Ensuring Quality in PACSs
Objectives
On completion of this chapter, you should be able to:
• Describe the differences between quality control (QC) and quality assurance activities.
• Define continuous quality improvement (CQI) and its uses in a radiology department.
• Describe the daily and monthly/quarterly QC monitoring activities.
• Discuss the process of daily/weekly QC on laser imagers.
• State the common QC activities used to measure system speed and data integrity.
• Describe several quality assurance (QA) activities used in a digital radiology department.
Key Terms
Acceptance testing
Continuous quality improvement (CQI)
Error maintenance
PACS administrator
Photometer
Quality assurance (QA)
Quality control (QC)
Routine maintenance
Super user
The Joint Commission (TJC)
Quality Aspects
When you think of quality, what is the first thing that comes to mind? Is it the service at a restaurant or the backpack purchased at the beginning of the semester? Both examples deal with quality in some way: one is people-centered, and the other is product-centered. Likewise, radiology has both a people-centered quality and a product-centered quality.
The traditional radiology department with film and chemistry has many quality procedures that must be followed. Many of these same protocols are used in the digital department, but they have been modified to be relevant to digital equipment and processes.
The next section introduces the terms used to talk about quality within a radiology department. The following sections introduce various routines that should be followed in the department to ensure that the picture archiving and communication system (PACS) is functioning properly and that the images are being produced at a certain quality level. Chapter 12 discusses quality issues related to digital radiographic equipment and processes.
Terms of Quality
Quality has always been a part of health care, whether as a service or product. Health care institutions pride themselves on providing the highest quality possible, and they put many measures into place to ensure that the highest quality is provided to each patient. The ultimate focus in health care is to improve patient care and provide a high-quality service so that patients will want to return. Most health care institutions are accredited by The Joint Commission (TJC), formerly known as the Joint Commission on the Accreditation of Healthcare Organizations (JCAHO). This accreditation is voluntary but necessary in many instances to obtain Medicaid certification, hold certain licenses, obtain reimbursements from insurance companies, and receive malpractice insurance. Rather than simply referring to quality, today TJC uses the more encompassing terms continuous quality improvement or total quality management. The next few sections define these concepts and provide examples of some basic applications in a digital department.
Quality Assurance
Quality assurance (QA) can be defined as a plan for the systematic observation and assessment of the different aspects of a project, service, or facility to make certain that standards of quality are being met. QA activities are focused around people and service. In a radiology department there are many processes involved in the day-to-day activities. For example, once a patient has been checked in at the front desk, there is a process that is followed to alert the technologist that a patient is waiting. If this process is not followed, the patient may have to wait for an extended period before a technologist arrives to check for waiting patients. This extended wait time affects patient care in a negative manner, and therefore it will be seen as poor quality of service. A QA measure should be in place to monitor patient wait times to ensure that the process of alerting a technologist that a patient is waiting is working properly.
Most QA activities will produce quantitative data that can be analyzed. These data can be used to monitor the processes and determine whether the process is working as it should and whether the standard of quality has been met.
Quality Control
Quality control (QC) can be defined as a comprehensive set of activities designed to monitor and maintain systems that produce a product. QC measures are instituted to ensure that radiologic procedures are performed safely, are appropriate for the patient, are performed efficiently, and produce a high-quality image. For example, tests are performed on the radiographic room to make sure that all of the parts, such as the collimator, generator, and focal spots, are functioning properly. All of these parts make up the whole of the room, and if one part is off, it can cause harm to the patient or reduce the quality of the examination.
QC measures are required by law to maintain the license for the room or department. The data from the various activities are kept by a designated individual within the department. Most QC activities are part of a QA program, and the data are used to improve the quality of the processes and department. There are three major categories of QC test that are used at various times:
Continuous Quality Improvement
Continuous quality improvement (CQI) tends to focus on the process rather than on the people or the service. The belief is that if the process is good, health care workers will follow it, and service will be good. The CQI process does not replace QA/QC programs. The QA/QC programs focus on maintaining a certain level of quality, not necessarily improving to a higher quality. CQI focuses on improving the process or system within which the people function as team members rather than focusing on an individual’s work.
One of the most important concepts to understand with CQI is that all levels of people within the organization must be involved in the process of improvement. Because CQI focuses not on individuals and their mistakes but rather on the process, each team member is more apt to participate in improving the organization. It is important that everyone participate because if one spoke is not involved, the wheel will fall off, and the quality cart cannot move forward.
PACS Equipment QC
When beginning a QC program, care must be taken to document all surrounding variables so that each quality measure can be repeated without harm to the process. Documentation is very important in any QC activity, and all paperwork must be kept up to date to make a valid performance measure. As with all quality activities, documentation is the most difficult part of the process and the easiest part to not complete. Without the documentation to back up the findings, it will be difficult to prove the need for repair or update of a system.
The next several sections focus on QC activities that should be monitored in a PACS environment, including display quality for both monitor and film, processing speed, network transfer speed, and the data integrity of data that are called back from the archive. This is not an all-encompassing list. Many vendors have suggestions for what should be monitored for their systems. It is very important that the vendor’s list and timetable for these various activities be followed. According to the American College of Radiology (ACR) Technical Standard for Digital Image Data Management: “Any facility using a digital image data management system must have documented policies and procedures for monitoring and evaluating the effective management, safety, and proper performance of acquisition, digitization, compression, transmission, display, archiving, and retrieval functions of the system. The quality control program should be designed to maximize the quality and accessibility of diagnostic information.”
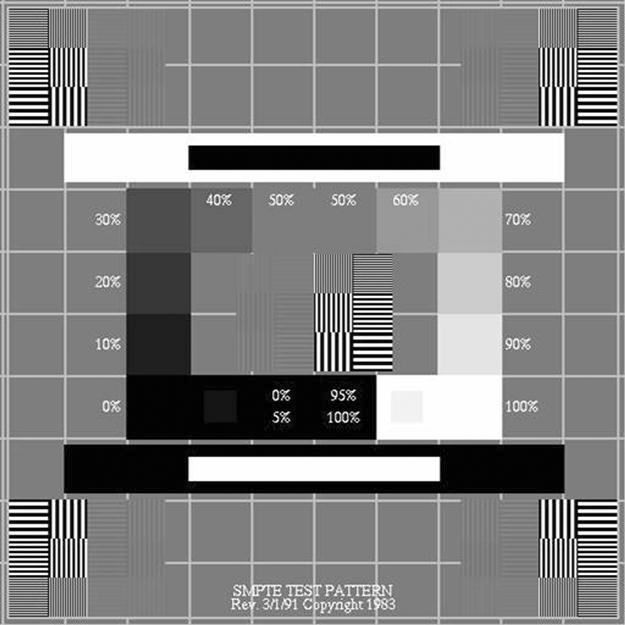
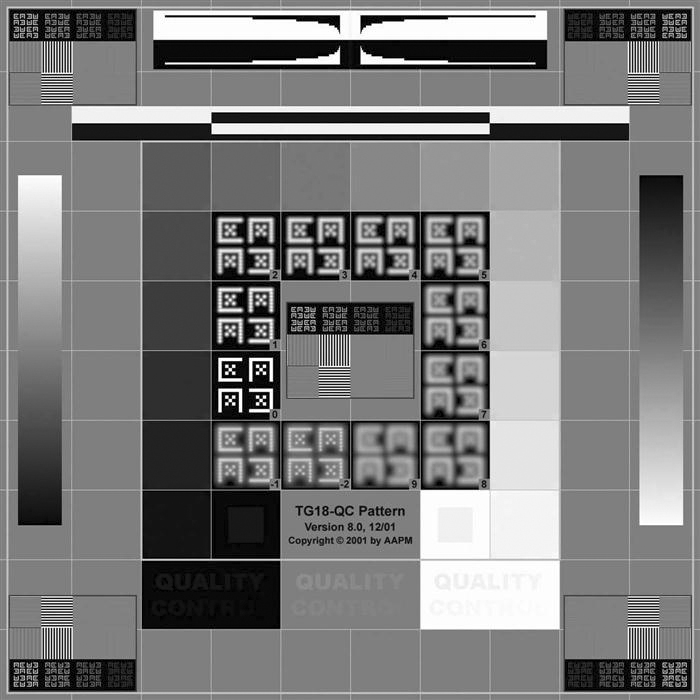
The ACR also suggests that all the quality tests described later in this chapter be carried out with a Society of Motion Pictures and Television Engineers (SMPTE) test pattern (Figure 11-1) to ensure continuity of measurements. A test pattern developed by the American Association of Physicists in Medicine (AAPM) Task Group 18 (TG18) (Figure 11-2) is also becoming more widely accepted for use in these QC tests. The ACR suggests that QC tasks be performed at least monthly, whereas the AAPM has a much more rigorous schedule. The AAPM suggests that the testing be performed on acceptance and annually by a trained physicist, and the daily and monthly/quarterly tests can be performed by a trained QC technologist or a physicist. If any of the following tests fails or produces out-of-range readings, corrective action and continued monitoring should be done. The technologist should follow the radiology department’s policy on equipment maintenance procedures. It may be necessary to contact a radiation physicist to follow up on the findings.
Monitor Quality
The monitor is often the weakest link in the digital imaging chain. The monitor has a direct effect on the quality of the image that is presented to the radiologist for reading or to the referring physician for review. Unfortunately, it is not cost-effective to provide the highest quality monitor for all viewing situations. As discussed in Chapter 2, the radiologist workstation will have the highest quality medical grade monitors, usually 2K or 3K for digital projection images, 1K or 2K for cross-sectional images, and up to 4K for mammography. As with digital cameras, the megapixel measurement may also be used to determine the appropriate monitor. Generally, the physician review workstations and the technologist QC workstations have high-quality commercial monitors; these usually have a resolution of 1K.
The following QC recommendations for display monitors come from the AAPM in its document titled “Assessment for Display Performance for Medical Imaging Systems.” This document outlines testing to be completed both by physicists and by technologists/users. The following sections outline the tasks to be completed by a trained technologist on a daily and monthly/quarterly basis on all monitors used to view images; annual and acceptance testing is conducted by a medical physicist.
Daily Monitor QC
• Turn on the monitor, and allow it ample time to warm up.
• Make sure that the monitor is dust-free on the viewing surface and near the airflow areas.
• Luminance, reflection, noise, and glare: Verify that all 16 luminance patches are clearly visible. If desired, measure their luminance using a luminance meter or photometer, a device used to measure the luminescence of areas on the monitor (Figure 11-3). Evaluate the results in comparison to previous measurements. Make sure that the 5% and 95% patches are clearly visible, and evaluate the appearance of low-contrast letters and the targets at the corners of all luminance patches with and without ambient lighting.

Monthly/Quarterly Monitor QC
• Turn on the monitor, and allow it ample time to warm up.
• Make sure that the monitor is dust-free on the viewing surface and near the airflow areas.
• Retrieve a QC monitor test pattern.
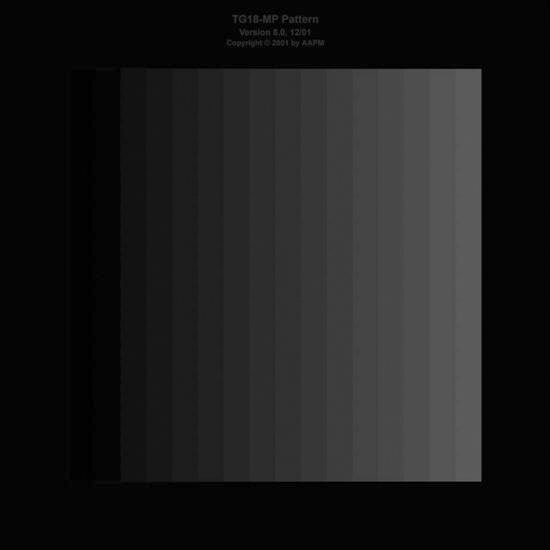
• Luminance response: Using the TG18-LN test patterns (Figures 11-4 to 11-6) and a photometer, measure the luminescence from the center of the monitor for each pattern, and record each reading. Also take a reading using the photometer with the monitor in power-save mode or turned off. This will give a baseline reading for the ambient luminance coming from the monitor. A cathode ray tube (CRT) monitor should have a luminance reading of greater than 170 cd/m2, and a liquid crystal display (LCD) should have a luminance reading of greater than 100 cd/m2. There should also be a greater than 250 cd/m2 difference between TG18-LN-01 and TG18-LN-18 test pattern readings (contrast ratio). Using the TG18-CT (Figure 11-7) pattern, the half-moon targets in the center and the four low-contrast objects at the corners of each of the 16 different luminance regions should be visible. Also the bit-depth resolution of the display should be assessed using the TG18-MP (Figure 11-8) test pattern. The assessment includes determining whether the horizontal contouring bands, their relative locations, and grayscale reversals are within limits. Both patterns should be examined from a normal viewing distance.
• Luminance dependencies: Nonuniformity—the visual method for determining display luminance uniformity—uses the TG18-UN10 and TG18-UN80 test patterns (Figure 11-9). The patterns are displayed, and the uniformity across the displayed pattern is assessed. The patterns should be observed from a normal viewing distance. Angular response may be assessed visually using the TG18-CT test pattern. The pattern should first be viewed on-axis to determine the visibility of all half-moon targets. The viewing angle at which any of the contrast thresholds become invisible should be noted. With the TG18-UNL10 and TG18-UNL80 test patterns (Figure 11-10), luminance is measured at five positions over the monitor (center and four corners) using a calibrated photometer. The five readings should be within 30% of one another.
• Resolution: Using the TG18-QC pattern and the magnifying glass within the PACS software, examine the displayed Cx patterns at the center and four corners of the monitor. The line pair patterns in the horizontal and vertical directions should also be evaluated in terms of visibility, and the average brightness of the patterns should also be assessed using the grayscale step pattern as a reference. Note any difference in appearance of the test patterns between the horizontal and vertical lines. The relative width of the black and white lines in these patches should also be examined using the magnifying glass. The resolution uniformity may be determined by using the TG18-CX (Figure 11-11) test pattern and magnifying glass in the same way that the Cx elements in the TG18-QC pattern were used.







As mentioned earlier, all annual testing and acceptance testing should be performed by a qualified medical physicist. They follow their own standard set of tests to make sure that the monitors are performing up to their capabilities.
Printer Image Quality
Besides the PACS, the printed image is another way to distribute images around the hospital enterprise. As with monitors, there are several steps that should be taken to ensure that the image being seen is of consistent quality.
Wet Laser Imager
Daily/Weekly QC
Dry Laser Imager
Daily/Weekly QC
Speed
Speed is always a concern in the radiology department, whether it is the speed at which patients are brought back for their x-rays or the speed at which the radiologist gets a final report signed. It is no different in a digital department, but there are other considerations when talking about speed: the processing speed of the workstation and the image retrieval/transfer rate.
Workstation Processing Speed.
The workstation processing speed can be measured or documented in many different ways. The following is a practical way for a technologist to monitor the speed of his or her workstation:
After acceptance of the workstation, this procedure should be followed weekly to establish a pattern. If no changes are seen, this procedure can then be done on a monthly basis. Any time the software or equipment is updated, the procedure should be done on a weekly basis until a pattern is established again.
Image Transfer Speed.
Image transfer speed should be monitored from the modality to PACS and from the archive to a workstation. The following steps are an easy way to monitor these transfer speeds:
After acceptance of the system, this procedure should be followed weekly to establish a pattern. If no changes are seen, this procedure can then be done on a monthly basis. Any time the software or equipment is updated, the procedure should be done on a weekly basis until a pattern is established again.
Data QC
Data Integrity.
A constant measure to be monitored is whether all images completed at the modality make it to the PACS. This is usually caught by the radiologist, but it is a good practice to have the technologist monitor this factor periodically. After initial installation, the technologist should check on a daily basis to make sure that all of the images that he or she sent to the PACS arrived on the PACS. If there are no missing images for several weeks, this practice can be scaled back to once a week. The technologist can randomly choose several studies that were sent during the week to determine whether all of the images made it to the PACS.
Another test for data integrity is to periodically pull up images from the archive to make sure the same images sent initially are still in the study after archival. This should also be done on a weekly basis.
After acceptance of the system, this procedure should be followed daily to establish a pattern. If no changes are seen, this procedure can then be done weekly and then on a monthly basis. Any time the software or equipment is updated, the procedure should be done on a daily basis until a pattern is established again.
Compression Recall.
Compression is used to reduce the size of the image files to increase the speed of the network transfer of the images. Compression protocols need to be established by the radiologists and radiation physicist. They will determine the level of compression that will be acceptable for the institution. The following steps are a practical way of observing compression recall of images:
PACS CQI
There are many processes used each day in a PACS environment, and each of these processes should be monitored to make sure that the PACS is functioning up to its capabilities. Remember that CQI activities revolve around process rather than people and systems. The next few sections describe several simple CQI activities that need to be monitored in a radiology department. Many activities that are monitored before the digital conversion of the department should continue after the conversion. Different PACS vendors may have different activities that they recommend. All of these activities should be adhered to so that the PACS will run up to its potential, and problems can be found before they cause major system downtime.
Recognition of Nondiagnostic Images
One CQI activity that should be monitored is the documentation of nondiagnostic images being forwarded to the PACS. This activity will be primarily carried out by the radiologists. If a poor-quality image is detected by the radiologist, the study and performing technologist are noted, and the information is shared with the lead technologist or the PACS administrator to follow up with the performing technologist.
The radiologist may note the areas and reasons for the poor-quality image. If the poor-quality image was caused by equipment malfunction, the appropriate QC test should be carried out, and the appropriate service protocol followed. If the poor-quality image was operator error, additional training or counseling by the supervisor may be required.
System Up-Time
Another common QA activity is the monitoring of how often the system is down for any reason. A log should be kept to note any time that the system is down. Also in the log note the reason, how long, what had to be done to fix the problem, and who fixed the problem. If the same problem continues to occur, this log can be used to prove either that a piece of equipment needs to be replaced or that additional service is needed.
System Training
System training is an important activity that must never stop. One of the early misconceptions related to installation of a PACS is that the vendor applications training will be sufficient for training all staff that interact with the PACS. This is far from the truth. The vendor applications personnel are usually on site for 1 to 2 weeks during initial installation. The vendor applications training is supposed to train several super users (people who are trained on all aspects of the system and are prepared to train others) and help set up the system to site specifications.
The super users and the PACS administrator (the person trained to oversee the PACS) need to set up an ongoing training program. The training program must include several skill levels, from the radiologist to the technologist to the ancillary personnel. Each new employee needs to be trained on the system. Each time that a new version of the software is installed, the training protocol needs to be revised; retraining of existing personnel may be necessary. Each department also has a list of skills that are tested and retrained each year. PACS skills should be included in this annual training. A training record should be kept for each employee to show proof of skills.
Summary