29 Endovascular Techniques for Giant Intracranial Aneurysms
Introduction
Giant aneurysms (fundus diameter ≥25 mm) comprise approximately 5% of the intracranial aneurysms in most published series.1–5 These lesions are slightly more common in women. Approximately two thirds are in the anterior circulation and one-third in the posterior circulation.1 The proportion of patients who present with rupture varies between 20% and 70%, with rebleeding rates similar to those seen for smaller aneurysms in patients presenting with subarachnoid hemorrhage (SAH).3,5,6 Other presenting signs and symptoms are related to mass effect and ischemic syndromes. Ischemic syndromes are seen in fewer than 10% of giant aneurysms.3,4
Giant aneurysms include saccular-shaped aneurysms with a demonstrable neck and fusiform-shaped aneurysms, which are fusiform dilatations of the entire vessel wall with a segmental defect in the parent artery.4 Saccular aneurysms are thought to develop from smaller saccular aneurysms at points of maximal hemodynamic stress, such as flow vector points or intracranial bifurcations. Fusiform aneurysms are thought to arise from atherosclerotic degeneration of the vessel wall that leads to aneurysmal defects in the parent vessel. They often involve entire segments of a first- or second-order intracranial vessel and incorporate branches and perforators.
Natural history
The natural history of a giant intracranial aneurysm, once diagnosed, is poor. Drake6 observed a group of 31 patients with untreatable intracranial aneurysms and found a mortality rate of 66% at 2 years and >80% at 5 years. In the first International Study of Unruptured Intracranial Aneurysms (ISUIA), the relative risk of rupture of an unruptured giant aneurysm when compared to an unruptured aneurysm <10 mm in size was 59.0 (p < 0.001).7 In the second ISUIA, 55 giant aneurysms were observed without treatment and the 5-year cumulative rupture risk by location was 6.4% in the cavernous carotid artery, 40% in other segments of the internal carotid artery (ICA), anterior cerebral artery, anterior communicating artery (AComA), and middle cerebral artery (MCA), and 50% in the posterior communicating artery (PComA) and posterior circulation.8 In the same study, giant aneurysms were treated with open surgery in 80 patients and endovascular methods in 55 patients. In the surgical group, the chances of poor outcomes (defined as a modified Rankin Scale [mRS] score of 3-5, or an impaired cognitive outcome) were 25% to 35% in the anterior circulation and 45% in the posterior circulation. In the endovascular group, the chances of poor outcomes were 12% to 15% in the anterior circulation and 40% in the posterior circulation. In giant aneurysms, a high-risk natural history is associated with a higher treatment risk. Accordingly, a decision to treat or not may in part be based on the decisions of patients and their physicians about whether risk is preferable immediately or over time; decisions might also be strongly influenced by the patient’s age and presence of medical comorbidities and aneurysmal mass effect.
Surgical management
A meta-analysis by Raaymakers et al.3 that examined the outcome of surgical treatment for unruptured aneurysms in studies from 1970 to 1996 showed posterior circulation aneurysms to have a 9.6% mortality and 37.9% morbidity and anterior circulation aneurysms to have a 7.4% mortality and 26.9% morbidity. The early reports of Drake6 and the more modern surgical series of Lawton et al.,9,10 Samson et al.,11 Surdell et al.,12 Piepgras et al.,13 Hanel et al.,14 and Sekhar et al.15–17 have shown improvement in the morbidity and mortality associated with surgically treating these lesions. Most surgical series report an operative mortality of at least 6% and a major morbidity of at least 20%. The results of endovascular therapies should always be compared to these surgical series.
Preoperative imaging
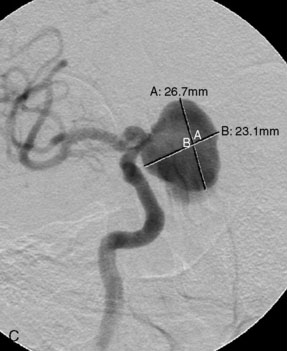
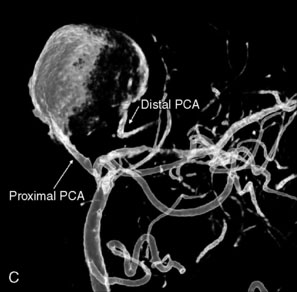
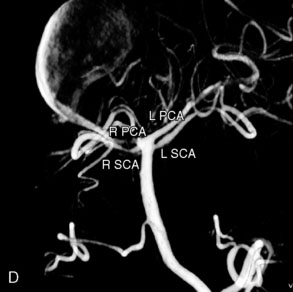
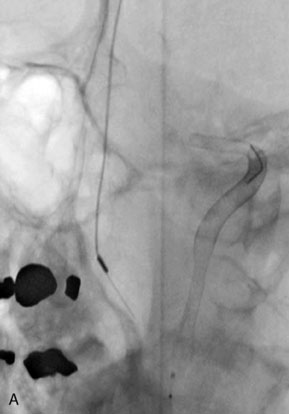
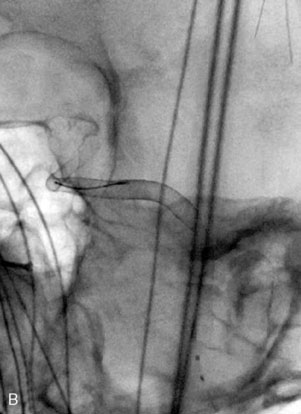
Catheter-based angiography, 3-D CT imaging, and MR angiography are helpful in confirming the diagnosis and/or consideration of an endovascular, surgical, or combination therapy approach. The performance of a six-vessel 3-D diagnostic cerebral angiogram is crucial before final decisions about treatment options are made. Catheter-based angiography provides critical information regarding not only the anatomic and morphologic features of the lesion but also the potential for collateral circulation should vessel occlusion be entertained as a treatment option. Cross-compression views can aid in determining the patency of PComAs and AComAs, as appropriate. In addition, potential donor arteries for surgical bypass can be assessed. Multiple angiographic projections or 3-D angiography can be extremely useful at delineating the relevant pathological anatomy. Balloon test occlusion is performed concurrently if permanent vessel occlusion (endovascular or surgical) is considered as a treatment option or as a bailout maneuver. Currently, microsurgical and endovascular deconstructive strategies without a bypass are used only for bailout when other treatment options are not available; this is because all the tests for collateral supply after temporary occlusion have false-negative results and a 16% to 20% chance of an ischemic event exists after carotid sacrifice, even if balloon occlusion tests were negative.18,19 If surgical bypass is planned, endovascular sacrifice should be performed promptly after the surgical procedure to minimize the risk of graft thrombosis owing to low flow.
Parent vessel preservation
Coil Embolization
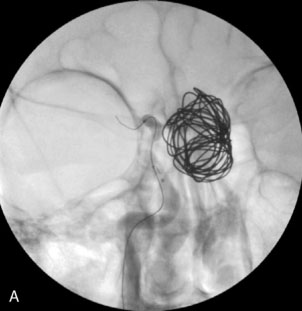
Some giant aneurysms may have a configuration amenable to pure endovascular coiling alone. The best angiographic projection of the aneurysm neck and parent vessel, or vessels, should be obtained. Placement of the microcatheter in a deep position and use of a larger microcatheter that will reduce catheter back-out may be helpful to improve the degree of coil packing. Ideally, 0.018-inch system coils should be used initially to provide the most stable framework from which to coil the bulk of the aneurysm. We prefer to continue to deposit sequentially smaller 3-D coils as feasible to increase the chances of good coverage of the aneurysm neck. Several series of results after simple coiling of giant aneurysms have been reported. Overall, the rate of complete occlusion is approximately 40%, and the rate of near-complete occlusion is approximately 66.7%.20–23 An extremely high recanalization rate of 40% to 60% that required retreatment was noted even in patients in whom complete occlusion was achieved during the primary procedure. With time, most aneurysms reopen by coil compaction, coil migration into intraluminal thrombus, or dissolution of intraluminal thrombus resulting in luminal enlargement.20–23 Clearly, according to these results, coil embolization alone typically is well tolerated clinically, but it is not sufficient to provide a complete and durable long-term result in most patients.
Balloon-Assisted Coil Embolization
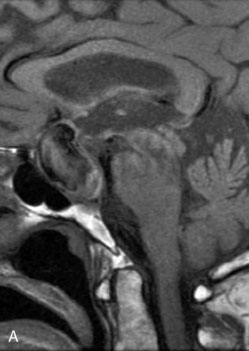
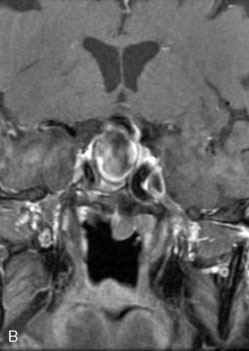
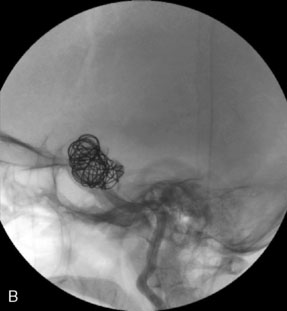
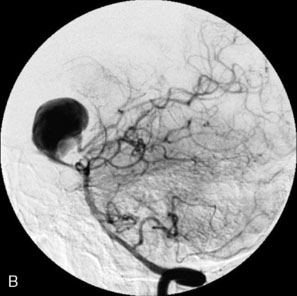
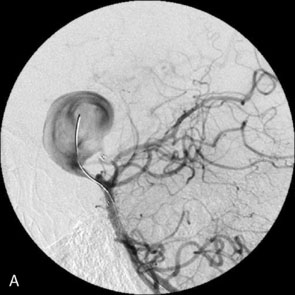
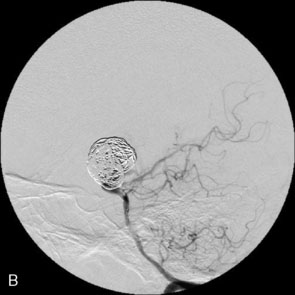
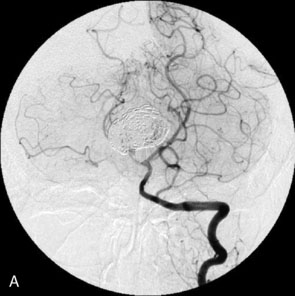
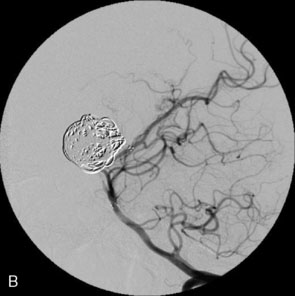
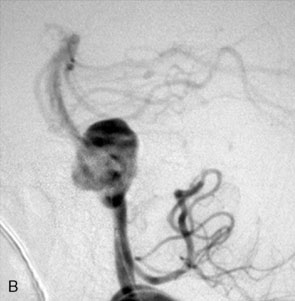
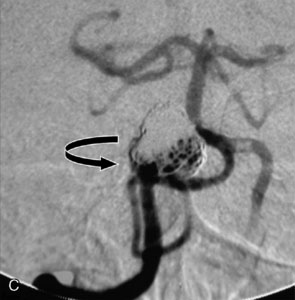
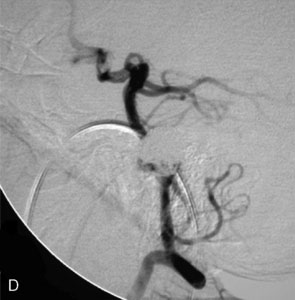
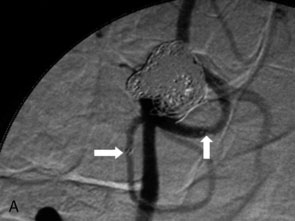
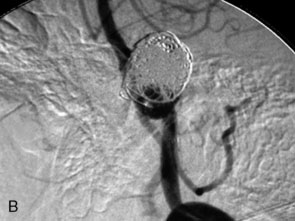
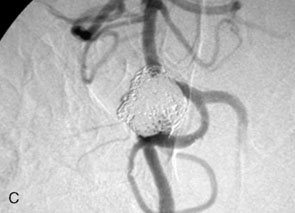
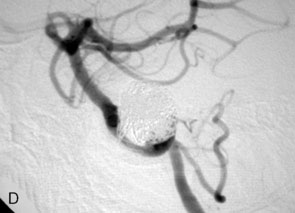
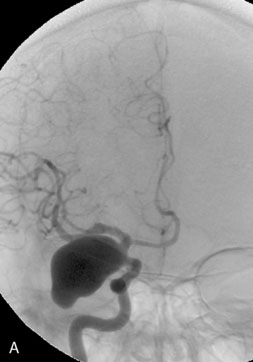
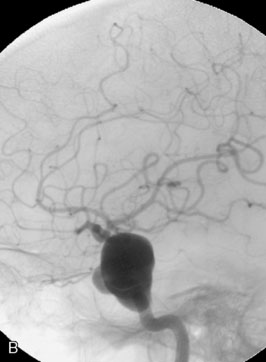
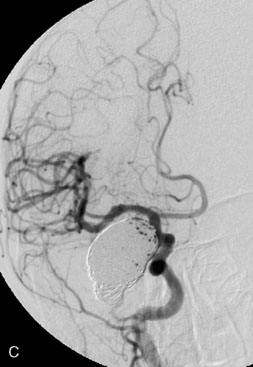
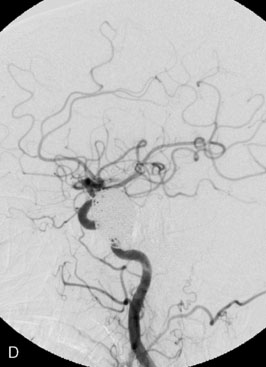
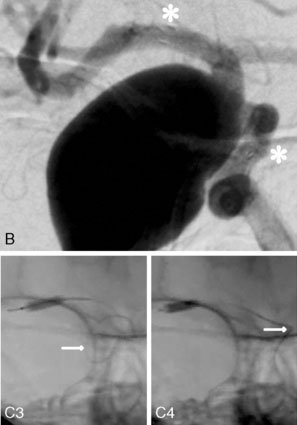
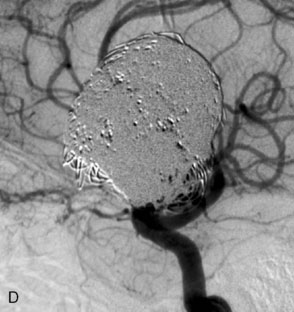
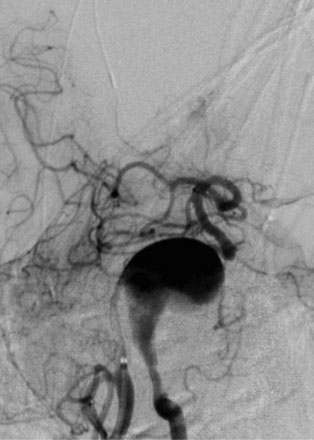
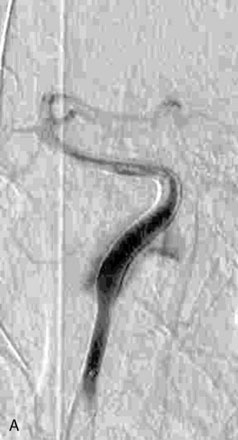
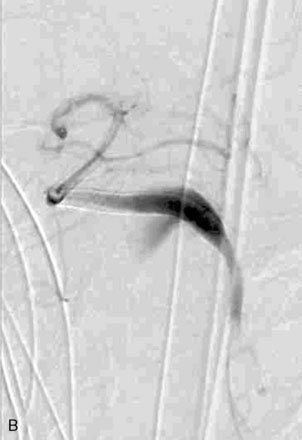
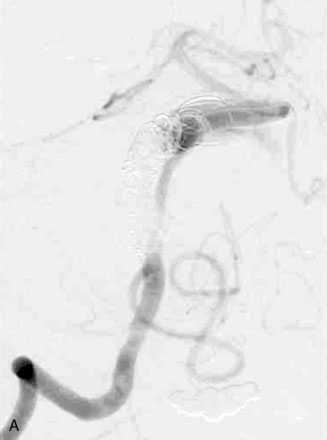
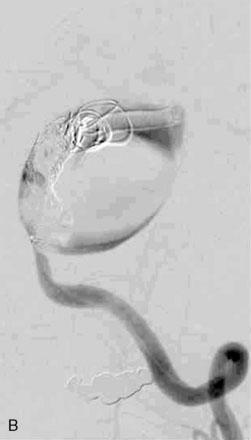
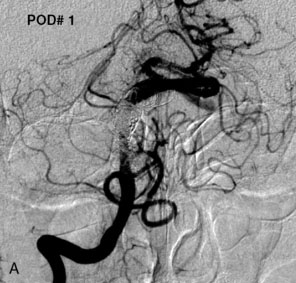
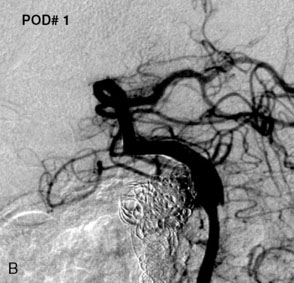
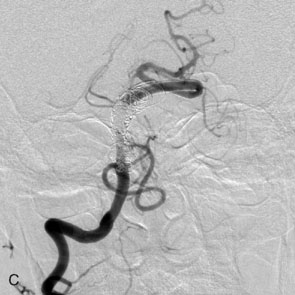
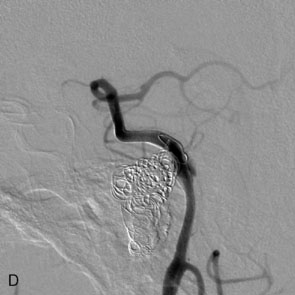
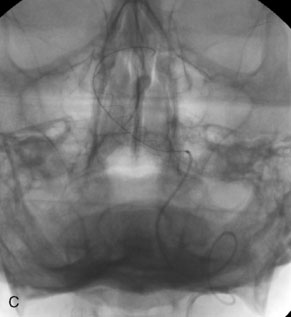
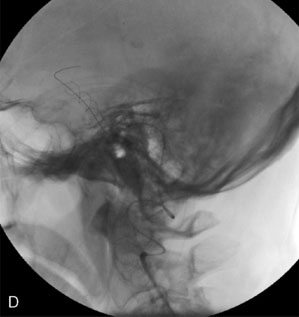
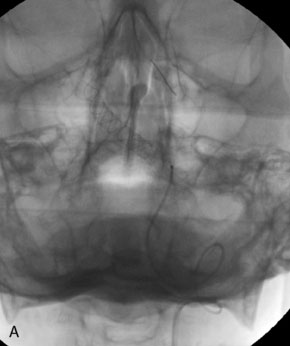
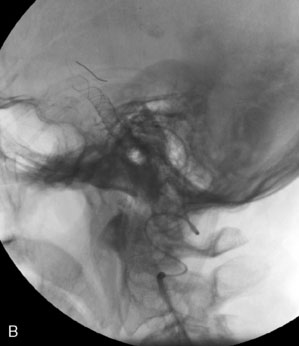
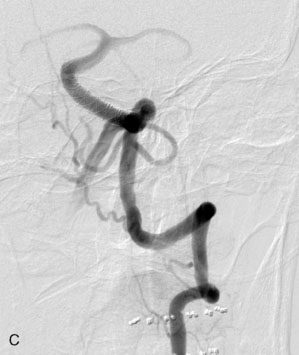
The use of balloons to occlude the aneurysm neck during coiling of wide-necked aneurysms was first described in 1994 by Moret et al.24 A 6-F or larger guide catheter is required to accommodate both a balloon catheter and a microcatheter. A microcatheter is placed into the aneurysm fundus, and a balloon catheter is centered over the aneurysm neck. The balloon is subsequently inflated during placement of a coil and then deflated intermittently in between coils to allow antegrade flow. Sequential inflations and deflations are performed as additional coils are placed, until the aneurysm is completely coiled, at which point the balloon is removed. The concept is that the balloon prevents distal embolization and conforms the coil mass to the shape of the balloon and that the coil mass shape becomes stable, thereby protecting the parent artery as the individual coils interlock. Care must be taken during the initial insertion of a coil to form a loop directing the distal end of the coil away from the aneurysm fundus to limit the risk of aneurysm perforation during balloon inflation (Figures 29-1 through 29-3).
Forty to 50 coils can be required to fill a giant aneurysm, leading to 40 to 50 cycles of balloon inflations, for which the risk may be prohibitive. In addition, the ability to protect the parent artery lumen by means of balloon occlusion during the coiling procedure, especially when there is extensive fusiform dilation, is minimal. Temporary balloon occlusion exposes the patient to an increased risk of cerebral ischemia resulting from thromboembolic complication and vessel rupture. The increase in thromboembolic complications occurs because of stasis of blood or temporary occlusion of local perforating end arteries covered by the balloon. The risk of vessel rupture stems from the compliant design of most balloons used for these purposes and is associated with dramatic changes in volume and pressure in the balloon with minimal inflation volume changes. Soeda et al.25 reported that the occurrence of diffusion-weighted imaging (DWI) lesions was significantly associated with the use of balloon remodeling. In that series, DWI was positive in 73% of all patients receiving a remodeling procedure, as compared to 49% in the control group. Other authors demonstrated lower rates of DWI lesions in 20% of patients treated with remodeling but did not provide a control group.26 Overall procedural balloon-assisted coiling morbidity and mortality range from 0% to 20.4% in all aneurysms. No results for specific series of balloon-assisted coiling for giant aneurysms have been reported.27
Balloon-Assisted Liquid Agent Embolization
In a multicenter study conducted by Molyneux et al.,28 permanent neurologic morbidity was 8.3% (eight of 97 patients), with two procedural deaths. In large and giant aneurysms, procedural time was long (up to 6 hours). Delayed occlusion of the carotid artery occurred in nine of 100 (9%) patients. At 12 months’ follow-up of 53 patients, 38 (72%) large and giant aneurysms were completely angiographically occluded. Retreatment was performed in nine instances. Although some single-center studies show slightly better results,29 in our opinion, the relatively high complication rate and high rate of delayed carotid artery occlusion do not justify this treatment in patients with unruptured aneurysms who cannot tolerate carotid artery occlusion. At present, the short-term results of Onyx occlusion for large and giant aneurysms are not better than those for selective coil occlusion, and the immediate and delayed complication rate is probably higher.
Stent-assisted coiling
In the early 1990s, we described the application of stents to treat experimental aneurysms.30 The basic principles of stent-supported therapy delineated by early experiments are (1) parent vessel protection by preventing coil prolapse30 and (2) parent vessel remodeling providing a scaffold for neointimal growth and producing flow-diversion that may facilitate and maintain aneurysm thrombosis.31,32 Balloon-expanding coronary stents were used initially to support coiling of wide-necked intracranial aneurysms.33–40 These stents were of limited benefit due to their rigid nature and the challenges involved in their delivery and deployment within the tortuous cerebrovasculature. The newer generation of balloon-mounted stents designed specifically for intracranial use (Pharos, Micrus Endovascular, Sunnyvale, CA) are more stable than the predicate devices designed for coronary applications and have indications for the treatment of both cerebral aneurysms and intracranial atherosclerosis.41–44
Self-expanding intracranial microstents (SEIMS)
The introduction of these devices led to a marked increase in the number of stent-assisted aneurysm treatments performed and greatly broadened the scope of lesions that were amenable to endovascular therapy. As practitioners gained experience with SEIMs, novel approaches to more complex lesions were innovated and the sophistication of endovascular reconstruction increased.45–52 Over the past decade, stenting has become a standard adjunctive technique used to facilitate the treatment of giant and complex aneurysms.
Neuroform stent
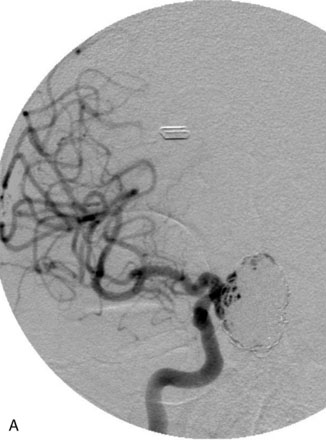
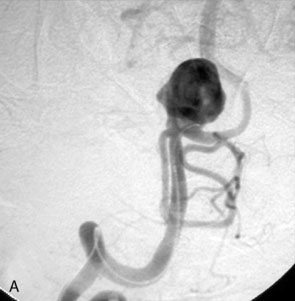
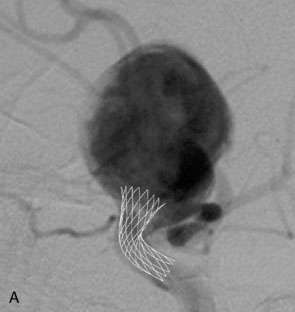
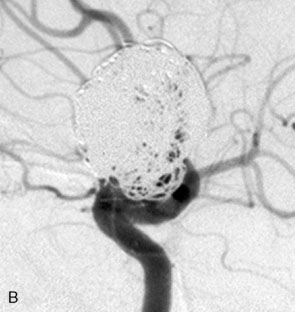
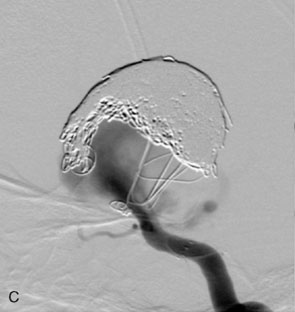
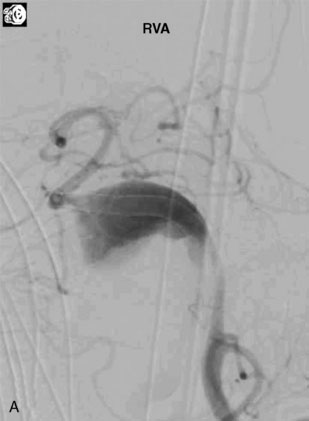
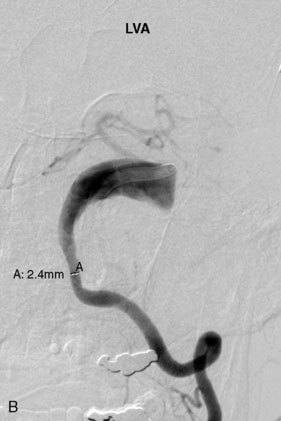
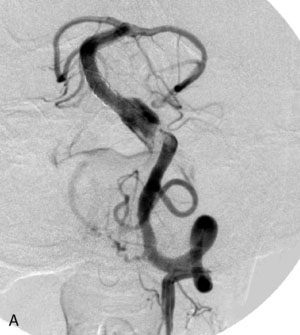
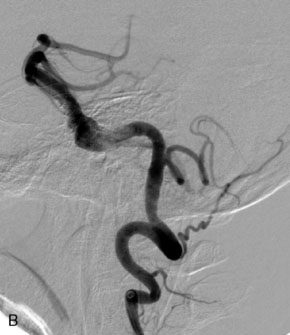
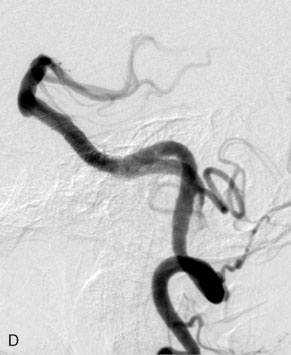
The Neuroform stent comes from the manufacturer preloaded in a 3-F microdelivery catheter. A “stabilizer” catheter, which is also preloaded in the microdelivery catheter, is then used to stabilize and deploy the stent as the microdelivery catheter is withdrawn. The stent consists of a fine wire mesh that cannot be seen during standard fluoroscopic imaging; however, each end of the stent is equipped with four radiopaque platinum marker bands. This device comes in sizes ranging between 2.5 and 4.5 mm in diameter and 10 and 30 mm in length. The diameter recommended by the manufacturer for placement is 0.5 mm greater than the largest diameter of the parent artery to be stented. The length is chosen such that the stent extends for at least 5 mm proximal and distal to the aneurysm neck. The struts composing the stent measure approximately 60 microns in thickness. The interstices of the fully expanded stent are large enough to accommodate a microcatheter tip that is 2.5 F or smaller (realistically, <2.0 F) for coiling. When the stent is placed in curved anatomy (in experimental models), the stent cells are prone to opening, producing gaps in stent coverage along the outer curvature of the vessel.53,54 These gaps could conceivably lead to incomplete coverage of the aneurysm neck and coil prolapse from the aneurysm into the parent artery during embolization (Figures 29-4 through 29-8).
Enterprise stent
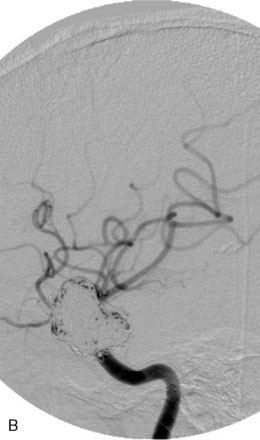
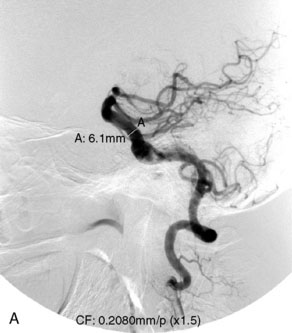
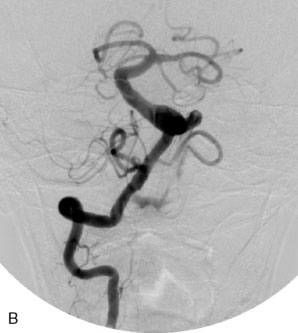
The Enterprise stent comes preloaded with a delivery wire, and both the stent and wire are enclosed within a plastic sheath (the “dispenser loop”). The delivery wire has three radiopaque zones: the proximal wire, the “stent-positioning marker” (which indicates where the undeployed stent is loaded and runs the length of the stent), and the distal tip. The Enterprise measures 4.5 mm in diameter when unconstrained, and as such, is only indicated for use in vessels measuring between 2.5 and 4 mm in diameter. The device comes in 14-, 22-, 28-, and 37-mm lengths. The struts of the Enterprise, like those of the Neuroform, are approximately 60 microns thick. The stent struts cannot be seen on standard fluoroscopy; each end of the four platinum marker bands can be seen, but these are considerably more difficult to see compared with the markers on the Neuroform stent. The interstices of the fully expanded Enterprise stent are large enough to accommodate a microcatheter tip with an outer diameter size of ≤2.3 F for coiling (Figures 29–9 and 29–10).
The closed-cell design of the Enterprise stent makes it reconstrainable. This design also prevents the stent from splaying open along the outer curvature of vascular bends and results in the incorporation of each cell into the entire device structure, making the individual cells more durable and less likely to become damaged during attempted traversal of the device with a microcatheter. At the same time, the loss of segmental flexibility created by the continuous closed-cell structure may result in the device “kinking” or forming a “cobra-head” configuration around tight vascular curves, potentially resulting in poor vessel wall apposition and suboptimal parent vessel protection.45,53 The interstices between the stent struts are also smaller and less deformable in some anatomic configurations, making microcatheter traversal more difficult in some situations. Finally, although the closed-cell structure provides higher radial resistive force (i.e., resistance to outward compression once deployed), it also exerts less chronic outward force (i.e., outward pressure upon the vessel wall), potentially making it more prone to migration during attempted catheterization of the aneurysm or, in some anatomic configurations, spontaneously.55
Indications for SEIMS
Stent-assisted coil embolization. The most common technique is trans-stent coiling in which an SEIM is placed across the aneurysm neck, and coil embolization is performed after microcatheter manipulation. Trans-stent coiling may be performed at the time of the initial procedure or during a second procedure (“staged technique”), typically 4 to 8 weeks after stenting. Some operators prefer the staged technique to allow endothelialization of the stent prior to attempted coiling. The advantages are that the stent is more stable after endothelialization, and the clopidogrel therapy is most often discontinued before coiling. Another technique used is a jailing technique in which a microcatheter is placed inside the aneurysm before stent deployment.56 This technique is less favored, because there is a chance of displacement of the microcatheter during stent placement and even a higher chance of coil stretching or breakage because the coil can get caught between the stent and the vessel wall.
Rescue during coil embolization. If detached coils or the entire mass of coils prolapse into the parent vessel during the procedure, the stent is placed in the vessel either to re-position the coils back in the aneurysm lumen or tack up the coils against the vessel wall and thus prevent further migration and distal emboli.57,58
Advanced seim techniques used in giant aneurysms
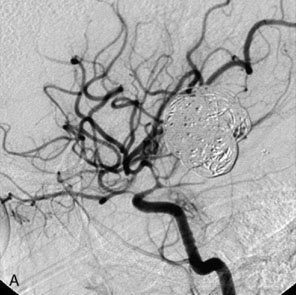
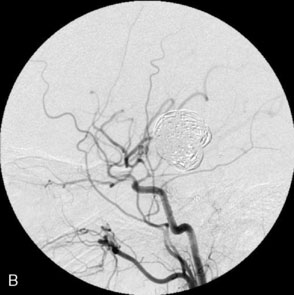
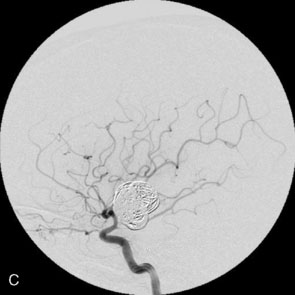
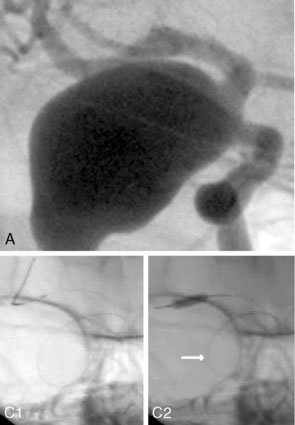
Balloon anchor technique. This technique is useful for the treatment of wide-necked aneurysms having a dominant flow jet that constantly directs any device used for distal vessel access into the aneurysm (Figures 29–11 and 29–12). This, coupled with the inability to achieve stable distal purchase of the access after it is obtained, often leads to abortion of the procedure. In a case treated with this technique at our center, distal parent vessel access was obtained by allowing the microwire to follow the local hemodynamics into a giant ICA aneurysm and around its dome into the distal vessel. An over-the-wire balloon inflated in the distal vessel followed by gentle retraction of the balloon catheter and microwire allowed only a wire bridge across the aneurysm neck, thereby allowing the stent catheter to be brought up in a standard fashion.
Y-stent. This technique is most commonly used for bifurcation aneurysms arising from the basilar tip or carotid terminus (carotid T). Two stents are placed, with the first extending out one limb of the bifurcation and the second introduced through the interstices of the first stent and extending into the other limb of the bifurcation. This configuration forms a “Y”-shaped construct at the bifurcation and provides very robust support for the coil embolization of terminal aneurysms.46,51,52 This Y-stent technique has also been applied to treat MCA aneurysms51 and AComA aneurysms.
Waffle-cone. In cases in which the application of the Y-stent technique is not feasible, a stent may be deployed from the parent artery directly into the aneurysm (i.e., “intra-extra-aneurysmal stent placement”) to achieve parent artery protection59 (Figure 29–13). Using this technique (called the waffle-cone technique because of the appearance of the stent-coil combination after treatment), a single stent can be used to stabilize an intra-aneurysmal coil mass.59 However, the final construct redirects flow into the terminal aneurysm sac and actually disrupts flow into the bifurcation branches. One might expect that such a construct could lead to high rates of recanalization. In addition, if this technique fails or leads to recanalization, other available means of treatment (surgical clipping, endovascular therapy with balloon remodeling, or Y-stent reconstruction) (see Figures 29–13C and 29–13D) are made considerably more difficult, if not impossible.
Trans–Circle of Willis stenting. In some cases where Y-stenting is not feasible for terminal aneurysms, a stent can be placed across the circle of Willis from P1 to P1 via the PComA, from A1 to M1 via the AComA, or from the ipsilateral A1 to contralateral A1 via the AComA.49 This technique provides a means by which to achieve protection of both limbs of the bifurcation with a single SEIM.
Flow-diverting devices
The concept of parent vessel reconstruction is quickly advancing with the very recent development of dedicated flow-diverting endovascular constructs designed for intracranial use. These devices primarily target parent vessel reconstruction, rather than endosaccular occlusion, as the means by which to achieve definitive aneurysm treatment. Thus, these devices can be used also in aneurysms with a fusiform component and a segmental aneurysmal defect in the parent vessel. Currently, these flow-diverting devices are high metal surface area coverage, stent-like constructs that are designed to provide enough flow redirection and endovascular remodeling to induce aneurysm thrombosis without the use of additional endosaccular occlusive devices (i.e., coils). At the same time, the pore size of the constructs is large enough to allow for the continued perfusion of branch vessels and perforators arising from the reconstructed segment of the parent vessel.60 Large or giant size or the presence of intra-aneurysmal thrombus, both of which are factors typically associated with coil compaction and aneurysm recurrence, are not an issue with the flow-diverting devices, because no endosaccular coils are placed. In fact, once the diseased segment is reconstructed and the construct fully endothelialized, the aneurysm and the diseased vascular segment could be considered “definitively” treated with the typical mechanisms of aneurysm recurrence or regrowth being essentially eliminated. In addition, as a purely “extrasaccular” treatment strategy, no direct catheterization or manipulation of the aneurysm sac is required, possibly reducing the likelihood of procedural rupture and potentially improving the safety of endovascular aneurysm treatment. The Pipeline Embolization device (PED; ev3) represents the first flow-diversion device used in humans. We have recently used the SILK flow-diverting device (BALT Extrusion, Montmorency, France). Multiple other flow-diverting devices are currently under development and in testing.
Pipeline embolization device
The pipeline embolization device (PED) is a cylindrical, stent-like construct composed of 48 braided strands of cobalt chromium and platinum. The device is packaged within an introducer sheath collapsed upon a delivery wire. The device is loaded into and delivered via the hub of a 0.027-inch internal diameter microcatheter that has been positioned across the neck of the aneurysm. Initially collapsed within the delivery sheath or microcatheter, the device is elongated approximately 2.5 times its deployed length when expanded to nominal diameter. As it is deployed, the device foreshortens toward its nominal length (which it achieves only if allowed to expand fully to its nominal diameter). Currently, the available devices range from 2.5 to 5 mm in diameter (in 0.25-mm increments) and 10 to 20 mm in length (in 2-mm increments). The deployed device is very flexible and conforms to the normal parent anatomy, even in very tortuous vascular anatomy (Figures 29-14 through 29-19).
To date, the PED has been implanted in more than 80 patients for the treatment of intracranial aneurysms—39 have been in the context of clinical studies, the Pipeline Embolization Device in the Intracranial Treatment of Aneurysms (PITA) trial or the single-center “Budapest post-PITA study.” The remainder have been performed under provisions for compassionate use for aneurysms that were otherwise untreatable or had failed numerous other conventional treatments.61
The PED has also been used to successfully treat nonsaccular (fusiform or circumferential) aneurysms. Three such cases have been performed by Fiorella et al.62 in North America under a Food and Drug Administration compassionate-use exemption. All three lesions, which were judged to be untreatable with existing endovascular or open surgical technologies, were angiographically cured with PED, without technical or neurologic complications. In two cases, eloquent perforators or side-branch vessels were covered by the PED construct; and in both cases, these remained patent at angiographic follow-up. Two of these patients now have more than 1 year of clinical and angiographic follow-up and remain angiographically cured of their lesions and are without neurological symptoms.62
Silk flow-diverting device
The SILK device is similar to the PED and has 48 braided wires (44 nitinol and four platinum). It is available in diameters of 2.0 to 5.5 mm (in 0.5-mm increments) and lengths of 15 to 40 mm (also in 5-mm increments). The SILK device shortens by at least 50% when deployed. It comes prepackaged with a delivery system comprised of a delivery wire and an introducer and a reinforced microcatheter for placement (Vasco+21 2.4 F for devices 2 to 4.5 mm in diameter and 3 F for devices 5 to 5.5 mm in diameter, preshaped with a multipurpose distal curve). The SILK device is preloaded on the delivery wire inside the introducer. After microcatheter access to the aneurysm has been obtained, working projections and vessel measurements are taken for device sizing. The SILK device is transferred from the introducer into the hub of the microcatheter using a Y connector and by gently advancing the delivery wire. The SILK device is positioned past the aneurysm neck at least 1.5 times the diameter of the parent vessel. A gentle push is given on the delivery wire until the distal radiopaque end is out of the distal marker on the microcatheter. Withdrawing the microcatheter to deploy the SILK device to approximately 1 cm creates a distal anchor of the SILK device on the vessel wall. This manipulation ensures that the SILK device does not move distally, because the wire struts of the SILK may cause damage to the vessel wall. After the SILK is deployed to approximately 1 cm, the distal tip is positioned by simultaneously pulling on the catheter and on the delivery wire. It is possible to move the SILK device by pulling the delivery wire, as long as the distal ring of the catheter does not superimpose the radiopaque marker on the delivery wire. If SILK repositioning is required, the catheter is gently advanced over the deployed SILK, the system is repositioned, and the device is re-deployed in the new location. The SILK is fully deployed when the marker of the delivery wire lines up with the distal ring of the catheter. It is then impossible to resheath the SILK. The delivery wire is pushed until its marker overpasses the catheter’s marker by a minimum of 2 mm, when it completely detaches. Once detached, the SILK proximal tip might be not fully deployed (particularly in a curve). The most effective technique is to push the proximal end with the microcatheter and make the device open. The microcatheter is positioned distal to the stent to maintain access through the stent before removal of the delivery wire. The main advantages of the SILK device over the PED is better translatability of the one-to-one movement of the microcatheter and the delivery system and the ability to recapture and reposition the system even up to 90% deployment. The ability to recapture a flow-diverting device in our experience is very important. These devices require frequent repositioning as they foreshorten during deployment, and we very often get to know what the final position of the stent would be only after partial deployment (Figures 29-20 through 29-22).
Covered stents
Newer generations of balloon-expandable covered, partially covered, and semiporous covered stents are currently under development, with modifications designed to overcome the aforementioned limitations of the predicate devices. The Willis covered stent (Micro-port, Shanghai, China) is built upon a cobalt chromium platform that incorporates a very thin layer of PTFE into its structure, designed to maximize its flexibility and deliverability. The limited clinical data available for the use of this stent in humans has been encouraging.63–65 The endovascular clip system (eCLIPs, Evasc Medical Systems, Vancouver, BC, Canada) is a partially covered, balloon-expandable stent that is designed such that the covered portion can be oriented to selectively cover an aneurysm neck,66 while leaving the remainder of the endoluminal surface of the parent artery uncovered, thus potentially allowing continued perfusion of regional perforators while occluding the aneurysm.
Complication avoidance and management
Stent Misplacement
Spontaneous delayed stent migration after proper placement has been observed only with the Enterprise.55 The closed-cell structure of the device results in its ability to migrate when placed into vessels of discrepant caliber. The force of the stent in the smaller vessel is transmitted to the part of the stent in the larger vessel, and the device can “watermelon seed” into the larger artery.
In-stent Stenosis
Delayed in-stent stenosis following implantation of a Neuroform stent represents a relatively uncommon (~5%) event.67 Neuroform-induced in-stent stenosis is usually asymptomatic; and, in more than half of the cases reported, spontaneously regressed at angiographic follow-up.67 Only in rare cases does this stenosis progress to the point where it can actually produce significant luminal compromise, perfusion failure, and clinical symptoms that may lead to ischemic stroke.
Imaging Follow Up
Given the high recurrence and retreatment rates associated with simple coiling in giant aneurysms, regular follow-up imaging is essential to monitor exclusion of the aneurysm from the circulation. Conventional angiography represents the only technique that provides a reliable assessment of the stented parent artery. As in-stent stenosis usually develops within 6 months, a 6-month conventional angiography is performed for assessment of the stented parent artery. Short echo time (TE) MR angiography sequences have been designed specifically to assess aneurysms after endovascular therapy.68 This sequence is designed to minimize susceptibility artifacts related to the coil mass and stents. Although very effective for imaging of the coil mass and assessing residual aneurysm, MR is limited to some extent for evaluation of the parent artery after stenting. CT angiography is of use in those cases treated with stenting alone, as the stents themselves produce much less artifact than the embolization coils.
Results of Endovascular Therapy
Most of the reported results of the endovascular management of giant aneurysms pertain to treatment via either parent vessel occlusion or simple coiling. We reported a series of 38 patients with 39 giant aneurysms treated between December 2001 and July 2007 in which stent-assisted coiling was used in 25 patients at some point during at least one treatment session.69 At the last angiographic follow-up examination (mean, 21.5 months; standard deviation [SD], ±22.9 months), 95% or higher and 100% occlusion rates were documented in 64% and 36% of aneurysms, respectively, with parent vessel preservation maintained in 74%. Twenty percent of treatment sessions resulted in permanent morbidity; death within 30 days occurred after 8% of treatment sessions. An average of 1.9 sessions (SD, ±1.1) were required to treat each aneurysm, with a resulting cumulative per-patient mortality of 16% and morbidity of 32%. At the last known clinical follow-up examination (mean, 24.8 months; SD, ±24.8 months), 24 (63%) patients had Glasgow Outcome Scale scores of 4 or 5 (good or excellent), 10 patients had worsened neurological function from baseline (26% morbidity), and 11 had died (29% mortality). Although the results in this series are comparable to microsurgical series, the endovascular technology used in this series has been outdated with the introduction of the Enterprise stent and dedicated flow-diversion devices.
The future
The current market is proliferating with numerous flow-diverting devices that are being designed and tested by multiple endovascular device manufacturers. The preliminary experience with the PED shows promise, although long-term results and the ability of these devices to preserve perforators and branch vessels need to be studied in detail. Asymmetric covered stents that can be positioned in such a way that they occlude only the circumference of the parent vessel involved by the aneurysm neck are being developed.70,71 In the future, stents with custom-made covers based on the morphology of the aneurysm neck-parent vessel interface may be available. Advances in endovascular device technology and imaging may allow the design of newer stents with increasing trackability and easy deployment in the tortuous cerebrovasculature. Stents specifically designed for terminal bifurcation aneurysms are being developed that will allow aneurysm occlusion with preservation of flow in both branches. Stents and systemic drugs that accelerate stent and orifice endothelialization need to be developed to reduce the duration of antiplatelet therapy, increase aneurysm occlusion rates, and allow more widespread use of these stents for ruptured aneurysms.
1 Locksley H.B. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. Based on 6368 cases in the cooperative study. J Neurosurg. 1966;25:219-239.
2 Morley T.P., Barr H.W. Giant intracranial aneurysms: diagnosis, course, and management. Clin Neurosurg. 1969;16:73-94.
3 Raaymakers T.W., Rinkel G.J., Limburg M., et al. Mortality and morbidity of surgery for unruptured intracranial aneurysms: a meta-analysis. Stroke. 1998;29:1531-1538.
4 Choi I.S., David C. Giant intracranial aneurysms: development, clinical presentation and treatment. Eur J Radiol. 2003;46:178-194.
5 Khanna R.K., Malik G.M., Qureshi N. Predicting outcome following surgical treatment of unruptured intracranial aneurysms: a proposed grading system. J Neurosurg. 1996;84:49-54.
6 Drake C.G. Giant intracranial aneurysms: experience with surgical treatment in 174 patients. Clin Neurosurg. 1979;26:12-95.
7 International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med. 1998;339:1725-1733.
8 Wiebers D.O., Whisnant J.P., Huston J.3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103-110.
9 Lawton M.T., Quinones-Hinojosa A., Sanai N., et al. Combined microsurgical and endovascular management of complex intracranial aneurysms. Neurosurgery. 2008;62(Suppl 3):1503-1515.
10 Lawton M.T., Spetzler R.F. Surgical strategies for giant intracranial aneurysms. Acta Neurochir Suppl. 1999;72:141-156.
11 Samson D., Batjer H.H., Kopitnik T.A.Jr. Current results of the surgical management of aneurysms of the basilar apex. Neurosurgery. 1999;44:697-704.
12 Surdell D.L., Hage Z.A., Eddleman C.S., et al. Revascularization for complex intracranial aneurysms. Neurosurg Focus. 2008;24:E21.
13 Piepgras D.G., Khurana V.G., Whisnant J.P. Ruptured giant intracranial aneurysms. Part II. A retrospective analysis of timing and outcome of surgical treatment. J Neurosurg. 1998;88:430-435.
14 Hanel R.A., Spetzler R.F. Surgical treatment of complex intracranial aneurysms. Neurosurgery. 2008;62(Suppl 3):1289-1297.
15 Mohit A.A., Sekhar L.N., Natarajan S.K., et al. High-flow bypass grafts in the management of complex intracranial aneurysms. Neurosurgery. 2007;60(Suppl 1):ONS105-ONS123.
16 Sekhar L.N., Stimac D., Bakir A., et al. Reconstruction options for complex middle cerebral artery aneurysms. Neurosurgery. 2005;56(Suppl 1):66-74.
17 Evans J.J., Sekhar L.N., Rak R., et al. Bypass grafting and revascularization in the management of posterior circulation aneurysms. Neurosurgery. 2004;55:1036-1049.
18 Larson J.J., Tew J.M.Jr, Tomsick T.A., et al. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: long-term follow-up of 58 patients. Neurosurgery. 1995;36:26-30.
19 Origitano T.C., al-Mefty O., Leonetti J.P., et al. Vascular considerations and complications in cranial base surgery. Neurosurgery. 1994;35:351-363.
20 Sluzewski M., Menovsky T., van Rooij W.J., et al. Coiling of very large or giant cerebral aneurysms: long-term clinical and serial angiographic results. AJNR Am J Neuroradiol. 2003;24:257-262.
21 Gruber A., Killer M., Bavinzski G., et al. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery. 1999;45:793-804.
22 van Rooij W.J., Sluzewski M. Coiling of very large and giant basilar tip aneurysms: midterm clinical and angiographic results. AJNR Am J Neuroradiol. 2007;28:1405-1408.
23 Murayama Y., Nien Y.L., Duckwiler G., et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg. 2003;98:959-966.
24 Moret J., Pierot L., Boulin A., et al. “Remodeling” of the arterial wall of the parent vessel in the endovascular treatment of intracranial aneurysms (abstr S83). Proceedings of the 20th Congress of the European Society of Neuroradiology. Neuroradiology. 1994;36(Suppl 1):S83.
25 Soeda A., Sakai N., Sakai H., et al. Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2003;24:127-132.
26 Albayram S., Selcuk H., Kara B., et al. Thromboembolic events associated with balloon-assisted coil embolization: evaluation with diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2004;25:1768-1777.
27 Kurre W., Berkefeld J. Materials and techniques for coiling of cerebral aneurysms: how much scientific evidence do we have? Neuroradiology. 2008;50:909-927.
28 Molyneux A.J., Cekirge S., Saatci I., et al. Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. AJNR Am J Neuroradiol. 2004;25:39-51.
29 Weber W., Siekmann R., Kis B., et al. Treatment and follow-up of 22 unruptured wide-necked intracranial aneurysms of the internal carotid artery with Onyx HD 500. AJNR Am J Neuroradiol. 2005;26:1909-1915.
30 Szikora I., Guterman L.R., Wells K.M., et al. Combined use of stents and coils to treat experimental wide-necked carotid aneurysms: preliminary results. AJNR Am J Neuroradiol. 1994;15:1091-1102.
31 Wakhloo A.K., Schellhammer F., de Vries J., et al. Self-expanding and balloon-expandable stents in the treatment of carotid aneurysms: an experimental study in a canine model. AJNR Am J Neuroradiol. 1994;15:493-502.
32 Wakhloo A.K., Tio F.O., Lieber B.B., et al. Self-expanding nitinol stents in canine vertebral arteries: hemodynamics and tissue response. AJNR Am J Neuroradiol. 1995;16:1043-1051.
33 Han P.P., Albuquerque F.C., Ponce F.A., et al. Percutaneous intracranial stent placement for aneurysms. J Neurosurg. 2003;99:23-30.
34 Lanzino G., Wakhloo A.K., Fessler R.D., et al. Efficacy and current limitations of intravascular stents for intracranial internal carotid, vertebral, and basilar artery aneurysms. J Neurosurg. 1999;91:538-546.
35 Lylyk P., Ceratto R., Hurvitz D., et al. Treatment of a vertebral dissecting aneurysm with stents and coils: technical case report. Neurosurgery. 1998;43:385-388.
36 Malek A.M., Halbach V.V., Phatouros C.C., et al. Balloon-assist technique for endovascular coil embolization of geometrically difficult intracranial aneurysms. Neurosurgery. 2000;46:1397-1407.
37 Mericle R.A., Lanzino G., Wakhloo A.K., et al. Stenting and secondary coiling of intracranial internal carotid artery aneurysm: technical case report. Neurosurgery. 1998;43:1229-1234.
38 Sekhon L.H., Morgan M.K., Sorby W., et al. Combined endovascular stent implantation and endosaccular coil placement for the treatment of a wide-necked vertebral artery aneurysm: technical case report. Neurosurgery. 1998;43:380-384.
39 Wilms G., van Calenbergh F., Stockx L., et al. Endovascular treatment of a ruptured paraclinoid aneurysm of the carotid siphon achieved using endovascular stent and endosaccular coil placement. AJNR Am J Neuroradiol. 2000;21:753-756.
40 Higashida R.T., Smith W., Gress D., et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case report and review of the literature. J Neurosurg. 1997;87:944-949.
41 Freitas J.M., Zenteno M., Aburto-Murrieta Y., et al. Intracranial arterial stenting for symptomatic stenoses: a Latin American experience. Surg Neurol. 2007;68:378-386.
42 Kurre W., Berkefeld J., Sitzer M., et al. Treatment of symptomatic high-grade intracranial stenoses with the balloon-expandable Pharos stent: initial experience. Neuroradiology. 2008;50:701-708.
43 Mocco J., Darkhabani Z., Levy E.I. Pharos neurovascular intracranial stent: elective use for a symptomatic stenosis refractory to medical therapy. Catheter Cardiovasc Interv. 2009;74:642-646.
44 Zenteno M.A., Santos-Franco J.A., Freitas-Modenesi J.M., et al. Use of the sole stenting technique for the management of aneurysms in the posterior circulation in a prospective series of 20 patients. J Neurosurg. 2008;108:1104-1118.
45 Benndorf G., Klucznik R.P., Meyer D., et al. “Cross-over” technique for horizontal stenting of an internal carotid bifurcation aneurysm using a new self-expandable stent: technical case report. Neurosurgery. 2006;58(Suppl 1):ONS-E172.
46 Chow M.M., Woo H.H., Masaryk T.J., et al. A novel endovascular treatment of a wide-necked basilar apex aneurysm by using a Y-configuration, double-stent technique. AJNR Am J Neuroradiol. 2004;25:509-512.
47 Cross D.T.3rd, Moran C.J., Derdeyn C.P., et al. Neuroform stent deployment for treatment of a basilar tip aneurysm via a posterior communicating artery route. AJNR Am J Neuroradiol. 2005;26:2578-2581.
48 Fiorella D., Albuquerque F.C., Masaryk T.J., et al. Balloon in-stent technique for the constructive endovascular treatment of “ultra-wide necked” circumferential aneurysms. Neurosurgery. 2005;57:1218-1227.
49 Kelly M.E., Turner R., Gonugunta V., et al. Stent reconstruction of wide-necked aneurysms across the circle of Willis. Neurosurgery. 2007;61(Suppl 2):249-255.
50 Moret J., Ross I.B., Weill A., et al. The retrograde approach: a consideration for the endovascular treatment of aneurysms. AJNR Am J Neuroradiol. 2000;21:262-268.
51 Sani S., Lopes D.K. Treatment of a middle cerebral artery bifurcation aneurysm using a double Neuroform stent “Y” configuration and coil embolization: technical case report. Neurosurgery. 2005;57(Suppl 1):E209.
52 Thorell W.E., Chow M.M., Woo H.H., et al. Y-configured dual intracranial stent-assisted coil embolization for the treatment of wide-necked basilar tip aneurysms. Neurosurgery. 2005;56:1035-1040.
53 Ebrahimi N., Claus B., Lee C.Y., et al. Stent conformity in curved vascular models with simulated aneurysm necks using flat-panel CT: an in vitro study. AJNR Am J Neuroradiol. 2007;28:823-829.
54 Hsu S.W., Chaloupka J.C., Feekes J.A., et al. In vitro studies of the Neuroform microstent using transparent human intracranial arteries. AJNR Am J Neuroradiol. 2006;27:1135-1139.
55 Kelly M.E., Turner R.D.4th, Moskowitz S.I., et al. Delayed migration of a self-expanding intracranial microstent. AJNR Am J Neuroradiol. 2008;29:1959-1960.
56 Hanel R.A., Boulos A.S., Sauvageau E.G., et al. Stent placement for the treatment of nonsaccular aneurysms of the vertebrobasilar system. Neurosurg Focus. 2005;18:E8.
57 Fiorella D., Albuquerque F.C., Han P., et al. Preliminary experience using the Neuroform stent for the treatment of cerebral aneurysms. Neurosurgery. 2004;54:6-17.
58 Lavine S.D., Larsen D.W., Giannotta S.L., et al. Parent vessel Guglielmi detachable coil herniation during wide-necked aneurysm embolization: treatment with intracranial stent placement: two technical case reports. Neurosurgery. 2000;46:1013-1017.
59 Horowitz M., Levy E., Sauvageau E., et al. Intra/extra-aneurysmal stent placement for management of complex and wide-necked-bifurcation aneurysms: eight cases using the waffle cone technique. Neurosurgery. 2006;58(Suppl 2):ONS-258–ONS-262.
60 Kallmes D.F., Ding Y.H., Dai D., et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007;38:2346-2352.
61 Fiorella D., Kelly M.E., Turner R.D.I., et al. Endovascular treatment of cerebral aneurysms. Endovascular Today. June, 2008;53-64. Available at www.evtoday.com/PDFarticles/0608/EVT0608_06.php Accessed January 5, 2009.
62 Fiorella D., Woo H.H., Albuquerque F.C., et al. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62:1115-1121.
63 Li M.H., Li Y.D., Gao B.L., et al. A new covered stent designed for intracranial vasculature: application in the management of pseudoaneurysms of the cranial internal carotid artery. AJNR Am J Neuroradiol. 2007;28:1579-1585.
64 Li M.H., Zhu Y.Q., Fang C., et al. The feasibility and efficacy of treatment with a Willis covered stent in recurrent intracranial aneurysms after coiling. AJNR Am J Neuroradiol. 2008;29:1395-1400.
65 Wang J.B., Li M.H., Fang C., et al. Endovascular treatment of giant intracranial aneurysms with Willis covered stents: technical case report. Neurosurgery. 2008;62:E1176-1177.
66 Marotta T.R., Gunnarsson T., Penn I., et al. A novel endovascular clip system for the treatment of intracranial aneurysms: technology, concept, and initial experimental results. Laboratory investigation. J Neurosurg. 2008;108:1230-1240.
67 Fiorella D., Albuquerque F.C., Woo H., et al. Neuroform in-stent stenosis: incidence, natural history, and treatment strategies. Neurosurgery. 2006;59:34-42.
68 Wallace R.C., Karis J.P., Partovi S., et al. Noninvasive imaging of treated cerebral aneurysms, part I: MR angiographic follow-up of coiled aneurysms. AJNR Am J Neuroradiol. 2007;28:1001-1008.
69 Jahromi B.S., Mocco J., Bang J.A., et al. Clinical and angiographic outcome after endovascular management of giant intracranial aneurysms. Neurosurgery. 2008;63:662-675.
70 Ionita C.N., Paciorek A.M., Dohatcu A., et al. The asymmetric vascular stent: efficacy in a rabbit aneurysm model. Stroke. 2009;40:959-965.
71 Ionita C.N., Paciorek A.M., Hoffmann K.R., et al. Asymmetric vascular stent: feasibility study of a new low-porosity patch-containing stent. Stroke. 2008;39:2105-2113.