Energy Devices
Electrosurgery—Laser—Harmonic Scalpel
Energy-releasing devices have been used in the past and currently are employed in pelvic surgery. The raison d’être for such tools consists of hemostasis and speed.
Compared with cold knife or scissor cutting, energy devices create a greater degree of surrounding tissue damage, usually in the form of thermal injury leading to necrosis, devitalization, subsequent fibrosis, and scar formation. Because of the aforesaid events, tissues neighboring the operative site are vulnerable to injury by a variety of mechanisms. The surgeon, his or her assistants, and supporting nursing staff must be fully acquainted with these devices and with the mechanisms by which each device produces desired and undesired actions. The aforesaid exercise is intended to protect a patient from unintended injury.
Electrosurgery
Two terms misused relative to electrosurgery are cautery and bovie. A cautery is rarely used in a modern operating room. It refers to heating of a conducting metal (e.g., an iron poker, a branding iron, an electric stove top heating element) until it has reached sufficient temperature such that the iron glows red. The heat of the device makes direct contact (e.g., severed limb stump), thereby cauterizing open vessels and quenching the flow of blood. In 1928, William Bovie, a physicist, and Harvey Cushing, a neurosurgeon, developed an electrosurgical unit capable of cutting and coagulating.
The Bovie unit was thus an early spark gap generator, which has been for many years obsolete. Contemporary microprocessor-controlled electrosurgical units are not Bovie units.
The following four terms are of key import for understanding the physics and tissue interactions of electrosurgery units:
current
voltage
resistance
power
Current refers to the flow of electrical charges. Without current flow, no electrosurgical action would happen. It is measured in amps (Amperes). The action of the electric generator produces a current within a complete electrical circuit. Current flows in the direction of positive charges.
For work to be accomplished, electrical charges must be moved from one point to another (i.e., the difference in potential between two points is expressed as volts [a potential force]). Impedance to the conduction of electrical current through a given medium is referred to as its resistance and is expressed in ohms. The relationship of current, potential, and resistance is expressed as Ohm’s law:
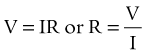
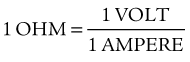
Power is equivalent to work performed over a period of time and is expressed in watts.
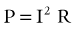
or
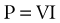
Two major types of current flow are described: direct and alternating. In the United States, electrosurgery utilizes radio-requency (RF) (>100,000 Hertz or cycles per second) alternating current to cut or coagulate tissue.
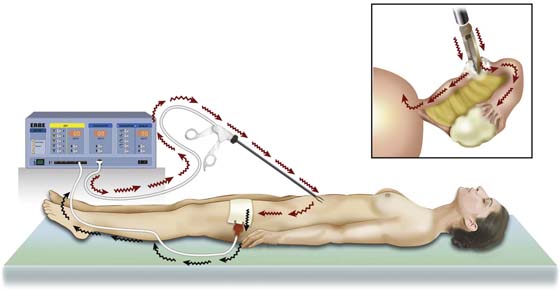
A monopolar circuit travels from the electrosurgical unit (ESU) via a copper wire to an electrode, where vaporization (100°C) [i.e., cutting or coagulation] (60°C) occurs.
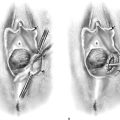
The current is then conducted through the patient’s body, usually via the great blood vessels, and is returned to the ESU via a neutral electrode (ground plate), which is also connected by a copper wire to the ESU (Fig. 5–1).
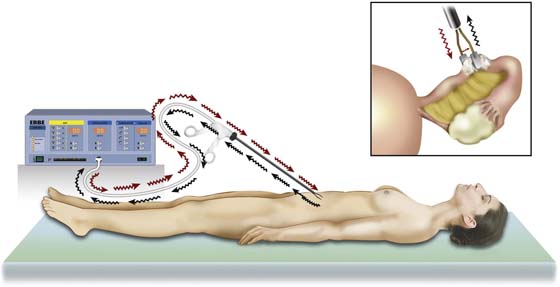
A bipolar circuit consists of two wires leaving the ESU; the first wire is connected through a two-part electrode to the portion that serves as the active electrode. The second portion, which serves as the return or neutral electrode, is connected to the second wire, which returns the current to the ESU. The advantage of the bipolar system is obvious. Electrical current flows only between active and neutral electrodes. Tissue action is observed only between the electrodes. Thus no current will traverse the patient’s entire body, as is the case with monopolar circuits (Fig. 5–2).
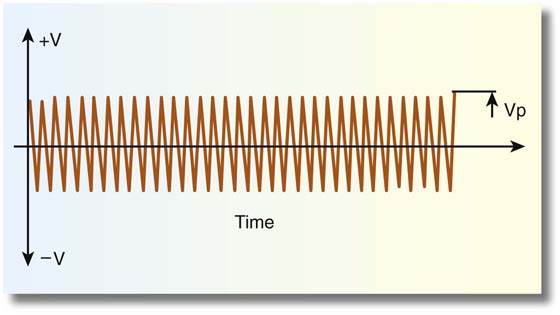
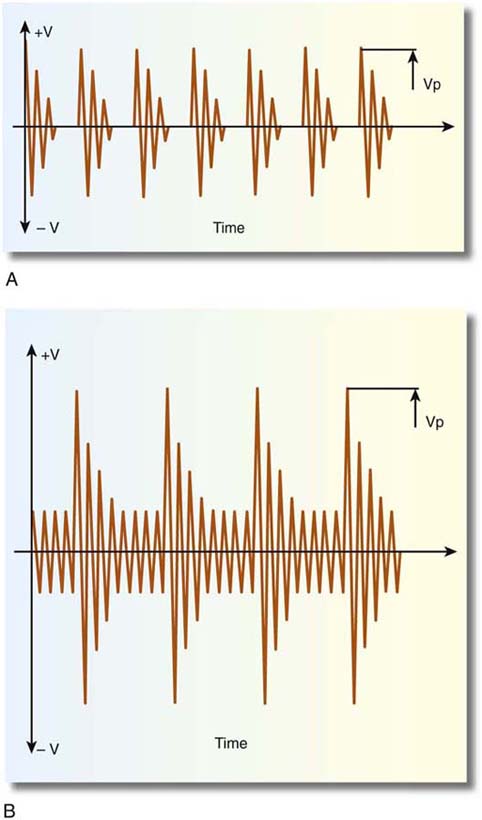
Cutting versus coagulation waveforms can be visualized on an oscilloscope (Fig. 5–3). Cutting is distinguished by (unmodulated) sine wave form characterized by high current flow, low peak-to-peak voltage, and rapid attainment of high tissue temperatures (e.g., 100°C) with attendant cellular vaporization. The best cutting and least coagulation artifacts occur with peak voltages ranging from 200 to 600 volts (Fig. 5–4A, B).
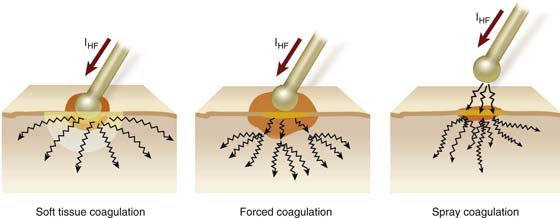
In contrast, electrocoagulation is modulated and exhibits lower current flow and higher voltages (Fig. 5–5). During coagulation, heating occurs less rapidly and at lower temperatures (60°C–70°C), rendering the cell dried or desiccated, because ions and water are driven out of the cells; resistance to flow increases as the cells lose conducting ions. Fulguration (spray coagulation) occurs when the coagulating electrode is held close to the tissue target but does not touch the tissue. Here very high voltages are required to allow the spark to jump across the air space and coagulate the cells. Typically, fulguration creates superficial coagulation as opposed to deeper penetrating contact coagulation (Fig. 5–6).
FIGURE 5–1 This illustration shows the electrical current flow with a monopolar circuit. Active current leaves the electrosurgical unit (ESU) and flows through the grasping forceps to create high current density where the forceps jaws close on the tissue (inset). The current is conducted through the patient’s body to exit over a large surface area (ground plate) and return to the ESU.
FIGURE 5–2 This illustrates a bipolar circuit. The current form in the electrosurgical unit (ESU) flows through an insulated conductor of the bipolar forceps to exert its thermal action on the tissue (inset). The current flows from the active jaw (electrode) to the inactive (neutral) jaw of the electrode. The current flows back to the ESU via the insulated neural limb of the bipolar forceps. Note that current flow to tissue is limited to that which is enclosed between active and neutral electrodes (forceps jaws).
FIGURE 5–3 A typical oscilloscopic pattern for “cutting current.” Note that the peak-to-peak voltage is relatively low and there is no modulation of amplitude. Current flow is high.
FIGURE 5–4 A. As voltage increases, the relative size of the electrical spark also increases. The effect on tissue of increased voltage is an increase in the area of coagulation artifact. B. A cutting loop electrode is illustrated here cutting into the cervix. The electrosurgical unit (ESU) foot pedal is activated just before the loop makes contact with the cervix. This creates an open circuit. Relatively high voltages are created as the electrode encounters the cervix. This is notable by high resistance and high thermal temperatures, thereby creating carbon formation (black). As voltage is diminished, current flow is picked up and the tissue is vaporized with little coagulation artifact. When the electrode exits, high temperatures again create thermal artifact.
FIGURE 5–5 Frequency modulation produces high-voltage (peak-to-peak) intermittent bursts (i.e., noncontinuous output). This results in less current flow and higher resistance. Temperatures are elevated more slowly and are at subvaporization levels (i.e., coagulating).
FIGURE 5–6 Constant-voltage electrosurgical units (ESUs) can precisely vary peak-to-peak voltages, thereby allowing a variety of coagulation modalities. Soft coagulation occurs at peak-to-peak voltages ≤200 volts. Deeper coagulation may be achieved at peak-to-peak voltages ≥600 volts (i.e., forced coagulation). Spray coagulation creates superficial coagulation. The electrical spark must transverse the air space between the electrode and the tissue. This requires peak-to-peak voltages ≥100 volts.
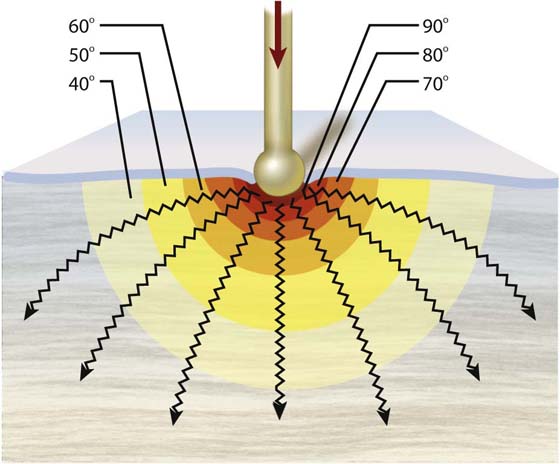
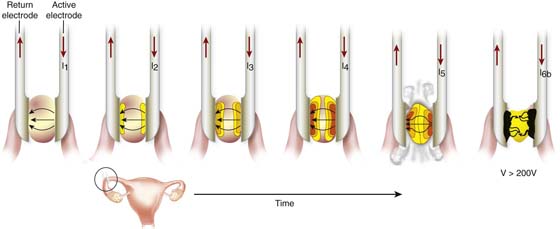
During the coagulation cycle, high temperatures are reached within close proximity to the electrode. Thermal conductivity spreads the heat action peripheral to the electrode-tissue interface. This is an important concept that surgeons must understand because structures in proximity to the coagulation target may be thermally damaged by spreading conductive heat (Figs. 5–7 and 5–8).
Several hazards related to electrosurgery are illustrated in the chapters that discuss endoscopic complications (laparoscopic and hysteroscopic).
Laser Surgery
A laser is a device that generates an energized light beam (light amplification by stimulated emission of radiation). This stimulated radiation in turn is utilized for surgery. Laser action on tissue is the result of conversion to heat (thermal), shock waves (fracture of tissue), or photochemical reactions (interaction with a dye or chemical compound).
Many actions of a laser depend on the ability of the light beam to be absorbed. Some beams are reflected from a tissue interface and exert no action. Depending on the energy of the incident laser beam, it will penetrate tissue to variable depths and will be stopped only when the incident energy has been fully absorbed.
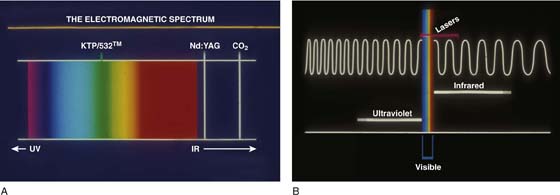
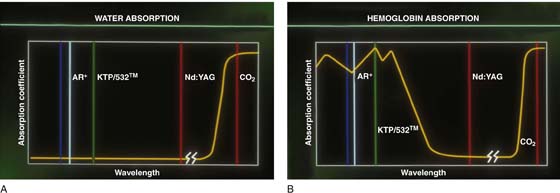
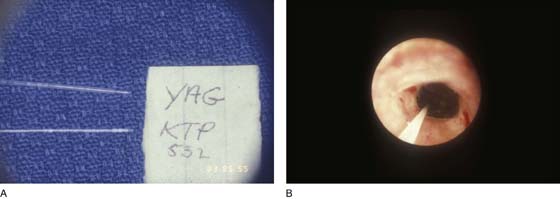
Because laser beams are produced across the electromagnetic spectrum, they may be absorbed selectively; this in turn is based on wavelength (Fig. 5–9A). For example, argon and KTP/532 lasers emit in the visible bands at 0.51 micron and will be selectively absorbed by hemoglobin-containing areas (e.g., varicosities, hemangiomas) (Fig. 5–9B), whereas a carbon dioxide (CO2) laser (10.6 microns) emitting in the far infrared is absorbed by water very efficiently and likewise by all tissues, regardless of color. The neodymium (Nd)-yttrium aluminum garnet (YAG) laser actually penetrates water (i.e., is not absorbed and principally coagulates tissue via front scatter). Several lasers are efficiently transmitted by flexible fibers (e.g., argon, KTP, Nd:YAG, holmium [Ho]-YAG). The CO2 laser is not transmitted well by fiber but traverses air and exerts its actions without directly touching the tissue (Fig. 5–10A, B).
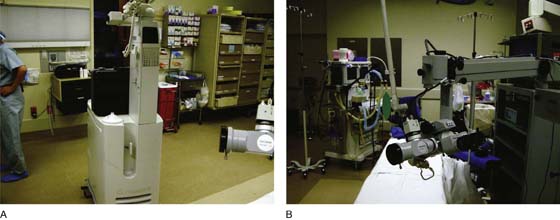
The CO2 laser has been used as a cutting, vaporizing coagulation tool for gynecologic surgery (Fig. 5–11). Through use of its property of being effectively absorbed by even small amounts of water, the penetration of a CO2 laser beam can be precisely controlled (heat sink action). The tissue actions of this laser depend on several variables.
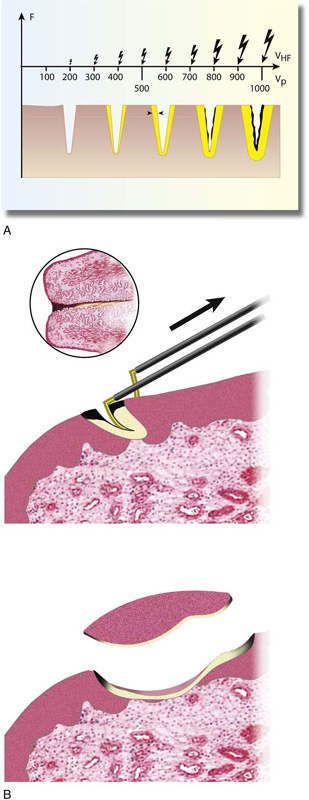
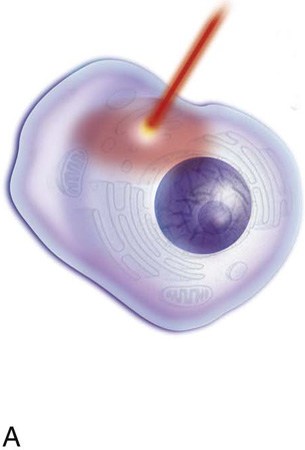
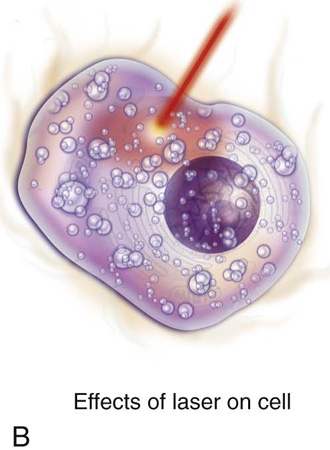
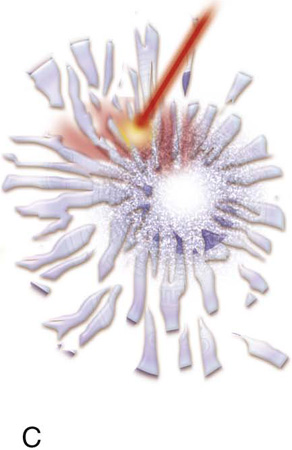
The diameter of a laser beam may be controlled by focusing it through a lens. A tightly focused beam (< 1 mm) will be rapidly absorbed by tissue cells. Light energy is instantaneously converted to heat energy, causing intracellular water to boil at 100°C; this is followed by conversion to steam, which causes the cell to literally blow up (Fig. 5–12). Explosive evaporation or vaporization results in the disappearance of a mass of cells. Moving the linear laser beam will produce an incision or cut. When the laser beam is out of focus (i.e., defocused or >2 mm in diameter), the absorbed beam is spread out over a larger area, which creates temperatures of 60°C to 80°C, thus (desiccating) coagulating tissues, rather than vaporizing them (Fig. 5–13). The CO2 laser may be delivered to tissue via a handpiece, a wave guide, or a micromanipulator (Fig. 5–14A, B).
The concept of expressing laser tissue effects in terms of power density (PD) is desirable:
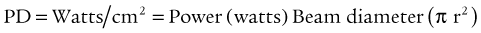
A simple empirical formula allows close and rapid calculation of the PD:
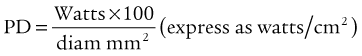
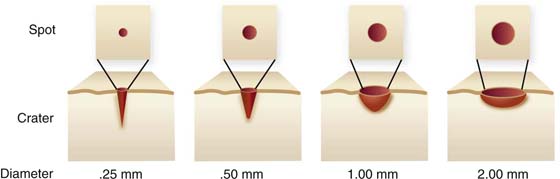
As the reader can readily understand, the most efficient way to raise the power density is to diminish laser beam diameter or spot size (see Fig. 5–13). Conversely, the most effective way to reduce penetration and decrease power density is by increasing the spot size (increasing beam diameter).
The Nd:YAG laser (10.6 microns) is commonly utilized for hysteroscopic and laparoscopic surgery because it penetrates water and other fluids, is a very effective coagulating device, and is efficiently transmitted by flexible fibers (e.g., quartz), which range from 0.5 mm to 1 mm or more in diameter (Fig. 5–15A, B). This allows the laser to be delivered through the operating channels of even small endoscopes. The same may be said for the Ho-YAG laser, which is an efficient cutting device.
Lasers are desirable tools because they do not conduct through tissues (e.g., electrosurgery) and are not dependent for fluid penetration/absorption on hypotonic fluids. There is no danger of electroshock. Clearly, lasers can accomplish certain things that other tools cannot.
FIGURE 5–7 Every surgeon must be cognizant that heat spreads via conductivity through tissue. The highest temperatures are recorded in the immediate vicinity of the electrode-tissue interface. As thermal energy spreads concentrically, the temperature decreases. Time of electrode contact is a critical factor relative to the distance to which harmful heating action affects tissues.
FIGURE 5–8 This illustration details the thermal action of bipolar electrodes. The tissue between the forceps arms heats to coagulation temperatures as a function of time-on-tissue. As a critical point, vaporization begins to happen as temperatures approach 100°C. Ions are driven out of the cells, thereby increasing resistance to current flow (i.e., a vapor barrier is created). If power is not increased, then electronic conduction ceases. If power is increased to permit sparks to penetrate the vapor barrier, then superheating of tissue results in carbonization when temperatures approach or exceed 400°C.
FIGURE 5–9 A. A schema of visible and invisible parts of the electromagnetic spectrum is shown here. Note that the KTP/532 laser emits in the visible green. The helium-neon laser emits in the visible red. The neodymium-yttrium aluminum garnet (Nd:YAG) laser and the carbon dioxide (CO2) laser emit in the infrared (near and far, respectively) and are not visible. B. This picture details the wavelengths of light within the spectrum. Note the very small visible band, which was magnified in Figure 5–9A.
FIGURE 5–10 A. Water absorption according to laser wavelength is detailed here. Note the high level of absorption for the carbon dioxide (CO2) laser. B. Selective absorption for hemoglobin occurs at the wavelengths where argon lasers and KTP/532 lasers operate.
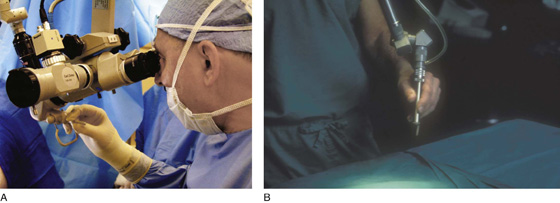
FIGURE 5–11 A. A high-power output carbon dioxide (CO2) laser is shown here. The vertical structure contains the CO2 laser tube. This laser is capable of superpulse and continuous modes. B. The laser arm couples to the operating microscope. The laser beam is precisely controlled via a micromanipulator. Note the cube-sized three-chip video cameras mounted to the beam splitter on the left side of the microscope.
FIGURE 5–12 The schematic pictures depict laser tissue interaction. The laser light beam is absorbed by the cell(s). A. Light energy is instantaneously converted to thermal energy. Cell water heats rapidly and begins to boil at 100°C. B. The water converts to a gaseous state (steam), which expands and explodes the cell and its contents. C. This process is referred to as explosive evaporation (vaporization).
FIGURE 5–13 The depth of a laser wound is controlled by a series of factors. The power setting of the laser beam is a clear factor. More important is the laser beam diameter or spot size. A tightly focused laser beam will create a deep conical crater because the power density is high. A defocused beam or spot will create a wider, shallower, bowel-shaped crater. The latter has a lower power density. The sharply focused beam creates less coagulation, whereas the defocused beam creates more coagulation.
FIGURE 5–14 A. This magnified view shows the surgeon controlling the laser beam by means of a micromanipulator. B. The laser handpiece provides the surgeon with an alternative delivery system for a carbon dioxide (CO2) laser beam.
FIGURE 5–15 A. Neodymium (Nd)-yttrium aluminum garnet (YAG), KTP/532, holmium-YAG, and argon laser beams may be delivered to tissue by means of fine optical fibers. These lasers will penetrate water rather than being absorbed by it. B. This hysteroscopic photo shows an Nd:YAG laser fiber, which is delivered via the operating channel of a hysteroscope to the interior of the uterus. The result of an endometrial ablation is clearly visible.
Ultrasonic Surgery
Ultrasonic radiation results in energy outputs, which may be applied to diagnosis (sonography) and to surgery. The latter requires much higher power density compared with the former.
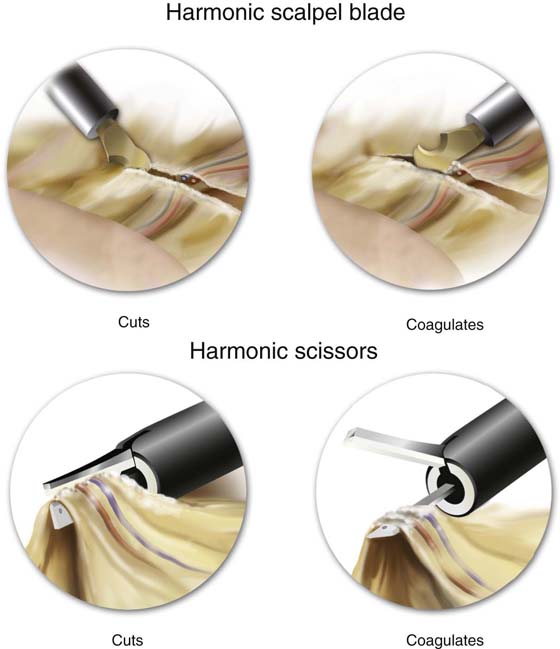
Two techniques and devices of surgical usage have been described: the cavitron ultrasonic surgical aspirator (CUSA) and the harmonic scalpel.
The ultrasonic aspirator has been used extensively for radical oncology surgery. This device dissects and creates hemostasis by coagulating vessels of up to 1 mm in diameter and atraumatically exposing vessels of larger diameter. Typically, tissues with higher water composition are selectively removed, whereas fibrous collagen, elastin-bearing tissues are not damaged. The CUSA simultaneously irrigates and suctions away debris, thereby maintaining a clear operative field. Unlike with electrosurgery or laser surgery, smoke vapor is not produced. However, a mist of fine particulate matter is produced, and the surgeon must take precautions to avoid contamination via contact or inspiration. Ultrasonic devices act on tissue by three mechanisms:
Viscous stress: Creates microbubbles, which may lead to cellular membrane disruption.
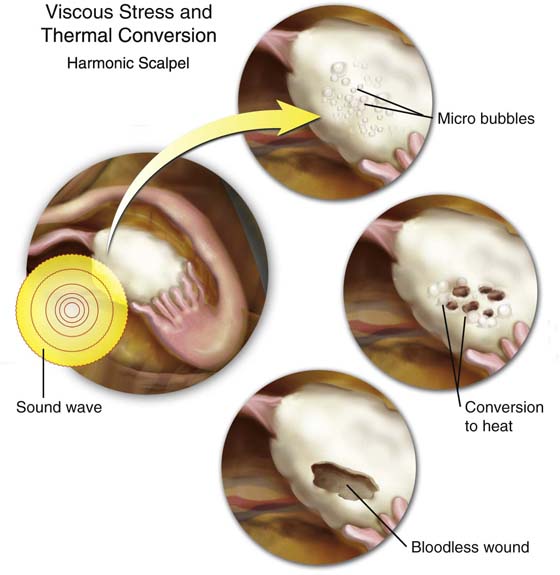
Thermal conversion: The sound wave is absorbed with conversion to heat. Fibrous, collagenous tissues absorb the waves more efficiently and demonstrate greater thermal coagulation effects. Additionally, the vibrating surgical tip of the transducer becomes hot as the result of friction (Fig. 5–16).
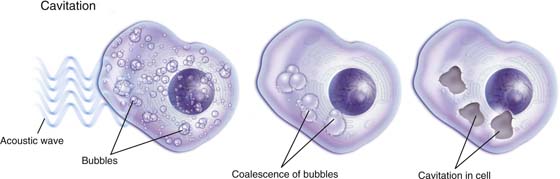
Cavitation: Fluid motion and shear stress perpetuate and reinforce ultrasonic wave absorption, creating progressively greater acoustic energy dissipation. This action results in alternate expansion and collapse of bubbles with similarly alternating conversion of liquid to gas (vapor) and back from gas to liquid. Because of the coinciding acute variance in pressure gradients, cellular cavities are created with an end point of cell disruption. The aforesaid events are clearly affected by increased exposure time to the sound waves (Fig. 5–17).
Both CUSA and harmonic scalpel utilize a piezoelectric crystal as the source of sound waves. The CUSA vibrates at 23 kHz, and the harmonic scalpel vibrates at 55.5 kHz with a linear blade motion of 50 to 100 microns (Fig. 5–18). Several variables determine the speed and action of the device (Fig. 5–19). These include the following:
Power setting (the highest power setting is associated with the greatest vertical excursion of the blade and the sharpest cutting effect).
Blade thinness (a honed or beveled blade surface will produce the most effective cutting action; in contrast, a thicker, nonsharp surface will result in inefficient cutting).
Tissue stretch (taut tissue is cut faster and with reduced coagulation artifact).
Grip pressure (the greater the grip pressure on a scissors-like device, the less the coagulation action).
As with electrosurgery and laser surgery, ultrasonic surgery is increasingly applied to endoscopic techniques. The harmonic scalpel tends to perform more slowly than comparable electrosurgery and laser devices. Nevertheless, it is an alternative energy device that has its peculiar set of advantages.
FIGURE 5–16 The harmonic scalpel delivers high-frequency sound waves to tissue. The effects of these sound waves are to cut tissue and coagulate small blood vessels. The actions of viscous stress and friction leading to heat (thermal) conversion are illustrated.
FIGURE 5–17 Cavitation is created by sound waves impinging on cells and creating microbubbles, which in turn coalesce into larger bubbles. The latter collapse and create holes or cavitation artifacts within the cell.
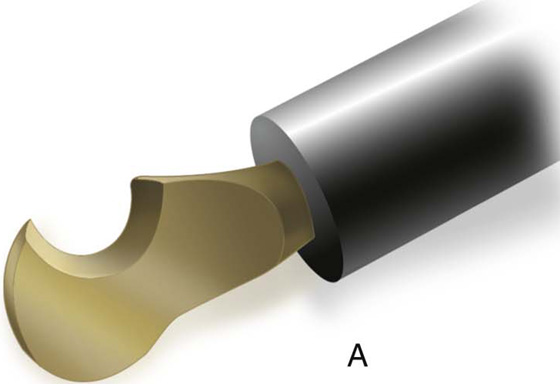
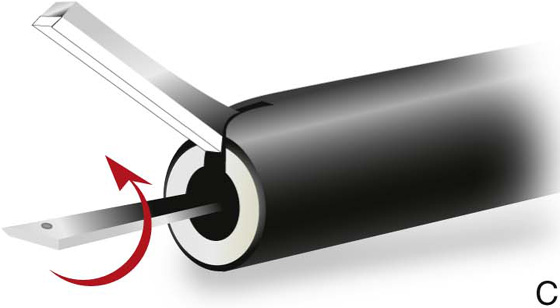
FIGURE 5–18 A. A harmonic scalpel hooked blade. The beveled surface is utilized for cutting. The convex, thicker, outer surface will coagulate tissue. B. The scissors-like device cuts tissue with the lower blade on edge. C. When the blade is rotated flat, the device coagulates tissue.
FIGURE 5–19 The tissue actions of the harmonic scalpel are illustrated here. Tissue stretch is an important factor for efficient cutting action and reduction of friction-generated heat.