Chapter 33 Endoscopic Therapy for Gastric Neoplasms
Introduction
The development and clinical application of endoscopic ultrasound (EUS) have enabled GI specialists to evaluate previously inaccessible intramural gastric lesions and to determine the layer of origin. With the ability to determine, with almost pinpoint accuracy, the depth of gastric wall layer involvement and to exclude lymph node metastasis, the endoscopist can safely proceed to endoscopic removal of a gastric neoplasm. Introduction of new techniques such as endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), and thermal ablation techniques such argon plasma coagulation (APC) have made it possible to remove or destroy certain types of gastric neoplasms safely and completely.1–7
A gastric tumor is defined as any mass lesion occurring in the wall of the stomach. Its metastatic potential defines the difference between benign and malignant neoplasms. Epithelial neoplasms include adenomas and carcinomas. Intramural lesions include gastric stromal cell tumors and lymphoma. Box 33.1 shows the classification of gastric neoplasms. The primary emphasis of this chapter is on endoscopic therapy for early gastric cancer (EGC).
Gastric Cancer
Gastric cancer is still the world’s second leading cause of cancer mortality after lung cancer, despite its worldwide decline in incidence and mortality. It has been estimated that 700,000 deaths per year from the disease occurred in 2002 accounting for more than 10% of all cancer deaths.8,9 Adenocarcinoma accounts for approximately 90% of gastric cancers, with the remainder being due to non-Hodgkin’s lymphoma (NHL) and leiomyosarcoma.
Epidemiology
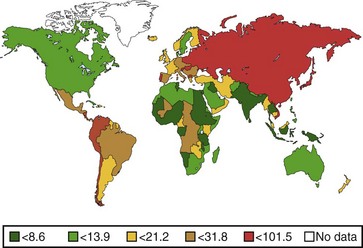
The annual incidence of gastric cancer varies worldwide. There is a difference in the prevalence of gastric cancer between the East and the West (Fig. 33.1).10 In Eastern Asian countries such as Japan and China and in many developing countries, gastric cancer is the most prevalent malignant neoplasm and the leading cause of cancer mortality.11 Fig. 33.2 shows the incidence of gastric cancer in Eastern Asia and the United States.10 There is a high incidence of gastric cancer in the East, the former Soviet Union, some portions of Central and Eastern Europe, and Central America (e.g., Costa Rica) and South America (e.g., Chile). The lowest rates occur in the United States and in some parts of Africa.
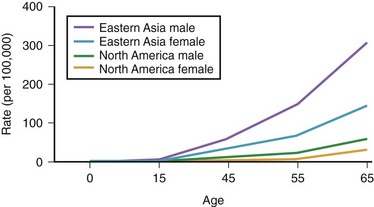
Fig. 33.2 Incidence of gastric cancer in East Asia and the United States according to gender and age.
The International Agency for Research on Cancer (IARC) data for 1996 reported an age-standardized incidence rate of 95.5/105 in Japanese men in Yamagata, Japan, and 7.5/105 in white men in the United States.11 Most of the geographic variation is due to differences in the incidence of gastric cancer not localized in the cardia (noncardia gastric cancer); cancer that is localized in the cardia has a more uniform distribution. Ethnic groups that migrate from a country with a high incidence to a country with a low incidence have an overall risk intermediate between that of their homeland and that of their new country. First-generation migrants tend to retain their high risk, whereas subsequent generations have risk levels approximating that of their host country.12
Both the incidence and the mortality rates for gastric cancer have declined sharply during the last several decades, particularly in the United States and in Western Europe. In the United States, gastric cancer is the 13th most common cancer and the 8th most common cause of cancer death. A steady decline in the incidence of noncardia gastric cancer has occurred since 1930 throughout the world.13 The incidence of adenocarcinoma involving the cardia or the esophagogastric junction, or both, has increased in developed countries such as the United States.14 The reasons for this trend are unknown.
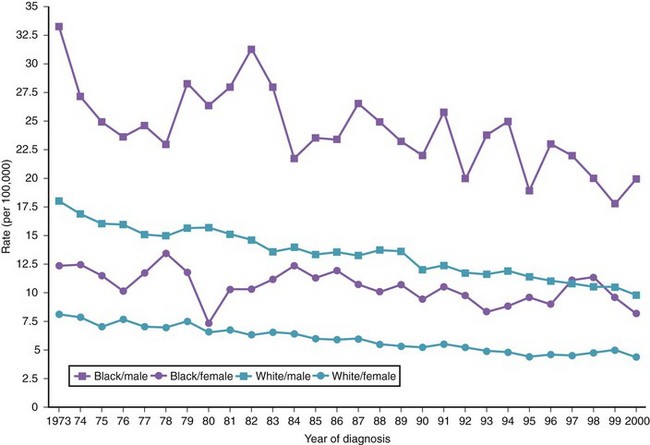
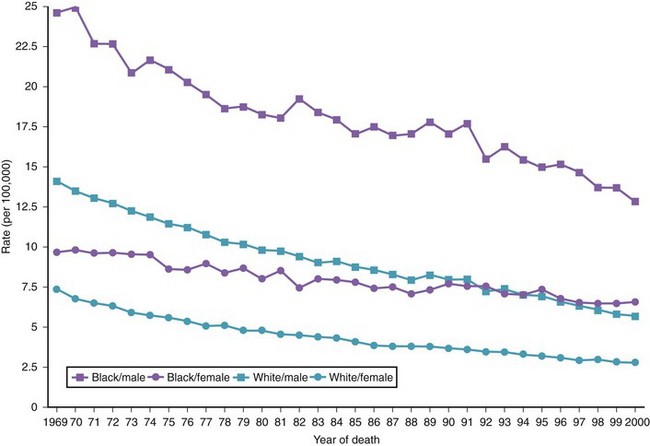
There are variations in the overall incidence and mortality of noncardia gastric cancer between gender and ethnic groups. In the United States, the incidence is greater among Native Americans, Hispanic whites, and African Americans.15 The ethnic distribution for cancer of the gastric cardia is also different, with a preponderance in American whites over African Americans.11 The incidence of gastric cancer increases with age, with most patients 50 to 70 years of age. The incidence of gastric cancer in patients younger than 36 years has increased from 1.8% before 1970 to 4.2% after 1970, with most (62.5%) occurring in Hispanics. Noncardia gastric cancer is more common in men than women by a ratio of approximately 2 : 1. Gastric cancer located in the cardia has an even higher male-to-female ratio of up to 6 : 1 in U.S. whites.16 Figs. 33-3 and 33-4 summarize the trends in the incidence and mortality of gastric cancer in the United States during the years 1975–2006 according to gender and ethnicity.17
Pathogenesis
Gastric cancer has a poor prognosis with a 5-year survival of less than 5%. This poor prognosis is mostly due to the fact that four out of five patients present at an advanced stage.18 Incidental diagnosis of gastric cancer has been reported to increase patient survival.19,20
Japan has the highest incidence of gastric cancer worldwide. However, the establishment of an aggressive mass screening policy has resulted in a remarkably high detection rate of EGC—40% to 66.4% of all gastric cancers diagnosed.21,22 Consequently, the most extensive experience with treatment of EGC has come from Japan.
The Japanese Society of Gastrointestinal Endoscopy defines EGC as cancer confined to the mucosa or submucosa, with or without lymph node metastasis. This designation was based on the observation that this subgroup of patients has an excellent prognosis, with a 5-year survival of greater than 90% after gastrectomy and removal of both the primary and the secondary lymph nodes.1,21 In the West, 10% to 20% of surgical resections for gastric carcinoma are for EGC23; in Japan, more than 50% of surgical specimens are classified as EGC.24,25
Helicobacter pylori
Since the bacterium Helicobacter pylori was first reported in 1983, a wealth of evidence has been gathered concerning its role in the etiology of gastric cancer.26–28 In 1994, the IARC classified H. pylori as carcinogenic to humans. Evidence supporting an etiologic association between H. pylori and gastric cancer can be found in ecologic studies, case-control studies, and prospective cohort studies.27,29–31 Meta-analyses of prospective studies suggest that the risk of gastric cancer is increased twofold to threefold in individuals with chronic H. pylori infection.32,33 One prospective, nested case-control study found a significant association between prior H. pylori infection and gastric adenocarcinoma overall, but there was no association with cancer of the cardia.34 Individuals without H. pylori colonization seem to have a minimal risk of gastric carcinoma.35
H. pylori contributes to the causation of gastric cancer via mechanisms that involve the development and progression of chronic gastritis.36 Infection causes chronic gastritis in almost all infected individuals and accounts for almost all cases of chronic gastritis.37 H. pylori–induced gastritis may progress over time from superficial nonatrophic gastritis to more severe forms, including severe atrophic gastritis with intestinal metaplasia.
Chronic gastritis is present in most cases of gastric cancer and is associated with an increased cancer risk.36 The risk of developing gastric cancer increases with the severity of gastritis, with reported risks greater than 10-fold for severe atrophic antral gastritis.36,38 Intestinal-type gastric cancer seems to be more strongly associated with severe atrophic gastritis, whereas the diffuse type is more common in nonatrophic gastritis.36
There is strong evidence for a role for virulence factors in H. pylori carcinogenesis. The Cag A virulence factor is strongly associated with the risk of adenocarcinoma. The absence of Cag A carries, at most, a low risk of developing diffuse-type adenocarcinoma.39
Peptic Ulcer Disease
H. pylori infection is a common risk factor for gastric ulcer, duodenal ulcer, and gastric cancer. An association between peptic ulcer disease and gastric cancer would be expected. However, studies have found duodenal ulceration to be inversely associated with gastric cancer risk.40
One possible explanation for this apparent paradox was suggested by Parsonnet,41 who argued that H. pylori infection can progress to gastric cancer or duodenal ulcer but seldom to both. Other factors, such as the genetic characteristics of the individual and the organism, may play a significant role in determining which disease would occur. The age at which infection was acquired may also influence the outcome. It has been suggested that infection early in life predisposes to atrophic gastritis and cancer but reduces duodenal ulcer risk owing to decreased acid production associated with the gastritis. If infection is acquired later in life, atrophic gastritis is less likely, and gastric cancer risk is decreased.41 More recent evidence supports a moderate association between gastric ulcer and noncardia gastric cancer.42,43
Dietary Factors
Several observational studies have shown a protective association with fresh fruits and vegetables, independent of other dietary factors. The association has been less impressive in limited cohort studies.44 Possible protective nutrients include vitamin E, carotenoids, selenium, and vitamin C.44 The evidence is stronger for vitamin C. In a case-control study, a high intake of vitamin C decreased the risk of gastric cancer by half compared with low vitamin C intake.45 However, a large 5-year intervention trial in China involving adults did not show any change in the risk of gastric cancer in individuals receiving supplemental vitamin C.46
High salt consumption has been fairly consistently associated with an increased risk of gastric cancer in ecologic and case-control studies.47–49 However, good quantitative data linking salt consumption with gastric cancer are still lacking.
N-nitroso compounds have been shown to be carcinogenic in animal studies.50 In the human stomach, these compounds may be formed from dietary nitrite or nitrate, leading to the hypothesis that a diet high in nitrite or nitrate predisposes to gastric cancer. Case-control studies examining dietary intake of nitrate and the risk of gastric cancer have consistently found a negative association. Because the major sources of nitrate and nitrite are vegetables and preserved meats, nitrate intake was probably an index of vegetable intake, and the negative association is not surprising.44 Case-control studies have reported a weak, statistically insignificant increased risk of gastric cancer (relative risk 1.12 to 1.28) for high versus low nitrite intake.51–54 It is difficult to isolate the effect of nitrate consumption in gastric cancer because of the fact that diets high in nitrite may also be high in antioxidants that are frequently found in vegetables and in fruits.54
It has been postulated that virulent strains of H. pylori release reactive oxygen metabolites that could destroy neighboring glandular cells leading to gastric glandular atrophy, hastened by other factors such as high salt intake but retarded by antioxidants such as vitamin C.55 The invention of refrigeration, the availability of fresh food, and the accompanying decreased consumption of preserved foods may have contributed to a decline in gastric cancer incidence in the second half of the 20th century.
Socioeconomic Status
Worldwide, a low socioeconomic status has been consistently shown to be associated with an increased risk of gastric cancer.16 The higher prevalence of gastric cancer among individuals with lower socioeconomic status is matched by a similar high prevalence of H. pylori infection in these individuals.56,57 An increased incidence of cancer of the cardia has been observed predominantly in individuals with higher socioeconomic status.14
Gastric Surgery
An increased risk of gastric cancer after gastric surgery has been reported, particularly after 15 or more years.58,59 The association is strongest for gastrectomy performed for gastric ulcer but less persuasive for either vagotomy or gastrectomy performed for duodenal ulcer. This association does not extend to cancer of the gastric cardia.40
Gastric Polyps
Single hyperplastic polyps are often found in the background of chronic gastritis, and they are mainly seen in the body of the stomach. At most, 0.3% of hyperplastic gastric polyps may contain a focus of adenocarcinoma. However, approximately 3% of multiple hyperplastic polyps are associated with a synchronous carcinoma elsewhere in the stomach.60 The presence of multiple hyperplastic polyps is considered a precancerous condition.
Fundic gland polyps do not have malignant potential when they occur sporadically or in association with proton pump inhibitor therapy. Hundreds of fundic gland polyps may be present in association with polyposis coli. Dysplasia has been reported in a few of these patients.61 Gastric adenocarcinoma associated with fundic gland polyposis in familial polyposis coli has been reported.62
Adenomatous polyps occur in the setting of intestinal metaplasia and associated chronic gastritis. Endoscopic features that increase the risk of malignant transformation include large size and a red, erosive surface color. A carcinoma may be contained in 40% of pyloric gland adenomas and generally 10% of tubular adenomas.60,63 Approximately 11% of patients with adenomatous polyps have a synchronous or metachronous gastric adenocarcinoma.63
Genetic Factors
Genetic predisposition may play a role in the development of gastric cancer, with a twofold to fourfold increased risk in first-degree relatives. Similarly, patients who have hereditary lesions, such as Lynch syndrome II, have an increased risk of gastric cancer.64,65
Miscellaneous Factors
A higher risk for gastric cancer has been reported in patients with Ménétrier’s disease, ataxia telangiectasia, and blood type group A.66–68 Pernicious anemia and its association with autoimmune chronic gastritis increases the risk of developing gastric cancer by twofold to threefold, according to population-based studies.69,70 Regular endoscopic surveillance in young patients with pernicious anemia has been advocated.71
Smoking may increase the incidence of premalignant gastric lesions and dysplasia; however a dose-response relationship has not been clearly shown.72–74 No clear relationship between alcohol consumption and gastric cancer has been shown.29,72,74
Molecular Abnormalities
Abnormalities at the molecular level have been described in patients who develop gastric cancer. These include allelic deletions of the atrial premature complex (APC) gene, the migrating motor complex (MCC) gene, and the tumor-suppressor gene TP53. Allelic deletions of TP53 can be found in 64% of gastric cancers. These deletions are common late events in gastric cancer.75 In addition, mutations of the APC gene and loss of heterozygosity on chromosomes 1q, 5q, and 17p have been reported in gastric carcinoma.76
Loss of E-cadherin–dependent cell-cell adhesion secondary to mutation of beta-catenin has been found in gastric cancer.77 Reduced expression of E-cadherin has been found to be significantly associated with recurrence of cancer and decreased survival.78 Microsatellite instability has also been found in gastric carcinoma.79 Higher expression of Sialyl-Tn has been associated with a poor prognosis in patients with gastric cancer.80 Patients with overexpression of c-erbB protein have been found to have a poorer prognosis than patients without c-erbB expression in poorly differentiated gastric adenocarcinoma.81 Germline truncating mutations in the E-cadherin gene (CDH1) have been found in several families with hereditary diffuse gastric cancer.82,83
Clinical Features
Advanced Gastric Cancer
Most patients with gastric adenocarcinoma present with advanced disease. Accompanying symptoms, such as weight loss, vomiting, anorexia, early satiety, abdominal pain, and anemia, may mimic peptic ulcer disease and other GI conditions. Typically, patients have been symptomatic for less than 12 months, and 40% of patients have been symptomatic for less than 3 months. Signs and symptoms are due to cancer invasion beyond the muscularis propria by direct extension or by distant metastasis. Liver and lungs are the most common sites of gastric cancer metastases (40%); bone and peritoneum are less frequent sites (10%). Occasionally, patients may present with a paraneoplastic syndrome, such as Trousseau’s syndrome (thrombosis), acanthosis nigricans (pigmented skin lesion, classically of the axilla), or dermatomyositis.84,85
Pathology
Most primary gastric cancers are adenocarcinoma, with an occasional squamous or adenosquamous carcinoma. Other rare gastric cancers include parietal cell carcinoma, choriocarcinoma, and rhabdoid tumor. Cancer metastasizing to the stomach is uncommon, with lung cancer being the most frequent primary tumor. A few cases of gastric carcinosarcoma and spindle cell carcinoma have been reported.86
Histologically, gastric adenocarcinoma can be divided into two categories87: (1) intestinal type, a well-differentiated neoplastic lesion that forms glandlike structures that ulcerate frequently, and (2) diffuse type, characterized by infiltration and thickening of the gastric wall without the formation of a discrete mass. Approximately 16% of gastric cancers cannot be categorized or are of the mixed type.86 The decline in incidence of gastric cancer has been predominantly in the distal stomach intestinal type, whereas a steady increase in the incidence of the proximal stomach diffuse type has been observed in the United States and in Europe.88,89 There has been inconsistent classification of gastric cardia and distal esophageal cancer in the literature because many, if not most, of these represent distal spread from specialized intestinal metaplasia of the distal esophagus or the esophagogastric junction.
Staging
Initial staging of gastric cancer should include spiral computed tomography (CT) of the abdomen to determine the presence or absence of metastatic disease. In the absence of metastasis, EUS is helpful for assessing resectability. Determination of surgical resectability for cure using EUS has been found to be 91% accurate.91
EGC is defined by the Japanese Society of Gastrointestinal Endoscopy as gastric cancer confined to the mucosa or submucosa, with or without lymph node metastasis. Patients with EGC have an excellent 5-year survival of greater than 90%.1,92 The risk of lymph node metastasis is 3% for intramucosal lesions (T1m) and 20% if there is submucosal involvement.93 Other independent risk factors for lymph node metastasis in EGC include lymphovascular invasion on histopathology, histologic ulceration, and tumor diameter more than 3 cm.94 For T1m tumors less than 3 cm without evidence of lymphovascular invasion or histologic ulceration, the risk of lymph node metastasis is only 0.36%. Gotoda and colleagues95 assessed the risk of lymph node metastasis of intramucosal cancer. None of the 1230 well-differentiated intramucosal cancers that were less than 30 mm in diameter regardless of ulceration findings were associated with metastases. None of the 929 lesions without ulceration were associated with nodal metastases regardless of tumor size. Similar to findings for intramucosal cancers, for submucosal lesions, there was a significant correlation between tumor size larger than 30 mm and lymphatic-vascular involvement with an increased risk of lymph node metastasis. None of the 145 differentiated adenocarcinomas less than 30 mm in diameter without lymphatic or venous permeation were associated with lymph node metastasis, provided that the lesion had invaded less than 500 µm into the submucosa.95
Treatment
Gastroscopy
A morphologic staging classification based on the macroscopic endoscopic appearance of EGC has been described by Japanese investigators, as follows96:
Types I, IIa, and IIc are considered to be endoscopically resectable. Certain adjuncts to morphologic staging also help to predict that a lesion is amendable to minimally invasive (endoscopic) therapy and is likely to have the same outcome as surgery: lesions less than 1 cm in size, absence of a scar or ulcer histologically and endoscopically (0.2% lymph metastasis), and a well-differentiated histology (<1% lymph metastasis).1,92
Narrow Band Imaging and Magnification Endoscopy
Diagnosis of gastric neoplasia using magnification endoscopy has been attempted in the past decades.97 However, compared with the colonic surface, standardized systemic classification of the magnified gastric surface has not been established owing to diverse histology of gastric carcinomas; the existence of three different types of proper gastric mucosa; and various changes on mucosal structures by atrophy, chronic inflammation, metaplasia, and erosions. The narrow band imaging system provides very clear images of fine superficial structure and microvasculature of the gastric mucosa and has improved the endoscopic diagnosis of superficial gastric cancers. Nakayoshi and coworkers98,99 reported that significant correlation between histopathology and microvascular pattern obtained with narrow band imaging magnification enables the sensitive diagnosis of superficial depressed gastric cancer, and the modality can assist in determining feasibility of EMR of gastric cancer.
Nakayoshi and coworkers98 measured the correlation between the magnified images obtained with narrow band imaging and the histologic findings, especially with regard to the microvascular pattern; 165 cases of superficial depressed gastric carcinoma (109 well-differentiated adenocarcinomas and 56 poorly differentiated adenocarcinomas) were evaluated. Microvascular patterns on the mucosal surface were classified into two patterns: fine network pattern and corkscrew pattern. The fine network pattern appears as a mesh, and abundant microvessels are well connected with one another. In contrast, the corkscrew pattern has isolated and tortuous microvessels and looks like a corkscrew. A fine network pattern was recognized in 72 cases (66.1%) of 109 well-differentiated adenocarcinomas (intestinal type). The corkscrew pattern was observed in 48 cases (85.7%) of 56 poorly differentiated adenocarcinomas (diffuse type). For the magnifying endoscopic diagnosis of superficial depressed gastric carcinoma, both the existence of irregular microvessels and the disappearance of fine superficial structure are essential.
Endoscopic Ultrasound and Endoscopic Ultrasound–Guided Fine Needle Aspiration Cytology
EUS is now established as the best local staging modality for esophageal, gastric, and rectal cancer with a T stage accuracy of about 90%.100,101 Kelly and associates91 reported that T staging by EUS was more accurate for gastric cancer than for esophageal cancer, although there was no difference for nodal staging. EUS has been shown to be the most accurate imaging modality for staging EGC,101,102 with an accuracy of greater than 90% for assessing the depth of tumor invasion.103,104
Long-term disease-free survival in GI malignancies is a function of lymphatic and distant tumor spread. The linear-array echoendoscope has enabled the endoscopist to place a needle precisely under real-time EUS guidance and obtain tissue samples both from the primary tumor and from transluminal lesions such as lymph nodes and liver metastasis. Other imaging modalities, such as CT scan, are still necessary to exclude more distant metastases. The overall performance of EUS with fine needle aspiration (FNA) cytology for benign versus malignant lymph node involvement has been found to have a sensitivity of 92% (range 84% to 97%), specificity of 93% (range 75% to 100%), and accuracy of 92% (range 82% to 98%).105 Disadvantages of dedicated echoendoscopes include inability to traverse critical stenoses and technical difficulty of evaluating superficial lesions in some areas of the stomach such as the cardia.
High-Frequency Ultrasound Probes
The miniature probe can also be used to evaluate a lesion after saline injection, to confirm complete separation of the lesion from the underlying muscularis propria layer of the gastric wall before EMR.106 Inability to separate the lesion from the muscularis propria after saline injection is a predictor of unresectability and obviates the need for additional imaging to determine endoscopic resectability.
High-Frequency Ultrasound Staging of Early Gastric Cancer
A classification system for EGC has been developed based on depth of tumor penetration into the mucosa (m) and submucosa (sm), as defined by using HFUS probes. The superficial cancers are divided further into the upper, middle, and lower third of the layer involvement as m1, m2, m3, sm1, sm2, and sm3.103
An overall T staging accuracy of about 80% has been reported using HFUS in patients with EGC.103,107,108 T staging accuracy is greater for Tm1 (92.1%) than for Tsm1 (62.8%) or T2 muscularis propria (42.9%).109 The curative effect of EMR is excellent for intramucosal cancers (m1, m2, and probably m3) but not for submucosal cancers (sm1 to sm3).14 The ability to distinguish between m3 and sm1 lesions is crucial. Using a 20-MHz frequency probe, Yanai and colleagues110 were able to distinguish EGC involving the mucosa from EGC involving the submucosa with an accuracy of 72.3%.
The usefulness of HFUS was evaluated in 33 patients referred for endoscopic management of superficial or submucosal neoplastic lesions.111 The depth of invasion was accurately predicted by HFUS before EMR in 25 of 26 patients (96%). Of the nine gastric lesions removed, eight had HFUS before EMR. There was 100% agreement between the depth of invasion determined by HFUS and pathologic staging. Almost half of the resected lesions were gastroduodenal, including gastric adenocarcinoma, gastric and duodenal adenoma, gastric and duodenal carcinoid, pancreatic rest, fibrovascular submucosal polyp, ampullary adenoma, and duodenal lipoma. The diagnostic accuracy of HFUS probes for small gastric lesions (<4 cm) has been found by some researchers to be comparable to dedicated (standard) echoendoscopes.112
Description of Techniques
Endoscopic Mucosal Resection
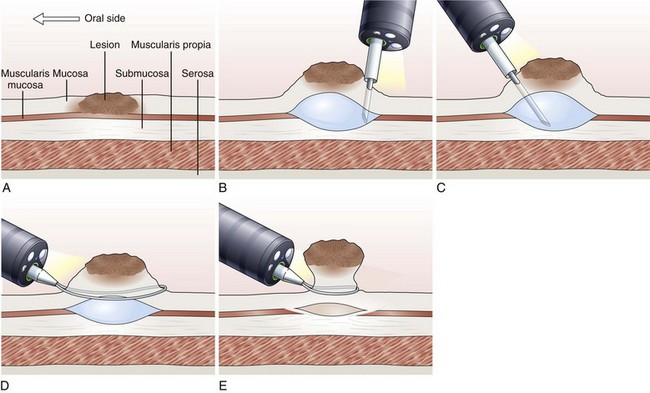
EMR involves the lifting of a lesion from the deep muscle layer of the gut wall, either by injection or by suction of the lesion into a cap fitted to the tip of the endoscope, followed by snare removal of the lesion (Fig. 33.5). Complete removal of the lesion en bloc during a single therapeutic procedure is ideal, although large lesions may require piecemeal resection. The availability of the resected specimen for pathologic examination is a distinct advantage of EMR over ablation therapies. Because EUS and HFUS cannot reliably distinguish between tumor infiltration and inflammation that may be associated with a malignant lesion, EMR provides both curative benefit and final staging confirmation.
Inject and Cut Technique
In the inject and cut technique, the lesion is lifted from the underlying muscularis propria by injecting a solution submucosally to produce a bleb beneath the lesion. The lesion is captured and resected by using an electrosurgical snare (Fig. 33.6). The required volume of submucosal injection varies according to the size of the lesion, provided that the bleb is sufficient to ensure a good lift of the entire lesion so that it can be safely captured and resected.
Various solutions are available for injection. A hypertonic saline and epinephrine solution is widely used in the hopes of decreasing the risk of bleeding116; other solutions have been used to obtain a more durable lift of the lesion.117 The use of hypertonic solutions except for glycerol has been questioned more recently because of concern about possible tissue damage. A study by Fujishiro and associates118 concluded that a combination of hyaluronate and glycerol is the most favorable submucosal injection solution, taking into consideration tissue damage and lesion-lifting ability. Also, various snares and currents (e.g., blended, ERBE, Tübingen, Germany) are preferred by different endoscopists. To date, no randomized trials have been conducted to compare the safety and effectiveness of different types of snares and injectants.
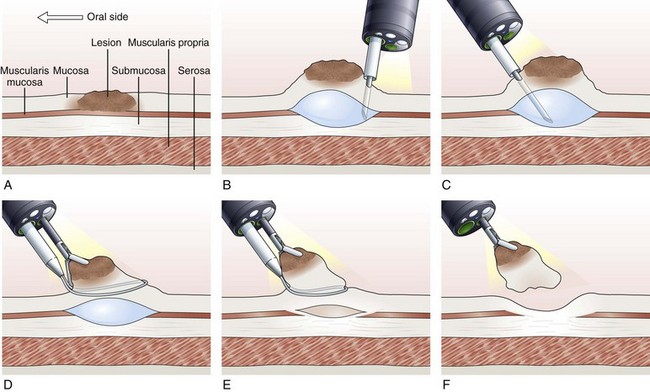
Inject, Lift, and Cut Technique (Strip Biopsy)
With the inject, lift, and cut technique, the submucosal injection is performed in the standard manner, as already described. A snare and grasping forceps are passed through the operating channels of a dual-channel endoscope. First, the grasping forceps is captured by the open snare, and the snare is closed over the forceps. Working as a unit, the forceps is used to grasp the lesion, the snare is opened, and the lesion is pulled through the open snare. The snare is closed over the lesion, and resection of the lesion is performed (Fig. 33.7).119–121 This technique is more cumbersome than the inject and cut technique and requires a dual-channel endoscope and two assistants to perform EMR.
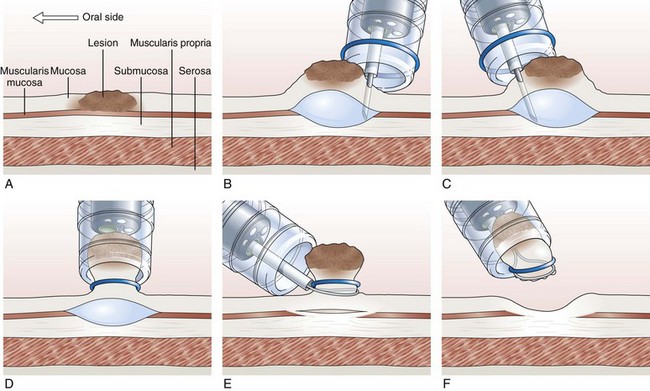
Endoscopic Mucosal Resection with Ligation
In the EMR with ligation technique, the lesion is removed with or without previous submucosal injection (Fig. 33.8).122–124 More recently, a conventional band ligation device (MBL; Wilson-Cook Medical, Inc, Winston-Salem, NC) was modified by widening the threading channel of the cranking device from 2 to 3.2 mm.125 This device allows for the insertion of a 7-Fr catheter through the threading channel of the cranking device into the 3.7-mm working channel of the endoscope. Band ligation can be performed with the polypectomy snare still within the working channel without any increased friction during winding of the thread; this enables sequential banding and snare resection of esophageal mucosa without the need to change the endoscope.125
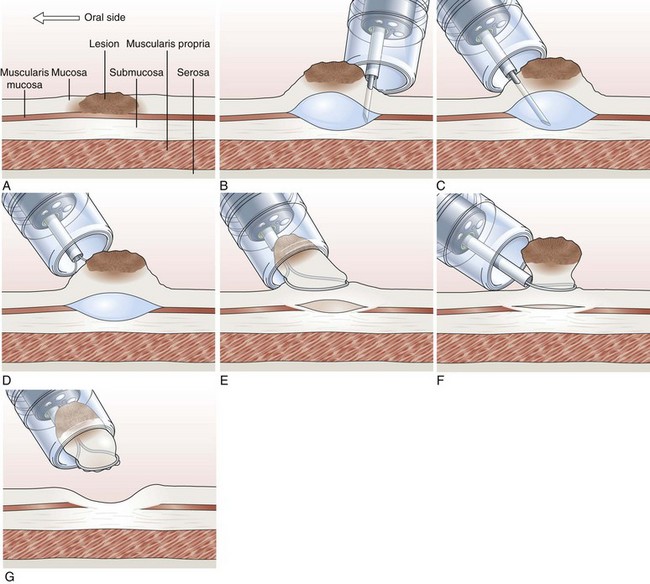
Cap-Assisted Endoscopic Mucosal Resection
In cap-assisted EMR, a specially designed transparent plastic cap is used.126,127 The plastic cap is fitted over the tip of the endoscope; various cap sizes are available. Submucosal injection of the lesion is performed in the standard manner. A crescent-shaped snare is prelooped into the groove of the rim of the specialized cap by gently suctioning normal mucosa into the cap and opening the snare to allow it to rest along the inside groove of the rim of the cap (SD-221L-25 or SD-7P-1; Olympus America, Inc). After prelooping the snare, the suction is turned off to release the normal mucosa. The cap is now used to suction the lesion while maintaining a constant medium to high vacuum. When the lesion is trapped completely inside the cap, the snare is closed over the lesion. After the lesion is tightly strangulated by the snare, the suction is turned off, and the lesion with the snare around it is allowed to leave the cap. Resection of the lesion is then performed with application of current. Gentle suction of the specimen into the cap allows safe and complete recovery of the specimen (Fig. 33.9). This technique is particularly useful for upper GI lesions.127–129 Matsuzaki and colleagues128 reported the use of a soft 18-mm diameter cap for en bloc resection of larger gastric lesions up to 1.4 times the size that can be removed with a standard cap.
For larger lesions greater than 3 cm, some endoscopists have used a more viscous material, sodium hyaluronate, for submucosal injection. A small-caliber tip transparent hood that accommodates a needle-knife or an insulated thermal knife with a ceramic cap is used to cut around the lesion on its entire circumference. The final step is the complete removal of the lesion using a large snare.130,131
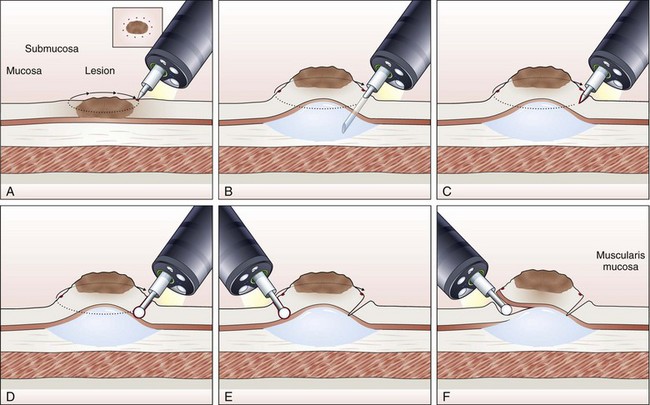
Endoscopic Submucosal Dissection
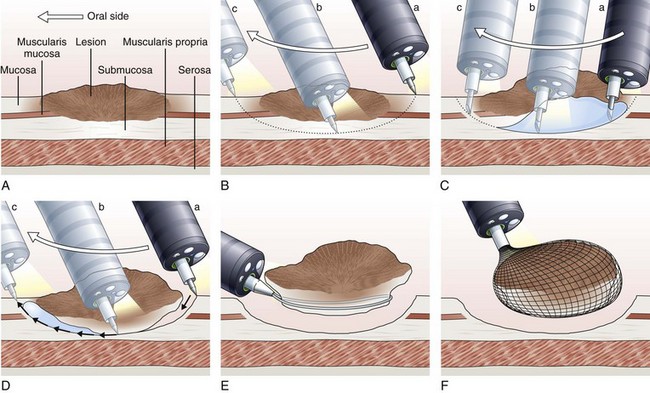
The concept and technique of ESD are markedly different from conventional EMR. The procedure begins by making circumferential markings all around the lesion at about a 5-mm distance, at 2-mm intervals. Injection of sodium hyaluronate or another solution to create a long-lasting submucosal fluid cushion between the lesion and the muscle layer is performed to prevent perforation during ESD (Fig. 33.10).117
Sodium hyaluronate is harmless to the injected tissue because of its isotonicity, but it needs to be diluted when used for ESD because of its high viscosity. Among different mixtures tested, a mixture of high-molecular-weight sodium hyaluronate with a sugar solution, with or without glycerin, seems to be the most cost-effective.132 Adding indigo carmine dye (0.004%) to the mixture is optional. To minimize resistance to injection, a high-flow injection needle with a large inner lumen should be used. The solution is first injected just outside the marking spots, where a circumferential incision to isolate the lesion to be resected from the normal mucosa is made using the electrosurgical knives selected for the procedure. For the insulated-tip (IT) knife, a small initial incision is made with a standard needle-knife to allow for its insertion into the submucosal layer. Several electrosurgical knives have been developed for ESD.
Needle-Knife
First used by Hirao and colleagues116 in 1988, the needle-knife (KD-10Q-1; Olympus) has a fine tip and a small contact area, which allows a sharp incision. The cutting power required for mucosal incision (150 W) is so high that perforation is likely to occur unless the operator is highly skilled.
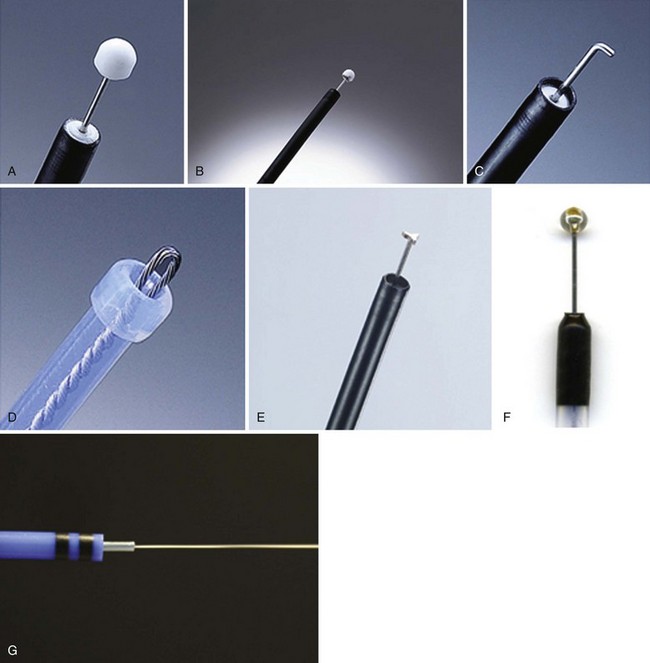
Insulated-Tip Knife
The IT knife (KD-610L; Olympus; Fig. 33.11A) is a needle-knife that is equipped with a ceramic ball at the top of the incising needle. The tip is blunted and insulated to prevent perforation; the knife was developed by Hosokawa and Yoshida133 for safe performance of ESD. The IT knife is most frequently used for ESD of the stomach; special care should be taken when operating in the colon. The second-generation IT knife (ITKnife2; KD-611L; Olympus; Fig. 33.11B) has an additional electrode at the back of its insulating tip that makes cutting easier, especially in the lateral direction.
Hook Knife
The hook knife (KD-620LR; Olympus; Fig. 33.11C) is a needle-knife with 1 mm of the tip bent to form a right angle. Submucosal tissue can be hooked and pulled before incision, which improves safety. Safety can be enhanced further by the concomitant use of a transparent hood. This knife has a rotating function so that operators can select any direction for hooking. Because of its high maneuverability, the hook knife is a useful endo-knife for esophageal ESD.134
Flex Knife
The flex knife (KD-630L; Olympus; Fig. 33.11D) has a rounded tip with a twisted wire similar to a snare, with a length that can be adjusted according to circumstances. The outer sheath of the knife is soft and flexible with a thick tip that functions as a stopper. Because of all these characteristics, perforation is less likely to occur with this needle, which can be used independently on the location of the lesion.135 A transparent hood for better visualization of the operating field is usually used.
Triangle-Tip Knife
The triangle-tip knife (KD-640L; Olympus; Fig. 33.11E) has a triangle-shaped conductive tip at the distal end, facilitating cutting of the mucosa. This knife can be used for any step in the ESD procedure: marking, precutting, incision, and dissection.
ESD Knife
The ESD knife (MTW, Fig. 33.11F) is a needle-knife that is equipped with a sapphire ball tip at the top of the incisioning needle. When the circumferential incision has been completed, additional solution is injected in the center of the lesion to lift up the mucosa adequately. Small lesions can be resected with an electrosurgical snare in the same way as with the standard polypectomy technique.136 Larger lesions require a more complex procedure, however, in which the submucosa is directly dissected using the electrosurgical knife until exfoliation of the entire tumor en bloc is obtained.131
Hybrid Knife
The hybrid knife (ERBE; Fig. 33.11G) is a stainless steel tube that incorporates a microcapillary lumen with a diameter of 150 mm to provide a water jet function.137 The flexible instrument has an outer diameter of 2.0 mm and a length of 2.2 mm and can be used with any standard endoscope. This flexible device allows injection, hydrodissection with preselected pressure (maximal water pressure 80 bar, adjustable from 0 to 80 bar), or dissection with any electrical current after usage of the saline-only solution without having to exchange the catheter. This novel device is the latest entry of clinically available ESP knives.
Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection for Early Gastric Cancer
A retrospective evaluation of 210 cases of EGC treated with EMR and followed up for 14 years reported a 5-year survival of 86% and a 10-year survival of 56%; there were no cancer-related deaths.119 EMR as a curative treatment has been evaluated in 102 patients.108 No distant or local metastases were seen during 9 years of follow-up.
A total of 106 patients with EGC up to 2 cm in diameter were treated with complete resection of the lesion in a single procedure, either by en bloc resection for lesions less than 10 mm (64%), or by piecemeal resection for larger lesions (36%).139 No recurrence after either technique was found in patients with tumor-negative margins. The overall recurrence rate of cancer in this particular study was 2.8%. Tumors that recurred all were greater than 15 mm initially and treated with piecemeal resection. Because histologic reconstruction to confirm complete resection by piecemeal method is often difficult, patients should be followed very closely after piecemeal resection.
In a retrospective study, Amano and coworkers140 evaluated endoscopic therapy in patients with EGC that does not meet the Japanese Research Society for Gastric Cancer morphologic criteria for lesions suitable for EMR. Endoscopic therapy consisted of EMR, thermal therapy, or both. The study included poorly differentiated and well-differentiated tumors 1 to 3 cm. Some patients with submucosal invasion limited to the most superficial layer (sm1) were included. Curative resection was achieved in 95%. The rate of cure in this group was statistically similar to the cure rate of cases that fulfilled the standard morphologic criteria for EMR resection (98%).140
Adequacy of EMR can be assessed by measuring the distance from the edge of the resected specimen to the margin of the cancer. In a prior study, no patient with a distance of more than 2 mm developed recurrence of the cancer, whereas 16% of patients with a distance of less than 2 mm developed recurrence.141 Presence of cancer at the edge of the specimen was associated with a recurrence of 45.8%. No recurrence was observed if the distance from the edge of the specimen to the cancer was more than 7 mm, suggesting that adequate distance (preferably at least 2 mm) between the edge of the specimen and the cancer needs to be achieved to ensure a complete resection.142 Margin-negative resections are more likely (81.2%) in cancers that are less than 1 cm in diameter than in cancers that are more than 2 cm in diameter.142
In a prospective analysis of 479 EGCs treated with EMR over an 11-year period at Tokyo National Cancer Center,130 the following selection criteria were used: well-differentiated or moderately well-differentiated gastric cancer; morphologic types I, IIa, and IIc; no histologic evidence of ulceration; diameter of less than 3 cm with histologic confirmation of intramucosal carcinoma; no lymphovascular invasion; and clean margins. EMR was used to treat 405 patients, and complete resection was achieved in 69%. The recurrence rate was only 2%, and all recurrences were treated successfully with a modified combination therapy of EMR and laser. There was no subsequent recurrence in any of the 278 patients after a median follow-up period of 38 months (range 3 to 120 months). No cancer-related deaths were reported. More recently, EMR and ESD have become established alternatives to surgical therapy for EGC in Japan and Korea.143–145
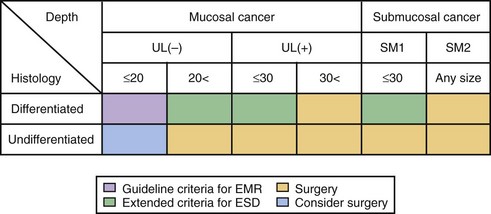
The current criteria for endoscopic therapy are (1) elevated-type intramucosal cancer (0 to IIa) less than 20 mm; (2) depressed-type mucosal cancer without ulceration (0 to IIb, 0 to IIc) less than 10 mm; and (3) well-differentiated or moderately well-differentiated intestinal-type ADC. Depressed cancers were associated with lymph node metastases (86%) when there was submucosal infiltration, size of 20 mm or greater, or lymphatic vessel involvement.146
Extended indications for EMR in EGC have been proposed as follows140: (1) well-differentiated lesions up to 30 mm, without an ulcer or ulcer scar; (2) mucosal cancers less than 20 mm, with an ulcer or ulcer scar; (3) sm1 lesions less than 20 mm, without an ulcer or ulcer scar; and (4) poorly differentiated lesions less than 10 mm.
The risk for node metastasis is approximately 0.4% for differentiated cancers. Undifferentiated mucosal cancers should not be treated by EMR because the risk for node metastasis is approximately 4%. Poorly differentiated and signet-ring cell carcinomas less than 5 mm in size could be managed, however.147 The proposed extended criteria for endoscopic resection in the ESD era are summarized in Fig. 33.12.
By expanding the criteria for EMR, the need for gastrectomy in EGC can be reduced. Because it is difficult to resect large and ulcerative lesions by conventional EMR, however, the technique of ESD was developed. Over the years, substantial experience in the use of this technique has been gained, and ESD with the IT knife has become a standard practice in Japan for the treatment of EGC.148,149
In Europe, ESD has been performed in 10 patients (9 EGCs and 1 adenoma, with a median diameter of 22 mm) using a new double-channel endoscope (the R-scope; Olympus Co, Tokyo, Japan). ESD was successful in six cases. Perforation occurred in two cases and were treated by surgery.150 In another study, ESD was performed in 19 patients with gastric superficial lesions (15 to 30 mm), with high-grade (n = 15) or low-grade (n = 4) noninvasive epithelial neoplasia. ESD was performed in all cases, with 89% R0 resection and 79% en bloc resection rates observed. Major bleeding was reported in 1 case (5%); there were no cases of perforation. With a median follow-up of 10 months, a single recurrence (5%) was observed.151
Endoscopic resection has also been performed for undifferentiated-type intramucosal cancer.152 In 38 patients with 42 undifferentiated intramucosal gastric cancers who declined surgical resection, ESD was performed with dedicated devices by experienced expert endoscopists. The en bloc resection rate was 83.3%; complete resection rate was 80.9%. Clinical remission was achieved in 92.8% with recurrence during follow-up seen in only 7.14%. In undifferentiated gastric cancer, grossly normal gastric mucosa surrounding the resected lesion could contain cancer cells beneath the epithelium153; this may explain why the complete resection rate of ESD for undifferentiated–type cancer is lower than that reported for well-differentiated cancer. The en bloc resection rate is better with ESD than with conventional EMR. The procedure time is longer in ESD, although this disadvantage might be improved with experience.154
Postoperative Care
Because of the extent of the resected area, a standard dose of proton pump inhibitor is administered for 8 weeks to prevent postoperative bleeding and to promote ulcer healing, and patients are typically placed on nothing per mouth (NPO) for 1 day, followed by clear liquids on the 2nd day and a soft diet for another 3 days.155 A large ulcer after ESD was reported to heal within 8 weeks after resection with the patient receiving antacid treatment.156,157
Complications of Endoscopic Resection
Bleeding
Bleeding is the most common complication of EMR and ESD of gastric tumors. Immediate bleeding, which can be brisk, seems to be more common with resection of tumors located in the upper third of the stomach. The reported incidence of bleeding after EMR has ranged from 0.38% to 16.1%.142 The discrepancy may be due to differences in definition of bleeding and to study methodology. Most institutions have reported the incidence of bleeding to be 10% to 16%, although studies based on surveys have reported a much lower incidence. In one large consecutive series, bleeding occurred in 7% of patients undergoing ESD.158
Most bleeding occurs during the procedure or within 24 hours after EMR. In the largest retrospective study dealing with predictors of bleeding after EMR of gastric tumors, the overall incidence of bleeding was 17.6%, with delayed bleeding occurring in 5.3%. In this study, the only statistically significant factor for predicting delayed bleeding was the occurrence of immediate bleeding during EMR.159 Delayed bleeding is probably not due to inadequate initial hemostasis but rather to insufficient coagulation during resection because in this study the sites of delayed bleeding were not the same as the sites of immediate bleeding.159 Size greater than 1 to 2 cm has been reported by some authors to be predictive of bleeding160; other studies have not found a positive correlation between size of lesion and risk of bleeding.159,161 No significant association has been found between the risk of bleeding and different techniques used for EMR, morphology of the lesion (flat, raised, or depressed), type of electrocautery current used, amount of saline used, or location of the lesion.
Management of Bleeding after Endoscopic Resection
No maneuver has been shown to help prevent bleeding. Bleeding during endoscopic resection usually stops spontaneously. If significant bleeding occurs, the standard methods of endoscopic hemostasis should be attempted. A few caveats need to be remembered when treating this complication endoscopically. Cautery should be applied cautiously keeping in mind that the site has already received a significant amount of energy. Overvigorous delivery of additional coagulation current may result in a transmural burn or a perforation. Injection of diluted epinephrine (1 in 10,000 or 1 in 20,000) can also be used to control the bleeding, either as the only measure or to prepare the bleeding site for another maneuver, such as cauterization or placement of mucosal hemoclips.159,160 One potential advantage of the latter maneuver is that hemoclips do not cause additional gastric wall injury to the resection bed. Application of clips during the procedure may preclude further endoscopic resection.
During ESD, immediate minor bleeding is common but can be successfully treated by grasping and coagulating the bleeding vessels with a hot biopsy forceps using 80-W soft mode coagulation (ICC200; ERBE).162 Endoclips are also often deployed for brisk bleeding. Delayed bleeding, manifested by hematemesis or melena at 0 to 30 days after the procedure, is treated by emergent endoscopy, performed after fluid resuscitation, using similar techniques. Most bleeding (75%) occurs within 12 hours after the procedure and has been reported to be strongly related to tumor location and size.159,163
Perforation
Perforation typically occurs when part of the muscularis proper is inadvertently resected in the specimen. Transmural burns secondary to aggressive cauterization may also result in delayed perforation. The rates of EMR-induced perforation are highest for gastric lesions at 2.5% to 5% (vs. lesions in the colon at <1%). Reported rates of perforation are higher when performing EMR with an IT knife (5.6%) compared with the endoscopic aspiration technique (0.8%).161 The overall risks of perforation during ESD is approximately 4%.6
A randomized controlled study in a pig model with a new water-jet hybrid knife reported no ESD perforations (0 of 12) and may be a promising modality to reduce complications in gastric ESD.137 The system is also convenient for the endoscopist to perform ESD without the need to exchange devices (knives and injection needles) during the procedure.
A surgical consultation should be obtained as soon as this complication is suspected. The earlier the diagnosis is established (within the first 6 hours), the better the prognosis. The standard of care for management of a recognized perforation is surgical; however, if the perforation is small and the patient is asymptomatic, endoclips could be used to close the defect, but this should be done early on.161,164,165 Patients should be placed NPO and treated with broad-spectrum antibiotics.
Transmural Burn Syndrome
Patients often present with symptoms and laboratory abnormalities that are indistinguishable from a perforation. It is crucial to exclude a perforation immediately before resorting to conservative management. A surgical consultation should be obtained. After a perforation has been excluded, patients should be placed on broad-spectrum antibiotics, intravenous hydration, and bowel rest. Serial abdominal x-rays should be ordered to monitor for the possibility of a late perforation. Most patients respond very well to conservative management.166
Luminal Stenosis
Luminal stenosis has been described as a delayed complication in patients who have had EMR mainly of an esophageal lesion,167 but it could potentially occur after EMR and ESD of tumors located in the gastroesophageal junction and prepyloric area. This complication tends to occur after extensive resection when the mucosa of more than three-fourths of the luminal circumference has been excised. The mechanism of retraction seems to be related to the healing process. Temporary metal stent placement168 and balloon dilations or a combination of both have been used effectively.167 Incremental resections in multiple treatment sessions may help to minimize contraction of the lumen.
Ablation Techniques
Neodymium:yttrium-aluminum-garnet (Nd:YAG) Laser
Laser energy can be delivered through flexible optic fibers at wavelengths of 1320 nm and 1064 nm. Because the emission is invisible, a helium-neon aiming beam is used in conjunction with Nd:YAG to visualize the focal target area.169,170 To obtain photoablation, an optical fiber is passed through the operating channel of the endoscope, and the transmitted laser beam can be delivered in a contact or noncontact fashion. Tangential irradiation is impossible with this technique, so the location of some lesions may be more difficult to target.171
When performing laser therapy, safety eyewear is used to avoid ocular damage to endoscopy personnel. Adequate local exhaust ventilation and use of respiratory filter masks have been recommended to avoid respiratory exposure to aerosolized infectious pathogens resulting from vaporization of tissue.172 Because of its limited portability, high cost, availability of less costly alternatives, and the need for specific training, laser therapy is not widely used today for endoscopic treatment of gastric neoplasms.
Photodynamic Therapy
Photodynamic therapy (PDT) also delivers energy via flexible optic fibers. The laser light activates a photosensitizing agent, releasing toxic singlet oxygen and causing tissue necrosis. The photosensitizer selectively accumulates in the target tissue. The only commercially available photosensitizer in the United States is porfimer sodium (Photofrin PDT).173 Other photosensitizers include 5-aminolevulinic acid (5-ALA), zinc II phthalocyanine, aluminum sulfonated phthalocyanine, benzoporphyrin, meta-tetrahydroxyphenylchlorin (mTHPC), N-aspartyl chlorine e6 (NPe6), and motexafin lutetium. Among these different photosensitizers, mTHPC, porfimer sodium, and 5-ALA have been used extensively in gastroenterology. mTHPC is a potent, highly selective drug that has been used in the treatment of neoplasms, whereas 5-ALA, which induces very superficial necrosis, has been used to treat Barrett’s esophagus.174,175
Porfimer sodium is administered at a recommended dose of 2 mg/kg intravenously and activated 48 hours later by a tunable dye laser at 630 nm.173 5-ALA is a heme pathway precursor that can be given orally or intravenously. 5-ALA is converted to the endogenous photosensitized protoporphyrin IX, which can be activated by red or green light.
Laser Therapy for Early Gastric Cancer
Endoscopic laser therapy has been used to treat inoperable patients with EGC. In 13 patients with EGC, a complete response was achieved with Nd:YAG or PDT in 85% of the patients.5 Sibille and colleagues141 used Nd:YAG laser to treat 18 nonoperative patients with EUS T1 gastric cancer until a complete response (negative endoscopic biopsies) was achieved. The number of sessions varied from 1 to 15 sessions, performed every 2 weeks. Initial complete response was achieved in 16 (89%) patients after a mean of 1.7 sessions (range 1 to 4); two patients (11%) did not respond to therapy.
PDT was shown to achieve a complete response in eight EGC lesions diagnosed in seven nonoperative patients.142 In another study, Takahira and associates145 reported on the use of PDT with an excimer dye laser in 10 patients with 11 EGCs. A complete response was observed in 10 of 11 tumors (91%). Surgery was required in only one patient who had residual tumor after PDT. Nd:YAG laser therapy has also been used for photoablation of residual tumor after an incomplete EMR.3,176
Laser Therapy for Advanced Gastric Cancer
Laser ablation therapy is more frequently used as a palliative modality in advanced gastric lesions. Successful palliation of bleeding or obstruction secondary to gastric cancer has been reported in 81% to 100%.5,177,178 Relief of obstructing cancer in the gastric cardia using Nd:YAG laser has been reported.179,180 Addition of external beam radiation and brachytherapy was found to increase the interval between laser treatments in advanced esophageal and gastric cardia adenocarcinoma.181–183
Complications of Ablation Therapy
Bleeding is one of the most common complications of Nd:YAG laser therapy. A major bleeding rate of 12.5% after laser treatment of gastric tumors has been reported.178 Perforations may occur in 1% to 9%, with a procedure-related mortality of up to 1%.178 Stricture formation as a late complication of laser therapy (Nd:YAG and PDT) has been observed in 5% to 13%.5 Pulmonary complications have been reported after PDT in up to 15%. Photosensitization, lasting up to 3 months, and severe sunburn have been reported in 5% to 7% of patients after PDT therapy.173,184
Argon Plasma Coagulation
APC uses a high-frequency current and an ionized argon gas for coagulating tissue. In the early 1990s, an APC delivery catheter that could be inserted through a flexible endoscope was invented by Farin and Grund.151 Initially introduced as a hemostatic device, the technology subsequently was used for ablation of superficial neoplastic lesions. A noncontact coagulation device, APC can deliver a tangential current to coagulate a target lesion uniformly.135
Argon Plasma Coagulation for Early Gastric Cancer
Compared with laser therapy, experience with APC for treatment of both early and advanced gastric cancer is limited with a shorter duration of follow-up.185 APC as a curative treatment for EGC was used by Sagawa and colleagues4 in 27 patients who were considered poor candidates for either surgical resection (17 patients) or EMR (10 patients) because of comorbidities including severe cardiac failure, marked thrombocytopenia, or anticoagulation therapy. No evidence of recurrence was observed in 26 treated patients (96%) at a median follow-up of 30 months. Only one patient had a tumor recurrence at 6 months, and this was successfully retreated with no evidence of recurrence after an additional follow-up of 39 months. Of the 27 patients, 12 (44%) had EGCs located in areas more difficult to access endoscopically, such as the posterior wall of the stomach or the cardia.4 APC is also commonly used to ablate any residual tumor after EMR and ESD.4,139
Argon Plasma Coagulation for Advanced Gastric Cancer
APC as part of a multimodality palliative approach for advanced gastric cancer has been reported.186 Ten patients with gastric carcinoma in whom surgery would not have been curative were treated with APC for debulking of a partially obstructing tumor; a mean of 4.9 treatment sessions was required to achieve effective palliation and symptom relief.186 In another study, 40 patients with superficial-type cancer were treated by APC only.187 Intestinal-type intramucosal carcinoma disappeared in all patients after one or two sessions of APC. Residual tumor or recurrence was noted in four patients (10%)—three with submucosal invasion and one with an extensive, diffuse-type carcinoma. However, such lesions were locally controlled by follow-up APC.187 APC has also been used to treat tumor ingrowth within self-expandable metal stents in patients with obstructing esophagogastric junction tumors.188
Complications of Argon Plasma Coagulation
APC therapy in 27 patients with EGC was not associated with any serious complications. Of 27 patients, 3 (11%) complained of abdominal fullness that was alleviated by intermittent suction or by continuous suction when a dual-channel upper endoscope was used.4
Enteral Stents
Enteral stents are indicated for malignant luminal obstruction of the GI tract. Patients who have gastric outlet obstruction secondary to gastric cancer can be treated with surgical palliative bypass (via laparoscopy in some centers).189 Patients who are not good candidates for surgical palliation may benefit from percutaneous gastrostomy for decompression and enteral feeding.190 Another alternative is the use of an enteral self-expandable metal stent (SEMS). SEMS have been used as palliative therapy for obstructing gastroesophageal junction tumors.191 SEMS are made of various metal alloys in varying sizes and shapes. Covered and uncovered SEMS are available.192 Many of the published series on placement of SEMS in the upper GI tract have used either modified or standard esophageal stents.193,194
A through the scope technique is used to place enteral SEMS, typically made of stainless steel or nitinol. Two important caveats for successful placement of SEMS are the ability to pass a guidewire across the stricture and the selection of a stent that is at least 3 to 4 cm longer than the obstruction, to allow an adequate margin on both sides of the obstruction.195
Other Gastric Neoplasms
Gastric Lymphoma
Epidemiology
GI lymphoma accounts for 4% to 20% of all NHL and 30% to 40% of all extranodal cases.196 The incidence rate for NHL has been increasing with this trend being more prevalent for extranodal disease. Even though primary gastric lymphoma accounts for less than 5% of all gastric malignant neoplasms, an increase in incidence of primary gastric lymphoma has been observed in the United States.197
Time trend analysis based on a population-based registry has shown increased incidence rates for gastric (6.3%) and small bowel (5.9%) NHL; there is a concomitant decrease in GI NHL of unknown site, suggesting that the increased incidence at least partly may be a result of more accurate diagnosis. In this particular study, the most common site of GI NHL for all age groups was gastric (43.3%), followed by small bowel (27.4%) and large bowel (11.1%); NHL of unknown site accounted for the remaining 16.1%.196
Pathogenesis
An association between H. pylori infection and gastric NHL has been shown in several studies.198,199 Acquired mucosa-associated lymphoid tissue (MALT) in the stomach provides the background for lymphoma to develop. H. pylori is the only well-established chronic antigenic stimulus that causes gastric MALT.200 Parsonnet and colleagues201 showed that prior H. pylori infection gives a statistically significant sixfold increased risk of developing gastric NHL, with the association being stronger for high-grade lymphoma. Complete regression of MALT after eradication of H. pylori infection has been described, with subsequent relapse of the lymphoma after reinfection with the organism.200,202,203 The link between H. pylori gastritis and low-grade gastric lymphoma of the MALT type has also been supported by published data from case-control and epidemiologic studies.202
Although the rate of H. pylori infection in patients with gastric lymphoma has been found to be higher (91%) than in the general population (64%) in some areas throughout the world, the incidence of gastric lymphoma only partially parallels the incidence of H. pylori gastritis.204 In some areas of Africa, the prevalence of H. pylori infection is very high, but the incidence of gastric lymphoma is very low. A study using population-based registries showed that the incidence of gastric lymphoma parallels the incidence of all NHL, indicating that H. pylori infection is not the only factor in the pathogenesis of MALT lymphoma.204 A 22% rate of H. pylori–negative status based on histologic and serologic tests has been reported in patients with low-grade gastric lymphoma.205 Rarely, low-grade lymphoma may develop in the background of Helicobacter heilmannii–associated gastritis.206
In some H. pylori–negative gastric MALT lymphomas, an association with autoimmune diseases such as Sjögren’s syndrome has been described, but no association with viruses known to be present in other types of lymphomas has been detected.207 Epstein-Barr virus infection, an early pathogen in the development of nodal lymphoma, is only very rarely found in gastric MALT lymphoma.208 Occupational exposure to solvents and pesticides was suggested to play a pathogenic role in some gastric lymphomas in an Italian study.198 The development of MALT that gives rise to gastric lymphoma is probably a multifactorial process involving both antigenic and host-related factors, but other mechanisms are unknown.
Clinical Features
Low-grade gastric lymphoma typically occurs in the fifth decade, whereas high-grade lymphoma occurs in the sixth decade, suggesting that the progression from low-grade to high-grade lymphoma takes about a decade.209 Patients with gastric lymphoma generally present with nonspecific dyspepsia or with symptoms suggestive of peptic ulcer disease. Some patients may present with GI bleeding or anemia. The finding of an abdominal mass at presentation is rare.209
Endoscopic Diagnosis
Endoscopically, patients with low-grade lymphoma may display normal-appearing gastric mucosa, nonspecific macroscopic gastritis, thickened gastric folds, or ulcerative lesions. High-grade lymphoma generally displays large ulcers on more protruding tumors.210 The diagnosis is based on gastric biopsies and enhanced by immunohistochemistry.211
Endoscopic Ultrasound Staging
EUS provides accurate staging of gastric lymphoma. Based on EUS criteria, gastric lymphoma can be divided into four types: superficially spreading (with thickening of the second or third layers or both), diffusely infiltrating (diffuse transmural irregular thickening of the gastric wall), mass-forming (localized hypoechoic mass with a clear margin located in the third layer or in the third and fourth layers), and mixed (combination of mass-forming and superficially spreading).212 Superficially spreading and diffusely infiltrating lymphomas are seen only in patients with low-grade MALT lymphoma. Mass-forming lymphomas are of the same histologic type as intermediate-grade lymphomas, either diffuse large cell or mixed cell type.212
EUS is considered the most accurate technique for locoregional staging of MALT lymphoma.212,213 EUS had a T staging accuracy of 91.5% when resected specimen histology was the “gold standard.”213 The accuracy of EUS for detection of lymph node metastasis was reported to be 83% compared with resected histology in the same study.213
A previous study evaluated the accuracy of a 12-MHz miniprobe for the staging of low-grade gastric MALT lymphoma compared with conventional EUS.214 The study retrospectively reviewed 39 patients before treatment who had histologically confirmed low-grade MALT lymphoma. The accuracy of T and N staging using miniprobe and conventional EUS were similar in this study. The advantage of performing staging with the miniprobe is that this examination can be performed as a single-step procedure during diagnostic gastroscopy. We believe, however, that conventional EUS and EUS-guided FNA is the procedure of choice for staging MALT lymphoma because miniprobes cannot detect distant metastatic lymph node involvement (e.g., nodes in the celiac region) or provide a specimen for cytologic evaluation.214,215
Pathology
NHL are malignant neoplasms of the B and T lymphocytes and their precursor cells.216 In Western countries, B-cell lymphoma is more common (>80%). In the Western world, B-cell lymphoma of the MALT type is the most common. Most of these lymphomas arise in the stomach.202,217–219 In southern Japan, T-cell lymphomas predominate, accounting for greater than 75% of GI cases.202,217–219
In the presence of H. pylori infection, B-lymphoid and T-lymphoid cells and neutrophils are brought to the gastric mucosa to form acquired MALT. In low-grade lymphoma, the autoreactive B-cell proliferation is secondary to a specific activation of reactive T cells by H. pylori and cytokines rather than to the bacteria per se.220 The two main groups of gastric lymphomas are extranodal marginal zone B-cell lymphoma, MALT-type lymphoma, and diffuse large B-cell lymphoma.221 Marginal zone B-cell lymphoma and MALT-type lymphoma are composed of a monotonous, diffuse infiltrate of small lymphoid cells, whereas diffuse large B-cell lymphoma is characterized by a diffuse infiltrate of large malignant lymphoid blasts resembling centroblasts, plasmablasts, and immunoblasts.222
Low-grade MALT lymphoma characteristically displays small cleaved cells or centrocytelike cells and is more frequently found in the stomach. As with nodal NHL, low-grade MALT lymphoma may progress to high-grade lymphoma. In the stomach, low-grade MALT lymphoma may extend over a large area or be multifocal.223 A subset of patients with low-grade B-cell MALT lymphoma can have a focal high-grade component histologically. A lower survival rate has been found in this subset of patients.224 Conventional biopsy specimens do not always yield a histologic diagnosis of low-grade B-cell MALT lymphoma.211 EUS detection with EMR histologic confirmation has been reported in one patient.225 Coexistence of gastric carcinoma and gastric lymphoma is uncommon but has been reported in the literature.226–228
Treatment
The strong association between H. pylori and B-cell lymphoma of MALT dictates that H. pylori eradication should be a routine treatment for superficial gastric lymphoma. A combination of two antibiotics (clarithromycin and metronidazole or amoxicillin) with a proton pump inhibitor results in more than 90% eradication of H. pylori.229 When H. pylori eradication is achieved, more than 70% of low-grade MALT-type lymphomas regress at an early stage. Complete histologic remission of lymphoma can be achieved 2 to 18 months after H. pylori eradication.203,205,230 In H. pylori–positive patients with localized gastric lymphoma who have no lymph node involvement assessed by EUS, a complete remission can be achieved in 79% of cases. A significant difference in response rate was found between lymphomas restricted to the mucosa and lymphomas involving the deeper layers.205 Patients who have persistent tumor and fail antibiotic treatment and patients with H. pylori–negative status could benefit from radiotherapy or radical surgery with curative intent.231 Elderly patients and patients in whom surgery is contraindicated should receive radiotherapy.209
Endoscopic Therapy
Present experience using EMR to treat gastric lymphoma is very limited. Toyoda and associates225 reported one patient with low-grade MALT lymphoma diagnosed by EUS and EMR to have a focal high-grade component. The ability of EMR to provide a histologic diagnosis and then confirm the depth of wall layer involvement allowed the appropriate therapy to be instituted (distal subtotal gastrectomy and extended lymph node [D2] dissection with Billroth I anastomosis). In a previous study, low-grade B-cell MALT lymphoma with a focal high-grade component was present in 27 of 233 (12%) patients. EUS and EMR may prove to be useful for identifying this subset of patients who have a worse postoperative 5-year survival rate than patients who have low-grade MALT lymphoma (80% vs. 96%).224
Carcinoid Tumor
Epidemiology
Epidemiologically, carcinoid tumor is a rare lesion, constituting only 0.5% of all malignancies. It is found in less than 1% of all cancer autopsy cases, and the approximate incidence is only 1 to 2 per 100,000 population.197,232 The largest epidemiologic series to date indicates that the incidence of gastric carcinoid, as a percentage of all carcinoid tumors, has increased from 2.25% (1950–1971) to 5.58% (1992–1999).233 The percentage of gastric carcinoid in relation to all gastric tumors also has increased from 0.4% in the early Surveillance, Epidemiology and End Results (SEER) data (1973–1999) to 1.77% in the late SEER data (1992–1999). It is unclear, however, if these findings represent a true increase in the incidence or are a result of increased awareness, more frequent use of endoscopy, or changes in reporting methods.233
Pathogenesis
Carcinoid tumors are slow-growing tumors that are derived from neuroendocrine cells known as enterochromaffinlike cells. The enterochromaffinlike cell is the main endocrine cell type of the corpus-fundus mucosa. Enterochromaffinlike cells are known to be highly sensitive to gastrin stimulus and able to trigger parietal cell acid secretion by releasing histamine.234 Gastrin, fibroblast growth factor, and H. pylori all have been shown to have trophic effects on enterochromaffinlike cells. These factors potentially may be relevant in the development of carcinoids.234,235
Nearly 67% of carcinoid tumors arise from the GI tract, with the tracheobronchopulmonary system being the most frequent site for carcinoid formation outside the GI tract.233 Most carcinoid tumors in the GI tract occur in the small intestines (41.8%), rectum (27.4%), or stomach (8.7%). A slight female predominance for this tumor has been found.233
The etiology of carcinoid tumor is unknown. Most tumors are considered to be due to sporadic somatic mutations, although there have been reports of a familial predisposition to the disease.236 Carcinoid tumors have been classified into three types: tumors associated with chronic atrophic gastritis type A (type 1), tumors associated with multiple endocrine neoplasia type I and Zollinger-Ellison syndrome (type 2), and sporadic gastric carcinoid tumors unaccompanied by hypergastrinemia or any specific gastric pathology (type 3).237
Clinical Features
Clinical manifestations of carcinoid tumors are often absent or vague. In approximately 8% to 10% of patients, these tumors manifest with the carcinoid syndrome (flushing, watery diarrhea, abdominal pain, and wheezing). This syndrome is attributed to secretion of the bioactive mediator serotonin (5-hydroxytryptamine) into the systemic circulation from the primary tumor or, more commonly, from metastatic sites.238
An association between GI carcinoids and second primary malignancies has been reported with incidence ranging from 12% to 46%.233,239 In the late SEER subset of data, carcinoid tumors in total were associated with other noncarcinoid tumors in 22.4%. Patients with gastric carcinoids had a decrease of 26% in the incidence of additional noncarcinoid neoplasms when the early SEER data (1973–1991) were compared with the late data (1992–1999). This findings prompted authors to speculate that the decrease might be related to higher identification and removal of these tumors endoscopically.233 The most common site of second primary malignancy is the GI tract, which is involved in 32% to 62% of cases, followed by the genitourinary tract (9% to 22%) and the lung and bronchial system (9% to 13%). Adenocarcinoma of the colon has been reported as the most common second primary malignancy.240
It has been speculated that some bioactive substances secreted by carcinoids, such as epidermal growth factor, CCK, VIP, secretin, bombesin, and gastrin, can promote the growth of tumor cells. It is probable that, over time, prolonged exposure to such growth factors may promote phenotypic changes in susceptible cells and induce neoplastic transformation.241 Metastases from carcinoid tumors have been reported to occur in approximately 29% of patients; most (61.2%) originate from the small intestine. After lymph node metastasis (89.9%), the liver is the most frequent site of metastasis (44.1%) followed by lung (13.6%), peritoneum (13.6%), and pancreas (6.8%).242
Endoscopic Diagnosis
Endoscopically, gastric carcinoid tumors may be present as polyplike lesions or, more frequently, as smooth, rounded submucosal lesions.243 The presence of an irregular erythematous depression or ulceration on the lesion has been considered characteristic but not pathognomonic of gastric carcinoids.244
Pathology
Type 1 tumors associated with chronic atrophic gastritis type A are the most common type of gastric carcinoids, characterized by multiple tumors and hypergastrinemia.237 The tumors are usually polypoid in appearance, are small (<1 cm), and are found in the body or fundus of the stomach. Type 1 tumors have a relatively benign course. Nodal involvement is reported in 16% of cases, and hepatic metastasis is reported in 4%.245,246 Type 2 gastric carcinoids are typically also small lesions, but the adjacent mucosa is not atrophic. They have a low potential for malignancy. Type 3 carcinoids are usually solitary tumors greater than 2 cm in size; 40% are present in the antrum and prepyloric area. There is no hypergastrinemia or chronic gastritis. Type 3 tumors are characterized by deep invasion and high potential for metastasis, even when primary lesions are small.237,247 Nodal metastasis is present in 55% of patients, and liver metastases are present in 25%. The 5-year survival is 50%. Histologically, tumors less than 1 cm or growth restricted to the mucosa are characterized as benign.248
Stromal Cell Tumor
Epidemiology
Gastrointestinal stromal tumors (GISTs) are rare; however, they are the most common mesenchymal tumors to arise in the GI tract. According to a population-based sample, the estimated incidence of GISTs is approximately 10 to 20 per 1 million per year.249 The annual incidence in the United States has been estimated to be 5000 to 6000 cases per year.250 The estimated incidence of malignant GISTs in southern Finland was 4 per 1 million.251 GISTs rarely occur before age 40 and are slightly more common in men than in women. An association between GISTs and von Recklinghausen’s disease has been suggested.252 GISTs are most commonly located in the stomach (60%), with the remainder being found in the small intestine (20% to 25%), colon and rectum (5%), and esophagus (<5%).250
Pathogenesis
Morphologically, GISTs are a heterogeneous group of neoplasms that arise anywhere in the GI tract. They represent a family of tumors that probably originates from the intestinal pacemaker cell, also known as the interstitial cell of Cajal (ICC).253 The ICC serves as a pacemaker system within the muscle layers of the gut and regulates GI motility.254 In the normal gut, these cells express vimentin, CD34, and CD117. The observation that immunohistochemical staining for several of these markers is identical between the ICC and GISTs prompted Kindblom and colleagues255 to propose that GISTs originate from the ICC or may originate from a pluripotential stem cell. The c-kit protein, or CD117, is now recognized as a very sensitive and specific marker for GI stromal cells.
Clinical Features
One-third of patients with GISTs are completely asymptomatic, and tumors are found incidentally during imaging, endoscopy, or surgical procedures being done for unrelated reasons.249,251 Patients may present with vague symptoms, but the most common manifestations are intestinal bleeding (20% to 50%), abdominal pain (40% to 50%), or a palpable mass (25% to 40%).251,256 Up to 30% of all GISTs are malignant. The most common site of extraintestinal metastasis is the liver, which is involved in 50% of malignant tumors, followed by lung (10%) and bone (<10%) involvement.249
Endoscopic Diagnosis
Most gastric stromal tumors appear as smooth, round, glistening masses covered with normal gastric mucosa. A defect in the overlying mucosa may occur when ulceration and resulting bleeding occur.256 Endoscopic mucosal biopsy specimens are inadequate to establish the histologic diagnosis in most patients, reemphasizing need for EUS in evaluating suspected GISTs and other subepithelial lesions.
Endoscopic Ultrasound Diagnosis
On EUS, GISTs appear as hypoechoic masses arising from the muscularis propria layer (fourth layer); infrequently, lesions may originate from the muscularis mucosa layer (second layer).257 When GISTs are occasionally found in the submucosal (third layer), they are thought to originate from the muscularis propria or the muscularis mucosa with subsequent extension into the submucosa.258
EUS features that may help to identify malignant tumors include size greater than 4 cm, irregular extraluminal borders, and presence of echogenic foci and cystic spaces. If at least two of these three features are present, the sensitivity of EUS for detecting malignancy is 80% to 100%.259 Palazzo and colleagues260 found that size less than 3 cm, homogeneous echo pattern, and regular margins were 100% specific for benign lesions. Whether EUS features alone can aid in the differentiation of high-risk versus low-risk tumors is less certain.261
EUS-guided FNA of suspected GISTs has been used widely to obtain a cytologic diagnosis. A single-center study by Ando and colleagues262 compared EUS-guided FNA with subsequently resected specimens. Using the surgical specimens as the “gold standard” for malignancy or benignity, they found that EUS-guided FNA had an accuracy of 91.3% and specificity of 100%. Sensitivity was only 66.7%, however, with two malignant lesions missed at FNA. Hunt and coworkers263 performed EUS-guided FNA in 40 patients for evaluation of spindle cell tumors. Of specimens, 17 stained positive for c-kit; tumors positive for c-kit were larger. Surgical resection was performed on 13 patients, 12 of whom had c-kit–positive tumors, and 3 of these 12 tumors had greater than 5 mitoses per 50 high-power fields. More recent data reinforce the idea that mutations in c-kit, high mitotic count, and larger tumor size are risk factors for poor prognosis in patients with localized GISTs and predict reduced relapse-free survival after curative resection.261
Pathology
The most common histologic variant among gastric GISTs is a cellular, spindle cell type of tumor consisting of uniform eosinophilic cells. Some of these tumors have a prominent nerve sheath tumorlike nuclear palisading pattern, whereas others show prominent perinuclear vacuolization with moderate to slight interstitial collagen.250 Malignant GISTs may have a spindled, round cell, or epithelioid pattern, or a combination of these. Some malignant GISTs histologically resemble leiomyosarcomas, although they usually have a less eosinophilic cytoplasm.250 Pathologic features that have been used to predict malignancy include mitotic activity, nuclear pleomorphism, degree of cellularity, nuclear-to-cytoplasmic ratio, tumor size (>5 cm have a high risk), mucosal invasion, ulceration, and tumor necrosis.264
Endoscopic Therapy of Submucosal Neoplasms
Submucosal tumors of the stomach are uncommon and are usually found incidentally during endoscopy. These tumors traditionally have been approached in one of two ways: (1) observation with or without an attempt to make the tissue diagnosis or (2) surgical resection. The advent of EUS has enabled the etiology of submucosal tumors to be determined noninvasively. Some tumors have EUS characteristics that are so pathognomonic that no additional diagnostic testing is needed. One example is a small lipoma that on EUS shows the classic hyperechoic texture of a lesion arising from the submucosa.265,266 In other situations, the EUS features alone are inadequate to establish the correct diagnosis. Mesenchymal tumors as a group typically arise from the fourth layer of the muscularis propria as a hypoechoic lesion; however, the EUS features alone do not distinguish gastric stromal tumors from other types of mesenchymal tumors. In this situation, cytopathology and immunocytochemistry obtained by EUS-guided FNA are quite helpful to establish the diagnosis and guide therapy. Patients with symptomatic disease and any submucosal tumors of the stomach with EUS features suggestive of local invasion require surgical consideration regardless of FNA results.
Endoscopic Ultrasound and High-Frequency Ultrasound Staging
Prior studies have shown the accuracy of EUS for the diagnosis of upper GI tract submucosal lesions.265 More recently, the efficacy and safety of endoscopic resection of submucosal tumors based on EUS or HFUS probe findings also have been reported.267 Prior reports and unpublished personal experience suggest that 20-MHz HFUS probes are useful for the evaluation of small submucosal lesions before EMR. Lesions larger than 2 cm may require the use of a lower frequency (12-MHz) probe or standard EUS-guided FNA for cytologic diagnosis and staging.267
Endoscopic Mucosal Resection
HFUS probe–assisted EMR has been performed in 26 of 28 submucosal tumors. A 20-MHz HFUS probe was used to evaluate the lesions and to confirm complete detachment of the lesion from the muscularis propria. All four gastric submucosal lesions (two carcinoids, one heterotopic pancreas, and one fibrovascular polyp) were successfully removed after saline solution injection into the submucosa. Two of the four (50%) benign gastric lesions involved the lower third of the submucosa (sm3), suggesting that deeply seated submucosal lesions are amendable to EMR therapy. Inability to separate the lesion completely from the muscularis propria by saline solution injection was noted in two rectal submucosal lesions, both of which were incompletely resected. Twenty-one of these lesions were removed by the lift-and-cut method, and six were removed using the cap technique. No complications occurred. There was no difference in complete resection rate with respect to location of the lesion in the submucosa (sm1, sm2, or sm3).106 No recurrence was observed during a median follow-up of 21.5 months. These results suggest that even deeply seated benign submucosal lesions (sm3), as delineated by HFUS probe, are equally amendable to complete removal by EMR as mucosal lesions.
More recently, Ichikawa and colleagues243 reported on the usefulness of EMR in the management of gastric carcinoid tumors. Five patients with type 1 gastric carcinoids underwent successful curative EMR. The depth of invasion was submucosal in three patients and mucosal in two patients by histologic evaluation of the EMR specimens. No evidence of recurrence was found during a mean follow-up of 32.6 months.243
Kojima and colleagues267 also reported on their experience with EMR of submucosal tumors. Of 54 lesions studied, 23 (43%) were gastric (10 leiomyomas, 2 lipomas, 6 aberrant pancreas, 1 neurofibroma, 1 neurinoma, 1 leiomyoblastoma, 1 leiomyosarcoma, and 1 granular cell tumor). EMR was performed to remove 18 (78%) of the 23 gastric lesions. The other five (22%) patients had evidence of muscularis propria involvement by EUS, and EMR was used in these patients to resect the mucosa and expose the tumor for forceps biopsies. In this series, only one patient had bleeding after gastric EMR; this patient was managed successfully with endoscopic therapy.
Future Trends
EMR implies a limitation of the technique to treatment of mucosal disease. ESD is technically more difficult to perform, but the technique enables en bloc resection of lesions greater than 2 cm and is likely to improve the complete resection rate of EGC and to expand the indications of endoscopic resection to include undifferentiated mucosal cancers. More recently, investigators have been “pushing the envelope” to show the safety and efficacy of extending endoscopic resection to submucosal lesions, although the cumulative experience to date is still limited.106,267 Concurrently, results in animal models indicate that full-thickness endoluminal resection and transluminal endoscopic surgery are technically feasible.268,269 Successful translation of such innovative techniques into endoscopic practice in the near future will redefine the limits of endoscopic therapy for gastric neoplasms and blur the boundary between therapeutic endoscopy and surgery further.
1 Okamura T, Tsujitani S, Korenaga D, et al. Lymphadenectomy for cure in patients with early gastric cancer and lymph node metastasis. Am J Surg. 1988;155:476-480.
2 Fujino MA, Morozumi A, Kojima Y, et al. Gastric carcinoma, an endoscopically curable disease. Bildgebung. 1994;61(Suppl 1):38-40.
3 Kojima T, Parra-Blanco A, Takahashi H, et al. Outcome of endoscopic mucosal resection for early gastric cancer: Review of the Japanese literature. Gastrointest Endosc. 1998;48:550-554.
4 Sagawa T, Takayama T, Oku T, et al. Argon plasma coagulation for successful treatment of early gastric cancer with intramucosal invasion. Gut. 2003;52:334-339.
5 Spinelli P, Mancini A, Dal Fante M. Endoscopic treatment of gastrointestinal tumors: Indications and results of laser photocoagulation and photodynamic therapy. Semin Surg Oncol. 1995;11:307-318.
6 Gotoda T. Endoscopic resection for premalignant and malignant lesions of the gastrointestinal tract from the esophagus to the colon. Gastrointest Endosc Clin N Am. 2008;18:435-450.
7 Larghi A, Waxman I. State of the art on endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc Clin N Am. 2007;17:441-469.
8 Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
9 Bertuccio P, Chatenoud L, Levi F, et al. Recent patterns in gastric cancer: A global overview. Int J Cancer. 2009;125:666-673.
10 Ferlay J, Bray F, Pisani P, et al. GLOBOCAN 2002: Cancer incidence, mortality and prevalence worldwide IARC CancerBase. Lyon, France: IARC Press; 2004. Available from http://www-dep.iarc.fr/
11 Parkin DM, Whelan SL, Ferlay J. Cancer incidence in five continents. International Agency for Research on Cancer. 1997. serial on the Internet
12 Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst. 1968;40:43-68.
13 Stolzenberg-Solomon RZ, Fraumeni JFJr, Wideroff L, et al. New malignancies following cancer of the digestive tract, excluding colorectal cancer. Silver Spring, MD: National Cancer Institute; 2008. pp 59–110. Available from http://seer.cancer.gov/publications/mpmono/Ch04_Digestive.pdf 2008-08-29; updated August 29, 2008; cited November 25, 2009
14 Powell J, McConkey CC. The rising trend in oesophageal adenocarcinoma and gastric cardia. Eur J Cancer Prev. 1992;1:265-269.
15 Wiggins CL, Becker TM, Key CR, et al. Stomach cancer among New Mexico’s American Indians, Hispanic whites, and non-Hispanic whites. Cancer Res. 1989;49:1595-1599.
16 Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: Epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1-27.
17 National Cancer Institute. SEER cancer statistics review 1975–2006. U.S. National Institutes of Health http://seer.cancer.gov/csr/1975_2006/index.html, 2006. Available from updated December 30, 2009; cited January 18, 2010
18 Allum WH, Powell DJ, McConkey CC, et al. Gastric cancer: A 25-year review. Br J Surg. 1989;76:535-540.
19 Hundahl SA, Stemmermann GN, Oishi A. Racial factors cannot explain superior Japanese outcomes in stomach cancer. Arch Surg. 1996;131:170-175.
20 Baba H, Maehara Y, Takeuchi H, et al. Effect of lymph node dissection on the prognosis in patients with node-negative early gastric cancer. Surgery. 1995;117:165-169.
21 Hisamichi S. Screening for gastric cancer. World J Surg. 1989;13:31-37.
22 Kampschoer GH, Fujii A, Masuda Y. Gastric cancer detected by mass survey: Comparison between mass survey and outpatient detection. Scand J Gastroenterol. 1989;24:813-817.
23 Jentschura D, Heubner C, Manegold BC, et al. Surgery for early gastric cancer: A European one-center experience. World J Surg. 1997;21:845-848.
24 Roukos DH. Current status and future perspectives in gastric cancer management. Cancer Treat Rev. 2000;26:243-255.
25 Hiki Y, Sakakibara Y, Mieno H, et al. Endoscopic treatment of gastric cancer. Surg Endosc. 1991;5:11-13.
26 Handa Y, Saitoh T, Kawaguchi M, et al. Production of secretory component and pathogenesis of gastric cancer in Helicobacter pylori-infected stomach. J Gastroenterol. 1999;34(Suppl 11):37-42.
27 . An international association between Helicobacter pylori infection and gastric cancer. The EUROGAST Study Group. Lancet, 341. 1359-1362:1993.
28 Handa Y, Misaka R, Kawaguchi M, et al. [Clinico-pathological study of Helicobacter pylori in early gastric cancer]. Nippon Rinsho. 1993;51:3249-3254.
29 Nomura AM, Stemmermann GN, Chyou PH. Gastric cancer among the Japanese in Hawaii. Jpn J Cancer Res. 1995;86:916-923.
30 Forman D, Newell DG, Fullerton F, et al. Association between infection with Helicobacter pylori and risk of gastric cancer: Evidence from a prospective investigation. BMJ. 1991;302:1302-1305.
31 Nomura A, Stemmermann GN, Chyou PH, et al. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132-1136.
32 Eslick GD, Lim LL, Byles JE, et al. Association of Helicobacter pylori infection with gastric carcinoma: A meta-analysis. Am J Gastroenterol. 1999;94:2373-2379.
33 Danesh J. Helicobacter pylori infection and gastric cancer: Systematic review of the epidemiological studies. Aliment Pharmacol Ther. 1999;13:851-856.
34 Siman JH, Forsgren A, Berglund G, et al. Association between Helicobacter pylori and gastric carcinoma in the city of Malmo, Sweden: A prospective study. Scand J Gastroenterol. 1997;32:1215-1221.
35 Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789.
36 Sipponen P, Riihela M, Hyvarinen H, et al. Chronic nonatropic (“superficial”) gastritis increases the risk of gastric carcinoma: A case-control study. Scand J Gastroenterol. 1994;29:336-340.
37 Valle J, Kekki M, Sipponen P, et al. Long-term course and consequences of Helicobacter pylori gastritis: Results of a 32-year follow-up study. Scand J Gastroenterol. 1996;31:546-550.
38 Sipponen P, Kekki M, Haapakoski J, et al. Gastric cancer risk in chronic atrophic gastritis: Statistical calculations of cross-sectional data. Int J Cancer. 1985;35:173-177.
39 Ponzetto A, Soldati T, De Giuli M. Helicobacter pylori screening and gastric cancer. Lancet. 1996;348:758.
40 Molloy RM, Sonnenberg A. Relation between gastric cancer and previous peptic ulcer disease. Gut. 1997;40:247-252.
41 Parsonnet J. Helicobacter pylori in the stomach—a paradox unmasked. N Engl J Med. 1996;335:278-280.
42 Hole DJ, Quigley EM, Gillis CR, et al. Peptic ulcer and cancer: An examination of the relationship between chronic peptic ulcer and gastric carcinoma. Scand J Gastroenterol. 1987;22:17-23.
43 Hansson LE, Nyren O, Hsing AW, et al. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med. 1996;335:242-249.
44 Kono S, Hirohata T. [A review of gastric cancer and life style]. Gan No Rinsho. 1990:257-267. (Spec No)
45 Neugut AI, Hayek M, Howe G. Epidemiology of gastric cancer. Semin Oncol. 1996;23:281-291.
46 Blot WJ, Li JY, Taylor PR, et al. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483-1492.
47 Buiatti E, Palli D, Decarli A, et al. A case-control study of gastric cancer and diet in Italy. Int J Cancer. 1989;44:611-616.
48 Ramon JM, Serra L, Cerdo C, et al. Dietary factors and gastric cancer risk: A case-control study in Spain. Cancer. 1993;71:1731-1735.
49 Hansson LE, Nyren O, Bergstrom R, et al. Diet and risk of gastric cancer: A population-based case-control study in Sweden. Int J Cancer. 1993;55:181-189.
50 Hasegawa R, Futakuchi M, Mizoguchi Y, et al. Studies of initiation and promotion of carcinogenesis by N-nitroso compounds. Cancer Lett. 1998;123:185-191.
51 Hansson LE, Nyren O, Bergstrom R, et al. Nutrients and gastric cancer risk: A population-based case-control study in Sweden. Int J Cancer. 1994;57:638-644.
52 La Vecchia C, Ferraroni M, D’Avanzo B, et al. Selected micronutrient intake and the risk of gastric cancer. Cancer Epidemiol Biomarkers Prev. 1994;3:393-398.
53 Gonzalez CA, Riboli E, Badosa J, et al. Nutritional factors and gastric cancer in Spain. Am J Epidemiol. 1994;139:466-473.
54 Buiatti E, Palli D, Decarli A, et al. A case-control study of gastric cancer and diet in Italy: II. Association with nutrients. Int J Cancer. 1990;45:896-901.
55 Dixon MF. Role of Helicobacter pylori on gastric mucosal damage, gastric cancer, and gastric MALT lymphoma. Gastroenterology. 1997;113(6 Suppl):S65-S66.
56 Webb PM, Forman D. Helicobacter pylori as a risk factor for cancer. Baillieres Clin Gastroenterol. 1995;9:563-582.
57 Banatvala N, Feldman R. The epidemiology of Helicobacter pylori: Missing pieces in a jigsaw. Commun Dis Rep CDR Rev. 1993;3:R56-R59.
58 Fisher SG, Davis F, Nelson R, et al. A cohort study of stomach cancer risk in men after gastric surgery for benign disease. J Natl Cancer Inst. 1993;85:1303-1310.
59 Stalnikowicz R, Benbassat J. Risk of gastric cancer after gastric surgery for benign disorders. Arch Intern Med. 1990;150:2022-2026.
60 Stolte M. Clinical consequences of the endoscopic diagnosis of gastric polyps. Endoscopy. 1995;27:32-37.
61 Attard TM, Giardiello FM, Argani P, et al. Fundic gland polyposis with high-grade dysplasia in a child with attenuated familial adenomatous polyposis and familial gastric cancer. J Pediatr Gastroenterol Nutr. 2001;32:215-218.
62 Hofgartner WT, Thorp M, Ramus MW, et al. Gastric adenocarcinoma associated with fundic gland polyps in a patient with attenuated familial adenomatous polyposis. Am J Gastroenterol. 1999;94:2275-2281.
63 Nakamura T, Nakano G. Histopathological classification and malignant change in gastric polyps. J Clin Pathol. 1985;38:754-764.
64 Benatti P, Sassatelli R, Roncucci L, et al. Tumour spectrum in hereditary non-polyposis colorectal cancer (HNPCC) and in families with “suspected HNPCC”: A population-based study in northern Italy. Colorectal Cancer Study Group. Int J Cancer. 1993;54:371-377.
65 La Vecchia C, Negri E, Franceschi S, et al. Family history and the risk of stomach and colorectal cancer. Cancer. 1992;70:50-55.
66 Ho SB. Premalignant lesions of the stomach. Semin Gastrointest Dis. 1996;7:61-73.
67 Bigalke KH, Dahm HH, Schiemoller M. [Menetrier’s disease and carcinoma]. Med Welt. 1977;28:1103-1106.
68 Stamatakis JD. Menetrier’s disease and carcinoma of stomach. Proc R Soc Med. 1976;69:264-265.
69 Hsing AW, Hansson LE, McLaughlin JK, et al. Pernicious anemia and subsequent cancer: A population-based cohort study. Cancer. 1993;71:745-750.
70 Brinton LA, Gridley G, Hrubec Z, et al. Cancer risk following pernicious anaemia. Br J Cancer. 1989;59:810-813.
71 Sjoblom SM, Sipponen P, Jarvinen H. Gastroscopic follow up of pernicious anaemia patients. Gut. 1993;34:28-32.
72 Stemmermann GN, Nomura AM, Chyou PH, et al. Impact of diet and smoking on risk of developing intestinal metaplasia of the stomach. Dig Dis Sci. 1990;35:433-438.
73 Nomura AM, Hankin JH, Kolonel LN, et al. Case-control study of diet and other risk factors for gastric cancer in Hawaii (United States). Cancer Causes Control. 2003;14:547-558.
74 Hansson LE, Baron J, Nyren O, et al. Tobacco, alcohol and the risk of gastric cancer: A population-based case-control study in Sweden. Int J Cancer. 1994;57:26-31.
75 Rhyu MG, Park WS, Jung YJ, et al. Allelic deletions of MCC/APC and p53 are frequent late events in human gastric carcinogenesis. Gastroenterology. 1994;106:1584-1588.
76 Sano T, Tsujino T, Yoshida K, et al. Frequent loss of heterozygosity on chromosomes 1q, 5q, and 17p in human gastric carcinomas. Cancer Res. 1991;51:2926-2931.
77 Kawanishi J, Kato J, Sasaki K, et al. Loss of E-cadherin-dependent cell-cell adhesion due to mutation of the beta-catenin gene in a human cancer cell line, HSC-39. Mol Cell Biol. 1995;15:1175-1181.
78 Mayer B, Johnson JP, Leitl F, et al. E-cadherin expression in primary and metastatic gastric cancer: Down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53:1690-1695.
79 Rhyu MG, Park WS, Meltzer SJ. Microsatellite instability occurs frequently in human gastric carcinoma. Oncogene. 1994;9:29-32.
80 Werther JL, Rivera-MacMurray S, Bruckner H, et al. Mucin-associated sialosyl-Tn antigen expression in gastric cancer correlates with an adverse outcome. Br J Cancer. 1994;69:613-616.
81 Yonemura Y, Ninomiya I, Tsugawa K, et al. Prognostic significance of c-erbB-2 gene expression in the poorly differentiated type of adenocarcinoma of the stomach. Cancer Detect Prev. 1998;22:139-146.
82 Guilford PJ, Hopkins JB, Grady WM, et al. E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum Mutat. 1999;14:249-255.
83 Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402-405.
84 Tonouchi H, Miki C, Masato K. [Gastric cancer associated with dermatomyositis accompanied by photoallergy]. Gan To Kagaku Ryoho. 2001;28:689-691.
85 Colombo E, Giorgi S, Sonzini E, et al. Disseminated intravascular coagulation and bone marrow metastases as presenting manifestations of gastric carcinoma. Haematologica. 1985;70:187.
86 Owen DA, Sternberg SS, editors. Diagnostic surgical pathology. Philadelphia: Lippincott Williams & Wilkins, 1999.
87 Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49.
88 Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1998;333:32-41.
89 Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287-1289.
90 Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma—2nd English edition. Gastric Cancer. 1998;1:10-24.
91 Kelly S, Harris KM, Berry E, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut. 2001;49:534-539.
92 Noguchi Y, Imada T, Matsumoto A, et al. Radical surgery for gastric cancer: A review of the Japanese experience. Cancer. 1989;64:2053-2062.
93 Sano T, Kobori O, Muto T. Lymph node metastasis from early gastric cancer: Endoscopic resection of tumour. Br J Surg. 1992;79:241-244.
94 Yamao T, Shirao K, Ono H, et al. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer. 1996;77:602-606.
95 Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: Estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225.
96 Nishi M, Omori Y, Miwa K, editors. Japanese classification of gastric carcinoma. Tokyo: Kanehara & Co, Ltd, 1995.
97 Sakaki N, Iida Y, Okazaki Y, et al. Magnifying endoscopic observation of the gastric mucosa, particularly in patients with atrophic gastritis. Endoscopy. 1978;10:269-274.
98 Nakayoshi T, Tajiri H, Matsuda K, et al. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: Correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084.
99 Sumiyama K, Kaise M, Nakayoshi T, et al. Combined use of a magnifying endoscope with a narrow band imaging system and a multibending endoscope for en bloc EMR of early stage gastric cancer. Gastrointest Endosc. 2004;60:79-84.
100 Rice TW, Boyce GA, Sivak MV, et al. Esophageal carcinoma: Esophageal ultrasound assessment of preoperative chemotherapy. Ann Thorac Surg. 1992;53:972-977.
101 Akahoshi K, Misawa T, Fujishima H, et al. Preoperative evaluation of gastric cancer by endoscopic ultrasound. Gut. 1991;32:479-482.
102 Dittler HJ, Siewert JR. Role of endoscopic ultrasonography in gastric carcinoma. Endoscopy. 1993;25:162-166.
103 Akahoshi K, Chijiwa Y, Hamada S, et al. Pretreatment staging of endoscopically early gastric cancer with a 15 MHz ultrasound catheter probe. Gastrointest Endosc. 1998;48:470-476.
104 Ohashi S, Nakazawa S, Yoshino J. Endoscopic ultrasonography in the assessment of invasive gastric cancer. Scand J Gastroenterol. 1989;24:1039-1048.
105 Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: Diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095.
106 Waxman I, Saitoh Y, Raju GS, et al. High-frequency probe EUS-assisted endoscopic mucosal resection: A therapeutic strategy for submucosal tumors of the GI tract. Gastrointest Endosc. 2002;55:44-49.
107 Maruta S, Tsukamoto Y, Niwa Y, et al. Evaluation of upper gastrointestinal tumors with a new endoscopic ultrasound probe. Gastrointest Endosc. 1994;40:603-608.
108 Hunerbein M, Ghadimi BM, Haensch W, et al. Transendoscopic ultrasound of esophageal and gastric cancer using miniaturized ultrasound catheter probes. Gastrointest Endosc. 1998;48:371-375.
109 Kida M, Tanabe S, Watanabe M, et al. Staging of gastric cancer with endoscopic ultrasonography and endoscopic mucosal resection. Endoscopy. 1998;30(Suppl 1):A64-A68.
110 Yanai H, Tada M, Karita M, et al. Diagnostic utility of 20-megahertz linear endoscopic ultrasonography in early gastric cancer. Gastrointest Endosc. 1996;44:29-33.
111 Waxman I, Saitoh Y. Clinical outcome of endoscopic mucosal resection for superficial GI lesions and the role of high-frequency US probe sonography in an American population. Gastrointest Endosc. 2000;52:322-327.
112 Akahoshi K, Chijiiwa Y, Sasaki I, et al. Pre-operative TN staging of gastric cancer using a 15 MHz ultrasound miniprobe. Br J Radiol. 1997;70:703-707.
113 Shim CS. Endoscopic mucosal resection. J Korean Med Sci. 1996;11:457-466.
114 Inoue H, Tani M, Nagai K, et al. Treatment of esophageal and gastric tumors. Endoscopy. 1999;31:47-55.
115 Rembacken BJ, Gotoda T, Fujii T, et al. Endoscopic mucosal resection. Endoscopy. 2001;33:709-718.
116 Hirao M, Masuda K, Asanuma T, et al. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269.
117 Yamamoto H, Yube T, Isoda N, et al. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc. 1999;50:251-256.
118 Fujishiro M, Yahagi N, Kashimura K, et al. Tissue damage of different submucosal injection solutions for EMR. Gastrointest Endosc. 2005;62:933-942.
119 Takekoshi T, Baba Y, Ota H, et al. Endoscopic resection of early gastric carcinoma: Results of a retrospective analysis of 308 cases. Endoscopy. 1994;26:352-358.
120 Tada M, Murakami A, Karita M, et al. Endoscopic resection of early gastric cancer. Endoscopy. 1993;25:445-450.
121 Karita M, Tada M, Okita K, et al. Endoscopic therapy for early colon cancer: The strip biopsy resection technique. Gastrointest Endosc. 1991;37:128-132.
122 Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2000;118:670-677.
123 Suzuki H. Endoscopic mucosal resection using ligating device for early gastric cancer. Gastrointest Endosc Clin N Am. 2001;11:511-518.
124 Suzuki Y, Hiraishi H, Kanke K, et al. Treatment of gastric tumors by endoscopic mucosal resection with a ligating device. Gastrointest Endosc. 1999;49:192-199.
125 Soehendra N, Seewald S, Groth S, et al. Use of modified multiband ligator facilitates circumferential EMR in Barrett’s esophagus (with video). Gastrointest Endosc. 2006;63:847-852.
126 Tada M, Inoue H, Yabata E, et al. Feasibility of the transparent cap-fitted colonoscope for screening and mucosal resection. Dis Colon Rectum. 1997;40:618-621.
127 Inoue H, Takeshita K, Hori H, et al. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58-62.
128 Matsuzaki K, Nagao S, Kawaguchi A, et al. Newly designed soft prelooped cap for endoscopic mucosal resection of gastric lesions. Gastrointest Endosc. 2003;57:242-246.
129 Tani M, Sakai P, Kondo H. Endoscopic mucosal resection of superficial cancer in the stomach using the cap technique. Endoscopy. 2003;35:348-355.
130 Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229.
131 Yamamoto H, Kawata H, Sunada K, et al. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690-694.
132 Fujishiro M, Yahagi N, Nakamura M, et al. Successful outcomes of a novel endoscopic treatment for GI tumors: Endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc. 2006;63:243-249.
133 Hosokawa K, Yoshida S. [Recent advances in endoscopic mucosal resection for early gastric cancer]. Gan To Kagaku Ryoho. 1998;25:476-483.
134 Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3(7 Suppl 1):S67-S70.
135 Kodashima S, Fujishiro M, Yahagi N, et al. Endoscopic submucosal dissection using flexknife. J Clin Gastroenterol. 2006;40:378-384.
136 Yamamoto H, Kawata H, Sunada K, et al. Success rate of curative endoscopic mucosal resection with circumferential mucosal incision assisted by submucosal injection of sodium hyaluronate. Gastrointest Endosc. 2002;56:507-512.
137 Neuhaus H, Wirths K, Schenk M, et al. Randomized controlled study of EMR versus endoscopic submucosal dissection with a water-jet hybrid-knife of esophageal lesions in a porcine model. Gastrointest Endosc. 2009;70:112-120.
138 Kitamura K, Yamaguchi T, Okamoto K, et al. Clinicopathologic features of synchronous multifocal early gastric cancers. Anticancer Res. 1997;17(1B):643-646.
139 Tanabe S, Koizumi W, Mitomi H, et al. Clinical outcome of endoscopic aspiration mucosectomy for early stage gastric cancer. Gastrointest Endosc. 2002;56:708-713.
140 Amano Y, Ishihara S, Amano K, et al. An assessment of local curability of endoscopic surgery in early gastric cancer without satisfaction of current therapeutic indications. Endoscopy. 1998;30:548-552.
141 Hamada T, Kondo K, Itagaki Y, et al. [Endoscopic mucosal resection for early gastric cancer]. Nippon Rinsho. 1996;54:1292-1297.
142 Mizumoto S, Misumi A, Harada K, et al. [Evaluation of endoscopic mucosal resection (EMR) as a curative therapy against early gastric cancer]. Nippon Geka Gakkai Zasshi. 1992;93:1071-1074.
143 Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235.
144 Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942.
145 Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11.
146 Abe N, Watanabe T, Suzuki K, et al. Risk factors predictive of lymph node metastasis in depressed early gastric cancer. Am J Surg. 2002;183:168-172.
147 Makuuchi H, Kise Y, Shimada H, et al. Endoscopic mucosal resection for early gastric cancer. Semin Surg Oncol. 1999;17:108-116.
148 Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol. 2005;3(7 Suppl 1):S71-S73.
149 Gotoda T, Kondo H, Ono H, et al. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: Report of two cases. Gastrointest Endosc. 1999;50:560-563.
150 Neuhaus H, Costamagna G, Deviere J, et al. Endoscopic submucosal dissection (ESD) of early neoplastic gastric lesions using a new double-channel endoscope (the “R-scope”). Endoscopy. 2006;38:1016-1023.
151 Dinis-Ribeiro M, Pimentel-Nunes P, Afonso M, et al. A European case series of endoscopic submucosal dissection for gastric superficial lesions. Gastrointest Endosc. 2009;69:350-355.
152 Ryu CB, Kim SG, Jung IS, et al. Is it possible to perform EMR in poorly differentiated type of early gastric cancer? Gastrointest Endosc. 61, 2005. AB180–AB
153 Kumarasinghe MP, Lim TK, Ooi CJ, et al. Tubule neck dysplasia: Precursor lesion of signet ring cell carcinoma and the immunohistochemical profile. Pathology. 2006;38:468-471.
154 Watanabe K, Ogata S, Kawazoe S, et al. Clinical outcomes of EMR for gastric tumors: Historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63:776-782.
155 Lee SY, Kim JJ, Lee JH, et al. Healing rate of EMR-induced ulcer in relation to the duration of treatment with omeprazole. Gastrointest Endosc. 2004;60:213-217.
156 Kakushima N, Fujishiro M, Yahagi N, et al. Helicobacter pylori status and the extent of gastric atrophy do not affect ulcer healing after endoscopic submucosal dissection. J Gastroenterol Hepatol. 2006;21:1586-1589.
157 Kakushima N, Fujishiro M, Kodashima S, et al. Histopathologic characteristics of gastric ulcers created by endoscopic submucosal dissection. Endoscopy. 2006;38:412-415.
158 Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: Technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005;17:54-58.
159 Okano A, Hajiro K, Takakuwa H, et al. Predictors of bleeding after endoscopic mucosal resection of gastric tumors. Gastrointest Endosc. 2003;57:687-690.
160 Ahmad NA, Kochman ML, Long WB, et al. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: A study of 101 cases. Gastrointest Endosc. 2002;55:390-396.
161 Tsunada S, Ogata S, Ohyama T, et al. Endoscopic closure of perforations caused by EMR in the stomach by application of metallic clips. Gastrointest Endosc. 2003;57:948-951.
162 Fujishiro M, Ono H, Gotoda T, et al. Usefulness of Maalox for detection of the precise bleeding points and confirmation of hemostasis on gastrointestinal hemorrhage. Endoscopy. 2001;33:196.
163 Shiba M, Higuchi K, Kadouchi K, et al. Risk factors for bleeding after endoscopic mucosal resection. World J Gastroenterol. 2005;11:7335-7339.
164 Ikehara H, Gotoda T, Ono H, et al. Gastric perforation during endoscopic resection for gastric carcinoma and the risk of peritoneal dissemination. Br J Surg. 2007;94:992-995.
165 Minami S, Gotoda T, Ono H, et al. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc. 2006;63:596-601.
166 Waye JD. Management of complications of colonoscopic polypectomy. Gastroenterologist. 1993;1:158-164.
167 Katada C, Muto M, Manabe T, et al. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc. 2003;57:165-169.
168 Ohmura K, Nagashima R, Takeda H, et al. Temporary stenting with metallic endoprosthesis for refractory esophageal stricture secondary to cylindrical resection of carcinoma. Gastrointest Endosc. 1998;48:214-217.
169 Polanyi TG. Laser physics. Otolaryngol Clin North Am. 1983;16:753-774.
170 Polanyi TG. Physics of surgery with lasers. Clin Chest Med. 1985;6:179-202.
171 Hiki Y, Shimao J, Yamao Y, et al. The concepts, procedures, and problems related in endoscopic laser therapy of early gastric cancer: A retrospective study on early gastric cancer. Surg Endosc. 1989;3:1-6.
172 Sliney DH. Laser safety. Lasers Surg Med. 1995;16:215-225.
173 Patrice T, Foultier MT, Yactayo S, et al. Endoscopic photodynamic therapy with hematoporphyrin derivative for primary treatment of gastrointestinal neoplasms in inoperable patients. Dig Dis Sci. 1990;35:545-552.
174 Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett’s esophagus with dysplasia and/or early stage carcinoma: Long-term results. Gastrointest Endosc. 2003;58:183-188.
175 Ell C, Gossner L, May A, et al. Photodynamic ablation of early cancers of the stomach by means of mTHPC and laser irradiation: Preliminary clinical experience. Gut. 1998;43:345-349.
176 Hiki Y, Shimao H, Mieno H, et al. Modified treatment of early gastric cancer: Evaluation of endoscopic treatment of early gastric cancers with respect to treatment indication groups. World J Surg. 1995;19:517-522.
177 Mathus-Vliegen EM, Tytgat GN. Laser photocoagulation in the palliative treatment of upper digestive tract tumors. Cancer. 1986;57:396-399.
178 Mathus-Vliegen EM, Tytgat GN. Analysis of failures and complications of neodymium:YAG laser photocoagulation in gastrointestinal tract tumors: A retrospective survey of 18 years’ experience. Endoscopy. 1990;22:17-23.
179 Fleischer D, Sivak MV. Endoscopic Nd:YAG laser therapy as palliative treatment for advanced adenocarcinoma of the gastric cardia. Gastroenterology. 1984;87:815-820.
180 Fleischer D, Sivak MVJr. Endoscopic Nd:YAG laser therapy as palliation for esophagogastric cancer: Parameters affecting initial outcome. Gastroenterology. 1985;89:827-831.
181 Spencer GM, Thorpe SM, Blackman GM, et al. Laser augmented by brachytherapy versus laser alone in the palliation of adenocarcinoma of the oesophagus and cardia: A randomised study. Gut. 2002;50:224-227.
182 Sargeant IR, Tobias JS, Blackman G, et al. Radiotherapy enhances laser palliation of malignant dysphagia: A randomised study. Gut. 1997;40:362-369.
183 Sargeant IR, Loizou LA, Tobias JS, et al. Radiation enhancement of laser palliation for malignant dysphagia: A pilot study. Gut. 1992;33:1597-1601.
184 McCaughan JSJr, Ellison EC, Guy JT, et al. Photodynamic therapy for esophageal malignancy: A prospective twelve-year study. Ann Thorac Surg. 1996;62:1005-1009.
185 Canard JM, Vedrenne B. Clinical application of argon plasma coagulation in gastrointestinal endoscopy: Has the time come to replace the laser? Endoscopy. 2001;33:353-357.
186 Wahab PJ, Mulder CJ, den Hartog G, et al. Argon plasma coagulation in flexible gastrointestinal endoscopy: Pilot experiences. Endoscopy. 1997;29:176-181.
187 Kitamura T, Tanabe S, Koizumi W, et al. Argon plasma coagulation for early gastric cancer: Technique and outcome. Gastrointest Endosc. 2006;63:48-54.
188 Grund KE, Storek D, Zindel C, et al. Highly flexible self-expanding metal mesh stents: A new kind of palliative therapy of malignant dysphagia. Z Gastroenterol. 1995;33:392-398.
189 Choi YB. Laparoscopic gatrojejunostomy for palliation of gastric outlet obstruction in unresectable gastric cancer. Surg Endosc. 2002;16:1620-1626.
190 Khulusi S, Morris T. Endoscopic palliation of gastrointestinal malignancy. Eur J Gastroenterol Hepatol. 2000;12:397-402.
191 Sihvo EI, Pentikainen T, Luostarinen ME, et al. Inoperable adenocarcinoma of the oesophagogastric junction: A comparative clinical study of laser coagulation versus self-expanding metallic stents with special reference to cost analysis. Eur J Surg Oncol. 2002;28:711-715.
192 Chan AC, Shin FG, Lam YH, et al. A comparison study on physical properties of self-expandable esophageal metal stents. Gastrointest Endosc. 1999;49(4 Pt 1):462-465.
193 Kim JH, Yoo BM, Lee KJ, et al. Self-expanding coil stent with a long delivery system for palliation of unresectable malignant gastric outlet obstruction: A prospective study. Endoscopy. 2001;33:838-842.
194 Maetani I, Tada T, Shimura J, et al. Technical modifications and strategies for stenting gastric outlet strictures using esophageal endoprostheses. Endoscopy. 2002;34:402-406.
195 Baron TH, Harewood GC. Enteral self-expandable stents. Gastrointest Endosc. 2003;58:421-433.
196 Gurney KA, Cartwright RA, Gilman EA. Descriptive epidemiology of gastrointestinal non-Hodgkin’s lymphoma in a population-based registry. Br J Cancer. 1999;79:1929-1934.
197 Severson RK, Davis S. Increasing incidence of primary gastric lymphoma. Cancer. 1990;66:1283-1287.
198 Fagioli F, Rigolin GM, Cuneo A, et al. Primary gastric lymphoma: Distribution and clinical relevance of different epidemiological factors. Haematologica. 1994;79:213-217.
199 Zaki M, Schubert ML. Helicobacter pylori and gastric lymphoma. Gastroenterology. 1995;108:610-612.
200 Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575-577.
201 Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267-1271.
202 Zucca E, Bertoni F, Roggero E, et al. The gastric marginal zone B-cell lymphoma of MALT type. Blood. 2000;96:410-419.
203 Steinbach G, Ford R, Glober G, et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue: An uncontrolled trial. Ann Intern Med. 1999;131:88-95.
204 Newton R, Ferlay J, Beral V, et al. The epidemiology of non-Hodgkin’s lymphoma: Comparison of nodal and extra-nodal sites. Int J Cancer. 1997;72:923-930.
205 Ruskone-Fourmestraux A, Lavergne A, Aegerter PH, et al. Predictive factors for regression of gastric MALT lymphoma after anti–Helicobacter pylori treatment. Gut. 2001;48:297-303.
206 Morgner A, Lehn N, Andersen LP, et al. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: Complete remission after curing the infection. Gastroenterology. 2000;118:821-828.
207 Royer B, Cazals-Hatem D, Sibilia J, et al. Lymphomas in patients with Sjogren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood. 1997;90:766-775.
208 Xu WS, Ho FC, Ho J, et al. Pathogenesis of gastric lymphoma: The enigma in Hong Kong. Ann Oncol. 1997;8(Suppl 2):41-44.
209 Ruskone-Fourmestraux A, Rambaud JC. Gastrointestinal lymphoma: Prevention and treatment of early lesions. Best Pract Res Clin Gastroenterol. 2001;15:337-354.
210 Taal BG, Boot H, van Heerde P, et al. Primary non-Hodgkin lymphoma of the stomach: Endoscopic pattern and prognosis in low versus high grade malignancy in relation to the MALT concept. Gut. 1996;39:556-561.
211 Strecker P, Eck M, Greiner A, et al. [Diagnostic value of stomach biopsy in comparison with surgical specimen in gastric B-cell lymphomas of the MALT type]. Pathologe. 1998;19:209-213.
212 Suekane H, Iida M, Yao T, et al. Endoscopic ultrasonography in primary gastric lymphoma: Correlation with endoscopic and histologic findings. Gastrointest Endosc. 1993;39:139-145.
213 Palazzo L, Roseau G, Ruskone-Fourmestraux A, et al. Endoscopic ultrasonography in the local staging of primary gastric lymphoma. Endoscopy. 1993;25:502-508.
214 Lugering N, Menzel J, Kucharzik T, et al. Impact of miniprobes compared to conventional endosonography in the staging of low-grade gastric malt lymphoma. Endoscopy. 2001;33:832-837.
215 Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, et al. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485-491.
216 . Introduction: Concepts and controversies in lymphoid neoplasias. I M IT M. The non-Hodgkin’s lymphomas, ed 2. London: Arnold. 1997:3-47.
217 Cogliatti SB, Schmid U, Schumacher U, et al. Primary B-cell gastric lymphoma: A clinicopathological study of 145 patients. Gastroenterology. 1991;101:1159-1170.
218 Fischbach W, Kestel W, Kirchner T, et al. Malignant lymphomas of the upper gastrointestinal tract: Results of a prospective study in 103 patients. Cancer. 1992;70:1075-1080.
219 Montalban C, Castrillo JM, Abraira V, et al. Gastric B-cell mucosa-associated lymphoid tissue (MALT) lymphoma: Clinicopathological study and evaluation of the prognostic factors in 143 patients. Ann Oncol. 1995;6:355-362.
220 Hussell T, Isaacson PG, Crabtree JE, et al. Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol. 1996;178:122-127.
221 Jaffe ES, Harris NL, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: A progress report. Am J Clin Pathol. 1999;111(1 Suppl 1):S8-S12.
222 de Jong D, Boot H, van Heerde P, et al. Histological grading in gastric lymphoma: Pretreatment criteria and clinical relevance. Gastroenterology. 1997;112:1466-1474.
223 Isaacson PG. Gastrointestinal lymphomas of T- and B-cell types. Mod Pathol. 1999;12:151-158.
224 Nakamura S, Akazawa K, Yao T, et al. A clinicopathologic study of 233 cases with special reference to evaluation with the MIB-1 index. Cancer. 1995;76:1313-1324.
225 Toyoda H, Ono T, Kiyose M, et al. Gastric mucosa-associated lymphoid tissue lymphoma with a focal high-grade component diagnosed by EUS and endoscopic mucosal resection for histologic evaluation. Gastrointest Endosc. 2000;51:752-755.
226 Kanamoto K, Aoyagi K, Nakamura S, et al. Simultaneous coexistence of early adenocarcinoma and low-grade MALT lymphoma of the stomach associated with Helicobacter pylori infection: A case report. Gastrointest Endosc. 1998;47:73-75.
227 Kelly SM, Geraghty JM, Neale G. H pylori, gastric carcinoma, and MALT lymphoma. Lancet. 1994;343:418.
228 Lin JI, Tseng CH, Chow S, et al. Coexisting malignant lymphoma and adenocarcinoma of the stomach. South Med J. 1979;72:619-622.
229 Isaacson PG, Diss TC, Wotherspoon AC, et al. Long-term follow-up of gastric MALT lymphoma treated by eradication of H. pylori with antibodies. Gastroenterology. 1999;117:750-751.
230 Neubauer A, Thiede C, Morgner A, et al. Cure of Helicobacter pylori infection and duration of remission of low-grade gastric mucosa-associated lymphoid tissue lymphoma. J Natl Cancer Inst. 1997;89:1350-1355.
231 Radaszkiewicz T, Dragosics B, Bauer P. Gastrointestinal malignant lymphomas of the mucosa-associated lymphoid tissue: Factors relevant to prognosis. Gastroenterology. 1992;102:1628-1638.
232 Richardson CT, Walsh JH. The value of a histamine H2-receptor antagonist in the management of patients with the Zollinger-Ellison syndrome. N Engl J Med. 1976;294:133-135.
233 Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959.
234 Hakanson R, Tielemans Y, Chen D, et al. The biology and pathobiology of the ECL cells. Yale J Biol Med. 1992;65:761-774.
235 Kidd M, Miu K, Tang LH, et al. Helicobacter pylori lipopolysaccharide stimulates histamine release and DNA synthesis in rat enterochromaffin-like cells. Gastroenterology. 1997;113:1110-1117.
236 Yeatman TJ, Sharp JV, Kimura AK. Can susceptibility to carcinoid tumors be inherited? Cancer. 1989;63:390-393.
237 Rindi G, Bordi C, Rappel S, et al. Gastric carcinoids and neuroendocrine carcinomas: Pathogenesis, pathology, and behavior. World J Surg. 1996;20:168-172.
238 Soga J, Yakuwa Y, Osaka M. Carcinoid syndrome: A statistical evaluation of 748 reported cases. J Exp Clin Cancer Res. 1999;18:133-141.
239 Sandor A, Modlin IM. A retrospective analysis of 1570 appendiceal carcinoids. Am J Gastroenterol. 1998;93:422-428.
240 Godwin JD2nd. Carcinoid tumors: An analysis of 2,837 cases. Cancer. 1975;36:560-569.
241 Oberg K. Expression of growth factors and their receptors in neuroendocrine gut and pancreatic tumors, and prognostic factors for survival. Ann N Y Acad Sci. 1994;733:46-55.
242 Berge T, Linell F. Carcinoid tumours: Frequency in a defined population during a 12-year period. Acta Pathol Microbiol Scand A. 1976;84:322-330.
243 Ichikawa J, Tanabe S, Koizumi W, et al. Endoscopic mucosal resection in the management of gastric carcinoid tumors. Endoscopy. 2003;35:203-206.
244 Nakamura S, Iida M, Yao T, et al. Endoscopic features of gastric carcinoids. Gastrointest Endosc. 1991;37:535-538.
245 Borch K. Atrophic gastritis and gastric carcinoid tumours. Ann Med. 1989;21:291-297.
246 Ahlman H, Kolby L, Lundell L, et al. Clinical management of gastric carcinoid tumors. Digestion. 1994;55(Suppl 3):77-85.
247 Rindi G, Luinetti O, Cornaggia M, et al. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: A clinicopathologic study. Gastroenterology. 1993;104:994-1006.
248 Rindi G, Azzoni C, La Rosa S, et al. ECL cell tumor and poorly differentiated endocrine carcinoma of the stomach: Prognostic evaluation by pathological analysis. Gastroenterology. 1999;116:532-542.
249 Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: Recent advances in understanding of their biology. Hum Pathol. 1999;30:1213-1220.
250 Miettinen M, Lasota J. Gastrointestinal stromal tumors—definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12.
251 Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumours. Ann Chir Gynaecol. 1998;87:278-281.
252 Schaldenbrand JD, Appelman HD. Solitary solid stromal gastrointestinal tumors in von Recklinghausen’s disease with minimal smooth muscle differentiation. Hum Pathol. 1984;15:229-232.
253 Sircar K, Hewlett BR, Huizinga JD, et al. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23:377-389.
254 Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492-515.
255 Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): Gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259-1269.
256 Davis GB, Blanchard DK, Hatch GF3rd, et al. Tumors of the stomach. World J Surg. 2000;24:412-420.
257 Tio TL, Tytgat GN, den Hartog Jager FC. Endoscopic ultrasonography for the evaluation of smooth muscle tumors in the upper gastrointestinal tract: An experience with 42 cases. Gastrointest Endosc. 1990;36:342-350.
258 Savides T, Gress F, Battacharya I, editors. Endoscopic ultrasonography. Oxford, UK: Blackwell Science, 2001.
259 Chak A, Canto MI, Rosch T, et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc. 1997;45:468-473.
260 Palazzo L, Landi B, Cellier C, et al. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88-92.
261 Shah R, Jonnalagadda S. The GIST of a stromal tumor. Gastroenterology. 2005;128:2170-2171.
262 Ando N, Goto H, Niwa Y, et al. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37-43.
263 Hunt GC, Rader AE, Faigel DO. A comparison of EUS features between CD-117 positive GI stromal tumors and CD-117 negative GI spindle cell tumors. Gastrointest Endosc. 2003;57:469-474.
264 Franquemont DW. Differentiation and risk assessment of gastrointestinal stromal tumors. Am J Clin Pathol. 1995;103:41-47.
265 Boyce GA, Sivak MVJr, Rosch T, et al. Evaluation of submucosal upper gastrointestinal tract lesions by endoscopic ultrasound. Gastrointest Endosc. 1991;37:449-454.
266 Nakamura S, Iida M, Suekane H, et al. Endoscopic removal of gastric lipoma: Diagnostic value of endoscopic ultrasonography. Am J Gastroenterol. 1991;86:619-621.
267 Kojima T, Takahashi H, Parra-Blanco A, et al. Diagnosis of submucosal tumor of the upper GI tract by endoscopic resection. Gastrointest Endosc. 1999;50:516-522.
268 Kantsevoy SV, Jagannath SB, Niiyama H, et al. Endoscopic gastrojejunostomy with survival in a porcine model. Gastrointest Endosc. 2005;62:287-292.
269 Rajan E, Gostout CJ, Burgart LJ, et al. First endoluminal system for transmural resection of colorectal tissue with a prototype full-thickness resection device in a porcine model. Gastrointest Endosc. 2002;55:915-920.