CHAPTER 20 Endoscopy of the upper and lower gastrointestinal tract
Indications
Types of endoscopy
Upper gastrointestinal endoscopy
To prepare for upper gastrointestinal endoscopy patients are asked not to eat or drink for 4–6 hours prior to the procedure. The procedure can be done under local anesthetic to the throat or under sedation. The gastroscope is passed via the mouth, through the cricopharyngeal sphincter and into the esophagus. Once intubation is achieved the examination of the esophagus, stomach and duodenum is relatively straightforward (Figures 20.3, 20.4 and 20.5, see color insert). There are several blind spots that merit special attention. The view of the esophageal mucosa is often lost at the point of passage through the sphincter and lesions can be missed. If this is suspected, the patient should undergo a barium swallow or a rigid esophagoscopy. Another potential blind spot is the superior part of the gastric antrum and angulus. It is important to retroflex the gastroscope in the antrum to gain a good view of this area.
Small bowel endoscopy
Enteroscopy is used to examine mainly the proximal small bowel. This is a much more involved procedure than gastroscopy. Enteroscopy is indicated for diagnosis of small bowel diseases including obscure gastrointestinal bleeding, malabsorption, obtaining tissue biopsies following abnormal fluoroscopic studies as well as therapeutic procedures for treating small bowel bleeding or polyps.
Colorectal endoscopy
Visualization of the lower bowel can be performed using a rigid or flexible endoscope. Rigid sigmoidoscopy and proctoscopy usually occur in the outpatient clinic. The Association of Coloproctology of Great Britain and Ireland recommends that a patient referred for barium enema should have had at least a rigid sigmoidoscopy prior to referral (Guidelines for Management of Colorectal Cancer, 2007). The reason for this is that lesions very low down in the rectum may be missed on barium enema but seen on proctoscopy or rigid sigmoidoscopy. A rigid examination is especially important in patients presenting with bright red rectal bleeding as common pathologies such as distal proctitis or hemorrhoids may not be demonstrated on barium enema.
For a flexible sigmoidoscopy, the left colon is examined to the splenic flexure. This is usually performed for rectal bleeding as red blood passed per rectum is usually coming from the left colon. If the origin of the bleeding is more proximal, it manifests as altered blood by the time it is passed per rectum (PR). The majority of cancers in patients presenting with rectal bleeding and/or change in bowel habit (CIBH) without any other significant diagnostic factors occur within 60 cm of the anal verge and can be diagnosed by flexible sigmoidoscopy (Guidelines for Management of Colorectal Cancer, 2007). Flexible sigmoidoscopy is also useful for indeterminate lesions detected on barium enema, e.g. strictures. It is mandatory for suspected rectal cancers where a tissue diagnosis is needed before starting neoadjuvant therapy (Guidelines for Management of Colorectal Cancer, 2007).
Colonoscopy requires the telescope to be inserted to the cecum. With a straight colonoscope, the cecum is approximately 80 cm from the anal verge. However, distance from the anal verge is notoriously unreliable in terms of judging position in the colon. This is because there may be redundant loops of colon causing the colonoscope to loop within the patient. Rather than using centimeters as a measure, the endoscopist uses the visual landmarks of the colon to judge position, for example the triangular appearance of the mature transverse colon (Figure 20.6, see color insert).
Identification of the cecum requires identification of the ileocecal valve, appendix orifice and tri-radiate fold (Figure 20.7, see color insert).
Trans-illumination is unreliable since the experienced fluoroscopist will know that the cecum can lie in a variety of positions. The gold standard for proof of a complete colonoscopy is intubation of the ileocecal valve with a terminal ileal biopsy but, in practice, this can be difficult. This may be clinically necessary where terminal ileal Crohn’s disease is suspected but the Guidelines for Management of Colorectal Cancer (2007) suggest that a printed picture of the ileocecal valve may be adequate for patients who are undergoing colonoscopy for colorectal cancer.
Although colonoscopy might be considered the gold standard for visualizing the colon, it is operator dependent. There are data from a UK study of 9223 colonoscopies demonstrating cecal intubation rates as low as 56% (Bowles et al., 2004) and one-third of 55 endoscopists failed to achieve a cecal intubation rate of 90% in 5905 colonoscopies (Taylor et al., 2004). It has been demonstrated that continuous quality assessment and training can increase standards (Ball et al., 2004) and there are now improved standards for training (Joint Advisory Group on Gastrointestinal Endoscopy, 2004). National quality standards for endoscopy have been set by the Department of Health using a global rating scale (GRS) (www.grs.nhs.uk) and by the British Society of Gastroenterology (www.BSG.org.uk).
Therapeutic endoscopic procedures
The simplest procedure is a biopsy where small fragments of tissue are sampled using forceps that are passed down the endoscope. This type of biopsy is used for benign or malignant lesions or to sample mucosal abnormalities such as colitis (Figure 20.8, see color insert).
Snare polypectomy is used for pedunculated polyps. This involves lassoing the polyp with an extendable wire loop. The wire loop is passed around the polyp stalk, pulled snug and diathermy is used to coagulate as the wire cuts through the tissue (Figure 20.9, see color insert). The polyp can then be retrieved with a variety of devices such as a basket. Argon beam (plasma) coagulation combines a jet (beam) of ionized argon gas and electrocoagulation to create rapid vaporization of tissue and coagulation with eschar formation. This can be used to destroy large carpeting rectal lesions in unfit patients or to ablate angiodysplasia, Barrett’s esophagus and gastric antral vascular ectasia (GAVE).
Endoscopic mucosal resection (EMR) is a technique proliferated in Japan for large mucosal as well as submucosal lesions. In its simplest form, a lesion can be raised by infiltration of adrenaline solution to create a pseudopolyp before the lesion is excised by snare polypectomy technique. Other variations involve using specialist attachments to the endoscope to remove lesions in a technique not too dissimilar to variceal banding (Soetikno et al., 2003).
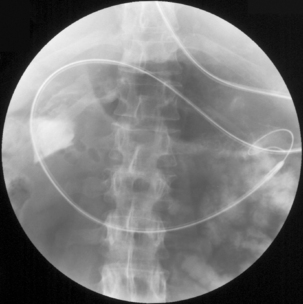
Dilatation and stent insertion can be carried out endoscopically for treating achalasia and strictures. Traditionally, dilatations have been done with Savoury-Gillard or Mallory bougies under semi-blinded or radiology control. Modern hydrostatic pneumatic balloons have allowed dilatation under direct vision and better control of the rate and extent of the dilatation (Figure 20.1). There is a risk of perforation associated with dilatations and the results have favored balloon dilatations over the other methods (Hernandez et al., 2000).
Complications of GI endoscopy
Endoscopy is a very safe procedure but, nonetheless, it is associated with its attendant risk and complications. Several large studies, including the National Confidential Enquiry into Patient Outcome and Death (2004), have highlighted cardiopulmonary and sedation-related complications as the major complications, together with a number of procedure-related complications.
The general complications include over-sedation that may induce respiratory depression with hypoxia, bleeding, perforation, hyper- or hypotension, aspiration, cardiac events including angina and myocardial infarction and strokes (Green, 2006). Cardiopulmonary complications account for 50% of serious morbidity and approximately 50% of all gastrointestinal endoscopy related deaths (Daneshmend et al., 1991). Respiratory depression from over-sedation from midazolam and opiates have an antidote in the form of flumazenil or naloxone, respectively, allowing the sedation to be reversed if an adverse event occurs (Bell and Quine, 2006).
The perforation risk in diagnostic gastroscopy is estimated to be 0.03%. Perforation can occur anywhere from the pharynx to the duodenum. This may be caused by pushing the gastroscope blindly, taking biopsy and esophageal tears from eosinophilic esophagitis. There is an increased perforation risk with all therapeutic procedures, e.g. dilatations, stent insertion, EMR and sclerotherapy. Mild bleeding following taking biopsy and polypectomy in the upper GI tract is not uncommon but major hemorrhage is rare. Bleeding (17%) is common after EMR, but the majority stop spontaneously without requiring further intervention. It is estimated that hemorrhage following EMR is approximately 2% (Riley and Alderson, 2006).
Perforation from colonoscopy is associated with greater risks than fluoroscopy. Full colonoscopy carries more risk than flexible sigmoidoscopy and therapeutic procedures produce more complications than diagnostic scopes. The main risks are perforation and bleeding (Epstein, 2006).
Perforation is rare following flexible sigmoidoscopy, but the risk increases with colonoscopy and/or therapeutic procedures. Perforation is more likely if transmural inflammation or ulceration is present and the risks are greater in the right colon where the bowel wall of the colon is thinner and subject to higher pressures (Epstein, 2006). Perforation rates have been quoted as 1.96 per 1000 procedures for colonoscopy and 0.88 per 1000 procedures for flexible sigmoidoscopy (Gatto et al., 2003) with similar figures of 1.9/1000 for 10 486 colonoscopy and 0.4/1000 out of 49 501 sigmoidoscopies (Anderson et al., 2000). However, the flexible sigmoidoscopy screening trial in the UK demonstrated a perforation rate of only one per 40 674 for flexible sigmoidoscopies and four out of 2131 colonoscopies (UK flexible screening trial investigators, 2002).
The perforation can be due either to direct trauma from the colonoscope or a thermal injury from diathermy and the diagnosis is often delayed (Hall et al., 2005; Epstein, 2006). If the bowel is perforated, free air may be seen on erect chest x-ray but a CT or contrast enema may be necessary to confirm the diagnosis. Some patients can be managed non-operatively as they have had full bowel preparation and hence have a clean colon limiting contamination, but others will require surgery. The surgery carried out depends on the extent of the injury and the bowel pathology. A resection ± stoma may be necessary or just oversewing of the perforation.
Barium enema, on the other hand, carries a risk of only one serious complication per 9000, perforation of the rectum in one in 25 000 and one death attributable to barium enema in one in 60 000 (Blakeborough et al., 1997).
Mild bleeding can occur after any biopsy or polypectomy with an incidence of 0.001–0.24% (Guidelines for Management of Colorectal Cancer, 2007). This is not usually severe and is usually self-limiting. More severe bleeding can occur after snare polypectomy, especially if the polyp has a thick stalk with a large vessel within it. Again, this is usually self-limiting but, if it persists or is severe, this can be treated endoscopically. Primary bleeding can be dealt with by coagulation, adrenaline injection or by applying a small metal clip to the bleeding vessel (endoclip).
Anderson M.L., Pasha T.M., Leighton J.A. Endoscopic perforation of the colon: lessons from a 10 year study. Am. J. Gastroenterol. 2000;95:3418-3422.
Ball J.E., Osborne J., Jowett S., et al. Quality improvement programme to achieve acceptable colonoscopy completion rates: prospective before and after study. Br. Med. J.. 2004;239(7467):665-667.
Bell G.D., Quine A.. Cardiopulmonary and sedation related complications. BSG guidelines in Gastroenterology.. 2006 http://www.bsg.org.uk/bsgdisp1.php?id = 48c1b0bcae9daa89d36a&m = 00023.
Blakeborough A., Sheridan M.B., Chapman A.H. Complications of barium enema examinations: a survey of UK consultant radiologists 1992–1994. Clin. Radiol.. 1997;52:142-148.
Bowles C.J., Leicester R., Romaya C., et al. A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow? Gut. 2004;53(2):277-283.
Daneshmend T.K., Bell G.D., Logan R.F.A. Sedation for upper gastrointestinal endoscopy: results of a nationwide survey. Gut. 1991;32:12-15.
Epstein O.. Complications of colonoscopy. BSG guidelines in Gastroenterology. 2006 http://www.bsg.org.uk/bsgdisp1.php?id = 48c1b0bcae9daa89d36a&m = 00023 Accessed 10.11.08
Gatto N.M., Frucht H., Sundararajan V., et al. Risk of perforation after colonoscopy and sigmoidoscopy: a population based study. J. Natl. Cancer. Inst.. 2003;95:230-236.
Green J.. Complications of gastrointestinal endoscopy. BSG guidelines in Gastroenterology. 2006 http://www.bsg.org.uk/bsgdisp1.php?id = 48c1b0bcae9daa89d36a&m = 00023 Accessed 10.1.08
Guidelines for Management of Colorectal Cancer. Association of Coloproctology of Great Britain and Ireland, third ed., 2007.
Hall C., Dorricott N.J., Donovan I.A., et al. Colon perforation during colonoscopy: surgical versus conservative management. Br. J. Surg.. 2005;78(5):542-544.
Hernandez L.V., Jacobson J.W., Harris M.S. Comparison among the perforation rates of Maloney, balloon, and savary dilation of esophageal strictures. Gastrointest. Endosc.. 2000;51(4 Pt 1):460-462.
Joint Advisory Group on Gastrointestinal Endoscopy. Guidelines for training, appraisal and assessment of trainees in gastrointestinal endoscopy and for the assessment of units for registration and re-registration. 2004.
National Confidential Enquiry into Patient Outcome and Death. Scoping our practice. 2004 http://www.ncepod.org.uk/reports2.htm Accessed 10.11.08
Riley S., Alderson D.. Complications of upper gastrointestinal endoscopy. British Society of Gastroenterology guidelines in Gastroenterology. 2006 http://www.bsg.org.uk/bsgdisp1.php?id = 48c1b0bcae9daa89d36a&m = 00023 Accessed 10.1.08
Soetikno R.M., Gotoda T., Nakanishi Y., et al. Endoscopic mucosal resection. Gastrointest. Endosc.. 2003;57:567-579.
Taylor K.M., Arajs K., Rouse T., et al. A prospective audit of colonoscopy quality in Kent and Medway, UK. Endoscopy. 2004;40(4):291-295.
Thomas R.D., Fairhurst J.J., Frost R.A. Wessex regional radiology audit: barium enema in colorectal carcinoma. Clin. Radiol.. 1995;50:647-650.
UK flexible screening trial investigators. Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomised trial. Lancet. 2002;359:1291-1300.