Chapter 92 Endovascular Treatment of Head and Neck Bleeding
The role of endovascular therapy in the treatment of neurologic disease has had a relatively short history. Since its initial introduction by Luessenhop and Spence in 1960, the technological improvements and subsequent indications for the use of endovascular techniques have evolved dramatically.1 The advances in polymer science, device design, and technique development have resulted in the maturation of this specialty and its integration into neurosurgical management. Indeed, the last 20 years have demonstrated endovascular surgical neuroradiology’s complementary role in several vascular disorders. One of the areas where this complementary relationship exists is in the treatment of traumatic injury to the vessels of the head and neck.
Though the incidence of blunt traumatic vascular injury is relatively rare—variably reported between 0.1% and 0.45% in trauma centers treating carotid injury—such injuries can be associated with high morbidity and mortality that have been reported from 20% to 40%.2 Intracranial injury secondary to blunt trauma or penetrating trauma can range from subintimal dissection with possible ischemia, or pseudoaneurysm formation with potential rupture, to acute or delayed traumatic aneurysm formation and frank transection with subsequent hemorrhage or arteriovenous fistula formation. The role of endovascular therapy in the setting of these pathologic entities not only has grown but also has expanded into areas where previous treatment options were very high risk or unavailable. This chapter thus discusses the role of endovascular therapy as it relates to the multidisciplinary treatment of acute vascular injury of the head and neck.
Evaluating Effects of Ischemia
Basic principles of surgical management should be followed prior to advancing to therapeutic interventions. Control of airway and breathing, along with establishment of venous access for appropriate fluid resuscitation, are a priority.3 The patient is usually under minimum sedation, allowing accurate neurologic assessment. If complete internal carotid artery (ICA) occlusion is necessary, collateral supply should be measured prior to occlusion to determine the effects of ischemia at the distal ICA segment. The standard procedure for assessing collateral sufficiency in the stable patient is balloon test occlusion (BTO), as introduced by Serbinenko.4–7 BTO of the ICA is designed to identify patients who are at risk for ischemic events following permanent ICA occlusion and to minimize the associated rates of complication.8 The location of the balloon at the time of inflation depends on the location of the ICA lesion and the type of balloon, but the procedure should always be performed under road map guidance. Modern BTO implies simultaneous anatomic and physiologic assessment before and after inflation.
Anatomic testing consists of visualization of collateral circulation through the circle of Willis. Collateral circulation may be assessed in a number of ways, including transcranial Doppler sonography, electroencephalography, quantitative cerebral blood flow (CBF) analyses, and qualitative CBF analyses. Any of these studies represent an attempt to identify those patients with compromised hemodynamics despite a normal examination.5,6 There has also been success with use of delayed venous phase protocols in assessing collateral adequacy.8 A negative test occlusion, according to venous phase protocol, is when the delay of venous drainage between the territory of the injected artery and the occluded hemisphere is 2 to 3 seconds or less. The major advantages of relying on venous phase criteria are that the neurologic assessment is unnecessary and the patient may be placed under general anesthesia.
Physiologic testing traditionally includes a neurologic assessment (standard Wada) with or without electroencephalogram (EEG) monitoring.5,9 EEG monitoring is primarily used for patients in whom the neurologic assessment may not be accurate. The addition of physiologic stressors—such as a hypotensive challenge, acetazolamide, or carbon dioxide challenge—represent an effort to identify patients who have a deficient circulatory reserve that would not be elucidated in a normal physical exam. A hypotensive challenge is performed with agents such as nitroprusside or labetalol, and blood pressure is brought down to 75% to 66% of baseline for 20 minutes. Confounding factors such as tumor-related deficits, hemorrhage- or vasospasm-related infarction, and embolic infarction have brought the predictive value of these challenges into question.4 In patients who tolerate BTO, the surgeon can proceed to carotid occlusion. If technical problems occur, or if the patient demonstrates evidence of ischemic deficits, patency of the carotid artery must be maintained.10
Blunt and Penetrating Injuries of the Head
Carotid–Cavernous Fistulas
CCF or caroticocavernous fistula is the result of a tear of the ICA that allows it to form a high-flow, low-resistance fistula with the venous system of the cavernous sinus.4,11 A CCF can be either direct or indirect. In a direct carotid–cavernous fistula (DCCF), blood is shunted from the ICA into the sinus; in an indirect carotid–cavernous fistula (ICCF), there is a dural arteriovenous communication and a slower flow rate. Barrow et al.12 classified direct, trauma-induced, high-flow shunts between the ICA and the cavernous sinus as a type A fistula. In contrast to dural-based fistulas, spontaneous cure of a type A CCF is rare.13,14 However, rare cases of spontaneous DCCF are found in cases of ruptured intracavernous ICA aneurysms and in patients with collagen vascular disease.15 A comprehensive description of the dural CCF is beyond the scope of this chapter.
The pressure gradient in a DCCF results in reversal of flow into the superior ophthalmic vein and superficial middle cerebral vein, with concomitant rapid shunting to the inferior petrosal sinus and the pterygoid vein.16,17 Classical presentation of DCCF is a pulsating exophthalmos with orbital bruit. Other symptoms may include visual changes, orbital pain, and proptosis. It is evident that most symptoms are a direct result of arterialization of the cavernous sinus and draining orbital veins.18–20 Some common sequelae encountered include venous congestion, hemorrhage, headache, tinnitus, vertigo, and cranial nerve palsies.21 In patients showing evidence of arterial steal phenomenon, such as cerebral hypoperfusion with subsequent focal neurologic deficits, urgent intervention is indicated.
Management of CCF depends on the stability of the patient, the anatomy of the fistula, and the hemodynamics involved in the system. Ideally, the focus of management should be on repair or obliteration of the tear or communication while preserving flow through the ICA.5 Sometimes, complete occlusion of the artery may be necessary. In 1973, Parkinson described a direct surgical repair of a traumatic CCF with preservation of the ICA. While any procedure in this anatomic region is delicate, open repair in the acute setting amid potential polytrauma carries a significant risk of morbidity, so endovascular repair, if tolerated by the patient, is the method of choice.22
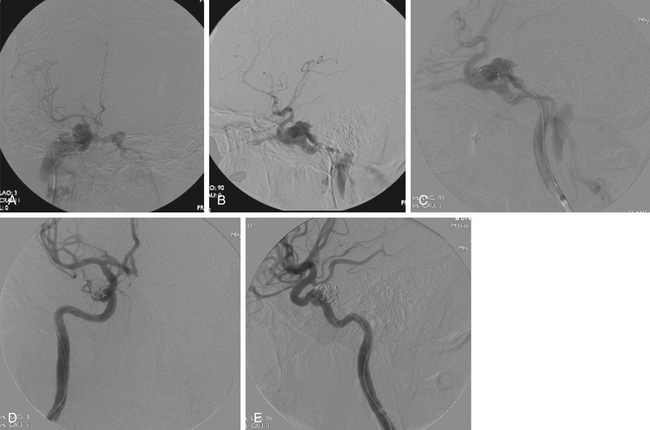
Approach of the CCF may be performed via transarterial or transvenous routes.11,18 The transvenous approach consists of retrograde catheterization and embolization of the venous structure draining the fistula. A venous route is only appropriate if the diseased, venous portion of the system is permanently occluded—and only if occlusion of this venous outflow does not compromise the drainage of the surrounding neural structures. Transarterial routes are more selective. Using a microcatheter, access to the fistula is provided by the arterial branches supplying it, and these pedicles are selectively occluded.23 The number of vessels occluded, and the route chosen to occlude them, are necessarily linked to the choice of embolic material. Today, the primary methods for endovascular embolization employ detachable coils or liquid embolic agents (Fig. 92-1).
Transarterial versus Transvenous Approach
Transvenous embolization is possible via the femoral vein or jugular vein and inferior petrosal sinus, or it may be possible by directly accessing the ophthalmic vein.24 The goal of treatment is to thrombose the cavernous sinus, with the assumption that spontaneous resolution of fistula will follow. If the draining sinus is occluded but the upstream fistula is neglected, the high flow system forces drainage through other veins, potentially draining into the cortical venous system.17,24–26 Cortical venous drainage in the setting of these shunts may result in potentially fatal intracranial hemorrhage.27
The transvenous approach for embolization, reported by Debrun in 1981, has become the treatment of choice for ICCF28 and for some traumatic dural arteriovenous fistulas. However, for reasons already discussed, it is not ideal in the management of DCCF.20 For instance, when treating traumatic dural fistulas of the cavernous sinus, the inferior petrosal sinus is the simplest and shortest venous route to the cavernous sinus. A guiding catheter is introduced via a femoral sheath and resides in the internal jugular vein near the inferior petrosal sinus. In cases of fistulas with feeders from the ICA, another guiding catheter is run through the ICA containing a balloon for BTO and a microcatheter for injection of the embolic.29 The balloon may also be inflated during embolization to prevent inadvertent reflux of the embolic agent into the ICA. Alternative access points are the basilar plexus, the pterygoid plexus, or the facial and angular veins.20 In cases of high-flow fistulas, coils may be placed at the confluence of the draining veins and within the cavernous sinus to act as a mesh, thus slowing the flow through the fistula and decreasing the efflux of embolic material through the veins.20
A transarterial approach allows the selective obliteration of individual pedicles feeding the CCF and makes the problem of venous rerouting less threatening.5,23 Problems with a transarterial approach may include several feeding arteries, anastomotic connections to cortical or nervous arterial supplies, or tortuous vessels that limit microcatheter access. For instance, catheterization of the small-caliber meningeal branches of ICCFs can be difficult or may supply several cranial nerves, thus limiting the success of this technique.20,30
In Barrow type A CCFs, a guide catheter is maneuvered into the ICA, followed by selective catheterization of feeding vessels. A microcatheter is navigated through the fistulous point into the cavernous sinus, and embolization is conducted. It may be necessary to simultaneously inflate a balloon in the cavernous ICA to prevent reflux into the parent artery.20 Again, an angiogram should be performed to assess progress and screen for dangerous anastomoses.
In some instances, a combination of arterial and venous approaches may be used.25,26 The transarterial component of therapy in this case decreases blood flow through the system, thus providing a less turbulent environment when deploying coils and allowing more precise targeting of the fistula from the venous side. During the procedure, the injection should be performed at a pace that allows the surgeon to monitor the evolving shape of the embolic. If a balloon is used, it should be deflated and any abnormal reflux should be noted prior to retrieval. Finally, a reference microguidewire should always be anchored in place to allow intraoperative navigation of the artery.29
Embolic Agents for Endovascular Occlusion
In choosing an embolic agent for CCF occlusion, consider what the desired properties of an ideal agent would be. The occlusion should be complete and permanent, and the delivery should be highly controllable. Radiopacity allows assessment of progress and precision of the occlusion. Finally, while some degree of proinflammatory properties may facilitate vessel occlusion, the material should not be antigenic. Modern embolics all attempt to strike a balance between these general themes, and some are more successful than others, depending on the task for which they were designed. Regardless of which embolic is chosen, all have inherent risks, including vessel rupture, vein occlusion, and embolization to normal parenchymal branches.23,31
The three broad categories of embolics are mechanical devices, such as balloons and detachable coils; particles; and liquids. Liquid embolics can further be divided into cyanoacrylates and polymers.32 Coils are principally used in the occlusion of larger vessels and aneurysms. Liquid embolic agents include n-butyl-2-cyanoacrylate (n-BCA) and ethylene–vinyl alcohol copolymer (Onyx, ev3 Neurovascular, Irvine, CA).
Balloons
For almost 30 years, the procedure of choice for the management and repair of type A CCFs was detachable balloon occlusion. While detachable balloons are no longer available in the United States, their description by Serbinenko in 1974 helped stimulate the growth of balloon technology in endovascular therapy.6 The balloon had many advantages, including low cost, easy navigability to the fistula, and the ability to intermittently inflate and deflate the balloon, which allowed constant reassessment of fistula anatomy. However, some difficulties encountered with permanent balloon occlusion were early detachment or deflation, rupture by bone fragments, and iatrogenic dilation of the ostium of the fistula or the cavernous sinus proper, causing a delayed recurrence of the fistula.33 If large balloons were used, or multiple balloons were deployed, de novo cranial nerve palsies sometimes resulted, or the resolution of preexisting palsies were sometimes hindered due to mass effect from the balloons.13
Particles
While particle embolic agents had been used for embolization of arteriovenous malformations,1 the first use of a permanent particle for CCF embolization was by Kosary et al. in 1968.34 Kosary et al. combined a porcelain bead with a Gelfoam technique described by Speakman in 1964.35 The Gelfoam—used to quicken the clotting process in the sinus and prevent “downward displacement” of the bead—would herald the use of balloons in preventing reflux of liquid and particle embolics in later years. Gelfoam can be used as either a macro- or a microparticle, depending on how it is prepared, but in both cases it is a temporary embolic agent.36 The choice of a porcelain bead was an important advancement, because it represented a rational choice of embolic particle size based on direct angiographic findings. Calibrated microparticles, such as Embospheres (BioSphere Medical, Rockland, MA) or polyvinyl alcohol particles of various sizes, now allow the physician to select a size based on the diameter of the vessels to be occluded, thus facilitating more accurate delivery of the embolics to the target vessel.
Coils
Detachable coils are easy to retrieve, reposition, or exchange when necessary, making them an appealing embolic agent. Endovascular treatment of CCF with coils is one of primary modes of treatment, with excellent results. In the setting of traumatic CCF, where the site of the fistula may be quite large, the coils may migrate or protrude into the ICA, thus risking carotid occlusion. Balloon-assist embolization, whereby the ICA is “protected” from coil migration and occlusion by the presence of a balloon that is inflated during coil delivery, has been quite efficacious in reducing this risk. Alternatively, similar to the principle of balloon assist, combining the use of coils with stents can prevent or mitigate such a risk.37 Complete obliteration of the fistula may be difficult, and there is a risk of impingement on the parent vessel and subsequent mass effects when excessive amounts of coil are packed.13,37 However, certain coated coils, such as Hydrocoils, the coating of which expands when placed in contact with blood, seem to require fewer coils to achieve occlusion than others.
n-Butyl-Cyanoacrylate
The first description of n-BCA as an embolic material was by Brothers et al. in 1989.38 This adhesive cyanoacrylate derivative polymerizes in the presence of anionic substances (e.g., blood) to form a solid cast that molds into the shape of the embolized region. The polymerization of n-BCA is immediate but can be prolonged by adding hydrophobic contrast agents (e.g., Ethiodol) or glacial acetic acid,38 rendering this embolic agent more controllable when depositing it into the intravascular system. During long injection times, great care must be taken to avoid microcatheter retention, which may result from the polymerization of the n-BCA along the outer wall of the microcatheter.
Onyx
Onyx is an ethylene–vinyl alcohol copolymer suspended in dimethyl sulfoxide with tantalum added for radiopacity.20,39,40 The first reported case of CCF embolization using Onyx was a type D fistula described by Arat et al. in 2004.39 In contrast to n-BCA, which rapidly polymerizes, this cohesive embolic agent precipitates slowly from its outer surface as the dimethyl sulfoxide slowly diffuses into the circulation and the Onyx precipitates. The result is the formation of a cast in the embolized vessel. The slow speed of precipitation allows deep penetration of Onyx with a slow, controlled injection.20,40 Because of these properties, Onyx permits the embolization of a venous sinus by a transarterial approach.23 Unique to this liquid embolic agent, it is possible to interrupt the injection during the procedure to allow assessment of the degree of embolization and occlusion. In this way, the endovascular surgeon can recognize the presence of dangerous anastomoses and avoid occlusion of normal vascular anatomy.
In contrast to n-BCA, Onyx’s nonadhesive characteristics allow a greater degree of reflux to be tolerated during embolization, leading to a reduced risk of catheter retention.20,40 Some limitations exist with the use of Onyx. The current microcatheter technology is somewhat limited in provided access to very distal vasculature secondary to the inherent stiffness of the microcatheters approved for use with Onyx. Furthermore, the solvent, dimethyl sulfoxide, may cause painful necrosis in the vasculature if rapidly infused. Like n-BCA, occlusion of the vasa nervorum can result in cranial neuropathies.
Stents
As briefly discussed, the advent of neurospecific stents represents a novel approach to the treatment of CCF and vascular lesions in general. As a nonocclusive scaffold along the ostium of the fistula or an aneurysm, stents provide the necessary parent vessel protection without intermittent interruption of flow within the parent vessel. Made of various alloys, a number of the stents are self-expanding, and though not approved for use for this indication, they have proved quite useful in specific situations. Coiling of the fistula can be performed in the same session as stent placement.41
The Pipeline embolization device (Chestnut Medical, Menlo Park, CA) is a flexible, self-expanding, stent used in the treatment of cerebral aneurysms. It is made out of platinum and cobalt chromium microfilaments in the form of a braided mesh cylinder. When deployed, the implants are designed to provide approximately 30% to 35% metal surface coverage at nominal expansion, which is a much higher percentage of coverage than that provided by uncovered intravascular stents.42
In addition to issues of flexibility, covered stents may occlude any small vessels in the region in which they are deployed. While this seriously limits their use in other areas of the neurovasculature, there seems to be little or no clinical significance to the occlusion of the perforators found within the cavernous sinus.13 Several varieties of covered stents have been used in neurovascular procedures; there is currently only one that is specifically designed for intracranial endovascular procedures: the Willis covered stent.13,43
Traumatic Aneurysms
The true incidence of traumatic intracranial aneurysm formation is unknown. Current reviews suggest that traumatic aneurysms after severe penetrating head trauma can have an incidence as high as 20% to 50%, though this literature may reflect severe injury secondary to military-grade weaponry.44–46 There is further suggestion that a proportion of these lesions grows and can ultimately rupture in the subacute to chronic stage, with resultant mortality concomitant to the mortality associated with berry aneurysms of the circle of Willis.44 Traumatic aneurysms have a higher propensity to form with penetrating injuries of the frontal or orbitobasilar region.46
Blunt and Penetrating Injuries of the Neck
The treatment of injury to the arteries of the neck has a limited recorded history, with the majority of treatment advances occurring within the last 300 to 500 years. The importance of the carotid artery was known since the days of the Greeks. Indeed, the term carotid is derived from the Greek karos, meaning “stupor,” demonstrating the Greeks’ knowledge of what happens when these arteries are compressed. Injury to these vessels was likely fatal the majority of times.
In 1522, Paré first reported the ligation of the common carotid artery and jugular vein in a soldier injured after a duel who tolerated common carotid occlusion for several days.47 The literature hereafter is incomplete, and subsequent reports are nonexistent until the early 19th century, when the surgeon aboard the HMS Tonnant ligated the carotid of a suicidal sailor.48 Soon thereafter, the first recorded treatment of an injured ICA was performed by Abernathy in 1804, when he tied the carotid artery of a man who had been gored by the horn of a cow.49 This early literature, and the medical war literature up until World War I, attests to the relatively poor outcomes that come from deconstruction of the carotid artery in the setting of injury.50
Pathophysiologic Correlates
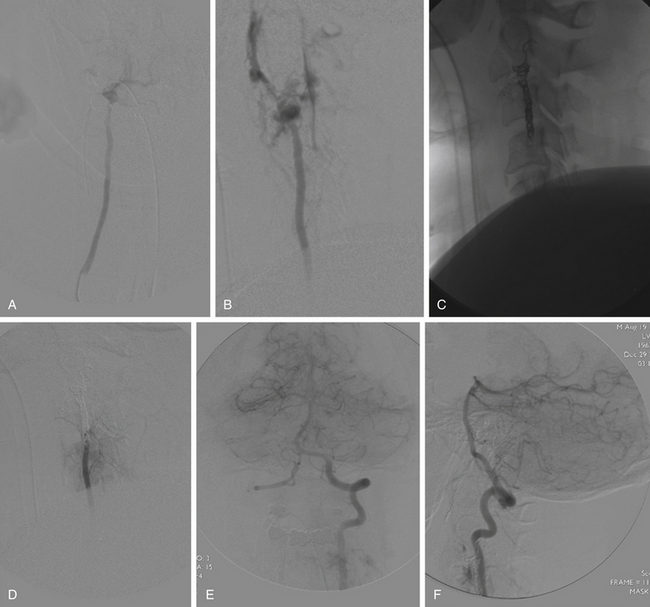
Direct trauma to the intracranial and extracranial circulation can result from both blunt and penetrating forces. Blunt trauma to both the carotid and the vertebral circulations can result in dissection and in one series has been reported as high as 0.45% of neck traumas, with a high degree of associated morbidity and mortality.51 Penetrating trauma can result in either complete transection of a vessel with concomitant extravasation or partial injury, which can then present immediately or after a delay. Injury to the tunica intima can result in dissection, intrusion of hematoma between layers of the vessel, and creation of true and false lumens. Compromise of all three arterial wall layers (the intima, media, and adventitia) can result in active extravasation and exsanguination in the field or containment of the hematoma by surrounding tissue planes. In this instance, a pseudoaneurysm results and can present with delayed bleeding subsequent to the inciting trauma. Penetrating trauma, particularly gunshot wounds, can also result in abnormal fistulas between native arteries and veins or venous sinuses (Fig. 92-2).
Cervical carotid injuries account for approximately 25% of all penetrating injuries to the neck, followed by the subclavian artery.52 As such, understanding the role of open and endovascular techniques for the treatment of vascular injuries to the neck is important from the moment of initial evaluation. Most clinicians employ Saletta et al.’s original description of the three-zone model of cervical injury, though the treatment approaches may now differ.53 Zone I injury is described as any vascular injury below the clavicle, which seems to be more akin to thoracic injury. Zone II is described as the region of the neck to the level of the angle of the mandible. Zone III is defined as the region above the angle of the mandible. Despite this model, penetrating or blunt injuries of the neck rarely present in isolation, with venous injury occurring in almost 30% of patients and tracheal or esophageal injury occurring in an additional 15%.
Noninvasive imaging has made substantial advances in the last 20 years and has been particularly useful in the diagnosis of cervical vascular injuries to the neck.54 However, despite advances in both computed tomography angiography (CTA) and magnetic resonance angiography, digital subtraction angiography remains the gold standard for diagnosis of traumatic vascular injuries to the vessels of the head and neck.55 In addition to the obvious ability to evaluate both the venous and the arterial systems during time of flight, this modality offers the potential for immediate therapeutic intervention if warranted. However, certain caveats must be borne in mind for the neurointerventionalist. The majority of referred trauma patients have already undergone evaluation in the emergency department, by either the traumatologist or the emergency department physician, and quite possibly in subspecialties such as otolaryngology and maxillofacial surgery. By definition, these patients have failed conservative therapy and possibly basic surgical maneuvers. In addition, the trauma team has likely determined that surgical exploration and treatment are not indicated in this situation. As stated by Morris, odds therefore are that “those sent for endovascular management likely have an unusual set of prevailing circumstances, physiologic, anatomic, or otherwise.”56 A thorough history must be taken from the referring team, and the location of the bleeding must be pinpointed as accurately as possible; nasal laterality should be established in the case of traumatic epistaxis, and attention should then be focused on that side. When possible, a radiopaque marker should be affixed to the site of any visible bleeding. Unless the patient is actively extravasating, it may be difficult to identify the exact site of bleeding on angiographic runs.
Embolic and particulate agents may be used in the case of small distal vessels. Older agents such as Gelfoam pledgets and polyvinyl alcohol particles can be used as in epistaxis cases. Newer embolics such as Embospheres provide a uniformity of particulate size not assured with older agents, which can be useful should concern exist for anastomotic communication between the intracranial and the extracranial circulation. In general, the size of nonvisualized anastomotic arteries ranges from 50 to 80 μm; therefore, particles that are larger than 150 μm will not penetrate these anastomoses and will avoid potential embolic complications.57
Larger vessels with lacerations and pseudoaneurysms require more definitive therapy via either coil occlusion or liquid embolic agents. When vessel sacrifice is not an option, for instance, when dealing with the common carotid artery, ICA, or dominant vertebral artery, covered or uncovered stents with coils can preserve luminal patency while ensuring maintenance of CBF. Surgical options for these injuries, such as resection and reanastomosis procedures or patch grafting techniques, should always be considered, because they may be more efficacious and safe. In the setting of suspected or known injury to the trachea or esophagus (zone II), surgical exploration is indicated.
Vortex coils (Boston Scientific, Natick, MA) provide an excellent and inexpensive way to occlude larger vessels when coil retrieval is not a concern. Liquid embolics such as n-BCA and Onyx are useful for dealing with flow-directed anomalies such as fistulas, as well as for rapid elimination of tributaries off of the external carotid circulation. n-BCA augmentation may also be used with fibered coils that are not generating sufficient stagnation of flow within the vessel lumen. Care must be taken when utilizing liquid embolics, because they can open and enter small anastomotic channels that are not visualized on the initial angiograms. However, as previously mentioned, increasing uses are being found for liquid embolic agents in the case of CCFs, severe epistaxis secondary to facial fractures (where bilateral internal maxillary artery sacrifice is typically performed), and ICA transection.58,59
Stent placement is increasingly being favored over surgical repair as an initial modality of treatment for ICA pseudoaneurysms caused by both blunt and penetrating trauma.60 Full anticoagulation is required during the procedure to minimize the risk of thromboembolic complications, and dual antiplatelet therapy is recommended postprocedure for 6 weeks while the stent endothelializes. While polytetrafluoroethylene-covered stents have traditionally been used for this purpose, data are emerging to support the use of specifically designed carotid artery stents. Stent-assisted vessel wall reconstruction may provide definitive treatment for traumatic pseudoaneurysms even if coil embolization proves unfeasible.61
Epistaxis
Endovascular treatment for control of epistaxis was first described in the literature by Sokoloff et al. in 1974 and then further refined by Lasjaunias et al., with particular emphasis on preoperative angiographic evaluation.62,63 As has been previously stated, some caveats regarding patient selection must be held in mind. Bleeding from the anterior septal (Little’s) area accounts for the majority of cases of epistaxis, a region that is vascularized by the Kiesselbach plexus and supplied primarily by the ethmoidal arteries, as well as branches off external carotid artery (sphenopalatine, descending palatine, and superior labial).64 Hemorrhage from this region typically responds well to direct pressure, topical hemostatic or cauterizing agents, direct electrocautery, or anterior packing. These patients normally do well with conservative therapy and therefore are not referred for endovascular evaluation in the absence of confounding factors.
In approximately 5% of epistaxis cases, however, the origin of bleeding lies more posteriorly on the nasal cavity.65 Posterior epistaxis typically presents with a more fulminant arterial bleeding and can result in a significant volume of blood loss in a short time. Causes can be varied and depend on individual patient comorbidities, but roughly 70% of cases are idiopathic.66 Initial treatment usually involves application of anterior and posterior packing by the otolaryngology team, frequently with direct pressure maintained by a clamped balloon catheter. Despite these measures, one third of posterior epistaxis patients either continues bleeding or recommences hemorrhage with the removal of tamponade.67 Endovascular treatment of such patients can serve as an alternative to open surgical treatment, which involves transantral ligation of the internal maxillary and anterior ethmoidal arteries.
Goals of endovascular therapy include diminishing flow to the affected nasal mucosa while avoiding necrosis of treated tissues and inadvertent embolization of critical structures. Due to the potential for airway compromise and the ongoing risk of hemodynamic instability, general anesthesia is preferred for such cases. Bilateral preoperative angiographic evaluation of both external and internal carotid arteries is essential for multiple reasons. The internal carotid arteries must be assessed to ensure normal origin and distribution of the ophthalmic arteries, rule out an ICA source of bleeding, and check the extent of blood supply to the nasal mucosa via the ethmoidal arteries. The external carotid arteries require careful angiographic runs to map the territory of the internal maxillary artery, check for hazardous ECA-ICA anastomoses, and rule out nonidiopathic causes of epistaxis (e.g., vascular malformations, neoplasms or traumatic pseudoaneurysms).68
If no overt lesion is evident on angiography, particle embolization of both internal maxillary arteries is carried out. Polyvinyl alcohol particles or Embospheres are typically used, with sizes between 150 and 500 μm preferred to avoid the risk of entering dangerous anastomoses. Gelfoam pledgets can be used to occlude the internal maxillary artery at the origin once near-stagnation of flow is seen; coil occlusion is another option, but it prevents the possibility of a repeat embolization if hemorrhage recurs.69 Light embolization of the facial artery ipsilateral to the bleeding can be carried out as well in cases of significant supply to the nasal mucosa via its angular branch. If embolization of the facial artery is to be performed, however, it should be carried out distal to the origin of the submandibular artery. In addition, the contralateral facial artery should not be embolized given the potential for nasal skin necrosis.
Technical success rates for bleeding control can range from 70% to 85% in selected patients.67,70 Major complications from the procedure can include embolic stroke, cranial nerve palsies, septal perforation, facial skin or mucosal necrosis, and blindness (frequently from unappreciated anastomoses between the distal internal maxillary branches and the ophthalmic artery). Fortunately, the incidence of such adverse events is low: less than 2% in reported series.69,71
Carotid Blowout
Rupture of the extracranial carotid artery or its major branches, commonly referred to as carotid blowout, remains one of the most feared and life-threatening complications associated with head and neck cancer and its treatment. The reported incidence of this complication in patients who have undergone neck dissection with or without tumor resection is 3% to 4%.72 While the morbidity and mortality associated with this condition were historically held to be quite high (60% and 40%, respectively), recent progress in diagnosis and management has resulted in a marked decrease.72 Radiation therapy has been strongly associated with this condition due to the obliteration of the vasa vasorum and the weakening of the arterial wall as a sequelae of treatment. It is estimated that patients who have been irradiated previously have a sevenfold increase in the risk of carotid blowout.73 Although traditionally associated with neoplastic processes, in particular squamous cell carcinoma, carotid blowout can result from direct blunt or penetrating trauma and may be iatrogenic as a result of endoscopic sinus surgery.
Carotid blowout is now considered to be a syndrome and can present as one of three separate entities: threatened, impending, and acute. Threatened carotid blowout is characterized by the suggestion of imminent hemorrhage, either due to exposure of the carotid artery via tissue necrosis or visualization of a pseudoaneurysm diagnostic angiography. Impending carotid blowout is indicated by the presence of a cervical or transoral sentinel hemorrhage that resolves spontaneously or with basic treatment. Acute carotid blowout is a life-threatening hemorrhage that cannot be controlled with packing or pressure and requires immediate surgical or endovascular intervention.74
The most common sites of hemorrhage on meta-analysis of multiple case studies were, in descending order of frequency, ICA (43.6%), external carotid artery (23.4%), common carotid artery (11.7%), buccal artery (3.2%), inferior thyroid artery (3.2%), superior thyroid artery (2.1%), internal mammary artery (2.1%), innominate artery (1.1%), facial artery (1.1%), and aorta (1.1%).3
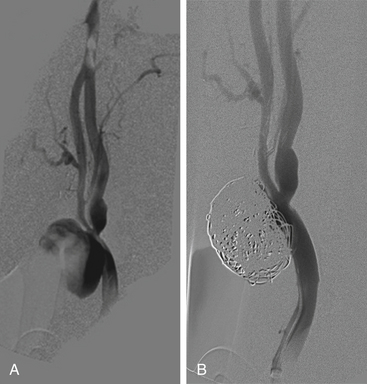
Retrospective case series have indicated that deconstructive management may be superior to constructive management. Stent occlusion in one series was 100% at 3 months, although technical issues and inadequate patient premedication with antiplatelet therapy may have accounted for this.75 The incidence of rebleeding may also be higher with constructive management. Furthermore, extensive tissue necrosis and direct exposure of the fistula or the carotid artery make stent placement into a contaminated field a matter for concern, and intracranial abscesses likely secondary to septic emboli have been reported.76 However, endovascular stent graft reconstruction may prove beneficial as a palliative measure, in patients who fail BTO in preparation for vessel sacrifice, or as an elective preventative measure for threatened blowout in the absence of active bleeding (Fig. 92-3).
Vertebral Artery Injury
Vertebral artery injury makes up less than 5% of all cervical arterial injuries, with penetrating trauma—most specifically, gunshot wounds—being the most common penetrating form of injury and motor vehicle accidents being the etiology of most blunt injuries to the vertebral artery. Almost 75% of injuries to the vertebral artery are asymptomatic because of its location and because the contralateral vertebral artery will most likely (85%) provide sufficient flow to the posterior circulation.77 Furthermore, the anatomic proximity of the epidural venous plexus along the C1 to C2 region, the most likely region of blunt vertebral artery injury, results in arteriovenous fistula formation. Physical and neurologic findings such as Horner syndrome, Wallenberg syndrome, cranial nerve palsies, and respiratory failure should lead the clinician to suspect possible vascular injury.78
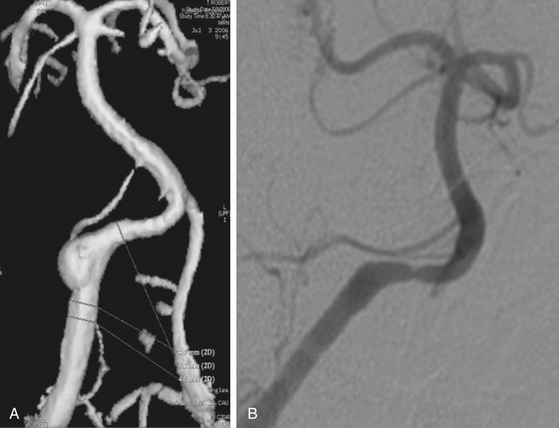
Because of the relative rarity of this injury, management strategies for vertebral artery injury have largely been adopted from the carotid injury data. Though accessible, the surgical approaches to the vertebral artery can be challenging. Surgical ligation of the proximal vertebral artery (at its origin) can be considered and performed by vascular surgeons in the acute trauma setting, especially in the setting of a nondominant vertebral artery injury. However, endovascular options of ligation and reconstruction should be considered first if injury to the mid- or distal vertebral artery (V2 and V3 segments) occurs (Fig. 92-4).
It is somewhat difficult to determine the true outcomes of patients sustaining vertebral artery injury, because it is rare to have severe, isolated injury. It is estimated that though mortality ranges from 11% to 25%, only 5% of that mortality is directly attributable to injury of the vertebral artery.77
Aarabi B. Traumatic aneurysms of brain due to high velocity missile head wounds. Neurosurgery. 1988;22:1056-1063.
Barrow D.L., Spector R.H., Braun I.F., et al. Classification and treatment of spontaneous carotid–cavernous sinus fistulas. J Neurosurg. 1985;62:248-256.
Brothers M.F., Kaufmann J.C., Fox A.J., Deveikis J.P. n-Butyl 2-cyanoacrylate—substitute for IBCA in interventional neuroradiology: histopathologic and polymerization time studies. AJNR Am J Neuroradiol. 1989;10:777-786.
Cohen J., Rad I. Contemporary management of carotid blowout. Curr Opin Otolaryngol Head Neck Surg. 2004;12:110-115.
Fiorella D., Woo H.H., Albuquerque F.C., Nelson P.K. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62:1115-1120.
Geibprasert S., Pongpech S., Armstrong D., et al. Dangerous Extracranial–Intracranial Anastomoses and Supply to the Cranial Nerves: Vessels the Neurointerventionalist Needs to Know. American Journal of Neuroradiology. 2009;30:1459-1468.
Houdart E., Gobin Y.P., Casasco A., et al. A proposed angiographic classification of intracranial arteriovenous fistulae and malformations. Neuroradiology. 1993;35:381-385.
Kosary I.Z., Lerner M.A., Mozes M., Lazar M. Artificial embolic occlusion of the terminal internal carotid artery in the treatment of carotid–cavernous fistula. Technical note. J Neurosurg. 1968;28:605-608.
Lasjaunias P., Marsot-Dupuch K., Doyon D. The radio-anatomic basis of arterial embolization for epistaxis. J Neuroradiol. 1979;6:45-53.
Luessenhop A.J., Spence W.T. Artificial embolization of cerebral arteries. The report of use in a case of AV malformation. JAMA. 1960;172:1153-1155.
Morris P.P. Practical Neuroangiography, 2nd ed. Philadelphia: Lippincott; 2007. 498-499
Morrissey D.D., Andersen P.E., Nesbit G.M., et al. Endovascular management of hemorrhage in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 1997;123:15-19.
Mulloy J.P., Flick P.A., Gold R.E. Blunt carotid injury: a review. Radiology. 1998;207:571-585.
Paré A. The collected works of Ambroise Paré (translated out of the Latin by Thomas Johnson, London, first English edition, 1634). Pound Ridge, NY: Milford House Inc; 1968.
Pearce W.H., Whitehill Ta. Carotid and vertebral artery injuries. Surg Clin North Am. 1988;68:705-723.
Prestigiacomo C.J. Surgical endovascular neuroradiology in the 21st century: what lies ahead? Neurosurgery. 2006;59(5 Suppl 3):S48-S55.
Rubio P.A., Reul G.J., Beall A.C., et al. Acute carotid artery injury: 25 years’ experience. J Trauma. 1974;14:967-973.
Saletta J.D., Folk F.A., Freeark R.J. Trauma to the neck region. Surg Clin North Am. 1973;53:73-85.
Schelhas K.P., Latchaw R.E., Wendling L.R., Gold L.H.A. Vertebrobasilar injuries following cervical manipulation. JAMA. 1980;224:1450-1453.
Serbinenko F.A. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974;41:125-145.
Sokoloff J., Wickbom I., McDonald D., et al. Therapeutic percutaneous embolization in intractable epistaxis. Radiology. 1974;111:285-287.
Stiefel M.F., Albuquerque F.C., Park M.S., et al. Endovascular treatment of intracranial dural arteriovenous fistulae using Onyx: a case series. Neurosurgery. 2009;65(Suppl 6):132-139.
Willems P.W.A., Farb R.I., Agid R. Endovascular treatment of epistaxis. American Journal of Neuroradiology. 2009;30:1637-1645.
Zenteno M., Santos-Franco J., Rodriguez-Parra V., et al. Management of direct carotid–cavernous sinus fistulas with the use of ethylene–vinyl alcohol (Onyx) only: preliminary results. J Neurosurg. 2009;112(3):595-602.
1. Luessenhop A.J., Spence W.T. Artificial embolization of cerebral arteries. The report of use in a case of AV malformation. JAMA. 1960;172:1153-1155.
2. Mulloy J.P., Flick P.A., Gold R.E. Blunt carotid injury: a review. Radiology. 1998;207:571-585.
3. Cohen J., Rad I. Contemporary management of carotid blowout. Curr Opin Otolaryngol Head Neck Surg. 2004;12:110-115.
4. Sorteberg A., Bakke S.J., Boysen M., Sorteberg W. Angiographic balloon test occlusion and therapeutic sacrifice of major arteries to the brain. Neurosurgery. 2008;63:651-660.
5. Mazumdar A., Derdeyn C.P., Holloway W., et al. Update on endovascular management of the carotid blowout syndrome. Neuroimaging Clin N Am. 2009;19:271-281.
6. Serbinenko F.A. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974;41:125-145.
7. Field M., Jungreis C.A., Chengelis N., et al. Symptomatic cavernous sinus aneurysms: management and outcome after carotid occlusion and selective cerebral revascularization. AJNR Am J Neuroradiol. 2003;24:1200-1207.
8. Abud D.G., Spelle L., Piotin M., et al. Venous phase timing during balloon test occlusion as a criterion for permanent internal carotid artery sacrifice. AJNR Am J Neuroradiol. 2005;26:2602-2609.
9. Chaloupka J.C., Roth T.C., Putman C.M., et al. Recurrent carotid blowout syndrome: diagnostic and therapeutic challenges in a newly recognized subgroup of patients. AJNR Am J Neuroradiol. 1999;20:1069-1077.
10. van Rooij W.J., Sluzewski M., Beute G.N. Ruptured cavernous sinus aneurysms causing carotid–cavernous fistula: incidence, clinical presentation, treatment, and outcome. AJNR Am J Neuroradiol. 2006;27:185-189.
11. Houdart E., Gobin Y.P., Casasco A., et al. A proposed angiographic classification of intracranial arteriovenous fistulae and malformations. Neuroradiology. 1993;35:381-385.
12. Barrow D.L., Spector R.H., Braun I.F., et al. Classification and treatment of spontaneous carotid–cavernous sinus fistulas. J Neurosurg. 1985;62:248-256.
13. Wang C., Xie X., You C., et al. Placement of covered stents for the treatment of direct carotid–cavernous fistulas. AJNR Am J Neuroradiol. 2009;30:1342-1346.
14. Madan A., Mujic A., Daniels K., et al. Traumatic carotid artery–cavernous sinus fistula treated with a covered stent. Report of two cases. J Neurosurg. 2006;104:969-973.
15. Yu J.S., Lei T., Chen J.C., et al. Diagnosis and endovascular treatment of spontaneous direct carotid–cavernous fistula. Chin Med J (Engl). 2008;121:1558-1562.
16. Luo C.B., Teng M.M., Chang F.C., et al. Bilateral traumatic carotid–cavernous fistulae: strategies for endovascular treatment. Acta Neurochir (Wien). 2007;149:675-680.
17. Oran I., Bozkaya H., Parildar M. Embolisation of both fistulae through the same carotid artery tear in a patient with bilateral traumatic caroticocavernous fistulae. Neuroradiology. 2004;46:234-237.
18. Gupta A.K., Purkayastha S., Krishnamoorthy T., et al. Endovascular treatment of direct carotid–cavernous fistulae: a pictorial review. Neuroradiology. 2006;48:831-839.
19. Karaman E., Isildak H., Haciyev Y., et al. Carotid–cavernous fistula after functional endoscopic sinus surgery. J Craniofac Surg. 2009;20:556-558.
20. Elhammady M.S., Wolfe S.Q., Farhat H., et al. Onyx embolization of carotid–cavernous fistulas. J Neurosurg. 2009;112(3):589-594.
21. Luo C.B., Teng M.M., Chang F.C., Chang C.Y. Endovascular treatment of intracranial high-flow arteriovenous fistulas by Guglielmi detachable coils. J Chin Med Assoc. 2006;69:80-85.
22. Parkinson D. Carotid–cavernous fistula: direct repair with preservation of the carotid artery. Technical note. J Neurosurg. 1973;38:99-106.
23. Stiefel M.F., Albuquerque F.C., Park M.S., et al. Endovascular treatment of intracranial dural arteriovenous fistulae using Onyx: a case series. Neurosurgery. 2009;65(Suppl 6):132-139.
24. McGuinness B.J., Moriarty M., Hope J.K. Interventional radiology in the treatment of intracranial vascular injuries and fistulae. Injury. 2008;39:1242-1248.
25. Yamashita K., Taki W., Nishi S., et al. Transvenous embolization of dural caroticocavernous fistulae: technical considerations. Neuroradiology. 1993;35:475-479.
26. Yoshida K., Melake M., Oishi H., et al. Transvenous embolization of dural carotid–cavernous fistulas: a series of 44 consecutive patients. AJNR Am J Neuroradiol. 2010;31(4):651-655.
27. Satomi J., van Dijk J.M., Terbrugge K.G., et al. Benign cranial dural arteriovenous fistulas: outcome of conservative management based on the natural history of the lesion. J Neurosurg. 2002;97:767-770.
28. Debrun G., Lacour P., Vinuela F., et al. Treatment of 54 traumatic carotid–cavernous fistulas. J Neurosurg. 1981;55:678-692.
29. Zenteno M., Santos-Franco J., Rodriguez-Parra V., et al. Management of direct carotid–cavernous sinus fistulas with the use of ethylene–vinyl alcohol (Onyx) only: preliminary results. J Neurosurg. 2009;112(3):595-602.
30. Shi Z.S., Ziegler J., Gonzalez N.R., et al. Transarterial embolization of clival dural arteriovenous fistulae using liquid embolic agents. Neurosurgery. 2008;62:408-415.
31. Prestigiacomo C.J. Surgical endovascular neuroradiology in the 21st century: what lies ahead? Neurosurgery. 2006;59(5 Suppl 3):S48-S55.
32. Oowaki H., Matsuda S., Sakai N., et al. Non-adhesive cyanoacrylate as an embolic material for endovascular neurosurgery. Biomaterials. 2000;21:1039-1046.
33. Archondakis E., Pero G., Valvassori L., et al. Angiographic follow-up of traumatic carotid–cavernous fistulas treated with endovascular stent graft placement. AJNR Am J Neuroradiol. 2007;28:342-347.
34. Kosary I.Z., Lerner M.A., Mozes M., Lazar M. Artificial embolic occlusion of the terminal internal carotid artery in the treatment of carotid–cavernous fistula. Technical note. J Neurosurg. 1968;28:605-608.
35. Speakman T.J. Internal occlusion of a carotid–cavernous fistula. J Neurosurg. 1964;21:303-305.
36. Abada H.T., Golzarian J. Gelatine sponge particles: handling characteristics for endovascular use. Tech Vasc Interv Radiol. 2007;10:257-260.
37. Ahn J.Y., Lee B.H., Joo J.Y. Stent-assisted Guglielmi detachable coils embolisation for the treatment of a traumatic carotid–cavernous fistula. J Clin Neurosci. 2003;10:96-98.
38. Brothers M.F., Kaufmann J.C., Fox A.J., Deveikis J.P. n-Butyl 2-cyanoacrylate—substitute for IBCA in interventional neuroradiology: histopathologic and polymerization time studies. AJNR Am J Neuroradiol. 1989;10:777-786.
39. Arat A., Cekirge S., Saatci I., Ozgen B. Transvenous injection of Onyx for casting of the cavernous sinus for the treatment of a carotid–cavernous fistula. Neuroradiology. 2004;46:1012-1015.
40. Gao K., Yang X.J., Mu S.Q., et al. Embolization of brain arteriovenous malformations with ethylene–vinyl alcohol copolymer: technical aspects. Chin Med J (Engl). 2009;122:1851-1856. 20
41. Moron F.E., Klucznik R.P., Mawad M.E., Strother C.M. Endovascular treatment of high-flow carotid–cavernous fistulas by stent-assisted coil placement. AJNR Am J Neuroradiol. 2005;26:1399-1404.
42. Fiorella D., Woo H.H., Albuquerque F.C., Nelson P.K. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62:1115-1120.
43. Li M.H., Li Y.D., Gao B.L., et al. A new covered stent designed for intracranial vasculature: application in the management of pseudoaneurysms of the cranial internal carotid artery. AJNR Am J Neuroradiol. 2007;28:1579-1585.
44. Bell R.S., Vo A.H., Roberts R., et al. Wartime traumatic aneurysms: acute presentation, diagnosis, and multimodal treatment of 64 craniocervical arterial injuries. Neurosurgery. 2010;66:66-79.
45. Aarabi B. Traumatic aneurysms of brain due to high velocity missile head wounds. Neurosurgery. 1988;22:1056-1063.
46. Amirjamshidi A., Rahmat H., Abbassioun K. Traumatic aneurysms and arteriovenous fistulas of intracranial vessels associated with penetrating head injuries occurring during war: principles and pitfalls in diagnosis and management. A survey of 31 cases and review of the literature. J Neurosurg. 1996;84:769-780.
47. Paré A. The collected works of Ambroise Paré (translated out of the Latin by Thomas Johnson, London, first English edition, 1634). Pound Ridge, NY: Milford House Inc; 1968.
48. Pearce W.H., Whitehill Ta. Carotid and vertebral artery injuries. Surg Clin North Am. 1988;68:705-723.
49. Abernathy J. Surgical Observations. London: Longman & Rees; 1804.
50. Rubio P.A., Reul G.J., Beall A.C., et al. Acute carotid artery injury: 25 years’ experience. J Trauma. 1974;14:967-973.
51. Feliciano D. Management of penetrating injuries to the carotid artery. World J Surg. 2001;25:1028-1035.
52. Carducci B., Lowe R.A., Dalsey W. Penetrating neck trauma: consensus and controversies. Ann Emerg Med. 1986;15:208-215.
53. Saletta J.D., Folk F.A., Freeark R.J. Trauma to the neck region. Surg Clin North Am. 1973;53:73-85.
54. Munera F., Soto J.A., Palacio D.M., et al. Penetrating neck injuries: helical CT angiography for initial evaluation. Radiology. 2002;224:366-372.
55. Wellwood J., Alcantara A., Michael D.B. Neurotrauma: the role of CT angiogram. Neurol Res. 2002;1(Suppl 24):S13-S16.
56. Morris P.P. Practical Neuroangiography, 2nd ed. Philadelphia: Lippincott; 2007. 498-99
57. Geibprasert S., Pongpech S., Armstrong D., Krings T. Dangerous extracranial-intracranial anastomoses and supply to the cranial nerves: vessels the neurointerventionalist needs to know. AJNR Am J Neuroradiol. 2009;30(08):1459-1468.
58. Lv X.L., Li Y.X., Liu A.H., et al. A complex cavernous sinus dural arteriovenous fistula secondary to covered stent placement for a traumatic carotid artery–cavernous sinus fistula: case report. J Neurosurg. 2008;108:588-590.
59. Diaz-Daza O., Arraiza F.J., Barkley J.M., Whigham C.J. Endovascular therapy of traumatic vascular lesions of the head and neck. Cardiovascular & Interventional Radiology. 2003;26:213-221.
60. Maras D., Lioupis C., Magoufis G., et al. Covered stent-graft treatment of traumatic internal carotid artery pseudoaneurysms: a review. Cardiovascular and Interventional Radiology. 2006;29:958-968.
61. Fiorella D., Albuquerue F.C., Deshmukh V.R., et al. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysms. Neurosurgery. 2006;59:291-300.
62. Sokoloff J., Wickbom I., McDonald D., et al. Therapeutic percutaneous embolization in intractable epistaxis. Radiology. 1974;111:285-287.
63. Lasjaunias P., Marsot-Dupuch K., Doyon D. The radio-anatomic basis of arterial embolization for epistaxis. J Neuroradiol. 1979;6:45-53.
64. Koh E., Frazzini V.I., Kagetsu N.J. Epistaxis: vascular anatomy, origins and endovascular treatment. AJR Am J Roentgenol. 2000;174:845-851.
65. Viducich R.A., Blanda M.P., Gerson L.W. Posterior epistaxis: clinical features and acute complications. Ann Emerg Med. 1995;25:592-596.
66. Tseng E.Y., Narducci C.A., Willing S.J., et al. Angiographic embolization for the treatment of epistaxis: a review of 114 cases. Laryngoscope. 1998;108:615-619.
67. Klotz D.A., Winkle M.R., Richmon J., et al. Surgical management of posterior epistaxis: a changing paradigm. Laryngoscope. 2002;112:1577-1582.
68. Willems P.W.A., Farb R.I., Agid R. Endovascular treatment of epistaxis. American Journal of Neuroradiology. 2009;30:1637-1645.
69. Christensen N.P., Smith D.S., Barnwell S.L., et al. Arterial embolization in the management of posterior epistaxis. Otolaryngol Head Neck Surg. 2005;133:748-753.
70. Andersen P.J., Kjeldsen A.D., Nepper-Rasmussen J. Selective embolization in the treatment of intractable epistaxis. Acta Otolaryngol. 2005;125:293-297.
71. Vokes D., McIvor N.P., Wattie W.J., et al. Endovascular treatment of epistaxis. ANZ J Surg. 2004;74:751-753.
72. Morrissey D.D., Andersen P.E., Nesbit G.M., et al. Endovascular management of hemorrhage in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 1997;123:15-19.
73. Maran A.G.D., Amin M., Wilson J. A radical neck dissection: a 19 year experience. J Laryngo Otol. 1989;10:760-764.
74. Warren F., Cohen J., Nesbit G., et al. Management of carotid “blowout” with endovascular stent grafts. The Laryngoscope. Mar 2002;112:428-433.
75. Chang F.C., Lirng J.F., Luo C.B., et al. Patients with head and neck cancers and associated postirradiated carotid blowout syndrome: endovascular therapeutic methods and outcomes. J Vasc Surg. May 2008;47(5):936-945.
76. Oweis Y., Gemmete J.J., Chaudhary N., et al. Delayed development of brain abscesses following stent-graft placement in a head and neck cancer patient presenting with carotid blowout syndrome. Cardiovasc Intervent Radiol. Dec 2009. [Epub ahead of print]
77. Biffl W.L., Moore E.E., Ryu R.K., et al. The devastating potential of blunt vertebral arterial injury. Ann Surg. 2000;231:672-681.
78. Schelhas K.P., Latchaw R.E., Wendling L.R., Gold L.H.A. Vertebrobasilar injuries following cervical manipulation. JAMA. 1980;224:1450-1453.