Chapter 91 Endovascular Management of Spinal Vascular Malformations
The first clinical report of a spinal vascular malformation was presented in 1890 by Berenbruch, who only recognized the lesion as a vascular malformation at autopsy.1 Heboldt and Gaupp were the first to recognize that spinal vascular malformations could cause subarachnoid hemorrhage (SAH).1 The first surgical treatment for a spinal vascular malformation was reported in 1916 by Elsberg.2 In 1943, Wyburn-Mason described two distinct types of spinal vascular malformations: a venous type consisting of dilated, tortuous blue pial veins that was typically located posteriorly in the thoracic cord; and an arteriovenous type with a fistulous capillary bed located anteriorly in the cervical or lumbar enlargements. This was the first differentiation of spinal intramedullary arteriovenous malformations (AVMs) from spinal dural fistulas.1 The first report of spinal angiography to characterize a spinal vascular malformation was published in 1962 by Djindjian and colleagues. Since that time, the use of spinal angiography as both a diagnostic and a therapeutic tool has become increasingly important in the management of spinal vascular malformations.
Spinal Vascular Anatomy
Each segmental artery accompanies its corresponding nerve into the neural foramen. Each segmental artery divides into dural and radicular arteries. The radicular artery provides supply to the dorsal and ventral nerve roots. Some segmental arteries provide supply to the spinal cord via branches connecting to the pial/coronal arterial network and are designated radiculopial arteries. Some segmental arteries provide direct supply to the anterior spinal artery and are designated radiculomedullary arteries. The two most significant radiculomedullary arteries are the artery of cervical enlargement and the artery of lumbar enlargement, also known as the arteria radicularis magna or the artery of Adamkiewicz.3
Classification of Spinal Vascular Malformations
The classification of spinal vascular malformations has evolved significantly over the last 150 years as the understanding of the complex pathophysiology of these lesions has continued to improve. The initial classification schemes that were proposed failed to properly categorize the lesions based on their anatomy. The first classification scheme for spinal vascular lesions was proposed by Virchow in 1858.4 Virchow subdivided vascular lesions into two types: angioma cavernosum (lesion without parenchyma between the blood vessels) and angioma racemosum (hamartoma: lesion with parenchyma between the blood vessels). Elsberg, in 1916, was the next to propose a classification scheme for spinal vascular lesions in which he divided them into three categories: aneurysm of spinal vessels, angioma in which a mass of dilated veins penetrates the spinal cord, and dilation of posterior spinal veins. In 1928, Cushing and Bailey proposed yet another classification system in which they divided spinal vascular lesions into two major groups: hemangioblastomas and vascular malformations. The broad group of vascular malformations included plexus of dilated veins, aneurismal varix, venous angioma, and telangiectasias.
Advances in neurointerventional surgery and microneurosurgery during the 1960s and 1970s combined with a clearer understanding of the true pathophysiology of spinal vascular lesions led to the development of a new classification system that divided spinal vascular malformations into types I to IV. This system, which is still widely used today, did not include neoplastic lesions (Table 91-1). The classification system proposed by Rodesch and colleagues divided spinal vascular malformations into three groups: AVMs, fistulas, and genetic classification of spinal cord arteriovenous shunts.5 The last group was further subdivided into three groups: genetic hereditary lesions (macrofistulas and hereditary hemorrhagic telangectasia), genetic nonhereditary lesions (multiple lesions with metameric or myelomeric associations), and single lesions (incomplete associations of either of the first two categories). The classification system for spinal vascular lesions proposed by Spetzler and colleagues also subdivided the lesions into four large groups: neoplasms, spinal aneurysms, spinal AVMs, and spinal fistulas.6 Arteriovenous fistulas (AVFs) were further subdivided into extradural and intradural (dorsal or ventral). AVMs were further classified into extradural-intradural or intradural. Intradural AVMs were further subclassified as intramedullary, compact, diffuse, and conus medullaris. This separate classification of conus medullaris AVMs is unique to the Spetzler system.6
TABLE 91-1 Common Classification of Spinal Vascular Malformations
| Type I: Dural AVF |
| Type IA: Single arterial feeder |
| Type IB: Multiple arterial feeders |
| Type II: True AVMs of the Spinal Cord |
| Structurally similar to cerebral AVMs |
| Type III: Juvenile AVMs or Metameric AVMs |
| More diffuse lesions than type II |
| Type IV: Pial AVF |
| Perimedullary, intradural |
| High-flow fistulas |
| Type IV A: Solitary AVF fed by the ASA |
| Type IV B: Small group of AVFs |
| • Supplied by anterior and posterior spinal arteries |
| • Located at the conus |
| Type IV C: Single large AVF |
| • Supplied by the anterior and posterior spinal arteries |
| • Located in the cervical or thoracic spinal cord |
AVF, Arteriovenous fistula; AVM, arteriovenous malformation; ASA, anterior spinal artery.
Of these classification systems, the type I through IV system remains the most commonly used. Type 1 spinal vascular malformations are actually dorsal intradural AVFs with the fistulas located in the proximal dura of the nerve root sleeve. These malformations are the most common spinal vascular malformation. They are subdivided into type A, in which the malformation is supplied by a single arterial feeder, and type B, in which the malformation is supplied by multiple arterial feeders.7 They occur most commonly in the lower thoracic and upper lumbar segments of the spinal cord, T4–L3, with the peak incidence between T7 and T12.8
In type IB malformations, there are anastomoses between branches of several adjacent radicular arteries and a radiculomedullary vein. The radiculomedullary vein becomes arterialized owing to increased flow and pressure, which it transmits to the valveless coronal plexus and the longitudinal veins. The radiculomedullary vein becomes enlarged and tortuous, leading to its classic angiographic appearance. Studies have shown that the mean intraluminal venous pressure is increased to 74% of the systemic arterial pressure.9 The normal pressure in the coronal venous plexus is approximately twice that of the epidural venous plexus. This significant pressure gradient is necessary for normal venous drainage. When it is compromised, as in the case of type I spinal AVMs, venous hypertension develops. Venous hypertension then leads to the development of progressive myelopathy due to transmission of increased venous pressure to the spinal cord parenchyma, resulting in multiple pathologic changes including demyelination.
Type II spinal vascular malformations are true AVMs of the spinal cord with multiple arterial feeders, a nidus, and draining vein(s). They are structurally similar to cerebral AVMs. They are the second most common spinal vascular malformation. They are high-flow, low-resistance, high-pressure lesions.6 The arterial feeders are usually branches of the anterior spinal artery or the posterior spinal arteries.
Spinal artery aneurysms or venous aneurysms are found in 20% to 40% of patients with intramedullary AVMs. The presence of a spinal artery aneurysm has been associated with an increased risk of hemorrhage.10
Type IV spinal vascular malformations are actually perimedullary AVFs and were first described by Djindjian and colleagues. The fistula occurs ventrally and in the midline between the anterior spinal artery and the coronal venous plexus.11 In contrast to type I dural AVFs, type IV lesions are high-flow fistulas. These lesions are further classified into three subtypes. Type A (Merland subtype I) is a solitary AVF fed by the anterior spinal artery (ASA) and located at the conus medullaris or the filum terminale. There is moderate venous hypertension without enlargement of the ASA. The ascending draining vein is only minimally dilated. Type B (Merland subtype II) is a small group of AVFs located at the conus and supplied by the anterior and posterior spinal arteries. The feeding arteries and draining veins are moderately dilated. Venous ectasia is present at the site of the fistula, and the ascending perimedullary veins are tortuous and enlarged. Type C (Merland subtype III) is a single large AVF supplied by the anterior and posterior spinal arteries and located in the cervical or thoracic spinal cord.12 The draining vein is significantly dilated and ectatic and may be embedded within the spinal cord.
Clinical Presentation and Natural History
Type IV spinal vascular malformations commonly manifest with progressive paraparesis secondary to myelopathy caused by venous congestion, but they can also manifest with acute neurologic deficit secondary to rupture of a feeding artery aneurysm. They occur more commonly in adults but can be seen in children.10 Patients typically present before the age of 40 years.
Osler-Weber-Rendu syndrome (hereditary hemorrhagic telangectasisa) type 1 is an autosomal dominant disorder associated with spinal arteriovenous shunts. The syndrome is typically associated with type IV, subtype C intradural AVFs. Klippel-Trenaunay and Parkes-Weber syndromes are associated with vascular malformations of the lower limbs and can involve spinal cord vascular malformations.3
The pathophysiology of spinal vascular malformations was elegantly detailed by Aminoff and Logue13 in a paper published in 1974 in which they refuted the previous theories of compression of the cord by the malformation being the primary cause of symptoms and argued that the most likely mechanism of neurologic deterioration was secondary to ischemia caused by venous hypertension. Aminoff and Logue further argued that support could be garnered for this theory by the fact most symptomatic spinal vascular malformations are located in the lower thoracic or thoracolumbar spine, where there are fewer large veins draining into the coronal plexus, compared to the cervical or upper thoracic spine. Histopathologic support for this theory has subsequently been provided by Matsuo and colleagues, who performed autopsies on three patients with progressive myelopathy and found evidence of venous congestive myelopathy. The spinal cord parenchyma at the affected levels demonstrated neuronal loss and gliosis with increased numbers of hyalinized vessels.14 Autopsy also demonstrated tortuous, dilated venous vessels on the dorsal surface of the spinal cord at the affected levels.
Endovascular Management
Technique
Spinal angiography is still the gold standard for the diagnosis of spinal vascular malformations, but recent advances in MRI and CT imaging have allowed diagnostic spinal angiograms to become more focused anatomically.15 However, if a vascular lesion is suspected, and MRI does not provide localizing information, a thorough spinal angiogram including possible evaluation of the aortic arch, the descending aorta, the abdominal aorta, the pelvic vasculature including the iliac arteries and the median sacral artery, the vertebral arteries, the thyrocervical trunk, and the deep and ascending cervical arteries should be considered in addition to injection of the segmental arteries at each spinal level. The artery of Adamkiewicz should also be identified. An aortogram is occasionally helpful in the setting of an atherosclerotic or dilated aorta where segmental artery catheterization may be difficult and may be performed by using a pigtail catheter. Selective catheterization of the segmental arteries may be accomplished by a variety of catheters including but not limited to a 5-Fr H1, C1, or C2. Spinal angiograms are typically acquired in an anteroposterior projection, but biplane angiography can be used for three-dimensional localization of pathologic vasculature. It is especially important to study both the arterial and venous phases during evaluation for a spinal vascular malformation. Prolonged imaging in the venous phase of the angiogram may be necessary to diagnose fistulas with slower flow (e.g., type I).
At our institution, the majority of embolizations of spinal cord vascular malformations are performed under general anesthesia with neurophysiologic monitoring. The use of intraprocedural motor evoked potentials (MEPs) and somatosenory evoked potentials (SSEPs) to monitor spinal cord function during embolization of spinal cord vascular malformations has been studied in the literature with good results.16,17 Intraprocedural neurophysiologic monitoring provides a method by which to assess the patient’s neurologic function during embolization under general anesthesia. In 2004, Niimi and colleagues reported their experience with MEP and SSEP monitoring in conjunction with provocative testing with amytal and lidocaine during embolization of spinal cord AVMs. The group also monitored BCRs (bulbocavernosus reflexes) in patients with conus lesions. Niimi and colleagues performed 84 angiographic procedures in 52 patients and found that the use of neurophysiologic monitoring in conjunction with provocative testing had a high negative predictive value (97.6%).16
Type I Fistulas
Surgical results are outstanding for this type of lesion; hence the bar for endovascular treatment is high. The first endovascular treatment of a spinal dural AVF was performed by Doppman and colleagues, who reported the embolization of a spinal dural AVF using metal pellets in 1968.18 Since then many other embolic agents have been used with varying degrees of success. Polyvinyl alcohol (PVA) was one of the initial agents used but soon fell out of favor because of high recurrence rates and was quickly replaced by n-butyl cyanoacrylate (nBCA) (Codman, Raynham, MA), which became increasingly popular as an embolic agent. More recently, the use of Onyx (ev3 Inc, Plymouth, MN) for treatment of spinal dural AVFs has gained favor.
Narvid and colleagues19 retrospectively reviewed their single institution experience over 20 years with the treatment of spinal dural AVFs. Between 1984 and 2005, they treated 63 patients for this condition. The diagnosis of spinal dural AVF was confirmed by spinal angiography in all patients. Thirty-nine patients underwent initial endovascular embolization, and 24 patients underwent surgical treatment initially. Of the 39 patients initially treated with embolization, 27 achieved complete obliteration of the fistula. Of the 12 patients who did not achieve complete obliteration, four were planned preoperative embolizations, and three were patients treated early in the series with PVA who required surgery for permanent obliteration. The remaining five patients demonstrated residual filling of the draining vein despite embolization. Of the 24 patients treated with surgery initially, 20 patients required no further treatment of their fistula. Of the four patients in the surgery group who required further treatment, one was successfully embolized and the other three required reoperation to obliterate the fistula. Embolization was not attempted in patients who had common origin of the feeding artery and the anterior spinal artery. The first four patients treated endovascularly were treated with PVA. All subsequent treatments were performed with nBCA and lipiodol. If embolization failed to occlude the fistula, patients were referred for surgical treatment.
Niimi and colleagues20 retrospectively reviewed 49 cases of spinal dural AVF treated by embolization at their institution between 1980 and 1995. The diagnosis of spinal dural AVF was made by spinal angiography in all cases. An acrylic material (isobutyl cyanoacrylate [IBCA] or nBCA) was used in 47 of the 49 cases, and variable stiffness microcatheters were used in 38 of the 49 cases. Initial embolization was deemed “adequate” in 39/49 cases (80%). Adequate embolization was defined as embolization with a liquid embolic agent in which the agent penetrated the fistula and draining vein with angiographic disappearance of the fistula without compromise of the venous drainage of the spinal cord. The initial success rate of embolization increased to 87% (33/38 cases) after variable stiffness microcatheters were introduced. Eight patients with initially “adequate” embolization required repeat embolization. Of the 10 cases that were initially “inadequately” embolized, 5 had been treated prior to the use of variable stiffness microcatheters and three demonstrated common origin of the arterial feeder and the ASA or posterior spinal artery (PSA), making “adequate” embolization difficult without incurring high risk. No patient developed neurologic complications as a result of embolization. No technical complications occurred after the introduction of variable stiffness microcatheters. Mean duration of follow-up post-treatment was 52 months for 29 patients. For 6 patients, follow-up was within 1 month of treatment, and for 14 patients follow-up was obtained between 1 and 12 months. No patient worsened clinically after treatment, and all except one improved clinically after treatment. A shorter duration of symptoms before treatment seemed to correlate with better clinical outcome, a finding that has since been noted in other similar studies.
Based on these results, Niimi and colleagues concluded that embolization with an acrylic material should be offered as the first-line treatment for spinal dural AVF unless there was common origin of the arterial feeder and the ASA or PSA and that patients should be followed long-term for potential recurrence. Niimi and colleagues argued that embolization was less invasive than surgery, that secondary to the advent of variable stiffness microcatheters and liquid embolic agents, embolization could be safely performed with a high cure rate, and that surgery could still be offered to a patient if embolization failed without increasing the difficulty or risk of the surgical procedure.
Ushikoshi and colleagues21 performed a retrospective review of 13 patients with spinal dural AVFs treated at their institution with surgery, embolization, or embolization and surgery. Six patients were treated with surgery alone. Seven patients were initially treated with embolization. In six of these patients, nBCA was the embolic agent used. Four of the six patients treated with nBCA achieved complete occlusion. Embolization was unsuccessful in two patients treated with nBCA and these patients subsequently underwent surgery. One patient was treated with PVA and coils as planned preoperative embolization to decrease flow in a high-flow spinal dural AVF. Functional outcome was measured using the ALS score 6 months after treatment. In the surgical group, permanent neurologic complication occurred in one patient who suffered from brainstem hemorrhage 1 day after surgical treatment of a dural AVF fed by the left C1 segmental artery. Gait improvement was demonstrated in 6 of 10 patients who presented with gait disturbances. No patient experienced worsening of gait disturbance. Of the six patients who presented with bladder dysfunction, three experienced improvement in their symptoms after treatment. Based on these results, Ushikoshi and colleagues concluded that embolization should be offered as a first-line treatment for spinal dural AVF when the feeding artery did not share a common origin with the ASA or PSA. If embolization did not achieve complete obliteration, surgery should be undertaken without delay.
Andres and colleagues22 performed a retrospective review of 21 patients at their institution who were treated for spinal dural AVFs with either surgery, embolization, or surgery after incomplete embolization. All patients underwent spinal angiography to confirm the presence of a spinal dural AVF. Thirteen patients were treated with embolization alone, four patients were treated with surgery, and four patients were treated with surgery after incomplete embolization. The four patients who were treated initially with surgery were deemed to be at high risk for endovascular treatment because of severe atherosclerosis, tortuous or small vessels that would be difficult to catheterize, or common origin of the anterior spinal artery and the feeding artery of the fistula. Median follow-up duration was 26 months after treatment. nBCA was the embolic agent employed. Patients were examined before and after treatment using the ALS score for myelopathy. Clinical outcome was also assessed using the modified Rankin scale (mRS). Patients treated with either embolization alone or surgery alone showed an improvement in both ALS and mRS scores after treatment. Patients treated with surgery after incomplete embolization showed improvement in only the ALS score after treatment. The failure rate of embolization was 23.5% (4/17). Based on these results, Andres and colleagues concluded that endovascular and surgical treatment both resulted in good, lasting clinical outcomes for patients and that the treatment modality for each patient should be determined by an interdisciplinary team after an initial spinal angiogram.
Sherif and colleagues23 performed a retrospective review of 26 patients with spinal dural AVFs treated at their institution. All spinal dural AVFs were demonstrated on spinal angiogram after the diagnosis was suspected on MR imaging. Embolization was offered as the primary treatment in 19 cases. Seven patients underwent surgical treatment. Embolization was performed with a mixture of 33% Histoacryl and 66% Lipiodol, which was slowly injected until the material penetrated the draining vein or reflux occurred into the feeding artery. Surgery was offered to patients who had severe atheromatous disease, patients with recurrent dural AVF after embolization, patients with common origin of the feeding artery and the ASA or PSA, and patients in whom it was felt that endovascular embolization was likely to be unsuccessful based on the angioarchitecture of the fistula. Clinical examinations were performed before and after treatment using the ALS and mRS scores. All patients underwent follow-up spinal angiography and follow-up MR imaging. Mean duration of radiographic follow-up was 91.5 months. Of the 19 patients who were initially treated with embolization, two patients required retreatment. One patient developed collateral feeding arteries requiring reembolization. Another patient demonstrated recanalization of the fistula owing to incomplete occlusion of the draining vein and underwent surgical occlusion of the fistula. Neither of these patients suffered a setback in their long-term clinical outcome secondary to fistula recurrence.
Song and colleagues24 performed a retrospective review of 30 patients with spinal dural AVFs treated at their institution between 1985 and 1999. Sixteen patients underwent embolization, seven patients underwent surgery, and seven patients underwent surgery after failed embolization. Of the seven patients who required surgery after embolization, two patients failed embolization during the initial treatment and were taken to surgery the next day. In the remaining five patients, the fistula recurred via collateral circulation as shown on follow-up angiography. nBCA was the embolic agent used in all procedures performed after 1990 (21/27 embolizations in 20/23 patients who underwent embolization). Before 1990, PVA, Gelfoam, or silicone were used as embolic agents. Twenty-six follow-up angiograms were performed in 19 patients. Seven of these angiograms demonstrated recurrence of the dural AVF, five of which were post-embolization and two of which were post-surgical. Mean clinical follow-up was 3.4 years. Clinical outcomes were assessed using the ALS score before and after treatment. Seventeen patients demonstrated improvement in gait, 12 were unchanged, and 1 demonstrated worsening of gait. There was no significant improvement in micturition scores unless treatment was initiated within 13 months of symptom onset. No significant differences in clinical outcome were noted between treatment groups: surgery alone, embolization alone, or combined embolization and surgery. Patients in all three groups demonstrated improvement in gait, which was sustained during the follow-up period.
Arteriovenous Malformations
Konan and colleagues25 reported five cases of embolization of spinal artery aneurysms associated with spinal cord AVMs in four patients. All four patients presented with hemorrhage: two with hematomyelia and two with SAH only. Three aneurysms were located on branches of the anterior spinal artery and two aneurysms were located in radiculopial arteries. All aneurysms were embolized with a mixture of nBCA and Lipiodol. Follow-up angiograms were obtained in all patients 3 to 6 months after treatment. No aneurysm recurrence was noted on follow-up angiography. Clinical follow-up ranged from 17 to 37 months. No rebleeding episodes or complications occurred during the follow-up period. Based on these results, Konan and colleagues concluded that aneurysms associated with spinal cord AVMs could be successfully embolized and that treatment of these aneurysms might decrease the risk of rebleeding in patients who could not be offered definitive surgical resection because of its high risk.
Spinal cord AVMs are notoriously difficult lesions to treat and often involve high risk of surgical morbidity. The poor natural history of these lesions combined with their relative high risk prompted neurointerventionalists to investigate possible endovascular approaches to the treatment of these lesions. In 1971, Doppman and colleagues reported on the use of percutaneous embolization for the treatment of seven patients.26 Doppman and colleagues used metallic pellets, Gelfoam, and muscle fragments to occlude feeding arteries to the spinal cord AVM. The procedure was performed under local anesthesia. Percutaneous embolization was successful in five of the seven patients. The two patients who failed embolization were treated early in the series prior to the development of a system of coaxial catheters that allowed the use of larger pellets for embolization, providing proper occlusion of the feeding arteries. None of the seven patients experienced any neurologic decline, and three of the seven experienced progressive neurologic improvement. Based on these results, Doppman and colleagues concluded that percutaneous embolization of spinal cord AVMs was a reasonable option when total excision was not surgically feasible.
In 1990, Biondi and colleagues27 reported their experience with particle embolization for the treatment of thoracic intramedullary AVMs. As expected with particle embolization, there were several instances of recanalization. They performed a retrospective review of 40 patients with thoracic intramedullary AVMs treated at their institution between 1978 and 1990. Of the 40 patients, 35 underwent embolization, 4 underwent surgery, and 1 did not receive either treatment. PVA particles were employed for the vast majority of the embolization procedures, but Gelfoam, silk threads, and microspheres were occasionally used. Follow-up angiography was performed 6 months after the initial embolization. The duration of mean follow-up after initial embolization was 6 years. Twenty-nine of the 35 patients required multiple sessions of embolization. The number of embolizations in each patient ranged from 1 to 15 (mean, 4.5).
Before liquid embolic agents were introduced, the initial results of embolization for treatment of spinal cord vascular malformations were dismal, with significantly high rates of recurrence leading many to conclude that embolization was an unsuccessful treatment with a high rate of failure. Hall and colleagues28 reported a series of three patients with spinal cord intramedullary glomus-type AVMs and three patients with spinal dural AVFs treated with PVA, five of whom demonstrated recurrence. Hall and colleagues concluded based on these results that embolization provided only temporary treatment and that surgery should be considered the treatment of choice when feasible. However, this study was conducted prior to the use of liquid embolic agents. More recent studies have demonstrated significantly better results for the treatment of spinal cord vascular malformations.29–32
The first report of the use of Onyx for treatment of a spinal vascular malformation was in 2000 from Molyneux and colleagues.2,33 Corkill and colleagues subsequently reported on the use of Onyx in a larger series of patients.2 In this retrospective review, Corkill and colleagues analyzed the records of 17 patients treated with Onyx embolization of spinal intramedullary AVMs. Thirteen patients underwent a single session of embolization and four patients underwent two sessions of embolization. There were two intraprocedural complications, which did not result in permanent neurologic sequelae. Mean follow-up interval was 24.3 months. Total obliteration of the AVM was achieved in six patients (37.5%), subtotal obliteration was achieved in five patients (31.25%), and partial obliteration was achieved in five patients (31.25%). Functional or neurologic status (or both) improved in 14 patients, resulting in a good clinical outcome for 82% of the study group. Based on these results, Corkill and colleagues concluded that Onyx embolization was a promising new treatment for spinal intramedullary AVMs.
Several studies have also examined the endovascular treatment of type IV spinal cord AVMs.12,34 In 1993, Mourier and colleagues reported their experience with surgical and endovascular treatment of type IV spinal cord AVMs. Of the 35 patients they treated between 1970 and 1990, 4 patients were classified as Merland type I, 9 patients were classified as Merland type II, and 22 patients were classified as Merland type III.12 Thirty-two of the patients presented with progressive neurologic symptoms and three patients presented with subarachnoid hemorrhage only. All of the Merland type III patients were treated with endovascular detachable silicone balloon occlusion of the fistula. Of the nine Merland type II patients, two were treated with embolization alone, and three were treated with surgery after incomplete embolization. One of the Merland type I patients was treated with endovascular embolization. Complete occlusion was achieved in all treated Merland type I and type II cases and in 15 of the type III cases. The 15 patients with Merland type III AVF that were completely embolized recovered completely. Two patients with Merland type III AVF worsened after incomplete occlusion, and one patient died after attempted endovascular obliteration of a cervical Merland type III AVF.
Halbach and colleagues also reported in 1993 on a series of 10 patients with Merland type II and type III perimedullary AVFs treated with embolization alone (three patients) or with embolization and surgical resection (seven patients).34 Of the 10 patients, five presented with progressive myelopathy, four presented with subarachnoid hemorrhage, and one presented with acute paraplegia. Two patients had Merland type II perimedullary fistulas and the remaining eight patients had Merland type III perimedullary fistulas. Sixteen embolization procedures were performed: 14 via transarterial routes, and 2 via transvenous routes. Complete occlusion was achieved in seven of the patients, incomplete occlusion was achieved in two patients, and one patient refused follow-up angiography. One patient suffered transient worsening of paraplegia after rupture of the anterior spinal artery by a detachable balloon. The mean follow-up period was 44.8 months (range, 3 to 112 months).
Type II and type III spinal cord AVMs are characteristically difficult to treat via conventional microsurgical and endovascular techniques. Hence, there has been significant interest in the use of stereotactic radiosurgery to treat these lesions. Stereotactic radiosurgery is thought to induce gradual endothelial hyperplasia, which eventually causes vessel narrowing and occlusion. Initial reluctance to use stereotactic radiosurgery to treat spinal cord vascular malformations stemmed from concern for spinal cord injury secondary to radiation toxicity. Sinclair and colleagues reported a series of 15 patients with intramedullary spinal cord AVMs treated with multisession cyberknife radiosurgery.35 Multisession radiosurgery was employed with the hope that allowing time between sessions would permit normal tissue repair, thus increasing the dose that could be administered to the AVM nidus because of increased radiation tolerance of the adjacent normal spinal cord. Patients were treated with an average marginal dose of 20.5 Gr over two to five sessions. Clinical and radiographic (MRI) follow-up were performed annually. Spinal angiography was repeated after 3 years. The mean follow-up period was 27.9 months (range, 3 to 59 months). Of the seven patients who were 3 years or more from treatment, six had significant reductions in the size of their lesions. Of the five patients who underwent posttreatment spinal angiograms, one patient showed complete obliteration of a conus medullaris AVM. No patient suffered a hemorrhage or neurologic deterioration after cyberknife treatment. Sinclair and colleagues were thus able to demonstrate that stereotactic radiosurgery for the treatment of intramedullary spinal cord AVMs is safe and feasible, but more studies are needed to further validate this approach.
Case Illustrations
Case 1
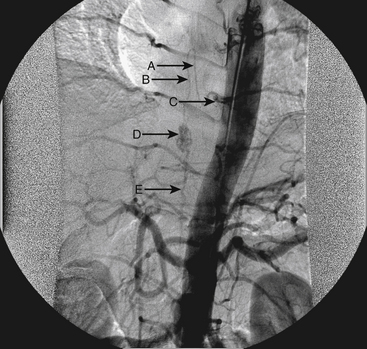
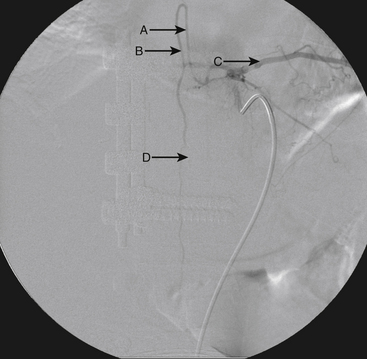
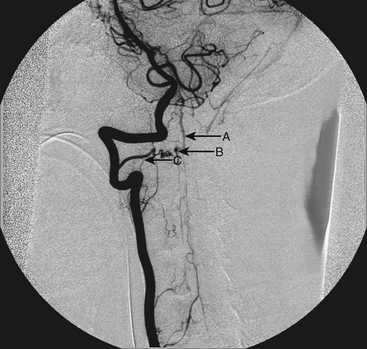
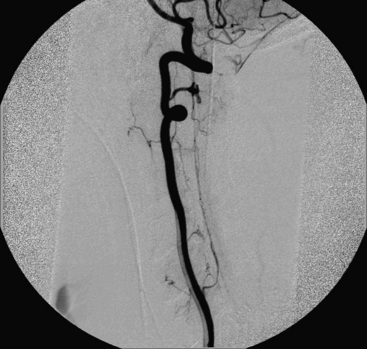
A 40-year-old man with cervical spinal cord AVM detected incidentally after motor vehicle accident (Figs. 91-1 to 91-5)underwent radiosurgery cyberknife treatment. A repeat angiogram demonstrated persistent AVM nidus (Figs. 91-6 to 91-8). The patient underwent surgical resection 5 years later.
Case 2
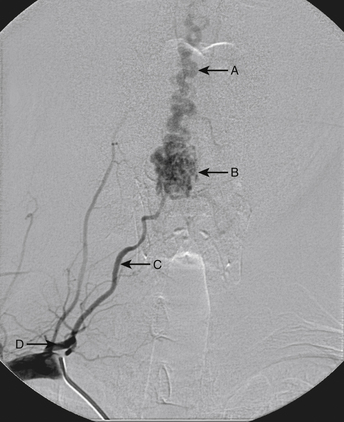
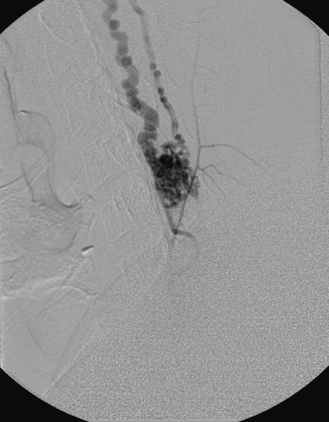
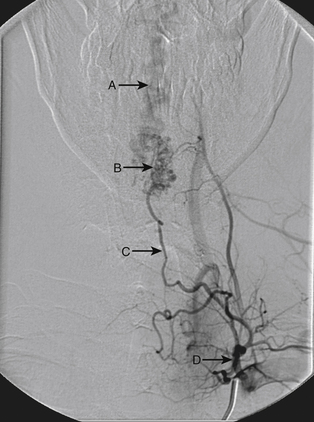
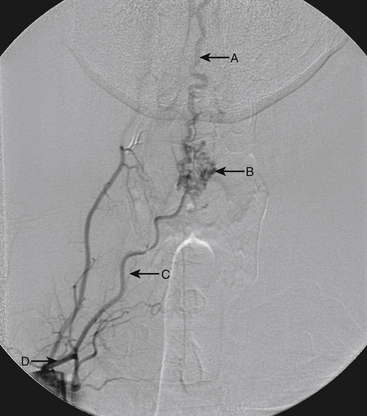
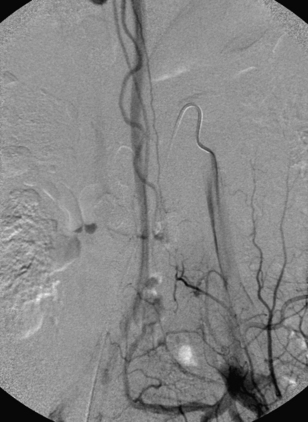
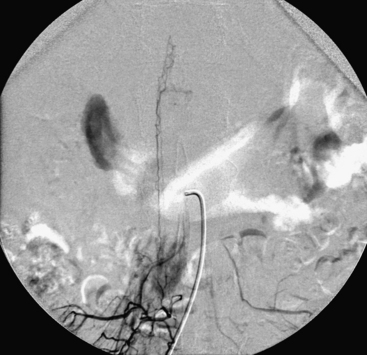
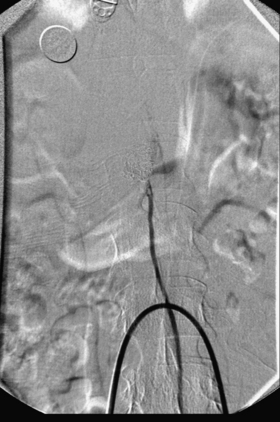
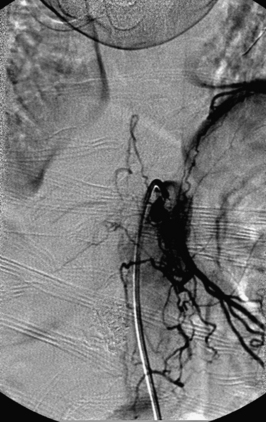
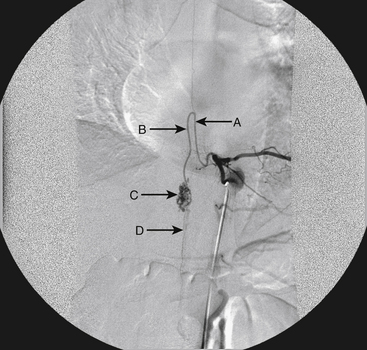
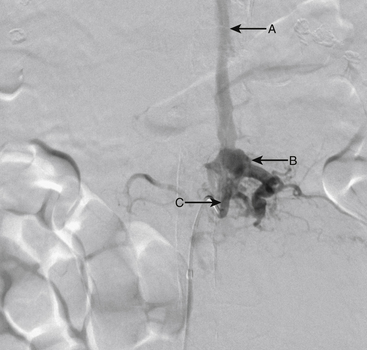
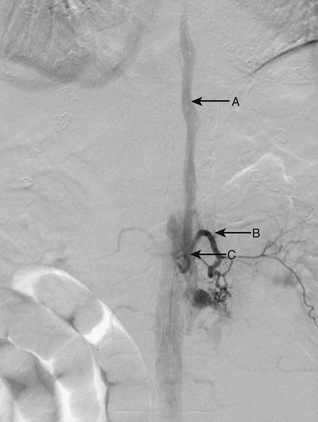
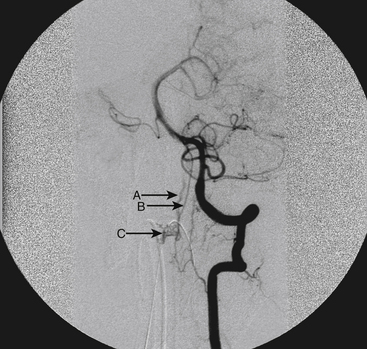
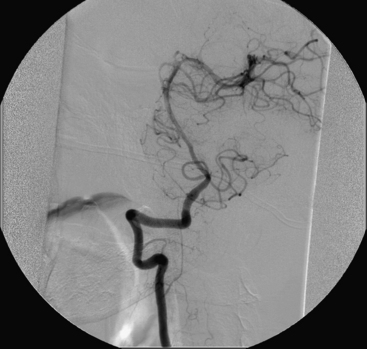
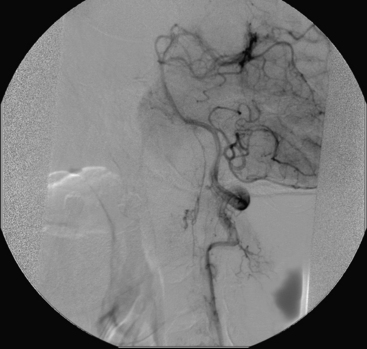
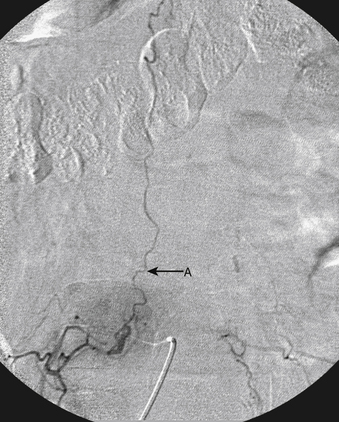
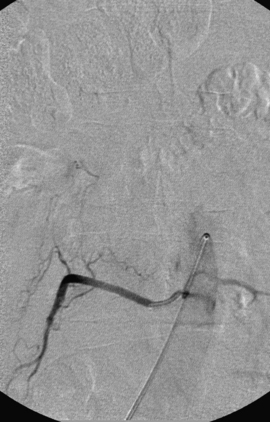
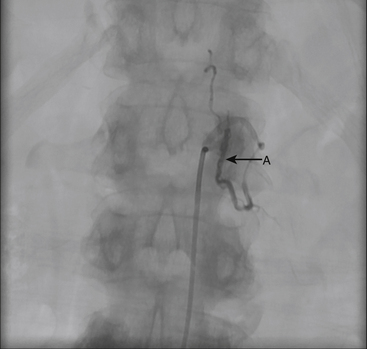
A 36-year-old woman with known Klippel-Trenaunay-Weber syndrome presented with increasing right lower extremity pain in March 2004. The patient was found to have a type IV arteriovenous pial fistula type B at the level of the conus, supplied by the anterior spinal artery, with two inferiorly draining veins and one superiorly draining vein arising from an anteriorly located venous varix (Figs. 91-9 to 91-11). She was treated with coil and glue embolization (Figs. 91-12 and 91-13).
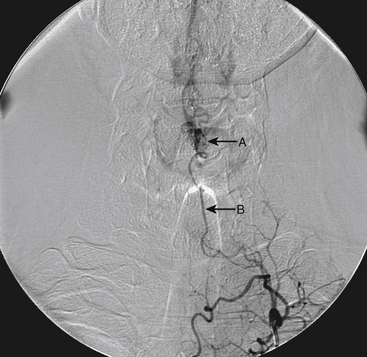
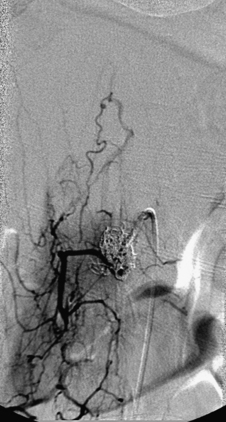
Although the patient’s symptoms continued to improve after embolization, follow-up MRI demonstrated residual fistula. The patient was brought back for repeat spinal angiography, which demonstrated that unlike previously thought, there were actually three type IV perimedullary fistulas: the previously embolized fistula at the conus and two other fistulas, which were centered at the inferior end plate of T11 (Figs. 91-14 and 91-15). These fistulas are supplied by the posterior spinal arteries and drain into short pial veins on the dorsal surface of the spinal cord and then subsequently converge into a large vein that drains inferiorly to the previously embolized venous varix at the conus.
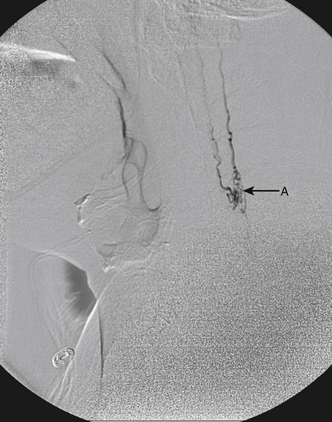
The patient was taken to the operating room for surgical treatment of the fistula. Postoperative angiography demonstrated no residual fistula.
Case 3
A 43-year-old man presented with sudden-onset back pain followed by lower extremity numbness and weakness. Spinal angiogram demonstrated a type II glomus AVM centered at T10 with a compact nidus (Figs. 91-16 and 91-17).
The patient was taken to the operating room for surgical resection of the lesion. Postoperative angiography demonstrated no evidence of residual malformation (Fig. 91-18).
Case 4
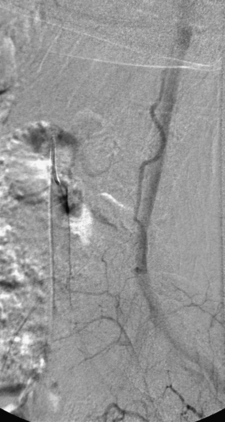
An 86-year-old woman was transferred from an outside institution with dense lower extremity paraparesis for several months. On workup she was found to have a left paraspinal mass and a spinal epidural AVF supplied primarily by the left L1 and L2 segmental arteries (Figs. 91-19 and 91-20). She was treated with Onyx embolization with mild residue filling. The patient and her family have not pursued further treatment options.
Case 5
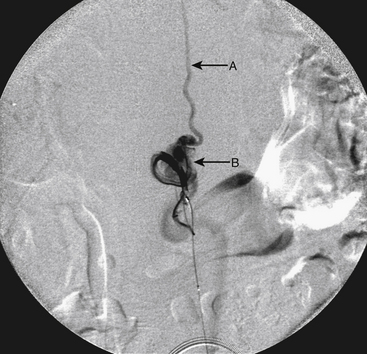
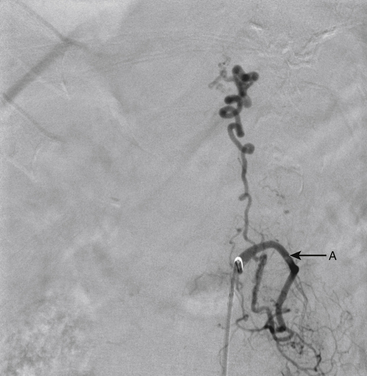
A 55-year-old man presented with SAH, Hunt/Hess grade 2. Angiography revealed an unusual spinal AVF at C2 with feeders from the right C2 segmental artery showing characteristics of a type 1 fistula and feeders from the anterior spinal artery arising from the left vertebral artery showing characteristics of a type 4A fistula (Figs. 91-21 to 91-23). Embolization (with nBCA) was performed via the right C2 segmental artery with extension of the nBCA into the draining vein. Angiography performed 4 days after embolization demonstrated complete occlusion of the fistula (Figs. 91-24 and 91-25).
Case 6
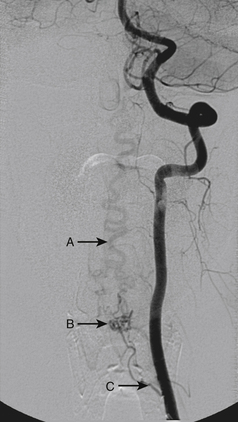
A 70-year-old man presented with progressive lower extremity weakness. MRI demonstrated a dural AVF. Angiogram demonstrated type 1 dural AVF fed by the right L3 segmental artery (Fig. 91-26). The patient was successfully treated with nBCA embolization (Fig. 91-27).
Case 7
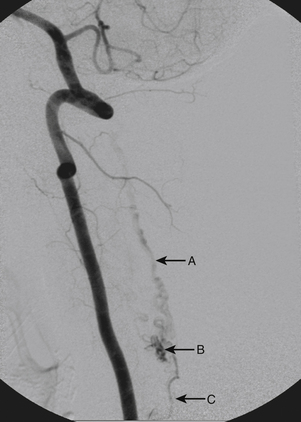
A 67-year-old woman presented with progressive history of lower extremity weakness. Workup including diagnostic spinal angiography demonstrated a type 1 spinal dural AVF supplied by the left L1 segmental artery (Fig. 91-28). The patient was treated with Onyx embolization of the spinal fistula with complete resolution of the fistula (Fig. 91-29).
Ali S., Cashen T.A., Carroll T.J., et al. Time-resolved spinal MR angiography: initial clinical experience in the evaluation of spinal arteriovenous shunts. AJNR Am J Neuroradiol. 2007;28(9):1806-1810.
Aminoff M.J., Barnard R.O., Logue V. The pathophysiology of spinal vascular malformations. J Neurol Sci. 1974;23(2):255-263.
Andres R.H., Barth A., Guzman R., et al. Endovascular and surgical treatment of spinal dural arteriovenous fistulas. Neuroradiology. 2008;50(10):869-876.
Caragine L.P.Jr., Halbach V.V., Ng P.P., Dowd C.F. Vascular myelopathies–vascular malformations of the spinal cord: presentation and endovascular surgical management. Semin Neurol. 2002;22(2):123-132.
Corkill R.A., Mitsos A.P., Molyneux A.J. Embolization of spinal intramedullary arteriovenous malformations using the liquid embolic agent, Onyx: a single-center experience in a series of 17 patients. J Neurosurg Spine. 2007;7(5):478-485.
da Costa L., Dehdashti A.R., terBrugge K.G. Spinal cord vascular shunts: spinal cord vascular malformations and dural arteriovenous fistulas. Neurosurg Focus. 2009;26(1):E6.
Dehdashti A.R., Da Costa L.B., terBrugge K.G., et al. Overview of the current role of endovascular and surgical treatment in spinal dural arteriovenous fistulas. Neurosurg Focus. 2009;26(1):E8.
Doppman J.L., Di Chiro G., Ommaya A. Obliteration of spinal-cord arteriovenous malformation by percutaneous embolisation. Lancet. 1968;1(7540):477.
Heros R.C., Debrun G.M., Ojemann R.G., et al. Direct spinal arteriovenous fistula: a new type of spinal AVM. Case report. J Neurosurg. 1986;64(1):134-139.
Konan A.V., Raymond J., Roy D. Transarterial embolization of aneurysms associated with spinal cord arteriovenous malformations. Report of four cases. J Neurosurg. 1999;90(Suppl 1):148-154.
Mathis J.M., Shaibani A., Wakhloo A.K. Spine anatomy. In: Mathis J.M., editor. Image-guided spine intervention. New York: Springer-Verlag; 2004:1-26.
Matsuo K., Kakita A., Ishizu N., et al. Venous congestive myelopathy: three autopsy cases showing a variety of clinicopathologic features. Neuropathology. Jun 2008;28(3):303-308.
Medel R., Crowley R.W., Dumont A.S. Endovascular management of spinal vascular malformations: history and literature review. Neurosurg Focus. 2009;26(1):E7.
Molyneux A.J., Coley S.C. Embolization of spinal cord arteriovenous malformations with an ethylene vinyl alcohol copolymer dissolved in dimethyl sulfoxide (Onyx liquid embolic system). Report of two cases. J Neurosurg. 2000;93(Suppl 2):304-308.
Mourier K.L., Gobin Y.P., George B., et al. Intradural perimedullary arteriovenous fistulae: results of surgical and endovascular treatment in a series of 35 cases. Neurosurgery. 1993;32(6):885-891.
Narvid J., Hetts S.W., Larsen D., et al. Spinal dural arteriovenous fistulae: clinical features and long-term results. Neurosurgery. 2008;62(1):159-166.
Niimi Y., Berenstein A., Setton A., Neophytides A. Embolization of spinal dural arteriovenous fistulae: results and follow-up. Neurosurgery. 1997;40(4):675-682.
Niimi Y., Sala F., Deletis V., et al. Neurophysiologic monitoring and pharmacologic provocative testing for embolization of spinal cord arteriovenous malformations. AJNR Am J Neuroradiol. 2004;25(7):1131-1138.
Rodesch G., Hurth M., Alvarez H., et al. Classification of spinal cord arteriovenous shunts: proposal for a reappraisal–the Bicetre experience with 155 consecutive patients treated between 1981 and 1999. Neurosurgery. 2002;51(2):374-379.
Shaibani A., Wakhloo A.K. Endovascular therapy of the spine. In: Mathis J.M., editor. Image-guided spine intervention. New York: Springer-Verlag; 2004:292-321.
Sherif C., Gruber A., Bavinzski G., et al. Long-term outcome of a multidisciplinary concept of spinal dural arteriovenous fistulae treatment. Neuroradiology. 2008;50(1):67-74.
Sinclair J., Chang S.D., Gibbs I.C., Adler J.R.Jr. Multisession CyberKnife radiosurgery for intramedullary spinal cord arteriovenous malformations. Neurosurgery. 2006;58(6):1081-1089.
Sivakumar W., Zada G., Yashar P., et al. Endovascular management of spinal dural arteriovenous fistulas. A review. Neurosurg Focus. 2009;26(5):E15.
Song J.K., Vinuela F., Gobin Y.P., et al. Surgical and endovascular treatment of spinal dural arteriovenous fistulas: long-term disability assessment and prognostic factors. J Neurosurg. 2001;94(Suppl 2):199-204.
Spetzler R.F., Detwiler P.W., Riina H.A., Porter R.W. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002;96(Suppl 2):145-156.
1. da Costa L., Dehdashti A.R., terBrugge K.G. Spinal cord vascular shunts: spinal cord vascular malformations and dural arteriovenous fistulas. Neurosurg Focus. 2009;26(1):E6.
2. Corkill R.A., Mitsos A.P., Molyneux A.J. Embolization of spinal intramedullary arteriovenous malformations using the liquid embolic agent, Onyx: a single-center experience in a series of 17 patients. J Neurosurg Spine. 2007;7(5):478-485.
3. Mathis J.M., Shaibani A., Wakhloo A.K. Spine anatomy. In: Mathis J.M., editor. Image-guided spine intervention. New York: Springer-Verlag; 2004:1-26.
4. Black P. Spinal vascular malformations: an historical perspective. Neurosurg Focus. 2006;21(6):E11.
5. Rodesch G., Hurth M., Alvarez H., et al. Classification of spinal cord arteriovenous shunts: proposal for a reappraisal–the Bicetre experience with 155 consecutive patients treated between 1981 and 1999. Neurosurgery. 2002;51(2):374-379.
6. Spetzler R.F., Detwiler P.W., Riina H.A., Porter R.W. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002;96(Suppl 2):145-156.
7. Shaibani A., Wakhloo A.K. Endovascular therapy of the spine. In: Mathis J.M., editor. Image-guided spine intervention. New York: Springer-Verlag; 2004:292-321.
8. Oldfield E.H., Bennett A.3rd, Chen M.Y., Doppman J.L. Successful management of spinal dural arteriovenous fistulas undetected by arteriography. Report of three cases. J Neurosurg. 2002;96(Suppl 2):220-229.
9. Eskandar E.N., Borges L.F., Budzik R.F.Jr., et al. Spinal dural arteriovenous fistulas: experience with endovascular and surgical therapy. J Neurosurg. 2002;96(Suppl 2):162-167.
10. Caragine L.P.Jr., Halbach V.V., Ng P.P., Dowd C.F. Vascular myelopathies–vascular malformations of the spinal cord: presentation and endovascular surgical management. Semin Neurol. 2002;22(2):123-132.
11. Heros R.C., Debrun G.M., Ojemann R.G., et al. Direct spinal arteriovenous fistula: a new type of spinal AVM. Case report. J Neurosurg. 1986;64(1):134-139.
12. Mourier K.L., Gobin Y.P., George B., et al. Intradural perimedullary arteriovenous fistulae: results of surgical and endovascular treatment in a series of 35 cases. Neurosurgery. 1993;32(6):885-891.
13. Aminoff M.J., Barnard R.O., Logue V. The pathophysiology of spinal vascular malformations. J Neurol Sci. 1974;23(2):255-263.
14. Matsuo K., Kakita A., Ishizu N., et al. Venous congestive myelopathy: three autopsy cases showing a variety of clinicopathologic features. Neuropathology. Jun 2008;28(3):303-308.
15. Ali S., Cashen T.A., Carroll T.J., et al. Time-resolved spinal MR angiography: initial clinical experience in the evaluation of spinal arteriovenous shunts. AJNR Am J Neuroradiol. 2007;28(9):1806-1810.
16. Niimi Y., Sala F., Deletis V., et al. Neurophysiologic monitoring and pharmacologic provocative testing for embolization of spinal cord arteriovenous malformations. AJNR Am J Neuroradiol. 2004;25(7):1131-1138.
17. Sala F., Niimi Y., Krzan M.J., et al. Embolization of a spinal arteriovenous malformation: correlation between motor evoked potentials and angiographic findings: technical case report. Neurosurgery. 1999;45(4):932-937.
18. Doppman J.L., Di Chiro G., Ommaya A. Obliteration of spinal-cord arteriovenous malformation by percutaneous embolisation. Lancet. 1968;1(7540):477.
19. Narvid J., Hetts S.W., Larsen D., et al. Spinal dural arteriovenous fistulae: clinical features and long-term results. Neurosurgery. 2008;62(1):159-166.
20. Niimi Y., Berenstein A., Setton A., Neophytides A. Embolization of spinal dural arteriovenous fistulae: results and follow-up. Neurosurgery. 1997;40(4):675-682.
21. Ushikoshi S., Hida K., Kikuchi Y., et al. Functional prognosis after treatment of spinal dural arteriovenous fistulas. Neurol Med Chir (Tokyo). 1999;39(3):206-212.
22. Andres R.H., Barth A., Guzman R., et al. Endovascular and surgical treatment of spinal dural arteriovenous fistulas. Neuroradiology. 2008;50(10):869-876.
23. Sherif C., Gruber A., Bavinzski G., et al. Long-term outcome of a multidisciplinary concept of spinal dural arteriovenous fistulae treatment. Neuroradiology. 2008;50(1):67-74.
24. Song J.K., Vinuela F., Gobin Y.P., et al. Surgical and endovascular treatment of spinal dural arteriovenous fistulas: long-term disability assessment and prognostic factors. J Neurosurg. 2001;94(Suppl 2):199-204.
25. Konan A.V., Raymond J., Roy D. Transarterial embolization of aneurysms associated with spinal cord arteriovenous malformations. Report of four cases. J Neurosurg. 1999;90(Suppl 1):148-154.
26. Doppman J.L., Di Chiro G., Ommaya A. Obliteration of spinal-cord arteriovenous malformation by percutaneous embolisation. Lancet. 1968;1(7540):477.
27. Biondi A., Merland J.J., Reizine D., et al. Embolization with particles in thoracic intramedullary arteriovenous malformations: long-term angiographic and clinical results. Radiology. 1990;177(3):651-658.
28. Hall W.A., Oldfield E.H., Doppman J.L. Recanalization of spinal arteriovenous malformations following embolization. J Neurosurg. 1989;70(5):714-720.
29. Dehdashti A.R., Da Costa L.B., terBrugge K.G., et al. Overview of the current role of endovascular and surgical treatment in spinal dural arteriovenous fistulas. Neurosurg Focus. 2009;26(1):E8.
30. Medel R., Crowley R.W., Dumont A.S. Endovascular management of spinal vascular malformations: history and literature review. Neurosurg Focus. 2009;26(1):E7.
31. Sivakumar W., Zada G., Yashar P., et al. Endovascular management of spinal dural arteriovenous fistulas. A review. Neurosurg Focus. 2009;26(5):E15.
32. Larsen D.W., Halbach V.V. Endovascular therapy: implications and general principles. In: Barrow D.L., Awad I.A. Spinal Vascular Malformations. Park Ridge, IL: American Association of Neurological Surgeons; 1999:85-101.
33. Molyneux A.J., Coley S.C. Embolization of spinal cord arteriovenous malformations with an ethylene vinyl alcohol copolymer dissolved in dimethyl sulfoxide (Onyx liquid embolic system). Report of two cases. J Neurosurg. 2000;93(Suppl 2):304-308.
34. Halbach V.V., Higashida R.T., Dowd C.F., et al. Treatment of giant intradural (perimedullary) arteriovenous fistulas. Neurosurgery. 1993;33(6):972-979.
35. Sinclair J., Chang S.D., Gibbs I.C., Adler J.R.Jr. Multisession CyberKnife radiosurgery for intramedullary spinal cord arteriovenous malformations. Neurosurgery. 2006;58(6):1081-1089.