PART 6: Nutrition and Weight Loss
95e |
Nutrient Requirements and Dietary Assessment |
Nutrients are substances that are not synthesized in sufficient amounts in the body and therefore must be supplied by the diet. Nutrient requirements for groups of healthy persons have been determined experimentally. The absence of essential nutrients leads to growth impairment, organ dysfunction, and failure to maintain nitrogen balance or adequate status of other nutrients. For good health, we require energy-providing nutrients (protein, fat, and carbohydrate), vitamins, minerals, and water. Requirements for organic nutrients include 9 essential amino acids, several fatty acids, glucose, 4 fat-soluble vitamins, 10 water-soluble vitamins, dietary fiber, and choline. Several inorganic substances, including 4 minerals, 7 trace minerals, 3 electrolytes, and the ultratrace elements, must also be supplied by diet.
The amounts of the essential nutrients that are required by individuals differ by age and physiologic state. Conditionally essential nutrients are not required in the diet but must be supplied to individuals who do not synthesize them in adequate amounts, such as those with genetic defects, those with pathologic conditions such as infection or trauma with nutritional implications, and developmentally immature infants. For example, inositol, taurine, arginine, and glutamine may be needed by premature infants. Many other organic and inorganic compounds that are present in foods, such as pesticides and lead, also have health effects.
ESSENTIAL NUTRIENT REQUIREMENTS
Energy For weight to remain stable, energy intake must match energy output. The major components of energy output are resting energy expenditure (REE) and physical activity; minor components include the energy cost of metabolizing food (thermic effect of food, or specific dynamic action) and shivering thermogenesis (e.g., cold-induced thermogenesis). The average energy intake is ~2600 kcal/d for American men and ~1800 kcal/d for American women, though these estimates vary with body size and activity level. Formulas for roughly estimating REE are useful in assessing the energy needs of an individual whose weight is stable. Thus, for males, REE = 900 + 10m, and for females, REE = 700 + 7m, where is m mass in kilograms. The calculated REE is then adjusted for physical activity level by multiplying by 1.2 for sedentary, 1.4 for moderately active, or 1.8 for very active individuals. The final figure, the estimated energy requirement (EER), provides an approximation of total caloric needs in a state of energy balance for a person of a certain age, sex, weight, height, and physical activity level. For further discussion of energy balance in health and disease, see Chap. 97.
Protein Dietary protein consists of both essential and nonessential amino acids that are required for protein synthesis. The nine essential amino acids are histidine, isoleucine, leucine, lysine, methionine/cystine, phenylalanine/tyrosine, threonine, tryptophan, and valine. Certain amino acids, such as alanine, can also be used for energy and gluconeogenesis. When energy intake is inadequate, protein intake must be increased, because ingested amino acids are diverted into pathways of glucose synthesis and oxidation. In extreme energy deprivation, protein-calorie malnutrition may ensue (Chap. 97).
For adults, the recommended dietary allowance (RDA) for protein is ~0.6 g/kg desirable body mass per day, assuming that energy needs are met and that the protein is of relatively high biologic value. Current recommendations for a healthy diet call for at least 10–14% of calories from protein. Most American diets provide at least those amounts. Biologic value tends to be highest for animal proteins, followed by proteins from legumes (beans), cereals (rice, wheat, corn), and roots. Combinations of plant proteins that complement one another in biologic value or combinations of animal and plant proteins can increase biologic value and lower total protein requirements. In healthy people with adequate diets, the timing of protein intake over the course of the day has little effect.
Protein needs increase during growth, pregnancy, lactation, and rehabilitation after injury or malnutrition. Tolerance to dietary protein is decreased in renal insufficiency (with consequent uremia) and in liver failure. Normal protein intake can precipitate encephalopathy in patients with cirrhosis of the liver.
Fat and Carbohydrate Fats are a concentrated source of energy and constitute, on average, 34% of calories in U.S. diets. However, for optimal health, fat intake should total no more than 30% of calories. Saturated fat and trans fat should be limited to <10% of calories and polyunsaturated fats to <10% of calories, with monounsaturated fats accounting for the remainder of fat intake. At least 45–55% of total calories should be derived from carbohydrates. The brain requires ~100 g of glucose per day for fuel; other tissues use about 50 g/d. Some tissues (e.g., brain and red blood cells) rely on glucose supplied either exogenously or from muscle proteolysis. Over time, adaptations in carbohydrate needs are possible during hypocaloric states. Like fat (9 kcal/g), carbohydrate (4 kcal/g), and protein (4 kcal/g), alcohol (ethanol) provides energy (7 kcal/g). However, it is not a nutrient.
Water For adults, 1–1.5 mL of water per kilocalorie of energy expenditure is sufficient under usual conditions to allow for normal variations in physical activity, sweating, and solute load of the diet. Water losses include 50–100 mL/d in the feces; 500–1000 mL/d by evaporation or exhalation; and, depending on the renal solute load, ≥1000 mL/d in the urine. If external losses increase, intakes must increase accordingly to avoid underhydration. Fever increases water losses by ~200 mL/d per °C; diarrheal losses vary but may be as great as 5 L/d in severe diarrhea. Heavy sweating, vigorous exercise, and vomiting also increase water losses. When renal function is normal and solute intakes are adequate, the kidneys can adjust to increased water intake by excreting up to 18 L of excess water per day (Chap. 404). However, obligatory urine outputs can compromise hydration status when there is inadequate water intake or when losses increase in disease or kidney damage.
Infants have high requirements for water because of their large ratio of surface area to volume, their inability to communicate their thirst, and the limited capacity of the immature kidney to handle high renal solute loads. Increased water needs during pregnancy are ~30 mL/d. During lactation, milk production increases daily water requirements so that ~1000 mL of additional water is needed, or 1 mL for each milliliter of milk produced. Special attention must be paid to the water needs of the elderly, who have reduced total body water and blunted thirst sensation and are more likely to be taking medications such as diuretics.
Other Nutrients See Chap. 96e for detailed descriptions of vitamins and trace minerals.
DIETARY REFERENCE INTAKES AND RECOMMENDED DIETARY ALLOWANCES
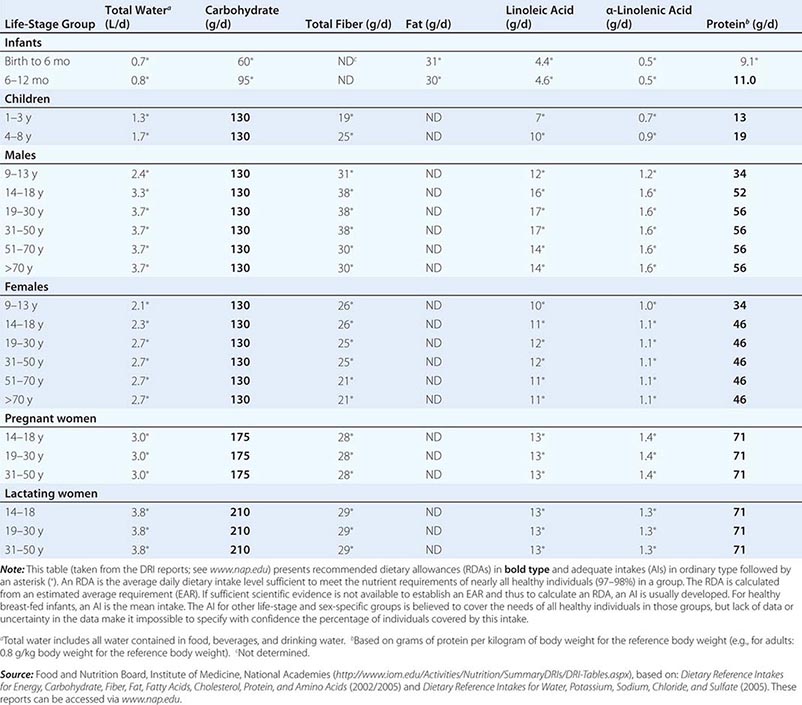
Fortunately, human life and well-being can be maintained within a fairly wide range for most nutrients. However, the capacity for adaptation is not infinite—too much, as well as too little, intake of a nutrient can have adverse effects or alter the health benefits conferred by another nutrient. Therefore, benchmark recommendations regarding nutrient intakes have been developed to guide clinical practice. These quantitative estimates of nutrient intakes are collectively referred to as the dietary reference intakes (DRIs). The DRIs have supplanted the RDAs—the single reference values used in the United States until the early 1990s. DRIs include the estimated average requirement (EAR) for nutrients as well as other reference values used for dietary planning for individuals: the RDA, the adequate intake (AI), and the tolerable upper level (UL). The DRIs also include acceptable macronutrient distribution ranges (AMDRs) for protein, fat, and carbohydrate. The current DRIs for vitamins and elements are provided in Tables 95e-1 and 95e-2, respectively. Table 95e-3 provides DRIs for water and macronutrients. EERs are discussed in Chap. 97 on energy balance in health and disease.
|
DIETARY REFERENCE INTAKES (DRIs): RECOMMENDED DIETARY ALLOWANCES AND ADEQUATE INTAKES FOR VITAMINS |

|
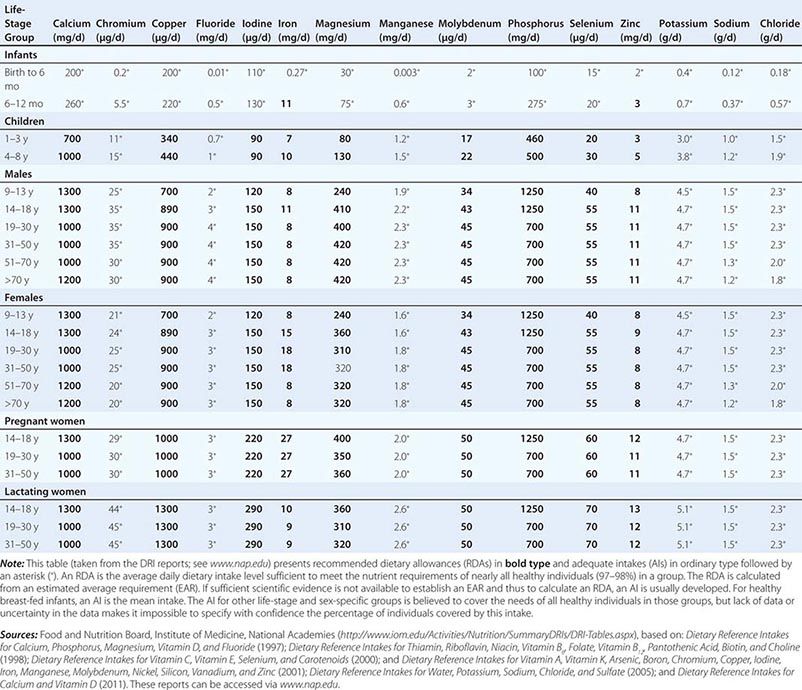
DIETARY REFERENCE INTAKES (DRIs): RECOMMENDED DIETARY ALLOWANCES AND ADEQUATE INTAKES FOR ELEMENTS |
|
DIETARY REFERENCE INTAKES (DRIs): RECOMMENDED DIETARY ALLOWANCES AND ADEQUATE INTAKES FOR TOTAL WATER AND MACRONUTRIENTS |
Estimated Average Requirement When florid manifestations of the classic dietary-deficiency diseases such as rickets (deficiency of vitamin D and calcium), scurvy (deficiency of vitamin C), xerophthalmia (deficiency of vitamin A), and protein-calorie malnutrition were common, nutrient adequacy was inferred from the absence of their clinical signs. Later, biochemical and other changes were found to be evident long before the deficiency became clinically apparent. Consequently, criteria of adequacy are now based on biologic markers when they are available. Priority is given to sensitive biochemical, physiologic, or behavioral tests that reflect early changes in regulatory processes; maintenance of body stores of nutrients; or, if available, the amount of a nutrient that minimizes the risk of chronic degenerative disease. Current efforts focus on this last variable, but relevant markers often are not available.
The EAR is the amount of a nutrient estimated to be adequate for half of the healthy individuals of a specific age and sex. The types of evidence and criteria used to establish nutrient requirements vary by nutrient, age, and physiologic group. The EAR is not an effective estimate of nutrient adequacy in individuals because it is a median requirement for a group; 50% of individuals in a group fall below the requirement and 50% fall above it. Thus, a person with a usual intake at the EAR has a 50% risk of inadequate intake. For these reasons, other standards, described below, are more useful for clinical purposes.
Recommended Dietary Allowances The RDA is the average daily dietary intake level that meets the nutrient requirements of nearly all healthy persons of a specific sex, age, life stage, or physiologic condition (e.g., pregnancy or lactation). The RDA, which is the nutrient-intake goal for planning diets of individuals, is defined statistically as two standard deviations above the EAR to ensure that the needs of any given individual are met. The online tool at http://fnic.nal.usda.gov/interactiveDRI/ allows health professionals to calculate individualized daily nutrient recommendations for dietary planning based on the DRIs for persons of a given age, sex, and weight. The RDAs are used to formulate food guides such as the U.S. Department of Agriculture (USDA) MyPlate Food Guide for individuals (www.supertracker.usda.gov/default.aspx), to create food-exchange lists for therapeutic diet planning, and as a standard for describing the nutritional content of foods and nutrient-containing dietary supplements.
The risk of dietary inadequacy increases as intake falls below the RDA. However, the RDA is an overly generous criterion for evaluating nutrient adequacy. For example, by definition, the RDA exceeds the actual requirements of all but ~2–3% of the population. Therefore, many people whose intake falls below the RDA may still be getting enough of the nutrient. On food labels, the nutrient content in a food is stated by weight or as a percent of the daily value (DV), a variant of the RDA used on the nutrition facts panel that, for an adult, represents the highest RDA for an adult consuming 2000 kcal.
Adequate Intake It is not possible to set an RDA for some nutrients that do not have an established EAR. In this circumstance, the AI is based on observed or experimentally determined approximations of nutrient intakes in healthy people. In the DRIs, AIs rather than RDAs are proposed for nutrients consumed by infants (up to age 1 year) as well as for chromium, fluoride, manganese, sodium, potassium, pantothenic acid, biotin, choline, and water consumed by persons of all ages. Vitamin D and calcium recommendations were recently revised, and more precise estimates are now available.
Tolerable Upper Levels of Nutrient Intake Healthy individuals derive no established benefit from consuming nutrient levels above the RDA or AI. In fact, excessive nutrient intake can disturb body functions and cause acute, progressive, or permanent disabilities. The tolerable UL is the highest level of chronic nutrient intake (usually daily) that is unlikely to pose a risk of adverse health effects for most of the population. Data on the adverse effects of large amounts of many nutrients are unavailable or too limited to establish a UL. Therefore, the lack of a UL does not mean that the risk of adverse effects from high intake is nonexistent. Nutrients in commonly eaten foods rarely exceed the UL. However, highly fortified foods and dietary supplements provide more concentrated amounts of nutrients per serving and thus pose a potential risk of toxicity. Nutrient supplements are labeled with supplement facts that express the amount of nutrient in absolute units or as the percentage of the DV provided per recommended serving size. Total nutrient consumption, including that in foods, supplements, and over-the-counter medications (e.g., antacids), should not exceed RDA levels.
Acceptable Macronutrient Distribution Ranges The AMDRs are not experimentally determined but are rough ranges for energy-providing macronutrient intakes (protein, carbohydrate, and fat) that the Institute of Medicine’s Food and Nutrition Board considers to be healthful. These ranges are 10–35% of calories for protein, 20–35% of calories for fat, and 45–65% of calories for carbohydrate. Alcohol, which also provides energy, is not a nutrient; therefore, no recommendations are not provided.
FACTORS ALTERING NUTRIENT NEEDS
The DRIs are affected by age, sex, rate of growth, pregnancy, lactation, physical activity level, concomitant diseases, drugs, and dietary composition. If requirements for nutrient sufficiency are close to levels indicating excess of a nutrient, dietary planning is difficult.
Physiologic Factors Growth, strenuous physical activity, pregnancy, and lactation all increase needs for energy and several essential nutrients. Energy needs rise during pregnancy due to the demands of fetal growth and during lactation because of the increased energy required for milk production. Energy needs decrease with loss of lean body mass, the major determinant of REE. Because lean tissue, physical activity, and health often decline with age, energy needs of older persons, especially those over 70, tend to be lower than those of younger persons.
Dietary Composition Dietary composition affects the biologic availability and use of nutrients. For example, the absorption of iron may be impaired by large amounts of calcium or lead; likewise, non-heme iron uptake may be impaired by a lack of ascorbic acid and amino acids in the meal. Protein use by the body may be decreased when essential amino acids are not present in sufficient amounts—a rare scenario in U.S. diets. Animal foods, such as milk, eggs, and meat, have high biologic values, with most of the needed amino acids present in adequate amounts. Plant proteins in corn (maize), soy, rice, and wheat have lower biologic values and must be combined with other plant or animal proteins or fortified with the amino acids that are deficient to achieve optimal use by the body.
Route of Intake The RDAs apply only to oral intakes. When nutrients are administered parenterally, similar values can sometimes be used for amino acids, glucose (carbohydrate), fats, sodium, chloride, potassium, and most vitamins because their intestinal absorption rate is nearly 100%. However, the oral bioavailability of most mineral elements may be only half that obtained by parenteral administration. For some nutrients that are not readily stored in the body or that cannot be stored in large amounts, timing of administration may also be important. For example, amino acids cannot be used for protein synthesis if they are not supplied together; instead, they will be used for energy production, although in healthy individuals eating adequate diets, the distribution of protein intake over the course of the day has little effect on health.
Disease Dietary deficiency diseases include protein-calorie malnutrition, iron-deficiency anemia, goiter (due to iodine deficiency), rickets and osteomalacia (vitamin D deficiency), and xeropthalmia (vitamin A deficiency), megaloblastic anemia (vitamin B12 or folic acid deficiency), scurvy (vitamin C/ascorbic acid deficiency), beriberi (thiamin deficiency), and pellagra (niacin and tryptophan deficiency) (Chaps. 96e and 97). Each deficiency disease is characterized by imbalances at the cellular level between the supply of nutrients or energy and the body’s nutritional needs for growth, maintenance, and other functions. Imbalances and excesses in nutrient intakes are recognized as risk factors for certain chronic degenerative diseases, such as saturated fat and cholesterol in coronary artery disease; sodium in hypertension; obesity in hormone-dependent endometrial and breast cancers; and ethanol in alcoholism. Because the etiology and pathogenesis of these disorders are multifactorial, diet is only one of many risk factors. Osteoporosis, for example, is associated with calcium deficiency, sometimes secondary to vitamin D deficiency, as well as with risk factors related to environment (e.g., smoking, sedentary lifestyle), physiology (e.g., estrogen deficiency), genetic determinants (e.g., defects in collagen metabolism), and drug use (chronic steroid and aromatase inhibitors) (Chap. 425).
DIETARY ASSESSMENT
In clinical situations, nutritional assessment is an iterative process that involves: (1) screening for malnutrition, (2) assessing the diet and other data to establish either the absence or the presence of malnutrition and its possible causes, (3) planning and implementing the most appropriate nutritional therapy, and (4) reassessing intakes to make sure that they have been consumed. Some disease states affect the bioavailability, requirements, use, or excretion of specific nutrients. In these circumstances, specific measurements of various nutrients or their biomarkers may be required to ensure adequate replacement (Chap. 96e).
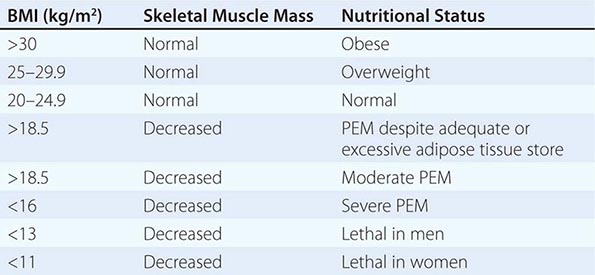
Most health care facilities have nutrition-screening processes in place for identifying possible malnutrition after hospital admission. Nutritional screening is required by the Joint Commission, which accredits and certifies health care organizations in the United States. However, there are no universally recognized or validated standards. The factors that are usually assessed include abnormal weight for height or body mass index (e.g., BMI <19 or >25); reported weight change (involuntary loss or gain of >5 kg in the past 6 months) (Chap. 56); diagnoses with known nutritional implications (e.g., metabolic disease, any disease affecting the gastrointestinal tract, alcoholism); present therapeutic dietary prescription; chronic poor appetite; presence of chewing and swallowing problems or major food intolerances; need for assistance with preparing or shopping for food, eating, or other aspects of self-care; and social isolation. The nutritional status of hospitalized patients should be reassessed periodically—at least once every week.
A more complete dietary assessment is indicated for patients who exhibit a high risk of or frank malnutrition on nutritional screening. The type of assessment varies with the clinical setting, the severity of the patient’s illness, and the stability of the patient’s condition.
Acute-Care Settings In acute-care settings, anorexia, various other diseases, test procedures, and medications can compromise dietary intake. Under such circumstances, the goal is to identify and avoid inadequate intake and to assure appropriate alimentation. Dietary assessment focuses on what patients are currently eating, whether or not they are able and willing to eat, and whether or not they experience any problems with eating. Dietary intake assessment is based on information from observed intakes; medical records; history; clinical examination; and anthropometric, biochemical, and functional status evaluations. The objective is to gather enough information to establish the likelihood of malnutrition due to poor dietary intake or other causes in order to assess whether nutritional therapy is indicated (Chap. 98e).
Simple observations may suffice to suggest inadequate oral intake. These include dietitians’ and nurses’ notes; observation of a patient’s frequent refusal to eat or the amount of food eaten on trays; the frequent performance of tests and procedures that are likely to cause meals to be skipped; adherence to nutritionally inadequate diet orders (e.g., clear liquids or full liquids) for more than a few days; the occurrence of fever, gastrointestinal distress, vomiting, diarrhea, or a comatose state; and the presence of diseases or use of treatments that involve any part of the alimentary tract. Acutely ill patients with diet-related diseases such as diabetes need assessment because an inappropriate diet may exacerbate these conditions and adversely affect other therapies. Abnormal biochemical values (serum albumin levels <35 g/L [<3.5 mg/dL]; serum cholesterol levels <3.9 mmol/L [<150 mg/dL]) are nonspecific but may indicate a need for further nutritional assessment.
Most therapeutic diets offered in hospitals are calculated to meet individual nutrient requirements and the RDA if they are eaten. Exceptions include clear liquids, some full-liquid diets, and test diets (such as those adhered to in preparation for gastrointestinal procedures), which are inadequate for several nutrients and should not be used, if possible, for more than 24 h. However, because as much as half of the food served to hospitalized patients is not eaten, it cannot be assumed that the intakes of hospitalized patients are adequate. Dietary assessment should compare how much and what kinds of food the patient has consumed with the diet that has been provided. Major deviations in intakes of energy, protein, fluids, or other nutrients of special concern for the patient’s illness should be noted and corrected.
Nutritional monitoring is especially important for patients who are very ill and who have extended lengths of hospital stay. Patients who are fed by enteral and parenteral routes also require special nutritional assessment and monitoring by physicians and/or dietitians with certification in nutritional support (Chap. 98e).
Ambulatory Settings The aim of dietary assessment in the outpatient setting is to determine whether or not the patient’s usual diet is a health risk in itself or if it contributes to existing chronic disease-related problems. Dietary assessment also provides the basis for planning a diet that fulfills therapeutic goals while ensuring patient adherence. The outpatient dietary assessment should review the adequacy of present and usual food intakes, including vitamin and mineral supplements, oral nutritional supplements, medical foods, other dietary supplements, medications, and alcohol, because all of these may affect the patient’s nutritional status. The assessment should focus on the dietary constituents that are most likely to be involved or compromised by a specific diagnosis as well as on any comorbidities that are present. More than one day’s intake should be reviewed to provide a better representation of the usual diet.
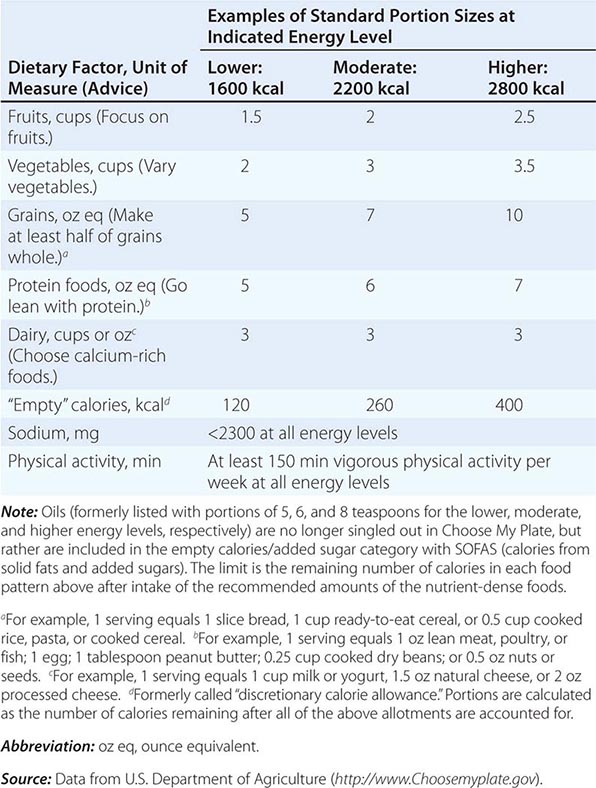
There are many ways to assess the adequacy of a patient’s habitual diet. These include use of a food guide, a food-exchange list, a diet history, or a food-frequency questionnaire. A commonly used food guide for healthy persons is the USDA’s Choose My Plate, which is useful as a rough guide for avoiding inadequate intakes of essential nutrients as well as likely excesses in the amounts of fat (especially saturated and trans fats), sodium, sugar, and alcohol consumed (Table 95e-4). The Choose My Plate graphic emphasizes a balance between calories and nutritional needs, encouraging increased intake of fruits and vegetables, whole grains, and low-fat milk in conjunction with reduced intake of sodium and high-calorie sugary drinks. The Web version of the guide provides a calculator that tailors the number of servings suggested for healthy patients of different weights, sexes, ages, and life-cycle stages to help them to meet their needs while avoiding excess (http://www.supertracker.usda.gov/default.aspx and www.ChooseMyPlate.gov). Patients who follow ethnic or unusual dietary patterns may need extra instruction on how foods should be categorized and on the appropriate portion sizes that constitute a serving. The process of reviewing the guide with patients helps them transition to healthier dietary patterns and identifies food groups eaten in excess of recommendations or in insufficient quantities. For persons on therapeutic diets, assessment against food-exchange lists may be useful. These include, for example, American Diabetes Association food-exchange lists for diabetes and the Academy of Nutrition and Dietetics food-exchange lists for renal disease.
|
CHOOSE MY PLATE: A GUIDE TO INDIVIDUALIZED DIETARY PLANNING |
NUTRITIONAL STATUS ASSESSMENT
Full nutritional status assessment is reserved for seriously ill patients and those at very high nutritional risk when the cause of malnutrition is still uncertain after the initial clinical evaluation and dietary assessment. It involves multiple dimensions, including documentation of dietary intake, anthropometric measurements, biochemical measurements of blood and urine, clinical examination, health history elicitation, and functional status evaluation. Therapeutic dietary prescriptions and menu plans for most diseases are available from most hospitals and from the Academy of Nutrition and Dietetics. For further discussion of nutritional assessment, see Chap. 97.
GLOBAL CONSIDERATIONS
![]() The DRIs (e.g., the EAR, the UL, and energy needs) are estimates of physiologic requirements based on experimental evidence. Assuming that appropriate adjustments are made for age, sex, body size, and physical activity level, these estimates should be applicable to individuals in most parts of the world. However, the AIs are based on customary and adequate intakes in U.S. and Canadian populations, which appear to be compatible with good health, rather than on a large body of direct experimental evidence. Similarly, the AMDRs represent expert opinion regarding the approximate intakes of energy-providing nutrients that are healthful in these North American populations. Thus these measures should be used with caution in other settings. Nutrient-based standards like the DRIs have also been developed by the World Health Organization/Food and Agricultural Organization of the United Nations and are available on the Web (http://www.who.int/nutrition/topics/nutrecomm/en/index.html). The European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies periodically publishes its recommendations in the EFSA Journal. Other countries have promulgated similar recommendations. The different standards have many similarities in their basic concepts, definitions, and nutrient recommendation levels, but there are some differences from the DRIs as a result of the functional criteria chosen, environmental differences, the timeliness of the evidence reviewed, and expert judgment.
The DRIs (e.g., the EAR, the UL, and energy needs) are estimates of physiologic requirements based on experimental evidence. Assuming that appropriate adjustments are made for age, sex, body size, and physical activity level, these estimates should be applicable to individuals in most parts of the world. However, the AIs are based on customary and adequate intakes in U.S. and Canadian populations, which appear to be compatible with good health, rather than on a large body of direct experimental evidence. Similarly, the AMDRs represent expert opinion regarding the approximate intakes of energy-providing nutrients that are healthful in these North American populations. Thus these measures should be used with caution in other settings. Nutrient-based standards like the DRIs have also been developed by the World Health Organization/Food and Agricultural Organization of the United Nations and are available on the Web (http://www.who.int/nutrition/topics/nutrecomm/en/index.html). The European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies periodically publishes its recommendations in the EFSA Journal. Other countries have promulgated similar recommendations. The different standards have many similarities in their basic concepts, definitions, and nutrient recommendation levels, but there are some differences from the DRIs as a result of the functional criteria chosen, environmental differences, the timeliness of the evidence reviewed, and expert judgment.
96e |
Vitamin and Trace Mineral Deficiency and Excess |
Vitamins are required constituents of the human diet since they are synthesized inadequately or not at all in the human body. Only small amounts of these substances are needed to carry out essential biochemical reactions (e.g., by acting as coenzymes or prosthetic groups). Overt vitamin or trace mineral deficiencies are rare in Western countries because of a plentiful, varied, and inexpensive food supply; food fortification; and use of supplements. However, multiple nutrient deficiencies may appear together in persons who are chronically ill or alcoholic. After gastric bypass surgery, patients are at high risk for multiple nutrient deficiencies. Moreover, subclinical vitamin and trace mineral deficiencies, as diagnosed by laboratory testing, are quite common in the normal population, especially in the geriatric age group. Conversely, because of the widespread use of nutrient supplements, nutrient toxicities are gaining pathophysiologic and clinical importance.
![]() Victims of famine, emergency-affected and displaced populations, and refugees are at increased risk for protein-energy malnutrition and classic micronutrient deficiencies (vitamin A, iron, iodine) as well as for overt deficiencies in thiamine (beriberi), riboflavin, vitamin C (scurvy), and niacin (pellagra).
Victims of famine, emergency-affected and displaced populations, and refugees are at increased risk for protein-energy malnutrition and classic micronutrient deficiencies (vitamin A, iron, iodine) as well as for overt deficiencies in thiamine (beriberi), riboflavin, vitamin C (scurvy), and niacin (pellagra).
Body stores of vitamins and minerals vary tremendously. For example, stores of vitamin B12 and vitamin A are large, and an adult may not become deficient until ≥1 year after beginning to eat a deficient diet. However, folate and thiamine may become depleted within weeks among those eating a deficient diet. Therapeutic modalities can deplete essential nutrients from the body; for example, hemodialysis removes water-soluble vitamins, which must be replaced by supplementation.
Vitamins and trace minerals play several roles in diseases: (1) Deficiencies of vitamins and minerals may be caused by disease states such as malabsorption. (2) Either deficiency or excess of vitamins and minerals can cause disease in and of itself (e.g., vitamin A intoxication and liver disease). (3) Vitamins and minerals in high doses may be used as drugs (e.g., niacin for hypercholesterolemia). Since they are covered elsewhere, the hematologic-related vitamins and minerals (Chaps. 126 and 128) either are not considered or are considered only briefly in this chapter, as are the bone-related vitamins and minerals (vitamin D, calcium, phosphorus, magnesium; Chap. 423).
VITAMINS
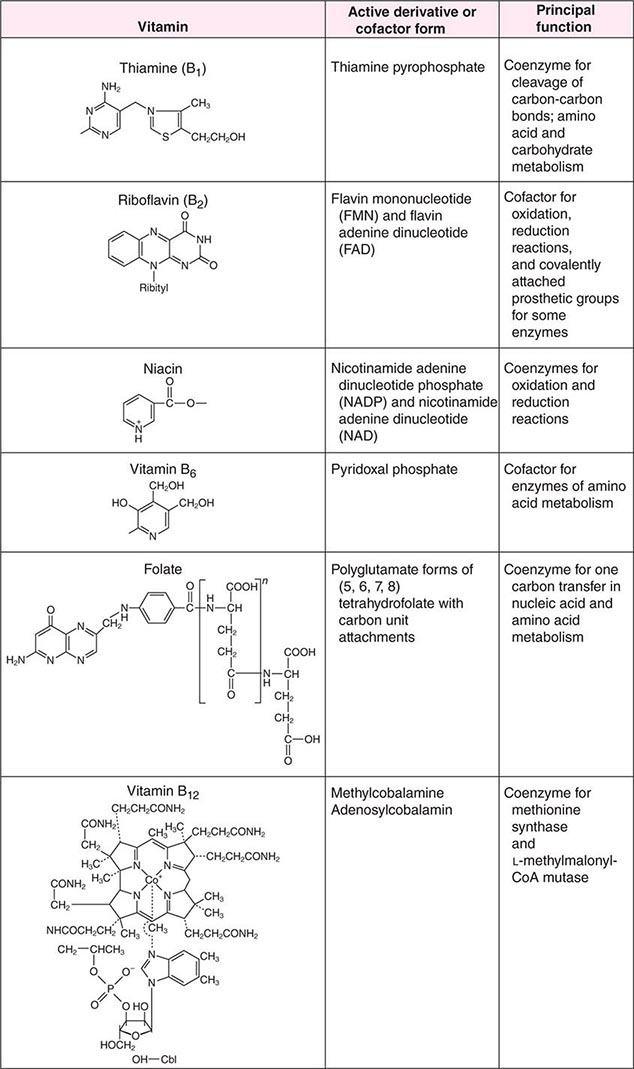
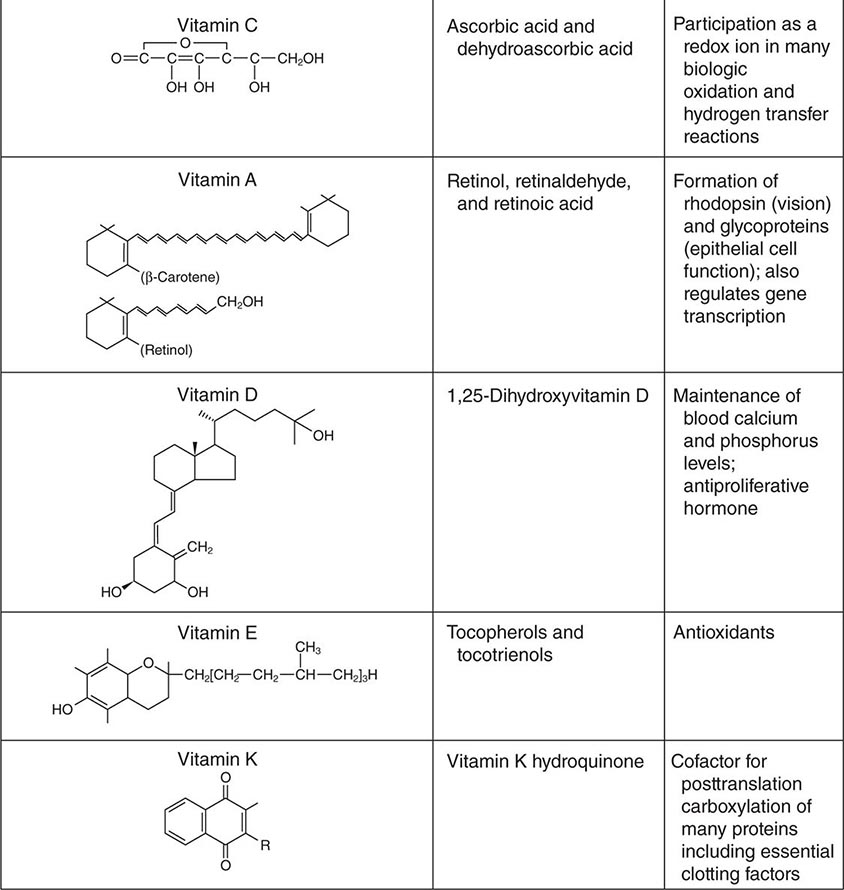
See also Table 96e-1 and Fig. 96e-1.
|
PRINCIPAL CLINICAL FINDINGS OF VITAMIN MALNUTRITION |
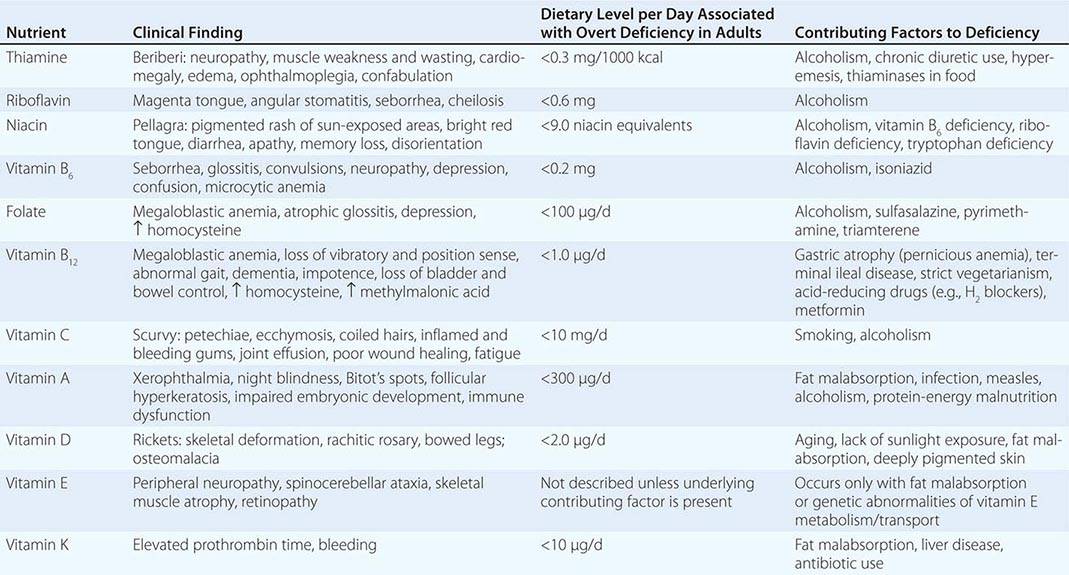
FIGURE 96e-1 Structures and principal functions of vitamins associated with human disorders.
THIAMINE (VITAMIN B1)
Thiamine was the first B vitamin to be identified and therefore is referred to as vitamin B1. Thiamine functions in the decarboxylation of α-ketoacids (e.g., pyruvate α-ketoglutarate) and branched-chain amino acids and thus is essential for energy generation. In addition, thiamine pyrophosphate acts as a coenzyme for a transketolase reaction that mediates the conversion of hexose and pentose phosphates. It has been postulated that thiamine plays a role in peripheral nerve conduction, although the exact chemical reactions underlying this function are not known.
Food Sources The median intake of thiamine in the United States from food alone is 2 mg/d. Primary food sources for thiamine include yeast, organ meat, pork, legumes, beef, whole grains, and nuts. Milled rice and grains contain little thiamine. Thiamine deficiency is therefore more common in cultures that rely heavily on a rice-based diet. Tea, coffee (regular and decaffeinated), raw fish, and shellfish contain thiaminases, which can destroy the vitamin. Thus, drinking large amounts of tea or coffee can theoretically lower thiamine body stores.
Deficiency Most dietary deficiency of thiamine worldwide is the result of poor dietary intake. In Western countries, the primary causes of thiamine deficiency are alcoholism and chronic illnesses such as cancer. Alcohol interferes directly with the absorption of thiamine and with the synthesis of thiamine pyrophosphate, and it increases urinary excretion. Thiamine should always be replenished when a patient with alcoholism is being refed, as carbohydrate repletion without adequate thiamine can precipitate acute thiamine deficiency with lactic acidosis. Other at-risk populations are women with prolonged hyperemesis gravidarum and anorexia, patients with overall poor nutritional status who are receiving parenteral glucose, patients who have had bariatric bypass surgery (bariatric Wernicke), and patients receiving chronic diuretic therapy (e.g., in hypertension or heart failure) due to increased urinary thiamine losses. Maternal thiamine deficiency can lead to infantile beriberi in breast-fed children. Thiamine deficiency could be an underlying factor in motor vehicle accidents and could be overlooked in the setting of head injury.
Thiamine deficiency in its early stage induces anorexia and nonspecific symptoms (e.g., irritability, decrease in short-term memory). Prolonged thiamine deficiency causes beriberi, which is classically categorized as wet or dry although there is considerable overlap between the two categories. In either form of beriberi, patients may complain of pain and paresthesia. Wet beriberi presents primarily with cardiovascular symptoms that are due to impaired myocardial energy metabolism and dysautonomia; it can occur after 3 months of a thiamine-deficient diet. Patients present with an enlarged heart, tachycardia, high-output congestive heart failure, peripheral edema, and peripheral neuritis. Patients with dry beriberi present with a symmetric peripheral neuropathy of the motor and sensory systems, with diminished reflexes. The neuropathy affects the legs most markedly, and patients have difficulty rising from a squatting position.
Alcoholic patients with chronic thiamine deficiency also may have central nervous system (CNS) manifestations known as Wernicke’s encephalopathy, which consists of horizontal nystagmus, ophthalmoplegia (due to weakness of one or more extraocular muscles), cerebellar ataxia, and mental impairment (Chap. 467). When there is an additional loss of memory and a confabulatory psychosis, the syndrome is known as Wernicke-Korsakoff syndrome. Despite the typical clinical picture and history, Wernicke-Korsakoff syndrome is underdiagnosed.
The laboratory diagnosis of thiamine deficiency usually is made by a functional enzymatic assay of transketolase activity measured before and after the addition of thiamine pyrophosphate. A >25% stimulation in response to the addition of thiamine pyrophosphate (i.e., an activity coefficient of 1.25) is interpreted as abnormal. Thiamine or the phosphorylated esters of thiamine in serum or blood also can be measured by high-performance liquid chromatography to detect deficiency.
Toxicity Although anaphylaxis has been reported after high intravenous doses of thiamine, no adverse effects have been recorded from either food or supplements at high doses. Thiamine supplements may be bought over the counter in doses of up to 50 mg/d.
RIBOFLAVIN (VITAMIN B2)
Riboflavin is important for the metabolism of fat, carbohydrate, and protein, acting as a respiratory coenzyme and an electron donor. Enzymes that contain flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN) as prosthetic groups are known as flavoenzymes (e.g., succinic acid dehydrogenase, monoamine oxidase, glutathione reductase). FAD is a cofactor for methyltetrahydrofolate reductase and therefore modulates homocysteine metabolism. The vitamin also plays a role in drug and steroid metabolism, including detoxification reactions.
Although much is known about the chemical and enzymatic reactions of riboflavin, the clinical manifestations of riboflavin deficiency are nonspecific and are similar to those of other deficiencies of B vitamins. Riboflavin deficiency is manifested principally by lesions of the mucocutaneous surfaces of the mouth and skin. In addition, corneal vascularization, anemia, and personality changes have been described with riboflavin deficiency.
Deficiency and Excess Riboflavin deficiency almost always is due to dietary deficiency. Milk, other dairy products, and enriched breads and cereals are the most important dietary sources of riboflavin in the United States, although lean meat, fish, eggs, broccoli, and legumes are also good sources. Riboflavin is extremely sensitive to light, and milk should be stored in containers that protect against photodegradation. Laboratory diagnosis of riboflavin deficiency can be made by determination of red blood cell or urinary riboflavin concentrations or by measurement of erythrocyte glutathione reductase activity, with and without added FAD. Because the capacity of the gastrointestinal tract to absorb riboflavin is limited (~20 mg after one oral dose), riboflavin toxicity has not been described.
NIACIN (VITAMIN B3)
The term niacin refers to nicotinic acid and nicotinamide and their biologically active derivatives. Nicotinic acid and nicotinamide serve as precursors of two coenzymes, nicotinamide adenine dinucleotide (NAD) and NAD phosphate (NADP), which are important in numerous oxidation and reduction reactions in the body. In addition, NAD and NADP are active in adenine diphosphate–ribose transfer reactions involved in DNA repair and calcium mobilization.
Metabolism and Requirements Nicotinic acid and nicotinamide are absorbed well from the stomach and small intestine. The bioavailability of niacin from beans, milk, meat, and eggs is high; bioavailability from cereal grains is lower. Since flour is enriched with “free” niacin (i.e., the non-coenzyme form), bioavailability is excellent. Median intakes of niacin in the United States considerably exceed the recommended dietary allowance (RDA).
The amino acid tryptophan can be converted to niacin with an efficiency of 60:1 by weight. Thus, the RDA for niacin is expressed in niacin equivalents. A lower-level conversion of tryptophan to niacin occurs in vitamin B6 and/or riboflavin deficiencies and in the presence of isoniazid. The urinary excretion products of niacin include 2-pyridone and 2-methyl nicotinamide, measurements of which are used in the diagnosis of niacin deficiency.
![]() Deficiency Niacin deficiency causes pellagra, which is found mostly among people eating corn-based diets in parts of China, Africa, and India. Pellagra in North America is found mainly among alcoholics; among patients with congenital defects of intestinal and kidney absorption of tryptophan (Hartnup disease; Chap. 434e); and among patients with carcinoid syndrome (Chap. 113), in which there is increased conversion of tryptophan to serotonin. The antituberculosis drug isoniazid is a structural analog of niacin and can precipitate pellagra. In the setting of famine or population displacement, pellagra results from the absolute lack of niacin but also from the deficiency of micronutrients required for the conversion of tryptophan to niacin (e.g., iron, riboflavin, and pyridoxine). The early symptoms of pellagra include loss of appetite, generalized weakness and irritability, abdominal pain, and vomiting. Bright red glossitis then ensues and is followed by a characteristic skin rash that is pigmented and scaling, particularly in skin areas exposed to sunlight. This rash is known as Casal’s necklace because it forms a ring around the neck; it is seen in advanced cases. Vaginitis and esophagitis also may occur. Diarrhea (due in part to proctitis and in part to malabsorption), depression, seizures, and dementia are also part of the pellagra syndrome. The primary manifestations of this syndrome are sometimes referred to as “the four D’s”: dermatitis, diarrhea, and dementia leading to death.
Deficiency Niacin deficiency causes pellagra, which is found mostly among people eating corn-based diets in parts of China, Africa, and India. Pellagra in North America is found mainly among alcoholics; among patients with congenital defects of intestinal and kidney absorption of tryptophan (Hartnup disease; Chap. 434e); and among patients with carcinoid syndrome (Chap. 113), in which there is increased conversion of tryptophan to serotonin. The antituberculosis drug isoniazid is a structural analog of niacin and can precipitate pellagra. In the setting of famine or population displacement, pellagra results from the absolute lack of niacin but also from the deficiency of micronutrients required for the conversion of tryptophan to niacin (e.g., iron, riboflavin, and pyridoxine). The early symptoms of pellagra include loss of appetite, generalized weakness and irritability, abdominal pain, and vomiting. Bright red glossitis then ensues and is followed by a characteristic skin rash that is pigmented and scaling, particularly in skin areas exposed to sunlight. This rash is known as Casal’s necklace because it forms a ring around the neck; it is seen in advanced cases. Vaginitis and esophagitis also may occur. Diarrhea (due in part to proctitis and in part to malabsorption), depression, seizures, and dementia are also part of the pellagra syndrome. The primary manifestations of this syndrome are sometimes referred to as “the four D’s”: dermatitis, diarrhea, and dementia leading to death.
Toxicity Prostaglandin-mediated flushing due to binding of the vitamin to a G protein–coupled receptor has been observed at daily nicotinic acid doses as low as 30 mg taken as a supplement or as therapy for dyslipidemia. There is no evidence of toxicity from niacin that is derived from food sources. Flushing always starts in the face and may be accompanied by skin dryness, itching, paresthesia, and headache. Pharmaceutical preparations of nicotinic acid combined with laropiprant, a selective prostaglandin D2 receptor 1 antagonist, or premedication with aspirin may alleviate these symptoms. Flushing is subject to tachyphylaxis and often improves with time. Nausea, vomiting, and abdominal pain also occur at similar doses of niacin. Hepatic toxicity is the most serious toxic reaction caused by sustained-release niacin and may present as jaundice with elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. A few cases of fulminant hepatitis requiring liver transplantation have been reported at doses of 3–9 g/d. Other toxic reactions include glucose intolerance, hyperuricemia, macular edema, and macular cysts. The combination of nicotinic acid preparations for dyslipidemia with 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors may increase the risk of rhabdomyolysis. The upper limit for daily niacin intake has been set at 35 mg. However, this upper limit does not pertain to the therapeutic use of niacin.
PYRIDOXINE (VITAMIN B6)
Vitamin B6 refers to a family of compounds that includes pyridoxine, pyridoxal, pyridoxamine, and their 5 ′-phosphate derivatives. 5 ′-Pyridoxal phosphate (PLP) is a cofactor for more than 100 enzymes involved in amino acid metabolism. Vitamin B6 also is involved in heme and neurotransmitter synthesis and in the metabolism of glycogen, lipids, steroids, sphingoid bases, and several vitamins, including the conversion of tryptophan to niacin.
Dietary Sources Plants contain vitamin B6 in the form of pyridoxine, whereas animal tissues contain PLP and pyridoxamine phosphate. The vitamin B6 contained in plants is less bioavailable than that in animal tissues. Rich food sources of vitamin B6 include legumes, nuts, wheat bran, and meat, although it is present in all food groups.
Deficiency Symptoms of vitamin B6 deficiency include epithelial changes, as seen frequently with other B vitamin deficiencies. In addition, severe vitamin B6 deficiency can lead to peripheral neuropathy, abnormal electroencephalograms, and personality changes that include depression and confusion. In infants, diarrhea, seizures, and anemia have been reported. Microcytic hypochromic anemia is due to diminished hemoglobin synthesis, since the first enzyme involved in heme biosynthesis (aminolevulinate synthase) requires PLP as a cofactor (Chap. 126). In some case reports, platelet dysfunction has been reported. Since vitamin B6 is necessary for the conversion of homocysteine to cystathionine, it is possible that chronic low-grade vitamin B6 deficiency may result in hyperhomocysteinemia and increased risk of cardiovascular disease (Chaps. 291e and 434e). Independent of homocysteine, low levels of circulating vitamin B6 have been associated with inflammation and elevated levels of C-reactive protein.
Certain medications, such as isoniazid, L-dopa, penicillamine, and cycloserine, interact with PLP due to a reaction with carbonyl groups. Pyridoxine should be given concurrently with isoniazid to avoid neuropathy. The increased ratio of AST to ALT seen in alcoholic liver disease reflects the relative vitamin B6 dependence of ALT. Vitamin B6 dependency syndromes that require pharmacologic doses of vitamin B6 are rare; they include cystathionine β-synthase deficiency, pyridoxine-responsive (primarily sideroblastic) anemias, and gyrate atrophy with chorioretinal degeneration due to decreased activity of the mitochondrial enzyme ornithine aminotransferase. In these situations, 100–200 mg/d of oral vitamin B6 is required for treatment.
High doses of vitamin B6 have been used to treat carpal tunnel syndrome, premenstrual syndrome, schizophrenia, autism, and diabetic neuropathy but have not been found to be effective.
The laboratory diagnosis of vitamin B6 deficiency is generally based on low plasma PLP values (<20 nmol/L). Vitamin B6 deficiency is treated with 50 mg/d; higher doses of 100–200 mg/d are given if the deficiency is related to medication use. Vitamin B6 should not be given with L-dopa, since the vitamin interferes with the action of this drug.
Toxicity The safe upper limit for vitamin B6 has been set at 100 mg/d, although no adverse effects have been associated with high intakes of vitamin B6 from food sources only. When toxicity occurs, it causes severe sensory neuropathy, leaving patients unable to walk. Some cases of photosensitivity and dermatitis have been reported.
FOLATE (VITAMIN B12)
See Chap. 128.
VITAMIN C
Both ascorbic acid and its oxidized product dehydroascorbic acid are biologically active. Actions of vitamin C include antioxidant activity, promotion of nonheme iron absorption, carnitine biosynthesis, conversion of dopamine to norepinephrine, and synthesis of many peptide hormones. Vitamin C is also important for connective tissue metabolism and cross-linking (proline hydroxylation), and it is a component of many drug-metabolizing enzyme systems, particularly the mixed-function oxidase systems.
Absorption and Dietary Sources Vitamin C is almost completely absorbed if <100 mg is administered in a single dose; however, only 50% or less is absorbed at doses >1 g. Enhanced degradation and fecal and urinary excretion of vitamin C occur at higher intake levels.
Good dietary sources of vitamin C include citrus fruits, green vegetables (especially broccoli), tomatoes, and potatoes. Consumption of five servings of fruits and vegetables a day provides vitamin C in excess of the RDA of 90 mg/d for men and 75 mg/d for women. In addition, ~40% of the U.S. population consumes vitamin C as a dietary supplement in which “natural forms” of the vitamin are no more bioavailable than synthetic forms. Smoking, hemodialysis, pregnancy, and stress (e.g., infection, trauma) appear to increase vitamin C requirements.
Deficiency Vitamin C deficiency causes scurvy. In the United States, this condition is seen primarily among the poor and the elderly, in alcoholics who consume <10 mg/d of vitamin C, and in individuals consuming macrobiotic diets. Vitamin C deficiency also can occur in young adults who eat severely unbalanced diets. In addition to generalized fatigue, symptoms of scurvy primarily reflect impaired formation of mature connective tissue and include bleeding into the skin (petechiae, ecchymoses, perifollicular hemorrhages); inflamed and bleeding gums; and manifestations of bleeding into joints, the peritoneal cavity, the pericardium, and the adrenal glands. In children, vitamin C deficiency may cause impaired bone growth. Laboratory diagnosis of vitamin C deficiency is based on low plasma or leukocyte levels.
Administration of vitamin C (200 mg/d) improves the symptoms of scurvy within several days. High-dose vitamin C supplementation (e.g., 1–2 g/d) may slightly decrease the symptoms and duration of upper respiratory tract infections. Vitamin C supplementation has also been reported to be useful in Chédiak-Higashi syndrome (Chap. 80) and osteogenesis imperfecta (Chap. 427). Diets high in vitamin C have been claimed to lower the incidence of certain cancers, particularly esophageal and gastric cancers. If proved, this effect may be due to the fact that vitamin C can prevent the conversion of nitrites and secondary amines to carcinogenic nitrosamines. However, an intervention study from China did not show vitamin C to be protective. A potential role for parenteral ascorbic acid in the treatment of advanced cancers has been suggested.
Toxicity Taking >2 g of vitamin C in a single dose may result in abdominal pain, diarrhea, and nausea. Since vitamin C may be metabolized to oxalate, it is feared that chronic high-dose vitamin C supplementation could result in an increased prevalence of kidney stones. However, except in patients with preexisting renal disease, this association has not been borne out in several trials. Nevertheless, it is reasonable to advise patients with a history of kidney stones not to take large doses of vitamin C. There is also an unproven but possible risk that chronic high doses of vitamin C could promote iron overload and iron toxicity. High doses of vitamin C can induce hemolysis in patients with glucose-6-phosphate dehydrogenase deficiency, and doses >1 g/d can cause false-negative guaiac reactions and interfere with tests for urinary glucose. High doses may interfere with the activity of certain drugs (e.g., bortezomib in myeloma patients).
BIOTIN
Biotin is a water-soluble vitamin that plays a role in gene expression, gluconeogenesis, and fatty acid synthesis and serves as a CO2 carrier on the surface of both cytosolic and mitochondrial carboxylase enzymes. The vitamin also functions in the catabolism of specific amino acids (e.g., leucine) and in gene regulation by histone biotinylation. Excellent food sources of biotin include organ meat such as liver or kidney, soy and other beans, yeast, and egg yolks; however, egg white contains the protein avidin, which strongly binds the vitamin and reduces its bioavailability.
Biotin deficiency due to low dietary intake is rare; rather, deficiency is due to inborn errors of metabolism. Biotin deficiency has been induced by experimental feeding of egg white diets and by biotin-free parenteral nutrition in patients with short bowels. In adults, biotin deficiency results in mental changes (depression, hallucinations), paresthesia, anorexia, and nausea. A scaling, seborrheic, and erythematous rash may occur around the eyes, nose, and mouth as well as on the extremities. In infants, biotin deficiency presents as hypotonia, lethargy, and apathy. In addition, infants may develop alopecia and a characteristic rash that includes the ears. The laboratory diagnosis of biotin deficiency can be established on the basis of a decreased concentration of urinary biotin (or its major metabolites), increased urinary excretion of 3-hydroxyisovaleric acid after a leucine challenge, or decreased activity of biotin-dependent enzymes in lymphocytes (e.g., propionyl-CoA carboxylase). Treatment requires pharmacologic doses of biotin–i.e., up to 10 mg/d. No toxicity is known.
PANTOTHENIC ACID (VITAMIN B5)
Pantothenic acid is a component of coenzyme A and phosphopantetheine, which are involved in fatty acid metabolism and the synthesis of cholesterol, steroid hormones, and all compounds formed from isoprenoid units. In addition, pantothenic acid is involved in the acetylation of proteins. The vitamin is excreted in the urine, and the laboratory diagnosis of deficiency is based on low urinary vitamin levels.
The vitamin is ubiquitous in the food supply. Liver, yeast, egg yolks, whole grains, and vegetables are particularly good sources. Human pantothenic acid deficiency has been demonstrated only by experimental feeding of diets low in pantothenic acid or by administration of a specific pantothenic acid antagonist. The symptoms of pantothenic acid deficiency are nonspecific and include gastrointestinal disturbance, depression, muscle cramps, paresthesia, ataxia, and hypoglycemia. Pantothenic acid deficiency is believed to have caused the “burning feet syndrome” seen in prisoners of war during World War II. No toxicity of this vitamin has been reported.
CHOLINE
Choline is a precursor for acetylcholine, phospholipids, and betaine. Choline is necessary for the structural integrity of cell membranes, cholinergic neurotransmission, lipid and cholesterol metabolism, methyl-group metabolism, and transmembrane signaling. Recently, a recommended adequate intake was set at 550 mg/d for men and 425 mg/d for women, although certain genetic polymorphisms can increase an individual’s requirement. Choline is thought to be a “conditionally essential” nutrient in that its de novo synthesis occurs in the liver and results in lesser-than-used amounts only under certain stress conditions (e.g., alcoholic liver disease). The dietary requirement for choline depends on the status of other nutrients involved in methyl-group metabolism (folate, vitamin B12, vitamin B6, and methionine) and thus varies widely. Choline is widely distributed in food (e.g., egg yolks, wheat germ, organ meat, milk) in the form of lecithin (phosphatidylcholine). Choline deficiency has occurred in patients receiving parenteral nutrition devoid of choline. Deficiency results in fatty liver, elevated aminotransferase levels, and skeletal muscle damage with high creatine phosphokinase values. The diagnosis of choline deficiency is currently based on low plasma levels, although nonspecific conditions (e.g., heavy exercise) may also suppress plasma levels.
Toxicity from choline results in hypotension, cholinergic sweating, diarrhea, salivation, and a fishy body odor. The upper limit for choline intake has been set at 3.5 g/d. Because of its ability to lower cholesterol and homocysteine levels, choline treatment has been suggested for patients with dementia and patients at high risk of cardiovascular disease. However, the benefits of such treatment have not been firmly documented. Choline- and betaine-restricted diets are of therapeutic value in trimethylaminuria (“fish odor syndrome”).
FLAVONOIDS
Flavonoids constitute a large family of polyphenols that contribute to the aroma, taste, and color of fruits and vegetables. Major groups of dietary flavonoids include anthocyanidins in berries; catechins in green tea and chocolate; flavonols (e.g., quercitin) in broccoli, kale, leeks, onions, and the skins of grapes and apples; and isoflavones (e.g., genistein) in legumes. Isoflavones have a low bioavailability and are partially metabolized by the intestinal flora. The dietary intake of flavonoids is estimated at 10–100 mg/d; this figure is almost certainly an underestimate attributable to a lack of information on their concentrations in many foods. Several flavonoids have antioxidant activity and affect cell signaling. From observational epidemiologic studies and limited clinical (human and animal) studies, flavonoids have been postulated to play a role in the prevention of several chronic diseases, including neurodegenerative disease, diabetes, and osteoporosis. The ultimate importance and usefulness of these compounds against human disease have not been demonstrated.
VITAMIN A
Vitamin A, in the strictest sense, refers to retinol. However, the oxidized metabolites retinaldehyde and retinoic acid are also biologically active compounds. The term retinoids includes all molecules (including synthetic molecules) that are chemically related to retinol. Retinaldehyde (11-cis) is the essential form of vitamin A that is required for normal vision, whereas retinoic acid is necessary for normal morphogenesis, growth, and cell differentiation. Retinoic acid does not function in vision and, in contrast to retinol, is not involved in reproduction. Vitamin A also plays a role in iron utilization, humoral immunity, T cell–mediated immunity, natural killer cell activity, and phagocytosis. Vitamin A is commercially available in esterified forms (e.g., acetate, palmitate), which are more stable than other forms.
There are more than 600 carotenoids in nature, ~50 of which can be metabolized to vitamin A. β-Carotene is the most prevalent carotenoid with provitamin A activity in the food supply. In humans, significant fractions of carotenoids are absorbed intact and are stored in liver and fat. It is estimated that ≥12 μg (range, 4–27 μg) of dietary all-trans β-carotene is equivalent to 1 μg of retinol activity, whereas the figure is ≥24 μg for other dietary provitamin A carotenoids (e.g., cryptoxanthin, α-carotene). The vitamin A equivalency for a β-carotene supplement in an oily solution is 2:1.
Metabolism The liver contains ~90% of the vitamin A reserves and secretes vitamin A in the form of retinol, which is bound to retinol-binding protein. Once binding has occurred, the retinol-binding protein complex interacts with a second protein, transthyretin. This trimolecular complex functions to prevent vitamin A from being filtered by the kidney glomerulus, thus protecting the body against the toxicity of retinol and allowing retinol to be taken up by specific cell-surface receptors that recognize retinol-binding protein. A certain amount of vitamin A enters peripheral cells even if it is not bound to retinol-binding protein. After retinol is internalized by the cell, it becomes bound to a series of cellular retinol-binding proteins, which function as sequestering and transporting agents as well as co-ligands for enzymatic reactions. Certain cells also contain retinoic acid–binding proteins, which have sequestering functions but also shuttle retinoic acid to the nucleus and enable its metabolism.
Retinoic acid is a ligand for certain nuclear receptors that act as transcription factors. Two families of receptors (retinoic acid receptors [RARs] and retinoid × receptors [RXRs]) are active in retinoid-mediated gene transcription. Retinoid receptors regulate transcription by binding as dimeric complexes to specific DNA sites—the retinoic acid response elements—in target genes (Chap. 400e). The receptors can either stimulate or repress gene expression in response to their ligands. RARs bind all-trans retinoic acid and 9-cis-retinoic acid, whereas RXRs bind only 9-cis-retinoic acid.
The retinoid receptors play an important role in controlling cell proliferation and differentiation. Retinoic acid is useful in the treatment of promyelocytic leukemia (Chap. 132) and also is used in the treatment of cystic acne because it inhibits keratinization, decreases sebum secretion, and possibly alters the inflammatory reaction (Chap. 71). RXRs dimerize with other nuclear receptors to function as coregulators of genes responsive to retinoids, thyroid hormone, and calcitriol. RXR agonists induce insulin sensitivity experimentally, perhaps because RXRs are cofactors for the peroxisome proliferator-activated receptors, which are targets for thiazolidinedione drugs such as rosiglitazone and troglitazone (Chap. 418).
Dietary Sources The retinol activity equivalent (RAE) is used to express the vitamin A value of food: 1 RAE is defined as 1 μg of retinol (0.003491 mmol), 12 μg of β-carotene, and 24 μg of other provitamin A carotenoids. In older literature, vitamin A often was expressed in international units (IU), with 1 μg of retinol equal to 3.33 IU of retinol and 20 IU of β-carotene, but these units are no longer in scientific use.
Liver, fish, and eggs are excellent food sources for preformed vitamin A; vegetable sources of provitamin A carotenoids include dark green and deeply colored fruits and vegetables. Moderate cooking of vegetables enhances carotenoid release for uptake in the gut. Carotenoid absorption is also aided by some fat in a meal. Infants are particularly susceptible to vitamin A deficiency because neither breast nor cow’s milk supplies enough vitamin A to prevent deficiency. In developing countries, chronic dietary deficiency is the main cause of vitamin A deficiency and is exacerbated by infection. In early childhood, low vitamin A status results from inadequate intakes of animal food sources and edible oils, both of which are expensive, coupled with seasonal unavailability of vegetables and fruits and lack of marketed fortified food products. Concurrent zinc deficiency can interfere with the mobilization of vitamin A from liver stores. Alcohol interferes with the conversion of retinol to retinaldehyde in the eye by competing for alcohol (retinol) dehydrogenase. Drugs that interfere with the absorption of vitamin A include mineral oil, neomycin, and cholestyramine.
![]() Deficiency Vitamin A deficiency is endemic in areas where diets are chronically poor, especially in southern Asia, sub-Saharan Africa, some parts of Latin America, and the western Pacific, including parts of China. Vitamin A status is usually assessed by measuring serum retinol (normal range, 1.05–3.50 μmol/L [30–100 μg/dL]) or blood-spot retinol or by tests of dark adaptation. Stable isotopic or invasive liver biopsy methods are available to estimate total body stores of vitamin A. As judged by deficient serum retinol (<0.70 μmol/L [20 μg/dL]), vitamin A deficiency worldwide is present in >90 million preschool-age children, among whom >4 million have an ocular manifestation of deficiency termed xerophthalmia. This condition includes milder stages of night blindness and conjunctival xerosis (dryness) with Bitot’s spots (white patches of keratinized epithelium appearing on the sclera) as well as rare, potentially blinding corneal ulceration and necrosis. Keratomalacia (softening of the cornea) leads to corneal scarring that blinds at least a quarter of a million children each year and is associated with fatality rates of 4–25%. However, vitamin A deficiency at any stage poses an increased risk of death from diarrhea, dysentery, measles, malaria, or respiratory disease. Vitamin A deficiency can compromise barrier, innate, and acquired immune defenses to infection. In areas where deficiency is widely prevalent, vitamin A supplementation can markedly reduce the risk of childhood mortality (by 23–34%, on average). About 10% of pregnant women in undernourished settings also develop night blindness (assessed by history) during the latter half of pregnancy, and this moderate vitamin A deficiency is associated with an increased risk of maternal infection and death.
Deficiency Vitamin A deficiency is endemic in areas where diets are chronically poor, especially in southern Asia, sub-Saharan Africa, some parts of Latin America, and the western Pacific, including parts of China. Vitamin A status is usually assessed by measuring serum retinol (normal range, 1.05–3.50 μmol/L [30–100 μg/dL]) or blood-spot retinol or by tests of dark adaptation. Stable isotopic or invasive liver biopsy methods are available to estimate total body stores of vitamin A. As judged by deficient serum retinol (<0.70 μmol/L [20 μg/dL]), vitamin A deficiency worldwide is present in >90 million preschool-age children, among whom >4 million have an ocular manifestation of deficiency termed xerophthalmia. This condition includes milder stages of night blindness and conjunctival xerosis (dryness) with Bitot’s spots (white patches of keratinized epithelium appearing on the sclera) as well as rare, potentially blinding corneal ulceration and necrosis. Keratomalacia (softening of the cornea) leads to corneal scarring that blinds at least a quarter of a million children each year and is associated with fatality rates of 4–25%. However, vitamin A deficiency at any stage poses an increased risk of death from diarrhea, dysentery, measles, malaria, or respiratory disease. Vitamin A deficiency can compromise barrier, innate, and acquired immune defenses to infection. In areas where deficiency is widely prevalent, vitamin A supplementation can markedly reduce the risk of childhood mortality (by 23–34%, on average). About 10% of pregnant women in undernourished settings also develop night blindness (assessed by history) during the latter half of pregnancy, and this moderate vitamin A deficiency is associated with an increased risk of maternal infection and death.
Toxicity The acute toxicity of vitamin A was first noted in Arctic explorers who ate polar bear liver and has also been seen after administration of 150 mg to adults or 100 mg to children. Acute toxicity is manifested by increased intracranial pressure, vertigo, diplopia, bulging fontanels (in children), seizures, and exfoliative dermatitis; it may result in death. Among children being treated for vitamin A deficiency according to the protocols outlined above, transient bulging of fontanels occurs in 2% of infants, and transient nausea, vomiting, and headache occur in 5% of preschoolers. Chronic vitamin A intoxication is largely a concern in industrialized countries and has been seen in otherwise healthy adults who ingest 15 mg/d and children who ingest 6 mg/d over a period of several months. Manifestations include dry skin, cheilosis, glossitis, vomiting, alopecia, bone demineralization and pain, hypercalcemia, lymph node enlargement, hyperlipidemia, amenorrhea, and features of pseudotumor cerebri with increased intracranial pressure and papilledema. Liver fibrosis with portal hypertension and bone demineralization may result from chronic vitamin A intoxication. Provision of vitamin A in excess to pregnant women has resulted in spontaneous abortion and in congenital malformations, including craniofacial abnormalities and valvular heart disease. In pregnancy, the daily dose of vitamin A should not exceed 3 mg. Commercially available retinoid derivatives are also toxic, including 13-cis-retinoic acid, which has been associated with birth defects. Thus contraception should be continued for at least 1 year and possibly longer in women who have taken 13-cis-retinoic acid.
In malnourished children, vitamin A supplements (30–60 mg), in amounts calculated as a function of age and given in several rounds over 2 years, are considered to amplify nonspecific effects of vaccines. However, for unclear reasons, there may be a negative effect on mortality rates in incompletely vaccinated girls.
High doses of carotenoids do not result in toxic symptoms but should be avoided in smokers due to an increased risk of lung cancer. Very high doses of β-carotene (~200 mg/d) have been used to treat or prevent the skin rashes of erythropoietic protoporphyria. Carotenemia, which is characterized by a yellowing of the skin (in creases of the palms and soles) but not the sclerae, may follow ingestion of >30 mg of β-carotene daily. Hypothyroid patients are particularly susceptible to the development of carotenemia due to impaired breakdown of carotene to vitamin A. Reduction of carotenes in the diet results in the disappearance of skin yellowing and carotenemia over a period of 30–60 days.
VITAMIN D
The metabolism of the fat-soluble vitamin D is described in detail in Chap. 423. The biologic effects of this vitamin are mediated by vitamin D receptors, which are found in most tissues; binding with these receptors potentially expands vitamin D actions on nearly all cell systems and organs (e.g., immune cells, brain, breast, colon, and prostate) as well as exerting classic endocrine effects on calcium metabolism and bone health. Vitamin D is thought to be important for maintaining normal function of many nonskeletal tissues such as muscle (including heart muscle), for immune function, and for inflammation as well as for cell proliferation and differentiation. Studies have shown that vitamin D may be useful as adjunctive treatment for tuberculosis, psoriasis, and multiple sclerosis or for the prevention of certain cancers. Vitamin D insufficiency may increase the risk of type 1 diabetes mellitus, cardiovascular disease (insulin resistance, hypertension, or low-grade inflammation), or brain dysfunction (e.g., depression). However, the exact physiologic roles of vitamin D in these nonskeletal diseases and the importance of these roles have not been clarified.
The skin is a major source of vitamin D, which is synthesized upon skin exposure to ultraviolet B radiation (UV-B; wavelength, 290–320 nm). Except for fish, food (unless fortified) contains only limited amounts of vitamin D. Vitamin D2 (ergocalciferol) is obtained from plant sources and is the chemical form found in some supplements.
Deficiency Vitamin D status has been assessed by measuring serum levels of 25-dihydroxyvitamin D (25[OH]2 vitamin D); however, there is no consensus on a uniform assay or on optimal serum levels. The optimal level might, in fact, differ according to the targeted disease entity. Epidemiologic and experimental data indicate that a 25(OH)2 vitamin D level of >20 ng/mL (≥50 nmol/L; to convert ng/mL to nmol/L, multiply by 2.496) is sufficient for good bone health. Some experts advocate higher serum levels (e.g., >30 ng/mL) for other desirable endpoints of vitamin D action. There is insufficient evidence to recommend combined vitamin D and calcium supplementation as a primary preventive strategy for reduction of the incidence of fractures in healthy men and premenopausal women.
Risk factors for vitamin D deficiency are old age, lack of sun exposure, dark skin (especially among residents of northern latitudes), fat malabsorption, and obesity. Rickets represents the classic disease of vitamin D deficiency. Signs of deficiency are muscle soreness, weakness, and bone pain. Some of these effects are independent of calcium intake.
The U.S. National Academy of Sciences recently concluded that the majority of North Americans are receiving adequate amounts of vitamin D (RDA = 15 μg/d or 600 IU/d; Chap. 95e). However, for people older than 70 years, the RDA is set at 20 μg/d (800 IU/d). The consumption of fortified or enriched foods as well as suberythemal sun exposure should be encouraged for people at risk for vitamin D deficiency. If adequate intake is impossible, vitamin D supplements should be taken, especially during the winter months. Vitamin D deficiency can be treated by the oral administration of 50,000 IU/week for 6–8 weeks followed by a maintenance dose of 800 IU/d (100 μg/d) from food and supplements once normal plasma levels have been attained. The physiologic effects of vitamin D2 and vitamin D3 are identical when these vitamins are ingested over long periods.
Toxicity The upper limit of intake has been set at 4000 IU/d. Contrary to earlier beliefs, acute vitamin D intoxication is rare and usually is caused by the uncontrolled and excessive ingestion of supplements or by faulty food fortification practices. High plasma levels of 1,25(OH)2 vitamin D and calcium are central features of toxicity and mandate discontinuation of vitamin D and calcium supplements; in addition, treatment of hypercalcemia may be required.
VITAMIN E
Vitamin E is the collective designation for all stereoisomers of tocopherols and tocotrienols, although only the RR tocopherols meet human requirements. Vitamin E acts as a chain-breaking antioxidant and is an efficient pyroxyl radical scavenger that protects low-density lipoproteins and polyunsaturated fats in membranes from oxidation. A network of other antioxidants (e.g., vitamin C, glutathione) and enzymes maintains vitamin E in a reduced state. Vitamin E also inhibits prostaglandin synthesis and the activities of protein kinase C and phospholipase A2.
Absorption and Metabolism After absorption, vitamin E is taken up from chylomicrons by the liver, and a hepatic α-tocopherol transport protein mediates intracellular vitamin E transport and incorporation into very low density lipoprotein. The transport protein has a particular affinity for the RRR isomeric form of α-tocopherol; thus, this natural isomer has the most biologic activity.
Requirement Vitamin E is widely distributed in the food supply, with particularly high levels in sunflower oil, safflower oil, and wheat germ oil; γ-tocotrienols are notably present in soybean and corn oils. Vitamin E is also found in meats, nuts, and cereal grains, and small amounts are present in fruits and vegetables. Vitamin E pills containing doses of 50–1000 mg are ingested by ~10% of the U.S. population. The RDA for vitamin E is 15 mg/d (34.9 μmol or 22.5 IU) for all adults. Diets high in polyunsaturated fats may necessitate a slightly higher intake of vitamin E.
Dietary deficiency of vitamin E does not exist. Vitamin E deficiency is seen only in severe and prolonged malabsorptive diseases, such as celiac disease, or after small-intestinal resection or bariatric surgery. Children with cystic fibrosis or prolonged cholestasis may develop vitamin E deficiency characterized by areflexia and hemolytic anemia. Children with abetalipoproteinemia cannot absorb or transport vitamin E and become deficient quite rapidly. A familial form of isolated vitamin E deficiency also exists; it is due to a defect in the α-tocopherol transport protein. Vitamin E deficiency causes axonal degeneration of the large myelinated axons and results in posterior column and spinocerebellar symptoms. Peripheral neuropathy is initially characterized by areflexia, with progression to an ataxic gait, and by decreased vibration and position sensations. Ophthalmoplegia, skeletal myopathy, and pigmented retinopathy may also be features of vitamin E deficiency. A deficiency of either vitamin E or selenium in the host has been shown to increase certain viral mutations and, therefore, virulence. The laboratory diagnosis of vitamin E deficiency is based on low blood levels of α-tocopherol (<5 μg/mL, or <0.8 mg of α-tocopherol per gram of total lipids).
Toxicity All forms of vitamin E are absorbed and could contribute to toxicity; however, the toxicity risk seems to be rather low as long as liver function is normal. High doses of vitamin E (>800 mg/d) may reduce platelet aggregation and interfere with vitamin K metabolism and are therefore contraindicated in patients taking warfarin and antiplatelet agents (such as aspirin or clopidogrel). Nausea, flatulence, and diarrhea have been reported at doses >1 g/d.
VITAMIN K
There are two natural forms of vitamin K: vitamin K1, also known as phylloquinone, from vegetable and animal sources, and vitamin K2, or menaquinone, which is synthesized by bacterial flora and found in hepatic tissue. Phylloquinone can be converted to menaquinone in some organs.
Vitamin K is required for the posttranslational carboxylation of glutamic acid, which is necessary for calcium binding to γ-carboxylated proteins such as prothrombin (factor II); factors VII, IX, and X; protein C; protein S; and proteins found in bone (osteocalcin) and vascular smooth muscle (e.g., matrix Gla protein). However, the importance of vitamin K for bone mineralization and prevention of vascular calcification is not known. Warfarin-type drugs inhibit γ-carboxylation by preventing the conversion of vitamin K to its active hydroquinone form.
Dietary Sources Vitamin K is found in green leafy vegetables such as kale and spinach, and appreciable amounts are also present in margarine and liver. Vitamin K is present in vegetable oils; olive, canola, and soybean oils are particularly rich sources. The average daily intake by Americans is estimated to be ~100 μg/d.
Deficiency The symptoms of vitamin K deficiency are due to hemorrhage; newborns are particularly susceptible because of low fat stores, low breast milk levels of vitamin K, relative sterility of the infantile intestinal tract, liver immaturity, and poor placental transport. Intracranial bleeding as well as gastrointestinal and skin bleeding can occur in vitamin K–deficient infants 1–7 days after birth. Thus, vitamin K (0.5–1 mg IM) is given prophylactically at delivery.
Vitamin K deficiency in adults may be seen in patients with chronic small-intestinal disease (e.g., celiac disease, Crohn’s disease), in those with obstructed biliary tracts, or after small-bowel resection. Broad-spectrum antibiotic treatment can precipitate vitamin K deficiency by reducing numbers of gut bacteria, which synthesize menaquinones, and by inhibiting the metabolism of vitamin K. In patients with warfarin therapy, the anti-obesity drug orlistat can lead to international normalized ratio changes due to vitamin K malabsorption. Vitamin K deficiency usually is diagnosed on the basis of an elevated prothrombin time or reduced clotting factors, although vitamin K may also be measured directly by high-pressure liquid chromatography. Vitamin K deficiency is treated with a parenteral dose of 10 mg. For patients with chronic malabsorption, 1–2 mg/d should be given orally or 1–2 mg per week can be taken parenterally. Patients with liver disease may have an elevated prothrombin time because of liver cell destruction as well as vitamin K deficiency. If an elevated prothrombin time does not improve during vitamin K therapy, it can be deduced that this abnormality is not the result of vitamin K deficiency.
Toxicity Toxicity from dietary phylloquinones and menaquinones has not been described. High doses of vitamin K can impair the actions of oral anticoagulants.
MINERALS
See also Table 96e-2.
|
DEFICIENCIES AND TOXICITIES OF METALS |
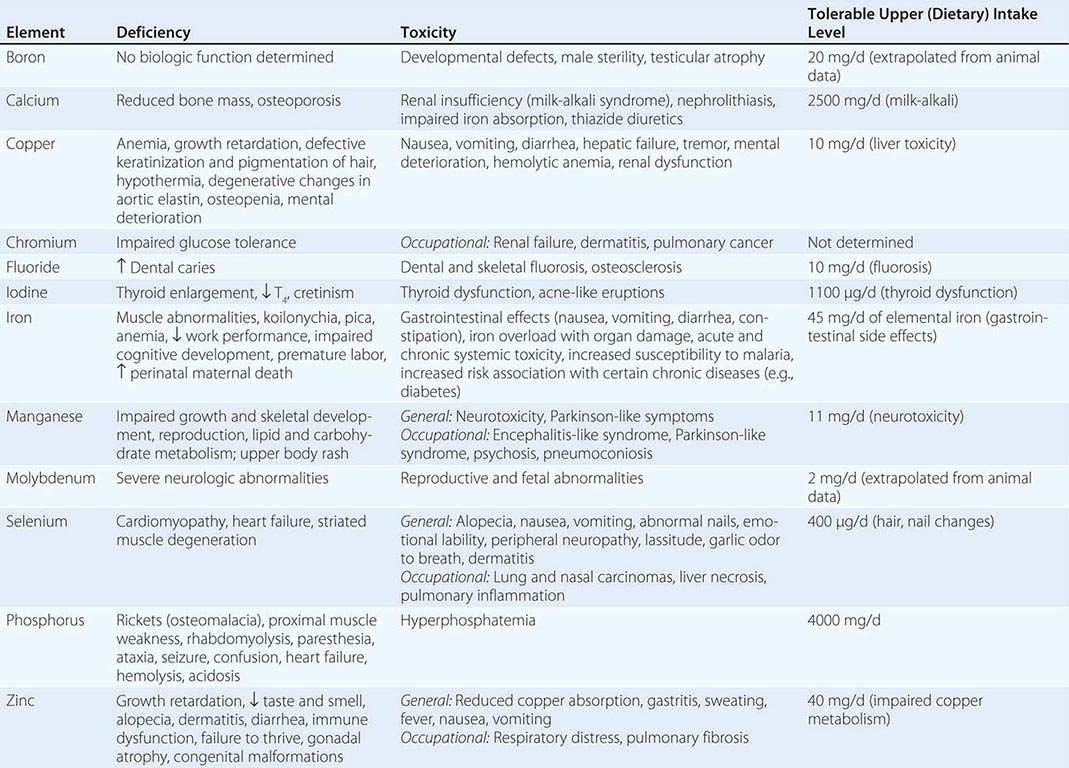
CALCIUM
See Chap. 423.
ZINC
Zinc is an integral component of many metalloenzymes in the body; it is involved in the synthesis and stabilization of proteins, DNA, and RNA and plays a structural role in ribosomes and membranes. Zinc is necessary for the binding of steroid hormone receptors and several other transcription factors to DNA. Zinc is absolutely required for normal spermatogenesis, fetal growth, and embryonic development.
Absorption The absorption of zinc from the diet is inhibited by dietary phytate, fiber, oxalate, iron, and copper as well as by certain drugs, including penicillamine, sodium valproate, and ethambutol. Meat, shellfish, nuts, and legumes are good sources of bioavailable zinc, whereas zinc in grains and legumes is less available for absorption.
![]() Deficiency Mild zinc deficiency has been described in many diseases, including diabetes mellitus, HIV/AIDS, cirrhosis, alcoholism, inflammatory bowel disease, malabsorption syndromes, and sickle cell disease. In these diseases, mild chronic zinc deficiency can cause stunted growth in children, decreased taste sensation (hypogeusia), and impaired immune function. Severe chronic zinc deficiency has been described as a cause of hypogonadism and dwarfism in several Middle Eastern countries. In these children, hypopigmented hair is also part of the syndrome. Acrodermatitis enteropathica is a rare autosomal recessive disorder characterized by abnormalities in zinc absorption. Clinical manifestations include diarrhea, alopecia, muscle wasting, depression, irritability, and a rash involving the extremities, face, and perineum. The rash is characterized by vesicular and pustular crusting with scaling and erythema. Occasional patients with Wilson’s disease have developed zinc deficiency as a consequence of penicillamine therapy (Chap. 429).
Deficiency Mild zinc deficiency has been described in many diseases, including diabetes mellitus, HIV/AIDS, cirrhosis, alcoholism, inflammatory bowel disease, malabsorption syndromes, and sickle cell disease. In these diseases, mild chronic zinc deficiency can cause stunted growth in children, decreased taste sensation (hypogeusia), and impaired immune function. Severe chronic zinc deficiency has been described as a cause of hypogonadism and dwarfism in several Middle Eastern countries. In these children, hypopigmented hair is also part of the syndrome. Acrodermatitis enteropathica is a rare autosomal recessive disorder characterized by abnormalities in zinc absorption. Clinical manifestations include diarrhea, alopecia, muscle wasting, depression, irritability, and a rash involving the extremities, face, and perineum. The rash is characterized by vesicular and pustular crusting with scaling and erythema. Occasional patients with Wilson’s disease have developed zinc deficiency as a consequence of penicillamine therapy (Chap. 429).
Zinc deficiency is prevalent in many developing countries and usually coexists with other micronutrient deficiencies (especially iron deficiency). Zinc (20 mg/d until recovery) may be an effective adjunctive therapeutic strategy for diarrheal disease and pneumonia in children ≥ 6 months of age.
The diagnosis of zinc deficiency is usually based on a serum zinc level <12 μmol/L (<70 μg/dL). Pregnancy and birth control pills may cause a slight depression in serum zinc levels, and hypoalbuminemia from any cause can result in hypozincemia. In acute stress situations, zinc may be redistributed from serum into tissues. Zinc deficiency may be treated with 60 mg of elemental zinc taken by mouth twice a day. Zinc gluconate lozenges (13 mg of elemental zinc every 2 h while awake) have been reported to reduce the duration and symptoms of the common cold in adults, but study results are conflicting.
Toxicity Acute zinc toxicity after oral ingestion causes nausea, vomiting, and fever. Zinc fumes from welding may also be toxic and cause fever, respiratory distress, excessive salivation, sweating, and headache. Chronic large doses of zinc may depress immune function and cause hypochromic anemia as a result of copper deficiency. Intranasal zinc preparations should be avoided because they may lead to irreversible damage of the nasal mucosa and anosmia.
COPPER
Copper is an integral part of numerous enzyme systems, including amine oxidases, ferroxidase (ceruloplasmin), cytochrome c oxidase, superoxide dismutase, and dopamine hydroxylase. Copper is also a component of ferroprotein, a transport protein involved in the basolateral transfer of iron during absorption from the enterocyte. As such, copper plays a role in iron metabolism, melanin synthesis, energy production, neurotransmitter synthesis, and CNS function; the synthesis and cross-linking of elastin and collagen; and the scavenging of superoxide radicals. Dietary sources of copper include shellfish, liver, nuts, legumes, bran, and organ meats.
Deficiency Dietary copper deficiency is relatively rare, although it has been described in premature infants who are fed milk diets and in infants with malabsorption (Table 96e-2). Copper-deficiency anemia (refractory to therapeutic iron) has been reported in patients with malabsorptive diseases and nephrotic syndrome and in patients treated for Wilson’s disease with chronic high doses of oral zinc, which can interfere with copper absorption. Menkes kinky hair syndrome is an X-linked metabolic disturbance of copper metabolism characterized by mental retardation, hypocupremia, and decreased circulating ceruloplasmin (Chap. 427). This syndrome is caused by mutations in the copper-transporting ATP7A gene. Children with this disease often die within 5 years because of dissecting aneurysms or cardiac rupture. Aceruloplasminemia is a rare autosomal recessive disease characterized by tissue iron overload, mental deterioration, microcytic anemia, and low serum iron and copper concentrations.
The diagnosis of copper deficiency is usually based on low serum levels of copper (<65 μg/dL) and low ceruloplasmin levels (<20 mg/dL). Serum levels of copper may be elevated in pregnancy or stress conditions since ceruloplasmin is an acute-phase reactant and 90% of circulating copper is bound to ceruloplasmin.
Toxicity Copper toxicity is usually accidental (Table 96e-2). In severe cases, kidney failure, liver failure, and coma may ensue. In Wilson’s disease, mutations in the copper-transporting ATP7B gene lead to accumulation of copper in the liver and brain, with low blood levels due to decreased ceruloplasmin (Chap. 429).
SELENIUM
![]() Selenium, in the form of selenocysteine, is a component of the enzyme glutathione peroxidase, which serves to protect proteins, cell membranes, lipids, and nucleic acids from oxidant molecules. As such, selenium is being actively studied as a chemopreventive agent against certain cancers, such as prostate cancer. Selenocysteine is also found in the deiodinase enzymes, which mediate the deiodination of thyroxine to triiodothyronine (Chap. 405). Rich dietary sources of selenium include seafood, muscle meat, and cereals, although the selenium content of cereal is determined by the soil concentration. Countries with low soil concentrations include parts of Scandinavia, China, and New Zealand. Keshan disease is an endemic cardiomyopathy found in children and young women residing in regions of China where dietary intake of selenium is low (<20 μg/d). Concomitant deficiencies of iodine and selenium may worsen the clinical manifestations of cretinism. Chronic ingestion of large amounts of selenium leads to selenosis, characterized by hair and nail brittleness and loss, garlic breath odor, skin rash, myopathy, irritability, and other abnormalities of the nervous system.
Selenium, in the form of selenocysteine, is a component of the enzyme glutathione peroxidase, which serves to protect proteins, cell membranes, lipids, and nucleic acids from oxidant molecules. As such, selenium is being actively studied as a chemopreventive agent against certain cancers, such as prostate cancer. Selenocysteine is also found in the deiodinase enzymes, which mediate the deiodination of thyroxine to triiodothyronine (Chap. 405). Rich dietary sources of selenium include seafood, muscle meat, and cereals, although the selenium content of cereal is determined by the soil concentration. Countries with low soil concentrations include parts of Scandinavia, China, and New Zealand. Keshan disease is an endemic cardiomyopathy found in children and young women residing in regions of China where dietary intake of selenium is low (<20 μg/d). Concomitant deficiencies of iodine and selenium may worsen the clinical manifestations of cretinism. Chronic ingestion of large amounts of selenium leads to selenosis, characterized by hair and nail brittleness and loss, garlic breath odor, skin rash, myopathy, irritability, and other abnormalities of the nervous system.
CHROMIUM
Chromium potentiates the action of insulin in patients with impaired glucose tolerance, presumably by increasing insulin receptor–mediated signaling, although its usefulness in treating type 2 diabetes is uncertain. In addition, improvement in blood lipid profiles has been reported in some patients. The usefulness of chromium supplements in muscle building has not been substantiated. Rich food sources of chromium include yeast, meat, and grain products. Chromium in the trivalent state is found in supplements and is largely nontoxic; however, chromium-6 is a product of stainless steel welding and is a known pulmonary carcinogen as well as a cause of liver, kidney, and CNS damage.
MAGNESIUM
See Chap. 423.
FLUORIDE, MANGANESE, AND ULTRATRACE ELEMENTS
An essential function for fluoride in humans has not been described, although it is useful for the maintenance of structure in teeth and bones. Adult fluorosis results in mottled and pitted defects in tooth enamel as well as brittle bone (skeletal fluorosis).
Manganese and molybdenum deficiencies have been reported in patients with rare genetic abnormalities and in a few patients receiving prolonged total parenteral nutrition. Several manganese-specific enzymes have been identified (e.g., manganese superoxide dismutase). Deficiencies of manganese have been reported to result in bone demineralization, poor growth, ataxia, disturbances in carbohydrate and lipid metabolism, and convulsions.
Ultratrace elements are defined as those needed in amounts <1 mg/d. Essentiality has not been established for most ultratrace elements, although selenium, chromium, and iodine are clearly essential (Chap. 405). Molybdenum is necessary for the activity of sulfite and xanthine oxidase, and molybdenum deficiency may result in skeletal and brain lesions.
97 |
Malnutrition and Nutritional Assessment |
Malnutrition can arise from primary or secondary causes, resulting in the former case from inadequate or poor-quality food intake and in the latter case from diseases that alter food intake or nutrient requirements, metabolism, or absorption. Primary malnutrition occurs mainly in developing countries and under conditions of political unrest, war, or famine. Secondary malnutrition, the main form encountered in industrialized countries, was largely unrecognized until the early 1970s, when it was appreciated that persons with adequate food supplies can become malnourished as a result of acute or chronic diseases that alter nutrient intake or metabolism, particularly diseases that cause acute or chronic inflammation. Various studies have shown that protein-energy malnutrition (PEM) affects one-third to one-half of patients on general medical and surgical wards in teaching hospitals. The consistent finding that nutritional status influences patient prognosis underscores the importance of preventing, detecting, and treating malnutrition.
Definitions for forms of PEM are in flux. Traditionally, the two major types of PEM have been marasmus and kwashiorkor. These conditions are compared in Table 97-1. Marasmus is the end result of a long-term deficit of dietary energy, whereas kwashiorkor has been understood to result from a protein-poor diet. Although the former concept remains essentially correct, evidence is accumulating that PEM syndromes are distinguished by two main features: insufficient dietary intake and underlying inflammatory processes. Energy-poor diets with minimal inflammation cause gradual erosion of body mass, resulting in classic marasmus. By contrast, inflammation from acute illnesses such as injury or sepsis or from chronic illnesses such as cancer, lung or heart disease, or HIV infection can erode lean body mass even in the presence of relatively sufficient dietary intake, leading to a kwashiorkor-like state. Quite often, inflammatory illnesses impair appetite and dietary intake, producing combinations of the two conditions.
|
COMPARISON OF MARASMUS/CACHEXIA AND KWASHIORKOR/ACUTE MALNUTRITION |
Consensus committees have proposed the following revised definitions. Starvation–related malnutrition is suggested for instances of chronic starvation without inflammation, chronic disease–related malnutrition when inflammation is chronic and of mild to moderate degree, and acute disease– or injury–related malnutrition when inflammation is acute and of a severe degree. However, because distinguishing diagnostic criteria for these conditions have not been universally adopted, this chapter integrates the older and newer terms.
MARASMUS (STARVATION–RELATED MALNUTRITION) AND CACHEXIA (CHRONIC DISEASE–RELATED MALNUTRITION)
Marasmus (starvation–related malnutrition) is a state in which virtually all available body fat stores have been exhausted due to starvation without systemic inflammation. Cachexia (chronic disease–related malnutrition) is a state that involves substantial loss of lean body mass in the presence of chronic systemic inflammation. Conditions that produce cachexia tend to be chronic and indolent, such as cancer and chronic pulmonary disease, whereas, in high-income countries, the classic setting for marasmus is in patients with anorexia nervosa. These conditions are relatively easy to detect because of the patient’s starved appearance. The diagnosis is based on fat and muscle wastage resulting from prolonged calorie deficiency and/or inflammation. Diminished skinfold thickness reflects the loss of fat reserves; reduced arm muscle circumference with temporal and interosseous muscle wasting reflects the catabolism of protein throughout the body, including in vital organs such as the heart, liver, and kidneys.
Routine laboratory findings in cachexia/marasmus are relatively unremarkable. The creatinine-height index (24-h urinary creatinine excretion compared with normal values based on height) is low, reflecting the loss of muscle mass. Occasionally, the serum albumin level is reduced, but it remains above 2.8 g/dL when systemic inflammation is absent. Despite a morbid appearance, immunocompetence, wound healing, and the ability to handle short-term stress are reasonably well preserved in most patients.
Pure starvation–related malnutrition is a chronic, fairly well adapted form of starvation rather than an acute illness; it should be treated cautiously in an attempt to reverse the downward trend gradually. Although nutritional support is necessary, overly aggressive repletion can result in severe, even life-threatening metabolic imbalances such as hypophosphatemia and cardiorespiratory failure (refeeding syndrome). When possible, oral or enteral nutritional support is preferred; treatment started slowly allows readaptation of metabolic and intestinal functions (Chap. 98e).
KWASHIORKOR (ACUTE DISEASE– OR INJURY–RELATED MALNUTRITION)
By contrast, kwashiorkor (acute disease– or injury–related malnutrition) in developed countries occurs mainly in connection with acute, life-threatening conditions such as trauma and sepsis. The physiologic stress produced by these illnesses increases protein and energy requirements at a time when intake is often limited. A classic scenario is an acutely stressed patient who receives only 5% dextrose solutions for periods as brief as 2 weeks. Although the etiologic mechanisms are not fully known, the protein-sparing response normally seen in starvation is blocked by the stressed state and by carbohydrate infusion.
In its early stages, the physical findings of kwashiorkor/acute malnutrition are few and subtle. Initially unaffected fat reserves and muscle mass give the deceptive appearance of adequate nutrition. Signs that support the diagnosis include easy hair pluckability, edema, skin breakdown, and poor wound healing. The major sine qua non is severe reduction of levels of serum proteins such as albumin (<2.8 g/dL) and transferrin (<150 mg/dL) or of iron-binding capacity (<200 μg/dL). Cellular immune function is depressed, as reflected by lymphopenia (<1500 lymphocytes/μL in adults and older children) and lack of response to skin test antigens (anergy).
The prognosis of adult patients with full-blown kwashiorkor/acute malnutrition is not good even with aggressive nutritional support. Surgical wounds often dehisce (fail to heal), pressure sores develop, gastroparesis and diarrhea can occur with enteral feeding, the risk of gastrointestinal bleeding from stress ulcers is increased, host defenses are compromised, and death from overwhelming infection may occur despite antibiotic therapy. Unlike treatment of marasmus, therapy for kwashiorkor entails aggressive nutritional support to restore better metabolic balance rapidly (Chap. 98e).
PHYSIOLOGIC CHARACTERISTICS OF HYPOMETABOLIC AND HYPERMETABOLIC STATES
The metabolic characteristics and nutritional needs of hypermetabolic patients who are stressed from injury, infection, or chronic inflammatory illness differ from those of hypometabolic patients who are unstressed but chronically starved. In both cases, nutritional support is important, but misjudgments in selecting the appropriate approach may have serious adverse consequences.
The hypometabolic patient is typified by the relatively less stressed but mildly catabolic and chronically starved individual who, with time, will develop cachexia/marasmus. The hypermetabolic patient stressed from injury or infection is catabolic (experiencing rapid breakdown of body mass) and is at high risk for developing acute malnutrition/kwashiorkor if nutritional needs are not met and/or the illness does not resolve quickly. As summarized in Table 97-2, the two states are distinguished by differing perturbations of metabolic rate, rates of protein breakdown (proteolysis), and rates of gluconeogenesis. These differences are mediated by proinflammatory cytokines and counterregulatory hormones—tumor necrosis factor, interleukins 1 and 6, C-reactive protein, catecholamines (epinephrine and norepinephrine), glucagon, and cortisol—whose levels are relatively reduced in hypometabolic patients and increased in hypermetabolic patients. Although insulin levels are also elevated in stressed patients, insulin resistance in the target tissues blocks insulin-mediated anabolic effects. Physiologic characteristics of patients at risk for chronic disease–related malnutrition are less predictable and likely represent a mixture of the two extremes depicted in Table 97-2.
|
PHYSIOLOGIC CHARACTERISTICS OF HYPOMETABOLIC AND HYPERMETABOLIC STATES |
Metabolic Rate In starvation and semistarvation, the resting metabolic rate falls between 10% and 30% as an adaptive response to energy restriction, slowing the rate of weight loss. By contrast, the resting metabolic rate rises in the presence of physiologic stress in proportion to the degree of the insult. The rate may increase by ~10% after elective surgery, 20–30% after bone fractures, 30–60% with severe infections such as peritonitis or gram-negative septicemia, and as much as 110% after major burns.
If the metabolic rate (energy requirement) is not matched by energy intake, weight loss results—slowly in hypometabolism and quickly in hypermetabolism. Losses of up to 10% of body mass are unlikely to be detrimental; however, greater losses in acutely ill hypermetabolic patients may be associated with rapid deterioration in body functions.
Protein Catabolism The rate of endogenous protein breakdown (catabolism) to supply energy needs normally falls during uncomplicated energy deprivation. After ~10 days of total starvation, an unstressed individual loses about 12–18 g of protein per day (equivalent to ~60 g of muscle tissue or ~2–3 g of nitrogen). In contrast, in injury and sepsis, protein breakdown accelerates in proportion to the degree of stress, reaching 30–60 g/d after elective surgery, 60–90 g/d with infection, 100–130 g/d with severe sepsis or skeletal trauma, and >175 g/d with major burns or head injuries. These losses are reflected by proportional increases in the excretion of urea nitrogen, the major by-product of protein breakdown.
Gluconeogenesis The major aim of protein catabolism during a state of starvation is to provide the glucogenic amino acids (especially alanine and glutamine) that serve as substrates for endogenous glucose production (gluconeogenesis) in the liver. In the hypometabolic/starved state, protein breakdown for gluconeogenesis is minimized, especially as ketones derived from fatty acids become the substrate preferred by certain tissues. In the hypermetabolic/stress state, gluconeogenesis increases dramatically and in proportion to the degree of the insult to increase the supply of glucose (the major fuel of reparation). Glucose is the only fuel that can be utilized by hypoxemic tissues (anaerobic glycolysis), white blood cells, and newly generated fibroblasts. Infusions of glucose partially offset a negative energy balance but do not significantly suppress the high rates of gluconeogenesis in catabolic patients. Hence, adequate supplies of protein are needed to replace the amino acids used for this metabolic response.
In summary, a hypometabolic patient is adapted to starvation and conserves body mass through reduction of the metabolic rate and use of fat as the primary fuel (rather than glucose and its precursor amino acids). A hypermetabolic patient also uses fat as a fuel but rapidly breaks down body protein to produce glucose, with consequent loss of muscle and organ tissue and danger to vital body functions.
MICRONUTRIENT MALNUTRITION
The same illnesses and reductions in nutrient intake that lead to PEM often produce deficiencies of vitamins and minerals as well (Chap. 96e). Deficiencies of nutrients that are stored in small amounts (such as the water-soluble vitamins) occur because of loss through external secretions, such as zinc in diarrhea fluid or burn exudate, and are probably more common than is generally recognized.
Deficiencies of vitamin C, folic acid, and zinc are relatively common in sick patients. Signs of scurvy, such as corkscrew hairs on the lower extremities, are found frequently in chronically ill and/or alcoholic patients. The diagnosis can be confirmed by determination of plasma vitamin C levels. Folic acid intakes and blood levels are often less than optimal, even among healthy persons; with illness, alcoholism, poverty, or poor dentition, these deficiencies are common. Low blood zinc levels are prevalent in patients with malabsorption syndromes such as inflammatory bowel disease. Patients with zinc deficiency often exhibit poor wound healing, pressure ulcer formation, and impaired immunity. Thiamine deficiency is a common complication of alcoholism but may be prevented by therapeutic doses of thiamine in patients treated for alcohol abuse.
Patients with low plasma vitamin C levels usually respond to the doses in multivitamin preparations, but patients with deficiencies should be supplemented with 250–500 mg/d. Folic acid is absent from some oral multivitamin preparations; patients with deficiencies should be supplemented with ~1 mg/d. Patients with zinc deficiencies resulting from large external losses sometimes require oral supplementation with 220 mg of zinc sulfate one to three times daily. For these reasons, laboratory assessments of the micronutrient status of patients at high risk are desirable.
Hypophosphatemia develops in hospitalized patients with remarkable frequency and generally results from rapid intracellular shifts of phosphate in underweight or alcoholic patients receiving intravenous glucose (Chap. 63). The adverse clinical sequelae are numerous; some, such as acute cardiopulmonary failure, are collectively called refeeding syndrome and can be life-threatening.
GLOBAL CONSIDERATIONS
![]() Many developing countries are still faced with high prevalences of the classic forms of PEM: marasmus and kwashiorkor. Food insecurity, which characterizes many poor countries, prevents consistent dietary sufficiency and/or quality and leads to endemic or cyclic malnutrition. Factors threatening food security include marked seasonal variations in agricultural productivity (rainy season–dry season cycles), periodic droughts, political unrest or injustice, and disease epidemics (especially of HIV/AIDS). The coexistence of malnutrition and disease epidemics exacerbates the latter and increases complications and mortality rates, creating vicious cycles of malnutrition and disease.
Many developing countries are still faced with high prevalences of the classic forms of PEM: marasmus and kwashiorkor. Food insecurity, which characterizes many poor countries, prevents consistent dietary sufficiency and/or quality and leads to endemic or cyclic malnutrition. Factors threatening food security include marked seasonal variations in agricultural productivity (rainy season–dry season cycles), periodic droughts, political unrest or injustice, and disease epidemics (especially of HIV/AIDS). The coexistence of malnutrition and disease epidemics exacerbates the latter and increases complications and mortality rates, creating vicious cycles of malnutrition and disease.
As economic prosperity improves, developing countries have been observed to undergo an epidemiologic transition, a component of which has been termed the nutrition transition. As improved economic resources make greater dietary diversity possible, middle-income populations (e.g., in southern Asia, China, and Latin America) typically begin to adopt lifestyle habits of industrialized nations, with increased consumption of energy and fat and decreased levels of physical activity. These changes lead to rising levels of obesity, metabolic syndrome, diabetes, cardiovascular disease, and cancer, sometimes coexisting in populations with persistent undernutrition.
Micronutrient deficiencies also remain prevalent in many countries of the world, impairing functional status and productivity and increasing mortality rates. Vitamin A deficiency impairs vision and increases morbidity and mortality rates from infections such as measles. Mild to moderate iron deficiency may be prevalent in up to 50% of the world, resulting from poor dietary diversity coupled with periodic blood loss and pregnancies. Iodine deficiency remains prevalent, causing goiter, hypothyroidism, and cretinism. Zinc deficiency is endemic in many populations, producing growth retardation, hypogonadism, and dermatoses and impairing wound healing. Fortunately, public health supplementation programs have substantially improved vitamin A and zinc status in developing countries during the past two decades, reducing mortality rates from measles, diarrheal diseases, and other manifestations. However, with the advancing nutrition transition and a shift toward nutritionally related chronic noncommunicable conditions, it is estimated that nutrition remains one of the three greatest contributors of risk for morbidity and mortality worldwide.
NUTRITIONAL ASSESSMENT
Because interactions between illness and nutrition are complex, many physical and laboratory findings reflect both underlying disease and nutritional status. Therefore, the nutritional evaluation of a patient requires an integration of history, physical examination, anthropometrics, and laboratory studies. This approach helps both to detect nutritional problems and to prevent the conclusion that isolated findings indicate nutritional problems when they do not. For example, hypoalbuminemia caused by an inflammatory illness does not necessarily indicate malnutrition.
Nutritional History Elicitation of a nutritional history is directed toward the identification of underlying mechanisms that put patients at risk for nutritional depletion or excess. These mechanisms include inadequate intake, impaired absorption, decreased utilization, increased losses, and increased requirements for nutrients.
Individuals with the characteristics listed in Table 97-3 are at particular risk for nutritional deficiencies.
|
NUTRITIONAL DEFICIENCY: THE HIGH-RISK PATIENT |
aNil per os (nothing by mouth).
Physical Examination Physical findings that suggest vitamin, mineral, and protein-energy deficiencies and excesses are outlined in Table 97-4. Most of the physical findings are not specific for individual nutrient deficiencies and must be integrated with historic, anthropometric, and laboratory findings. For example, follicular hyperkeratosis on the back of the arms is a fairly common, normal finding. However, if it is widespread in a person who consumes few fruits and vegetables and smokes regularly (increasing ascorbic acid requirements), vitamin C deficiency is likely. Similarly, easily pluckable hair may be a consequence of chemotherapy but suggests acute malnutrition/kwashiorkor in a hospitalized patient who has poorly healing surgical wounds and hypoalbuminemia.
|
PHYSICAL FINDINGS OF NUTRITIONAL DEFICIENCIES |
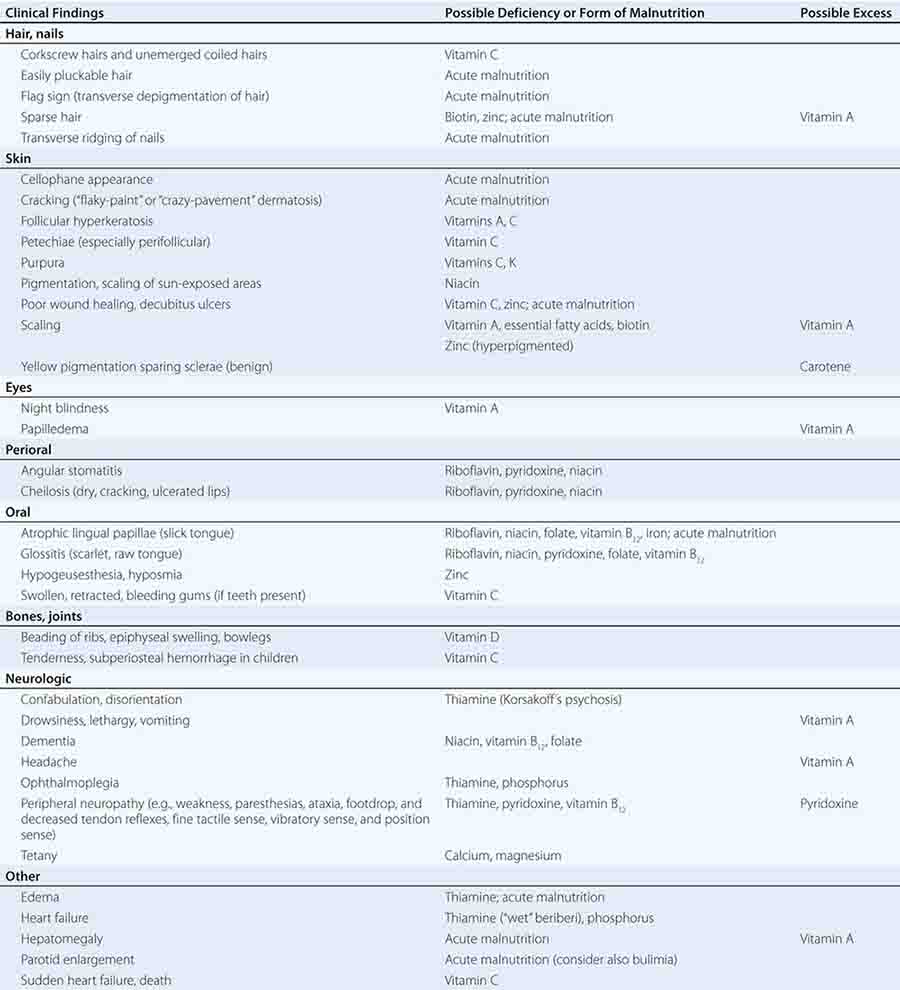
Anthropometric Measurements Anthropometric measurements provide information on body muscle mass and fat reserves. The most practical and commonly used measurements are body weight, height, triceps skinfold (TSF), and midarm muscle circumference (MAMC). Body weight is one of the most useful nutritional parameters to follow in patients who are acutely or chronically ill. Unintentional weight loss during illness often reflects loss of lean body mass (muscle and organ tissue), especially if it is rapid and is not caused by diuresis. Such weight loss can be an ominous sign since it indicates use of vital body protein stores for metabolic fuel. The reference standard for normal body weight, body mass index (BMI: weight in kilograms divided by height, in meters, squared), is discussed in Chap. 416. BMI values <18.5 are considered underweight; <17, significantly underweight; and <16, severely wasted. Values of 18.5–24.9 are normal; 25–29.9, overweight; and ≥30, obese.
Measurement of skinfold thickness is useful for estimating body fat stores, because ~50% of body fat is normally located in the subcutaneous region. This measurement can also permit discrimination of fat mass from muscle mass. The triceps is a convenient site that is generally representative of the body’s overall fat level. A thickness <3 mm suggests virtually complete exhaustion of fat stores. The MAMC can be used to estimate skeletal muscle mass, calculated as follows:
MAMC (cm) = upper arm circumference (cm) – [0.314 × TSF (mm)]
Laboratory Studies A number of laboratory tests used routinely in clinical medicine can yield valuable information about a patient’s nutritional status if a slightly different approach to their interpretation is used. For example, abnormally low serum albumin levels, low total iron-binding capacity, and anergy may have a distinct explanation, but collectively they may represent kwashiorkor. In the clinical setting of a hypermetabolic, acutely ill patient who is edematous and has easily pluckable hair and inadequate protein intake, the diagnosis of acute malnutrition/kwashiorkor is clear-cut. Commonly used laboratory tests for assessing nutritional status are outlined in Table 97-5. The table also provides tips to avoid the assignment of nutritional significance to tests that may be abnormal for nonnutritional reasons.
|
LABORATORY TESTS FOR NUTRITIONAL ASSESSMENT |
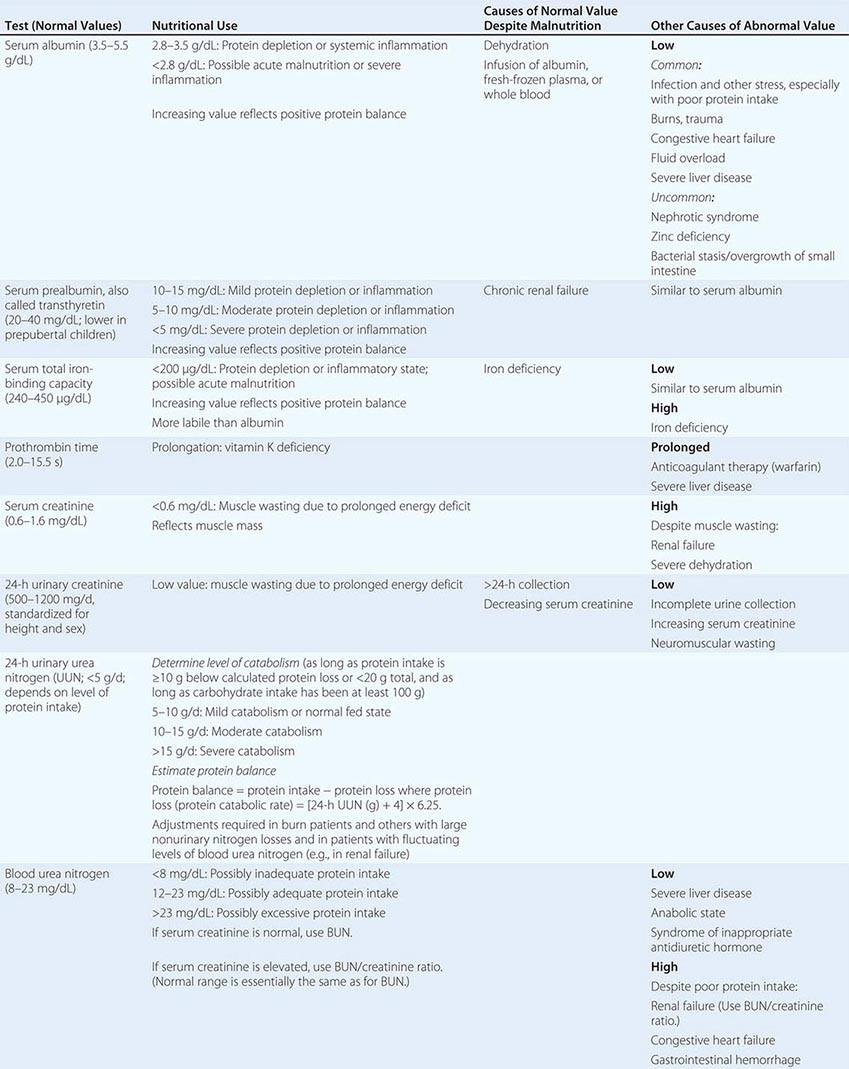
ASSESSMENT OF CIRCULATING (VISCERAL) PROTEINS The serum proteins most commonly used to assess nutritional status include albumin, total iron-binding capacity (or transferrin), thyroxine-binding prealbumin (or transthyretin), and retinol-binding protein. Because they have different synthesis rates and half-lives (the half-life of serum albumin is ~21 days, whereas those of prealbumin and retinol-binding protein are ~2 days and ~12 h, respectively), some of these proteins reflect changes in nutritional status more quickly than do others. However, rapid fluctuations can also make shorter-half-life proteins less reliable.
Levels of circulating proteins are influenced by their rates of synthesis and catabolism, “third spacing” (loss into interstitial spaces), and, in some cases, external loss. Although an adequate intake of calories and protein is necessary for optimal circulating protein levels, serum protein levels generally do not reflect protein intake. For example, a drop in the serum level of albumin or transferrin often accompanies significant physiologic stress (e.g., from infection or injury) and is not necessarily an indication of malnutrition or poor intake. A low serum albumin level in a burned patient with both hypermetabolism and increased dermal losses of protein may not indicate malnutrition. However, adequate nutritional support of the patient’s calorie and protein needs is critical for returning circulating proteins to normal levels as stress resolves. Thus low values by themselves do not define malnutrition, but they often point to increased risk of malnutrition because of the hypermetabolic stress state. As long as significant physiologic stress persists, serum protein levels remain low, even with aggressive nutritional support. However, if the levels do not rise after the underlying illness improves, the patient’s protein and calorie needs should be reassessed to ensure that intake is sufficient.
ASSESSMENT OF VITAMIN AND MINERAL STATUS The use of laboratory tests to confirm suspected micronutrient deficiencies is desirable because the physical findings for those deficiencies are often equivocal or nonspecific. Low blood micronutrient levels can predate more serious clinical manifestations and also may indicate drug-nutrient interactions.
ESTIMATING PROTEIN AND ENERGY REQUIREMENTS
A patient’s basal energy expenditure (BEE, measured in kilocalories per day) can be estimated from height, weight, age, and sex with the Harris-Benedict equations:
Men: BEE = 66.47 + 13.75W + 5.00H – 6.76A
Women: BEE = 655.10 + 9.56W + 1.85H – 4.68A
In these equations, W is weight in kilograms, H is height in centimeters, and A is age in years. After these equations are solved, total energy requirements are estimated by multiplying BEE by a factor that accounts for the stress of illness. Multiplying by 1.1–1.4 yields a range 10–40% above basal that estimates the 24-h energy expenditure of the majority of patients. The lower value (1.1) is used for patients without evidence of significant physiologic stress; the higher value (1.4) is appropriate for patients with marked stress such as sepsis or trauma. The result is used as a 24-h energy goal for feeding.
When it is important to have a more accurate assessment, energy expenditure can be measured at the bedside by indirect calorimetry. This technique is useful in patients who are thought to be hypermetabolic from sepsis or trauma and whose body weight cannot be ascertained accurately. Indirect calorimetry can also be useful in patients who have difficulty weaning from a ventilator and whose energy needs therefore should not be exceeded to avoid excessive CO2 production. Patients at the extremes of weight (e.g., obese persons) and/or age are good candidates as well, because the Harris-Benedict equations were developed from measurements in adults with roughly normal body weights.
Because urea is a major by-product of protein catabolism, the amount of urea nitrogen excreted each day can be used to estimate the rate of protein catabolism and determine whether protein intake is adequate to offset it. Total protein loss and protein balance can be calculated from urinary urea nitrogen (UUN) as follows:
Protein catabolic rate (g/d) = [24-h UUN (g) + 4]
× 6.25 (g protein/g nitrogen)
The value of 4 g added to the UUN represents a liberal estimate of the unmeasured nitrogen lost in the urine (e.g., creatinine and uric acid), sweat, hair, skin, and feces. When protein intake is low (e.g., less than ~20 g/d), the equation indicates both the patient’s protein requirement and the severity of the catabolic state (Table 97-5). More substantial protein intakes can raise the UUN because some of the ingested (or intravenously infused) protein is catabolized and converted to UUN. Thus, at lower protein intakes, the equation is useful for estimating requirements, and at higher protein intakes it is useful for assessing protein balance.
Protein balance (g/d) = protein intake – protein catabolic rate
98e |
Enteral and Parenteral Nutrition Therapy |
When correctly implemented, specialized nutritional support (SNS) plays a major and often life-saving role in medicine. SNS is used for two main purposes: (1) to provide an appropriate nutritional substrate in order to maintain or replenish the nutritional status of patients unable to voluntarily ingest or absorb sufficient amounts of food, and (2) to maintain the nutritional and metabolic status of adequately nourished patients who are experiencing systemic hypercatabolic effects of severe inflammation, injury, or infection in the course of persistent critical illness. Patients with permanent major loss of intestinal length or function often require lifelong SNS. Many patients who require treatment in chronic-care facilities receive enteral SNS, most often because their voluntary food intake is deemed insufficient or because impaired chewing and swallowing create a high risk of aspiration pneumonia.
Enteral SNS is the provision of liquid formula meals through a tube placed into the gut. Parenteral SNS is the direct infusion of complete mixtures of crystalline amino acids, dextrose, triglyceride emulsions, and micronutrients into the bloodstream through a central venous catheter or (rarely in adults) via a peripheral vein. The enteral route is almost always preferred because of its relative simplicity and safety, its low cost, and the benefits of maintaining digestive, absorptive, and immunologic barrier functions of the gastrointestinal tract. Pliable, small-bore feeding tubes make placement relatively easy and acceptable to patients. Constant-rate infusion pumps increase the reliability of nutrient delivery. The chief disadvantage of enteral SNS is that many days may be required to meet the patient’s nutrient requirements.
For short-term use, the feeding tube can be placed via the nose into the stomach, duodenum, or jejunum. For long-term use, these sites may be accessed through the abdominal wall by endoscopic or surgical procedures. The chief disadvantage of tube feeding in acute illness is intolerance due to gastric retention, risk of vomiting, or diarrhea. The presence of severe coagulopathy is a relative contraindication to the insertion of a feeding tube. In adults, parenteral nutrition (PN) almost always requires aseptic insertion of a central venous catheter with a dedicated port. Many circumstances can delay or slow the progression of enteral SNS, whereas parenteral SNS can provide a complete substrate mix easily and promptly. This practical advantage is mitigated by the need to infuse relatively large fluid volumes and the real risk of inadvertent toxic overfeeding.
THE DESIGN OF INDIVIDUAL REGIMENS
FLUID REQUIREMENTS
Normal adults require ~30 mL of fluid/kg of body weight from all sources each day as well as the replacement of abnormal losses such as those caused by diuretic therapy, nasogastric tube drainage, wound output, high rates of perspiration (which can be several liters per day during periods of extreme heat), and diarrhea/ostomy losses. Electrolyte and mineral losses can be estimated or measured and need to be replaced (Table 98e-2). Fluid restriction may be necessary in patients with fluid overload. Total fluid input can usually be limited to 1200 mL/d as long as urine is the only significant source of fluid output. In severe fluid overload, a 1-L central vein PN solution of 7% crystalline amino acids (70 g) and 21% dextrose (210 g) can temporarily provide an acceptable amount of glucose and protein substrate in the absence of significant catabolic stress.
|
APPROXIMATE VOLUME AND COMPOSITION OF FLUID SECRETIONSa |
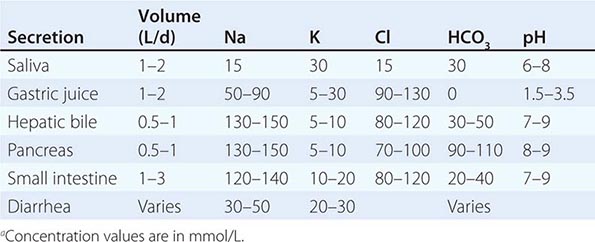
Patients who require PN or EN in the acute-care setting generally have associated hormonal adaptations to their underlying disease (e.g., increased secretion of antidiuretic hormone, aldosterone, insulin, glucagon, or cortisol), and these signals promote fluid retention and hyperglycemia. In critical illness, body weight is invariably increased due to fluid resuscitation and fluid retention. Lean-tissue accretion is minimal in the acute phase of critical illness, no matter how much protein and or how many calories are provided. Because excess fluid removal can be difficult, limiting fluid intake to allow for balanced intake and output is more effective.
ENERGY REQUIREMENTS
Total energy expenditure comprises resting energy expenditure, activity energy expenditure, and the thermal effect of feeding (Chap. 97). Resting energy expenditure accounts for two-thirds of total energy expenditure, activity energy expenditure for one-fourth to one-third, and the thermal effect of feeding for ~10%. For normally nourished, healthy individuals, the total energy expenditure is ~30–35 kcal/kg. Critical illness increases resting energy expenditure, but this increase is significant only in initially well-nourished individuals with a robust SRI who experience, for example, severe multiple trauma, extensive burns, sepsis, sustained high fever, or closed head injury. In these situations, total energy expenditure can reach 40–45 kcal/kg. The chronically starved patient with adapted PEM has a reduced energy expenditure and is inactive, with a usual total energy expenditure of ~20–25 kcal/kg. Very few patients with adapted PEM require as much as 30 kcal/kg for energy balance. Because providing ~50% of measured energy expenditure as SNS is at least as effective as 100% for the first 10 days of critical illness, actual measurement of energy expenditure generally is not necessary in the early period of SNS. However, in patients who remain critically ill beyond several weeks, in patients with severe PEM for whom estimates of energy expenditure are unreliable, and in patients who are difficult to wean from ventilators, it is reasonable to measure energy expenditure directly when the technique is available, targeting an energy intake of 100–120% of the measured energy expenditure.
Insulin resistance due to the SRI is associated with increased gluconeogenesis and reduced peripheral glucose utilization, with resulting hyperglycemia. Hyperglycemia is aggravated by excessive exogenous carbohydrate administration from SNS. In critically ill patients receiving SNS, normalization of blood glucose levels by insulin infusion reduces morbidity and mortality risk. In mildly or moderately malnourished patients, it is reasonable to provide metabolic support in order to improve protein synthesis and maintain metabolic homeostasis. Hypocaloric nutrition, with provision of ~1000 kcal and 70 g protein per day for up to 10 days, requires less fluid and reduces the likelihood of poor glycemic control, although a higher protein intake would be optimal. During the second week of SNS, energy and protein provision can be advanced to 20–25 kcal/kg and 1.5 g/kg per day, respectively, as metabolic conditions permit. As mentioned above, patients with multiple trauma, closed head injury, and severe burns often have greatly elevated energy expenditures, but there is little evidence that providing >30 kcal/kg daily confers further benefit, and such high caloric intake may well be harmful as it substantially increases the risk of hyperglycemia.
As a rule, amino acids and glucose are provided in an increasing dose until energy provision matches estimated resting energy expenditure. At this point, it becomes beneficial to add fat. A surfeit of glucose merely stimulates de novo lipogenesis—an energy-inefficient process. Polyunsaturated long-chain triglycerides (e.g., in soybean oil) are the chief ingredient in most parenteral fat emulsions and provide the majority of the fat in enteral feeding formulas. These vegetable oil–based emulsions provide essential fatty acids. The fat content of enteral feeding formulas ranges from 3% to 50% of energy. Parenteral fat is provided in separate containers as 20% and 30% emulsions that can be infused separately or mixed in the sterile pharmacy as an all-in-one or total nutrient admixture of amino acids, glucose, lipid, electrolytes, vitamins, and minerals. Although parenteral fat needs to make up only ~3% of the energy requirement in order to meet essential fatty acid requirements, when provided daily as an all-in-one mixture of carbohydrate, fat, and protein, the complete admixture has a fat content of 2–3 g/dL and provides 20–30% of the total energy requirement—an acceptable level that offers the advantage of ensuring emulsion stability. When given as a separate infusion, parenteral fat should not be provided at rates exceeding 0.11 g/kg of body mass or 100 g over 12 h—equivalent to 500 mL of 20% parenteral fat.
Medium-chain triglycerides containing saturated fatty acids with chain lengths of 6, 8, 10, or 12 carbons (>95% of which are C8 and C10) are included in a number of enteral feeding formulas because they are absorbed preferentially. Fish oil contains polyunsaturated fatty acids of the omega 3 family, which improve immune function and reduce the inflammatory response. At this time, fish oil injectable emulsions are available in the United States as an investigational new drug.
PN formulations provide carbohydrate as hydrous glucose (3.4 kcal/g). In enteral formulas, glucose is the carbohydrate source for so-called monomeric diets. These diets provide protein as amino acids and fat in minimal amounts (3%) to meet essential fatty acid requirements. Monomeric formulas are designed to optimize absorption in the seriously compromised gut. These formulas, like immune-enhancing diets, are expensive. In polymeric diets, the carbohydrate source is usually an osmotically less active polysaccharide, the protein is usually soy or casein protein, and fat is present at concentrations of 25–50%. Such formulas are usually well tolerated by patients with normal intestinal length, and some are acceptable for oral consumption.
PROTEIN OR AMINO ACID REQUIREMENTS
The daily protein recommendation for healthy adults is 0.8 g/kg, but body proteins are replenished faster with 1.5 g/kg in patients with PEM, and net protein catabolism is reduced in critically ill patients when 1.5–2.0 g/kg is provided. In patients who are not critically ill but who require SNS in the acute-care setting, at least 1 g of protein/kg is recommended, and larger amounts up to 1.5 g/kg are appropriate when volume, renal, and hepatic tolerances allow. The standard parenteral and enteral formulas contain protein of high biologic value and meet the requirements for the eight essential amino acids when nitrogen needs are met. Parenteral amino acid mixtures and elemental enteral mixtures consist of hydrated individual amino acids. Because of their hydrated status, elemental amino acid solutions deliver 17% less protein substrate than intact proteins. In protein-intolerant conditions such as renal and hepatic failure, modified amino acid formulas may be considered. In hepatic failure, higher branched-chain, amino acid–enriched formulas appear to improve outcomes. Conditionally essential amino acids like arginine and glutamine may also have some benefit in supplemental amounts.
Protein (nitrogen) balance provides a measure of the efficacy of parenteral or enteral SNS. This balance is calculated as protein intake/6.25 (because proteins are, on average, 16% nitrogen) minus the 24-h urine urea nitrogen plus 4 g of nitrogen (the latter reflecting other nitrogen losses). In critical illness, a mild negative nitrogen balance of 2–4 g/d is often achievable. A similarly mild positive nitrogen balance is observed in the nonstressed recuperating patient. Each gram of nitrogen lost or gained represents ~30 g of lean tissue.
MINERAL AND VITAMIN REQUIREMENTS
Parenteral electrolyte, vitamin, and trace mineral requirements are summarized in Tables 98e-3, 98e-4, and 98e-5, respectively. Electrolyte modifications are necessary with substantial gastrointestinal losses from nasogastric drainage or intestinal losses from fistulas, diarrhea, or ostomy outputs. Such losses also imply extra calcium, magnesium, and zinc losses. Zinc losses are high in secretory diarrhea. Secretory diarrhea contains ~12 mg of zinc/L, and patients with intestinal fistulas or chronic diarrhea require an average of ~12 mg of parenteral zinc/d (equivalent to 30 mg of oral elemental zinc) to maintain zinc balance. Excessive urinary potassium losses with amphotericin or magnesium losses with cisplatin or in renal failure necessitate adjustments in sodium, potassium, magnesium, phosphorus, and acid-base balance. Vitamin and trace element requirements are met by the daily provision of a complete parenteral vitamin supplement and trace elements via PN and by the provision of adequate amounts of enteral feeding formulas that contain these micronutrients.
|
USUAL DAILY ELECTROLYTE ADDITIONS TO PARENTERAL NUTRITION |
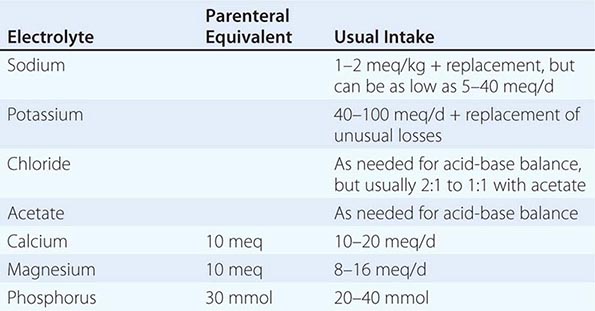
|
PARENTERAL MULTIVITAMIN REQUIREMENTS FOR ADULTS |
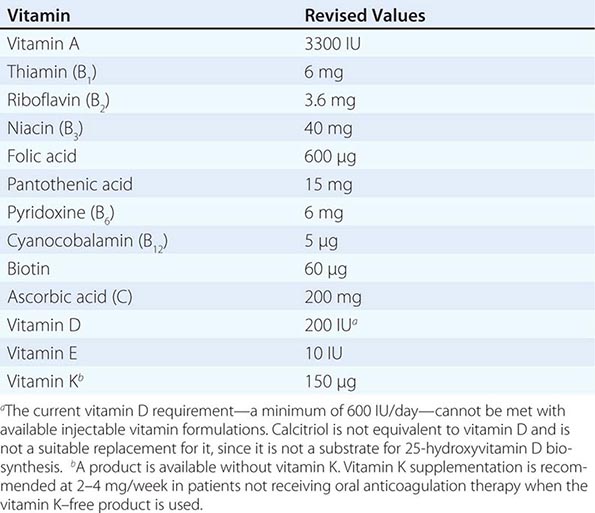
|
PARENTERAL TRACE METAL SUPPLEMENTATION FOR ADULTSa |
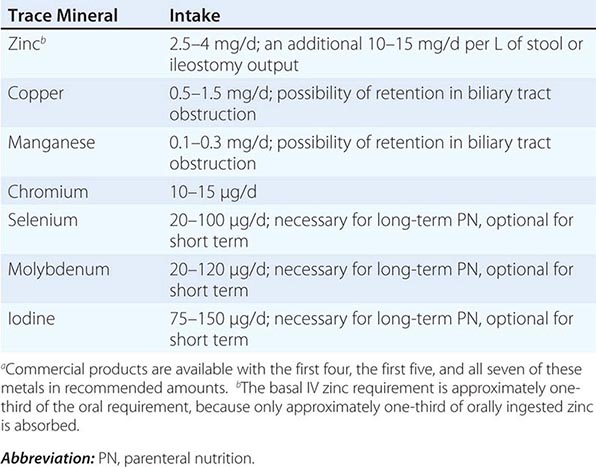
Iron is a highly reactive catalyst of oxidative reactions and thus is not included in PN mixtures. The parenteral iron requirement is normally only ~1 mg/d. Iron deficiency occurs with considerable frequency in acutely ill hospitalized patients, especially those with PEM and gastrointestinal tract disease, and in patients subjected to frequent blood withdrawals. Iron deficiency is sometimes inadequately considered in hospitalized patients because there are commoner causes: the inflammation-mediated anemia of chronic disease (with an associated increase in serum ferritin, an acute-phase protein) and redistribution of the intravascular fluid volume during prolonged bed rest. Iron deficiency should be considered in every patient receiving SNS. A falling mean red cell volume, even if still in the low-normal range, together with an intermediate serum ferritin concentration is suggestive of iron deficiency. Intravenous iron infusions follow standard guidelines, always with a termination order and never as a standing order because of the risk of inadvertent iron overdosing. Major iron replacement during critical illness is of some concern because of the possibility that a substantial rise in the serum iron concentration may increase susceptibility to some bacterial infections.
PARENTERAL NUTRITION
INFUSION TECHNIQUE AND PATIENT MONITORING
Parenteral feeding through a peripheral vein is limited by osmolarity and volume constraints. Solutions with an osmolarity >900 mOsm/L (e.g., those which contain >3% amino acids and 5% glucose [290 kcal/L]) are poorly tolerated peripherally. Parenteral lipid emulsions (20%) can be given to increase the calories delivered. The total volume required for a marginal amino acid provision rate of 60 g (equivalent to 50 g of protein) and a total of 1680 kcal is 2.5 L. Moreover, the risk of significant morbidity and mortality from incompatibilities of calcium and phosphate salts is greatest in these low-osmolarity, low-glucose regimens. For short-term infusions, calcium may be temporarily limited or even omitted from the mixture. Parenteral feeding via a peripheral vein is generally intended as a supplement to oral feeding; it is not suitable for the critically ill. Peripheral PN may be enhanced by small amounts of heparin (1000 U/L) and co-infusion with parenteral fat to reduce osmolarity, but volume constraints still limit the value of this therapy, especially in critical illness.
PICCs may be used to infuse solutions of 20–25% dextrose and 4–7% amino acids, thus avoiding the traumatic complications of percutaneous central vein catheter placement. With PICC lines, however, flow can be position-related, and the lines cannot be exchanged over a wire for infection monitoring. It is important to withdraw blood samples carefully and appropriately from a dual-port PICC because intermixing of the blood sample with even tiny volumes of nutrient infusate will falsely indicate hyperglycemia and hyperkalemia. For all these reasons, centrally placed catheters are preferred in critical illness. The subclavian approach is best tolerated by the patient and is the easiest to dress. The jugular approach is less likely to cause a pneumothorax. Femoral vein catheterization is strongly discouraged because of the risk of catheter infection. For long-term feeding at home, tunneled catheters and implanted ports are used to reduce infection risk and are more acceptable to patients. Tunneled catheters require placement in the operating room.
Catheters are made of Silastic®, polyurethane, or polyvinyl chloride. Silastic catheters are less thrombogenic and are best for tunneled catheters. Polyurethane is best for temporary catheters. To avoid infection, dressing changes with dry gauze should be performed at regular intervals by nurses skilled in catheter care. Chlorhexidine solution is more effective than alcohol or iodine compounds. Appropriate monitoring for patients receiving PN is summarized in Table 98e-6.
|
MONITORING THE PATIENT RECEIVING PARENTERAL NUTRITIONa |
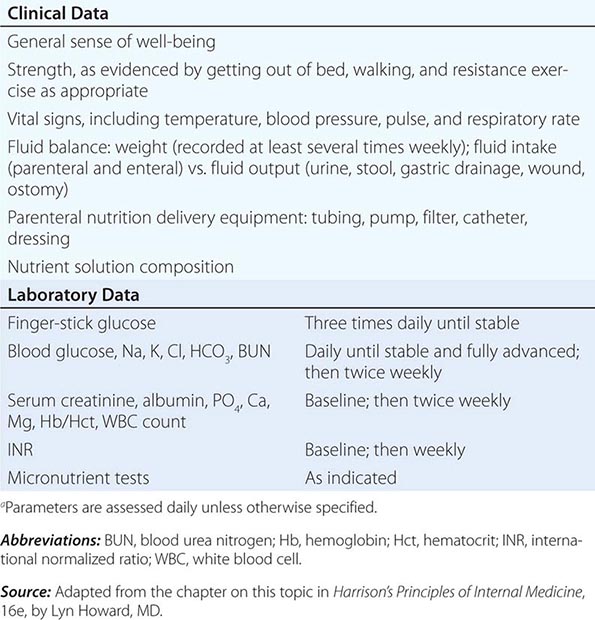
STANDARD VERSUS INDIVIDUALIZED NUTRIENT PROVISION
Even though premixed solutions of crystalline amino acids and dextrose are in common use, the future of evidence-based PN lies in computer-controlled sterile compounders that rapidly and inexpensively generate personalized solutions that meet the specific protein and calorie goals for different patients in different clinical situations. For example, 1 L of a standard mixture of 5% amino acids/25% dextrose solution provides 50 g of amino acids (41.5 g of protein substrate) and 1000 kcal; the use of this solution to meet the 1.5–to 2.0-g/kg protein requirement of an acutely ill 70-kg patient requires the infusion of 2.5–3.4 L of fluid and a potentially excessively high energy dose of 2500-3300 kcal. When the body fat store is adequate, clinical evidence increasingly supports the greater safety and efficacy of high-protein, moderately hypocaloric SNS in such patients. A sterile compounder can accurately generate an appropriate recipe for such a patient. For example, 1 L of a solution including 600 mL of 15% amino acids, 300 mL of 50% dextrose, and 100 mL of electrolyte/micronutrient mix contains 75 g of protein substrate and 800 kcal; thus it is feasible to meet the patient’s protein requirement with only 1.4–1.9 L of solution and a more appropriate 1100–1520 kcal; any mild gap in energy provision is easily filled by use of intravenous lipid.
COMPLICATIONS
Mechanical The insertion of a central venous catheter should be performed by trained and experienced personnel using aseptic techniques to limit the major common complications of pneumothorax and inadvertent arterial puncture or injury. The catheter’s position should be radiographically confirmed to be in the superior vena cava distal to the junction with the jugular or subclavian vein and not directly against the vessel wall. Thrombosis related to the catheter may occur at the site of entry into the vein and extend to encase the catheter. Catheter infection predisposes to thrombosis, as does the SRI. The addition of 6000 U of heparin to the daily parenteral formula for hospitalized patients with temporary catheters reduces the risk of fibrin sheath formation and catheter infection. Temporary catheters that develop a thrombus should be removed and, according to clinical findings, treated with anticoagulants. Thrombolytic therapy can be considered for patients with permanent catheters, depending on the ease of replacement and the presence of alternative, reasonably acceptable venous access sites. Low-dose warfarin therapy (1 mg/d) reduces the risk of thrombosis in permanent catheters used for at-home parenteral SNS, but full anticoagulation may be required for patients who have recurrent thrombosis related to permanent catheters. A recent U.S. Food and Drug Administration mandate to reformulate parenteral multivitamins to include vitamin K at a dose of 150 μg/d may affect the efficacy of low-dose warfarin therapy. A “no vitamin K” version is available for patients receiving this therapy. Catheters can become occluded due to mechanical factors; by fibrin at the tip; or by fat, minerals, or drugs intraluminally. These occlusions can be managed with low-dose alteplase for fibrin, with indwelling 70% alcohol for fat, with 0.1 N hydrochloric acid for mineral precipitates, and with either 0.1 N hydrochloric acid or 0.1 N sodium hydroxide for drugs, depending on the pH of the drug.
Metabolic The most common problems caused by parenteral SNS are fluid overload and hyperglycemia (Table 98e-7). Hypertonic dextrose stimulates a much higher insulin level than meal feeding. Because insulin is a potent antinatriuretic and antidiuretic hormone, hyperinsulinemia leads to sodium and fluid retention. Consequently, in the absence of gastrointestinal losses or renal dysfunction, net fluid retention is likely when total fluid intake exceeds 2000 mL/d. Close monitoring of body mass as well as of fluid intake and output is necessary to prevent this complication. In the absence of significant renal impairment, the sodium content of the urine is likely to be <10 meq/L. Provision of sodium in limited amounts (40 meq/d) and the use of both glucose and fat in the PN mixture will reduce serum glucose levels and help reduce fluid retention. The elevated insulin level also increases the intracellular transport of potassium, magnesium, and phosphorus, which can precipitate a dangerous re-feeding syndrome if the total glucose content of the PN solution is advanced too quickly in severely malnourished patients. To assess glucose tolerance, it is generally best to start PN with <200 g of glucose/d. Regular insulin can be added to the PN formula to establish glycemic control, and the insulin doses can be increased proportionately as the glucose content is advanced. As a general rule, patients with insulin-dependent diabetes require about twice their usual at-home insulin dose when receiving PN at 20–25 kcal/kg, largely as a consequence of parenteral glucose administration and some loss of insulin to the formula’s container. As a rough estimate, the amount of insulin provided can be proportionately similar to the number of calories provided as total parenteral nutrition (TPN) relative to full feeding, and the insulin can be placed in the TPN formula. Subcutaneous regular insulin can be provided to improve glucose control as assessed by measurements of blood glucose every 6 h. About two-thirds of the total 24-h amount can be added to the next day’s order, with SC insulin supplements as needed. Advances in the TPN glucose concentration should be made when reasonable glucose control is established, and the insulin dose can be adjusted proportionately to the calories added as glucose and amino acids. These are general rules, and they are conservative. Given the adverse clinical impact of hyperglycemia, it may be necessary to use intensive insulin therapy as a separate infusion with a standard protocol to initially establish control. Once control is established, this insulin dose can be added to the PN formula. Acid-base imbalance is also common during parenteral SNS. Amino acid formulas are buffered, but critically ill patients are prone to metabolic acidosis, often due to renal tubular impairment. The use of sodium and potassium acetate salts in the PN formula may address this problem. Bicarbonate salts should not be used because they are incompatible with TPN formulations. Nasogastric drainage produces hypochloremic alkalosis that can be managed by attention to chloride balance. Occasionally, hydrochloric acid may be required for a more rapid response or when diuretic therapy limits the ability to provide substantial sodium chloride. Up to 100 meq/L and up to 150 meq of hydrochloric acid per day may be placed in a fat-free TPN formula.
|
SELECTED METABOLIC DISTURBANCES CAUSED BY PN AND THEIR CORRECTION |
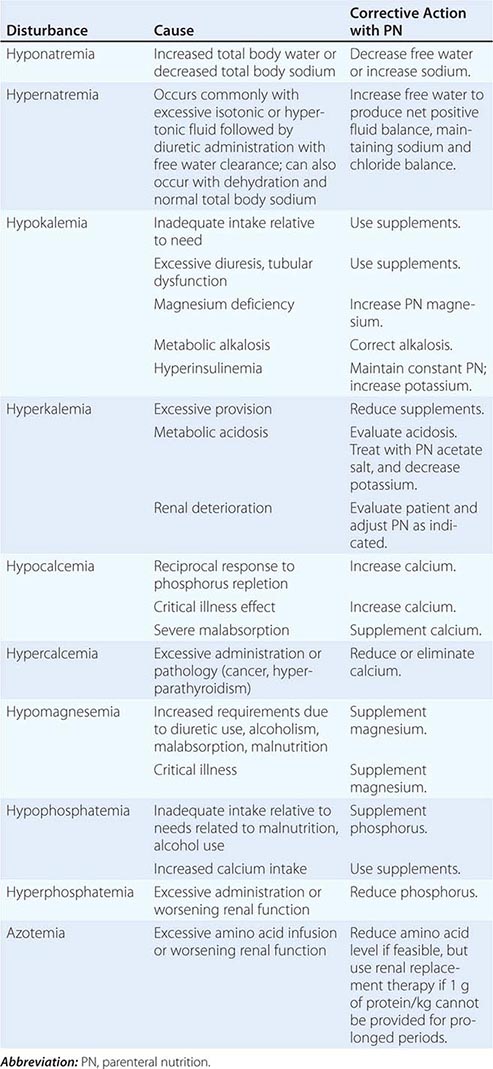
Infectious Infections of the central access catheter rarely occur in the first 72 h. Fever during this period is usually attributable to infection elsewhere or another cause. Fever that develops during parenteral SNS can be addressed by checking the catheter site and, if the site looks clean, exchanging the catheter over a wire, with cultures taken through the catheter and at the catheter tip. If these cultures are negative, as they usually are, the new catheter can continue to be used. If a culture is positive for a relatively nonpathogenic bacterium like Staphylococcus epidermidis, a second exchange over a wire with repeat cultures or replacement of the catheter can be considered in light of the clinical circumstances. If cultures are positive for more pathogenic bacteria or for fungi like Candida albicans, it is generally best to replace the catheter at a new site. Whether antibiotic treatment is required is a clinical decision, but C. albicans grown from the blood culture in a patient receiving PN should always be treated with an antifungal drug because the consequences of failure to treat can be dire.
Catheter infections can be minimized by dedicating the feeding catheter to TPN, without blood sampling or medication administration. Central catheter infections are a serious complication, with an attributed mortality rate of 12–25%. Fewer than three infections per 1000 catheter-days should occur in central venous catheters dedicated to feeding. At-home TPN catheter infections may be treated through the catheter without its removal, particularly if the offending organism is S. epidermidis. Clearing of the biofilm and fibrin sheath by local treatment of the catheter with indwelling alteplase may increase the likelihood of eradication. Antibiotic lock therapy with high concentrations of antibiotic, with or without heparin in addition to systemic therapy, may improve efficacy. Sepsis with hypotension should precipitate catheter removal in either the temporary or the permanent TPN setting.
ENTERAL NUTRITION
TUBE PLACEMENT AND PATIENT MONITORING
The types of enteral feeding tubes, methods of insertion, their clinical uses, and potential complications are outlined in Table 98e-8. The different types of enteral formulas are listed in Table 98e-9. Patients receiving EN are at risk for many of the same metabolic complications as those who receive PN and should be monitored in the same manner. EN can be a source of similar problems, but not to the same degree, because the insulin response to EN is about half of that to PN. Enteral feeding formulas have fixed electrolyte compositions that are generally modest in sodium and somewhat higher in potassium. Acid-base disturbances can be addressed to a more limited extent with EN. Acetate salts can be added to the formula to treat chronic metabolic acidosis. Calcium chloride can be added to treat mild chronic metabolic alkalosis. Medications and other additives to enteral feeding formulas can clog the tubes (e.g., calcium chloride may interact with casein-based formulas to form insoluble calcium caseinate products) and may reduce the efficacy of some drugs (e.g., phenytoin). Since small-bore tubes are easily displaced, tube position should be checked at intervals by aspirating and measuring the pH of the gut fluid (normal: <4 in the stomach, >6 in the jejunum).
|
ENTERAL FEEDING TUBES |
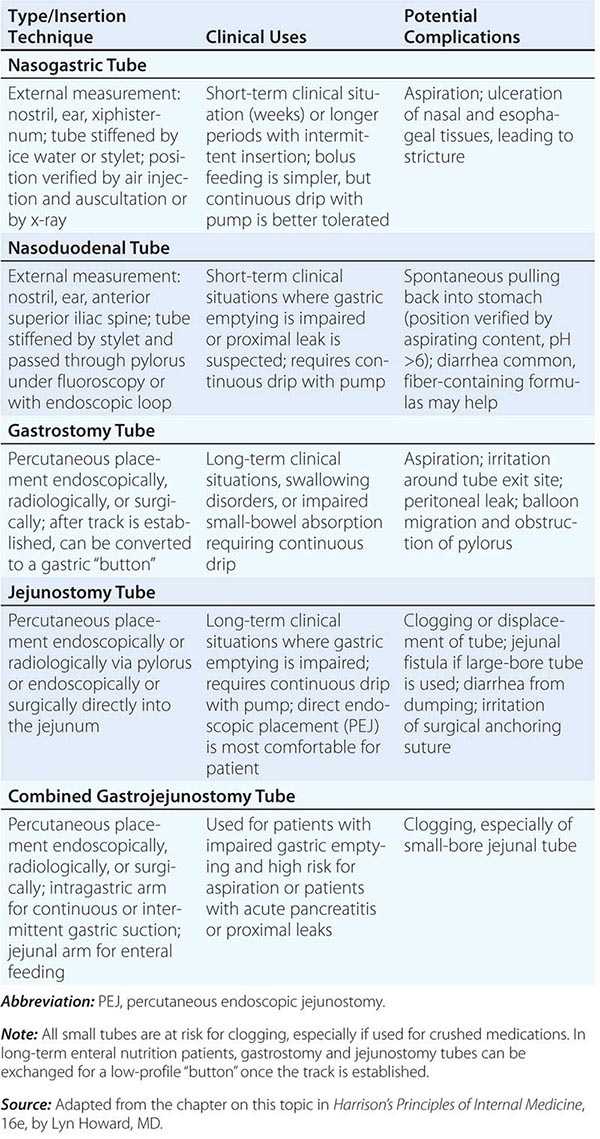
|
ENTERAL FORMULAS |
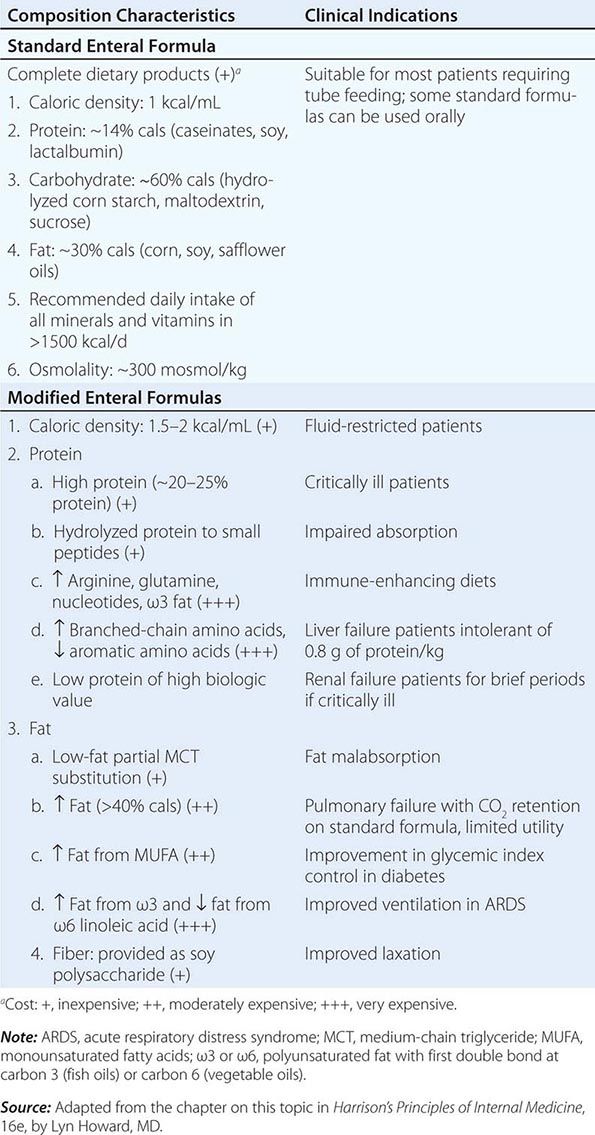
COMPLICATIONS
Aspiration The debilitated patient with poor gastric emptying and impairment of swallowing and cough is at risk for aspiration; this complication is particularly common among patients who are mechanically ventilated. Tracheal suctioning induces coughing and gastric regurgitation, and cuffs on endotracheal or tracheostomy tubes seldom protect against aspiration. Preventive measures include elevating the head of the bed to 30°, using nurse-directed algorithms for formula advancement, combining enteral with parenteral feeding, and using post–ligament of Treitz feeding. Tube feeding should not be discontinued for gastric residuals of <300 mL unless there are other signs of gastrointestinal intolerance, such as nausea, vomiting, or abdominal distention. Continuous feeding using pumps is better tolerated intragastrically than bolus feeding and is essential for feeding into the jejunum. For small-bowel feeding, residuals are not assessed, but abdominal pain and distention should be monitored.
Diarrhea Enteral feeding often leads to diarrhea, especially if bowel function is compromised by disease or drugs (most often, broad-spectrum antibiotics). Sorbitol used to flavor some medications can also cause diarrhea. Diarrhea may be controlled by the use of a continuous drip, with a fiber-containing formula, or by the addition of an antidiarrheal agent to the formula. However, Clostridium difficile, which is a common cause of diarrhea in patients being tube-fed, should be ruled out as the etiology before antidiarrheal agents are used. H2 blockers may help reduce the net volume of fluid presented to the colon. Diarrhea associated with enteral feeding does not necessarily imply inadequate absorption of nutrients other than water and electrolytes. Amino acids and glucose are particularly well absorbed in the upper small bowel except in the most diseased or shortest bowel. Since luminal nutrients exert trophic effects on the gut mucosa, it is often appropriate to persist with tube feeding despite diarrhea, even when this course necessitates supplemental parenteral fluid support. Apart from conditions with drastically diminished small-intestinal absorptive function, there are no established indications for short peptide–based or elemental formulas.
GLOBAL CONSIDERATIONS
![]() In the United States, the only parenteral lipid emulsion available is made with soybean oil, whose constituent fatty acids have been suggested to be immunosuppressive under certain circumstances. In Europe and Japan, a number of other lipid emulsions are available, including those containing fish oil only; mixtures of fish oil, medium-chain triglycerides, and long-chain triglycerides as olive oil and/or soybean oil; mixtures of medium-chain triglycerides and long-chain triglycerides as soybean oil; and long-chain triglyceride mixtures as olive oil and soybean oil, which may be more beneficial in terms of metabolism and hepatic and immune function. Furthermore, a glutamine-containing dipeptide for inclusion in TPN formulas is available in Europe and may be helpful in terms of immune function and resistance to infection, although a recent study using a larger-than-recommended dose was associated with net harm.
In the United States, the only parenteral lipid emulsion available is made with soybean oil, whose constituent fatty acids have been suggested to be immunosuppressive under certain circumstances. In Europe and Japan, a number of other lipid emulsions are available, including those containing fish oil only; mixtures of fish oil, medium-chain triglycerides, and long-chain triglycerides as olive oil and/or soybean oil; mixtures of medium-chain triglycerides and long-chain triglycerides as soybean oil; and long-chain triglyceride mixtures as olive oil and soybean oil, which may be more beneficial in terms of metabolism and hepatic and immune function. Furthermore, a glutamine-containing dipeptide for inclusion in TPN formulas is available in Europe and may be helpful in terms of immune function and resistance to infection, although a recent study using a larger-than-recommended dose was associated with net harm.
ACKNOWLEDGMENT
The authors acknowledge the contributions of Lyn Howard, MD, the author in earlier editions of HPIM, to material in this chapter.