Chapter 27 Endoscopic Treatment of Superficial Esophageal Cancers
Biology of Early Esophageal Cancer
In contrast to Asia, where the high incidence of esophageal cancer has led to screening programs, esophageal cancer in the West has primarily been found when patients become symptomatic. Although it has been established that older white men with reflux symptoms are at greatest risk of esophageal adenocarcinoma and Barrett’s esophagus, the high prevalence of Barrett’s esophagus in patients without symptoms makes screening for this condition in only symptomatic patients problematic.1,2 Surveillance endoscopy finds patients with earlier staged malignancies that are suitable for endoscopic therapies.3
Cancers related to Barrett’s esophagus apparently arise from chronically inflamed areas of the mucosa. The mechanism of carcinogenesis seems to be related to inflammation producing increased levels of prostaglandin E2, which is an inflammatory mediator that can cause increased cell proliferation.4 Cell proliferation has been used as a marker of neoplasia and is an early event in cancer development. Increased cell proliferation drives the cell cycle, and progression to cancer in Barrett’s esophagus usually involves the loss of cell cycle checkpoint gene products such as p16. The loss of p16 function through either promoter inactivation via hypermethylation or loss of heterozygosity occurs early in carcinogenesis and is found almost universally in dysplastic Barrett’s mucosa.5 This loss of cell cycle control leads to further acceleration of the cell cycle, which allows further genetic events to occur including loss of p53, which is well recognized to be an important tumor suppressor gene that is also involved in promoting apoptosis of cells that have accumulated genetic defects.6 The loss of p53, which is important for elimination of cells with chromosomal abnormalities, and the increase in proliferation of cells caused by the loss of p16 lead to further chromosomal instability, which is manifested by the appearance of aneuploidy, or abnormal amounts of DNA.7 These large-scale changes of chromosomal loss are related to the degree of histologic changes of dysplasia in the tissue with minimal DNA loss in nondysplastic tissue, moderate loss in low-grade dysplasia, and large degrees of loss in patients with high-grade dysplasia or cancer.
The important aspect of the biology of Barrett’s esophagus to the endoscopist is that these genetic changes do not always correlate with histologic changes, especially when ablative therapies have been applied. Ablative therapy has the ability to decrease histologic changes of dysplasia, while allowing genetic abnormalities to persist.8 These persistent genetic abnormalities over time lead to recurrence of dysplasia and cancer. These findings suggest that histologically benign Barrett’s esophagus after ablative therapy may be precancerous, and long-term control of the neoplastic risk in Barrett’s esophagus may involve elimination of any of the tissue with genetic abnormalities.
These findings were verified by data from a randomized prospective study of photodynamic therapy for Barrett’s esophagus with high-grade dysplasia. In this study of 208 patients randomly assigned to either photodynamic therapy in combination with omeprazole or omeprazole alone, patients in whom complete elimination of Barrett’s mucosa was achieved did not progress to cancer, whereas patients with any residual Barrett’s mucosa did have a significant chance of developing cancer.9 The complete elimination of the intestinal metaplasia may be difficult even with newer therapies such as radiofrequency ablation; in a randomized trial of patients with low-grade and high-grade dysplasia, the dysplasia could be eliminated in greater than 90% of patients, but the intestinal metaplasia remained in 77%.10
Staging of Early Esophageal Cancer
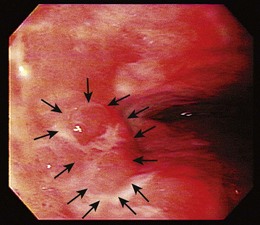
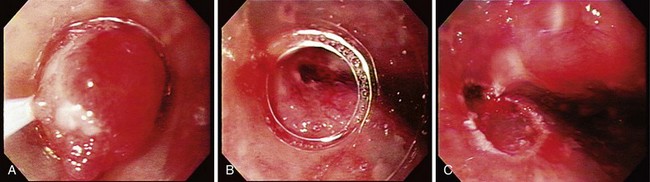
Endoscopic treatment of early esophageal cancer must involve careful and accurate staging of the malignancy. Previously, early esophageal cancer was primarily defined endoscopically on the basis of size. An example of an early cancer in Barrett’s esophagus is shown in Fig. 27.1. Cancers that were 2 cm or less in diameter were generally thought to be early cancers.11 This definition was inaccurate because visualization of local lymph nodes and assessment of the depth of tumor invasion were impossible without surgical resection during this time period. However, surgical resections have shown physicians that early mucosally based cancers above the muscularis mucosae are rarely associated with metastatic disease. In a survey of European centers that performed 253 esophagectomies for early squamous cell carcinomas, it was found that patients with disease confined to the epithelium had a survival rate of 92.8%.12 With penetration into the submucosa, the 5-year survival rate decreased to 72.8%. The overall mortality rate for esophagectomy for early cancers in this series was 9.1%. Similar results for squamous cell carcinomas were reported from Japan, where there was increased chance of metastasis if squamous cell carcinoma was found to penetrate beyond the muscularis mucosae.13
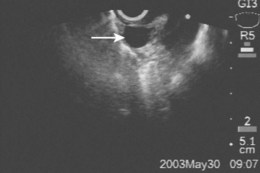
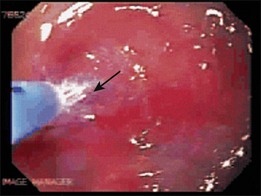
The advent of endoscopic ultrasound (EUS) has affected the need for surgical resection to define the depth of cancer invasion accurately. EUS can be performed with either dedicated echoendoscopes or high-frequency ultrasound probes. The echoendoscopes allow the endoscopist to visualize at 7.5- to 12-MHz frequencies, which permits examination of the periesophageal lymph nodes (N1 stage), celiac lymph nodes (M1a stage), nodes of the lesser curve of the stomach (N1 stage), most of the liver (M1b stage), and pleural or vascular involvement (T4 stage) by the tumor. An example of a periesophageal lymph node on EUS is shown in Fig. 27.2. A lymph node is suspicious on EUS if it is hypoechoic, rounded, in proximity to the tumor, and more than 1 cm in diameter. If suspicious lymph nodes are found, they should be sampled by fine needle aspiration performed using a linear array instrument.
For mucosally based lesions, ultrasound probes are more desirable because they can image at 20 to 30 MHz allowing the endoscopist to image at the resolution needed to resolve the muscularis mucosae to a greater extent. In addition, the use of probes allows visualization using water filling the esophagus, which is necessary to view small mucosal cancers without the artifact caused by the compression by a balloon that is traditionally used with echoendoscopes. Ultrasound probes cannot image deep into the tissue to visualize lymph nodes, however, so to stage an early cancer accurately, both probes and echoendoscopes are needed.14 Despite the use of careful ultrasound examination, lymph nodes can be missed or inaccessible because of Barrett’s mucosa that is in the field of view. It is believed that false-positive findings can be caused by contamination with epithelial cells.15
Small case series have been performed of patients with Barrett’s esophagus and early cancers staged by EUS before esophagectomy. At one center, the technique was 100% sensitive for detection of submucosal invasion but was only 90% specific for invasion in a group of 22 patients.16 Inflammatory changes are virtually impossible to differentiate from early cancer invasion by EUS techniques. Overall, EUS seems to have a tendency to overstage the depth of tumor invasion. A retrospective review of the experience of a single center with EUS staging of esophageal cancer from 1991–2001 in 222 patients found that the accuracy of EUS in tumor staging was only 54%, and the accuracy of EUS in lymph node staging was 65%.17 These results did not seem to be related to a “learning curve” in EUS interpretation because the accuracy in the first half of the time period studied was similar to the accuracy in the second half of the study period. More recent publications emphasize that although EUS may not be as accurate as previously hoped in staging, the information provided from having a negative evaluation for lymph nodes or for resectability was nonetheless valuable.14
Endoscopic mucosal resection (EMR) has been used to help define the depth of cancer penetration and as a primary treatment for early cancer in Barrett’s esophagus. EMR involves the use of a friction fitted cap that attaches to the tip of a standard endoscope. This technique was pioneered in Japan, where EMR has become the standard of care for early mucosal esophageal cancers. A survey of more than 145 Japanese hospitals found that 76% used EMR for the treatment of early esophageal cancers.18
The cap technique is the predominant technique used in the United States because of its commercial availability. The cap is available in the two styles shown in Fig. 27.3; one is completely level, whereas the other has the lip of the cap at an oblique angle. The cap also varies in terms of the consistency of the material from which it is made. Generally, hard plastic caps are favored when there is a need to try to suction more scarred tissue, whereas soft caps are more useful when passing the caps through the upper pharynx. The oblique cap is favored in resection of larger pieces of tissue, whereas the straight cap seems to be more favored when precision is required in the amount of tissue necessary for resection.
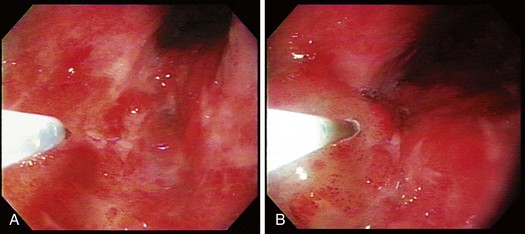
The technique of EMR is performed in a similar fashion. For early esophageal cancers, it is critical that the endoscopist is able to obtain adequate lifting of the lesion to be removed using an injection of a saline-epinephrine solution. Depending on the visibility of the lesion, the borders may need to be marked using a cautery device such as a multipolar coagulator before injection to ensure that the area to be removed can be visualized after injection. The area of carcinoma has usually been previously established by biopsy, and the area is lifted by positioning an injection needle proximal to the lesion. Generally, it is not advisable to inject into the lesion because it is theoretically possible to disseminate cancer cells into the submucosa. The sequence of an injection with adequate lifting of the target lesion is shown in Fig. 27.4.
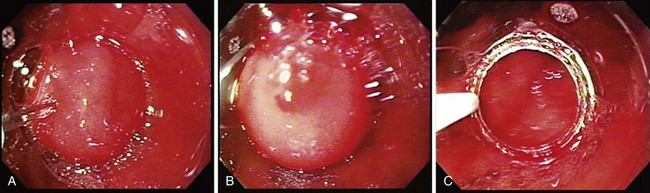
The sequence of positioning a snare around the lip of a mucosal resection cap is shown in Fig. 27.5. This is often the most difficult portion of the mucosal resection because if the snare is not properly formed, suctioning the tissue into the cap can result in dislodgment of the snare and improper tissue resection. In addition, the snare is easily deformed and can be twisted during the process of forming the loop around the lip of the resection cap resulting in the need for a new crescent snare. When the snare is positioned, it is important that the technician assisting with the snare not move it because even slight disruptions in the position of the snare can cause it to dislodge.
The tissue resection is completed by suctioning the tissue into the cap as shown in Fig. 27.6. To accomplish a wider resection, more tissue must be suctioned into the cap similar to what is done in variceal band ligation. The submucosa is often resected with the mucosa during EMR. When sufficient tissue is suctioned into the cap, the snare is closed, and cautery is applied until the tissue is transected. This process takes several more seconds than with polypectomy because the amount of tissue to be resected is much greater. The average diameter of resected mucosa is about 1 cm when assessed by the pathologist, and the defect left behind by the resection is often about 2 to 3 cm in diameter. The resection and the residual ulcer are also shown in Fig. 27.6. Because tumors are often larger than the size of a single EMR, a second resection can be performed directly adjacent to the first resection; care must be taken not to suction the muscularis propria exposed at the site of the first resection into the cap. Multiple resections in the same area should be attempted only by endoscopists familiar with the mucosal resection technique.
EMR can be used for diagnosis of unusual-appearing lesions within Barrett’s esophagus. In our experience, regions of nodularity or mucosal irregularity have a significantly higher incidence of carcinoma being present. A study of agreement among expert pathologists on distinguishing intramucosal carcinoma versus high-grade dysplasia found only moderate agreement with a κ score of less than .6 even when the pathologists could agree to standard definitions.19 It was thought that limited tissue from pinch biopsies often obscured the pathologist’s ability to determine invasion. Using EMR, our group found that the diagnosis of adenocarcinoma increased by 40% in a group of 25 patients with Barrett’s esophagus because EMR furnishes larger specimens.20 EMR is also an excellent tool for staging esophageal carcinoma as has been shown in Japan.21 It has been established that if the tumor can be found to be m1 or m2 stage (confined to the lamina propria), the tumor can be safely resected with only rare incidence of metastasis. Metastasis was found in 6% of patients who had cancers that penetrated to the muscularis mucosae or superficially into the submucosa. The technique of EMR gives the endoscopist similar tools to evaluate cancer curability as surgeons have had in the past—the ability to obtain histology and pathologic depth of staging.
Methods of Endoscopic Treatment of Esophageal Cancer
Thermal Lasers
An early method of treating esophageal cancer was the application of thermal energy. These techniques originated from therapies developed for palliation of esophageal cancer, which involved the application of laser or intensive thermal energy to create a new lumen through obstructing cancers. Because 80% to 90% of esophageal cancers manifest as obstructive disease, this was naturally the starting point of most esophageal cancer therapies. Thermal techniques were ideal for this application because they could offer immediate tumor ablation and allowed the endoscopist to fashion a lumen that could pass food easily. However, laser therapies were found to be costly and required several applications to achieve long-term palliation in contrast to expandable metal stents.22
Laser therapy was used as primary treatment of superficial cancers of the esophagus and stomach. The choice of lasers has varied, with the initial choice being neodymium:yttrium-aluminum-garnet (Nd:YAG), which produces infrared laser light (1063 nm) that can penetrate 2 cm through tissue. This therapy was used in the 1980s with relative success against predominately superficial squamous cell esophageal carcinomas. The results of a smaller series suggested that cancers could be eliminated in 73% of 33 patients with superficial cancers of the esophagus and gastric cardia with most patients followed for at least 2 years.23 The treatment could be repeated 6 times, although the mean frequency of retreatment was 2.6 times.
More recent studies using Nd:YAG laser therapy in combination with multipolar coagulation have involved very small numbers of patients. One study enrolled only six patients over 7 years and found that the treatment failed in one patient and left residual Barrett’s mucosa in three others.24 Three Nd:YAG sessions and three multipolar coagulation treatment sessions were required on average to treat each patient. Although this form of therapy could be effective, it requires multiple endoscopic treatment sessions and can fail. One case report in the literature suggests that with Nd:YAG therapy, failure could occur underneath normal-appearing squamous tissue and may be difficult to detect.25
Other laser therapies that have been employed to treat superficial cancers include potassium titanyl phosphate:yttrium-aluminum-garnet (KTP:YAG) and argon lasers because they have a more limited depth of penetration than Nd:YAG lasers. These lasers all operate in the visible green light region (532 nm) spectrum and penetrate tissue to a depth of only about 2 mm. These lasers have been advocated for the treatment of vascular lesions because of their limited depth of penetration and their intense absorption by hemoglobin. Several more recent series have reported that these lasers can be used to treat Barrett’s esophagus because they offer the safety of superficial therapy. These lasers have been used more for ablation of dysplastic lesions in Barrett’s esophagus rather than cancers, but these green lasers may be effective for some very superficial cancers. One case series found that KTP:YAG laser was able to destroy early cancers in two patients, although this required multiple treatments and had evidence of intestinal epithelium under squamous mucosa in 2 in 10 patients treated (for Barrett’s mucosa).26
Argon Plasma Coagulation
APC was originally developed as a hemostatic device that could cauterize bleeding lesions in a noncontact fashion that were awkward to reach with traditional probes. APC has the ability to treat superficially and has been thought to represent decreased risks of perforation. Mucosal ablative therapy for Barrett’s esophagus has been investigated by several investigators who reported good to excellent results.27–35 The technique is shown in Fig. 27.7. The primary goal of APC is to apply a current to the target lesion without producing a perforation. The argon gas is released under pressure. The endoscopist must be vigilant against allowing the tip of the probe to become embedded into the mucosa during treatment; this could result in submucosa air or, worse, a perforation. For this reason, coagulation performed with the argon plasma coagulator should be done only while the probe is being withdrawn toward the endoscope; this should ensure that the tip of the probe is not embedded in the mucosa. Settings for treating adenocarcinoma should be in the higher power ranges; 80 to 90 W has been cited in the literature. Higher output powers seem to be associated with improved outcomes in Barrett’s esophagus with high-grade dysplasia, although complications such as perforation and strictures are also seen at these dosages.33,36,37
Treatment of intraepithelial carcinoma in Barrett’s esophagus has been reported in a limited series of patients. In one series of only three intraepithelial cancers, treatment with APC was ineffective and resulted in invasive disease.37 This cancer also failed treatment with subsequent photodynamic therapy. Similar results were observed in another small series of three patients with early esophageal cancers who were treated with APC, with one recurrence noted that was subsequently treated with photodynamic therapy.38 In the Asian literature, early squamous cell carcinomas and high-grade dysplasia were treated with the APC with good results.39,40 In one series, 29 patients with early squamous carcinomas and 42 patients with high-grade dysplasia were first treated with EMR with any residual treated with APC.40 The early results seem promising with only three of the cancers (10%) found to recur after 4 months. Esophageal strictures were found in four of the cases after EMR of most of the diameter of the esophagus. Overall, it seems that although APC is well tolerated with few described complications, its efficacy in elimination of early esophageal cancer is limited with significant (33%) failure rates in small numbers of Barrett’s esophagus–related cancers and lower failure rates (10%) in superficial squamous cell carcinomas. This finding is not surprising given the decreased depth of injury associated with this treatment.
Endoscopic Mucosal Resection for Early Cancers
Since its inception in Japan, EMR has been used to treat early esophageal and gastric cancers.41 The technique was performed by Japanese surgeons who had excellent endoscopic skills and anatomic understanding of cancer surgery. Initial resections were performed with an overtube that was used to anchor tissue. A standard endoscope was placed within the lumen of the overtube and used to remove the tissue with a snare from the endoscope or one that was preformed around the overtube. The large ulcers that these procedures produced were worrisome, but these ulcers all were found to heal within 2 months.42 The most commonly used of these devices was the Makuuchi tube, which had been used in 152 cases of superficial esophageal cancer in 1992.43 These pioneers of mucosal resection established that vascular invasion or metastasis was extremely rare when cancers were either intraepithelial or confined to the upper two-thirds of the mucosa. Only when cancers penetrated into the bottom one-third of the mucosa did vascular invasion or lymph nodes become apparent in 25% of the patients. In their hands, patients with intraepithelial disease had 5-year survivals approaching 100%. However, once the cancers penetrated into the submucosa, the 5-year survival rate decreased to about 55% to 59%.
Initially, mucosal resection was recommended only for esophageal cancers that were less than 2 cm in size and occupied less than one-third of the circumference of the esophagus in patients who were not ideal surgical candidates.44 In one study that correlated the lymph node metastasis to depth of cancer invasion, the presence of lymph node metastasis was found to be zero if the lesion was confined to the mucosa, 10% if the muscularis mucosae became invaded, and 43% if the submucosa was invaded.45 Over time, recommendations concerning treatment of early esophageal cancers were altered because about one-quarter of all esophageal cancers were classified as early stage owing to intensive screening programs.46 By the late 1990s, a survey of Japanese institutions found that EMR was favored as the treatment of choice for all esophageal cancers confined to the upper two-thirds of the mucosa.47 All patients with mucosally confined disease survived, but disease that penetrated to the submucosa had a significantly worse prognosis than disease that remained mucosal.
The results of using EMR for the treatment of adenocarcinoma within Barrett’s esophagus have been limited to a few series. In most cases, EMR is used as a staging or treatment technique, and additional therapy is applied to remove the remainder of the dysplastic tissue. In a large retrospective comparison study, EMR combined with photodynamic therapy was compared with surgical treatment in a series in 178 patients with T1a cancers. Cumulative survival in the endoscopically treated patients was 17% versus 20% in the surgical patients.48 Cancer-related deaths occurred in both groups (<2%). In a series from Germany, 100 patients with early esophageal adenocarcinoma were managed with EMR with a 99% local response rate but with an 11% rate of metachronous or recurrent cancer rate.49 It has been found that endoscopically favorable lesions are lesions that are polypoid, elevated, or flat, which are characteristics that occur in most patients.50 The results of these series have led to recommendations about which lesions can be approached with EMR (Table 27.1).
Table 27.1 Characteristics of Esophageal Cancers Amenable to Endoscopic Mucosal Resection
| Characteristic | Favorable Outcome |
|---|---|
| Size | <2 cm |
| Depth of penetration | No penetration of muscularis mucosa |
| Grade of cancer | Well-differentiated cancer |
| Appearance | Polypoid, elevated, or flat |
Endoscopic Submucosal Dissection
Endoscopic submucosal dissection has been popularized in Asia as a method for complete excision of neoplastic lesions. A disadvantage of EMR has been the presence of neoplastic tissue remaining at the margins of resection, which generally indicates that neoplastic tissue remains in the tissue despite the use of cautery.51 These findings have led to an attempt to perform en bloc resections using endoscopic mucosal dissection techniques to try to define areas of tissue that require large resections. In particular in Japan, large superficial gastric cancers have been approached in this fashion.52 Retrospective studies comparing EMR with endoscopic submucosal dissection in terms of esophageal cancer have found that endoscopic submucosal dissection increases the ability to cure early cancer from 71% to 97% in squamous cell carcinoma.53 This difference was not apparent if the cancers were less than 1.5 cm in diameter, which is probably the limit of resection using EMR cap techniques. Similar findings have been described with gastric cancers approached with either EMR or endoscopic submucosal dissection with 1.5 cm being the a priori cutoff between the two techniques with similar complications and success rates of cancer removal.54 Even cancers of the esophagogastric junction can be resected with reasonable success rates using these techniques, although this resection is more technically difficult.55
More recent studies have compared EMR and endoscopic submucosal dissection in animal models and found that endoscopic submucosal dissection, particularly with a water jet assistance, can achieve resection faster than traditional EMR.56 The first steps in endoscopic submucosal dissection are to mark the area to be resected, usually by using cautery, to ensure that the complete lesion and at least a 3-mm margin are resected (Fig. 27.8).
A submucosal bleb is formed similar to with EMR except that usually a solution that is designed to persist is used, such as sodium carboxymethylcellulose, sodium hydroxypropyl methylcellulose, sodium hyaluronate, hypertonic dextrose solution, hypertonic saline, glycerol, or fibrinogen. The goal is to allow dissection in the submucosal plane, which can be very difficult depending on the technique used. The devices available for endoscopic submucosal dissection vary greatly, and it would not be prudent for any endoscopist to master all of these. However, several of these “knives” can be applied by a skilled endoscopist with appropriate training so long as certain principles are followed. Japanese experts perform the entire dissection with a single knife. However, the knives vary in their applicability, and some are best suited for certain portions of the procedure (Fig. 27.9).
The lesions are generally circumscribed with a knife to free the lesion completely from the rest of the normal mucosa. Sharp-edged knives, such as the needle knife or IT knife, have an advantage to allow easier application through the tissue. The cut is made carefully to avoid penetrating the entire wall, and once the defect is made, knives similar to the IT knife have an advantage by not having a sharp tip to penetrate through the muscularis layer. The submucosal dissection is performed using a small dissection cap to lift the mucosa and cut the fibrous tissue that is underneath. This is the most challenging portion of the dissection and requires careful control. The hook knife has an advantage because the knife can “hook” the fibrous tissue from underneath the mucosa, and the cutting can be done more in the endoscopic field of vision (Fig. 27.10). The dissection is a substantial undertaking, although a typical lesion can be removed in 1 to 2 hours by an experienced endoscopist.
Photodynamic Therapy for Early Esophageal Cancer
Photodynamic therapy has been investigated since its origins in 1961 as a treatment for cancer.57 The therapy has traditionally involved the use of a combination of a drug termed a photosensitizer and light of a specific wavelength that is required to activate the drug. The typical practice is to administer the drug days or hours before light delivery to allow the photosensitizer to concentrate into the neoplastic tissue. Light from a laser is applied to the mucosa, which causes general tissue destruction and cell death.
Photoradiation is usually performed by placing an optical fiber through the biopsy channel of the endoscope. The tip of this optical fiber has a cylindrical diffusing fiber that can be placed in the lumen of the esophagus. Generally, in the case of an early tumor, the fiber is pressed against the tumor as shown in Fig. 27.11. Treatment parameters for photodynamic therapy for Barrett’s esophagus with a cancer using porfimer sodium involve a drug dosage of 2 mg/kg body weight. The drug is given 48 hours before photodynamic therapy. Red light is delivered through a diffusing fiber at a power output of 400 mW/cm fiber for a total energy of around 300 J/cm fiber. If there is no endoscopically apparent disease and mucosal resection has completely removed the lesion, a smaller dose of light such as 200 J/cm fiber can be used to treat the remaining Barrett’s mucosa.
Patients can experience numerous complications. After injection of the drug, cutaneous photosensitivity can occur and persist for 30 to 90 days. After photoradiation, patients can experience severe chest pain within 24 hours. This pain can require narcotic administration to achieve pain relief. Transdermal administration of narcotics usually is preferred because patients have odynophagia from the therapy. Dehydration occurs because of the inability to obtain adequate fluid intake and may require intravenous fluids to resolve. Nausea occurs in the first 1 to 3 days after photoradiation and can require administration of antiemetics. Rare side effects include injury of organs that are located near the esophagus, resulting in atrial fibrillation or pleural effusions.58,59 Esophageal strictures are a major problem because they occur in about one-third of patients treated. These strictures are often quite fibrotic and require multiple dilations to large diameters to resolve the constriction.
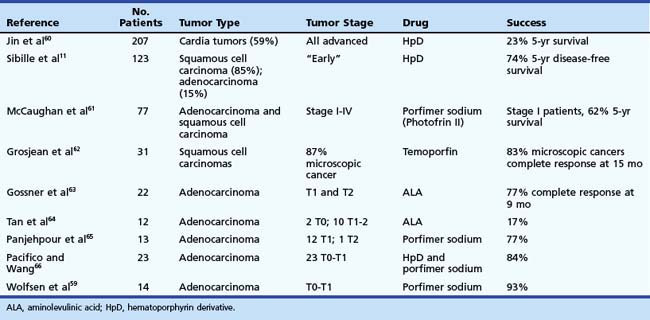
Results of photodynamic therapy for the treatment of esophageal cancer are shown in Table 27.2.11,59–66 The overall results are quite impressive if only the early cancers are considered. Significant (23%) 5-year survivals were reported in patients who had advanced stage esophageal cancer. Most of the series involved similar photosensitizers, such as hematoporphyrin derivative or porfimer sodium II, although a few involved temoporfin, which is unavailable in the United States and is believed to have a deeper depth of penetration than porfimer sodium. Among the series using porphyrin compounds, the survival rates range from 62% to 93% with a median response of about 75%. An important factor illustrated best by the study by Sibille and colleagues11 is that although disease-free survival was excellent, overall survival over 5 years was only 25%, indicating that these are medically ill patients who have severe comorbidities. These results are very good considering that most of the series contain patients with more invasive disease with penetration of the submucosa and even the muscularis propria. Complications that have been reported in these series are similar to the complications discussed earlier with strictures occurring in about one-third of the patients.
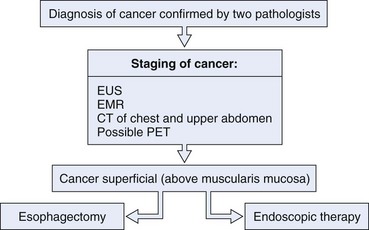
These results indicate that endoscopic therapy for early cancers may be a viable alternative to traditional esophagectomy. An algorithm for consideration when evaluating patients with esophageal cancer is shown in Fig. 27.12. If a patient has early stage cancer, endoscopic therapy should be considered as a possible option.
Combinational Therapy
If the resection is complete, the cancer should be inspected for depth of invasion, and cancers that penetrate beyond the muscularis mucosae should be considered for some other type of therapy. Cancers that infiltrate a duplicated muscularis mucosae are at increased risk of metastasis.67 However, cancers that infiltrate the lymphovascular system also seem to be at a high risk of metastasis if they already invade the submucosa.68
Complete elimination of all preneoplastic mucosa can be difficult in situations such as squamous cell carcinomas. In these cases, careful observation and elimination of new neoplastic lesions is probably needed. Overall success of endoscopic therapies is excellent, and more recent data show that endoscopic therapy compares favorably with surgical therapy in terms of survival in adenocarcinoma in Barrett’s esophagus, although there is a 12% recurrence rate.48 The success of endoscopic therapy in early squamous cell carcinoma is also excellent.
Acknowledgments
The author would like to acknowledge the support of NIH grants CA85992-01 and R01CA097048-01.
1 Sharma P, Sidorenko EI. Are screening and surveillance for Barrett’s oesophagus really worthwhile? Gut. 2005;54(Suppl 1):i27-i32.
2 Tomizawa Y, Wang KK. Screening, surveillance, and prevention for esophageal cancer. Gastroenterol Clin North Am. 2009;38:59-73.
3 Corley DA, Levin TR, Habel LA, et al. Surveillance and survival in Barrett’s adenocarcinomas: A population-based study. Gastroenterology. 2002;122:633-640.
4 Buttar NS, Wang KK, Anderson MA, et al. The effect of selective cyclooxygenase-2 inhibition in Barrett’s esophagus epithelium: An in vitro study. J Natl Cancer Inst. 2002;94:422-429.
5 Galipeau PC, Prevo LJ, Sanchez CA, et al. Clonal expansion and loss of heterozygosity at chromosomes 9p and 17p in premalignant esophageal (Barrett’s) tissue. J Natl Cancer Inst. 1999;91:2087-2095.
6 Prevo LJ, Sanchez CA, Galipeau PC, et al. p53-mutant clones and field effects in Barrett’s esophagus. Cancer Res. 1999;59:4784-4787.
7 Paulson TG, Maley CC, Li X, et al. Chromosomal instability and copy number alterations in Barrett’s esophagus and esophageal adenocarcinoma. Clin Cancer Res. 2009;15:3305-3314.
8 Krishnadath K, Wang K, Liu W, et al. Persistent genetic abnormalities in Barrett’s esophagus after photodynamic therapy. Gastroenterology. 2000;119:624-630.
9 Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: International, partially blinded, randomized phase III trial [erratum appears in Gastrointest Endosc 2006 Feb;63(2):359]. Gastrointest Endosc. 2005;62:488-498.
10 Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277-2288.
11 Sibille A, Lambert R, Souquet JC, et al. Long-term survival after photodynamic therapy for esophageal cancer. Gastroenterology. 1995;108:337-344.
12 Bonavina L. Early oesophageal cancer: Results of a European multicentre survey. Group Europeen pour l’Etude des Maladies de l’Oesophage. Br J Surg. 1995;82:98-101.
13 Noguchi H, Naomoto Y, Kondo H, et al. Evaluation of endoscopic mucosal resection for superficial esophageal carcinoma. Surg Laparosc Endosc Percutan Tech. 2000;10:343-350.
14 Rampado S, Bocus P, Battaglia G, et al. Endoscopic ultrasound: Accuracy in staging superficial carcinomas of the esophagus. Ann Thorac Surg. 2008;85:251-256.
15 Schwartz DA, Unni KK, Levy MJ, et al. The rate of false-positive results with EUS-guided fine-needle aspiration. Gastrointest Endosc. 2002;56:868-872.
16 Scotiniotis IA, Kochman ML, Lewis JD, et al. Accuracy of EUS in the evaluation of Barrett’s esophagus and high-grade dysplasia or intramucosal carcinoma. Gastrointest Endosc. 2001;54:689-696.
17 Bosing N, Schumacher B, Frieling T, et al. [Endoscopic ultrasound in routine clinical practice for staging adenocarcinomas of the stomach and distal esophagus]. Chirurg. 2003;74:214-223.
18 Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: A summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery. 1998;123:432-439.
19 Ormsby AH, Petras RE, Henricks WH, et al. Observer variation in the diagnosis of superficial oesophageal adenocarcinoma. Gut. 2002;51:671-676.
20 Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett’s esophagus. Gastrointest Endosc. 2000;52:328-332.
21 Yoshida MMK. [Endoscopic evaluation of the depth of invasion in cases of superficial esophageal cancer in determining indications for endoscopic mucosal resection]. Nippon Geka Gakkai Zasshi. 2002;103:337-342.
22 Dallal HJ, Smith GD, Grieve DC, et al. A randomized trial of thermal ablative therapy versus expandable metal stents in the palliative treatment of patients with esophageal carcinoma. Gastrointest Endosc. 2001;54:549-557.
23 Yang GR, Zhao LQ, Li SS, et al. Endoscopic Nd:YAG laser therapy in patients with early superficial carcinoma of the esophagus and the gastric cardia. Endoscopy. 1994;26:681-685.
24 Sharma P, Jaffe PE, Bhattacharyya A, et al. Laser and multipolar electrocoagulation ablation of early Barrett’s adenocarcinoma: Long-term follow-up. Gastrointest Endosc. 1999;49:442-446.
25 Fremond L, Bouche O, Diebold MD, et al. [Partial regression of Barret esophagus with high grade dysplasia and adenocarcinoma after photocoagulation and endocurietherapy under antisecretory treatment]. Gastroenterol Clin Biol. 1995;19:112-116.
26 Gossner L, May A, Stolte M, et al. KTP laser destruction of dysplasia and early cancer in columnar-lined Barrett’s esophagus. Gastrointest Endosc. 1999;49:8-12.
27 Dumoulin FL, Terjung B, Neubrand M, et al. Treatment of Barrett’s esophagus by endoscopic argon plasma coagulation. Endoscopy. 1997;29:751-753.
28 Maass S, Martin WR, Spiethoff A, Riemann JF. [Barrett esophagus with severe dysplasia in argon beam therapy]. Zeitschrift Gastroenterol. 1998;36:301-306.
29 Mork H, Barth T, Kreipe HH, et al. Reconstitution of squamous epithelium in Barrett’s oesophagus with endoscopic argon plasma coagulation: A prospective study. Scand J Gastroenterol. 1998;33:1130-1134.
30 Van Laethem JL, Cremer M, Peny MO, et al. Eradication of Barrett’s mucosa with argon plasma coagulation and acid suppression: Immediate and mid term results. Gut. 1998;43:747-751.
31 Byrne JP, Armstrong GR, Attwood SE. Restoration of the normal squamous lining in Barrett’s esophagus by argon beam plasma coagulation. Am J Gastroenterol. 1998;93:1810-1815.
32 Grade AJ, Shah IA, Medlin SM, et al. The efficacy and safety of argon plasma coagulation therapy in Barrett’s esophagus. Gastrointest Endosc. 1999;50:18-22.
33 Pereira-Lima JC, Busnello JV, Saul C, et al. High power setting argon plasma coagulation for the eradication of Barrett’s esophagus. Am J Gastroenterol. 2000;95:1661-1668.
34 Martin WR, Jakobs R, Spiethoff A, et al. [Treatment of Barrett esophagus with argon plasma coagulation with acid suppression—a prospective study]. Zeitschrift Gastroenterol. 1999;37:779-784.
35 Tigges H, Fuchs KH, Maroske J, et al. Combination of endoscopic argon plasma coagulation and antireflux surgery for treatment of Barrett’s esophagus. J Gastrointest Surg. 2001;5:251-259.
36 Schulz H, Miehlke S, Antos D, et al. Ablation of Barrett’s epithelium by endoscopic argon plasma coagulation in combination with high-dose omeprazole. Gastrointest Endosc. 2000;51:659-663.
37 Van Laethem JL, Jagodzinski R, Peny MO, et al. Argon plasma coagulation in the treatment of Barrett’s high-grade dysplasia and in situ adenocarcinoma. Endoscopy. 2001;33:257-261.
38 May A, Gossner L, Gunter E, et al. Local treatment of early cancer in short Barrett’s esophagus by means of argon plasma coagulation: Initial experience. Endoscopy. 1999;31:497-500.
39 Katsuta M, Tajiri T, Nomura T, et al. [Treatment of superficial esophageal cancer by argon plasma coagulation]. Nihon Ika Daigahu Zasshi. 2002;69:383-385.
40 Wang GQ, Wei WQ, Hao CQ, et al. [Minimal invasive treatment of early esophageal cancer and its precancerous lesion: Endoscopic mucosal resection using transparent cap-fitted endoscope]. Chin Med J. 2003;83:306-308.
41 Inoue H, Endo M. Endoscopic esophageal mucosal resection using a transparent tube. Surg Endosc. 1990;4:198-201.
42 Inoue M, Shiozaki H, Tamura S, et al. [Endoscopic mucosal resection for early esophageal cancer]. Jpn J Clin Med. 1996;54:1286-1291.
43 Makuuchi H, Machimura T, Mizutani K, et al. [Controversy in the treatment of superficial esophageal carcinoma—indications and problems of the procedures]. Nippon Geka Gakkai Zasshi. 1992;93:1059-1062.
44 Endo M, Takeshita K, Inoue H. [Endoscopic mucosal resection of esophageal cancer]. Jpn J Cancer Chemother. 1995;22:192-195.
45 Yoshida M, Hanashi T, Momma K, et al. [Endoscopic mucosal resection for radical treatment of esophageal cancer]. Jpn J Cancer Chemother. 1995;22:847-854.
46 Kato H. Diagnosis and treatment of esophageal neoplasms. Jpn J Cancer Res. 1995;86:993-1009.
47 Kodama M, Kakegawa T. [Treatment of superficial carcinoma of the esophagus—a review of responses to questionnaire on superficial carcinoma of the esophagus collected at the 49th conference of Japanese Society for Esophageal Diseases]. Nippon Geka Gakkai Zasshi. 1996;97:683-690.
48 Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology. 2009;137:815-823.
49 Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc. 2007;65:3-10.
50 Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2000;118:670-677.
51 Prasad GA, Buttar NS, Wongkeesong LM, et al. Significance of neoplastic involvement of margins obtained by endoscopic mucosal resection in Barrett’s esophagus. Am J Gastroenterol. 2007;102:2380-2386.
52 Yamamoto H. Endoscopic submucosal dissection of early cancers and large flat adenomas. Clin Gastroenterol Hepatol. 2005;3:S74-S76.
53 Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc. 2008;68:1066-1072.
54 Yamaguchi Y, Katusmi N, Aoki K, et al. Resection area of 15 mm as dividing line for choosing strip biopsy or endoscopic submucosal dissection for mucosal gastric neoplasm. J Clin Gastroenterol. 2007;41:472-476.
55 Yoshinaga S, Gotoda T, Kusano C, et al. Clinical impact of endoscopic submucosal dissection for superficial adenocarcinoma located at the esophagogastric junction. Gastrointest Endosc. 2008;67:202-209.
56 Neuhaus H, Wirths K, Schenk M, et al. Randomized controlled study of EMR versus endoscopic submucosal dissection with a water-jet hybrid-knife of esophageal lesions in a porcine model. Gastrointest Endosc. 2009;70:112-120.
57 Wang KK. Current status of photodynamic therapy of Barrett’s esophagus. Gastrointest Endosc. 1999;49:S20-S23.
58 Overholt BF, Panjehpour M, Ayres M. Photodynamic therapy for Barrett’s esophagus: Cardiac effects. Lasers Surg Med. 1997;21:317-320.
59 Wolfsen HC, Woodward TA, Raimondo M. Photodynamic therapy for dysplastic Barrett esophagus and early esophageal adenocarcinoma. Mayo Clin Proc. 2002;77:1176-1181.
60 Jin M, Yang B, Zhang W, et al. Photodynamic therapy for upper gastrointestinal tumours over the past 10 years. Semin Surg Oncol. 1994;10:111-113.
61 McCaughan JSJr, Ellison EC, Guy JT, et al. Photodynamic therapy for esophageal malignancy: A prospective twelve-year study. Ann Thorac Surg. 1996;62:1005-1010.
62 Grosjean P, Savary JF, Mizeret J, et al. Photodynamic therapy for cancer of the upper aerodigestive tract using tetra(m-hydroxyphenyl)chlorin. J Clin Laser Med Surg. 1996;14:281-287.
63 Gossner L, Stolte M, Sroka R, et al. Photodynamic ablation of high-grade dysplasia and early cancer in Barrett’s esophagus by means of 5-aminolevulinic acid. Gastroenterology. 1998;114:448-455.
64 Tan WC, Fulljames C, Stone N, et al. Photodynamic therapy using 5-aminolaevulinic acid for oesophageal adenocarcinoma associated with Barrett’s metaplasia. J Photochem Photobiol B. 1999;53:81-90.
65 Panjehpour M, Overholt BF, Haydek JM, et al. Results of photodynamic therapy for ablation of dysplasia and early cancer in Barrett’s esophagus and effect of oral steroids on stricture formation. Am J Gastroenterol. 2000;95:2177-2184.
66 Pacifico RJ, Wang KK. Role of mucosal ablative therapy in the treatment of the columnar-lined esophagus. Chest Surg Clin North Am. 2002;12:185-203.
67 Lewis JT, Wang KK, Abraham SC. Muscularis mucosae duplication and the musculo-fibrous anomaly in endoscopic mucosal resections for Barrett esophagus: Implications for staging of adenocarcinoma. Am J Surg Pathol. 2008;32:566-571.
68 Cen P, Hofstetter WL, Correa AM, et al. Lymphovascular invasion as a tool to further subclassify T1b esophageal adenocarcinoma. Cancer. 2008;112:1020-1027.