CHAPTER 102 Enteric Microbiota and Small Intestinal Bacterial Overgrowth
COMPOSITION AND MOLECULAR ANALYSIS OF THE ENTERIC MICROBIOTA
Most human enteric bacteria cannot be cultured, because of a lack of truly selective growth media. Nonetheless, molecular profiling has shown that whereas the microbiota appear distinct in different persons, the composition of each person’s microbiota is relatively stable after infant weaning and throughout adulthood. Evidence from studies of twins suggests that the individuality of human microflora may be genetically controlled,1 but environmental variables including diet and sanitation appear to have profound effects on early intestinal colonization with bacteria.2,3 In adulthood, dietary fluctuations appear to induce changes in bacterial enzymes and metabolic activity rather than changes in the relative populations of the microflora.2,4,5
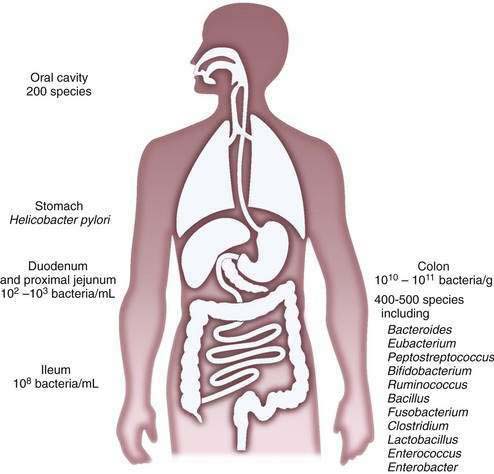
The composition of the microflora varies quantitatively and qualitatively over the longitudinal and the cross-sectional axes of the alimentary tract. Beyond the oral cavity, which harbors approximately 200 different bacterial species, the size and diversity of the microflora increase distally along the digestive tract (Fig. 102-1). Gastric acid restricts bacterial numbers within the stomach to fewer than 103 colony-forming units (CFU)/mL. The gradient in bacterial density is greatest across the ileocecal valve, with approximately 108 bacteria per gram of ileal contents and up to 1012 bacteria per gram of colonic contents, comprising more than 1000 different bacterial species.2,4–6 More than 99% of the culturable bacteria in the ileum and the colon are obligate anaerobes, but the composition of the flora at the mucosal surface differs from that within the lumen; ratios of anaerobes to aerobes are lower at mucosal surfaces. Culture-independent methods, such as the various molecular approaches described later, suggest that mucosa-associated bacteria differ from those recovered from feces, thus supporting the idea that host-related factors have a role in determining the enteric microflora7 and implying that bacterial aspirates from the lumen may be an incomplete reflection of mucosa-associated bacteria.
The microbiota of the proximal small intestine consist predominantly of Gram-positive facultative bacteria—bacteria that can survive under aerobic or anaerobic conditions—although enterobacteria and Bacteroides species also may be present. Peristalsis is the principal factor restricting bacterial numbers in the small intestine. In the distal small intestine, the composition of the microflora resembles that of the colon, with a preponderance of Gram-negative anaerobes. The most prominently represented genera in the distal bowel include Bacteroides, Clostridium, Lactobacillus, Fusobacterium, Bifidobacterium, Eubacterium, Peptococcus, and Escherichia species.2,6
Detailed analysis of the enteric microflora has been confounded by the limitations of traditional culture-dependent microbiology.8 First, obtaining representative material from different niches within the intestine is problematic; because most of the indigenous microflora are obligate anaerobes, major methodologic difficulties are encountered with sampling, contamination, transport, and storage. Second, the lack of truly selective growth media precludes culture of most components of the microflora. In this respect, it is noteworthy that culture of H. pylori and C. difficile was accomplished only within the past two to three decades. This difficulty has led to a shift in emphasis from conventional bacterial phenotyping toward genotyping and molecular approaches to study the unculturables.4,9,10
For rapid profiling of the dominant culturable and nonculturable organisms within a complex ecosystem such as that in the intestinal tract, 16S rRNA can be amplified by polymerase chain reaction (PCR) with universal primers spanning conserved and variable regions. The mixture of hypervariable RNA fragments can then be separated by a chemical denaturing gradient or a temperature gradient gel electrophoresis (DGGE and TGGE). Complete denaturation of the RNA fragments is prevented by incorporating a GC-rich 5′ end to one of the primers (a GC clamp).11 Variations in migration distance through the denaturing gradients reflect the diversity of 16S species in the sample (Fig. 102-2). Theoretically, the technique is semiquantitative, because the more dominant the organism, the more abundant the specific PCR product. The specific PCR product can be cut from the gel and further amplified, cloned, and sequenced to identify individual bacterial strains without requiring a conventional culture step. Further refinements of the technique can be achieved by using species-specific PCR primers.
Other molecular techniques for analysis of specific bacterial species now are possible because of the increasing availability of genomic sequence data for the major components of the bacterial flora. These techniques include fluorescence in situ hybridization (FISH) flow cytometry (FISH-flow) and bacterial DNA microarrays. In disorders such as inflammatory bowel disease, immunologic reactivity against components of the microflora has been used to identify microbes that may be etiologic in the disease. Marker antibodies generated by hybridoma or phage-display technology have been used as reagents to identify microbial antigens. For example, antineutrophil cytoplasmic antibody (pANCA), which is associated with ulcerative colitis, has been used to identify colonic bacteria expressing a pANCA-related epitope.12,13 In addition, candidate microbes can be identified by the presence of unique bacterial nucleic acid sequences associated with a particular lesion or disease location, by subtractive cloning using genomic representational difference analysis. With this technique, a sequence representing a bacterial transcription factor from an apparent commensal organism, Pseudomonas fluorescens,14–16 was found in lesions of Crohn’s disease but not in adjacent nonlesional mucosa. Serologic expression cloning also has been used to identify bacterial flagellin as a dominant antigen in Crohn’s disease.17
The new science of metagenomics—the sequencing of genes from whole microbial environments at once—promises to address many of the unresolved questions about the microbiota. The microbiota comprise a repository of genetic information (microbiome) that greatly exceeds that of the host genome. By combining metagenomics with bioinformatics, biochemistry, and traditional bioassays, new insights into the metabolic capacity of the human intestinal microbiota can be achieved.4 Major consortia (including the human microbiome project4) around the globe are under way using metagenomics as a tool for bioprospecting the intestinal microbiota in health and disease.
INTERACTIONS BETWEEN HOST AND MICROBES
The microbiota exert both positive and negative regulatory effects on the development and function of the intestine. These complex influences first were shown in comparative studies of germ-free and conventionally colonized animals. A sterile intestine is associated with reductions in mucosal cell turnover, digestive enzyme activity, cytokine production, lymphoid tissue, lamina propria cellularity, vascularity, muscle wall thickness, and motility, but with an increase in enterochromaffin cell area.18 The molecular events underpinning this regulatory signaling from the lumen currently are being explored using modern techniques such as laser capture microdissection and gene array analysis; such studies promise to reveal new molecular targets to be exploited for the design of novel therapeutics.19,20 Thus, for example, when applied to animals colonized with only a single bacterial strain, Bacteroides thetaiotaomicron, this combined approach has illustrated the impact of bacteria-derived signaling on the expression of host genes controlling mucosal barrier function, nutrient absorption, angiogenesis, and development of the enteric nervous system.
Incoming bacterial signals include secreted chemoattractants, such as the formylated peptide f-Met-Leu-Phe, cellular constituents such as lipopolysaccharide (LPS) and peptidoglycans, flagellin, and bacterial nucleic acids (i.e., CpG DNA). Detection of bacterial stimuli by the host and discrimination of pathogens from commensals are mediated in part by pattern recognition receptors such as Toll-like receptors (TLRs) that are present on epithelial and immune (dendritic) cells. In health, engagement of TLRs by ligands from the commensal microbiota appears to be required for mucosal homeostasis.21,22 Thus, not only are bacterial signals required for optimal mucosal and immune development, but they also actually are required to maintain and condition the mucosa for responses to injury.22
The immune system mediates the sense of microbial danger and responses to injury. Although the primary lymphoid organs are developed at birth, mucosal immune functions require continual education and fine-tuning of cytokine balances and T-cell responses; this process is achieved by microbial colonization and sporadic mucosal infections. Without the microbiota, mucosal lymphoid tissue is rudimentary, and induction of mucosal immune responses and tolerance is suboptimal.23,24
With a surface area similar to that of a tennis court (approximately 400 m2) and only one cell layer separating the internal milieu from the lumen, the enteric mucosa is well adapted to immunologic sampling of the intraluminal microbial community. Sampling of the microbiota across the epithelial barrier is mediated by M cells, which deliver particulate and microbial antigens to underlying immune cells, and by mucosal dendritic cells, which appear to extend processes into the lumen between the surface enterocytes without disrupting tight junctions.25 It appears that intestinal dendritic cells can ingest and retain intact live commensal bacteria and then transit to the mesenteric lymph node, where immune responses to commensals are induced locally.26 Thus, the mesenteric lymph node acts as a gatekeeper, preventing access of commensal bacteria to the internal milieu and protecting the host from harmful systemic immune reactivity. The immunosensory function of dendritic cells is facilitated by their plasticity and versatility of responses,27 depending on whether they are presented with commensals or pathogens; moreover, they appear to exhibit tissue-specific specialization in the intestine.23,24
In addition to specific immune responses to enteric bacteria, the surface epithelial cells serve a sensory function to detect microbial danger by producing chemokines that activate the host immune response and recruit it to any breach in the mucosal barrier caused by pathogenic infection.28
Transduction of bacterial signals into host immune responses after engagement of TLRs may proceed along more than one molecular pathway. The transcription factor nuclear factor-κB (NF-κB) is the pivotal regulator of epithelial responses to invasive pathogens, but nonpathogenic bacteria can attenuate inflammatory responses by delaying the degradation of IκB, which is counter-regulatory to NF-κB.29 Other signal transduction pathways are likely to emerge to account for the anti-inflammatory effects of probiotics and other commensal organisms such as Bacteroides thetaiotaomicron. This anaerobic commensal can antagonize the proinflammatory effects of NF-κB within the epithelial cell by enhancing the nuclear export of its transcriptionally active subunit (RelA) in a peroxisome proliferator-activated receptor-γ (PPAR-γ)–dependent manner.30
METABOLIC ACTIVITY OF THE MICROBIOTA
The enteric microbiota are tantamount to a hidden metabolic organ (Table 102-1). Although our understanding of indigenous bacterial metabolites is still superficial, coevolution with this living inner mass of bacteria has several apparent benefits for the host. In addition to the production of regulatory signals for mucosal homeostasis as discussed earlier, the microbiota exhibit important metabolic properties not possessed by the host. These include biotransformation of bile acids; degradation of oxalate; breakdown of otherwise indigestible dietary components, such as plant polysaccharides; and production of short-chain fatty acids, a major energy source for colonic epithelium, from fermentable carbohydrates. Other activities include synthesis of biotin, folate, and vitamin K.2,20 Clinicians also have exploited enteric bacterial enzymes such as azoreductase to convert prodrugs such as sulfasalazine to active drug metabolites (e.g., aminosalicylate). Other examples of bacterial action on drug bioavailability include the metabolism of l-dopa to dopamine and degradation of digoxin. Not all of the metabolic changes induced by the enteric microbiota are beneficial to the host, however, and although bacteria probably degrade some carcinogens, they also might promote the production of carcinogens from dietary procarcinogens.31
Table 102-1 Examples of Metabolic Activities of Intestinal Microbiota
A striking example of the importance of bacterial metabolism is exemplified by the regulatory effect that the enteric microbiota exert on fat storage.32 It has long been known that germ-free animals need a significantly greater caloric intake to sustain a body weight similar to that of normal colonized animals. Thus, the normal host-microbiota relationship has nutritional benefit, in contrast with the negative nutritional effect associated with bacterial overgrowth syndromes. Elegant studies with germ-free mice have shown that upon colonization, body weight increases despite a reduced caloric intake. The bacteria in the microbiota colonizing the intestine promote storage of dietary calories in fat by increasing absorption of monosaccharides and suppressing epithelial-derived fasting-induced adipocyte factor (FIAF).32 Thus, the composition and activity of the intestinal microbiota should be considered as a diet-influenced variable that can influence susceptibility to obesity.
One of the outcomes of bacterial metabolic activity is gas production. Of the five gases—N2, O2, CO2, H2, CH4—that constitute 99% of flatus, the latter three are produced by the enteric bacteria, and bacteria are the sole source of hydrogen and methane in the intestine. Hydrogen production by bacterial action on carbohydrates, and to lesser extent on protein, normally occurs in the colon. In patients with small intestinal bacterial overgrowth, however, the small intestine also becomes a site of H2 production. Bacterial methanogens occur in the colon and produce methane from H2 and CO2, with significantly detectable excretion in approximately 30% of humans.33–37 The principal gases produced are odorless, but bacterial metabolism also is responsible for producing various trace and odiferous gases in flatus such as hydrogen disulfide.38,39 Qualitative and quantitative variability in gas production with diet illustrates the fluctuations in bacterial metabolic activity despite the apparent stability of the microbiota in adulthood.
SMALL INTESTINAL BACTERIAL OVERGROWTH
Small intestinal bacterial overgrowth (SIBO) is characterized by malabsorption and overgrowth of bacteria in the small intestine. The syndrome often is referred to as blind loop syndrome because of recognition of the disorder in patients with predisposing anatomic abnormalities. Other terms that have been used to describe the disorder include stagnant loop syndrome, contaminated small bowel, small intestinal colonization, and small bowel stasis. In 1939, Barker and Hummel40 reported macrocytic anemia in association with intestinal strictures and anastomoses and postulated that the anemia was secondary to bacterial overgrowth, or “putrefaction.” SIBO is not confined to humans and is well recognized in dogs.41 The syndrome is associated with a variety of anatomic disturbances, such as blind loops,42 and motility disorders, such as scleroderma,43 but it can occur in the absence of any specific predisposing factor. It is likely that the condition is underdiagnosed, particularly in the elderly.44
Patients with SIBO do not necessarily present with a florid malabsorption syndrome, and symptoms may be minor and nonspecific. Considerable debate has concerned the relationship between irritable bowel syndrome (IBS) and SIBO (see Chapter 118).45 Bacterial overgrowth has been documented in asymptomatic elderly persons in the community,46,47 in whom it is debatable whether the phenomenon is of any significance48; in the absence of malabsorption or related symptoms, such overgrowth probably should not be considered to represent true SIBO. Asymptomatic SIBO may be termed simple colonization and probably results from achlorhydria and abnormal fasting intestinal motility (see later).
The diagnosis of SIBO usually is made by noninvasive breath testing,49 even though studies on the accuracy of these tests report very variable results. For this reason, culture of a small intestinal aspirate must be regarded as the diagnostic gold standard. Unfortunately, much of the published literature on SIBO is based on breath tests, rather than on culture, and the findings must be interpreted with caution.
ETIOLOGY AND PREDISPOSING FACTORS
The upper small intestine is an environment of relatively low bacterial counts because of the combined effects of gastric acid and peristalsis. Bacterial counts in aspirates from the normal upper small intestine generally are less than 1000/mL. Pathophysiology and predisposing conditions are listed in Table 102-2.
Table 102-2 Pathophysiology and Some Conditions Associated with Small Intestinal Bacterial Overgrowth
| PATHOPHYSIOLOGY | CONDITION |
|---|---|
| Anatomic abnormalities |
Anatomic Abnormalities
A variety of anatomic abnormalities, including iatrogenic and disease-related abnormalities, lead to stagnation of small intestinal contents, resulting in bacterial overgrowth. The bacteria in SIBO are similar to those found in the normal colon, and certain organisms are common. Common aerobic organisms include Escherichia coli and Streptococcus, Staphylococcus, Micrococcus, Klebsiella, and Proteus species. Common anaerobic species include Lactobacillus, Bacteroides, Clostridium, Veillonella, Fusobacterium, and Peptostreptococcus.50 The classic anatomic cause of SIBO is a blind loop resulting from abdominal surgery, such as Billroth II partial gastrectomy; other anatomic abnormalities that can result in SIBO include intestinal strictures and small bowel diverticulosis.
Motility Disorders
Disorders affecting small intestinal peristalsis, such as scleroderma,43 diabetes mellitus,51 and chronic idiopathic intestinal pseudo-obstruction52 constitute the next most common cause of SIBO after anatomic abnormalities.
SIBO is well recognized in scleroderma and occurs mainly in patients with small intestinal involvement72 who have limited cutaneous systemic sclerosis. Diarrhea is the most important symptom. The somatostatin analog octreotide is effective in the management of SIBO associated with scleroderma.73
Although small intestinal dysmotility is thought to be the main predisposing factor in diabetes, SIBO in diabetics is not especially associated with autonomic neuropathy.74 Treatment of SIBO in diabetics improves orocecal transit time.75
Fistula or Ileocecal Valve Resection
The ileocecal valve prevents reflux of colonic bacteria into the small intestine, and resection of the valve or development of fistulas between the colon and upper gastrointestinal tract can lead to reflux of colonic contents into the small intestine, with ensuing bacterial overgrowth.53,54
Reduced Gastric Acid Secretion
Achlorhydria is known to be a predisposing factor for SIBO, and SIBO has been described in patients after vagotomy,55 in those with atrophic gastritis, and in those taking acid suppressants.56–59 SIBO occurs more often in patients taking proton-pump inhibitors (PPIs) than in those taking histamine H2 receptor antagonists,58 but clinical malabsorption does not appear to occur in this situation.59
Aging
Advancing age seems to be an independent risk factor for SIBO, but it is not clear if overgrowth results from the aging process itself and age-related changes in intestinal motility or if it is a consequence of achlorhydria. Early studies in this area found that SIBO was a common (and commonly unrecognized) cause of malabsorption in the elderly44,60 and that many such patients did not have an obvious predisposing factor, such as a blind loop. More-recent studies have reported SIBO in asymptomatic elderly persons residing in the community. These patients, although asymptomatic, had lower weights and body mass indices (BMI) than expected, and treatment with antibiotics increased both weight and BMI.46,47 In contrast, a Japanese study reported SIBO (diagnosed by glucose hydrogen breath test) in 25.6% of disabled older adults but in none of the healthy older adults.61
Chronic Liver Disease
SIBO appears to be common in patients with chronic liver disease,62,63 is more common in patients with advanced (Child class C) liver disease,63 and may be an independent risk factor for spontaneous bacterial peritonitis,64 although this association is controversial.65 No association with any particular cause of chronic liver disease has been found,66 but SIBO does not occur in cirrhotic patients who do not have portal hypertension.67 The etiology of SIBO in patients with chronic liver disease is likely to be related to disturbances in gastrointestinal motility67 and possibly to the use of antacids,65 both of which can foster proliferation of bacteria. Small intestinal dysmotility is more severe in cirrhotic patients with a history of spontaneous bacterial peritonitis,64 and treatment of SIBO improves motility.68 Liver transplantation improves small bowel dysmotility in cirrhotic patients.68 Antibiotics and prokinetic agents are effective in reducing the SIBO associated with cirrhosis.69 SIBO in cirrhosis is associated with systemic endotoxemia.70 Oral conjugated bile acids reduce bacterial overgrowth and endotoxemia in cirrhotic rats, suggesting a contributory role for cholestasis in cirrhotic patients with SIBO.71
Other Causes
SIBO is present in many patients with celiac disease who have persistent symptoms despite their adherence to a gluten-free diet.76 It is not clear why this is so, but a motility disturbance seems the most likely explanation.
SIBO is common in Crohn’s disease, particularly in patients who have had previous intestinal resection, and orocecal transit time has been reported to be prolonged in Crohn’s patients with SIBO.77 Positive results on glucose hydrogen breath tests are particularly associated with the presence of a small bowel stricture.78
SIBO is common in chronic pancreatitis.79 SIBO in this setting may be caused by small bowel dysmotility resulting from chronic opioid use and achlorhydria. Furthermore, pancreatic juice may have an antibacterial effect, so its absence might allow enteric bacteria to proliferate more freely.80
SIBO in rheumatoid arthritis is associated with high disease activity and does not appear to be related to achlorhydria.81
SIBO occurs in late radiation enteritis and appears to be related to intestinal dysmotility.82
SIBO is common in chronic kidney disease, which is associated with neuropathic-type abnormalities of small intestinal motility.83
SIBO occurs commonly in cystic fibrosis (CF). Use of azithromycin is paradoxically associated with an increased risk of a positive breath test for SIBO.84 In a murine model of CF, eradication of SIBO decreased intestinal mucus secretion.85
SIBO has been reported in patient populations with interstitial cystitis,86 acne rosacea,87 morbid obesity,88 fibromyalgia,89 acromegaly,90 and focal segmental ischemia (see Chapter 114.)
MECHANISMS OF MALABSORPTION
SIBO classically causes a combination of megaloblastic anemia (due to vitamin B12 deficiency) and steatorrhea (due to fat malabsorption). Megaloblastic anemia was described in association with intestinal strictures as long ago as 1897.91 Vitamin B12 deficiency is caused by bacterial utilization of the vitamin within the intestinal lumen before it can be absorbed across the mucosa.92 Anaerobic organisms mainly are responsible for the vitamin B12 deficiency, and in animal studies, only therapy directed against anaerobes reversed the deficiency.93 Unlike aerobic bacteria, anaerobes can use vitamin B12 both in its free form and complexed with intrinsic factor.94 Anaerobic bacteria deprive the host of ingested vitamin B12 and exacerbate B12 deficiency by using the vitamin to produce inactive cobamides, which then can compete with dietary B12 for ileal binding sites, thereby decreasing absorption of the vitamin.95 Deficiencies of thiamine96 and nicotinamide also have been reported in SIBO.97 Folate levels tend to be high in SIBO, because the bacteria synthesize folate,98 which then is absorbed and used by the host.99
Much of our knowledge on the mechanisms of malabsorption in SIBO is derived from animal models of blind loops.100,101 Malabsorption of fat and fat-soluble vitamins results mainly from deconjugation of bile acids,102 and administration of conjugated bile acids has been reported to reverse steatorrhea in human and animal studies.103 Deficiencies of vitamins A, D,104 and E105 have been reported, but vitamin K deficiency is uncommon because production of vitamin K by luminal bacteria offsets any deficiencies attributed to fecal fat loss.
SIBO leads to carbohydrate malabsorption by reducing brush border disaccharidase levels.106–108 In animal studies, bacterial extracts of cultures from experimentally created blind loops contain proteases that can remove components of the intestinal surface membrane.109 These proteases appear to have elastase-like substrate specificity and may be etiologic in disaccharidase deficiency. Lactose intolerance is common and contributes to the diarrhea that typifies SIBO. Bacterial fermentation of carbohydrates contributes to abdominal discomfort and bloating in SIBO and is the basis for the various breath tests used to diagnose the condition.
Protein malabsorption in SIBO is caused by a number of factors: decreased absorption of amino acid and peptides, which has been described in animal models and can result from mucosal damage110; low levels of enterokinase, which can impair the activation of pancreatic proteases111; and protein-losing enteropathy.112 Although hypoproteinemia is common in SIBO, manifestations of severe hypoproteinemia, such as edema, are rare.
Small intestinal histologic findings generally are normal in patients with SIBO, and in one study, morphometric findings in the small intestine also were described as normal.113 Abnormalities of small intestinal mucosa (e.g., villus atrophy, cellular infiltration of the lamina propria, intraepithelial lymphocytosis) have been described in some patients with SIBO, and these changes revert to normal following treatment with antibiotics.114 Electron microscopy studies of experimental animals with SIBO have described enterocyte abnormalities, such as vacuolization of microvillus membranes and mitochondrial swelling.115
CLINICAL FEATURES
SIBO may be difficult to diagnose because symptoms associated with the predisposing disorder can predominate. The classic clinical presentation of SIBO is that of a malabsorptive state characterized by steatorrhea and vitamin B12 deficiency that is not reversible with intrinsic factor. Patients with vitamin B12 deficiency can present with neurologic symptoms, central or peripheral neuropathy, and symptoms of anemia, such as fatigue, breathlessness, and chest pain. Patients with steatorrhea can report weight loss, diarrhea, and abdominal bloating and discomfort. Associated fat-soluble vitamin deficiency can occur, leading to night blindness (in vitamin A deficiency) and metabolic bone disease (in vitamin D deficiency). Osteoporosis is another well-recognized complication of SIBO.116,117
The clinical presentation of SIBO appears to be changing. Older references to clinical features of SIBO emphasized steatorrhea, megaloblastic anemia, and a history of surgery leading to blind loop syndrome. In a somewhat more modern series, Toskes and Kumar reported data for 100 consecutive, albeit highly selected, symptomatic patients referred for 14C-xylose breath testing118 and found a history of gastrointestinal surgery in only 15%. The three most common associated conditions, which accounted for more than 90% of the positive results on 14C-xylose breath tests for the patients in their referral center, were gastroparesis, chronic pancreatitis, and irritable bowel syndrome. Diarrhea, bloating, and flatulence were the most common symptoms. More recent studies demonstrate that the clinical presentation of SIBO may be less dramatic than the classic descriptions of SIBO and have milder symptoms. The wide use of breath tests is one reason we see this newer type of SIBO patient.
Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome
Many patients with SIBO fulfill the Rome Criteria for IBS, and considerable debate on the relationship between IBS and SIBO has ensued since a study in 2000 reported that 78% of patients with Rome criteria-positive IBS tested positive for SIBO by lactulose hydrogen breath testing.119 Antibiotic therapy led to clinical improvement and normalization of the breath test. This study also provoked criticism of its selection criteria and study design and raised concerns regarding the accuracy of the lactulose hydrogen breath test.120 Two subsequent studies found no difference in lactulose hydrogen breath test positivity between IBS patients and controls.121,122 A Swedish study, using cultures of intestinal aspirate to diagnose SIBO, reported a prevalence of SIBO of 4% in both IBS patients and controls.123 The hypothesis that SIBO is a significant etiological factor in IBS remains unproved.
Nonetheless, the putative relationship between SIBO and IBS has stimulated a number of clinical trials of antibiotic therapy in IBS. Three relatively small controlled trials reported a modest benefit of antibiotic therapy in IBS patients,124–126 although the design and statistical analysis of these trials have been criticized.45 The major benefit appeared to be in patients with abdominal bloating, suggesting that the predominant effect of antibiotic therapy is to reduce gas-forming bacteria. Symptom improvement in these studies did not consistently correlate with normalization of the lactulose hydrogen breath test, however, suggesting that the benefit of antibiotic therapy might be reduction of colonic, rather than small intestinal, gas-forming bacteria.
Small Intestinal Bacterial Overgrowth and Nonalcoholic Steatohepatitis
Considerable interest has arisen in the putative association between SIBO and nonalcoholic steatohepatitis (NASH). It has been postulated that SIBO might play a role in the pathogenesis of NASH,127 because NASH is a common complication of jejunoileal bypass surgery for morbid obesity and can be reversed with metronidazole treatment. Antibiotic treatment prevents hepatic and bile duct injury in genetically susceptible rats with surgically created blind loops and SIBO,128,129 although the pattern of hepatic and biliary injury in this experimental situation was histologically and radiologically more compatible with primary sclerosing cholangitis than with NASH. Wigg and colleagues130 postulated that SIBO might lead to NASH by altering small intestinal permeability and thereby increasing absorption of endotoxin. They studied 22 patients with NASH and found SIBO (by lactulose hydrogen breath testing) in 50% of subjects; serum endotoxin levels and small intestinal permeability, measured by the lactulose-rhamnose test, however, were normal in the patients with SIBO.
DIAGNOSIS
The diagnosis of SIBO should be considered in any patient with malabsorption and a predisposing condition. As mentioned earlier, most patients today do not have predisposing surgically induced anatomic abnormalities. It is likely that SIBO is commonly overlooked in patients without known predisposing factors and in patients who have nonspecific symptoms. Blood tests in patients with SIBO typically reveal a macrocytic anemia: Vitamin B12 levels are low, and folate levels may be high. Steatorrhea may be confirmed by three-day quantitative fecal fat collection; this test has understandably fallen from favor with patients and laboratory staff, and qualitative microscopic examination of fresh stool for fat globules usually is performed.131,132 If an anatomic defect is suspected as the cause of SIBO, appropriate barium studies may be used to define the anatomy.
Aspiration
The gold standard test for the diagnosis of SIBO is aspiration of small intestinal fluid with culture and bacterial counts of the aspirate; presence of more than 105 CFU/mL of duodenal aspirate is considered diagnostic. Unfortunately, such aspiration is invasive and time-consuming. Moreover, although it still is recommended by most experts, some investigators have raised concerns that the test might miss bacterial overgrowth occurring more distally in the small intestine. Corazza and colleagues,133 however, collected intestinal juice at two different levels of the proximal jejunum and reported a highly significant correlation between the bacterial counts at these sites. Other potential problems with aspiration of small intestinal fluid include contamination of the aspirate with bacteria from the mouth and technical difficulties with transport and culture of the aspirate. Contamination with oropharyngeal bacteria may be controlled for by simultaneous culture of saliva and jejunal aspirate.134
Several techniques for collecting small intestinal contents have been described, including duodenal intubation with fluoroscopic guidance and endoscopic collection of fluid,135 and brushing of the duodenal mucosa with a cytology brush.136 Culture of unwashed small intestinal mucosal biopsy specimens is an alternative to culture of a small intestinal aspirate, although the former method appears to have a lower sensitivity compared with culture of aspirates.137 Aspirate can be collected easily during routine endoscopy, and this is probably the easiest method in routine clinical practice. Small intestinal aspirate is collected by placing a sterile suction catheter inside a sterile overtube, which is passed through the suction channel of the endoscope. The aspirate should be placed immediately in aerobic and anaerobic transport vials, and the aspirate should be plated for aerobic and anaerobic organisms as soon as possible.
High levels of jejunal fluid volatile fatty acids, such as acetate and propionate, have been reported in SIBO.138 These acids may be measured by gas-liquid chromatography; although the technique is highly specific, the sensitivity is low,133 and this test is rarely used.
Breath Tests
A variety of noninvasive tests have been developed for diagnosing SIBO. The 14C-glycocholic acid breath test was one of the first breath tests used for this purpose and is based on the ability of bacteria to deconjugate bile salts. 14C-glycine is produced and metabolized, resulting in a peak of 14CO2 in the expired air. The test has a low sensitivity, because not all bacteria are capable of such deconjugation, and the test has a low specificity, because increased colonic deconjugation of bile salts can occur with ileal disease or following ileal resection.139 The test therefore cannot distinguish between SIBO and ileal malabsorption and has largely fallen out of favor.
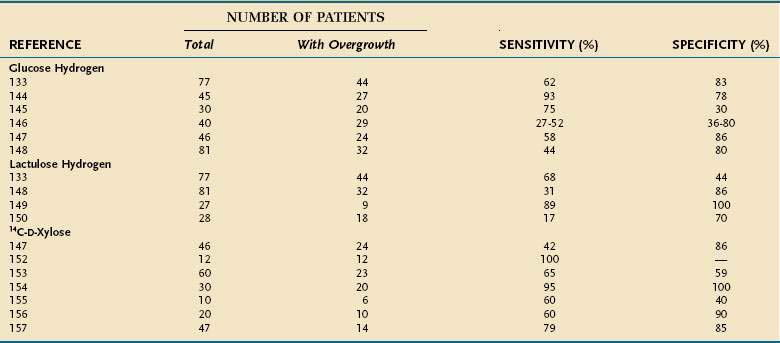
The currently used breath tests are based on the ability of bacteria to produce hydrogen or radiolabeled carbon dioxide after metabolizing a substrate such as glucose, lactulose, or xylose. Breath tests are simple and noninvasive and therefore are more attractive than is duodenal intubation or endoscopy for collecting intestinal aspirates. These breath tests, however, do have several potential problems49: About 15% of the population are methane producers (in persons who are colonized with Methanobrevibacter smithii, hydrogen reacts with carbon dioxide to form methane, so less hydrogen is produced than in non-methane producers). Both slow and rapid small intestinal transit can affect the accuracy of these tests. An acidic environment in the colon, such as occurs with ingestion of nonabsorbable carbohydrates (e.g., lactulose), inhibits bacterial carbohydrate metabolism.140 Several patient-related factors, such as recent diet, smoking, and exercise, can influence baseline levels of breath hydrogen (see later). The literature on breath tests in SIBO is confusing, with wide variations in sensitivity and specificity (Table 102-3).
Table 102-3 Sensitivity and Specificity of Breath Testing in the Diagnosis of Small Intestinal Bacterial Overgrowth: Summary of Clinical Studies
Glucose Hydrogen
The glucose hydrogen breath test probably is the most widely used breath test in clinical practice: The substrate is inexpensive, and the hydrogen meter is economical, portable, and easy to use. The glucose hydrogen breath test first was reported as a diagnostic test for SIBO in 1972 by Bond and Levitt.141 Normally, glucose is absorbed completely in the upper small intestine; with bacterial overgrowth, however, the glucose is cleaved by bacteria into carbon dioxide and hydrogen. The hydrogen is measured in the exhaled breath (at baseline and then every 30 minutes for 2 hours); a rise of 20 parts per million (ppm) above the baseline is regarded as diagnostic of SIBO. Fasting breath hydrogen levels of more than 20 ppm also are considered positive. High baseline hydrogen levels also are common in untreated celiac disease and normalize after gluten withdrawal for as-yet-unknown reasons.142
Patient preparation is important for the glucose hydrogen test143: Patients must avoid smoking and ingestion of nonfermentable carbohydrates, such as pasta and bread, the night before the test, because these factors can raise baseline breath hydrogen values. Exercise can induce hyperventilation, thereby reducing baseline breath hydrogen values, and should be avoided for two hours before the test. Some authors recommend an antibacterial mouth rinse before testing to prevent premature hydrogen or carbon dioxide production from the action of the oral flora on the glucose substrate.
A number of studies have compared the glucose hydrogen breath test against the gold standard of culture of intestinal aspirate (see Table 102-3). Sensitivity levels from 27% to 52% have been reported, with specificity rates between 30% and 83%133,144–148; the largest study, by Corazza and colleagues,133 reported sensitivity of 62% and specificity of 83%. Very rapid intestinal transit can lead to a false-positive test result, because glucose can reach the colon before it can be absorbed.
Lactulose Hydrogen
The lactulose hydrogen breath test is based on a principle similar to that of the glucose hydrogen breath test: Lactulose is a disaccharide that is not absorbed in the small intestine but is metabolized by bacteria in the proximal colon, producing a late peak in exhaled hydrogen. In the presence of bacterial overgrowth, an early hydrogen peak is observed. Results of this test may be difficult to interpret with either slow or fast intestinal transit, and sensitivity and specificity have been disappointing133,149,150 (see Table 102-3); Corazza and associates reported sensitivity and specificity rates of 68% and 44%, respectively.133 Sensitivity of the test may be increased by the addition of scintigraphy to correct for abnormalities of intestinal transit,150 but the lactulose hydrogen breath test cannot be recommended for routine clinical use.
Xylose
The 14C-xylose and 13C-xylose breath tests measure labeled carbon dioxide that is produced by breakdown of labeled substrates by bacteria. The isotope may be radioactive (14C) or stable (13C); the stable isotope has been used in children.151 d-Xylose is the most widely used substrate and is a good substrate for breath testing for SIBO because it is absorbed completely in the small intestine, is metabolized minimally, and is catabolized by Gram-negative bacteria. The 14C-d-xylose breath test appears to perform better than the glucose or lactulose hydrogen breath test (see Table 102-3) but, as with these other breath tests, widely differing levels of accuracy have been reported, with sensitivity rates ranging from 42% to 95% and specificity rates between 40% and 100%.147,152–157 The 14C-d-xylose breath test result is considered positive when the “cumulated dose at four hours exceeds 4.5% of the administered radioactivity.”49 Disturbances in intestinal transit particularly affect the performance of this test, and accuracy may be improved by the addition of a transit marker (such as barium or diatrizoate meglumine–diatrizoate sodium [Gastrografin]) and radiologic imaging.156
Other Tests
Other noninvasive tests described for SIBO include measurement of urinary cholyl-para-aminobenzoic acid (PABA) and serum bile acids. Cholyl-PABA is a synthetic substrate made by conjugating cholic acid with PABA, which is hydrolyzed by the bacterial enzyme cholyl hydrolase to release PABA158; this PABA-based test, however, does not accurately distinguish between SIBO and other causes of malabsorption.159 Elevated free serum bile acids have been reported in SIBO, but the test depends on the presence of bacteria that deconjugate bile salts, such as Bacteroides.160
TREATMENT
Attention should be given to the patient’s nutritional state, and any vitamin deficiency should be corrected (see Chapters 4, 5, and 100). A lactose-free diet can ameliorate the diarrhea. If possible, any predisposing anatomic or functional abnormality should be corrected, but in practice, this is unlikely to be an option. Acid-lowering medication should be discontinued, if possible.
A variety of antibiotics have been reported to be effective in SIBO, but little evidence exists to favor one agent over another. Antibiotics that have been reported to be effective include metronidazole, amoxicillin, amoxicillin-clavulanate, ciprofloxacin, tetracycline, and cotrimoxazole. One randomized crossover trial reported that norfloxacin and amoxicillin-clavulanate were effective in SIBO.161 In another study, rifaximin and chlortetracycline normalized results on glucose hydrogen breath testing in 70% and 27%, respectively, of patients with SIBO.162 Both ciprofloxacin and metronidazole were found to be highly effective in SIBO associated with Crohn’s disease, and although these antibiotics have been used for primary therapy in Crohn’s disease, normalization of breath tests occurred in most of the patients in this study.163
There have been several reports on the use of the nonabsorbable antibiotic rifaximin in SIBO. Rifaximin at a dose of 1.2 g/day and 1.6 g/day leads to normalization of the glucose hydrogen breath test in 58% and 80% of patients, respectively.164 Metronidazole is more effective than rifaximin, at least in patients with SIBO associated with the blind-loop syndrome.165 Recurrence of SIBO after rifaximin treatment is common.166
The somatostatin analog octreotide stimulates intestinal motor activity when administered in low dosage. Given subcutaneously at 50 µg once daily for three weeks, it has been reported to be effective in SIBO associated with scleroderma.73 At higher doses (200 µg three times daily), octreotide paradoxically can cause SIBO by inducing hypomotility.167 The prokinetic agent cisapride has been reported to be effective in SIBO associated with cirrhosis,168 but the drug is no longer available in the United States, and its use in several countries is strictly controlled because of risk of drug interactions and cardiac arrhythmias.
Probiotic therapy is a logical and attractive approach to the management of SIBO, but it has been examined in only a few studies. Saccharomyces boulardii does not appear to be effective, and in one double-blind crossover study, Lactobacillus fermentum KLD showed no advantage over placebo.169 A small uncontrolled trial showed that Lactobacillus plantarum 299V and Lactobacillus GG benefited children who had SIBO associated with short bowel syndrome.170
Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411-20. (Ref 28.)
Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718-23. (Ref 32.)
Bratten J, Spanier J, Jones MP. Lactulose hydrogen breath testing does not discriminate patients with irritable bowel syndrome from healthy controls. Am J Gastroenterol. 2008;103:958-63. (Ref 122.)
Castiglione F, Rispo A, Di Girolamo E, et al. Antibiotic treatment of small bowel bacterial overgrowth in patients with Crohn’s disease. Aliment Pharmacol Ther. 2003;18:1107. (Ref 163.)
Di Sefano M, Miceli E, Missanelli M, et al. Absorbable vs non-absorbable antibiotics in the treatment of small intestine bacterial overgrowth in patients with blind-loop syndrome. Aliment Pharmacol Ther. 2005;21:985. (Ref 165.)
Marchesi J, Shanahan F. The normal intestinal microbiota. Curr Opin Infectious Dis. 2007;20:508-13. (Ref 8.)
O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688-93. (Ref 2.)
Lauritano EC, Gabrielli M, Scarpellini E, et al. Small intestinal bacterial overgrowth recurrence after antibiotic therapy. Am J Gastroenterol. 2008;103:2031-5. (Ref 166.)
Parlesak A, Klein B, Schecher K, et al. Prevalence of small bowel bacterial overgrowth and its association with nutrition intake in nonhospitalised older adults. J Am Geriatr Soc. 2003;51:768-73. (Ref 47.)
Pimentel M, Park S, Mirocha J, et al. The effect of a non-absorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: A randomized trial. Ann Intern Med. 2006;145:557-63. (Ref 125.)
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229-41. (Ref 21.)
Riordan SM, McIver CJ, Wakefield D, et al. Small intestinal mucosal immunity and morphometry in luminal growth of indigenous gut flora. Am J Gastroenterol. 2001;96:494-500. (Ref 113.)
Romagnuolo J, Schiller D, Bailey RJ. Using breath tests wisely in a gastroenterology practice: An evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol. 2002;97:2113-26. (Ref 49.)
Scarpellini E, Gabrielli M, Lauritano CE, et al. High dose rifaximin for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2007;25:781-6. (Ref 164.)
Sharara AI, Aoun E, Abdul-Baki H, et al. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101:326-33. (Ref 126.)
Turnbaugh PJ, Ley RE, Hamady M, et al. The human microbiome project. Nature. 2007;449:804-10. (Ref 4.)
Vanner S. The lactulose breath test for diagnosing SIBO in IBS patients: Another nail in the coffin. Am J Gastroenterol. 2008;103:964-65. (Ref 120.)
Vanner S. The small intestinal bacterial overgrowth/irritable bowel syndrome hypothesis: Implications for treatment. Gut. 2008;57:1315-21. (Ref 45.)
Wigg AJ, Roberts-Thomson IC, Dymock RB. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206-11. (Ref 130.)
1. Zoetendal EG, Akkermans ADL, Akkermans-van Vilet WM, et al. The host genotype affects the bacterial community in the human gastrointestinal tract. Microb Ecol Health Dis. 2001;13:129-34.
2. O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688-93.
3. Sonnenburg JL, Angenent LT, Gordon JI. Getting a grip on things: How do communities of bacterial symbionts become established in our intestine? Nat Immunol. 2004;5:569-73.
4. Turnbaugh PJ, Ley RE, Hamady M, et al. The human microbiome project. Nature. 2007;449:804-10.
5. Gordon JI, Hooper LV, McNevin SM, et al. Epithelial cell growth and differentiation III. Promoting diversity in the intestine: Conversations between the microflora, epithelium, and diffuse GALT. Am J Physiol Gastrointest Liver Physiol. 1997;273:G565-70.
6. Xu J, Gordon JI. Honor thy symbionts. Proc Natl Acad Sci U S A. 2003;100:10452-9.
7. Zoetendal EG, Von Wright A, Vilpponen-Salmela T, et al. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from the feces. Appl Environ Microbiol. 2002;68:3401-7.
8. Marchesi J, Shanahan F. The normal intestinal microbiota. Curr Opin Infectious Dis. 2007;20:508-13.
9. Vaughan EE, Schut F, Heilig HG, et al. A molecular view of the intestinal ecosystem. Curr Issues Intest Microbiol. 2000;1:1-12.
10. Tzeneva VA, Heilig HG, van Vliet WA, et al. 16SrRNA targeted DGGE fingerprinting of microbial communities. Methods Mol Biol. 2008;410:335-49.
11. Collins K, O’Mahony J. The “unculturables.”. In: Hart AL, Stagg AJ, Graffner H, et al, editors. Gut ecology. London: Martin Dunitz; 2002:25-33.
12. Dalwadi H, Wei B, Braun J. Defining new pathogens and non-culturable infectious agents associated with inflammatory bowel disease. Curr Opin Gastroenterol. 2000;16:56-9.
13. Cohavy O, Bruckner D, Gordon LK, et al. Colonic bacteria express an ulcerative colitis pANCA-related protein epitope. Infect Immun. 2000;68:1542-8.
14. Wei B, Dalwadi H, Gordon LK, et al. Molecular cloning of a Bacteroides caccae TonB-linked outer membrane protein identified by an inflammatory bowel disease marker antibody. Infect Immun. 2001;69:6044-54.
15. Dalwadi H, Wei B, Kronenberg M, et al. The Crohn’s disease–associated bacterial protein I2 is a novel enteric T cell superantigen. Immunity. 2001;15:149-58.
16. Sutton CL, Kim J, Yamane A, et al. Identification of a novel bacterial sequence associated with Crohn’s disease. Gastroenterology. 2000;119:23-31.
17. Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296-1306.
18. Midtvedt T. Microbial functional activities. In: Hanson LA, Yolken RH, editors. Intestinal microflora. Nestle Nutrition Workshop Series No. 42. Philadelphia: Lippincott-Raven; 1999:79.
19. Hooper LV, Wong MH, Thelin A, et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881-4.
20. Hooper LV, Midvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283-307.
21. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229-41.
22. Madara J. Building an intestine—architectural contributions of commensal bacteria. N Engl J Med. 2004;351:1685-6.
23. Shanahan F. Nutrient tasting and signaling mechanisms in the gut: V. Mechanisms of immunologic sensation of intestinal contents. Am J Physiol Gastrointest Liver Physiol. 2000;278:G191-6.
24. Shanahan F. Pathophysiologic basis and prospects for probiotic therapy in inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2005;288:G417-21.
25. Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361-7.
26. Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662-5.
27. Huang Q, Liu D, Majewski P, et al. The plasticity of dendritic cell responses to pathogens and their components. Science. 2001;294:870-5.
28. Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411-20.
29. Neish AS, Gewirtz AT, Zeng H, et al. Prokaryotic regulation of epithelial responses by inhibition of IκB-α ubiquitination. Science. 2000;289:1560-3.
30. Kelly D, Campbell JI, King TP, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104-12.
31. Rafter J. Probiotics and colon cancer. Best Pract Res Clin Gastroenterol. 2003;17:849-59.
32. Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718-23.
33. Levitt MD. Volume and composition of human intestinal gas determined by means of an intestinal washout technic. N Engl J Med. 1971;284:1394-98.
34. Levine AS, Bond JH, Prentiss RA, et al. Metabolism of carbon monoxide by the colonic flora of humans. Gastroenterology. 1982;83:633-7.
35. Levitt MD, Engel RR. Intestinal gas. Adv Intern Med. 1975;20:151-65.
36. Levitt MD. Intestinal gas production—recent advances in flatology. N Engl J Med. 1980;302:1474-5.
37. Levitt MD. Methane production in the gut. N Engl J Med. 1974;291:528-9.
38. Moore JG, Jessop LD, Osborne DN. Gas-chromatographic and mass-spectrometric analysis of the odor of human feces. Gastroenterology. 1987;93:1321-9.
39. Suarez FL, Springfield J, Levitt MD. Identification of gases responsible for the odour of human flatus and evaluation of a device purported to reduce this odour. Gut. 1998;43:100.
40. Barker WH, Hummel LE. Macrocytic anemia in association with intestinal strictures and anastomoses. Bull Johns Hopkins Hosp. 1939;46:215.
41. Batt RM, McLean L, Riley JE. Response of the jejunal mucosa of dogs with aerobic and anaerobic overgrowth to antibiotic therapy. Gut. 1988;29:473-82.
42. Wirts CW, Goldstein F. Studies of the mechanism of postgastrectomy steatorrhea. Ann Intern Med. 1963;58:25-36.
43. Kahn IJ, Jeffries GH, Sleisenger MH. Malabsorption in intestinal scleroderma: Correction by antibiotics. N Engl J Med. 1966;274:1339-44.
44. Roberts SH, James O, Jarvis EH. Bacterial overgrowth without “blind loop”: A cause for malnutrition in the elderly. Lancet. 1977;10:1193-5.
45. Vanner S. The small intestinal bacterial overgrowth/irritable bowel syndrome hypothesis: Implications for treatment. Gut. 2008;57:1315-21.
46. Lewis SJ, Potts LF, Malhotra R, Mountford R. Small bowel bacterial overgrowth in subjects living in residential care homes. Age Ageing. 1999;28:181-5.
47. Parlesak A, Klein B, Schecher K, et al. Prevalence of small bowel bacterial overgrowth and its association with nutrition intake in nonhospitalised older adults. J Am Geriatr Soc. 2003;51:768-73.
48. Lipski PS, Kelly PJ, James OFW. Bacterial contamination of the small bowel in elderly people: Is it necessarily pathological? Age Ageing. 1992;21:5-12.
49. Romagnuolo J, Schiller D, Bailey RJ. Using breath tests wisely in a gastroenterology practice: An evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol. 2002;97:1113-26.
50. Bouhnik Y, Alain S, Attar A, et al. Bacterial populations contaminating the upper gut in patients with small intestinal bacterial overgrowth syndrome. Am J Gastroenterol. 1999;94:1327-31.
51. Goldstein F, Wirts CW, Kowlessar OD. Diabetic diarrhea and steatorrhea: Microbiologic and clinical observations. Ann Intern Med. 1970;72:215.
52. Pearson AJ, Brezechwa-Ajdukiewicz A, McCarthy CF. Intestinal pseudo-obstruction with bacterial overgrowth in the small intestine. Am J Dig Dis. 1969;14:200.
53. Griffin WOJr, Richardson JD, Medley ES. Prevention of small bowel contamination by ileocecal valve. South Med J. 1971;64:1056.
54. Atwater JS, Butt HR, Priestly JT. Gastrojejunocolic fistulae with special reference to associated nutritional deficiencies and certain surgical aspects. Ann Surg. 1943;117:414.
55. Browning GG, Buchan KA, Mackay C. The effect of vagotomy and drainage on the small bowel flora. Gut. 1974;15:139.
56. Ruddell WSJ, Losowsky MS. Severe diarrhoea due to small intestinal colonization during cimetidine treatment. BMJ. 1980;281:273.
57. Fried M, Siegrist H, Frei R, et al. Duodenal bacterial overgrowth during treatment in outpatients with omeprazole. Gut. 1994;35:23.
58. Thorens J, Froehlich F, Schwizer W, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: A prospective randomised double blind study. Gut. 1996;39:54.
59. Pereira SP, Gainsborough N, Dowling RH. Drug-induced hypochlorhydria causes high duodenal bacterial counts in the elderly. Aliment Pharmacol Ther. 1998;12:99.
60. McEvoy A, Dutton J, James OF. Bacterial contamination of the small intestine is an important cause of occult malabsorption in the elderly. Br Med J (Clin Res Ed). 1983;287:789.
61. Mitsui T, Shimaoka K, Goto Y, et al. Small bowel bacterial overgrowth is not seen in healthy adults but is in disabled older adults. Hepatogastroenterology. 2006;53:82.
62. Shindo K, Machida M, Miyakawa K, et al. A syndrome of cirrhosis, achlorhydria, small intestinal bacterial overgrowth, and fat malabsorption. Am J Gastroenterol. 1993;88:2084.
63. Morencos FC, de las Heras Castano G, Martin Ramos L, et al. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1995;40:1252.
64. Chang CS, Chen GH, Lien HC, et al. Small intestinal dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1998;28:1187.
65. Bauer TM, Steinbruckner B, Brinkmann FE, et al. Small intestinal bacterial overgrowth in patients with cirrhosis: Prevalence and relation with spontaneous bacterial peritonitis. Am J Gastroenterol. 2001;96:2962.
66. Yang CY, Chang CS, Chen GH. Small-intestinal bacterial overgrowth in patients with liver cirrhosis, diagnosed with glucose H2 and CH4 breath tests. Scand J Gastroenterol. 1998;33:867.
67. Gunnarsdottir SA, Sadik R, Shev S, et al. Small intestinal motility disturbances and bacterial overgrowth in patients with liver cirrhosis and portal hypertension. Am J Gastroenterol. 2003;98:1362.
68. Madrid AM, Hurtado C, Venegas M, et al. Long-term treatment with cisapride and antibiotics in liver cirrhosis: Effect on small intestinal motility, bacterial overgrowth and liver function. Am J Gastroenterol. 2001;96:1251.
69. Madrid AM, Brahm J, Buckel E, et al. Orthotopic liver transplantation improves small bowel motility disorders in cirrhotic patients. Am J Gastroenterol. 1997;92:1044.
70. Bauer TM, Schawacha H, Steinbruckner B, et al. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol. 2002;97:2364.
71. Lorenzo-Zuniga V, Bartoli R, Planas R. Oral bile acids reduce bacterial overgrowth, bacterial translocation and endotoxemia in cirrhotic rats. Hepatology. 2003;37:551.
72. Kay SA, Lim SG, Taylor M, et al. Small bowel bacterial overgrowth in systemic sclerosis: Detection using direct and indirect methods and treatment outcome. Br J Rheumatol. 1995;34:265.
73. Soudah HC, Hasler WL, Owyang C. Effect of octreotide on intestinal motility and bacterial overgrowth in scleroderma. N Engl J Med. 1991;325:1461.
74. Virally-Monod M, Tielmans D, Kevorkian JP, et al. Chronic diarrhoea and diabetes mellitus: Prevalence of small intestinal bacterial overgrowth. Diabetes Metab. 1998;24:530.
75. Cuoco L, Montalto M, Jorizzo RA, et al. Eradication of small intestinal bacterial overgrowth and oro-cecal transit in diabetics. Hepatogastroenterology. 2002;49:1582.
76. Tursi A, Brandimarte G, Giorgetti G. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am J Gastroenterol. 2003;98:839.
77. Castiglione F, Del Vecchio Blanco G, Rispo A, et al. Orocaecal transit time and bacterial overgrowth in patients with Crohn’s disease. J Clin Gastroenterol. 2000;31:63.
78. Mishkin D, Boston FM, Blank D, et al. The glucose breath-test: A diagnostic test for small bowel strictures in Crohn’s disease. Dig Dis Sci. 2002;47:489.
79. Lembeke B, Kraus B, Lankisch PG. Small intestinal function in chronic relapsing pancreatitis. Hepatogastroenterology. 1985;32:149.
80. Rubinstein E, Mark Z, Haspel J, et al. Antibacterial activity of the pancreatic fluid. Gastroenterology. 1985;88:927.
81. Henriksson AE, Blomquist L, Nord CE, et al. Small intestinal bacterial overgrowth in patients with rheumatoid arthritis. Ann Rheum Dis. 1993;52:503.
82. Husebye E, Skar V, Hoverstad T, et al. Abnormal intestinal motor patterns explain enteric colonization with gram-negative bacilli in late radiation enteropathy. Gastroenterology. 1995;109:1078.
83. Strid H, Simren M, Stotzer PO, et al. Patients with chronic renal failure have abnormal small intestinal motility and a high prevalence of small intestinal bacterial overgrowth. Digestion. 2003;67:129.
84. Fridge JL, Conrad C, Gerson L, et al. Risk factors for small bowel bacterial overgrowth in cystic fibrosis. J. Pediatr Gastroenterol Nutr. 2007;44:212.
85. De Lisle RC, Roach EA, Norkina O. Eradication of small intestinal bacterial overgrowth in the cystic fibrosis mouse reduces mucus accumulation. J Paediatr Gastroenterol Nutr. 2006;42:46.
86. Weinstock LB, Klutke CG, Lin HC. Small intestinal bacterial overgrowth in patients with interstitial cystitis and gastrointestinal symptoms. Dig Dis Sci. 2007;53:1246.
87. Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: Clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759.
88. Sabate JM, Jouet P, Harnois F, et al. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: A contributor to severe hepatic steatosis. Obes Surg. 2008;18:371.
89. Pimentel M, Wallace D, Hallegua D, et al. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann Rheum Dis. 2004;63:450.
90. Resmini E, Parodi A, Savarino V, et al. Evidence of prolonged orocecal transit time and small bowel bacterial overgrowth in acromegalic patients. J Clin Endocrinol Metab. 2007;92:2119.
91. Faber K. Perniciöse Anämie bei Dunndarmstricturen. Berl Klin Wochenschr. 1897;34:643.
92. King CE, Toskes PP. Small intestinal bacterial overgrowth. Gastroenterology. 1979;76:1035.
93. Giannella RA, Broitman SA, Zamchek N. Competition between bacteria and intrinsic factor for vitamin B12: Implications for vitamin B12 malabsorption in intestinal bacterial overgrowth. Gastroenterology. 1972;62:255.
94. Welkos SL, Toskes PP, Baer H. Importance of anaerobic bacteria in the cobalamin malabsorption of the experimental rat blind loop syndrome. Gastroenterology. 1981;80:313.
95. Brandt LJ, Bernstein LH, Wagle A. Production of vitamin B12 analogues in patients with small bowel bacterial overgrowth. Ann Intern Med. 1977;87:546.
96. Larvol L, Eugène C, Anciaux ML, Quevauvilliers J. [Polyneuritis complicating chronic bacterial colonization of the small intestine in jejunal diverticulosis]. Gastroenterol Clin Biol. 1988;12(6-7):585-6.
97. Tabaqchali S, Pallis C. Reversible nicotinamide deficiency encephalopathy in a patient with jejunal diverticulosis. Gut. 1970;11:1024.
98. Hoffbrand AV, Tabaqchali S, Moilin DL. High serum folate levels in intestinal blind loop syndrome. Lancet. 1966;1:1339.
99. Camilo E, Zimmerman J, Mason JB, et al. Folate synthesized by bacteria in the human upper small intestine is assimilated by the host. Gastroenterology. 1996;110:991.
100. Cameron DG, Watson GM, Witts LJ. The experimental production of macrocytic anemia by operations of the intestinal tract. Blood. 1949;4:803.
101. King CE, Toskes PP. The experimental rat blind loop preparation: A model for small-intestine bacterial overgrowth in man. In: Pfeiffer CJ, editor. Animal models for intestinal disease. Boca Raton, Fla: CRC Press; 1985:217.
102. Tabaqchali S, Hatzioanuou J, Booth CC. Bile salt deconjugation and steatorrhoea in patients with the stagnant loop syndrome. Lancet. 1968;2:12.
103. Kim YS, Spritz N, Blum M, et al. The role of altered bile acid metabolism in the steatorrhea of experimental blind-loop syndrome. J Clin Invest. 1966;45:956.
104. Schonsby H. Osteomalacia in the stagnant loop syndrome. Acta Med Scand Suppl. 1977;603:39.
105. Brin MF, Fetell MR, Green PHA, et al. Blind loop syndrome, vitamin E malabsorption and spinocerebellar degeneration. Neurology. 1985;35:338.
106. Riepe SP, Goldstein J, Alpers DH. Effect of secreted Bacteroides proteases on human intestinal brush border hydrolases. J Clin Invest. 1980;66:314.
107. Sherman P, Wesley A, Forstner G. Sequential disaccharidase loss in rat intestinal blind loops: Impact of malnutrition. Am J Physiol. 1985;248:G626.
108. Giannella RA, Rout WR, Toskes PP. Jejunal brush border injury and impaired sugar and amino acid uptake in the blind loop syndrome. Gastroenterology. 1974;67:95.
109. Jonas A, Krishnan C, Forstner G. Pathogenesis of mucosal injury in the blind loop syndrome: Release of disaccharidases from brush border membrane extracts of bacteria obtained from intestinal blind loops in rats. Gastroenterology. 1978;75:791.
110. Jones EA, Craigie A, Tavill AS, et al. Protein metabolism in the intestinal stagnant loop syndrome. Gut. 1968;9:466.
111. Rutgeerts L, Mainguet P, Tytgat G, et al. Enterokinase in contaminated small-bowel syndrome. Digestion. 1974;10:249.
112. King CE, Toskes PP. Protein-losing enteropathy in the human and experimental rat blind-loop syndrome. Gastroenterology. 1981;80:834.
113. Riordan SM, McIver CJ, Wakefield D, et al. Small intestinal mucosal immunity and morphometry in luminal growth of indigenous gut flora. Am J Gastroenterol. 2001;96:494-500.
114. Haboubi NY, Lee GS, Montgomery RD. Duodenal mucosal morphometry of elderly patients with small intestinal bacterial overgrowth: Response to antibiotic treatment. Age Ageing. 1991;20:29.
115. Toskes PP, Giannella RA, Jervis HR, et al. Small intestinal mucosal injury in the experimental blind loop syndrome. Light- and electron-microscopic and histochemical studies. Gastroenterology. 1975;68:193.
116. Di Stefano M, Veneto G, Malservisi S, Corazza GR. Small intestinal bacterial overgrowth and metabolic bone disease. Dig Dis Sci. 2001;46:1077.
117. Stotzer PO, Johansson C, Mellstrom D, et al. Bone mineral density in patients with small intestinal bacterial overgrowth. Hepatogastroenterology. 2003;50:1415.
118. Kumar A, Forsmark C, Toskes P. Small bowel bacterial overgrowth, the changing face of an old disease. Gastroenterology. 1996;110:A340.
119. Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503.
120. Vanner S. The lactulose breath test for diagnosing SIBO in IBS patients: Another nail in the coffin. Am J Gastroenterol. 2008;103:964-65.
121. Walters B, Vanner SJ. Detection of bacterial overgrowth in IBS using the lactulose H2 breath test: Comparison with 14C-d-xylose and healthy controls. Am J Gastroenterol. 2005;100:1566.
122. Bratten J, Spanier J, Jones MP. Lactulose hydrogen breath testing does not discriminate patients with irritable bowel syndrome from healthy controls. Am J Gastroenterol. 2008;103:958-63.
123. Posserud M, Stotzer PO, Björnsson E, et al. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802.
124. Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. A double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412.
125. Pimentel M, Park S, Mirocha J, et al. The effect of a non-absorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: A randomized trial. Ann Intern Med. 2006;145:557-63.
126. Sharara AI, Aoun E, Abdul-Baki H, et al. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101:326-33.
127. Farrell GC. Is bacterial ash the flash that ignites NASH? Gut. 2001;48:148.
128. Lichtman SN, Keku J, Clark RL, et al. Biliary tract disease in rats with experimental small bowel bacterial overgrowth. Hepatology. 1991;13:766.
129. Lichtman SN, Keku J, Schwab JH, et al. Hepatic injury associated with small bowel bacterial overgrowth in rats is prevented by metronidazole and tetracycline. Gastroenterology. 1991;100:513.
130. Wigg AJ, Roberts-Thomson IC, Dymock RB. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206-11.
131. Simco V. Fecal fat microscopy. Acceptable predictive value in screening for steatorrhea. Am J Gastroenterol. 1981;75:204.
132. Fine KD, Ogunji F. A new method of quantitative fecal fat microscopy and its correlation with chemically measured fecal fat output. Am J Clin Pathol. 2000;113:528.
133. Corazza GR, Menozzi MG, Strocchi A, et al. The diagnosis of small bowel bacterial overgrowth. Reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology. 1990;98:302.
134. Hamilton I, Worsley BW, Cobden I, et al. Simultaneous culture of saliva and jejunal aspirate in the investigation of small bowel bacterial overgrowth. Gut. 1982;23:847.
135. Bardhan PK, Gyr K, Beglinger C, et al. Diagnosis of bacterial overgrowth after culturing proximal small bowel aspirate obtained during routine upper gastrointestinal endoscopy. Scand J Gastroenterol. 1992;27:253.
136. Leon-Barua R, Gilman RH, Rodriguez C, et al. Comparison of three methods to obtain upper small bowel contents for culture. Am J Gastroenterol. 1993;88:925.
137. Riordan SM, McIver CJ, Duncombe VM, et al. Bacteriologic analysis of mucosal biopsy specimens for detecting small-intestinal bacterial overgrowth. Scand J Gastroenterol. 1995;30:681.
138. Chernov AJ, Doe WF, Gompertz D. Intrajejunal volatile fatty acids in the stagnant loop syndrome. Gut. 1972;13:103.
139. Ferguson J, Walker K, Thomson AB. Limitations in the use of 14C-glycocholate breath and stool bile acid determinations in patients with chronic diarrhea. J Clin Gastroenterol. 1986;8:258.
140. Perman JA, Modler S, Olson AC. Role of pH in production of hydrogen from carbohydrates by colonic bacterial flora. J Clin Invest. 1981;67:643.
141. Bond JH, Levitt MD. Use of pulmonary hydrogen [H2] measurements to quantitate carbohydrate absorption. Study of partially gastrectomized patients. J Clin Invest. 1972;51:1219.
142. Corazza GR, Strocchi A, Gasbarrini G. Fasting breath hydrogen in celiac disease. Gastroenterology. 1987;93:53.
143. Thompson DG, Binfoeld P, DeBelder A, et al. Extraintestnal influences on exhaled breath hydrogen measurements during the investigation of gastrointestinal disease. Gut. 1985;26:1349.
144. Kerlin P, Wong L. Breath hydrogen testing in bacterial overgrowth of the small intestine. Gastroenterology. 1988;95:982.
145. MacMahon M, Gibbons N, Mullins E, et al. Are breath hydrogen tests valid in the elderly? Gerontology. 1996;42:40.
146. Bauer TM, Schwacha H, Steinbruckner B, et al. Diagnosis of small intestinal bacterial overgrowth in patients with cirrhosis of the liver: Poor performance of the glucose breath hydrogen test. J Hepatol. 2000;33:382.
147. Stotzer PO, Kilander AF. Comparison of the 1-gram 14C-d-xylose breath test and the 50 gram hydrogen glucose breath test for diagnosis of small intestinal bacterial overgrowth. Digestion. 2000;61:165.
148. Ghosal UC, Ghosal U, Misra A. Utility of hydrogen breath tests in diagnosis of small intestinal bacterial overgrowth in malabsorption syndrome and its relationship with oro-cecal transit time. Indian J Gastroenterol.. 2006;25:6.
149. Rhodes JM, Middleton P, Jewell DP. The lactulose hydrogen breath test as a diagnostic test for small bowel bacterial overgrowth. Scand J Gastroenterol. 1979;14:333.
150. Riordan SM, McIver CJ, Walker BM, et al. The lactulose breath hydrogen test and small intestinal bacterial overgrowth. Am J Gastroenterol. 1996;91:1795.
151. Dellert SF, Nowicki MJ, Farrell MK, et al. The 13C-xylose breath test for the diagnosis of small bowel bacterial overgrowth in children. J Pediatr Gastroenterol Nutr. 1997;25:153.
152. King CE, Toskes PP, Guilarte TR, et al. Comparison of the one-gram d-[14C]xylose breath test to the [14C]bile acid breath test in patients with small-intestine bacterial overgrowth. Dig Dis Sci. 1980;25:53.
153. Rumessen JJ, Gudmand-Hoyer E, Bachmann E, et al. Diagnosis of bacterial overgrowth of the small intestine. Comparison of the 14C-d-xylose breath test and jejunal cultures in 60 patients. Scand J Gastroenterol. 1985;20:1267.
154. King CE, Toskes PP. Comparison of the 1-gram [14C]xylose, 10-gram lactulose-H2, and 80-gram glucose-H2 breath tests in patients with small intestinal bacterial overgrowth. Gastroenterology. 1986;91:1447.
155. Valdovinos MA, Camilleri M, Thomforde GM, et al. Reduced accuracy of 14C-d-xylose breath test for detecting bacterial overgrowth in gastrointestinal motility disorders. Scand J Gastroenterol. 1993;28:963.
156. Chang CS, Chen GH, Kao CH, et al. Increased accuracy of the carbon-14 d-xylose breath test in detecting small-intestinal bacterial overgrowth by correction with the gastric emptying rate. Eur J Nucl Med. 1995;22:1118.
157. Lewis SJ, Young G, Mann M, et al. Improvement in specificity of [14C]d-xylose breath test for bacterial overgrowth. Dig Dis Sci. 1997;42:1587.
158. Bardhan PK, Feger A, Kogon M, et al. Urinary cholyl-PABA excretion in diagnosing small intestinal bacterial overgrowth: Evaluation of a new non-invasive method. Dig Dis Sci. 2000;45:474.
159. Toskes P. Bacterial overgrowth of the gastrointestinal tract. Adv Intern Med. 1993;38:387.
160. Setchell KD, Harrison DL, Gilbert JM, et al. Serum unconjugated bile acids: Qualitative and quantitative profiles in ileal resection and bacterial overgrowth. Clin Chem Acta. 1985;152:297.
161. Attar A, Flourie B, Rambaud JC, et al. Antibiotic efficacy in small intestinal bacterial overgrowth-related chronic diarrhea: A crossover, randomised trial. Gastroenterology. 1999;117:794-7.
162. Di Stefano M, Malservisi S, Veneto G, et al. Rifaximin versus chlortetracycline in the short-term treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2000;14:551.
163. Castiglione F, Rispo A, Di Girolamo E, et al. Antibiotic treatment of small bowel bacterial overgrowth in patients with Crohn’s disease. Aliment Pharmacol Ther. 2003;18:1107.
164. Scarpellini E, Gabrielli M, Lauritano CE, et al. High dose rifaximin for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2007;25:781-6.
165. Di Sefano M, Miceli E, Missanelli M, et al. Absorbable vs non-absorbable antibiotics in the treatment of small intestine bacterial overgrowth in patients with blind-loop syndrome. Aliment Pharmacol Ther. 2005;21:985.
166. Lauritano EC, Gabrielli M, Scarpellini E, et al. Small intestinal bacterial overgrowth recurrence after antibiotic therapy. Am J Gastroenterol. 2008;103:2031-5.
167. Witt K, Pedersen NT. The long-acting somatostatin analogue SMS 201-995 causes malabsorption. Scand J Gastroenterol. 1989;24:1248.
168. Pardo A, Bartoli R, Lorenzo-Zuniga V, et al. Effect of cisapride on intestinal bacterial overgrowth and bacterial translocation in cirrhosis. Hepatology. 2000;31:858.
169. Stotzer PO, Blomberg L, Conway PL, et al. Probiotic treatment of small intestinal bacterial overgrowth by Lactobacillus fermentum KLD. Scand J Infect Dis. 1996;28:615.
170. Vanderhoof JA, Young RJ, Murray N, et al. Treatment strategies for small bowel bacterial overgrowth in short bowel syndrome. J Pediatr Gastroenterol Nutr. 1998;27:155.