Chapter 42 Endoscopic Ultrasound–Guided Fine Needle Aspiration of Pancreaticobiliary Lesions
Endoscopic Ultrasound–Guided Fine Needle Aspiration in the Diagnosis of Pancreatic Tumors
Pancreatic Cancer
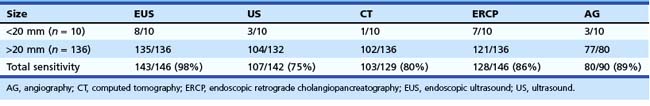
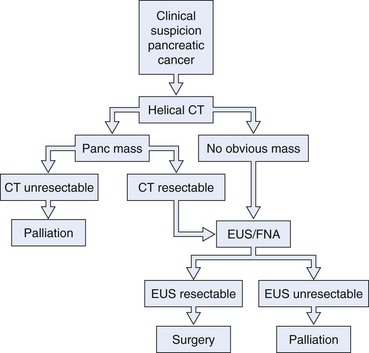
EUS is considered one of the most useful diagnostic procedures among the body imaging tools for detecting pancreatic cancer. EUS was shown to be superior (sensitivity of 98%) to other imaging modalities, including computed tomography (CT), in 146 patients with pancreatic cancer (Table 42.1).1 With the more recent introduction of spiral CT with dual-phase contrast, the detection rate for CT is improving. However, more recent comparisons between dual-phase spiral CT and EUS still favor EUS. The ability to obtain cytologic specimen by EUS-guided FNA has greatly aided in differentiating benign versus malignant lesions seen on EUS alone.
The application of EUS-guided FNA to the pancreas in particular has great clinical utility. CT-guided and ultrasound-guided percutaneous FNA have previously been the most commonly used methods for diagnosing pancreatic cancer. The sensitivity of percutaneous FNA ranges from 45% to 100%, with a specificity of up to 100%. However, obtaining a tissue diagnosis with CT or ultrasound guidance is limited by the ability to visualize the lesion. In our previous multicenter trial, 56% of patients with pancreatic carcinoma had CT scans that did not show a mass or revealed nonspecific enlargement of the pancreas.2 Endoscopic retrograde cholangiopancreatography (ERCP) with cytologic brushing also has historically had a relatively low yield, with sensitivities between 30% and 56%. The overall sensitivity, specificity, diagnostic accuracy, negative predictive value, and positive predictive value of EUS-guided FNA for pancreatic cancer in this study were 83%, 90%, 85%, 80%, and 100%. These values were superior to CT alone (without FNA): 56%, 37%, 50%, 28%, and 65% (P < .05). There were four complications in 164 patients (2%), including two major (perforation, bleeding) and two minor (fever) complications. Comparison among the four centers showed that institutions in which a cytologist was present during the procedure had a significantly higher cytologic yield, sensitivity, and diagnostic accuracy.
Advantages of EUS-guided FNA include procuring a tissue diagnosis while obtaining additional tumor and nodal staging information, the avoidance of additional diagnostic testing or surgery, and the prognostic information gained from the staging information (Figs. 42.1 and 42.4). Another report from a large single-institution study of 144 pancreatic lesions undergoing EUS-guided FNA showed sensitivity, specificity, and diagnostic accuracy of 82%, 100%, and 85%.3 More recently, helical or spiral CT has improved imaging of the pancreas. However, preliminary studies still show superiority of EUS compared with spiral CT.4
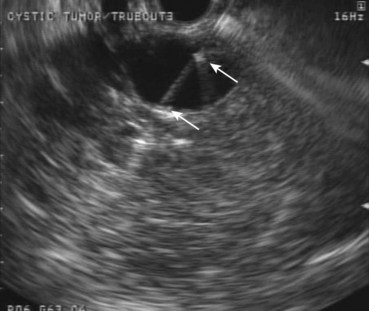
Fig. 42.3 Quick-Core (Cook Medical, Orange, CA) 19-gauge needle used to evaluate a pancreatic cyst.
(Courtesy of Cook Medical, Orange, CA.)
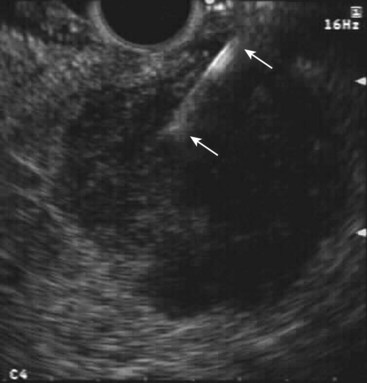
Fig. 42.4 Fine needle aspiration (FNA) (25-gauge) of metastatic liver lesion from pancreatic cancer.
Despite these data, EUS and EUS-guided FNA still possess limitations. The most difficult diagnostic problem for any imaging test, including EUS-guided FNA, is the differentiation between pancreatic carcinoma and chronic pancreatitis. Although a positive FNA is almost 100% accurate, a negative FNA is only about 80% accurate. A multicenter, retrospective study of 20 cancers missed on EUS by nine experienced endosonographers found that although 60% (12 cases) of the cancers were missed because of underlying chronic pancreatitis, other factors associated with missed lesions included a diffusely infiltrating carcinoma (3 cases), a prominent ventral or dorsal split (2 cases), and a recent episode (<4 weeks) of acute pancreatitis (1 case).5 A study of 116 pancreatic malignancies identified in patients with and without chronic pancreatitis found a sensitivity of EUS-guided FNA for malignancy of 89% in patients without chronic pancreatitis compared with 54% in patients with chronic pancreatitis.6 An additional study of 282 patients with pancreatic mass lesions undergoing 300 EUS-guided FNA procedures also found reduced sensitivity in patients with chronic pancreatitis compared with patients without chronic pancreatitis (73.9% vs. 91.3%).7 However, it was thought that this reduced sensitivity could be overcome with an increased number of FNA passes (median of five passes in patients with chronic pancreatitis patients vs. two passes in patients without chronic pancreatitis). An additional prospective study aiming to assess the number of passes to perform to maximize cytologic yield suggested that seven passes be made to optimize yield in pancreatic and miscellaneous (non–lymph node) lesions.8
These data have significantly affected the clinical algorithm of patients with known or suspected pancreatic malignancies. A clinical outcomes study was performed at a single center comparing the management and survival of 136 patients with pancreatic cancer between the pre-EUS and post-EUS eras.9 EUS detected carcinomas that were either not seen or only possibly seen by CT in 34% of patients, and there were 75% fewer required operations for diagnosis. The median survival without liver metastases was also longer during the EUS period (102 days vs. 205 days; P < .02, log-rank test), probably secondary to lead-time bias.
We believe that all patients thought to have operable disease based on initial CT imaging should undergo EUS with or without FNA before surgical intervention (Fig. 42.5). Considering the possibility of a false-negative result (up to 20%, especially in the setting of chronic pancreatitis), we also believe that surgical intervention should not be precluded in a patient with a high suspicion of resectable pancreatic carcinoma and a negative FNA cytology. EUS-guided FNA of pancreatic lesions is also worthwhile in patients with a prior negative tissue diagnosis by ERCP or CT of the abdomen. Gress and colleagues10 reported their experience with EUS-guided FNA of pancreatic mass lesions in 102 patients who had negative cytologic tissue diagnosis by ERCP sampling or CT-guided FNA. Among their patients, 57 of the 61 patients (93.4%) with a final diagnosis of pancreatic cancer had positive cytology results for adenocarcinoma by EUS-guided FNA. The false-positive results were zero.
The resulting changes in clinical algorithms can result in significant economic savings. In an earlier report, we reviewed a series of 44 consecutive patients who underwent EUS with or without FNA as part of their pancreatic cancer evaluation.11 Surgery and further diagnostic testing were avoided in 41% and 57% of patients. A substantial cost saving of $3300 per patient was calculated. In a series of 216 consecutive patients, Erickson and Garza12 studied the use of EUS with EUS-guided FNA as the initial approach to patients with obstructive jaundice. EUS and EUS-guided FNA not only proved useful as a diagnostic and staging modality but also served in directing the need for subsequent therapeutic ERCP, saving approximately $1007 to $1313 per patient. In addition, if EUS and EUS-guided FNA were not used at all, an extra $2200 would be spent per patient. In contrast to CT-guided FNA, EUS-guided FNA of the pancreas can be performed during the initial EUS procedure. The overall complication rate of EUS-guided FNA was reported to be 0.5% to 2.9%. An additional study comparing ERCP with brushing with EUS-guided FNA, laparoscopic biopsy, and CT-guided or ultrasound-guided FNA found that EUS-guided FNA is the best and most cost-effective initial method and the preferred secondary alternative method for the diagnosis of suspected pancreatic cancer.13
Several case reports have described malignant seeding of the needle tract after transcutaneous FNA. However, the true incidence has yet to be established. Theoretically, EUS-guided FNA of pancreatic cancers should have a lower chance of malignant seeding because of the short needle tract. A retrospective study comparing 46 patients diagnosed with pancreatic cancer via EUS-guided FNA with 43 patients diagnosed via CT-guided FNA seemed to confirm this hypothesis.14 All patients underwent neoadjuvant therapy, followed by restaging CT and attempt at surgical resection if disease progression was absent. Despite no differences in tumor characteristics between the groups, the frequency of peritoneal metastases was 2.2% in the EUS group compared with 16.3% in the CT group (P < .025).
Cystic Neoplasms
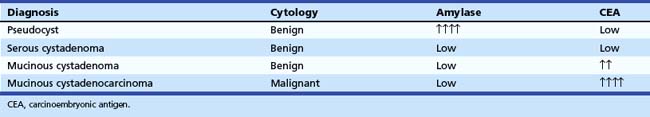
EUS can be helpful in distinguishing cystic neoplasms from pancreatic pseudocysts, although the specificity is not perfect.15 The more problematic discernment is between serous and mucinous cysts, with the latter considered premalignant. The interobserver agreement for the interpretation of cystic lesions in the pancreas is quite low. The interobserver agreement on 31 pancreatic cyst cases among eight expert endosonographers was shown to be “fair” between endosonographers for diagnosis of neoplastic versus nonneoplastic lesions (κ = 0.24).16 Agreement for individual types of lesions was moderately good for serous cystadenomas (κ = 0.46) but fair for the remainder. Accuracy rates of EUS for the diagnosis of neoplastic versus nonneoplastic lesions ranged from 40% to 93%.
EUS imaging alone is often inadequate for the clinical management of these patients. EUS-guided FNA of cystic contents can be analyzed for cytology, biochemistry, and tumor markers to aid in cyst classification. Because cytology is a relatively insensitive test, cyst fluid tumor markers such as carcinoembryonic antigen (CEA) have been employed to improve the sensitivity for the detection of malignancy. Cyst fluid CEA values are uniformly low in serous cystadenomas, higher in mucinous lesions, and markedly elevated in mucinous cystadenocarcinomas.17 A multicenter European study reported a series of 67 patients who underwent EUS-guided FNA of pancreatic cysts and subsequently underwent surgery.18 EUS alone (no FNA) correctly identified 49 cases (73%), whereas FNA correctly identified 65 cases (97%). Sensitivity, specificity, positive predictive value, and negative predictive value of EUS and EUS-guided FNA to indicate whether a lesion needed further surgery were 71% and 97%, 30% and 100%, 49% and 100%, and 40% and 95%. A value of the tumor marker for colorectal and pancreatic carcinomas (CA 19.9) greater than 50,000 U/mL had 15% sensitivity and 81% specificity to distinguish mucinous cysts from other cystic lesions and 86% sensitivity and 85% specificity to distinguish cystadenocarcinoma from other cystic lesions.
The most definitive data on cyst fluid analysis come from the Cooperative Pancreatic Cyst study, a multicenter U.S. trial that assessed 341 patients undergoing EUS-guided FNA of a pancreatic cystic lesion.19 Surgical resection was performed in 112 patients, with a final histologic diagnosis of the cysts as follows: 68 mucinous, 7 serous, 27 inflammatory, 5 endocrine, and 5 other. The results of EUS, cyst fluid cytology, and cyst fluid tumor markers (CEA, marker for gastrointestinal (GI) and ovarian carcinomas [CA 72.4], marker for ovarian and endometrial carcinomas [CA 125], CA 19.9, and marker for breast carcinoma [CA 15.3]) were prospectively collected and compared. Receiver operator curve analysis of the tumor markers showed that cyst fluid CEA (optimal cutoff of 192 ng/mL) showed the greatest area under the curve (0.79) for differentiating mucinous versus nonmucinous cystic lesions and was the most useful cyst fluid tumor marker. The accuracy of CEA (88 of 111 [79%]) was significantly greater than the accuracy of EUS morphology (57 of 112 [51%]) or cytology (64 of 109 [59%]) (P < .05). No combination of tests provided greater accuracy than CEA alone (P < .0001). The study concluded that of the tested markers, cyst fluid CEA is the most accurate test available for the diagnosis of mucinous cystic lesions of the pancreas.
Based on these data, we routinely send cyst fluid for cytology, amylase, and CEA (Table 42.2). Pseudocysts have very high amylase levels (often >50,000 IU/L) with normal CEA and benign cytology. Serous cystadenomas usually have benign cytology, normal CEA, and normal amylase. However, the specific detection of serous epithelial cells on cytology to make a definitive diagnosis in these lesions is rare (<20%) with EUS-guided FNA.20 Mucinous cystadenomas usually differ from serous cystadenoma in having a high CEA. Mucinous cystadenocarcinoma classically has malignant cytology, a low amylase, and a very elevated CEA. Although there is still some overlap of the CEA levels in these three entities, we have found the recommended cutoff value of CEA greater than 192 U/mL to be very helpful in stratifying patients to surgical versus conservative management.
Several more recent studies have aimed to characterize pancreatic cysts via use of molecular markers within the cyst fluid. A small study of 27 patients comparing CEA with the presence of k-ras-2 or loss of heterozygosity mutations using surgical histology or cytopathology as a “gold standard” found CEA to be the most predictive of histology, with concordance among the three tests in only 35% of patients.21 A larger study of 100 patients compared CEA with molecular criteria including DNA quantity, k-ras-2 point mutations, or two or more allelic imbalance mutations with pathology as the “gold standard.” The study concluded there was poor agreement (κ = 0.2) between CEA levels and molecular analysis for diagnosis of mucinous cysts. The diagnostic sensitivity for CEA was 82% compared with 77% for molecular analysis but improved to 100% when results of CEA levels and molecular analysis were combined.22
A more recent large, prospective multicenter study of 113 patients presenting for EUS-guided FNA of a pancreatic cyst assessed cytology and CEA levels compared with a detailed FNA analysis that incorporated DNA quantification, k-ras mutation and multiple allelic loss analysis, mutational amplitude, and sequence determination.23 Of 113 patients, 40 had malignant, 48 had premalignant, and 25 had benign cysts. Mucinous cysts were associated with a k-ras mutation (odds ratio 20.9) and with CEA levels greater than 148 ng/mL and allelic loss amplitudes of greater than 65%. Malignant cysts were associated with a DNA analysis that detected an allelic loss amplitude of greater than 82% and a high DNA amount (optical density ratio >10). The combination of a high-amplitude k-ras mutation followed by an allelic loss showed a specificity of 96% for malignancy, and all 10 of the malignant cysts with negative cytology were diagnosed as malignant by DNA analysis. The authors concluded that elevated amounts of pancreatic cyst fluid DNA, high-amplitude mutations, and specific mutation acquisition sequences are indicators of malignancy and that the presence of a k-ras mutation is also indicative of a mucinous cyst. They recommended DNA analysis should be considered when clinically suspicious cysts have a cytologic examination that is negative for malignancy.
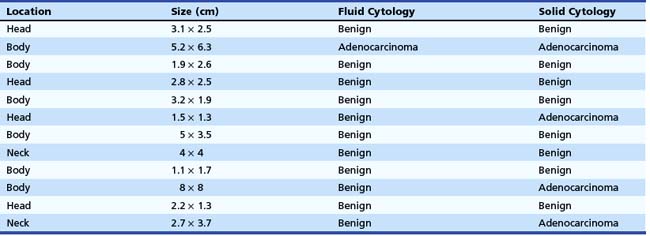
The cytology from the fluid of malignant cysts is usually nondiagnostic. For analysis of the cyst fluid to be most useful, several methods have been developed to improve the cytologic yield obtained from EUS-guided FNA. We have found that targeting any solid component, including the cyst wall, may enhance the yield on FNA cytology. We examined 42 pancreatic cystic lesions on which EUS-guided FNA was performed.24 The needle was advanced under EUS guidance into the cyst in the direction of the solid component and aspirated completely. Without withdrawal, the needle was advanced directly into the solid component. Fluid and solid cytologic samples were analyzed separately. All patients received prophylactic antibiotics. Of the 42 cysts, 12 were found to have a solid component (Table 42.3). Eight patients had both fluid and solid cytology showing benign cells. A single patient had malignant cells on both fluid and solid cytology. Three patients had benign fluid cytology but malignant (consistent with cystadenocarcinoma) solid cytology. The results of this study suggest an enhanced cytologic yield if the solid component is targeted.
In two small studies, the use of EUS-guided Tru-Cut needle biopsy (TCB) also seemed to enhance the diagnostic capability in cystic pancreatic tumors and lymphoepithelial cysts (see Fig. 42.3).25,26 In addition, the use of a cytology brush was found to be superior to conventional FNA in 7 of 10 patients undergoing EUS-guided FNA of cystic neoplastic lesions of the pancreas in a single-center study.27 Complications from this technique included one major and one minor intracystic bleed, with no infection or pancreatitis observed. Future techniques to enhance cytologic yield along with improved molecular methods of fluid analysis are needed to aid further our ability to characterize pancreatic cysts accurately.
Endocrine Tumors
EUS is very accurate in the detection of neuroendocrine tumors of the pancreas. Zimmer and associates28 reported their results in localizing and staging neuroendocrine tumors of the foregut in 40 patients examined by EUS, somatostatin receptor scintigraphy (SRS), CT, magnetic resonance imaging (MRI), and transabdominal ultrasound. EUS showed the highest sensitivity in localizing insulinomas compared with SRS, ultrasound, CT, and MRI. The authors suggested that ultrasound and EUS should be the first-line diagnostic procedures if insulinoma has been proven by a fasting test. Further diagnostic procedures were unnecessary in most cases. Further diagnostic procedures such as CT or MRI to search for distant metastases are necessary in large tumors or local invasive tumors. EUS shows the highest accuracy to detect or exclude pancreatic gastrinomas, but it fails to detect extrapancreatic gastrinomas in about 50%. The combination of EUS and SRS may give additional information. Zimmer and associates28 recommended that the first-line diagnostic procedures in patients with gastrinoma should be SRS and CT or MRI. If no metastases are detected, EUS should be the next preoperative imaging procedure. In nonfunctional neuroendocrine tumors, EUS provides the best information on local tumor invasion and regional lymph node involvement.
EUS has also been shown to be cost-effective in the preoperative localization of pancreatic endocrine tumors. Bansal and colleagues29 reported a case-control study of 36 patients who underwent preoperative EUS with a matched group of 36 patients who underwent surgical exploration immediately before the introduction of EUS. The EUS group had reduced charges for preoperative localization studies: $2620 versus $4846 per patient (P < .05). The lower cost was largely because of reductions in the number of diagnostic angiograms and venous sampling procedures performed. Surgical and total anesthesia times were decreased, as were the number of preoperative admissions for angiographic procedures. The cost-effectiveness ratio for the EUS group was $3144 per tumor localized compared with $5628 per tumor localized for the group treated before EUS became available (P < .05). The more specific utility of EUS-guided FNA in these patients was reported more recently (see Fig. 42.1).30 EUS-guided FNA was performed in 10 patients with clinically suspected functioning neuroendocrine tumors (hormonal disturbances) to determine the location and to confirm the diagnosis cytologically. EUS identified 14 tumors in these 10 patients. In all but one patient, CT did not show the tumor or missed at least one of multiple lesions. Mean tumor size was 12 mm (range 4 to 25 mm). Tumor locations were pancreas (n = 13) and duodenal wall (n = 1). Of the 14 detected lesions, 11 were aspirated under EUS-guided FNA with accurate diagnosis in all cases. Surgical confirmation of EUS-guided FNA findings was available in seven patients. There were no complications related to EUS-guided FNA.
More recently, an additional study of 30 patients with 33 lesions identified intraoperatively found sensitivity, specificity, positive predictive value, negative predictive value, and accuracy rate of EUS in conjunction with FNA of 82.6%, 85.7%, 95%, 60%, and 83.3%.31 EUS-guided FNA also seems to be highly effective and accurate in detecting the less common presentation of these lesions as cystic neuroendocrine tumors.32 In addition to diagnosing neuroendocrine tumors via EUS-guided FNA, EUS may also be useful in marking these subtle lesions using EUS-guided fine needle “tattooing” before surgery to assist in intraoperative localization.33 EUS-guided FNI may also be used in a therapeutic capacity for these lesions; a case of EUS-guided alcohol ablation of an insulinoma has been reported.34
Endoscopic Ultrasound–Guided Fine Needle Aspiration in the Staging of Pancreatic Cancer
In a prospective analysis, Mortensen and coworkers35 found a 30% overall impact of EUS-guided FNA on clinical management in 99 consecutive patients with pancreatic cancer of whom 20 patients underwent EUS-guided FNA for staging purposes: 5 liver lesions, 1 malignant ascites, 13 lymph nodes, and 1 aspiration from retroperitoneal tumor infiltration. The remaining 25 patients had diagnostic FNA: 22 pancreatic and 3 duodenal. EUS-guided FNA was performed only if positive results would have a clinically relevant impact on the subsequent management of the patient. The clinical impact of EUS-guided FNA was 12% (12 of 99) for staging purposes and 86% (18 of 21) for diagnostic purposes.
The economic impact of EUS-guided FNA in the preoperative staging of patients with pancreatic head adenocarcinoma was shown in a decision analysis model.36 The use of EUS-guided FNA prevented 16 surgeries per 100 patients compared with 8 surgeries per 100 patients if CT-guided FNA was performed for nonperitumoral lymph nodes. If the frequency of nonperitumoral lymph nodes was greater than 4%, EUS-guided FNA was the least costly procedure: $15,938 versus $16,378 for CT-guided FNA and $18,723 for surgery.
Lymph Node Assessment
According to a multivariate analysis, lymph node metastasis, intrapancreatic perineural invasion, and portal vein invasion are significant prognostic factors in patients with pancreatic cancer after curative resection.37 A retrospective analysis of patients who underwent curative resection was conducted. Of 193 patients, 38 (20%) survived for more than 5 years; 5-year survival rates for stages I, II, III, and IV disease were 41%, 17%, 11%, and 6%. Subsequently, a subgroup analysis of nodal metastasis and intrapancreatic perineural invasion was performed in 126 patients with records of these histologic findings. In the group of patients without nodal metastasis, the 5-year survival rate for patients without perineural invasion was 75%, whereas the 5-year survival rate for patients with perineural invasion was 29%; the difference in survival of these subgroups was significant (P < .02). In the group of patients with nodal metastasis, the 5-year survival rate for patients without perineural invasion was 17%, whereas the 5-year survival rate for patients with perineural invasion was 10%.
EUS imaging alone cannot fully distinguish malignant from inflammatory nodes, limiting its specificity in lymph node staging. Various EUS criteria have been described to distinguish malignant from benign nodes. These parameters, including size, shape, borders, and echotexture have lacked specificity, however. We previously conducted a study correlating EUS features of lymph nodes with the respective EUS-guided FNA diagnosis (Fig. 42.6).38 Computer analysis of EUS images of 48 lymph nodes in 47 patients using both linear array and radial scanning transducers was performed. Parameters included lymph node area, longest diameter, shape factor, and gray scale. There were 22 malignant and 26 benign nodes. When correlated with the FNA cytology results for each node, the only single criterion that was 100% specific for predicting malignancy was a longest diameter greater than 2.5 cm or an area greater than 2.5 cm2. However, using this size cutoff, the sensitivity declined to only 18%. No single criterion has an acceptable sensitivity and specificity to circumvent the need for a tissue diagnosis by EUS-guided FNA.
A multicenter study of 171 patients undergoing EUS-guided FNA of 192 lymph nodes (46 benign, 146 malignant) has been performed.39 The final diagnosis was ascertained by clinical follow-up (108 lymph nodes) or histopathology correlation (84 lymph nodes). The mean long axis dimension of benign lymph nodes was less than malignant lymph nodes (18 mm [5 to 37 mm] vs. 27 mm [5 to 80 mm]; P < .001). On average, two to three needle passes were made for each lymph node. The overall performance of EUS-guided FNA in lymph node assessment was sensitivity 92% (84% to 97% among four centers), specificity 93% (75% to 100%), and overall accuracy 92% (82% to 98%). If a long axis dimension of 15 mm was used for determining benign (≤15 mm) versus malignant (>15 mm) lymphadenopathy, EUS alone had sensitivity (67%), specificity (50%), and accuracy (63%) all inferior to EUS-guided FNA (P < .05). In 89 patients, a total of 101 lymph nodes underwent EUS-guided FNA for the staging of lung cancer (14 patients) or primary GI or pancreatic malignancies (75 patients). When comparing EUS-guided FNA with EUS size criteria (≤10 mm = benign), the sensitivity (90% vs. 91%; P = not significant) and accuracy (92% vs. 83%; P = not significant) for EUS-guided FNA were similar, whereas the specificity was superior to that of EUS size criteria alone (100% vs. 47%; P < .001).
Not only can EUS-guided FNA improve the specificity of lymph node metastasis with cytologic confirmation, but also more recently FNA has been used to detect genetic alterations in cytologic negative nodes. A prospective study was conducted to assess the clinical value of genetic staging of lymph node metastasis in patients with pancreatic adenocarcinoma who underwent curative surgery.40 In the primary tumors in 18 of 25 patients with pancreatic adenocarcinoma, k-ras gene mutations were detected. Among these 18 patients, a mutated k-ras gene was also found in at least one lymph node in 13 patients. Of these 13 patients, 7 had no evidence of histologic nodal involvement, and 6 had histologic lymph node metastasis. Although there was no significant difference in overall survival rates between the pathologic node-negative and node-positive patients, overall survival of the five patients with nodes negative for the mutated k-ras gene was significantly better than overall survival of the 13 patients with genetically metastasis-positive nodes (P < .001). Overall survival of the six patients with genetically metastasis-positive nodes limited to the peripancreatic area was significantly better than overall survival of the seven patients with genetic metastasis in lymph nodes beyond the peripancreatic areas (P = .018). These findings suggest that detection of k-ras gene mutations in lymph nodes may be clinically useful to assess the accurate tumor staging and to stratify patients who may be at higher risk for recurrence after curative resection.
Finally, in addition to abdominal lymphadenopathy, a more recent study of 160 patients with pancreatic and periampullary cancers undergoing EUS staging found that 5% had malignant mediastinal adenopathy.41 Only one of the eight patients with malignant adenopathy in the mediastinum had other sites of documented distal metastases by CT or positron emission tomography scan, although seven patients had a locally advanced cancer. These findings emphasize the importance of carefully examining the mediastinum for suspicious adenopathy routinely as a part of every EUS examination done to stage pancreatic cancer.
Liver Metastasis
EUS is not traditionally thought to be clinically applicable in liver imaging. However, more recent data have suggested otherwise. A prospective study was conducted in which 574 consecutive patients with a history or suspicion of GI or pulmonary malignant tumor undergoing upper EUS examinations underwent EUS evaluation of the liver.42 Focal liver lesions were found in 14 (2.4%) patients, and they underwent EUS-guided FNA (see Fig. 42.4). Before EUS, CT depicted liver lesions in only 3 of 14 (21%) patients. Seven of 14 patients had a known cancer diagnosis. For the other seven patients, the initial diagnosis of cancer was made by means of EUS-guided FNA of the liver. There were no immediate or late complications. This study showed that EUS can detect small focal liver lesions that are not detected at CT.
Findings of EUS-guided FNA can confirm a cytologic diagnosis of liver metastasis and establish a definitive M stage that may change clinical management. A retrospective questionnaire study regarding indications, complications, and findings of EUS-guided FNA of the liver was reported more recently, which included 21 EUS and FNA centers around the world.43 There were 167 cases of EUS-guided FNA of the liver. A complication was reported in 6 (4%) of 167 cases, including death in 1 patient with an occluding biliary stent and biliary sepsis, bleeding in 1 patient, fever in 2 patients, and pain in 2 patients. EUS-guided FNA diagnosed malignancy in 23 of 26 (89%) patients who had a prior nondiagnostic FNA under transabdominal ultrasound guidance. EUS also localized an unrecognized primary tumor in 17 of 33 (52%) cases in which CT had shown only liver metastases. EUS imaging characteristics were not predictive of malignant versus benign lesions. The authors concluded that EUS-guided FNA of the liver apparently is a safe procedure with a major complication rate of approximately 1%. Additional studies have confirmed that EUS can detect previously undetected or additional lesions not visualized by other modalities44–48 and correspondingly alter planned clinical management.44,45 In addition, EUS-guided FNA achieves results comparable to or better than CT-guided FNA of hepatic lesions.49
Liver lesions typically have a much higher cytologic yield (requiring fewer needle passes owing to less inflammatory and fibrotic reaction compared with a primary pancreatic neoplasm) and give the highest staging information (see section on FNA technique). A study supporting these statements found a 94% diagnostic yield with EUS-guided FNA (31 of 33 lesions confirmed positive) of hepatic lesions with a mean of only 1.4 FNA passes taken.50
Ascites
The utility of EUS-guided FNA was evaluated for detection and aspiration of scant ascites among patients undergoing EUS for diagnosis and staging of GI malignancies.51 EUS found ascites in 85 patients (15% of a series of 571 patients). CT performed before EUS identified ascites in only 18% of patients with ascites on EUS. Of the 85 patients, 31 underwent EUS-guided FNA paracentesis, and malignant ascites was diagnosed by EUS-guided FNA in 5 patients. The clinical impact was great in these patients because surgery was avoided.
Two additional studies have corroborated these findings. Kaushik and colleagues52 identified ascites in 25 patients undergoing EUS for suspected or proven malignancy and diagnosed 16 cancers (64% of patients). Six of the nine patients with negative ascites on EUS-guided FNA had a diagnosed malignancy, but only one false-negative ascites cytology result occurred in these patients, yielding 94% sensitivity and 89% negative predictive value. DeWitt and colleagues53 detected ascites on EUS in 60 patients being staged with known or suspected malignancy. Of these, MRI and CT detected ascites in only about 50% of patients who also had either of these examinations, and ascites was detected in only 27% of patients undergoing transabdominal ultrasound. Cancer was diagnosed in 16 (27%) patients. Of the eight patients who underwent subsequent surgery, three were found to have malignant ascites, illustrating the fact that a negative fluid cytology does not exclude the possibility of peritoneal carcinomatosis.
Two more recent articles have highlighted the fact that peritoneal implants may also be identified on EUS and that these can successfully undergo EUS-guided FNA with adequate specimen to confirm malignancy.54,55 Some investigators have suggested that in patients with a small amount of ascites, a transrectal approach may be more sensitive in identifying and diagnosing malignant carcinomatosis.56
Endoscopic Ultrasound–Guided Fine Needle Aspiration in Biliary Lesions
Diagnosis and Staging of Cholangiocarcinoma
Cholangiocarcinoma is associated with a high mortality, and it is often difficult to obtain an accurate tissue diagnosis, with ERCP and brushings of the bile duct being the preferred modality for this purpose. The currently reported diagnostic yield from ERCP ranges from only 30% to 60%, and the diagnosis of malignant biliary stricture remains a challenge. EUS-guided FNA is now being used to diagnose and stage cholangiocarcinoma (see Fig. 42.6).57,58 In the original case series describing the use of EUS in diagnosis of cholangiocarcinoma, 10 patients with bile duct strictures at the hepatic hilum, diagnosed by CT or ERCP or both, underwent EUS-guided FNA. Adequate material was obtained in nine patients. Cytology revealed cholangiocarcinoma in seven patients and hepatocellular carcinoma in one patient. One benign inflammatory lesion identified on cytology proved to be a false-negative finding by frozen section. Metastatic locoregional hilar lymph nodes were detected in two patients, and in one patient the celiac and paraaortic lymph nodes were aspirated to obtain tissue proof of distant metastasis.
These studies suggest that EUS with FNA is safe and effective in evaluating proximal biliary strictures. Several additional studies, totaling 142 patients, have reported on the effect of EUS-guided FNA in evaluating biliary (including hilar) strictures, most of which had prior negative cytology with other tissue sampling modalities.59–62 These studies have shown high sensitivities (80% to 90%) and overall accuracy rates (80% to 90%) with this technique. Even though the overall accuracy was high, this was due to the high percentage of malignancies in these series. One-quarter to one-third of patients with nonmalignant cytology on EUS-guided FNA were also ultimately diagnosed with malignancy. Given the low negative predictive values observed, a negative FNA result does not reliably exclude the possibility of malignancy. Nevertheless, this technique, when used in combination with ERCP, is extremely helpful in distinguishing benign from malignant strictures and in facilitating a definitive diagnosis by increasing tissue yield.
Another use of EUS-guided FNA is staging of cholangiocarcinoma via looking for perihepatic and distal lymphadenopathy. Assessing lymphadenopathy is not only important for staging patients before planned resection or chemotherapy, but it is also crucial in evaluating patients who are being considered for liver transplantation. A study comprising 44 patients with cholangiocarcinoma being evaluated with EUS before liver transplantation found 70 regional lymph nodes, with 9 of 70 nodes positive for malignancy63; this represented 8 of the 47 patients (18%). EUS detected 12 patients with lymph nodes not seen on standard imaging (CT or MRI). Of the 22 patients with negative lymph nodes by EUS and EUS-guided FNA, 20 (91%) had the EUS findings regarding their lymph node status confirmed. Two patients were found to have malignant perigastric lymph nodes at the time of surgery. An important additional finding of this study was that lymph node morphology and echo features did not predict malignant involvement and that EUS-guided FNA of all visualized lymph nodes in such patients is advised. Finally, in addition to cholangiocarcinoma, EUS-guided FNA has been found to be useful in diagnosing gallbladder masses, with sensitivity rates of 80% or greater for diagnosing malignancies.62,64
Diagnosis and Staging of Ampullary Cancer
Conventional abdominal imaging studies such as CT, MRI, and transabdominal ultrasound frequently fail to detect ampullary lesions. EUS is a sensitive modality for detecting and staging ampullary tumors. Accurate staging may be affected by biliary stent placement, which is frequently performed in these patients with obstructive jaundice. Combined data from two centers reported the accuracy of ampullary tumor staging with multiple imaging modalities in patients with and patients without endobiliary stents.65 Preoperative staging was performed in 50 consecutive patients with ampullary neoplasms by EUS plus CT (37 patients), MRI (13 patients), or angiography (10 patients) over a 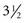 -year period. Of the 50 patients, 25 had a transpapillary endobiliary stent present at the time of EUS examination. EUS was shown to be more accurate than CT and MRI in the overall assessment of the T stage of ampullary neoplasms (EUS 78%, CT 24%, MRI 46%). No significant difference in N stage accuracy was noted between the three imaging modalities (EUS 68%, CT 59%, MRI 77%). EUS T stage accuracy was reduced from 84% to 72% in the presence of a transpapillary endobiliary stent. This was most prominent in the understaging of T2 and T3 carcinomas.
-year period. Of the 50 patients, 25 had a transpapillary endobiliary stent present at the time of EUS examination. EUS was shown to be more accurate than CT and MRI in the overall assessment of the T stage of ampullary neoplasms (EUS 78%, CT 24%, MRI 46%). No significant difference in N stage accuracy was noted between the three imaging modalities (EUS 68%, CT 59%, MRI 77%). EUS T stage accuracy was reduced from 84% to 72% in the presence of a transpapillary endobiliary stent. This was most prominent in the understaging of T2 and T3 carcinomas.
A second retrospective study was published in which the role of EUS-guided FNA in the diagnosis and staging of ampullary lesions was reported.66 EUS-guided FNA was performed in 20 of 27 (74%) patients with suspected ampullary tumors. EUS-guided FNA made the initial ampullary tissue diagnosis in seven patients (adenocarcinoma in five patients, adenoma in one patient, neuroendocrine tumor in one patient). In addition, EUS-guided FNA resulted in a change of the diagnosis from adenoma to adenocarcinoma in one patient. In one patient, EUS-guided FNA detected a liver metastasis not seen on CT. Overall, EUS-guided FNA provided new histologic information in 9 of 27 patients (33%).
Another study of 35 patients who underwent EUS-guided FNA of ampullary lesions, with follow-up available in 27 patients, revealed 13 patients with adenocarcinoma, 6 with atypical cells (4 suspicious for cancer and 2 consistent with reactive atypia), 2 with adenomas, 1 with carcinoid, and 13 with no evidence of malignancy.67 Three false-negative studies were identified, yielding sensitivity 82.4%, specificity 100%, negative predictive value 76.9%, and overall accuracy 88.8% for EUS-guided FNA in diagnosing ampullary lesions.
A more recent study of 40 patients with adenoma and ampullary adenocarcinoma suggested that the use of intraductal ultrasound showed a trend toward improved T staging compared with standard EUS (78% vs. 63%), although this was nonsignificant (P = .14).68 There was some concern that intraductal ultrasound may overstage lesions. Two recent studies comprising 68 patients found that EUS was comparable to CT or MRI in T and N staging of ampullary lesions.69,70 Both studies showed about a 25% improvement in T staging accuracy with EUS, but this was not statistically significant because of the small number of subjects in each study.
Endoscopic Ultrasound–Guided Therapy for Pancreaticobiliary Lesions
Celiac Plexus Block and Celiac Plexus Neurolysis
Patients with significant abdominal pain who have unresectable pancreatic cancer may be candidates for EUS-guided celiac plexus neurolysis (CPN). Wiersema and Wiersema71 described this novel technique and the impact on pain management of patients with pancreatic cancer. After visualizing the celiac trunk by the linear array echoendoscope and using a 22-gauge needle, injection of bupivacaine (0.25%) followed by ethyl alcohol (98%) can be performed on either side of the vessel. Of patients, 88% had persistent improvement in their pain score. Only minor complications were seen and consisted of transient diarrhea in four patients. This anterior transgastric approach for performing CPN is theoretically considered safer compared with the traditional CT-guided posterior method. Rare reported cases of paraplegia occurred with the posterior approach because of its proximity to the spinal column.
The effect of EUS-guided celiac plexus block (CPB) in controlling abdominal pain from chronic pancreatitis is less evident. Gress and colleagues72 performed EUS-guided CPB in 80 patients with chronic pancreatitis. A mixture of bupivacaine 0.25% and 80 mg of triamcinolone (the substitution of steroid for alcohol distinguishes CPB from CPN) was injected using the above-described technique. Only 10% had benefit beyond 24 weeks. There were two major complications: peripancreatic abscess and bleeding from the celiac artery secondary to ethanol-induced arterial pseudoaneurysm. This technique was less effective in younger patients (<45 years old) and patients who had previous surgery for chronic pancreatitis. There was a slight economical advantage of EUS-guided CPB over the CT-guided approach ($1200 vs. $1400).
The findings of superior results with EUS-guided CPN in patients with cancer compared with EUS-guided CPB in patients with chronic pancreatitis were corroborated by a meta-analysis of eight studies of EUS-guided CPN comprising 283 patients and nine studies of EUS-guided CPB comprising 376 patients.73 The results showed pain relief in 80.12% of patients with cancer and in only 59.45% of patients with chronic pancreatitis. A randomized trial of single versus bilateral injections of the same amount of medication during EUS-guided CPB in 51 patients with chronic pancreatitis was performed to determine if the suboptimal response rate in these patients could be improved. The results showed no difference between the injection techniques with regard to the overall response rates (56.5% for single injection, 53.6% for bilateral injection), the total duration of pain relief, or the onset of pain relief.74
Another study comparing single with bilateral injections that included patients with both chronic pancreatitis and pancreatic cancer and CPB and CPN in each group reached a different conclusion. This prospective, consecutive, nonrandomized study evaluated 89 patients receiving bilateral injections and compared them with 71 patients receiving a central injection. The group receiving bilateral injections had a mean pain reduction of 70.4%, which was significantly better than the 45.9% achieved by the group receiving a central injection.75 Both groups had roughly equal numbers of patients with cancer and chronic pancreatitis and were similar in the percentages in each group undergoing CPB versus CPN. The only observed complication was in a single anticoagulated patient who developed self-limited bleeding secondary to a laceration of the left adrenal artery after a bilateral injection.
Routine bilateral injection may be preferred to routine central injection when performing a CPN or CPB in patients with abdominal pain secondary to pancreatic cancer or chronic pancreatitis. However, based on the available data, it is unclear whether this approach is beneficial in patients with benign pancreatic disease. The current technique of either bilateral or central EUS-guided CPN or CPB seems to be safe because others have more recently reported similarly low rates (1.8% [4 of 220]) of known complications including asymptomatic hypotension (n = 1), self-limited postprocedural pain (n = 2), or retroperitoneal abscess (n = 1).76
In 2006, two groups showed the ability of EUS to identify the celiac ganglia accurately.77,78 This development potentially allows for a more focused delivery of medication to achieve neurolysis or nerve block (Fig. 42.7). In addition, it may allow for consideration of alcohol injection (CPN) for patients with pain from chronic pancreatitis. A subsequent prospective study of 200 patients undergoing EUS for various indications found that 81% had identifiable celiac ganglia at the time of EUS.79 Female sex and no prior history of abdominal surgery were predictive of ganglion visualization. Among patients with visualized ganglia, linear echoendoscopes identified more ganglia per patient than radial echoendoscopes.
A retrospective study of 33 patients undergoing 36 ganglia injections (18 in patients with cancer, 18 in patients with chronic pancreatitis) found that 94% (16 of 17) of patients with cancer and 80% (4 of 5) of patients with chronic pancreatitis who received EUS-guided CPN into the ganglia reported pain relief compared with 0% (0 of 1) and 38% (5 of 13) when EUS-guided CPB was performed.80 Patients with initial pain exacerbation secondary to injection of the ganglia seemed to have a significantly improved therapeutic response. These findings suggest that intraganglionic injections may improve response rates to EUS-guided CPN in patients with both benign and malignant disease. Prospective, randomized trials evaluating EUS-guided CPN and CPB into the ganglia are either under way or being planned. The results of these trials in patients with pain from benign and malignant pancreatic disease will serve to delineate better the efficacy and safety of this novel technique and to clarify its indications.
Cyst Gastrostomy
Endoscopic therapy for pseudocyst drainage is an established alternative for surgical and radiologic approaches. Since the initial report of EUS-assisted pseudocyst drainage,81 the use of EUS as the sole tool for draining pseudocysts has been evolving. This approach became feasible with the development of larger accessory channel echoendoscopes. EUS-guided pseudocyst drainage technique overcomes many obstacles associated with the conventional endoscopic approach. In the absence of an apparent intraluminal bulge, common in cysts in the region of the pancreatic tail, cyst drainage can still be safely performed after an accurate measurement of the distance between the cyst and the GI wall, with a distance of less than 1 cm between the GI wall and cyst lumens recommended. EUS imaging with color flow and Doppler techniques can be used to define and avoid any intervening vessels.
These advantages theoretically can reduce the risk of hemorrhage and perforation. However, significant bleeding after EUS-guided cyst gastrostomy requiring angiographic embolization has been reported.82 In cases where cystic neoplasms are a concern, cyst fluid aspiration should be performed before drainage. The level of tumor markers in the fluid, mainly CEA, and the concentration of amylase may assist in differentiating inflammatory from neoplastic cysts.83,84 Careful assessment of the cyst is important and can exclude patients with cysts that may be malignant, patients with cysts that are unlikely to be technically feasible, or patients who are poor candidates for successful endoscopic therapy. Previous studies suggest that EUS may exclude 5% to 37% of patients referred for cyst gastrostomy for these reasons.85,86
After the first stent is successfully deployed and confirmed in proper position via endoscopy and fluoroscopy, we reinsert the guidewire into the cyst alongside the first stent, and once it is properly coiled in the cyst on fluoroscopy, we place a second 10-Fr double-pigtail stent before completing the procedure. Nasocystic drainage is appropriate if evidence of infection or solid or thick debris is present within the cyst. Some authors have proposed placing fully covered self-expanding metal stents instead of one or two plastic stents and reported a 95% (17 of 18) technical success rate with 14 of 18 patients (78%) achieving complete resolution of pancreatic fluid collections with a mean time to final resolution of 77 days.87
Antibiotics should be administered during and after the procedure. Complications from this technique occur in less than 5% of patients undergoing the EUS technique, which appears reduced compared with direct endoscopic or surgical techniques.86,88–90 Complications typically have included hemorrhage, infection, perforation, or pneumoperitoneum. Stent migration into the cyst should occur rarely if pigtailed stents are used; however, should this occur, endoscopic stent retrieval and repositioning is feasible and has been reported.91
Several published studies have reported the efficacy of this technique. Giovannini and coworkers92 drained 35 pancreatic cysts under EUS guidance, of whom 15 had pseudocysts and 20 had pancreatic abscesses. Of the 33 cysts drained via a transgastric approach, an extrinsic compression was seen in only one patient using a forward-viewing gastroscope. No major complications occurred except for a single case of a pneumoperitoneum, which was successfully managed medically. No bleeding was encountered. A 7-Fr nasocystic drain was placed in 18 of 20 cases of pancreatic abscess. Surgery was performed in the two other patients. In the pseudocyst group, placement of an 8.5-Fr stent was successful in 10 patients, and placement of a nasocystic drain was done in 5 patients. In one case, only cyst puncture and aspiration was performed. Over a mean follow-up of 27 months (range 6 to 48 months), one recurrence among the 15 pancreatic pseudocysts and two relapses of the 18 pancreatic abscesses were observed. The EUS-guided drainage success rate was 88.5% (31 of 35); only 4 patients with pancreatic abscesses underwent surgery.
In a related article, Seifert and associates93 evaluated a new one-step device for stent placement using a large-channel echoendoscope (3.2 mm) for pseudocyst drainage in six patients. One of these patients had a pancreatic abscess. Transmural drainage was successfully done using modified 7-Fr diameter stents. No complications were encountered with the endoscopic interventions. One patient with necrotizing pancreatitis, who denied surgery, died secondary to sepsis. At follow-up of 3 to 13 months, the cysts had completely resolved in four patients. This study confirmed the feasibility and effectiveness of the EUS-guided one-step technique in draining various cystic lesions; however, larger studies are needed. In a subsequent study and using the one-step device through a 3.7-mm channel echoendoscope, the same group was able to place a 10-Fr stent in three patients and a 7-Fr stent in one patient, all with peripancreatic cystic lesions. One of the cysts had persisted for more than 3 months and was found to be a ganglioneuroma after surgical enucleation.94
Several comparative trials have been reported more recently evaluating endoscopic, EUS-guided, and surgical cyst gastrostomy. A retrospective study comparing 10 patients who had undergone a surgical approach with 20 patients who underwent EUS-guided cyst gastrostomy found no differences in clinical outcomes between the modalities but reported a reduction in postprocedure hospital stay (6.5 days for surgery vs. 2.65 days for the EUS approach), which resulted in a significant cost savings of $5738 per patient.95
A randomized controlled trial comparing two groups of 15 patients undergoing cyst gastrostomy has also been performed.96 In the group undergoing pseudocyst drainage with conventional endoscopy, technical success was achieved in only 5 of 15 patients, with two bleeding complications and one death. All 10 patients with technical failures were crossed over to EUS-guided cyst gastrostomy, and technical success was achieved. The EUS-guided group had 14 of 14 patients achieve technical success (one cyst was correctly diagnosed as a biliary cystadenoma at EUS and excluded from drainage). No significant differences in clinical success rates or complications among the groups were noted, although the study appeared underpowered to make this determination.
Delivery of Antitumor Agents
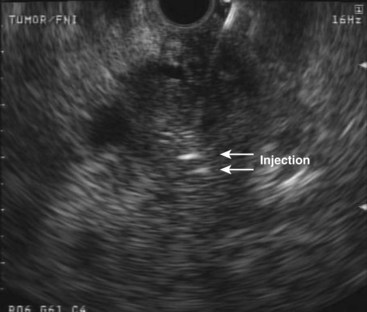
We have examined the feasibility and safety of direct injection of allogeneic mixed lymphocyte culture (cytoimplant) in pancreatic adenocarcinoma under EUS guidance.97 In a phase I clinical trial, eight patients with unresectable pancreatic adenocarcinoma underwent EUS-guided FNI of cytoimplants. Four patients were in stage II, three were in stage III, and one was in stage IV. Escalating doses of cytoimplants of 3 billion, 6 billion, or 9 billion cells were implanted using a novel EUS-guided FNI technique. The median survival was 13.2 months with two partial responders and one minor response. Major complications including bone marrow toxicity, hemorrhage, infections, renal toxicity, or cardiopulmonary toxicity were absent. Low-grade fever was encountered in seven of the eight patients and was symptomatically treated with acetaminophen.
Our study showed that local immunotherapy is feasible and safe. The technique of EUS-guided FNI was later applied to deliver antitumor viral therapy.98 ONYX-015 (dl1520) is an E1B-55kD gene-deleted replication-selective adenovirus that preferentially replicates in and kills malignant cells. Over 8 weeks, 21 patients with locally advanced adenocarcinoma of the pancreas or with metastatic disease but minimal or absent liver metastases underwent eight sessions of ONYX-015 delivered by EUS injection into the primary pancreatic tumor. The final four treatments were given in combination with gemcitabine (1000 mg/m2 intravenously). After combination therapy, 2 patients had partial regressions of the injected tumor, 2 had minor responses, 6 had stable disease, and 11 had progressive disease. No clinical pancreatitis occurred despite mild, transient elevations in lipase in a few patients. Two patients had sepsis before the institution of prophylactic oral antibiotics. Two patients had duodenal perforations from the rigid endoscope tip. No perforations occurred after the protocol was changed to transgastric injections only.
The most recent EUS-guided antitumor therapy involves novel gene therapies.99 TNFerade, which involves a novel gene transfer approach, is the newest EUS-guided antitumor therapy (Fig. 42.8).99–101 The attractiveness of this new approach is the potential to maximize local antitumor activity and minimize systemic toxicity. TNFerade was constructed as a second-generation (E1-deleted, partial E3-deleted, and E4-deleted) adenovector, expressing the cDNA encoding human tumor necrosis factor (TNF). To optimize local effectiveness further and minimize systemic toxicity, the radiation-inducible immediate response Egr-1 (early growth response) promoter was placed upstream of the transcriptional start site of the human TNF cDNA. This vector was engineered to ensure that maximal gene expression and subsequent TNF secretion are constrained in space and time by radiation therapy. A synergistic “triple threat” is formulated: (1) 5-Fluorouracil chemotherapy is directly toxic to cancer cells and is a radiosensitizer, (2) external beam radiation destroys cancer cells and upregulates TNF production, and (3) TNFerade causes cancer cell death and is itself a radiosensitizer.
TNFerade in combination with radiation therapy has been studied in preclinical and early clinical (phase I) trials with encouraging results.102,103 The study design consisted of a 5-week treatment of weekly intratumoral injections of TNFerade (4 × 109, 1010, and 1011 particle units in 2 mL). EUS-guided FNI was compared with percutaneous approaches (CT or ultrasound). TNFerade was combined with continuous intravenous 5-fluorouracil (200 mg/m2/day × 5 days/wk) and radiation (50.4 Gy). TNFerade was delivered with a single needle pass at a single site in the tumor for percutaneous approaches; up to four injections were given by EUS. The long-term results from a cohort of 50 patients showed that toxicities potentially related to TNFerade were mild and well tolerated.100 Compared with two lower dose cohorts (n = 30), the higher dose group (n = 11) was associated with greater locoregional control of treated tumors, longer progression-free survival, a greater proportion of patients with stable or decreasing levels of CA 19.9, a greater percentage (45%) of patients undergoing resection, and improved median survival (6.6 months, 8.8 months, 11.2 months, and 10.9 months, in the 4 × 109, 4 × 1010, 4 × 1011, or 1 × 1012 particle units cohorts). At the 4 × 1011 dose, four of five patients whose tumors became surgically resectable achieved pathologically negative margins, and three survived more than 24 months.
Among patients receiving TNFerade who underwent resection, we achieved a complete pathologic response with no residual tumor at surgical resection.101 However, this patient developed hepatic and bilateral pulmonary metastases slightly more than 1 year after resection and died 19 months after receiving the initial TNFerade injection.
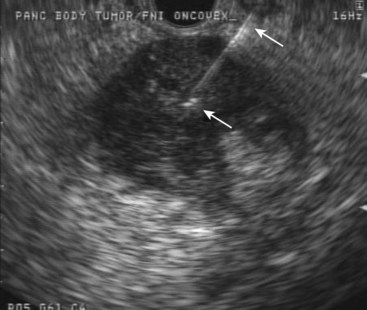
Another oncolytic viral therapy for pancreatic cancer using a herpes simplex virus carrying the granulocyte-macrophage colony-stimulating factor gene (OncoVEXGM-CSF) has been developed (Fig. 42.9).104 It remains to be seen which virus and encoded product combination will achieve the best results in this nascent field of viral oncolytic therapy.
Two additional therapies delivered by EUS-guided FNI have also been reported more recently. The first involves injecting immature dendritic cells into patients with pancreatic cancer to induce a T cell–dependent immune response.105 Dendritic cells are powerful antigen-presenting cells that can initiate a strong tumor-specific immune response. Injections were done on days 1, 8, and 15 of each 28-day cycle. Seven patients with metastatic pancreatic cancer who were refractory to gemcitabine chemotherapy underwent EUS-guided FNI of immature dendritic cells; no complications were observed, and technical success was achieved in all cases. The median survival of 9.9 months in these patients was thought to be encouraging given their advanced disease at the time of the initiation of this therapy.
The second therapy involves treating cystic pancreatic tumors with ethanol lavage and paclitaxel injection.106 This technique involves aspirating the cyst with a 22-gauge needle until it collapses while maintaining the needle tip within the cyst. The cyst is irrigated and lavaged with pure (99%) ethanol for 3 to 5 minutes, followed by reaspiration of all the injected ethanol and injection of the cyst cavity with a 3 mg/mL solution of paclitaxel diluted 1 : 1 with 0.9% saline. The amount of paclitaxel and saline solution reinjected was equal to the amount of fluid originally withdrawn from the cyst. A study of 14 patients with a median cyst size of 25.5 mm who were followed for a median of 9 months showed complete cyst resolution in 11 patients, partial resolution in 2 patients, and a persistent cyst in 1 patient. This technique holds promise for patients with indeterminate or mucinous cysts as an alternative to surgery or surveillance.
Accessing the Pancreaticobiliary System
EUS-guided FNA and FNI can be used to access dilated pancreatic and biliary systems to perform cholangiography or pancreatography through contrast agent injection and provide decompression and drainage via the ampulla using rendezvous techniques or through the creation of transenteric fistulas. The initial EUS-guided injection of contrast agent through the duodenal wall into the common bile duct was first reported by Wiersema and colleagues107 in 1996 as an alternative method for performing cholangiopancreatography in patients in whom ERCP had failed. This technique also has been used to provide ductal imaging and locate the minor papilla when it had not been identifiable at ERCP, using a combination of contrast agent and methylene blue.108 Although technically feasible, direct injection of contrast agent into the bile duct or pancreatic duct for diagnostic purposes in the era of magnetic resonance cholangiopancreatography should be avoided unless subsequent therapy or drainage during the procedure is planned.
EUS-guided contrast agent injection followed by guidewire placement through either the duodenum or the stomach may also salvage difficult ERCP cannulations via EUS-guided rendezvous techniques, first reported by Mallery and colleagues in 2004.109 In these techniques, a wire is passed across the major papilla to achieve biliary drainage or the major or minor papilla to achieve pancreatic duct drainage. Although the approach is usually transduodenal for biliary access and transgastric for pancreatic duct access, transhepatic cholangiography performed from the stomach with subsequent rendezvous ERCP and stent placement has been reported.110 Once the wire traverses the papilla, the EUS scope is replaced with a duodenoscope, and a second wire is passed adjacent to the EUS-placed wire into the desired duct, if possible. If this is not achievable, the EUS-placed wire is grabbed and brought through the biopsy channel of the scope, and the procedure is completed in the standard ERCP fashion.
A series of 23 patients undergoing EUS-guided cholangiography (13 via a transhepatic approach and 10 via a transcholedochal approach) with subsequent rendezvous ERCP found that drainage could be achieved in 21 patients, with transpapillary drainage being achieved in 18 and choledochoenteric fistula creation being required in 3. Complications included one bile leak, two cases of self-limited pneumoperitoneum, and one case of minor bleeding. The transhepatic approach was believed to be safer than the transcholedochal approach, possibly owing to reduced intraperitoneal spillage of bile.111
There are special situations after failed ERCP cannulation where EUS-guided passage of a wire into the pancreatic duct or biliary tree is needed to assist in transmural drainage as an alternative to percutaneous or surgical approaches. These cases usually occur when the major and minor papillae are inaccessible or not identifiable or when EUS-guided rendezvous attempts have failed, necessitating the need for alternative drainage techniques. Two reports showed the feasibility of therapeutic EUS-guided cholangiography or pancreatography with stent placement in patients with failed cannulation at ERCP. Burmester and coworkers112 successfully performed EUS-guided cholangiography-assisted drainage in three of four patients with malignant biliary strictures in which ERCP was impossible. They used a modification of the one-step method for performing pancreatic pseudocyst drainage described by Seifert and colleagues.93 The technique was performed by transgastric, transduodenal, and transjejunal approaches. Access and direct drainage via the proximal biliary tree has been described with EUS-guided hepaticogastrostomy.111,112 These preliminary reports show that EUS-guided cholangiopancreatography with duct decompression can be successfully performed in patients in whom ERCP has failed.
Pancreatic duct decompression and drainage can also be achieved through the creation of enteropancreatic fistulas. Kahaleh and associates113 performed EUS-guided pancreatography with subsequent gastropancreatic duct stent placement in two patients. These patients had complicated pancreatitis with pancreatic duct strictures where surgical reconstruction precluded access to the papilla. One patient developed upper GI bleeding 24 hours after the procedure, which was thought to be related to the needle tract. The bleeding was treated endoscopically. The second patient had no procedure-related complications. Both patients had improvement in symptoms 1 month after the procedure. Two larger series evaluating this technique in a combined 49 patients both achieved success rates of greater than 80%.114,115 Four complications occurred (8% of patients): bleeding in two patients, pancreatitis in one patient, and a perforation in one patient.
Brachytherapy and Placement of Fiducial Markers
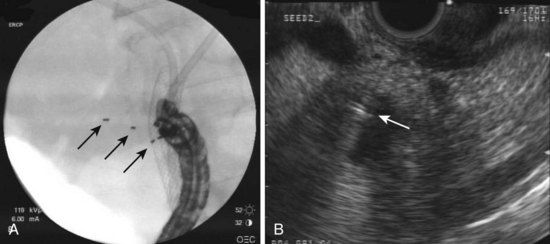
EUS-guided FNI has increasingly been used to facilitate or provide radiation therapy to malignant lesions. This technique has been used to place radiographic, or fiducial, markers within focal lesions in the mediastinum and abdomen to facilitate image-guided radiation therapy (Fig. 42.10). EUS-guided FNI is an attractive alternative to surgical or percutaneous placement of these markers because it has fewer risks and limitations. It particularly seems useful in accessing lesions in the posterior mediastinum and abdomen.
EUS-guided fiducial marker placement was reported to have a high rate of success (85% [11 of 13]) in one more recent series of mediastinal and abdominal lesions for which fiducial marker placement was requested.116 Fiducial markers could not be placed on two lesions because of the inability of the EUS needle to access the target lesion adequately or safely. In one lesion, an intervening blood vessel (the aorta) precluded needle advancement, and in the second lesion, a gastric outlet obstruction precluded accessing the lesion anatomically. The authors noted some difficulty passing a 5 mm long marker through the stiff 19-gauge needle when angulation of the tip of the scope was required, necessitating the placement of shorter 3-mm seeds in some cases.
A report of a prototype forward-viewing linear echoendoscope (XGIF-UCT 160 J-AL5; Olympus America Inc, Center Valley, PA) that was used to place fiducial markers in a perirectal lesion suggested this echoendoscope may facilitate advancement of the stiffer 19-gauge needle into targeted lesions, especially when the lesions are firm, allowing routine placement of the preferred 5 mm long seeds.117 This facilitation with this echoendoscope may be due to the fact that the working channel is in line with the shaft of the echoendoscope, allowing for needle advancement and fiducial marker placement to be performed in a straight plane rather than tangentially.
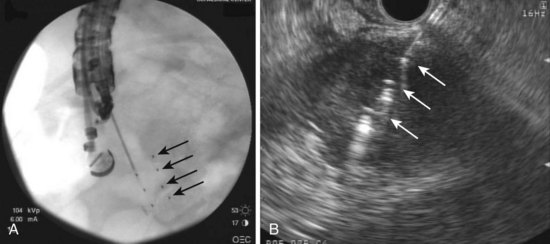
EUS-guided FNI may also be used to place radioactive seeds within lesions to provide brachytherapy (Fig. 42.11). This technique also uses a 19-gauge needle in which the seeds are backloaded into the needle and sealed with bone wax before insertion into the echoendoscope. We initially reported on the successful placement of radioactive iodine (iodine 125) seeds via EUS-guided FNI in a patient with recurrent malignant lymphadenopathy after esophagectomy that was not visualized on CT imaging and required EUS for radioactive seed placement118; several more recent studies have focused on this technique for the treatment of advanced pancreatic cancer. EUS-guided brachytherapy was reported to have a 100% technical success rate in a series of 22 patients with locally advanced pancreatic cancer who received this treatment in conjunction with chemotherapy.119 No complications occurred, and the authors noted a transient improvement in pain but no long-term survival benefit with this approach.
A second study of 15 patients with unresectable pancreatic cancer using EUS-guided FNI of iodine-125 seeds found 30% showed some clinical benefit, whereas 20% (3 of 15) had localized complications of pancreatitis and pseudocyst formation.120 A more recent report in a porcine model suggests placement of iodine-125 seeds adjacent to the celiac ganglia via EUS-guided FNI may induce neuronal apoptosis and may provide palliation of pain in patients with advanced pancreatic cancer.121 Future studies are needed to determine the utility and indications for this use of this novel technique.
Technical Considerations
The technique of EUS-guided FNA has been well described in the literature.39,122–127 Specifically, the area of interest is visualized by EUS and placed within the center (or just slightly left of center on the monitor) of the imaging field. Doppler imaging is used as needed to identify vascularity of the lesion and to assess adjacent vascular structures. The needle, with the stylet slightly withdrawn to provide a sharp needle tip, is advanced through the endoscope biopsy channel and advanced into the lesion under direct ultrasound visualization, avoiding any possible intervening vascular structures. The central stylet is advanced to remove any tissue that may be present in the needle as a result of the transluminal puncture and subsequently completely removed. A 10-mL syringe is attached to the hub of the needle, and suction is applied as the needle is moved back and forth within the lesion (we typically perform 10 to-and-fro cycles). The suction is slowly released, the needle is retracted in the catheter, and the entire assembly is removed from the biopsy channel.
Studies regarding whether the choice of needle affects overall diagnostic yield or accuracy have yielded mixed results (Fig. 42.12). A study of 24 patients with solid pancreatic masses who underwent attempted tissue sampling with a 25-gauge, 22-gauge, and TCB (discussed subsequently) showed similar diagnostic accuracy rates of 91.7%, 95%, and 91.7% when technical success was achieved with each needle. However, technical success rates were significantly lower (50%) with the TCB, owing to its difficulty in accessing head and uncinate lesions, which lead to overall accuracy rates of 91.7%, 79.7%, and 54.1%. These findings suggested no statistically significant difference in overall diagnostic accuracy between the 25-gauge and 22-gauge needles, although the authors commented that the 25-gauge needle was easier to use and was associated with significantly higher technical success rates compared with the 22-gauge needle for uncinate lesions.128
A similar study of 12 patients with pancreatic masses who underwent FNA with both 25-gauge and 22-gauge needles found no difference in cellularity with either needle, but three needle failures were seen in the 25-gauge group. A diagnosis was made in all cases.129 A prospective, randomized controlled trial of 25-gauge (n = 67) versus 22-gauge (n = 64) needles for solid pancreatic masses found diagnostic accuracy rates of 95.5% versus 87.5% for the two needles. A similar number of passes was needed to achieve a diagnosis for each needle, and the findings were not statistically significant. The authors noted that the study may have been underpowered to detect small differences (up to 10%) in diagnostic accuracy.
In contrast to these data, two more recent larger studies have found significant improvement with the use of a 25-gauge needle in the diagnosis of solid pancreatic masses via EUS-guided FNA. The first study comprised 842 patients with pancreatic masses on EUS over a 6-year period who underwent EUS-guided FNA with a 22-gauge (n = 540) or 25-gauge (n = 302) needle. Sensitivity, specificity, positive predictive value, and negative predictive value were 84%, 100%, 100%, and 49% for the 22-gauge group and 92%, 97%, 98%, and 89% for the 25-gauge group. No complications were seen in the 25-gauge group; pancreatitis was reported in 2% of the 22-gauge group.130 Our group reported on 100 patients with pancreatic masses undergoing EUS-guided FNA in each of two distinct time groups. The 22-gauge needle had sensitivity, specificity, positive predictive value, and negative predictive value of 88%, 100%, 100%, and 67% compared with 99%, 100%, 100%, and 93% for the 25-gauge needle. In patients ultimately diagnosed with a pancreatic malignancy, 95% achieved a diagnosis within two passes using a 25-gauge needle compared with only 88% achieving a diagnosis after six passes with a 22-gauge needle. No complications occurred in either group.131
A more recent advance in our tissue sampling armamentarium was the introduction of the EUS-guided TCB in 2002 (Fig. 42.13). This needle containing an 18-mm tissue tray allows for histologic, rather than cytologic, analysis owing to the ability of EUS-guided TCB to obtain tissue cores that enable the assessment of both tissue architecture and cellularity. EUS-guided TCB seems to allow improved diagnosis of autoimmune pancreatitis compared with standard EUS-FNA.132,133 Other suggested indications where EUS-guided TCB may be preferred over EUS-guided FNA include in the diagnosis of well-differentiated neoplasms, vascular lesions, and cystic or metastatic pancreatic tumors.134
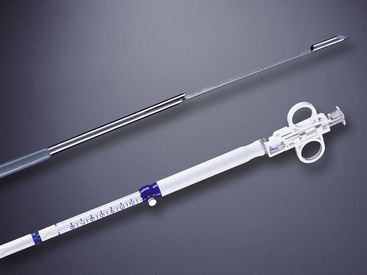
Fig. 42.13 Quick-Core (Cook Medical, Orange, CA) 19-gauge needle.
(Courtesy of Cook Medical, Orange, CA.)
Although EUS-guided TCB offers the theoretical advantage of diagnosing early chronic pancreatitis owing to its ability to obtain a core tissue specimen, the only study to date looking at this issue found that only 1 of 16 patients with suspected nonfocal chronic pancreatitis had tissue confirmation with EUS-TCB, leading the authors to discourage its use for this indication.135 In addition, several studies have reported that although no significant difference was noted in diagnostic yields or accuracy between FNA and TCB,136,137 the combination of the two modalities may be better than either alone.138 As mentioned earlier, a comparative study of 25-gauge, 22-gauge, and TCB needles in 24 patients with solid pancreatic lesions showed EUS-guided TCB achieved the lowest accuracy rates, a result almost entirely due to the fact that EUS-guided TCB was deployed successfully in only 11 of 24 patients and in only 1 of 12 patients with head or uncinate lesions.128
The most recent and largest study of 113 patients undergoing EUS-guided TCB of pancreatic lesions also showed that EUS-guided TCB had a relatively poor sensitivity and overall diagnostic accuracy of 62% and 67.5%.139 In contrast to prior reports, the size or location of the lesion within the pancreas in this study did not seem to affect the diagnostic yield. A multivariate analysis showed that only a transgastric approach for EUS-guided TCB and obtaining more than two passes improved diagnostic yield. No pancreatitis-associated complications were observed.
Although EUS-guided TCB offers the ability to obtain information about tissue structure, it seems to have a similar (and most likely reduced) diagnostic accuracy in assessing pancreatic lesions compared with standard FNA needles, likely owing to technical issues related to its deployment in the duodenum. It is best used for lesions greater than 2 cm in size that can be accessed via a transgastric approach. It seems relatively safe, especially with operator experience. Overall complication rates reported with EUS-guided TCB appear to be less than 2%, similar to complication rates reported for EUS-guided FNA.140 We limit our use of this device to cases where information regarding the pancreatic tissue architecture is essential to the patient’s clinical management or when a patient with a highly suspicious pancreatic lesion has a nondiagnostic EUS-guided FNA.
Prioritizing Lesions for Fine Needle Aspiration
There are situations when more than one lesion in a patient may be targeted for EUS-guided FNA. The priority and sequence for multiple lesions in a patient with a pancreatic primary are summarized in Table 42.4. The sequence priority is predicated on the principle of confirming the most advanced stage and economizing the number of passes.
Table 42.4 Sequence Priority for Endoscopic Ultrasound–Guided Fine Needle Aspiration in a Patient with a Pancreatic Tumor
| Target Site | Average No. Passes | Sequence Priority |
|---|---|---|
| Mediastinal lymph nodes | 2 (range 1–5) | 1 |
| Ascites or pleural fluid | 1 | 2 |
| Liver | 2 (range 1–5) | 3 |
| Distant (e.g., celiac) lymph node | 2 (range 1–10) | 4 |
| Proximal lymph node | 2 (range 1–10) | 5 |
| Pancreatic tumor | 3–5 (range 1–19) | 6 |
Number of Passes
Pancreatic adenocarcinoma generally requires the greatest number of FNA passes to obtain an adequate specimen. Approximately three to five passes are required (range 1 to 19).2 Pancreatic tumors may have extensive fibrosis (desmoplastic reaction) or necrosis, which decreases the cellularity of malignant cells. The number of passes required for pancreatic tumors may be related to the differentiation of the tumor. A study designed to assess prospectively whether any patient or EUS characteristics could predict the number of EUS-guided FNA passes needed for diagnosis of pancreatic malignancy has been performed.141 Among 95 patients undergoing EUS-guided FNA of a pancreatic mass, the average number of needle passes into the mass (includes head, neck, body, and tail) was 3.44 ± 2.19 (range 1 to 10). Tumors that were well differentiated required an average of 5.5 passes to obtain an adequate specimen; this is significantly different from 2.7 passes for moderately differentiated tumors and 2.3 passes for poorly differentiated tumors (P < .001).
Based on this study, it was recommended that without a cytopathologist in attendance, five to six passes should be made for pancreatic masses. However, this approach would still be associated with a 10% to 15% reduction in definitive cytologic diagnoses, extra procedure time, increased risk, and additional needles compared with having “real-time” cytopathology interpretations. Lymph nodes and liver lesions generally require much fewer passes. In an earlier series of 171 patients, the median number of passes for lymph node was 2 (range 1 to 10).39 Liver lesions in one series showed that the average number of passes was similarly 2 (range 1 to 5).42 Lymph node and liver metastases generally do not exhibit the desmoplastic or necrotic reaction that is common for primary tumors of the pancreas.
Ascites and pleural fluid on average require only a single FNA pass to obtain a specimen for cytologic diagnosis.51 The endosonographer should try to obtain as much fluid as possible (preferably >10 mL). The fluid is spun down to concentrate the cells on a slide. Making the diagnosis of peritoneal metastasis from ascitic fluid has a lower yield than solid lesions (approximately 50% false-negative rate, especially with small amounts of fluid). However, a positive cytology is still very helpful in staging the tumor as unresectable. Ascitic fluid, if present, should be aspirated first before any other abdominal lesions, especially given the possibility of contamination of the ascites from other lesions if they were to undergo FNA first. In the presence of ascites, FNA of the primary pancreas tumor, lymph node metastasis, or liver metastasis theoretically could contaminate the fluid. In addition, the process of preparing cytology slides from ascitic fluid requires an additional step of concentrating the cells by centrifugation. We often aspirate the fluid first, even before performing vascular staging, to allow sufficient time for cytologic interpretation before moving on to the second lesion. For the reasons of most relevant staging information and efficiency of FNA passes, the priority sequence for FNA is suspicious mediastinal adenopathy, ascites, liver metastases, distant and local lymph nodes, and primary pancreatic tumor.
The importance of dynamic “real-time” cytologic interpretation has been emphasized in numerous studies.2,39,123,142 These studies have shown that centers with an attendant cytopathologist had higher cytologic yield and diagnostic accuracy compared with centers that performed passes on an empiric basis. Increasing the number of empiric passes may increase cytologic accuracy but at the expense of performing unnecessary passes with associated cost, time, and safety issues. A more recent abstract reported the experience of a single endosonographer practicing in two clinical sites, one with an attendant cytopathologist and one without.143 In the site without an attendant cytopathologist, 17% of patients required repeat procedures compared with 2% of patients requiring repeat procedures in the site with a cytopathologist present (P = .015). This study further confirmed that on-site cytopathologic interpretation during EUS-guided FNA has a significant clinical impact by increasing the diagnostic yield of the FNA and suggests that EUS centers should allocate resources for on-site cytopathologic evaluation.
Needle Insertion and Suction
The technique of needle advancement can vary considerably from very fine motion of the needle handle, using only the fine motor pincher muscles of the thumb and index finger, to very large motions using a full hand grasp on the needle handle with gross motor elbow and shoulder movements similar to that of downward thrust with an ice pick. The optimal technique of advancing the needle varies according to three factors: (1) the consistency of the GI wall (wall parameter), (2) the size and consistency of the lesion targeted (lesion parameter), and (3) the proximity of surrounding vessels (vessel parameter). Table 42.5 summarizes the needle advancement technique with respect to these parameters.
The amount of pressure to apply to the suction syringe also needs to be considered. For most lesions, 5 mL of continuous suction applied to a 10-mL syringe is optimal. One report assessing various syringe sizes and continuous versus intermittent suction of lymph nodes from an autopsy specimen showed that continuous rather than intermittent suction with smaller syringes (5- to 10-mL) provided optimal cellularity and that use of larger (20- to 30-mL) syringes did not improve the rate of obtaining a diagnostic specimen.144 If a large amount of blood is present on the smear after the first pass, one should consider very little (2 to 3 mL) or no suction. Too much suction in a vascular lesion sometimes may result in an inadequate cytology specimen owing to the overwhelming amount of red blood cells.
Complications of Endoscopic Ultrasound–Guided Fine Needle Aspiration of Pancreaticobiliary Lesions
An important part of maximizing the yield of EUS-guided FNA is being aware of and avoiding potential complications. These complications include pancreatitis, bleeding, infection, seeding of malignant cells, and bile peritonitis. Initial data on the overall complication rates of EUS-guided FNA came from three large published series comprising more than 1000 patients.3,39,145 One multicenter trial showed that complications associated with the procedure (457 patients) seem to arise predominantly from infectious or hemorrhagic events after puncturing pancreatic cystic lesions.39 Five nonfatal complications occurred for a rate of 0.5% (95% confidence interval 0.1% to 0.8%) in solid lesions versus 14% (95% confidence interval 6% to 21%) in cystic lesions (P < .001). Another single-institution study among 333 patients who underwent EUS-guided FNA experienced only one complication (0.3%)—a streptococcal sepsis after puncture of a cystic pancreatic lesion.3 A small risk (1 in 121 patients) of developing pancreatitis after EUS-guided FNA of the pancreas has been reported.146 The overall risk of FNA is extremely low. Several more recent studies have reported on the specific risks of FNA of pancreaticobiliary lesions and are discussed subsequently.
The overall risk of pancreatitis from EUS-guided FNA appears to be less than 1%. A large retrospective series of 4909 patients undergoing EUS-guided FNA of solid pancreatic lesions from 19 centers over a 4-year period showed a 0.29% risk of pancreatitis.147 This risk was 0.64% from the two centers that prospectively collected data and 0.26% from the 17 centers that retrospectively collected data, suggesting an underreporting of complications in the latter group. A subsequent prospective single-center study by the same lead author revealed a pancreatitis rate for solid pancreatic lesions of 0.85% (3 of 355).148 The rates of pancreatitis are similarly low for cystic lesions; a large study of 603 patients with 651 pancreatic cysts undergoing EUS-guided FNA revealed only six cases of pancreatitis (0.92%) and an overall complication rate of only 2.2%.149 Similarly, the complication rate from EUS-guided TCB is less than 2% and not significantly different from EUS-guided FNA.
Hemorrhage is a rare complication of EUS and EUS-guided FNA. Gress and colleagues146 initially reported two cases of hemorrhage, resulting in one death, in 208 patients who underwent 705 FNA passes using both radial and linear echoendoscopes. In both cases, EUS was performed using a radial echoendoscope, which precluded visualization of the needle tip during FNA. In one case, bleeding extended into the gastric lumen, whereas bleeding occurred in the pancreatic head region in the other case.
A more recent study from Denmark reported on 3324 consecutive patients who underwent EUS procedures using curvilinear echoendoscopes over an 11-year period, of which 670 underwent EUS-guided FNA and 136 received EUS-guided interventions.150 Only a single case of GI bleeding was reported in a patient with widespread pancreatic cancer who died 6 hours after EUS-guided FNA from massive GI bleeding. However, at autopsy, the FNA puncture site showed no signs of bleeding and no vessels in the puncture route, and the cause of bleeding could not be established.
A third study described self-limited, non–transfusion-requiring intraluminal bleeding occurring in 3 of 90 patients undergoing EUS-guided FNA of solid pancreatic masses.151 These consisted of a small rim of blood after FNA, and one additional patient developed an intramural duodenal hematoma for an overall bleeding complication rate of 4.4%.
Limited data on extramural hemorrhage exist. One study reported 3 of 227 patients (1.3%) undergoing FNA had extraluminal hemorrhage at the site of aspiration.152 The bleeding lesions included a large pancreatic islet cell tumor, a benign lymph node in a patient with esophageal cancer, and a recurrent benign pancreatic cyst in a patient with a history of a mucinous cystadenoma that had been surgically resected. The authors described the bleeding as an expanding echo-poor region adjacent to the sampled lesions, although no clinically recognized sequelae of bleeding were noted. No predictive factors for bleeding were identified. The investigators applied 15 to 25 minutes of pressure at the puncture site via balloon inflation and echoendoscope tip deflection, although it is unclear if this had a tamponade effect on extraluminal bleeding.
A case of self-limited retroperitoneal bleeding after EUS-guided FNA of the pancreas has also been reported.153 Self-limited intracystic hemorrhage after EUS-guided FNA of pancreatic cysts has been reported to occur in 6% (3 of 50) patients in one series,154 and clinically evident hemosuccus pancreaticus has also been observed after EUS-guided FNA of a pancreatic cyst.155 However, a more recent and larger series of 651 cysts undergoing EUS-guided FNA revealed only a single retroperitoneal bleed.149 These data indicate that clinically significant bleeding after EUS-guided FNA of solid or cystic pancreaticobiliary lesions is extremely uncommon with current techniques.
As stated previously, EUS-guided FNA seems primarily to be associated with infectious complications when cystic lesions are aspirated. Initial studies reported infectious complications in 14% (3 of 22) of patients undergoing EUS-guided FNA of pancreatic cystic lesions.39 As a result, routine preprocedure and postprocedure antibiotic prophylaxis was adopted for FNA of such lesions. A single case of streptococcal sepsis after EUS-guided FNA of a pancreatic cystadenoma was reported in a large single-center report of 317 patients undergoing FNA of 327 sites3; this infection occurred despite the use of prophylactic antibiotics and corresponded to an infectious complication rate of 0.3%. Fever, although infrequent, has also been reported in several studies after EUS-guided FNA. We reported 1 patient of 44 (2%) undergoing EUS-guided FNA for pancreatic lesions who developed fever after sampling of a pancreatic cystic lesion.11 This patient did not receive antibiotics before FNA.
Another series showed that 2 patients developed fever and infection out of 355 patients with solid pancreatic masses undergoing EUS-guided FNA, yielding an infectious complication rate of 0.56%.148 One of these patients had cystic spaces within a pancreatic adenocarcinoma and the other, who required surgical débridement, had acute pancreatitis with a focal lesion in the pancreatic tail. Bournet and colleagues156 reported 1 patient of 224 undergoing EUS-guided FNA (0.45%) who developed fever and abdominal pain after aspiration of a mucinous cystadenoma, despite administration of antibiotics before the procedure. Similarly, Lee and colleagues149 reported 1 of 603 patients undergoing aspiration of 651 pancreatic cysts who developed fever, pain, and leukocytosis, although it is unclear if this patient received antibiotics before the procedure. As a result of these data, current guidelines recommend the use of antibiotic prophylaxis for planned FNA of any cystic lesion but not for solid pancreaticobiliary lesions.
The risk of malignant seeding from EUS-guided FNA is also believed to be very low. However, a case of a focal gastric intramural recurrence at the site of an EUS-guided FNA of a pancreatic tail lesion that underwent distal pancreatectomy was reported 21 months after a surgical resection with negative margins.157 Although seeding may occur with this technique, EUS-guided FNA seems to have a significantly lower potential for peritoneal seeding (2.2% vs. 16.3%) compared with CT-guided FNA, as mentioned earlier in the section on EUS-guided FNA of pancreatic lesions.14
EUS-guided FNA of the bile duct and gallbladder has been reported to cause bile peritonitis. A case of this complication requiring laparotomy was reported after FNA of a pancreatic head mass in which the obstructed bile duct was traversed with the FNA needle.158 Another study evaluating EUS-guided FNA to diagnose gallbladder microlithiasis was halted after two of the first three patients developed bile peritonitis.159 FNA of solid gallbladder masses in which the gallbladder lumen was avoided seems to be safe.64 The specific complications associated with EUS-guided FNI and other therapeutic EUS interventions have been discussed earlier separately in the sections describing these techniques.
1 Rosch T, Lorenz R, Braig C, et al. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc. 1991;37:347-352.
2 Chang K, Wiersema M, Giovannini M, et al. Multi-center collaborative study on endoscopic ultrasound (EUS) guided fine needle aspiration (FNA) of the pancreas. Gastrointest Endosc. 1996;43:A507.
3 Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: A large single centre experience. Gut. 1999;44:720-726.
4 DeWitt J, Devereaux B, Chriswell M, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753-763.
5 Bhutani MS, Gress FG, Giovannini M, et al. The No Endosonographic Detection of Tumor (NEST) study: A case series of pancreatic cancers missed on endoscopic ultrasonography. Endoscopy. 2004;36:385-389.
6 Fritscher-Ravens A, Brand L, Knofel WT, et al. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol. 2002;97:2768-2775.
7 Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728-736.
8 LeBlanc JK, Ciaccia D, Al-Assi MT, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475-481.
9 Erickson RA, Garza AA. Impact of endoscopic ultrasound on the management and outcome of pancreatic carcinoma. Am J Gastroenterol. 2000;95:2248-2254.
10 Gress F, Gottlieb K, Sherman S, et al. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med. 2001;134:459-464.
11 Chang KJ, Nguyen P, Erickson RA, et al. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387-393.
12 Erickson RA, Garza AA. EUS with EUS-guided fine-needle aspiration as the first endoscopic test for the evaluation of obstructive jaundice. Gastrointest Endosc. 2001;53:475-484.
13 Chen VK, Arguedas MR, Kilgore ML, et al. A cost-minimization analysis of alternative strategies in diagnosing pancreatic cancer. Am J Gastroenterol. 2004;99:2223-2234.
14 Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690-695.
15 Song MH, Lee SK, Kim MH, et al. EUS in the evaluation of pancreatic cystic lesions. Gastrointest Endosc. 2003;57:891-896.
16 Ahmad NA, Kochman ML, Brensinger C, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc. 2003;58:59-64.
17 Brugge WR. Role of endoscopic ultrasound in the diagnosis of cystic lesions of the pancreas. Pancreatology. 2001;1:637-640.
18 Frossard JL, Amouyal P, Amouyal G, et al. Performance of endosonography-guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. Am J Gastroenterol. 2003;98:1516-1524.
19 Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336.
20 Belsley NA, Pitman MB, Lauwers GY, et al. Serous cystadenoma of the pancreas: Limitations and pitfalls of endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer. 2008;114:102-110.
21 Sreenarasimhaiah J, Lara LF, Jazrawi SF, et al. A comparative analysis of pancreas cyst fluid CEA and histology with DNA mutational analysis in the detection of mucin producing or malignant cysts. JOP. 2009;10:163-168.
22 Sawhney MS, Devarajan S, O’Farrel P, et al. Comparison of carcinoembryonic antigen and molecular analysis in pancreatic cyst fluid. Gastrointest Endosc. 2009;69:1106-1110.
23 Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: A report of the PANDA study. Gastrointest Endosc. 2009;69:1095-1102.
24 Powis ME, Nguyen P, Chang K. A novel endoscopic ultrasound (EUS) guided fine needle aspiration (FNA) technique for the diagnosis of malignant cystic lesions of the pancreas. Gastrointest Endosc. 2000;51:164.
25 Levy MJ, Smyrk TC, Reddy RP, et al. Endoscopic ultrasound-guided Trucut biopsy of the cyst wall for diagnosing cystic pancreatic tumors. Clin Gastroenterol Hepatol. 2005;3:974-979.
26 Ali S, Wilkinson N, Jensen CS, et al. EUS-guided Trucut biopsies may enable the diagnosis of lymphoepithelial cysts of the pancreas: Report of two cases. JOP. 2009;10:409-412.
27 Al-Haddad M, Raimondo M, Woodward T, et al. Safety and efficacy of cytology brushings versus standard FNA in evaluating cystic lesions of the pancreas: A pilot study. Gastrointest Endosc. 2007;65:894-898.
28 Zimmer T, Scherubl H, Faiss S, et al. Endoscopic ultrasonography of neuroendocrine tumours. Digestion. 2000;62(Suppl 1):45-50.
29 Bansal R, Tierney W, Carpenter S, et al. Cost effectiveness of EUS for preoperative localization of pancreatic endocrine tumors. Gastrointest Endosc. 1999;49:19-25.
30 Gines A, Vazquez-Sequeiros E, Soria MT, et al. Usefulness of EUS-guided fine needle aspiration (EUS-FNA) in the diagnosis of functioning neuroendocrine tumors. Gastrointest Endosc. 2002;56:291-296.
31 Ardengh JC, de Paulo GA, Ferrari AP. EUS-guided FNA in the diagnosis of pancreatic neuroendocrine tumors before surgery. Gastrointest Endosc. 2004;60:378-384.
32 Kongkam P, Al-Haddad M, Attasaranya S, et al. EUS and clinical characteristics of cystic pancreatic neuroendocrine tumors. Endoscopy. 2008;40:602-605.
33 Gress FG, Barawi M, Kim D, et al. Preoperative localization of a neuroendocrine tumor of the pancreas with EUS-guided fine needle tattooing. Gastrointest Endosc. 2002;55:594-597.
34 Jurgensen C, Schuppan D, Neser F, et al. EUS-guided alcohol ablation of an insulinoma. Gastrointest Endosc. 2006;63:1059-1062.
35 Mortensen MB, Pless T, Durup J, et al. Clinical impact of endoscopic ultrasound-guided fine needle aspiration biopsy in patients with upper gastrointestinal tract malignancies: A prospective study. Endoscopy. 2001;33:478-483.
36 Harewood GC, Wiersema MJ. A cost analysis of endoscopic ultrasound in the evaluation of pancreatic head adenocarcinoma. Am J Gastroenterol. 2001;96:2651-2656.
37 Ozaki H, Hiraoka T, Mizumoto R, et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today. 1999;29:16-22.
38 Durbin TE, Chang KJ. Endoscopic ultrasound (EUS) criteria for predicting malignant lymph nodes using linear array and radial scans—correlation with EUS-guided fine needle aspiration (FNA). Gastrointest Endosc. 1996;43:418.
39 Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: Diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095.
40 Yamada T, Nakamori S, Ohzato H, et al. Outcome of pancreatic cancer patients based on genetic lymph node staging. Int J Oncol. 2000;16:1165-1171.
41 Agarwal B, Gogia S, Eloubeidi MA, et al. Malignant mediastinal lymphadenopathy detected by staging EUS in patients with pancreaticobiliary cancer. Gastrointest Endosc. 2005;61:849-853.
42 Nguyen P, Feng JC, Chang KJ. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (FNA) of liver lesions. Gastrointest Endosc. 1999;50:357-361.
43 tenBerge J, Hoffman BJ, Hawes RH, et al. EUS-guided fine needle aspiration of the liver: Indications, yield, and safety based on an international survey of 167 cases. Gastrointest Endosc. 2002;55:859-862.
44 Awad SS, Fagan S, Abudayyeh S, et al. Preoperative evaluation of hepatic lesions for the staging of hepatocellular and metastatic liver carcinoma using endoscopic ultrasonography. Am J Surg. 2002;184:601-604.
45 DeWitt J, LeBlanc J, McHenry L, et al. Endoscopic ultrasound-guided fine needle aspiration cytology of solid liver lesions: A large single-center experience. Am J Gastroenterol. 2003;98:1976-1981.
46 Prasad P, Schmulewitz N, Patel A, et al. Detection of occult liver metastases during EUS for staging of malignancies. Gastrointest Endosc. 2004;59:49-53.
47 Singh P, Erickson RA, Mukhopadhyay P, et al. EUS for detection of the hepatocellular carcinoma: Results of a prospective study. Gastrointest Endosc. 2007;66:265-273.
48 Singh P, Mukhopadhyay P, Bhatt B, et al. Endoscopic ultrasound versus CT scan for detection of the metastases to the liver: Results of a prospective comparative study. J Clin Gastroenterol. 2009;43:367-373.
49 Crowe DR, Eloubeidi MA, Chhieng DC, et al. Fine-needle aspiration biopsy of hepatic lesions: Computerized tomographic-guided versus endoscopic ultrasound-guided FNA. Cancer. 2006;108:180-185.
50 Hollerbach S, Willert J, Topalidis T, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy of liver lesions: Histological and cytological assessment. Endoscopy. 2003;35:743-749.
51 Nguyen PT, Chang KJ. EUS in the detection of ascites and EUS-guided paracentesis. Gastrointest Endosc. 2001;54:336-339.
52 Kaushik N, Khalid A, Brody D, et al. EUS-guided paracentesis for the diagnosis of malignant ascites. Gastrointest Endosc. 2006;64:908-913.
53 DeWitt J, LeBlanc J, McHenry L, et al. Endoscopic ultrasound-guided fine-needle aspiration of ascites. Clin Gastroenterol Hepatol. 2007;5:609-615.
54 Schmulewitz N, Singh P, Safa M, et al. Diagnosis of peritoneal carcinomatosis by EUS-guided FNA. Gastrointest Endosc. 2007;66:825-826.
55 Peter S, Eltoum I, Eloubeidi MA. EUS-guided FNA of peritoneal carcinomatosis in patients with unknown primary malignancy. Gastrointest Endosc. 2009;70:1266-1270.
56 Matsushita M, Hajiro K, Takakuwa H, et al. Transvaginal versus transrectal ultrasonography in the diagnosis of peritoneal carcinomatosis. Am J Gastroenterol. 2000;95:1381.
57 Fritscher-Ravens A, Broering DC, Sriram PV, et al. EUS-guided fine-needle aspiration cytodiagnosis of hilar cholangiocarcinoma: A case series. Gastrointest Endosc. 2000;52:534-540.
58 Sharma A, Chang KJ, Nguyen PT. The role of endoscopic ultrasound (EUS) and EUS-guided fine needle aspiration (FNA) in the diagnosis of proximal biliary strictures. Gastrointest Endosc. 2003;57:AB681.
59 Eloubeidi MA, Chen VK, Jhala NC, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209-213.
60 Fritscher-Ravens A, Broering DC, Knoefel WT, et al. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol. 2004;99:45-51.
61 DeWitt J, Misra VL, Leblanc JK, et al. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest Endosc. 2006;64:325-333.
62 Meara RS, Jhala D, Eloubeidi MA, et al. Endoscopic ultrasound-guided FNA biopsy of bile duct and gallbladder: Analysis of 53 cases. Cytopathology. 2006;17:42-49.
63 Gleeson FC, Rajan E, Levy MJ, et al. EUS-guided FNA of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointest Endosc. 2008;67:438-443.
64 Jacobson BC, Pitman MB, Brugge WR. EUS-guided FNA for the diagnosis of gallbladder masses. Gastrointest Endosc. 2003;57:251-254.
65 Cannon ME, Carpenter SL, Elta GH, et al. EUS compared with CT, magnetic resonance imaging, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest Endosc. 1999;50:27-33.
66 Muthusamy R, Jafri SF, Jivcu C, et al. Endoscopic ultrasound (EUS) guided fine needle aspiration (FNA) in the diagnosis and staging of ampullary neoplasms. Gastrointest Endosc. 2001;53:176.
67 Defrain C, Chang CY, Srikureja W, et al. Cytologic features and diagnostic pitfalls of primary ampullary tumors by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer. 2005;105:289-297.
68 Ito K, Fujita N, Noda Y, et al. Preoperative evaluation of ampullary neoplasm with EUS and transpapillary intraductal US: A prospective and histopathologically controlled study. Gastrointest Endosc. 2007;66:740-747.
69 Artifon EL, Couto DJr, Sakai P, et al. Prospective evaluation of EUS versus CT scan for staging of ampullary cancer. Gastrointest Endosc. 2009;70:290-296.
70 Chen CH, Yang CC, Yeh YH, et al. Reappraisal of endosonography of ampullary tumors: Correlation with transabdominal sonography, CT, and MRI. J Clin Ultrasound. 2009;37:18-25.
71 Wiersema M, Wiersema L. Endosonography guided celiac plexus neurolysis (EUS-CPN) in patients with pain due to intra-abdominal malignancy (IAM). Gastrointest Endosc. 1996;43:A565.
72 Gress F, Schmitt C, Sherman S, et al. Endoscopic ultrasound-guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: A prospective single center experience. Am J Gastroenterol. 2001;96:409-416.
73 Puli SR, Reddy JB, Bechtold ML, et al. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: A meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330-2337.
74 LeBlanc JK, DeWitt J, Johnson C, et al. A prospective randomized trial of 1 versus 2 injections during EUS-guided celiac plexus block for chronic pancreatitis pain. Gastrointest Endosc. 2009;69:835-842.
75 Sahai AV, Lemelin V, Lam E, et al. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: A comparative study of short-term effectiveness. Am J Gastroenterol. 2009;104:326-329.
76 O’Toole TM, Schmulewitz N. Complication rates of EUS-guided celiac plexus blockade and neurolysis: Results of a large case series. Endoscopy. 2009;41:593-597.
77 Gerke H, Silva RGJr, Shamoun D, et al. EUS characteristics of celiac ganglia with cytologic and histologic confirmation. Gastrointest Endosc. 2006;64:35-39.
78 Levy M, Rajan E, Keeney G, et al. Neural ganglia visualized by endoscopic ultrasound. Am J Gastroenterol. 2006;101:1787-1791.
79 Gleeson FC, Levy MJ, Papachristou GI, et al. Frequency of visualization of presumed celiac ganglia by endoscopic ultrasound. Endoscopy. 2007;39:620-624.
80 Levy MJ, Topazian MD, Wiersema MJ, et al. Initial evaluation of the efficacy and safety of endoscopic ultrasound-guided direct ganglia neurolysis and block. Am J Gastroenterol. 2008;103:98-103.
81 Grimm H, Binmoeller KF, Soehendra N. Endosonography-guided drainage of a pancreatic pseudocyst. Gastrointest Endosc. 1992;38:170-171.
82 Brandon JL, Ruden NM, Turba UC, et al. Angiographic embolization of arterial hemorrhage following endoscopic US-guided cystogastrostomy for pancreatic pseudocyst drainage. Diagn Interv Radiol. 2008;14:57-60.
83 Brugge WR. The role of EUS in the diagnosis of cystic lesions of the pancreas. Gastrointest Endosc. 2000;52:S18-S22.
84 Lewandrowski KB, Southern JF, Pins MR, et al. Cyst fluid analysis in the differential diagnosis of pancreatic cysts: A comparison of pseudocysts, serous cystadenomas, mucinous cystic neoplasms, and mucinous cystadenocarcinoma. Ann Surg. 1993;217:41-47.
85 Fockens P, Johnson TG, van Dullemen HM, et al. Endosonographic imaging of pancreatic pseudocysts before endoscopic transmural drainage. Gastrointest Endosc. 1997;46:412-416.
86 Varadarajulu S, Wilcox CM, Tamhane A, et al. Role of EUS in drainage of peripancreatic fluid collections not amenable for endoscopic transmural drainage. Gastrointest Endosc. 2007;66:1107-1119.
87 Talreja JP, Shami VM, Ku J, et al. Transenteric drainage of pancreatic-fluid collections with fully covered self-expanding metallic stents (with video). Gastrointest Endosc. 2008;68:1199-1203.
88 Ahlawat SK, Charabaty-Pishvaian A, Jackson PG, et al. Single-step EUS-guided pancreatic pseudocyst drainage using a large channel linear array echoendoscope and cystotome: Results in 11 patients. JOP. 2006;7:616-624.
89 Ramesh J, Varadarajulu S. Interventional endoscopic ultrasound. Dig Dis. 2008;26:347-355.
90 Kruger M, Schneider AS, Manns MP, et al. Endoscopic management of pancreatic pseudocysts or abscesses after an EUS-guided 1-step procedure for initial access. Gastrointest Endosc. 2006;63:409-416.
91 Varadarajulu S. EUS-guided retrieval of a migrated transgastric pancreatic stent. Endoscopy. 2007;39(Suppl 1):E18-E19.
92 Giovannini M, Pesenti C, Rolland AL, et al. Endoscopic ultrasound-guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy. 2001;33:473-477.
93 Seifert H, Dietrich C, Schmitt T, et al. Endoscopic ultrasound-guided one-step transmural drainage of cystic abdominal lesions with a large-channel echo endoscope. Endoscopy. 2000;32:255-259.
94 Seifert H, Faust D, Schmitt T, et al. Transmural drainage of cystic peripancreatic lesions with a new large-channel echo endoscope. Endoscopy. 2001;33:1022-1026.
95 Varadarajulu S, Lopes TL, Wilcox CM, et al. EUS versus surgical cyst-gastrostomy for management of pancreatic pseudocysts. Gastrointest Endosc. 2008;68:649-655.
96 Varadarajulu S, Christein JD, Tamhane A, et al. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2008;68:1102-1111.
97 Chang KJ, Nguyen PT, Thompson JA, et al. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound-guided fine-needle injection in patients with advanced pancreatic carcinoma. Cancer. 2000;88:1325-1335.
98 Hecht JR, Bedford R, Abbruzzese JL, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555-561.
99 Chang KJ, Senzer N, Chung T, et al. A novel gene transfer therapy against pancreatic cancer (TNFerade) delivered by endoscopic ultrasound (EUS) and percutaneous guided fine needle injection (FNI). Gastrointest Endosc. 2004.
100 Farrell JJ, Senzer N, Hecht JR, et al. Long-term data for endoscopic ultrasound (EUS) and percutanous (PTA) guided intratumoral TNFerade gene delivery combined with chemoradiation in the treatment of locally advanced pancreatic cancer (LAPC). Gastrointest Endosc. 2006;63:AB93.
101 Chang KJ, Lee JG, Holcombe RF, et al. Endoscopic ultrasound delivery of an antitumor agent to treat a case of pancreatic cancer. Nat Clin Pract Gastroenterol Hepatol. 2008;5:107-111.
102 Mundt AJ, Vijayakumar S, Nemunaitis J, et al. A phase I trial of TNFerade biologic in patients with soft tissue sarcoma in the extremities. Clin Cancer Res. 2004;10:5747-5753.
103 McLoughlin JM, McCarty TM, Cunningham C, et al. TNFerade, an adenovector carrying the transgene for human tumor necrosis factor alpha, for patients with advanced solid tumors: Surgical experience and long-term follow-up. Ann Surg Oncol. 2005;12:825-830.
104 Kasuya H, Takeda S, Nomoto S, et al. The potential of oncolytic virus therapy for pancreatic cancer. Cancer Gene Ther. 2005;12:725-736.
105 Irisawa A, Takagi T, Kanazawa M, et al. Endoscopic ultrasound-guided fine-needle injection of immature dendritic cells into advanced pancreatic cancer refractory to gemcitabine: A pilot study. Pancreas. 2007;35:189-190.
106 Oh HC, Seo DW, Lee TY, et al. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc. 2008;67:636-642.
107 Wiersema MJ, Sandusky D, Carr R, et al. Endosonography-guided cholangiopancreatography. Gastrointest Endosc. 1996;43:102-106.
108 Dewitt J, McHenry L, Fogel E, et al. EUS-guided methylene blue pancreatography for minor papilla localization after unsuccessful ERCP. Gastrointest Endosc. 2004;59:133-136.
109 Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest Endosc. 2004;59:100-107.
110 Kahaleh M, Wang P, Shami VM, et al. EUS-guided transhepatic cholangiography: Report of 6 cases. Gastrointest Endosc. 2005;61:307-313.
111 Kahaleh M, Hernandez AJ, Tokar J, et al. Interventional EUS-guided cholangiography: Evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52-59.
112 Burmester E, Niehaus J, Leineweber T, et al. EUS-cholangio-drainage of the bile duct: Report of 4 cases. Gastrointest Endosc. 2003;57:246-251.
113 Kahaleh M, Yoshida C, Yeaton P. EUS antegrade pancreatography with gastropancreatic duct stent placement: Review of two cases. Gastrointest Endosc. 2003;58:919-923.
114 Kahaleh M, Hernandez AJ, Tokar J, et al. EUS-guided pancreaticogastrostomy: Analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest Endosc. 2007;65:224-230.
115 Tessier G, Bories E, Arvanitakis M, et al. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest Endosc. 2007;65:233-241.
116 Pishvaian AC, Collins B, Gagnon G, et al. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64:412-417.
117 Trevino JM, Varadarajulu S. Initial experience with the prototype forward-viewing echoendoscope for therapeutic interventions other than pancreatic pseudocyst drainage (with videos). Gastrointest Endosc. 2009;69:361-365.
118 Lah JJ, Kuo JV, Chang KJ, et al. EUS-guided brachytherapy. Gastrointest Endosc. 2005;62:805-808.
119 Jin Z, Du Y, Li Z, et al. Endoscopic ultrasonography-guided interstitial implantation of iodine-125 seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: A prospective pilot study. Endoscopy. 2008;40:314-320.
120 Sun S, Xu H, Xin J, et al. Endoscopic ultrasound-guided interstitial brachytherapy of unresectable pancreatic cancer: Results of a pilot trial. Endoscopy. 2006;38:399-403.
121 Wang K, Jin Z, Du Y, et al. Evaluation of endoscopic-ultrasound-guided celiac ganglion irradiation with iodine-125 seeds: A pilot study in a porcine model. Endoscopy. 2009;41:346-351.
122 Vilmann P, Hancke S, Henriksen FW, et al. Endosonographically-guided fine needle aspiration biopsy of malignant lesions in the upper gastrointestinal tract. Endoscopy. 1993;25:523-527.
123 Chang KJ, Albers CG, Erickson RA, et al. Endoscopic ultrasound-guided fine needle aspiration of pancreatic carcinoma. Am J Gastroenterol. 1994;89:263-266.
124 Wiersema MJ, Wiersema LM, Khusro Q, et al. Combined endosonography and fine-needle aspiration cytology in the evaluation of gastrointestinal lesions. Gastrointest Endosc. 1994;40:199-206.
125 Giovannini M, Seitz JF, Monges G, et al. Fine-needle aspiration cytology guided by endoscopic ultrasonography: Results in 141 patients. Endoscopy. 1995;27:171-177.
126 Rosch T. Staging of pancreatic cancer: Analysis of literature results. Gastrointest Endosc Clin N Am. 1995;5:735-739.
127 Erickson RA, Sayage-Rabie L, Avots-Avotins A. Clinical utility of endoscopic ultrasound-guided fine needle aspiration. Acta Cytol. 1997;41:1647-1653.
128 Sakamoto H, Kitano M, Komaki T, et al. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384-390.
129 Lee JH, Stewart J, Ross WA, et al. Blinded prospective comparison of the performance of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of the pancreas and peri-pancreatic lesions. Dig Dis Sci. 2009;54:2274-2281.
130 Yusuf TE, Ho S, Pavey DA, et al. Retrospective analysis of the utility of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in pancreatic masses, using a 22-gauge or 25-gauge needle system: A multicenter experience. Endoscopy. 2009;41:445-448.
131 Nguyen TT, Whang CS, Ashida R, et al. A comparison of the diagnostic yield and specimen adequacy between 22 and 25 gauge needles for endoscopic ultrasound guided fine-needle aspiration (EUS-FNA) of solid pancreatic lesions (SPL): Is bigger better? Gastrointest Endosc. 2008;67:AB100.
132 Levy MJ, Reddy RP, Wiersema MJ, et al. EUS-guided Trucut biopsy in establishing autoimmune pancreatitis as the cause of obstructive jaundice. Gastrointest Endosc. 2005;61:467-472.
133 Mizuno N, Bhatia V, Hosoda W, et al. Histological diagnosis of autoimmune pancreatitis using EUS-guided Trucut biopsy: A comparison study with EUS-FNA. J Gastroenterol. 2009;44:742-750.
134 Levy MJ. Endoscopic ultrasound-guided trucut biopsy of the pancreas: Prospects and problems. Pancreatology. 2007;7:163-166.
135 DeWitt J, McGreevy K, LeBlanc J, et al. EUS-guided Trucut biopsy of suspected nonfocal chronic pancreatitis. Gastrointest Endosc. 2005;62:76-84.
136 Varadarajulu S, Fraig M, Schmulewitz N, et al. Comparison of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397-401.
137 Levy MJ, Jondal ML, Clain J, et al. Preliminary experience with an EUS-guided Trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101-106.
138 Wittmann J, Kocjan G, Sgouros SN, et al. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and Trucut needle biopsy: A prospective study. Cytopathology. 2006;17:27-33.
139 Thomas T, Kaye PV, Ragunath K, et al. Efficacy, safety, and predictive factors for a positive yield of EUS-guided Trucut biopsy: A large tertiary referral center experience. Am J Gastroenterol. 2009;104:584-591.
140 Pungpapong S, Wallace MB. EUS-guided Trucut needle biopsy: Is more tissue really better? Gastrointest Endosc. 2005;62:602-604.
141 Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184-190.
142 Chang KJ. Endoscopic ultrasound-guided fine needle aspiration in the diagnosis and staging of pancreatic tumors. Gastrointest Endosc Clin N Am. 1995;5:723-734.
143 Klapman J, Logrono R, Dye C, et al. Clinical impact of on-site cytopathology interpretation during endoscopic ultrasound-guided fine needle aspiration (EUS-guided FNA). Gastrointest Endosc. 2002:A1513.
144 Bhutani MS, Suryaprasad S, Moezzi J, et al. Improved technique for performing endoscopic ultrasound guided fine needle aspiration of lymph nodes. Endoscopy. 1999;31:550-553.
145 O’Toole D, Palazzo L, Arotcarena R, et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470-474.
146 Gress FG, Hawes RH, Savides TJ, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy using linear array and radial scanning endosonography. Gastrointest Endosc. 1997;45:243-250.
147 Eloubeidi MA, Gress FG, Savides TJ, et al. Acute pancreatitis after EUS-guided FNA of solid pancreatic masses: A pooled analysis from EUS centers in the United States. Gastrointest Endosc. 2004;60:385-389.
148 Eloubeidi MA, Tamhane A, Varadarajulu S, et al. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: A prospective evaluation. Gastrointest Endosc. 2006;63:622-629.
149 Lee LS, Saltzman JR, Bounds BC, et al. EUS-guided fine needle aspiration of pancreatic cysts: A retrospective analysis of complications and their predictors. Clin Gastroenterol Hepatol. 2005;3:231-236.
150 Mortensen MB, Fristrup C, Holm FS, et al. Prospective evaluation of patient tolerability, satisfaction with patient information, and complications in endoscopic ultrasonography. Endoscopy. 2005;37:146-153.
151 Voss M, Hammel P, Molas G, et al. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244-249.
152 Affi A, Vazquez-Sequeiros E, Norton ID, et al. Acute extraluminal hemorrhage associated with EUS-guided fine needle aspiration: Frequency and clinical significance. Gastrointest Endosc. 2001;53:221-225.
153 Lim WC, Leblanc JK. Retroperitoneal bleeding after EUS-guided FNA of a pancreatic mass. Gastrointest Endosc. 2006;63:499-500.
154 Varadarajulu S, Eloubeidi MA. Frequency and significance of acute intracystic hemorrhage during EUS-FNA of cystic lesions of the pancreas. Gastrointest Endosc. 2004;60:631-635.
155 Singh P, Gelrud A, Schmulewitz N, et al. Hemosuccus pancreaticus after EUS-FNA of pancreatic cyst (with video). Gastrointest Endosc. 2008;67:543.
156 Bournet B, Migueres I, Delacroix M, et al. Early morbidity of endoscopic ultrasound: 13 years’ experience at a referral center. Endoscopy. 2006;38:349-354.
157 Paquin SC, Gariepy G, Lepanto L, et al. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610-611.
158 Chen HY, Lee CH, Hsieh CH. Bile peritonitis after EUS-guided fine-needle aspiration. Gastrointest Endosc. 2002;56:594-596.
159 Jacobson BC, Waxman I, Parmar K, et al. Endoscopic ultrasound-guided gallbladder bile aspiration in idiopathic pancreatitis carries a significant risk of bile peritonitis. Pancreatology. 2002;2:26-29.