Chapter 41 Endoscopic Ultrasound of Pancreatic and Biliary Diseases
Introduction
Endoscopic ultrasound (EUS) is a powerful technique that has changed the clinical approach to biliary and pancreatic disease. Because it provides detailed images of the extrahepatic biliary tree and pancreas with very little risk to the patient, EUS is useful in the evaluation of obstructive jaundice,1 biliary or pancreatic ductal dilation,2 pancreatic masses, and pancreatitis.3 EUS and endoscopic retrograde cholangiopancreatography (ERCP) can be performed under the same sedation, with EUS identifying patients likely to benefit from therapeutic ERCP. EUS-guided therapeutic interventions have an evolving role in selected patients, including celiac plexus block (CPB),4 drainage of pancreas fluid collections, and EUS-guided drainage of inaccessible biliary and pancreatic ducts.5 EUS is an increasingly important tool for biliary and pancreatic endoscopy. This chapter discusses the use of EUS for diagnosis of common biliary and pancreatic diseases and emerging methods of EUS-guided pancreaticobiliary therapy.![]() The included video clips on the accompanying website show station-based techniques for performance of biliary and pancreatic EUS. The techniques of EUS-guided fine needle aspiration (FNA) and tissue biopsy are presented in a subsequent chapter.
The included video clips on the accompanying website show station-based techniques for performance of biliary and pancreatic EUS. The techniques of EUS-guided fine needle aspiration (FNA) and tissue biopsy are presented in a subsequent chapter.
Gallbladder Stones, Sludge, and Polyps
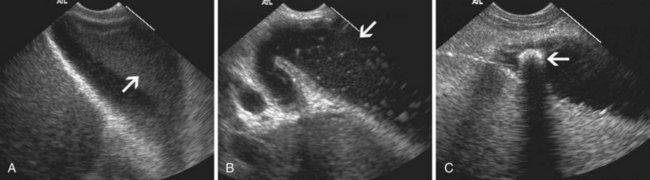
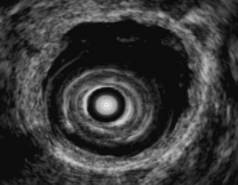
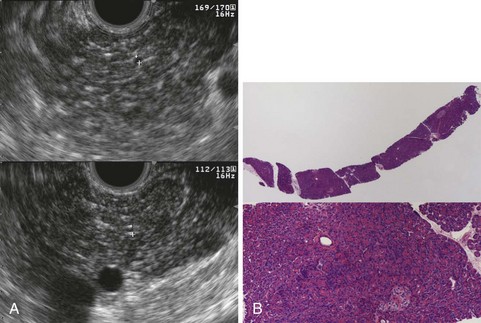
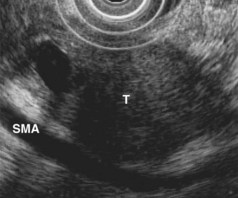
EUS is useful for diagnosis of gallbladder sludge or stones missed by transabdominal ultrasound and is more sensitive than bile microscopy in such patients (Fig. 41.1).6,7 It may be especially useful in obese patients and patients with stones in the gallbladder neck, settings in which transabdominal ultrasound is less sensitive for diagnosis. Sludge is visualized as echogenic, nonshadowing, layering material. It should not be confused with gain artifact or “ring-down” artifact, circular bright lines parallel to the transducer seen with mechanical radial scopes. A clear-cut sludge-bile interface is helpful for diagnosis of sludge. Cholesterol crystals have straight edges and are highly echogenic; they appear as bright flecks in bile, sometimes casting “comet tails.” Calcium bilirubinate granules are rounded and much less echogenic and can be missed by EUS unless they are present in sufficient quantity to form layering sludge.
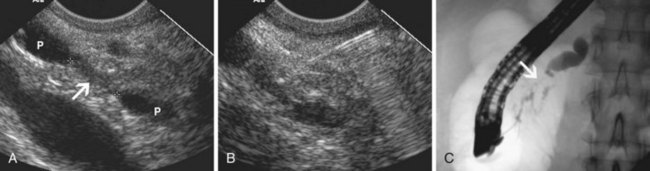
EUS has been used for differential diagnosis of gallbladder polyps. The best clinical studies have been reported from Asia, and their applicability to Western populations has not been well studied. Most gallbladder polyps are readily imaged, although the fundus and cap of the gallbladder may be difficult or impossible to visualize in some patients. Adherent sludge can mimic a gallbladder polyp, but sludge can usually be distinguished from polyp by gently shaking the patient’s abdomen or turning the patient to the right decubitus position during EUS to determine whether the lesion moves (Fig. 41.2). The differential diagnosis of gallbladder polyps is presented in Table 41.1. The size of gallbladder polyps is the most important consideration in differential diagnosis. In Asian populations, neoplasm is very unlikely in polyps with a diameter of 5 mm or less but is usually present in polyps greater than 15 mm in diameter.8–10
| Nonneoplastic | Neoplastic |
|---|---|
| Cholesterol | Adenoma |
| Hyperplastic | Adenocarcinoma |
| Inflammatory | Adenosquamous carcinoma |
| Fibrous | Neuroendocrine tumor |
| Adenomyomatosis |
Particular echo features that predict the type of polyp have been identified. Neoplastic polyps may contain hypoechoic foci,11 whereas cholesterol polyps often contain bright, echogenic spots or show “comet tail” artifacts caused by cholesterol crystals in the lesion. Adenomyomatosis typically contains multiple small cystic or anechoic spaces and may show evidence of cholesterol deposits in the lesion. When these distinctive findings are seen, it may be reasonable to follow larger gallbladder polyps, at least polyps less than 20 mm in diameter,10 although neoplastic polyps having EUS features of cholesterolosis or adenomyomatosis have been reported.12 Neoplasm should be considered when the characteristic findings of a nonneoplastic lesion are absent and the lesion is greater than 5 mm, even in lesions confined to the mucosa. Loss of gallbladder wall architecture is suggestive of an invasive cancer.13
Bile Duct Stones
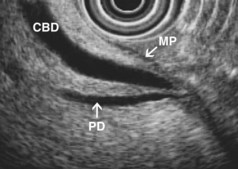
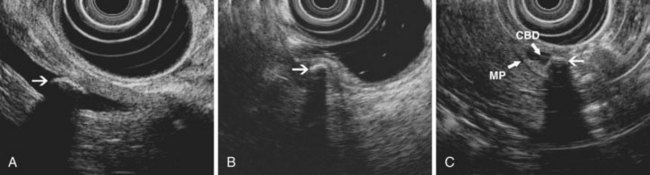
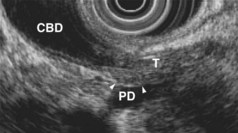
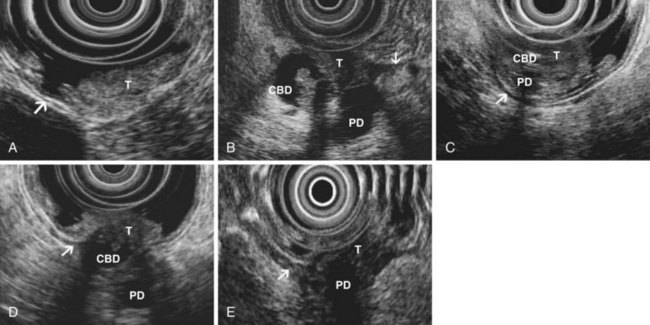
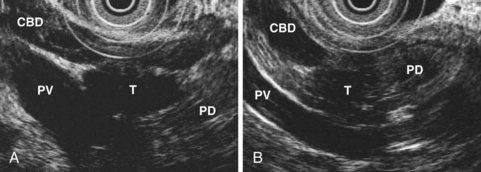
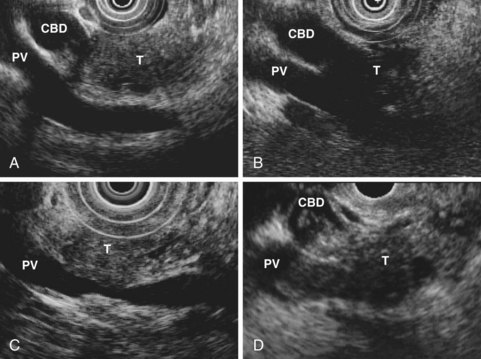
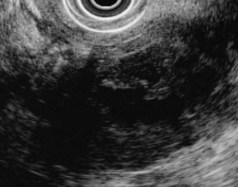
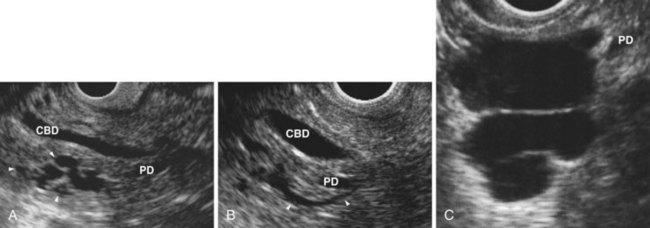
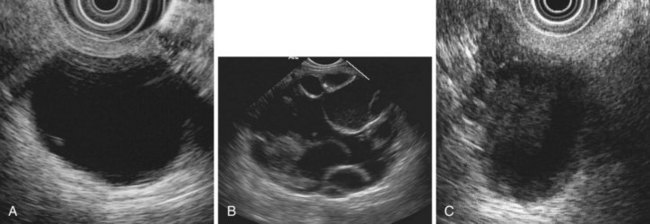
EUS is highly accurate for diagnosis of choledocholithiasis. EUS is especially useful in patients with a low or intermediate risk of bile duct stones, refining the use of ERCP and decreasing the overall risks of an endoscopic approach. The accuracy of EUS for diagnosis of bile duct stones and sludge relies on both endoscopic and patient factors. The common duct is best visualized from the duodenal bulb, where it can be followed from the common hepatic duct to the periampullary region. The biliary confluence is sometimes visualized from the bulb, more often with linear array echoendoscopes. The bile duct is distinguished from adjacent vessels by identifying its convergence with the pancreatic duct as both ducts taper into the duodenal wall—the “stack” sign (Fig. 41.3). The cystic duct insertion is another useful landmark. In addition, the bile duct wall has an inner hypoechoic mucosal layer that is not present in adjacent vessels. Imaging should also be performed with the endoscope opposite the ampulla in the second duodenum for detection of stones in the ampulla or periampullary bile duct.
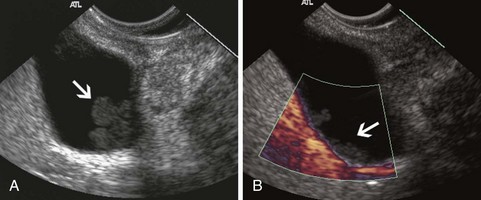
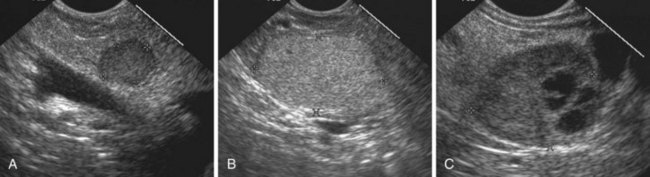
Stones are identified as echogenic structures casting dark acoustic shadows (Fig. 41.4). Air bubbles in the duct also appear as echogenic, rounded structures but cast hyperechoic acoustic reverberations instead of shadows. Sludge or cholesterol crystals can be visualized in the bile duct much as they are visualized in the gallbladder (see Fig. 41.1). Diagnosis of ductal stones is easiest when small stones are present in a dilated duct—the very situation in which cholangiography can miss stones. Conversely, ultrasound diagnosis may be challenging when a diminutive duct is present or when the common duct is completely filled with stones, obliterating a visible ductal lumen. Care should be taken to visualize the entire bile duct, not skipping over portions. It is important to recognize a technically inadequate or incomplete EUS examination and to consider other imaging tests rather than concluding that no stones are present.
EUS has been compared with ERCP for diagnosis of bile duct stones. Prat and colleagues14 performed EUS and ERCP in 119 patients with suspected bile duct stones, performing sphincterotomy and balloon sweeps of the bile duct in all subjects to obtain an independent “gold standard.” The sensitivity and specificity of EUS were 93% and 97% compared with 89% and 100% for ERCP. This study suggested that radial EUS was more sensitive than cholangiography for diagnosis of stones. A study of similar design using linear array echoendoscopes reported that the sensitivity and specificity of EUS were both 93%.15 Magnetic resonance cholangiopancreatography (MRCP) and EUS are both good tests for detection of bile duct stones, with roughly equivalent overall accuracy.16 EUS seems to be more accurate for diagnosis of stones less than 5 mm in diameter and may be preferable for diagnosis of ampullary stones,17 whereas intrahepatic duct stones not visualized by EUS can be diagnosed on MRCP.
EUS can be used as the sole imaging study to exclude choledocholithiasis before laparoscopic cholecystectomy. When EUS showed no bile duct stones, recurrent symptoms owing to ductal stones did not occur during almost 3 years of follow-up in a European cohort.18 Several prospective randomized trials compared clinical outcomes when either EUS or ERCP was used to evaluate the bile duct in patients at intermediate risk for bile duct stones, such as patients with uncomplicated biliary pancreatitis or elevated serum liver tests.19–22 Taken together, these studies show higher diagnostic accuracy, fewer overall complications, and less resource use in patients evaluated with EUS.17 Most patients enrolled in the EUS arm of these studies did not have bile duct stones and did not require ERCP; patients who did have ductal stones usually underwent ERCP immediately after EUS, under the same sedation. EUS has emerged as a preferred alternative to ERCP in patients at intermediate risk of bile duct stones.
Biliary intraductal ultrasound (IDUS) is also accurate for diagnosis of bile duct stones and sludge. IDUS requires deep cannulation of the bile duct with the intraductal probe, which can be passed over a guidewire without sphincterotomy. Most investigators have performed IDUS after obtaining a cholangiogram during ERCP. To minimize trauma to the probe and extend its useful life, the operator should use as little elevator as possible and image only during slow probe withdrawal. Because IDUS uses a high-frequency probe placed directly in the duct, it is probably the best available imaging technique for diagnosis of small stones and ductal sludge. In one direct comparison of cholangiography and IDUS, the sensitivity of IDUS for stones was 97% compared with 81% for ERCP.23 Because it is performed during ERCP, IDUS is probably best used to clarify diagnosis in patients with equivocal findings at cholangiography, such as small filling defects, possible air bubbles or polyps in the bile duct, or a dilated bile duct. IDUS improves diagnostic accuracy in about one-third of these patients.24 The need to diagnose and treat small (<5 mm) stones detected only with IDUS has been questioned, however, because such stones often pass spontaneously.25,26
Choledochoscopy is also useful for diagnosis of ductal stones; direct comparisons with IDUS have not been reported. Direct cannulation of the papilla via a linear array echoendoscope, with EUS confirmation of deep bile duct cannulation and subsequent sphincterotomy, is technically feasible and seems to have similar efficacy and complications as standard ERCP for sphincterotomy and stone extraction in patients with a solitary small bile duct stone.27 This technique may be desirable in pregnant patients because it avoids use of fluoroscopy.
Bile Duct Strictures and Cholangiocarcinoma
Biliary strictures may be of indeterminate etiology, typically when cross-sectional imaging and intraductal biopsy specimens and brushings obtained during ERCP are nondiagnostic. The cholangiographic appearance of a stricture and the patient’s clinical history traditionally determined whether unexplained bile duct strictures should be resected on suspicion of malignancy. EUS and IDUS may be used to evaluate biliary strictures and may aid clinical decision making by suggesting a benign or malignant process. EUS can also be used for local staging of malignant biliary strictures. The bile duct wall appears to have two or three layers on EUS and IDUS (Fig. 41.5). An internal, hyperechoic layer representing an interface echo is sometimes seen. Deep to this layer is a hypoechoic layer corresponding to the mucosa, subepithelial connective tissue, muscularis propria, and the fibrous layer of the subserosa. The amount of muscularis varies, with little or no muscularis propria in the proximal bile duct. Deep to this hypoechoic layer is an outer hyperechoic layer formed by the adipose layer of the subserosa, the serosa, and the interface with surrounding tissue.28 The normal bile duct wall is less than 1 mm thick on EUS,29 although the presence of a stent or drain in the duct may lead to thickening of the wall up to 2.8 mm.30 The bile duct wall layers can be identified with either EUS or IDUS. IDUS probes can be passed into the central intrahepatic ducts, visualizing portions of the biliary tree not usually accessible to transduodenal EUS; IDUS also provides high-resolution images of the bile duct wall and adjacent vessels and tissue. EUS with a dedicated echoendoscope can image the extrahepatic biliary tree, including Klatskin’s tumors,31 and its deeper depth of penetration permits a thorough assessment of the gallbladder, pancreatic head, and regional nodes. The two techniques are complementary.
EUS has been used for differential diagnosis of unexplained bile duct strictures. During IDUS, malignant strictures typically appear hypoechoic with a thickened wall and irregular margins, whereas postoperative strictures are usually relatively hyperechoic with smooth edges.32 Both primary sclerosing cholangitis and IgG4-associated cholangitis can mimic malignant strictures on IDUS. Studies have shown IDUS and EUS to be more accurate than ERCP and intraductal tissue sampling for diagnosis of malignant bile duct strictures,33–35 although these were retrospective studies that included few or no patients with primary sclerosing cholangitis or IgG4-associated cholangitis. In a large prospective study, IDUS was as useful as advanced cytology techniques (including fluorescent in situ hybridization) for diagnosis of indeterminate strictures.36
The relative utility of IDUS and direct cholangioscopy has not been widely studied, but in one report percutaneous cholangioscopy with directed biopsy was more accurate than IDUS.37 The current TNM (primary tumor, regional nodes, metastases) staging system for carcinoma of the extrahepatic bile ducts is presented in Box 41.1. Both EUS and IDUS have been used to stage cholangiocarcinoma. The two techniques have similar accuracy of about 80% for T stage, differentiating T1 lesions confined to the bile duct wall (involving the hypoechoic layer) from T2 lesions invading beyond the bile duct wall (with disruption of the outer hyperechoic layer) (Fig. 41.6).28
Box 41.1
Staging of Extrahepatic Cholangiocarcinoma
From American Joint Committee on Cancer (AJCC): AJCC cancer staging manual, ed 7, New York, 2010, Springer-Verlag (www.springer-ny.com).
IDUS is more useful than EUS for lesions of the proximal biliary tree. IDUS has also been used to estimate the longitudinal extent of cholangiocarcinoma because cholangiography is well known to underestimate the longitudinal extent of ductal involvement. Nonspecific thickening of the bile duct wall owing to the presence of a stent or drain limits the value of IDUS in patients who have had a drain placed.38 Intravenous administration of ultrasound contrast medium may improve the specificity of IDUS for malignancy, showing hyperperfusion of inflammatory lesions and hypoperfusion of tumor,39 but confirmatory studies have not been reported. IDUS is probably a sensitive modality for diagnosis of early cholangiocarcinoma in choledochal cysts. The technique should be considered in adult patients with choledochal cysts, especially if surgical resection of the cyst is not otherwise planned.
Ampulla of Vater
The submucosal apparatus of the papilla can be visualized as a round hypoechoic structure in duodenal submucosa composed of the sphincter of Oddi and the intramural ducts. The normal submucosal mound of the papilla is usually less than 6 mm in transverse cross-sectional diameter. The lumens of the bile duct and pancreatic duct are usually not visible within the papilla; they generally taper and disappear from view as they reach the duodenal wall. The finding of a visible ductal lumen in the papilla suggests obstruction of the papilla by a stone (see Fig. 41.4), stenosis, or tumor, but a ductal lumen can also be seen in choledochocele and intraductal papillary mucinous neoplasm (IPMN). IDUS has been used to study the ampulla and may aid in the local staging of some ampullary tumors. It identifies the sphincter mechanism and permits accurate measurement of its length. Ultrasound features do not distinguish normal from hypertensive sphincters.40
Ampullary Neoplasms
The TNM staging of ampullary cancers is presented in Box 41.2 and illustrated in Fig. 41.7. T1 carcinoma may be limited to the mucosal surfaces of the ampulla and intraampullary ducts but may also involve the sphincter mechanism of the ampulla. The presence of an irregular outer edge of the submucosal ampullary apparatus suggests a T2 lesion invading the duodenal submucosa or muscularis propria. T3 cancers invade the pancreas, extending either through the duodenal wall or directly from the periampullary ducts. A T4 tumor extends into peripancreatic soft tissue or other adjacent structures. Regional lymph nodes include not only nodes adjacent to pancreatic head but also porta hepatic and celiac nodes.
Box 41.2
Staging of Ampullary Carcinoma
From American Joint Committee on Cancer (AJCC): AJCC cancer staging manual, ed 7, New York, 2010, Springer-Verlag (www.springer-ny.com).
In one large series, EUS accuracy for T staging of ampullary malignancies was 78%.41 Adenomas were considered T1 lesions, highlighting the difficulty in distinguishing adenoma from T1 cancer with EUS. Most errors in staging involved overstaging of T2 lesions or understaging of T3 lesions because of difficulty in assessing the presence of invasion into the pancreas. The presence of peritumoral pancreatitis and edema and shadowing and tissue thickening secondary to an indwelling biliary stent were the major factors limiting the accuracy of EUS. Tumor may be difficult to distinguish from the normally hypoechoic ventral pancreas, and invasion of the duodenal muscularis propria may be difficult to detect because in normal persons the muscularis propria is interrupted by the ducts as they cross into the papilla. Despite these limitations, EUS is more accurate than computed tomography (CT) or magnetic resonance imaging (MRI).41–43
IDUS is probably more accurate that transduodenal EUS for T staging of ampullary neoplasms. In one large series, IDUS had an overall accuracy of 89%.42 IDUS visualized small tumors missed by EUS and was more accurate than endoscopic biopsies for diagnosis of ampullary neoplasm. IDUS was also accurate for differentiation of adenoma from T1 carcinoma. These results were achieved by experienced endosonographers, using IDUS at the patient’s initial ERCP and before sphincterotomy, stent placement, or biopsy—an optimal algorithm for tumor imaging but difficult to replicate in most EUS referral centers.
Acute Pancreatitis
One prospective trial investigating EUS in gallstone pancreatitis reported that it was accurate for diagnosis of gallbladder and ductal stones and predicted longer hospital stay in patients found to have peripancreatic fluid by EUS.44 In another large series in which ERCP was used selectively, on the basis of EUS findings, patient outcomes were good, and recurrent biliary pancreatitis was uncommon.45 EUS is also a useful tool in the evaluation of idiopathic pancreatitis, showing abnormalities in most patients.3,46,47 Findings include missed biliary stones or sludge (see Fig. 41.1), chronic pancreatitis, pancreas divisum, pancreatic or ampullary malignancy, and pancreatic duct stones. EUS does not diagnose pancreatic sphincter dysfunction but may nevertheless supplant ERCP by diagnosing or excluding previously unsuspected gallbladder pathology, chronic pancreatitis, or pancreatic malignancy. EUS and MRCP seemed to have similar utility in one study.48
Chronic Pancreatitis
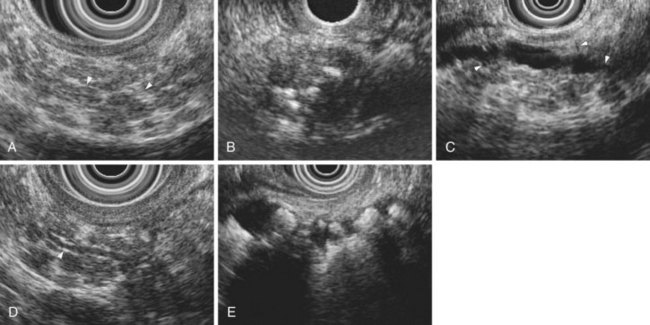
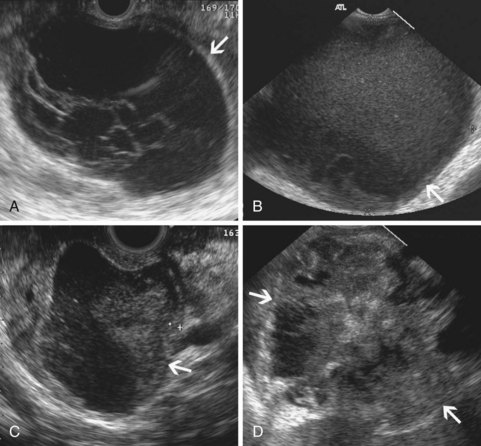
The traditional EUS features of chronic pancreatitis are listed in Table 41.2 and illustrated in Fig. 41.8. This list of consensus criteria uses minimal standard terminology adopted by an international working group,49 and good interobserver agreement has been shown for these criteria among experienced American endosonographers.50 Investigators have also described other features not included in this list, including honeycombing (in which hyperechoic strands form a honeycomb pattern), heterogeneous echotexture, focal areas of hypoechogenicity, tortuous pancreatic duct, thickened pancreatic duct wall, and narrowing of the main pancreatic duct. The traditional EUS approach to diagnosis of chronic pancreatitis gives each feature equal weight and sums the number of features present.
Table 41.2 Traditional Endoscopic Ultrasound Features of Chronic Pancreatitis
| Parenchymal Features | Ductal Features |
|---|---|
| Hyperechoic strands | Stones |
| Hyperechoic foci | Main duct irregularity |
| Lobularity | Hyperechoic main duct |
| Cysts | Visible side branches |
| Main duct dilation |
From The International Working Group for Minimal Standard Terminology in Gastrointestinal Endosonography: Minimal standard terminology in gastrointestinal endosonography. Dig Endosc 10:159–184, 1998.
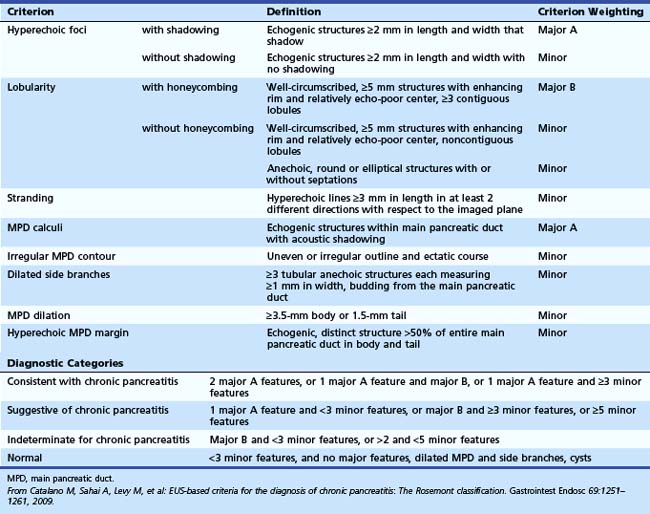
The Rosemont criteria, proposed in 2009, offer an alternative approach to diagnosis based on major and minor criteria (Table 41.3).51 In one study, the Rosemont criteria resulted in improved specificity and decreased sensitivity, although these changes were not statistically significant.52 Definitions vary for some criteria. Hyperechoic foci have been defined as greater than 3 mm by some investigators53 but as 1 to 2 mm by most others.54,55 Main pancreatic duct dilation has been variably defined, often as a diameter of greater than 2 mm in the body or greater than 1 mm in the tail.50 The Rosemont criteria offer semiquantitative definitions for many criteria (see Table 41.3). Criteria have been considered abnormal when visualized at either 12 MHz or 7.5 MHz by some investigators but at only 7.5 MHz by others. Findings must be interpreted with considerable caution when imaging the pancreatic head because some features (e.g., hyperechoic strands and visible side branches) are often seen in the normal pancreatic head, whereas others (e.g., cysts and stones) are not. Diagnosis is best made based on features seen in the pancreatic body and tail. Some investigators have seen visible duct side branches in the normal pancreatic body.53
There are caveats regarding the specificity of EUS criteria for diagnosis of pancreatitis. EUS features of chronic pancreatitis have been reported in members of pancreatic cancer kindreds, in whom lobularity may correlate with the presence of pancreatic intraepithelial neoplasia in pancreatic branch ducts.56–58 Focal areas of pancreatic hypoechogenicity can be due to focal inflammation but may also be due to neoplasm (Fig. 41.9). Acute pancreatitis may cause decreased parenchymal echogenicity (owing to edema), accentuating the echogenicity of the pancreatic duct wall and the interlobular septa of the pancreas. For diagnosis of chronic pancreatitis, EUS should be performed after an acute episode of pancreatitis has resolved. Finally, ductal dilation and pancreatic fibrosis occur in older persons without clinical pancreatic disease.
Some EUS findings may be attributable to the effects of age, cigarette smoking, and alcohol on the pancreas rather than chronic pancreatitis. Although studies in normal volunteers have generally shown no parenchymal EUS abnormalities in young, asymptomatic individuals who do not use alcohol,53,55,59 older patients with no history of pancreatic disease undergoing EUS for other indications had on average two EUS features of chronic pancreatitis.60 Autopsy data show that most alcoholic cirrhotics without a clinical history of pancreatic disease have pancreatic fibrosis.61,62 EUS findings are strongly correlated with extent of ethanol ingestion; also, cigarette smoking correlates with increased number of EUS criteria.63
The accuracy of EUS for diagnosis of “early” or “minimal change” chronic pancreatitis is debated, and EUS has been compared with pancreatography, functional tests, and histology. Early studies comparing EUS with pancreatography concluded that the presence of three or more traditional criteria was the best threshold for EUS diagnosis of chronic pancreatitis.53–5564 These studies used pancreatography as a “gold standard”; however, the validity of pancreatography for diagnosis of early chronic pancreatitis is poorly validated: The commonly used Cambridge criteria for pancreatography interpretation are based on expert opinion, and autopsy studies have shown abnormalities on pancreatography in most people without a clinical history of pancreatic disease.62,65
Histologic comparisons have reached conflicting conclusions, with a retrospective study of surgical resection specimens concluding that the best threshold for diagnosis was the presence of three EUS criteria66 and a prospective study of EUS-guided Tru-Cut needle biopsy concluding that histologic abnormalities were uncommon in individuals with three or four traditional EUS criteria (Fig. 41.10).67 This discrepancy likely has several causes, including variations in EUS criteria, tissue sampling error, and differences in patient populations; the first study included patients with chronic pancreatitis of sufficient magnitude to require surgical resection, whereas the second study enrolled patients with chronic abdominal pain and a paucity of other objective findings. These studies are limited by the lack of consensus criteria for histologic diagnosis of chronic pancreatitis. There is histologic overlap between chronic pancreatitis and age-related pancreatic atrophy and fibrosis,62 yet the presence of mild fibrosis was considered sufficient for histologic diagnosis of chronic pancreatitis in the first study.66
EUS has also been compared with pancreatic function testing (PFT) with conflicting results. In one early study, EUS and PFT correlated well in patients with advanced disease, but PFT was often normal in patients with early chronic pancreatitis by pancreatography and EUS; it seemed that PFT was likely insensitive for diagnosis of early disease.55 In a subsequent comparison from a large-volume PFT center, EUS fared poorly, appearing less sensitive than PFT for diagnosis of early disease.68 More recently, endoscopic PFT has been compared with EUS; discordance between EUS and PFT was most likely in patients with two, three, or four traditional EUS criteria.69 In some cases, EUS findings should be deemed indeterminate for chronic pancreatitis. This category, introduced by the Rosemont criteria (which rate the presence of three or four minor criteria as indeterminate), reflects clinical and histologic realities and the current state of knowledge. In such cases, PFT and repeat evaluation over time may be useful.
Solid Pancreatic Neoplasms
EUS is commonly used for diagnosis of pancreatic neoplasms. It images small tumors missed by other diagnostic modalities, provides local staging information, and permits immediate aspiration or biopsy of pancreatic masses and lymph nodes under real-time ultrasound guidance. This section reviews the role of EUS in patients with known or suspected pancreatic adenocarcinoma. Cystic neoplasms are discussed elsewhere in this chapter, and pancreatic neuroendocrine tumors are discussed in another chapter. To diagnose and stage pancreatic neoplasms accurately, the endosonographer must have a detailed understanding of pancreatic anatomy on ultrasound and the ability to identify important adjacent vessels reliably, including the portal vein, portal confluence, hepatic and gastroduodenal arteries, splenic vessels, and superior mesenteric vessels. Educational materials that teach this EUS anatomy are available.70
Adenocarcinoma
Pancreatic adenocarcinoma typically appears as a hypoechoic mass with poorly defined, irregular edges. The lesion often appears to obstruct the pancreatic duct. Large tumors may show poor echo penetration, making assessment for vascular invasion difficult or impossible.71 Infiltrating adenocarcinomas may cause a heterogeneous or hypoechoic parenchymal echotexture without a discrete mass. The finding of a hypoechoic pancreatic mass is not specific for adenocarcinoma and may be caused by focal pancreatitis. Acute or chronic pancreatitis can cause this appearance, and a focal mass is often seen in autoimmune pancreatitis. Benign pancreatic masses tend to have better defined edges than malignancies (see Fig. 41.9), and computerized image analysis has been used as an aid in differential diagnosis.72 Analysis of blood flow in the mass using power Doppler and an echo-enhancing intravenous contrast agent has been reported to distinguish inflammatory masses from malignant masses accurately, but criteria vary,73,74 and the technique seems unlikely to replace tissue acquisition for definitive diagnosis. EUS elastography of the pancreas does not seem to distinguish benign from malignant pancreatic masses reliably.75
EUS-guided aspiration of focal lesions is useful for preoperative differential diagnosis of a hypoechoic pancreatic mass, although a negative cytologic result does not fully exclude malignancy. EUS aspiration cytology has a sensitivity of about 80% for diagnosis of pancreatic cancer.76,77 Analysis of cytologic aspirates for mutations and allele loss may improve the diagnostic yield further.78,79 Early studies comparing CT and EUS showed that EUS was more sensitive for detection of pancreatic adenocarcinoma, particularly lesions less than 2 cm in diameter. Subsequent studies comparing contrast-enhanced helical CT with EUS, taken in aggregate, suggest that EUS remains superior.80–82 Patients suspected to have pancreatic carcinoma but with no mass on CT should undergo EUS. In patients with an infiltrative rather than mass-forming malignancy, EUS may also fail to show a discrete lesion in the pancreas, although diffuse parenchymal changes are often seen. EUS-guided FNA of the pancreatic parenchyma may yield a diagnosis of malignancy in such cases, particularly if the aspirate is obtained near a ductal stricture.
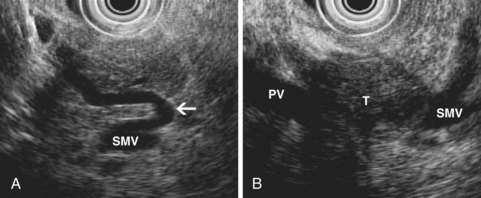
EUS also permits assessment of the relationship between a pancreatic tumor and adjacent vascular structures. The finding of normal tissue between a tumor and a vessel, or an intact echo-rich interface (echo plane) between the two, reliably excludes tumor invasion of the visualized vessel. Conversely, other findings suggest possible vascular involvement (Fig. 41.11), including loss of the echo plane between tumor and vessel, irregularity of the vessel wall, narrowing of the vessel lumen, echogenic material within the vessel lumen, and the presence of peripancreatic venous collaterals (Fig. 41.12). Although numerous studies have reported EUS accuracy of 75% to 100% for diagnosis of vascular invasion,81 these studies relied on a reference standard of intraoperative assessment by dissection and palpation. The surgeon’s intraoperative suspicion of vascular invasion may be incorrect, however, particularly if a tumor is densely adherent to a vessel without actual invasion.83 Surgical resection of portal vein or superior mesenteric vein at the time of pancreatectomy has become an accepted technique, and patients undergoing venous resection have a survival comparable to patients not requiring venous resection.84
In a study comparing EUS with surgical histology of resected pancreatic masses, including masses undergoing venous resection, loss of echo plane was a poor predictor of vascular involvement (specificity <30%), and only half of resectable tumors with more advanced ultrasound features of vascular invasion required vascular resection.85 A blinded review of EUS videotapes suggested that endosonographers were less accurate in their assessment of vascular invasion when they were denied access to other clinical information about the patient, including results of other imaging studies.86 Taken together, these data suggest that EUS features of venous invasion are sensitive but not specific. EUS combined with a cross-sectional imaging study may provide more accurate local staging than either study alone.87
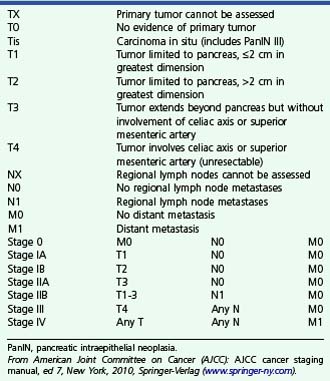
The 2002 revision of the TNM staging system for pancreatic carcinoma (Box 41.3) reflects current surgical philosophy and categorizes tumors as unresectable if there is local arterial invasion (stage III) or distant metastases (stage IV).88 The classification distinguishes between tumors limited to the pancreas (T1 if ≤2 cm, T2 if >2 cm) and tumors that extend beyond the pancreas (T3 if not involving celiac axis or superior mesenteric artery, T4 if involving these arteries). Arterial but not venous invasion is considered when assigning T stage. T3 tumors, which extend beyond the pancreas, are considered resectable. Extension of tumor beyond the edge of the pancreas has become an important criterion for T staging of pancreatic cancer. Tumor extension is best seen during EUS when the pancreatic parenchyma has a different echotexture than surrounding fat. When the pancreatic parenchyma is isoechoic with surrounding fat, the endosonographer is able only to identify the edge of the pancreas where it abuts major peripancreatic veins or other organs. In such cases, it may be impossible to arrive at an accurate T stage on ultrasound, and CT or MRI may be more useful. When assessing the edge of the pancreas, particular attention should be given to the retroperitoneal margin of the pancreatic head abutting the connective tissue between pancreas and superior mesenteric artery; this is the most likely site for a positive microscopic margin and subsequent local recurrence after resection of pancreatic head cancers (Fig. 41.13).88
Box 41.3
Staging of Pancreatic Adenocarcinoma
PanIN, pancreatic intraepithelial neoplasia.
From American Joint Committee on Cancer (AJCC): AJCC cancer staging manual, ed 7, New York, 2010, Springer-Verlag (www.springer-ny.com).
EUS features of arterial invasion may suggest T4 disease, although the true specificity of these features for arterial invasion is unknown. EUS aids in the detection of nodal and liver metastases from pancreatic cancer. Compared with CT, EUS has equal or greater sensitivity for identification of abnormal lymph nodes and is usually the procedure of choice for sampling abnormal nodes. Regional lymph nodes (considered N1 when malignant) are the peripancreatic nodes, including nodes along the hepatic artery, celiac axis, pyloric region, and splenic region.88 The presence of N1 disease is not considered a contraindication to resection in many referral centers because a “complete” resection seems to lengthen survival in such patients.84,88 The finding of malignant nodes distant from the primary tumor is considered metastatic disease and generally excludes surgery. In this regard, EUS identifies mediastinal nodal metastases in up to 7% of patients with pancreatic adenocarcinoma.89A standard clinical approach to patients with suspected pancreatic cancer begins with a high-quality, contrast-enhanced CT or MRI examination. If liver lesions are shown, percutaneous or EUS-guided biopsy is the most direct approach to staging.
If a locally unresectable pancreatic mass is shown, EUS can provide confirmation of local staging and permits aspiration for tissue diagnosis. If an apparently resectable mass is shown, EUS may change staging by detecting liver or distant nodal metastases in a few patients, and FNA of an apparently resectable mass shows a diagnosis other than adenocarcinoma in up to one-fourth of patients, potentially changing the management plan.81,90 EUS-guided tissue acquisition is required to exclude cancer in some cases of autoimmune pancreatitis.91 If pancreatic cancer is suspected, but no mass is shown on CT or MRI, EUS should be strongly considered for diagnosis of a small lesion missed by other modalities. Patients with small masses missed by other imaging techniques are probably the most likely patients to benefit from surgery.
Despite its overall excellent sensitivity for diagnosis of pancreatic cancer, EUS may fail to identify cancer arising in the setting of underlying chronic pancreatitis90 or severe acute pancreatitis. Tumor may be difficult to differentiate from areas of inflammation. FNA is more useful than EUS imaging alone in this setting, although more needle passes may be required to make a diagnosis of malignancy.92 Use of an intravenous ultrasound contrast agent may improve diagnosis in this setting; positron emission tomography also has been advocated. Infiltrating neoplasms that do not form a circumscribed mass can also be missed by EUS, as can early ductal malignancies (Fig. 41.14). FNA cytology is a reasonable diagnostic strategy in the absence of a mass, particularly when performed adjacent to an obstructed duct. When suspicion for malignancy remains high despite a negative EUS examination, other investigations (e.g., ERCP) may be warranted.
Other Solid Tumors
The most common variant of pancreatic adenocarcinoma is colloid carcinoma (or mucinous cyst adenocarcinoma), a solid tumor containing pools of mucus that may arise from a preexisting IPMN or mucinous cystadenoma. Colloid carcinoma may contain demonstrable cystic spaces on EUS (Fig. 41.15) and has a better prognosis after resection than typical adenocarcinoma. Metastatic cancer may manifest in the pancreas, including melanoma, breast, and renal cell carcinomas. Other uncommon solid tumors of the pancreas include lymphoma, acinar cell carcinoma, medullary carcinoma, osteogenic giant cell tumors, and pancreatoblastoma. The EUS features of these rarer tumors are not well described.
In contrast to adenocarcinoma, pancreatic neuroendocrine tumors typically appear as uniform, rounded, homogeneous masses with discrete edges. They may also appear cystic (Fig. 41.16). EUS is the most sensitive preoperative imaging test for diagnosis of pancreatic insulinomas and is an important modality in patients with multiple endocrine neoplasia type I who have numerous pancreatic gastrinomas, some of which are visualized only by EUS. EUS features suggestive of a malignant neuroendocrine tumor include an irregular central echogenic area and displacement or obstruction of the main pancreatic duct.73 EUS-guided FNA is an accurate means of diagnosing functioning neuroendocrine tumors.74 EUS of neuroendocrine tumors is discussed in more detail in Chapter 42.
Intraductal Papillary Mucinous Neoplasm
IPMN is a papillary growth of neoplastic epithelium in the main pancreatic duct or branch ducts. IPMN shares histologic and genetic features with pancreatic intraepithelial neoplasia, and both are precursor lesions for pancreatic adenocarcinoma, but IPMN is characterized by ductal dilation, mucin production, and more extensive ductal involvement.93 IPMN occurs most commonly in the pancreatic head, but this neoplasm may be seen anywhere in the pancreas and may be multifocal. Histologically, IPMN ranges from hyperplasia of ductal mucosa (not a true neoplasia), to adenoma with low- or high-grade dysplasia, carcinoma in situ, and invasive carcinoma. Carcinoma is seen in more than 50% of cases of resected main duct IPMN but only about 15% of cases of resected branch duct IPMN,94–97 and branch duct lesions appear less likely to progress over time.95,98,99
Diagnosis of main duct IPMN is clear-cut in a symptomatic patient with a markedly dilated main pancreatic duct containing polypoid filling defects, in which mucus is seen disgorging into the duodenum through a gaping ampullary orifice. Other, less classic presentations of main duct IPMN are increasingly recognized, including focal dilation or ectasia of the main pancreatic duct mimicking chronic pancreatitis or isolated polyps within the pancreatic duct. EUS shows the ductal dilation that is a hallmark of IPMN (Fig. 41.17). In main duct lesions, there is typically marked dilation of the duct without an obstructing mass, stricture, or stone. The main duct wall may be irregular or thickened, with visible side branches that are also involved. Faintly echogenic material may be seen in the duct lumen corresponding to mucin, and polypoid thickening of the duct mucosa may be evident, but these findings may be subtle or absent.
The differential diagnosis of main duct dilation includes chronic pancreatitis and downstream ductal obstruction by stone, stricture, or tumor, and the pancreatic duct should be traced during EUS looking for an obstructing lesion. Some branch duct IPMNs have a tubular branching structure that is clearly recognizable as a dilated ductal system, but others appear cystic rather than ductal (Fig. 41.18). EUS features that may suggest a diagnosis of branch duct IPMN include a connecting duct visible on ultrasound leading to the main pancreatic duct, pancreatic parenchyma rather than septa between the locules of a lesion, proximity to the pancreatic duct, or the presence of a branching architecture. In some cases, these features are absent, and the branch duct lesion appears to be a cyst without communication to the main pancreatic duct. IPMN should be considered in the differential diagnosis of any mucus-containing pancreatic cyst, particularly in men (in whom mucinous cystic neoplasm [MCN] is rare). Infiltrating parenchymal malignancy may be present in patients with IPMN and should always be suspected when a patient with IPMN has obstructive jaundice. Hypoechoic pancreatic parenchyma associated with IPMN can be due to inflammation or malignancy, and EUS-guided FNA may clarify the diagnosis in such cases.
Pancreas Cysts and Fluid Collections
EUS is commonly used as an aid in the diagnosis of pancreatic cystic lesions. Although the differential diagnosis is extensive (Table 41.4), this discussion focuses on the most common cystic lesions of the pancreas. EUS features of pancreatic cystic lesions are listed in Table 41.5. On ultrasound, all cystic structures are characterized by increased through-transmission, with the appearance of enhanced echogenicity of the tissue beyond the cyst. The overall cyst architecture, the cyst wall, and the cyst lumen should be assessed during EUS. Unilocular cysts are usually round and have one cyst cavity, with or without incomplete septa. Oligocystic cysts contain more than one cystic space or locule separated by septa, but the individual locules are easily seen during EUS and can be counted. Microcystic cysts contain innumerable cystic spaces, some of which may be too small to be resolved by EUS.
| Architecture | Wall | Lumen |
|---|---|---|
| Shape | Presence of a wall | Echogenicity (anechoic, echogenic) |
| Unilocular, oligocystic, microcystic | Mural thickening (>1 mm) | Heterogeneity |
| Septa | Mural nodularity, mass | Sludge (layering, mobile) |
| Branching structure | Mural calcification | Intracystic mass |
| Irregularity | Intracystic calcification |
Inflammatory Fluid Collections
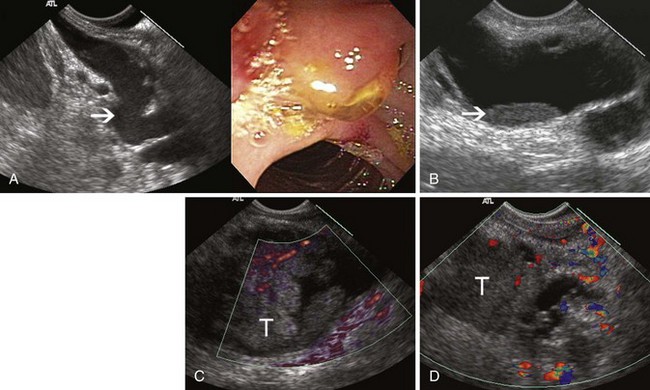
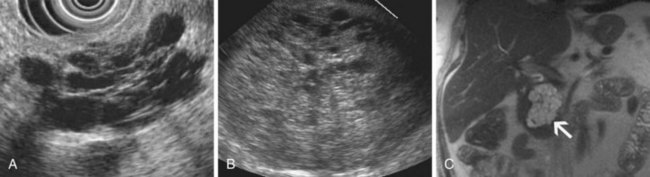
Inflammatory fluid collections arise as complications of pancreatitis. Pathologically, they are distinguished from cysts by the absence of an epithelial lining. Acute fluid collections are less than 4 weeks old and do not replace pancreatic parenchyma; they commonly occur after acute pancreatitis and usually resolve spontaneously. Pseudocysts develop from an acute fluid collection or arise as complications of chronic pancreatitis; they are more than 4 weeks old and have a discrete wall. Necrosis is a nonperfused area of pancreatic parenchyma; when a wall develops around the necrotic region, it may be termed “organized” or “walled-off” pancreatic necrosis. Acute fluid collections often have a complex, heterogeneous appearance on EUS and may appear solid. They may have ill-defined borders, but a developing wall may be seen at the periphery of the collection as it matures. Pseudocysts are usually unilocular and have a well-defined wall; the pseudocyst lumen may be anechoic or may contain layering echogenic material (Fig. 41.19). Necrosis contains variable amounts of echogenic material, which sometimes fills the entire lesion, giving a solid appearance on EUS (see Fig. 41.19). The distinction between a cystic neoplasm and an inflammatory collection may be difficult to make with certainty by EUS appearance alone. Mural calcification can be seen in both chronic pseudocysts and cystic neoplasms. Echogenic material in the cyst lumen may layer, suggesting sludge, but may also have the appearance of an intracystic mass.
Serous Cystic Neoplasm
Serous cystic neoplasms are cystic growths that likely arise from centroacinar cells. Most serous cystic neoplasms are serous cystadenomas, although a few serous cystadenocarcinomas have been reported in the medical literature. In contrast to mucinous cystic lesions and IPMNs, these tumors lack k-ras mutations and have very low malignant potential.93 Mutations of the von Hippel-Lindau (VHL) tumor suppressor gene have been implicated in their pathogenesis.100 Most serous cystadenomas are microcystic, although they may also be oligocystic, unilocular, or (uncommonly) solid. A fibrous capsule or wall is usually not present.
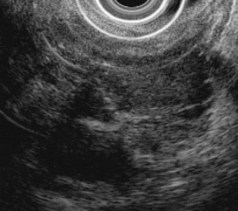
Microcystic serous cystadenoma has a characteristic ultrasound appearance (Fig. 41.20). The lesion is rounded and contains innumerable small cystic spaces, with the largest individual locule usually less than 2 cm in diameter. Central calcification may be seen in the lesion with a “sunburst” configuration. There is a discrete edge but typically without an identifiable wall. The absence of a wall is an important feature because cystic malignancies (e.g., colloid carcinoma and papillary and cystic neoplasm) may appear microcystic but often have a wall. In some portions of the lesion, the individual cysts are too small to resolve with EUS; in these areas, septa predominate, and the tissue has a relatively hypoechoic, solid appearance. Sometimes most of the lesion has this appearance, leading to misdiagnosis of a solid mass, but MRI can show the cystic nature of the lesion (see Fig. 41.20). Branch duct IPMNs may appear similar to oligocystic serous cystadenoma, and the two lesions can be confused with each other, especially when small. In contrast to the microcystic form, serous cystadenomas that are oligocystic or unilocular do not have ultrasound features that distinguish them from mucinous cystadenoma or inflammatory fluid collections.
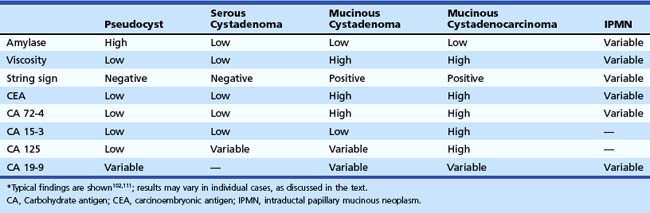
Fluid aspirated from a serous cystadenoma is typically clear and watery and does not form a string. Little or no fluid may be obtained when the microcystic form is aspirated. Amylase level, CEA, and other tumor marker levels in the fluid are low, even in the macrocystic form.101 CEA is low and is highly suggestive of serous cystadenoma or pseudocyst when less than 5 ng/mL.102 Cytology may show glycogen-containing cells but is often nondiagnostic.103
Mucinous Cystic Neoplasm
MCNs are cystic growths with well-recognized malignant potential. Histologically, they are characterized by the presence of ovarian stroma. Ninety percent of MCNs occur in women. They are usually oligocystic, although a single large cyst may dominate. MCNs may be benign (mucinous cystadenoma) or malignant (mucinous cystadenocarcinoma) or have borderline features (MCN with moderate dysplasia).93 Epidemiologic data suggest that these lesions begin as cystadenomas and grow slowly because patients with cystadenocarcinoma on average are 10 years older than patients with cystadenoma. In one large series, invasive carcinoma was present in only 18% of resected MCNs, and all such lesions were greater than 4 cm in diameter or had mural nodules.104
MCNs may appear oligocystic or unilocular on ultrasound (Fig. 41.21). When MCNs are oligocystic, some locules are generally greater than 2 cm in diameter. When the rim and septa of the lesion are thin and smooth, invasive malignancy is unlikely. Thickening, nodularity, or irregularity of these structures suggests the presence of mucinous cystadenocarcinoma (see Fig. 41.21), but these findings are nonspecific. Fluid aspirated from MCNs is often cloudy and tenacious, and a drop placed between two gloved fingers usually forms a string. Amylase level in the cystic fluid is usually low, and tumor marker levels in cystic fluid are elevated. CEA levels are generally elevated in fluid from MCNs.102
Intraductal Papillary Mucinous Neoplasm
Branch duct IPMNs may have the appearance of a cystic lesion on EUS. The lesion typically appears oligocystic and may be rounded. Clues to diagnosis include a branching architecture suggestive of a dilated ductal system and the presence of pancreatic tissue between the loculi of the lesion, rather than true septa. Careful examination may show the connecting duct leading from the lesion to the main pancreatic duct (see Fig. 41.18). These features are not always present, however. The Sendai criteria, which are consensus guidelines for resection of branch duct IPMNs, suggest that resection should be considered if one or more of the following criteria are present: cyst-related symptoms, main pancreatic duct diameter 10 mm or greater, cyst diameter 30 mm or greater, presence of intramural nodules, or cystic fluid cytology suspicious or positive for malignancy.105,106 Patients with branch duct IPMNs may be at increased risk for development of typical pancreatic adenocarcinoma separate from the cystic lesion,107 and the entire pancreas should be examined during follow-up examinations.
Other Cystic Lesions
Solid pancreatic adenocarcinomas may undergo cystic degeneration, perhaps secondary to necrosis and liquefaction of portions of the tumor. About 15% of pancreatic adenocarcinomas are colloid carcinomas containing gelatinous pools of extracellular mucin108; these lesions often arise from MCNs or IPMNs and may appear heterogeneous or cystic on EUS. Uncommon cystic neoplasms of the pancreas include cystic neuroendocrine tumors (see Fig. 41.16), acinar cell cystadenocarcinomas, and papillary and cystic tumors (Fig. 41.22). These lesions typically have a discrete, thickened wall and a complex internal architecture, raising concern for an invasive neoplasm. The EUS appearance of these lesions is not specific for diagnosis. Acinar cell cystadenocarcinoma may be associated with elevated serum alpha fetoprotein levels. Little information is available regarding the results of cystic fluid analysis in these less common cystic neoplasms. Among nonneoplastic cystic lesions, lymphangioma may be distinguished by the finding of milky white lymph fluid when the cyst is aspirated. The finding of squamous cells on cytology may suggest a lymphoepithelial cyst or teratoma.
Accuracy of Endoscopic Ultrasound for Differential Diagnosis
The ultrasound appearance of a pancreatic cystic lesion usually does not provide a specific diagnosis. Characteristic ultrasound features suggest a likely diagnosis when the classic features of microcystic serous cystadenoma or branch duct IPMN are seen. In oligocystic or unilocular lesions, however, ultrasound appearance alone is nonspecific for final diagnosis, and EUS appearance alone does not discriminate cysts of little or no malignant potential (pseudocysts and serous cystadenomas) from cysts with significant malignant potential over time (mucinous cystadenomas or branch duct IPMNs).109 In one large surgical series, only 3% of asymptomatic cysts less than 2 cm in size were malignant, but half of the lesions were premalignant (MCNs or IPMN).110 Follow-up is warranted in small, benign-appearing cystic lesions that are not resected.
Cystic fluid analysis may aid in the differential diagnosis of cystic lesions. Lesions containing thick mucinous fluid or numerous septa may be difficult to aspirate, with a plug of mucin or tissue filling the needle lumen. When sufficient fluid can be aspirated, multiple diagnostic studies should be obtained, including cytology, amylase, and CEA. Viscosity should also be assessed, either by measuring fluid viscosity or by placing a drop of fluid between two gloved fingers and determining if the fluid forms a string (the “string” sign). The typical results of fluid analysis for various cystic lesions are shown in Table 41.6. Routine cystic fluid analysis alone does not provide a confident diagnosis in many cases. Cytology is specific but insensitive for diagnosis of MCNs and IPMNs. A cystic fluid CEA value of greater than 800 mg/L is highly suggestive of a mucinous lesion, but only a few mucinous lesions show such high values102; an optimal cutoff of 192 ng/mL has an accuracy of 79% for diagnosis of a mucinous lesion, and extent of CEA elevation does not distinguish premalignant from malignant mucinous lesions.111 Because IPMNs arise from the pancreatic ductal system, they show variable results of fluid analysis depending in part on the ratio of normal pancreatic juice to mucin in the aspirated specimen. The absence of typical findings on fluid analysis does not exclude a mucinous lesion.
A promising alternative approach to cystic fluid analysis involves analysis of cystic fluid DNA, including the optical density of cystic fluid (said to be a measure of DNA concentration), the presence of k-ras mutations, the amplitude of loss of selected alleles, and the presumed acquisition sequence of selected mutations.112 In one more recent report, this approach distinguished mucinous from nonmucinous cysts and malignant from nonmalignant mucinous lesions with more accuracy than routine cytology and cystic fluid CEA concentration.112 Most malignant lesions in this study had other indicators of malignancy, including presence of a solid component in 75% and positive EUS-guided FNA cytology in 75%. The investigators suggested that DNA analysis should be considered when cystic fluid cytology is negative, but the performance characteristics of DNA analysis were not reported in this subset of all study patients. Additional studies are needed before cystic fluid DNA analysis can be recommended as part of the clinical evaluation of pancreas cystic lesions.
Interventional Endoscopic Ultrasound of the Biliary Tree and Pancreas
Many EUS-guided therapeutic interventions for treatment of biliary and pancreatic disease have been described. CPB and pseudocyst drainage were the earliest and are now widely practiced modalities. More recent interventions include drainage of inaccessible biliary or pancreatic ducts, ablation of pancreatic cystic neoplasms, injection therapy of pancreatic malignancies, drainage of abscesses, placement of fiducial markers in pancreatic masses, retrieval of migrated pancreatic stents, thrombosis of pancreatic pseudoaneurysms, and transduodenal gallbladder drainage. Educational materials are available that show detailed EUS techniques for some of these procedures.113 This section reviews selected interventions.
Celiac Plexus and Celiac Ganglion Block and Neurolysis
Treatment of pain by blockade or neurolysis of the celiac plexus has been performed for more than 100 years by percutaneous or surgical techniques. More recently, EUS-guided CPB or celiac plexus neurolysis (CPN) have been widely adopted. In both procedures, a local anesthetic is injected; for CPB, a steroid is usually added, and for CPN, ethanol is usually injected. Various injection methods and injectants may be used and are well summarized elsewhere.114,115
Traditional CPB and CPN procedures involve injections of the soft tissue surrounding the celiac axis, with diffusion of injected fluid into and around the celiac plexus; because celiac ganglia can be directly visualized by EUS in about 80% of persons,116 direct ganglion injection has also been described.117 Few randomized, controlled trials of CPB or CPN have been done. In one prospective, randomized, double-blind trial, percutaneous CPN was more effective than sham injections.118 Two randomized, prospective, nonblinded trials have compared EUS-guided CPB with percutaneous CPB, with both studies reporting better pain relief with the EUS approach.119,120 A more recent prospective, randomized trial comparing two EUS techniques (central vs. bilateral injections) reported no difference in pain relief.121
Common side effects of CPB and CPN include orthostatic hypotension and diarrhea. Complications are uncommon and include retroperitoneal abscess and adrenal artery laceration.122,123 Some experts administer prophylactic antibiotics before CPB. Spinal cord injury, with resultant paralysis and loss of bowel and bladder control, is a rare complication of CPB and CPN that probably occurs owing to spasm of a spinal artery. This complication has not been reported with EUS-guided approaches but probably rarely occurs with this modality as well. Direct injection of the celiac ganglia may theoretically decrease the risk of this complication.117
Drainage of Fluid Collections
Fluid collections are complications of pancreatitis and include acute fluid collections, pseudocysts, and walled-off necrosis. Endoscopic drainage is a preferred treatment modality for many of these collections, and a Seldinger technique for transgastric or transduodenal drainage is often preferred.124 EUS may be used to guide endoscopic drainage of a pancreatic fluid collection, and the technique has been described in detail elsewhere.113,125 Theoretical advantages of EUS guidance over standard endoscopic drainage include EUS examination of the lesion, possibly with FNA, to identify a cystic neoplasm mimicking a pseudocyst; avoidance of intervening vessels or varices that may lie in the path of planned transmural drainage; and better access to fluid collections that do not indent the lumen of the stomach or duodenum. In one large retrospective series, patients assigned to EUS-guided drainage because of a nonbulging collection or portal hypertension had similar outcomes to patients without these risk factors.126 In two prospective, randomized trials, overall success of drainage was significantly higher with an EUS-guided approach compared with conventional endoscopic drainage, largely because nonbulging collections could be drained with EUS.127,128
Endoscopic drainage of pancreatic necrosis has traditionally had lower overall success rates than drainage of pseudocysts owing to the presence of solid necrotic material in the collection and the tendency for infection to occur when the collection has been inadequately drained. The emerging technique of endoscopic necrosectomy seems to improve the overall success of endoscopic treatment. Bleeding from vessels in the gut wall or retroperitoneum is the most common complication of endoscopic necrosectomy.129 EUS miniprobes introduced into regions of pancreatic necrosis through a standard endoscope may identify vessels in the wall of the collection, theoretically decreasing the risk of vascular injury during endoscopic necrosectomy.
Ablation of Cystic Neoplasms
Cystic neoplasms of the pancreas are increasingly diagnosed, and some histologic types, including MCNs and branch duct IPMNs, have malignant potential. Standard therapy for these lesions is either observation or surgical resection. EUS-guided therapy of these lesions has been reported, including ethanol lavage of the cyst130 and ethanol lavage followed by instillation of paclitaxel gel.131 These therapies apparently result in decrease in the overall size of the cystic lesion; in one prospective study, ethanol lavage was superior to saline lavage.132 It is unclear, however, whether these therapies ablate all neoplastic epithelium in the lesions or decrease the risk of pancreatic carcinoma. Most series have not used a standardized follow-up imaging protocol optimized to detect small remnant cysts. At the present time, EUS-guided ablation of pancreatic cysts remains investigational.
Drainage of Biliary and Pancreatic Ducts
EUS can facilitate access to the biliary tree and pancreas and drainage of otherwise inaccessible ducts. EUS techniques may be used when ERCP fails or is unlikely to succeed (e.g., pancreatic duct access after a Whipple procedure). Techniques and instrumentation are evolving, and numerous procedural variations and technical subtleties are presented elsewhere.113 It is possible to obtain an EUS-guided cholangiogram or pancreatogram in most patients requiring an EUS approach.5,133,134 The goals of the procedure typically extend beyond ductal visualization, however, and injection of contrast material into an obstructed ductal system may be complicated by infection or leakage unless a drainage procedure is also performed. Passage of a guidewire through an EUS needle and across the site of ductal obstruction can be achieved in most patients.5,133,134 A “rendezvous” procedure may be performed by leaving the guidewire in place, removing the echoendoscope, and passing another endoscope to grasp the end of the guidewire in the gut lumen and place a stent across the ductal obstruction over the guidewire.5,133–135
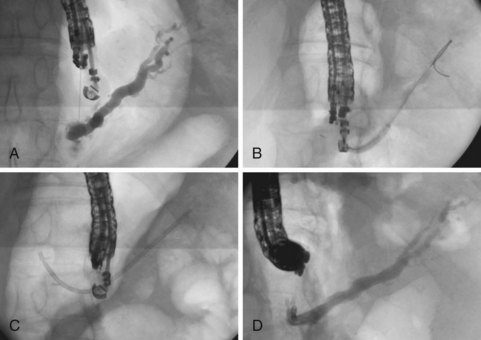
Alternatively, the drainage procedure may be performed through the echoendoscope, by dilation of the tract to the duct system over the guidewire and subsequent antegrade transgastric stent placement. If the site of ductal obstruction can be crossed with a guidewire, the stent may traverse the stomach wall, pancreas, and ductal obstruction, with the far end of the stent residing in the small bowel. If the site of ductal obstruction cannot be crossed with a guidewire, a stent may still be placed into the obstructed ductal system through the echoendoscope, accomplishing transgastric drainage (Fig. 41.23).5 In patients with pancreatic duct obstruction after a Whipple procedure, placement of a transgastric stent that traverses the stomach wall and pancreas may facilitate future ductal access and stent exchange via the transgastric tract. Complications are frequent after EUS-guided ductal drainage procedures and include abdominal pain requiring hospitalization, infectious complications, and bleeding.5,133,134 Urgent percutaneous or surgical procedures may be required if successful ductal drainage is not achieved. At the present time, these procedures are best reserved for patients whose clinical condition mandates ductal drainage, in whom other modalities (e.g., ERCP, percutaneous drainage, or surgery) are unfavorable.
1 Erickson RA, Garza AA. EUS with EUS-guided fine-needle aspiration as the first endoscopic test for evaluation of obstructive jaundice. Gastrointest Endosc. 2001;53:475-484.
2 Songur Y, Temucin G, Sahin B. Endoscopic ultrasonography in the evaluation of dilated common bile duct. J Clin Gastroenterol. 2001;33:302-305.
3 Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol. 2001;96:705-709.
4 Michaels A, Draganov P. Endoscopic ultrasonography guided celiac plexus neurolysis and celiac plexus block in the management of pain due to pancreatic cancer and chronic pancreatitis. World J Gastroenterol. 2007;13:3575-3580.
5 Kahaleh M, Hernandez A, Tokar J, et al. EUS-guided pancreaticogastrostomy: Analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest Endosc. 2006;65:224-230.
6 Dill JE, Hill S, Callis J, et al. Combined endoscopic ultrasound and stimulated biliary drainage in the diagnosis of cholecystitis and microlithiasis [letter]. Endoscopy. 1995;27:218.
7 Dahan P, Andant C, Levy P, et al. Prospective evaluation of endoscopic ultrasonography and microscopic examination of duodenal bile in the diagnosis of cholecystolithiasis in 45 patients with normal conventional ultrasonography. Gut. 1996;38:277-281.
8 Choi WB, Lee SK, Kim MH, et al. A new strategy to predict the neoplastic polyps of the gallbladder based on a scoring system using EUS. Gastrointest Endosc. 2000;52:372-379.
9 Azuma T, Yoshikawa T, Araida T, et al. Differential diagnosis of polypoid lesions of the gallbladder by endoscopic ultrasonography. Am J Surg. 2001;181:65-70.
10 Sugiyama M, Atomi Y, Yamato T. Endoscopic ultrasonography for differential diagnosis of polypoid gall bladder lesions: Analysis in surgical and follow up series. Gut. 2000;46:250-254.
11 Cho J, Park J, Kim Y, et al. Hypoechoic foci on EUS are simple and strong predictive factors for neoplastic gallbladder polyps. Gastrointest Endosc. 2009;69:1244-1250.
12 Akatsu T, Aiura K, Shimazu M, et al. Can endoscopic ultrasonography differentiate nonneoplastic from neoplastic gallbladder polyps? Dig Dis Sci. 2006;51:416-421.
13 Mizuguchi M, Kudo S, Fukahori T, et al. Endoscopic ultrasonography for demonstrating loss of multiple-layer pattern of the thickened gallbladder wall in the preoperative diagnosis of gallbladder cancer. Eur Radiol. 1997;7:1323-1327.
14 Prat F, Amouyal G, Amouyal P, et al. Prospective controlled study of endoscopic ultrasonography and endoscopic retrograde cholangiography in patients with suspected common-bile duct liithiasis. Lancet. 1996;347:75-79.
15 Kohut M, Nowakowska-Dulawa E, Marek T, et al. Accuracy of linear endoscopic ultrasonography in the evaluation of patients with suspected common bile duct stones. Endoscopy. 2002;34:299-303.
16 Verma D, Kapadia A, Eisen G. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc. 2006;64:248-254.
17 Savides T. EUS-guided ERCP for patients with intermediate probability for choledocholithiasis: Is it time for all of us to start doing this? Gastrointest Endosc. 2008;67:669-672.
18 Berdah SV, Orsoni P, Bege T, et al. Follow-up of selective endoscopic ultrasonography and/or endoscopic retrograde cholangiography prior to laparoscopic cholecystectomy: A prospective study of 300 patients. Endoscopy. 2001;33:216-220.
19 Polkowski M, Regula J, Tilszer A. Endoscopic ultrasound versus endoscopic retrograde cholangiography for patients with intermediate probability of bile duct stones: A randomized trial comparing two management strategies. Endoscopy. 2007;39:296-303.
20 Karakan T, Cindoruk M, Alagozlu H, et al. EUS versus endoscopic retrograde cholangiography for patients with intermediate probability of bile duct stones: A prospective randomized trial. Gastrointest Endosc. 2009;69:244-252.
21 Lee Y, Chan F, Leung W. Comparison of EUS and ERCP in the investigation with suspected biliary obstruction caused by choledocholithiasis: A randomized study. Gastrointest Endosc. 2008;67:660-668.
22 Liu C, Fan S, Lo C. Comparison of early endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in the management of acute biliary pancreatitis: A prospective randomized study. Clin Gastroenterol Hepatol. 2005;3:1238-1244.
23 Ueno N, Nishizono M, Tamada K, et al. Diagnosing extrahepatic bile duct stones using intraductal ultrasonography: A case series. Endoscopy. 1997;29:356-360.
24 Catanzaro A, Pfau P, Isenberg GA, et al. Clinical utility of intraductal US for evaluation of choledocholithiasis. Gastrointest Endosc. 2003;57:648-652.
25 Haber GB. Is seeing believing? Gastrointest Endosc. 2003;57:712-714.
26 Frossard JL, Hadengue A, Amouyal G, et al. Choledocholithiasis: A prospective study of spontaneous common bile duct stone migration. Gastrointest Endosc. 2000;51:175-179.
27 Artifon E, Kumar A, Eloubeidi M, et al. Prospective randomized trial of EUS versus ERCP-guided common bile duct stone removal: An interim report (with video). Gastrointest Endosc. 2009;69:238-243.
28 Fujita N, Noda Y, Kobayashi G, et al. Staging of bile duct carcinoma by EUS and IDUS. Endoscopy. 1998;30:A132-A134.
29 Tamada K, Tomiyama T, Oohashi A, et al. Bile duct wall thickness measured by intraductal US in patients who have not undergone previous biliary drainage. Gastrointest Endosc. 1999;48:199-203.
30 Tamada K, Tomiyama T, Ichiyama M, et al. Influence of biliary drainage catheter on bile duct wall thickness as measured by intraductal ultrasonography. Gastrointest Endosc. 1998;47:28-33.
31 Fritscher-Ravens A, Broering DC, Sriram PVJ, et al. EUS-guided fine-needle aspiration cytodiagnosis of hilar cholangiocarcinoma: A case. Gastrointest Endosc. 2000;52:534-540.
32 Inui K, Nakazawa S, Yoshino J, et al. Ultrasound probes for biliary lesions. Endoscopy. 1998;390:A120-A123.
33 Domagk D, Poremba C, Dietl KH, et al. Endoscopic transpapillary biopsies and intraductal ultrasonography in the diagnostics of bile duct strictures: A prospective study. Gut. 2002;51:240-244.
34 Lee JH, Salem R, Aslanian H, et al. Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am J Gastroenterol. 2004;99:1069-1073.
35 Vazquez-Sequeiros E, Baron TH, Clain JE, et al. Evaluation of indeterminate bile duct strictures. Gastrointest Endosc. 2002;53:372-379.
36 Levy M, Baron T, Clayton A, et al. Prospective evaluation of advanced molecular markers and imaging techniques in patients with indeterminate bile duct strictures. Am J Gastroenterol. 2008;103:1263-1273.
37 Tamada K, Ueno N, Tomiyama T, et al. Characterization of biliary strictures using intraductal ultrasonography: Comparison with percutaneous cholangioscopic biopsy. Gastrointest Endosc. 1998;47:341-349.
38 Tamada K, Kanai N, Wada S, et al. Utility and limitations of intraductal ultrasonography in distinguishing longitudinal cancer extension along the bile duct from inflammatory wall thickening. Abdom Imaging. 2001;26:623-631.
39 Hyodo T, Hyodo N, Yamanaka T, et al. Contrast-enhanced intraductal ultrasonography for thickened bile duct wall. J Gastroenterol. 2001;36:557-559.
40 Wehrmann T, Stergiou N, Riphaus A, et al. Correlation between sphincter of Oddi manometry and intraductal ultrasound morphology in patients with suspected sphincter of Oddi dysfunction. Endoscopy. 2001;33:773-777.
41 Cannon ME, Carpenter SL, Elta GH, et al. EUS compared with CT, magnetic resonance imaging, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest Endosc. 1999;50:27-33.
42 Menzel J, Hoepffner N, Sulkowski U, et al. Polypoid tumors of the major duodenal papilla: Preoperative staging with intraductal US, EUS, and CT—a prospective, histopathologically controlled study. Gastrointest Endosc. 1999;49:349-357.
43 Skordilis P, Mouzas IA, Dimoulios PD, et al. Is endosonography an effective method for detection and local staging of the ampullary carcinoma? A prospective study. BMC Surg. 2002;2:1-8.
44 Chak A, Hawes RH, Cooper GS, et al. Prospective assessment of the utility of EUS in the evaluation of gallstone pancreatitis. Gastrointest Endosc. 1999;49:599-604.
45 Prat F, Edery J, Meduri B, et al. Early EUS of the bile duct before endoscopic sphincterotomy for acute biliary pancreatitis. Gastrointest Endosc. 2001;54:724-729.
46 Coyle WJ, Pineau BC, Tarnasky PR, et al. Evaluation of unexplained acute and acute recurrent pancreatitis using endoscopic retrograde cholangiopancreatography, sphincter of Oddi manometry and endoscopic ultrasound. Endoscopy. 2002;34:617-623.
47 Norton SA, Alderson D. Endoscopic ultrasonography in the evaluation of idiopathic acute pancreatitis. Br J Surg. 2000;87:1650-1655.
48 Mariani A, Arcidiacono P, Curioni S, et al. Diagnostic yield of ERCP and secretin-enhanced MRCP and EUS in patients with acute recurrent pancreatitis of unknown aetiology. Dig Liver Dis. 2009;41:753-758.
49 The International Working Group for Minimal Standard Terminology in Gastrointestinal Endosonography. Minimal standard terminology in gastrointestinal endosonography. Dig Endosc. 1998;10:159-184.
50 Wallace MB, Hawes RH, Durkalski V, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: Interobserver agreement among experienced endosonographers. Gastrointest Endosc. 2001;53:294-299.
51 Catalano M, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: The Rosemont classification. Gastrointest Endosc. 2009;69:1251-1261.
52 Stevens T, Lopez R, Adler D, et al. Multicenter comparison of the interobserver agreement of standard endoscopic ultrasound scoring and Rosemont classification for diagnosis of chronic pancreatitis. Gastrointest Endosc. 2010;71:519-526.
53 Wiersema MJ, Hawes RH, Lehman GA, et al. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy. 1993;25:555-564.
54 Sahai AV, Zimmerman M, Aabakken L, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48:18-25.
55 Catalano MF, Lahoti S, Geenen JE, et al. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and Secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc. 1998;48:11-17.
56 Brune K, Abe T, Canto M, et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol. 2006;30:1067-1076.
57 Canto M, Goggins M, Hruban R, et al. Screening for early pancreatic neoplasia in high-risk individuals: A prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766-781.
58 Kimmey MB, Bronner MP, Byrd DR, et al. Screening and surveillance for hereditary pancreatic cancer. Gastrointest Endosc. 2002;56:S82-S86.
59 Bhutani MS. Endoscopic ultrasonography: Changes of chronic pancreatitis in asymptomatic and symptomatic alcoholic patients. J Ultrasound Med. 1999;18:455-462.
60 Sahai AV, Mishra G, Penman ID, et al. EUS to detect evidence of pancreatic disease in patients with persistent or nonspecific dyspepsia. Gastrointest Endosc. 2000;52:153-159.
61 Martin E, Bedossa P. Diffuse fibrosis of the pancreas: A peculiar pattern of pancreatitis in alcoholic cirrhosis. Gastroenterol Clin Biol. 1989;13:579-584.
62 Pitchumoni CS, Glasser M, Saran RM, et al. Pancreatic fibrosis in chronic alcoholics and nonalcoholics without clinical pancreatitis. Am J Gastroenterol. 1984;79:382-388.
63 Yusoff I, Sahai A. A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin Gastroenterol Hepatol. 2004;2:405-409.
64 Hollerbach S, Klamann A, Topalidis T, et al. Endoscopic ultrasonography and fine-needle aspiration cytology for diagnosis of chronic pancreatitis. Endoscopy. 2001;33:824-831.
65 Hastier P, Buckley M, Dumas R, et al. A study of the effect of age on pancreatic duct morphology. Gastrointest Endosc. 1998;48:53-57.
66 Chong A, Hawes R, Hoffman B, et al. Diagnostic performance of EUS for chronic pancreatitis: A comparison with histopathology. Gastrointest Endosc. 2007;65:808-814.
67 DeWitt J, McGreevy K, LeBlanc J, et al. EUS-guided Trucut biopsy of suspected nonfocal chronic pancreatitis. Gastrointest Endosc. 2005;62:76-84.
68 Chowdhury R, Bhutani M, Mishra G, et al. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas. 2005;31:63-68.
69 Stevens T, Dumot J, Zuccaro GJr, et al. Evaluation of duct-cell and acinar-cell function and endosonographic abnormalities in patients with suspected chronic pancreatitis. Clin Gastroenterol Hepatol. 2009;7:114-119.
70 Topazian M: Endoscopic ultrasound of the pancreas . American Society for Gastrointestinal Endoscopy Learning Center and Video Library, 2004.
71 Yasuda K, Mukai H, Fugimoto S, et al. The diagnosis of pancreatic cancer by endoscopic ultrasonography. Gastrointest Endosc. 1988;34:1-8.
72 Norton ID, Zheng Y, Wiersema MJ, et al. Neural network analysis of EUS images to differentiate between pancreatic malignancy and pancreatitis. Gastrointest Endosc. 2001;54:625-629.
73 Hocke M, Schulze E, Gottschalk P, et al. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol. 2006;12:246-250.
74 Becker D, Strobel D, Bernatik T, et al. Echo-enhanced color- and power-Doppler EUS for the discrimination between focal pancreatitis and pancreatic carcinoma. Gastrointest Endosc. 2001;53:784-789.
75 Hirche T, Ignee A, Barreiros A, et al. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy. 2008;40:910-917.
76 Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: A large single center experience. Gut. 1999;44:720-726.
77 Voss M, Hammel P, Molas G, et al. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244-249.
78 Khalid A, Nodit L, Zahid M, et al. Endoscopic ultrasound fine needle aspirate DNA analysis to differentiate malignant and benign pancreatic masses. Am J Gastroenterol. 2006;101:2493-2500.
79 Salek C, Benesova L, Zavoral M, et al. Evaluation of clinical relevance of examining K-ras, p16 and p53 mutations along with allelic losses at 9p and 18q in EUS-guided fine needle aspiration samples of patients with chronic pancreatitis and pancreatic cancer. World J Gastroenterol. 2007;13:3714-3720.
80 Jemaa Y, Houissa F, Trabelsi S, et al. Endoscopic ultrasonography versus helical CT in diagnosis and staging of pancreatic cancer. Tunis Med. 2008;86:346-349.
81 Hunt GC, Faigel DO. Assessment of EUS for diagnosing, staging, and determining the resectability of pancreatic cancer: A review. Gastrointest Endosc. 2002;55:232-237.
82 Dewitt J, Ciaccia D, Leblanc J, et al. Prospective comparison of endoscopic ultrasound (EUS) and dual-phase helical computed tomography (DPHCT) for the preoperative evaluation of known or suspected pancreatic malignancy: Final results. Gastrointest Endosc. 2003;57:AB98.
83 Bold RJ, Charnsangavej C, Cleary K, et al. Major vascular resection as part of pancreaticoduodenectomy for cancer: Radiologic, intraoperative and pathologic analysis. J Gastrointest Surg. 1999;3:233-243.
84 Leach SD, Lee JE, Charnsangavej C, et al. Survival following pancreaticoduodenectomy with resection of the superior mesenteric-portal vein confluence for adenocarcinoma of the pancreatic head. Br J Surg. 1998;85:611-617.
85 Aslanian H, Salem R, Lee J, et al. EUS criteria for vascular involvement by pancreatic cancer: Comparison with intra-operative venous resection. Am J Gastroenterol. 2005;100:1381-1385.
86 Rosch T, Dittler HJ, Strobel K, et al. Endoscopic ultrasound criteria for vascular invasion in the staging of cancer of the head of the pancreas: A blind reevaluation of videotapes. Gastrointest Endosc. 2000;52:469-477.
87 Ahmad NA, Lewis JD, Siegelman ES, et al. Role of endoscopic ultrasound and magnetic resonance imaging in the preoperative staging of pancreatic adenocarcinoma. Am J Gastroenterol. 2000;95:1926-1931.
88 Greene FL. Exocrine pancreas. In AJCC cancer staging manual, ed 6, New York: Springer-Verlag; 2002:179-187.
89 Hahn M, Faigel D. Frequency of mediastinal lymph node metastases in patients undergoing EUS evaluation of pancreaticobiliary masses. Gastrointest Endosc. 2001;54:331-335.
90 Fritscher-Ravens A, Brand L, Knöfel W, et al. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol. 2002;97:2768-2775.
91 Chari S, Takahashi N, Levy M, et al. A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7:1097-1103.
92 Varadarajulu S, Tamhane A, Eloubeidi M. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728-736.
93 Compton C. Histology of cystic tumors of the pancreas. Gastrointest Endosc Clin N Am. 2002;12:673-696.
94 Terris B, Ponsot P, Paye F, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372-1377.
95 Matsumoto T, Aramaki M, Yada K, et al. Optimal management of the branch duct type intraductal papillary mucinous neoplasm of the pancreas. J Clin Gastroenterol. 2003;36:261-265.
96 Kobari M, Egawa S, Shibuya K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: Differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131-1136.
97 Bernard P, Scoazec J, Joubert M, et al. Intraductal papillary-mucinous tumors of the pancreas: Predictive criteria of malignancy according to pathological examination of 53 cases. Arch Surg. 2002;137:1274-1278.
98 Yamaguchi K, Sugitani A, Chijiiwa K, et al. Intraductal papillary-mucinous tumor of the pancreas: Assessing the grade of malignancy from natural history. Am Surg. 2001;67:400-406.
99 Das A, Wells C, Nguyen CC. Incidental cystic neoplasms of pancreas: What is the optimal interval of imaging surveillance? Am J Gastroenterol. 2008;103:1657-1662.
100 Vortmeyer A, Lubensky I, Fogt F, et al. Allelic deletion and mutation of the von Hippel-Lindau (VHL) tumor suppressor gene in pancreatic microcystic adenomas. Am J Pathol. 1997;151:951-956.
101 Chatelain D, Hammel P, O’Toole D, et al. Macrocystic form of serous pancreatic cystadenoma. Am J Gastroenterol. 2002;97:2566-2571.
102 van der Waaij L, van Dullemen H, Porte R. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: A pooled analysis. Gastrointest Endosc. 2005;62:383-389.
103 Centeno B. Role of cytology in the diagnosis of cystic and intraductal papillary mucinous neoplasms. Gastrointest Endosc Clin N Am. 2002;12:697-708.
104 Crippa S, Salvia R, Warshaw A, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: Lessons from 163 resected patients. Ann Surg. 2008;247:580-582.
105 Pelaez-Luna M, Chari S, Smyrk T, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102:1759-1764.
106 Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32.
107 Uehara H, Nakaizumi A, Ishikawa O, et al. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut. 2008;57:1561-1565.
108 Adsay N, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26-42.
109 Ahmad N, Kochman M, Brensinger C, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc. 2003;58:59-64.
110 Fernández-del Castillo C, Targarona J, Thayer S, et al. Incidental pancreatic cysts: Clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427-430.
111 Brugge W, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336.
112 Khalid A, Zahid M, Finkelstein S, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: A report of the PANDA study. Gastrointest Endosc. 2009;69:1095-1102.
113 Levy M, Topazian M: Therapeutic EUS . American Society for Gastrointestinal Endoscopy Learning Center and Video Library, 2009.
114 Penman I, Rösch T, Group EW. EUS 2008 Working Group document: Evaluation of EUS-guided celiac plexus neurolysis/block (with video). Gastrointest Endosc. 2009;69:S28-S31.
115 Levy M, Wiersema M. EUS-guided celiac plexus neurolysis and celiac plexus block. Gastrointest Endosc. 2003;57:923-930.
116 Gleeson F, Levy M, Papachristou G, et al. Frequency of visualization of presumed celiac ganglia by endoscopic ultrasound. Endoscopy. 2007;39:620-624.
117 Levy M, Topazian M, Wiersema M, et al. Initial evaluation of the efficacy and safety of endoscopic ultrasound-guided direct ganglia neurolysis and block. Am J Gastroenterol. 2008;103:98-103.
118 Wong G, Schroeder D, Carns P, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: A randomized controlled trial. JAMA. 2004;291:1092-1099.
119 Santosh D, Lakhtakia S, Gupta R, et al. Clinical trial: A randomized trial comparing fluoroscopy guided percutaneous technique vs. endoscopic ultrasound guided technique of coeliac plexus block for treatment of pain in chronic pancreatitis. Aliment Pharmacol Ther. 2009;29:979-984.
120 Gress F, Schmitt C, Sherman S, et al. A prospective randomized comparison of endoscopic ultrasound- and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroenterol. 1999;94:900-905.
121 LeBlanc J, DeWitt J, Johnson C, et al. A prospective randomized trial of 1 versus 2 injections during EUS-guided celiac plexus block for chronic pancreatitis pain. Gastrointest Endosc. 2009;69:835-842.
122 Sahai A, Lemelin V, Lam E, et al. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: A comparative study of short-term effectiveness. Am J Gastroenterol. 2009;104:326-329.
123 O’Toole T, Schmulewitz N. Complication rates of EUS-guided celiac plexus blockade and neurolysis: Results of a large case series. Endoscopy. 2009;41:593-597.
124 Mönkemüller K, Baron T, Morgan D. Transmural drainage of pancreatic fluid collections without electrocautery using the Seldinger technique. Gastrointest Endosc. 1998;48:195-200.
125 Varadarajulu S. EUS followed by endoscopic pancreatic pseudocyst drainage or all-in-one procedure: A review of basic techniques (with video). Gastrointest Endosc. 2009;69:S176-S181.
126 Kahaleh M, Shami V, Conaway M, et al. Endoscopic ultrasound drainage of pancreatic pseudocyst: A prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355-359.
127 Varadarajulu S, Christein J, Tamhane A, et al. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2008;68:1102-1111.
128 Park D, Lee S, Moon S, et al. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: A prospective randomized trial. Endoscopy. 2009;41:842-848.
129 Gardner T, Chahal P, Papachristou G, et al. A comparison of direct endoscopic necrosectomy with transmural endoscopic drainage for the treatment of walled-off pancreatic necrosis. Gastrointest Endosc. 2009;69:1085-1094.
130 Gan S, Thompson C, Lauwers G, et al. Ethanol lavage of pancreatic cystic lesions: Initial pilot study. Gastrointest Endosc. 2005;61:746-752.
131 Oh H, Seo D, Lee T, et al. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc. 2008;67:636-642.
132 DeWitt J, McGreevy K, Schmidt C, et al. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: A randomized, double-blind study. Gastrointest Endosc. 2009;70:710-723.
133 Will U, Fueldner F, Thieme A, et al. Transgastric pancreatography and EUS-guided drainage of the pancreatic duct. J Hepatobiliary Pancreat Surg. 2007;14:377-382.
134 Kahaleh M, Hernandez A, Tokar J, et al. Interventional EUS-guided cholangiography: Evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52-59.
135 Larghi A, Lecca P, Mutignani M, et al. EUS-directed transpapillary self-expandable metallic stent placement after successful interventional EUS-guided cholangiography. Gastrointest Endosc. 2008;67:996-998.