandt EB, et al. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2001;2:353-60. (Ref 26.)
Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: A 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3:1198-1206. (Ref 44.)
Loscher T, Saathoff E. Eosinophilia during intestinal infection. Best Pract Res Clin Gastroenterol. 2008;22:511-36. (Ref 126.)
Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997;100:444-51. (Ref 108.)
Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: A randomized trial in children. Clin Gastroenterol Hepatol. 2008;6:165-73. (Ref 168.)
Sicherer SH. Food protein-induced enterocolitis syndrome: Case presentations and management lessons. J Allergy Clin Immunol. 2005:115149-56. (Ref 95.)
Stein ML, Collins MH, Villanueva JM, et al. Anti–IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118:1312-19. (Ref 181.)
Talley NJ. Gut eosinophilia in food allergy and systemic and autoimmune diseases. Gastroenterol Clin North Am. Jun 2008;37:307-32. (Ref 6.)
1. Mueller S. Classification of eosinophilic gastrointestinal diseases. Best Pract Res Clin Gastroenterol. 2008;22:425-40.
2. Morris CD, Wilkinson J, Fox D, et al. Diffuse esophageal leiomyomatosis with localized dense eosinophilic infiltration. Dis Esophagus. 2002;15:85-7.
3. Bischoff SC, Ulmer FA. Eosinophils and allergic diseases of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2008;22:455-79.
4. Guajardo JR, Plotnick LM, Fende JM, et al. Eosinophil-associated gastrointestinal disorders: A world-wide-web based registry. J Pediatr. Oct 2002;141:576-81.
5. Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342-63.
6. Talley NJ. Gut eosinophilia in food allergy and systemic and autoimmune diseases. Gastroenterol Clin North Am. 2008;37:307-32.
7. Shakeer VK, Devi SR, Chettupuzha AP, et al. Carbamazepine-induced eosinophilic enteritis. Indian J Gastroenterol. 2002;21:114-15.
8. Lee JH, Park HY, Choe YH, et al. The development of eosinophilic colitis after liver transplantation in children. Pediatr Transplant. 2007;11:518-23.
9. Saeed SA, Integlia MJ, Pleskow RG, et al. Tacrolimus-associated eosinophilic gastroenterocolitis in pediatric liver transplant recipients: Role of potential food allergies in pathogenesis. Pediatr Transplant. 2006;10:730-5.
10. Fulkerson PC, Rothenberg ME. Origin, regulation and physiological function of intestinal oeosinophils. Best Pract Res Clin Gastroenterol. 2008;22:411-23.
11. Straumann A, Simon HU. The physiological and pathophysiological roles of eosinophils in the gastrointestinal tract. Allergy. Jan 2004;59:15-25.
12. DeBrosse CW, Case JW, Putnam PE, et al. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol. 2006;9:210-18.
13. Forbes E, Hulett M, Ahrens R, et al. ICAM-1-dependent pathways regulate colonic eosinophilic inflammation. J Leukoc Biol. 2006;80:330-41.
14. Mishra A, Hogan SP, Brandt EB, Rothenberg ME. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol. 2002;168:2464-9.
15. Hogan SP, Rothenberg ME, Forbes E, et al. Chemokines in eosinophil-associated gastrointestinal disorders. Curr Allergy Asthma Rep. 2004;4:74-82.
16. Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107:83-90.
17. Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003;125:1419-27.
18. Bhattacharya B, Carlsten J, Sabo E, et al. Increased expression of eotaxin-3 distinguishes between eosinophilic esophagitis and gastroesophageal reflux disease. Hum Pathol. 2007;38:1744-53.
19. Chehade M. Translational research on the pathogenesis of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:145-56.
20. Bullock JZ, Villanueva JM, Blanchard C, et al. Interplay of adaptive Th2 immunity with eotaxin-3/c-C chemokine receptor 3 in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:22-31.
21. Straumann A, Bauer M, Fischer B, et al. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108:954-61.
22. Fujiwara H, Morita A, Kobayashi H, et al. Infiltrating eosinophils and eotaxin: Their association with idiopathic eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2002;89:429-32.
23. Blanchard C, Mingler MK, Vicario M, et al. IL-13 involvement in eosinophilic esophagitis: Transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292-300.
24. Aceves SS, Newbury RO, Dohil R, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206-12.
25. Mishra A, Wang M, Pemmaraju VR, et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134:204-14.
26. Hogan SP, Mishra A, Brandt EB, et al. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2001;2:353-60.
27. Cousins L, Graham M, Tooze R, et al. Eosinophilic bowel disease controlled by the BB rat-derived lymphopenia/Gimap5 gene. Gastroenterology. 2006;131:1475-85.
28. Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536-47.
29. Ngo P, Furuta GT, Antonioli DA, Fox VL. Eosinophils in the esophagus—peptic or allergic eosinophilic esophagitis? Case series of three patients with esophageal eosinophilia. Am J Gastroenterol. 2006;101:1666-70.
30. Cozen W, Ferreira MA. Understanding the asthma epidemic: Can twin studies help? Twin Res Hum Genet. 2008;11:111.
31. Holgate ST. The epidemic of asthma and allergy. J R Soc Med. 2004;97:103-10.
32. Vanderheyden AD, Petras RE, DeYoung BR, Mitros FA. Emerging eosinophilic (allergic) esophagitis: increased incidence or increased recognition? Arch Pathol Lab Med. 2007;131:777-9.
33. Muller S, Puhl S, Vieth M, Stolte M. Analysis of symptoms and endoscopic findings in 117 patients with histological diagnoses of eosinophilic esophagitis. Endoscopy. 2007;39:339-44.
34. Mihai C, Prelipcean CC, Gogalniceanu P, et al. Eosinophilic esophagitis—from a rare pediatric disease to the forefront of adult gastroenterology. Rev Med Chir Soc Med Nat Iasi. 2007;111:811-17.
35. Katzka DA. Demographic data and symptoms of eosinophilic esophagitis in adults. Gastrointest Endosc Clin N Am. 2008;18:25-32.
36. Straumann A, Bussmann C, Zuber M, et al. Eosinophilic esophagitis: Analysis of food impaction and perforation in 251 adolescent and adult patients. Clin Gastroenterol Hepatol. 2008;6:598-600.
37. Kapel RC, Miller JK, Torres C, et al. Eosinophilic esophagitis: A prevalent disease in the United States that affects all age groups. Gastroenterology. 2008;134:1316-21.
38. Pasha SF, DiBaise JK, Kim HJ, et al. Patient characteristics, clinical, endoscopic, and histologic findings in adult eosinophilic esophagitis: A case series and systematic review of the medical literature. Dis Esophagus. 2007;20:311-19.
39. Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: Clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63:3-12.
40. Orenstein SR, Shalaby TM, Di Lorenzo C, et al. The spectrum of pediatric eosinophilic esophagitis beyond infancy: A clinical series of 30 children. Am J Gastroenterol. 2000;95:1422-30.
41. Straumann A, Spichtin HP, Grize L, et al. Natural history of primary eosinophilic esophagitis: A follow-up of 30 adult patients for up to 11.5 years. Gastroenterology. 2003;125:1660-9.
42. Wang FY, Gupta SK, Fitzgerald JF. Is there a seasonal variation in the incidence or intensity of allergic eosinophilic esophagitis in newly diagnosed children? J Clin Gastroenterol. 2007;41:451-3.
43. Cherian S, Smith NM, Forbes DA. Rapidly increasing prevalence of eosinophilic oesophagitis in Western Australia. Arch Dis Child. 2006;91:1000-4.
44. Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: A 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3:1198-1206.
45. Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940-1.
46. Straumann A, Simon HU. Eosinophilic esophagitis: Escalating epidemiology? J Allergy Clin Immunol. 2005;115:418-19.
47. Khan S, Orenstein SR, Di Lorenzo C, et al. Eosinophilic esophagitis: Strictures, impactions, dysphagia. Dig Dis Sci. 2003;48:22-9.
48. Rothenberg ME, Mishra A, Collins MH, Putnam PE. Pathogenesis and clinical features of eosinophilic esophagitis. J Allergy Clin Immunol. 2001;108:891-4.
49. Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2008;6:531-5.
50. Spergel JM, Shuker M. Nutritional management of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:179-94.
51. Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol. 2003;98:777-82.
52. Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002;109:363-8.
53. Onbasi K, Sin AZ, Doganavsargil B, et al. Eosinophil infiltration of the oesophageal mucosa in patients with pollen allergy during the season. Clin Exp Allergy. 2005;35:1423-31.
54. Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol. 2003;112:796-7.
55. Landres RT, Kuster GG, Strum WB. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology. 1978;74:1298-301.
56. Kelly KJ, Lazenby AJ, Rowe PC, et al. Eosinophilic esophagitis attributed to gastroesophageal reflux: Improvement with an amino acid-based formula. Gastroenterology. 1995;109:1503-12.
57. Cury EK, Schraibman V, Faintuch S. Eosinophilic infiltration of the esophagus: Gastroesophageal reflux versus eosinophilic esophagitis in children—discussion on daily practice. J Pediatr Surg. 2004;39:e4-7.
58. Pentiuk SP, Miller CK, Kaul A. Eosinophilic esophagitis in infants and toddlers. Dysphagia. 2007;22:44-8.
59. Aceves SS, Newbury RO, Dohil R, et al. Distinguishing eosinophilic esophagitis in pediatric patients: Clinical, endoscopic, and histologic features of an emerging disorder. J Clin Gastroenterol. 2007;41:252-6.
60. Lucendo AJ, Castillo P, Martin-Chavarri S, et al. Manometric findings in adult eosinophilic oesophagitis: A study of 12 cases. Eur J Gastroenterol Hepatol. 2007;19:417-24.
61. Thompson DM, Orvidas LJ. Otorhinolaryngologic manifestations of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:91-8.
62. Assa’ad AH, Putnam PE, Collins MH, et al. Pediatric patients with eosinophilic esophagitis: An 8-year follow-up. J Allergy Clin Immunol. 2007;119:731-8.
63. Helou EF, Simonson J, Arora AS. 3-yr-follow-up of topical corticosteroid treatment for eosinophilic esophagitis in adults. Am J Gastroenterol. 2008;103:2194-9.
64. Straumann A. The natural history and complications of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:99-118.
65. Lucendo AJ, De Rezende L. Endoscopic dilation in eosinophilic esophagitis: A treatment strategy associated with a high risk of perforation. Endoscopy. 2007;39:376.
66. Wolfsen HC, Hemminger LL, Achem SR. Eosinophilic esophagitis and Barrett’s esophagus with dysplasia. Clin Gastroenterol Hepatol. 2007;5:A18.
67. Francalanci P, De Angelis P, Minnei F, et al. Eosinophilic esophagitis and Barrett’s esophagus: An occasional association or an overlap disease? Esophageal “double trouble” in two children. Digestion. 2008;77:16-9.
68. Khan S, Orenstein SR. Eosinophilic gastroenteritis. Gastroenterol Clin North Am. 2008;37:333-48.
69. Kim N, Kim JW, Hwang JH, et al. Visualization of jejunal bleeding by capsule endoscopy in a case of eosinophilic enteritis. Korean J Intern Med. Mar 2005;20:63-7.
70. Biswas S, Hoo W, Katsoulas N, et al. Eosinophilic enteritis: A rare cause of abdominal pain. Int J Colorectal Dis. 2007;22:87-8.
71. Gallagher TK, Winter DC. Diarrhoea, ascites and eosinophilia: An unusual triad. Scand J Gastroenterol. 2007;42:1509-11.
72. Yun MY, Cho YU, Park IS, et al. Eosinophilic gastroenteritis presenting as small bowel obstruction: A case report and review of the literature. World J Gastroenterol. 2007;13:1758-60.
73. Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: A clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut. 1990;31:54-8.
74. Chen MJ, Chu CH, Lin SC, et al. Eosinophilic gastroenteritis: Clinical experience with 15 patients. World J Gastroenterol. 2003;9:2813-16.
75. Chehade M, Sicherer SH, Magid MS, et al. Multiple exudative ulcers and pseudopolyps in allergic eosinophilic gastroenteritis that responded to dietary therapy. J Pediatr Gastroenterol Nutr. 2007;45:354-7.
76. Shin WG, Park CH, Lee YS, et al. Eosinophilic enteritis presenting as intussusception in adult. Korean J Intern Med. Mar 2007;22:13-17.
77. Zheng X, Cheng J, Pan K, et al. Eosinophilic enteritis: CT features. Abdom Imaging. 2008;33:191-5.
78. Chehade M, Magid MS, Mofidi S, et al. Allergic eosinophilic gastroenteritis with protein-losing enteropathy: Intestinal pathology, clinical course, and long-term follow-up. J Pediatr Gastroenterol Nutr. 2006;42:516-21.
79. von Wattenwyl F, Zimmermann A, Netzer P. Synchronous first manifestation of an idiopathic eosinophilic gastroenteritis and bronchial asthma. Eur J Gastroenterol Hepatol. 2001;13:721-5.
80. Straumann A. Idiopathic eosinophilic gastrointestinal diseases in adults. Best Pract Res Clin Gastroenterol. 2008;22:481-96.
81. Mazokopakis E, Vrentzos G, Spanakis E, et al. A case of eosinophilic gastroenteritis with severe peripheral eosinophilia. Mil Med. 2006;171:331-2.
82. Klein NC, Hargrove RL, Sleisenger MH, Jeffries GH. Eosinophilic gastroenteritis. Medicine (Baltimore). 1970;49:299-319.
83. Sandrasegaran K, Rajesh A, Maglinte DD. Eosinophilic gastroenteritis presenting as acute abdomen. Emerg Radiol. 2006;13:151-4.
84. Charalabopoulos A, Charalabopoulos K, Avuzuklidou M, et al. Eosinophilic gastroenteritis: presentation of two patients with unusual affect of terminal ileum and caecum with manifestations of acute abdomen and literature review. Int J Clin Pract. 2004;58:413-16.
85. Tursi A, Rella G, Inchingolo CD, Maiorano M. Gastric outlet obstruction due to gastroduodenal eosinophilic gastroenteritis. Endoscopy. 2007;39(Suppl 1):E184.
86. Bachmeyer C, Ammouri W, Ravet N, et al. Pyloric stenosis in an adult with eosinophilic gastroenteritis. Rev Med Interne. 2006;27:430-1.
87. Khan S, Orenstein SR. Eosinophilic gastroenteritis masquerading as pyloric stenosis. Clin Pediatr (Phila). 2000;39:55-7.
88. Kellermayer R, Tatevian N, Klish W, Shulman RJ. Steroid-responsive eosinophilic gastric outlet obstruction in a child. World J Gastroenterol. 2008;14:2270-1.
89. Schappi MG, Smith VV, Milla PJ, Lindley KJ. Eosinophilic myenteric ganglionitis is associated with functional intestinal obstruction. Gut. 2003;52:752-5.
90. Zhou HB, Chen JM, Du Q. Eosinophilic gastroenteritis with ascites and hepatic dysfunction. World J Gastroenterol. 8 2007;13:1303-5.
91. Fenoglio LM, Benedetti V, Rossi C, et al. Eosinophilic gastroenteritis with ascites: A case report and review of the literature. Dig Dis Sci. 2003;48:1013-20.
92. Nowak-Wegrzyn A, Sampson HA, Wood RA, Sicherer SH. Food protein–induced enterocolitis syndrome caused by solid food proteins. Pediatrics. 2003;111(t 1):829-35.
93. Sicherer SH, Sampson HA. Food allergy: Recent advances in pathophysiology and treatment. Annu Rev Med. 2009;60:261-77.
94. Hojsak I, Kljaic-Turkalj M, Misak Z, Kolacek S. Rice protein-induced enterocolitis syndrome. Clin Nutr. 2006;25:533-6.
95. Sicherer SH. Food protein-induced enterocolitis syndrome: Case presentations and management lessons. J Allergy Clin Immunol. 2005;115:149-56.
96. Anand RK, Appachi E. Case report of methemoglobinemia in two patients with food protein–induced enterocolitis. Clin Pediatr (Phila). 2006;45:679-82.
97. Maloney J, Nowak-Wegrzyn A. Educational clinical case series for pediatric allergy and immunology: Allergic proctocolitis, food protein–induced enterocolitis syndrome and allergic eosinophilic gastroenteritis with protein-losing gastroenteropathy as manifestations of non-IgE–mediated cow’s milk allergy. Pediatr Allergy Immunol. 2007;18:360-7.
98. Fogg MI, Brown-Whitehorn TA, Pawlowski NA, Spergel JM. Atopy patch test for the diagnosis of food protein-induced enterocolitis syndrome. Pediatr Allergy Immunol. 2006;17:351-5.
99. Lake AM. Food-induced eosinophilic proctocolitis. J Pediatr Gastroenterol Nutr. 2000;30(Suppl):S58-60.
100. Arvola T, Ruuska T, Keranen J, et al. Rectal bleeding in infancy: Clinical, allergological, and microbiological examination. Pediatrics. 2006;117:e760-8.
101. Xanthakos SA, Schwimmer JB, Melin-Aldana H, et al. Prevalence and outcome of allergic colitis in healthy infants with rectal bleeding: A prospective cohort study. J Pediatr Gastroenterol Nutr. 2005;41:16-22.
102. Clark PJ. Utility of eosinophilia as a diagnostic clue in lower abdominal pain in northern Australia: A retrospective case-control study. Intern Med J. 2008;38:278-80.
103. Wolthers OD, Heuck C. Circadian variations in serum eosinophil cationic protein, and serum and urine eosinophil protein X. Pediatr Allergy Immunol. 2003;14:130-3.
104. Gupta SK. Noninvasive markers of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:157-67.
105. Canani RB, Ruotolo S, Auricchio L, et al. Diagnostic accuracy of the atopy patch test in children with food allergy–related gastrointestinal symptoms. Allergy. 2007;62:738-43.
106. Spergel JM, Andrews T, Brown-Whitehorn TF, et al. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol. 2005;95:336-43.
107. Spergel JM, Brown-Whitehorn T, Beausoleil JL, et al. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:509-11.
108. Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997;100:444-51.
109. Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097-102.
110. Fox VL, Nurko S, Teitelbaum JE, et al. High-resolution EUS in children with eosinophilic “allergic” esophagitis. Gastrointest Endosc. 2003;57:30-6.
111. Teele RL, Katz AJ, Goldman H, Kettell RM. Radiographic features of eosinophilic gastroenteritis (allergic gastroenteropathy) of childhood. AJR Am J Roentgenol. 1979;132:575-80.
112. Horton KM, Corl FM, Fishman EK. CT of nonneoplastic diseases of the small bowel: Spectrum of disease. J Comput Assist Tomogr. 1999;23:417-28.
113. Straumann A, Rossi L, Simon HU, et al. Fragility of the esophageal mucosa: A pathognomonic endoscopic sign of primary eosinophilic esophagitis? Gastrointest Endosc. 2003;57:407-12.
114. Lim JR, Gupta SK, Croffie JM, et al. White specks in the esophageal mucosa: An endoscopic manifestation of non-reflux eosinophilic esophagitis in children. Gastrointest Endosc. 2004;59:835-8.
115. Fox VL. Eosinophilic esophagitis: Endoscopic findings. Gastrointest Endosc Clin N Am. 2008;18:45-57.
116. Straumann A, Spichtin HP, Bucher KA, et al. Eosinophilic esophagitis: Red on microscopy, white on endoscopy. Digestion. 2004;70:109-16.
117. Liguori G, Cortale M, Cimino F, Sozzi M. Circumferential mucosal dissection and esophageal perforation in a patient with eosinophilic esophagitis. World J Gastroenterol. 2008;14:803-4.
118. Collins MH. Histopathologic features of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:59-71.
119. Ruchelli E, Wenner W, Voytek T, et al. Severity of esophageal eosinophilia predicts response to conventional gastroesophageal reflux therapy. Pediatr Dev Pathol. 1999;2:15-18.
120. Jimenez-Rivera C, Ngan B, Jackson R, Ahmed N. Gastric pseudopolyps in eosinophilic gastroenteritis. J Pediatr Gastroenterol Nutr. 2005;40:83-6.
121. Treiber GG, Weidner S. Eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2007;5:e16.
122. Lowichik A, Weinberg AG. A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Mod Pathol. 1996;9:110-14.
123. Alamo Martinez JM, Ibanez Delgado F, Galindo Galindo A, et al. Intestinal obstruction by eosinophilic jejunitis. Rev Esp Enferm Dig. 2004;96:279-83.
124. Repiso Ortega A, Alcantara Torres M, Gonzalez de Frutos C, et al. [Gastrointestinal anisakiasis. Study of a series of 25 patients. Gastroenterol Hepatol. 2003;26:341-6.
125. Hong ST, Lim HS, Kim DH, Kim SJ. A case of gastroenteritis associated with gastric trichuriasis. J Korean Med Sci. 2003;18:429-32.
126. Loscher T, Saathoff E. Eosinophilia during intestinal infection. Best Pract Res Clin Gastroenterol. 2008;22:511-36.
127. Cruz AT, Franklin GY, Kaplan SL. Toxocariasis causing eosinophilic ascites. Pediatr Infect Dis J. 2008;27:563-4.
128. Chira O, Badea R, Dumitrascu D, et al. Eosinophilic ascites in a patient with toxocara canis infection. A case report. Rom J Gastroenterol. 2005;14:397-400.
129. Lawate P, Singh SP. Eosinophilic ascites due to Strongyloides stercoralis. Trop Gastroenterol. 2005;26:91-2.
130. Turhan O, Korkmaz M, Saba R, et al. Seroepidemiology of fascioliasis in the Antalya region and uselessness of eosinophil count as a surrogate marker and portable ultrasonography for epidemiological surveillance. Infez Med. 2006;14:208-12.
131. Saba R, Korkmaz M, Inan D, et al. Human fascioliasis. Clin Microbiol Infect. 2004;10:385-7.
132. Montalto M, Miele L, Marcheggiano A, et al. Anisakis infestation: A case of acute abdomen mimicking Crohn’s disease and eosinophilic gastroenteritis. Dig Liver Dis. 2005;37:62-4.
133. Esteve C, Resano A, Diaz-Tejeiro P, Fernandez-Benitez M. Eosinophilic gastritis due to Anisakis: A case report. Allergol Immunopathol (Madr). 2000;28:21-3.
134. Gomez B, Tabar AI, Tunon T, et al. Eosinophilic gastroenteritis and Anisakis. Allergy. 1998;53:1148-54.
135. Kawaguchi Y, Mine T, Yasuzaki H, et al. Eosinophilic gastroenteritis cured with Helicobacter pylori eradication. J Clin Gastroenterol. 2008;42:1063-4.
136. Papadopoulos AA, Tzathas C, Polymeros D, Ladas SD. Symptomatic eosinophilic gastritis cured with Helicobacter pylori eradication. Gut. 2005;54:1822.
137. Muller MJ, Sewell GS. Coexistence of eosinophilic gastroenteritis and Helicobacter pylori gastritis: Causality versus coincidence. Dig Dis Sci. 2001;46:1784-6.
138. Morimoto T, Sato T, Matsuoka A, et al. Trimethoprim-sulfamethoxazole–induced hypersensitivity syndrome associated with reactivation of human herpesvirus-6. Intern Med. 2006;45:101-5.
139. Balatsinou C, Milano A, Caldarella MP, et al. Eosinophilic esophagitis is a component of the anticonvulsant hypersensitivity syndrome: Description of two cases. Dig Liver Dis. 2008;40:145-8.
140. Lee JY, Medellin MV, Tumpkin C. Allergic reaction to gemfibrozil manifesting as eosinophilic gastroenteritis. South Med J. 2000;93:807-8.
141. Barak N, Hart J, Sitrin MD. Enalapril-induced eosinophilic gastroenteritis. J Clin Gastroenterol. 2001;33:157-8.
142. Kakumitsu S, Shijo H, Akiyoshi N, et al. Eosinophilic enteritis observed during alpha-interferon therapy for chronic hepatitis C. J Gastroenterol. 2000;35:548-51.
143. Sunkureddi PR, Luu N, Xiao SY, et al. Eosinophilic enteritis with systemic lupus erythematosus. South Med J. 2005;98:1049-52.
144. Giouleme O, Tsiaousi E, Theodoridis A, et al. A case of Churg-Strauss syndrome revealed by eosinophilic gastroenteritis. Dig Dis Sci. 2009;54:174-7.
145. Ozolek JA, Sasatomi E, Swalsky PA, et al. Inflammatory fibroid polyps of the gastrointestinal tract: Clinical, pathologic, and molecular characteristics. Appl Immunohistochem Mol Morphol. 2004;12:59-66.
146. Wysocki AP, Taylor G, Windsor JA. Inflammatory fibroid polyps of the duodenum: A review of the literature. Dig Surg. 2007;24:162-8.
147. Moezzi J, Gopalswamy N, Haas RJJr, et al. Stromal eosinophilia in colonic epithelial neoplasms. Am J Gastroenterol. 2000;95:520-3.
148. Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37.
149. Sheikh J, Weller PF. Clinical overview of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27:333-55.
150. Quintas-Cardama A, Cortes J. Therapeutic options for patients with clonal and idiopathic hypereosinophia. Expert Opin Investig Drugs. 2008;17:1039-50.
151. Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215-28.
152. Garrett JK, Jameson SC, Thomson B, et al. Anti–interleukin-5 (mepolizumab) therapy for hypereosinophilic syndromes. J Allergy Clin Immunol. 2004;113:115-19.
153. Wedemeyer J, Vosskuhl K. Role of gastrointestinal eosinophils in inflammatory bowel disease and intestinal tumours. Best Pract Res Clin Gastroenterol. 2008;22:537-49.
154. Carvalho AT, Elia CC, de Souza HS, et al. Immunohistochemical study of intestinal eosinophils in inflammatory bowel disease. J Clin Gastroenterol. 2003;36:120-5.
155. Chen W, Paulus B, Shu D, et al. Increased serum levels of eotaxin in patients with inflammatory bowel disease. Scand J Gastroenterol. 2001;36:515-20.
156. D’Arienzo A, Manguso F, Astarita C, et al. Allergy and mucosal eosinophil infiltrate in ulcerative colitis. Scand J Gastroenterol. 2000;35:624-31.
157. Mir A, Minguez M, Tatay J, et al. Elevated serum eotaxin levels in patients with inflammatory bowel disease. Am J Gastroenterol. 2002;97:1452-7.
158. Peterson CG, Eklund E, Taha Y, et al. A new method for the quantification of neutrophil and eosinophil cationic proteins in feces: Establishment of normal levels and clinical application in patients with inflammatory bowel disease. Am J Gastroenterol. 2002;97:1755-62.
159. Talley NJ, Kephart GM, McGovern TW, et al. Deposition of eosinophil granule major basic protein in eosinophilic gastroenteritis and celiac disease. Gastroenterology. 1992;103:137-45.
160. Heine RG. Eosinophilic esophagitis in children with celiac disease: New diagnostic and therapeutic dilemmas. J Gastroenterol Hepatol. 2008;23(Pt 1):993-4.
161. Ooi CY, Day AS, Jackson R, et al. Eosinophilic esophagitis in children with celiac disease. J Gastroenterol Hepatol. 2008;23(Pt 1):1144-8.
162. Romero R, Abramowsky CR, Pillen T, et al. Peripheral eosinophilia and eosinophilic gastroenteritis after pediatric liver transplantation. Pediatr Transplant. Dec 2003;7:484-8.
163. Pratt CA, Demain JG, Rathkopf MM. Food allergy and eosinophilic gastrointestinal disorders: Guiding our diagnosis and treatment. Curr Probl Pediatr Adolesc Health Care. 2008;38:170-88.
164. Liacouras CA, Wenner WJ, Brown K, Ruchelli E. Primary eosinophilic esophagitis in children: Successful treatment with oral corticosteroids. J Pediatr Gastroenterol Nutr. 1998;26:380-5.
165. Teitelbaum JE, Fox VL, Twarog FJ, et al. Eosinophilic esophagitis in children: Immunopathological analysis and response to fluticasone propionate. Gastroenterology. 2002;122:1216-25.
166. Konikoff MR, Noel RJ, Blanchard C, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131:1381-91.
167. Lindberg GM, Van Eldik R, Saboorian MH. A case of herpes esophagitis after fluticasone propionate for eosinophilic esophagitis. Nat Clin Pract Gastroenterol Hepatol. 2008;5:527-30.
168. Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: A randomized trial in children. Clin Gastroenterol Hepatol. 2008;6:165-73.
169. Aceves SS, Bastian JF, Newbury RO, Dohil R. Oral viscous budesonide: A potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol. 2007;102:2271-9.
170. Elsing C, Placke J, Gross-Weege W. Budesonide for the treatment of obstructive eosinophilic jejunitis. Z Gastroenterol. 2007;45:187-9.
171. Siewert E, Lammert F, Koppitz P, et al. Eosinophilic gastroenteritis with severe protein-losing enteropathy: Successful treatment with budesonide. Dig Liver Dis. 2006;38:55-9.
172. Suzuki J, Kawasaki Y, Nozawa R, et al. Oral disodium cromoglycate and ketotifen for a patient with eosinophilic gastroenteritis, food allergy and protein-losing enteropathy. Asian Pac J Allergy Immunol. 2003;21:193-7.
173. Bolukbas FF, Bolukbas C, Uzunkoy A, et al. A dramatic response to ketotifen in a case of eosinophilic gastroenteritis mimicking abdominal emergency. Dig Dis Sci. 2004;49:1782-5.
174. Katsinelos P, Pilpilidis I, Xiarchos P, et al. Oral administration of ketotifen in a patient with eosinophilic colitis and severe osteoporosis. Am J Gastroenterol. 2002;97:1072-4.
175. Friesen CA, Sandridge L, Andre L, et al. Mucosal eosinophilia and response to H1/H2 antagonist and cromolyn therapy in pediatric dyspepsia. Clin Pediatr (Phila). 2006;45:143-7.
176. Sinharay R. Eosinophilic oesophagitis: Treatment using Montelukast. Gut. 2003;52:1228-9.
177. Quack I, Sellin L, Buchner NJ, et al. Eosinophilic gastroenteritis in a young girl—long-term remission under Montelukast. BMC Gastroenterol. 2005;5:24.
178. Daikh BE, Ryan CK, Schwartz RH. Montelukast reduces peripheral blood eosinophilia but not tissue eosinophilia or symptoms in a patient with eosinophilic gastroenteritis and esophageal stricture. Ann Allergy Asthma Immunol. 2003;90:23-7.
179. Urek MC, Kujundzic M, Banic M, et al. Leukotriene receptor antagonists as potential steroid-sparing agents in a patient with serosal eosinophilic gastroenteritis. Gut. 2006;55:1363-4.
180. Mepolizumab: 240563, anti-IL-5 monoclonal antibody—GlaxoSmithKline, anti-interleukin-5 monoclonal antibody—GlaxoSmithKline, SB 240563. Drugs R D. 2008;9:125-30.
181. Stein ML, Collins MH, Villanueva JM, et al. Anti–IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118:1312-19.
182. Foroughi S, Foster B, Kim N, et al. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J Allergy Clin Immunol. 2007;120:594-601.
183. Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303-10.
184. Schoepfer AM, Gschossmann J, Scheurer U, et al. Esophageal strictures in adult eosinophilic esophagitis: Dilation is an effective and safe alternative after failure of topical corticosteroids. Endoscopy. 2008;40:161-4.
185. Potter JW, Saeian K, Staff D, et al. Eosinophilic esophagitis in adults: An emerging problem with unique esophageal features. Gastrointest Endosc. 2004;59:355-61.
186. Uenishi T, Sakata C, Tanaka S, et al. Eosinophilic enteritis presenting as acute intestinal obstruction: A case report and review of the literature. Dig Surg. 2003;20:326-9.
187. Alexander P, Jacob S, Paul V. Laparoscopy in eosinophilic jejunitis presenting as subacute bowel obstruction: A case report. Trop Gastroenterol. 2003;24:97-8.