Chapter 40 Endoscopic Retrograde Cholangiopancreatography Tissue Sampling Techniques
![]() Video related to this chapter’s topics: Tissue Sampling of Bile Duct Stricture
Video related to this chapter’s topics: Tissue Sampling of Bile Duct Stricture
Introduction
Nevertheless, ERCP may present a unique opportunity to establish a definite diagnosis of malignancy during a drainage procedure, which may save the patient subsequent unnecessary, painful, and expensive procedures.1 Despite many years of study, imaging alone cannot make the diagnosis of malignancy.2 This chapter covers this controversial topic including historical background, pathogenesis, techniques in tissue sampling, complications, and future trends and potential.
History
Specificity in early reports was uniformly 100%. Despite early enthusiasm, clinicians noted low yields when the technique was used in a clinical setting. Reports of sensitivity of only 6% to 32% in six published studies3–8 have caused this technique to fall from practice in favor of the newer, higher yield approaches of brush cytology, fine needle aspiration (FNA) cytology, and endobiliary forceps biopsy.
Pathogenesis
Obstruction of the biliary tree by benign or malignant stricturing requiring temporary or palliative stent placement in the bile duct remains a major indication for ERCP, now that diagnostic ERCP has been largely replaced by lower risk imaging techniques of helical computed tomography (CT) and magnetic resonance cholangiopancreatography. As discussed elsewhere in this textbook, endoscopic ultrasound (EUS) has an important role in examining patients with pancreatic neoplasms in which the resectability remains uncertain after radiologic imaging. Many gastroenterologists use EUS largely to perform tissue sampling in lieu of ERCP, often because of poor yields at their center with ERCP techniques, and to permit an advance tissue diagnosis before metal stent placement. Increasingly, cross-training of endoscopists has permitted EUS and ERCP to be performed concomitantly during the same procedure to shorten hospital time, avoid repeat procedures, and reduce cost.9
These three types of malignant obstruction are pathologically distinct and represent special problems when attempting tissue sampling. The first major pathologic factor influencing biopsy or cytologic yield is tumor cellularity. Pancreatic carcinoma, in particular, often stimulates an intense desmoplastic and fibrotic reaction, making the tumor very dense and of low cellularity. Sampling often produces acellular or false-negative specimens.10,11 Maximizing yield requires repeated, deep, or large specimen sampling. Occasionally, an immune response or relative ischemia produces ulceration, bleeding, exudate, or debris that can obscure the rare malignant cell recovered in an endoscopic specimen. Cholangiocarcinoma of the primary type begins in the mucosa of the primary or secondary bile ducts. It is a relatively cellular cancer, and cells are more often shed in bile and can be more readily collected by sampling the superficial epithelium. These tumors pose difficult access problems making EUS FNA yields lower, although the procedure is technically possible in selected patients.12
Hepatocellular carcinoma often can invade and extend intraductally. Superficial sampling generally obtains diagnostic cells in this setting as well. As with pancreatic cancer, gallbladder cancer and, especially, metastatic cancer encase or compress the biliary tree, often while preserving intact benign biliary epithelium. Establishing a tissue diagnosis often requires sampling deeper than the surface epithelium.10,13–15 Very well-differentiated tumors represent a significant minority of malignant pancreaticobiliary tumors and prove very difficult to diagnose by cytologic criteria. Large specimens are often necessary to permit the pathologist to examine and compare these tumors to differentiate them from normal tissue. This fact likely explains why no biopsy technique, even open surgical wedge biopsy, has a 100% yield. These pathogenetic factors demand refined techniques and devices if adequate specimens are to be obtained to permit a positive cytologic or histologic diagnosis to be made in most cases.
Finally, there is increasing recognition of idiopathic autoimmune pancreatitis and cholangitis—a condition that can closely mimic cancer.16 This benign, IgG subtype 4–associated disorder responds to corticosteroids. It should be distinguished from malignancy if possible. A high index of suspicion for elevated IgG subtype 4 levels and negative tissue may preclude operative resection. Progress is being made to permit accurate nonoperative diagnosis of this increasingly recognized condition.17
Techniques of Tissue Sampling
Collecting adequate samples for cytologic and histologic review remains a major challenge for endoscopists during ERCP. The primary goal of planned ERCP is to provide endoscopic drainage for a patient with jaundice and obstruction; this involves obtaining ductal access, negotiating the obstruction with a guidewire, usually performing sphincterotomy, and placing a biliary endoprosthesis. Tissue sampling has always assumed a secondary position in this sequence, likely explaining the reason for the limited experience generally reported by most endoscopists. Alternatively, EUS can be scheduled with FNA as the only or at least a major goal. A cytopathologist can be present at the EUS procedure, and up to 16 needle passes have been used to establish a tissue diagnosis.18
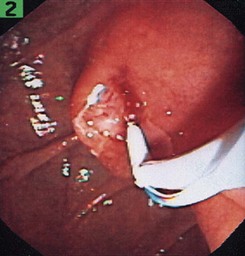
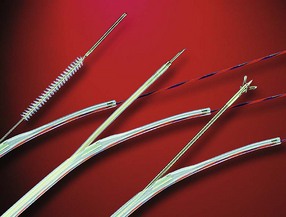
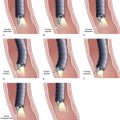
Inadequate tissue acquisition at ERCP remains the most common reason for failing to establish an accurate pathologic diagnosis. Technical difficulty, time consideration, patient restlessness, and the need to proceed with the primary goal of biliary drainage all contribute to limit the time and thoroughness of tissue collection for many endoscopists. Because of these factors, brush cytology has been the only sampling technique adopted widely into clinical practice. Initially, standard endoscopic brushes were inserted, usually after sphincterotomy, in an attempt to sample from within a malignant-appearing stricture (Fig. 40.1A). These devices can be “groomed” by manually curving their end portions before placement. Most have a blunt smooth metal tip to minimize trauma and the risk of perforation. Nevertheless, negotiation through the stricture was often problematic. This factor and the very superficial nature of this technique of sampling produced disappointing yields, and it never became popular. Manufacturers responded to these problems by producing a variety of cytology brushes, some of which could be inserted over a guidewire placed through the malignant-appearing stricture before attempted sampling (see Fig. 40.1). Because most endoscopists concentrate on negotiating a guidewire through the stricture as the first major step in the therapeutic goal of stent placement, tissue sampling may be done at this appropriate time without changing or interrupting this sequence.
Two popular guided brushes are Combocath (Microvasive Boston Scientific, Natick, MA) and Cytomax (Wilson-Cook Medical, Winston Salem, NC) (see Fig. 40.1C). Disadvantages of these guided devices are their relative large size of 8-Fr and their stiffness. In addition, the length of the bristles must be quite short to fit within the small channel size in these double-lumen devices, a feature that may limit specimen collection. Smaller versions of these two devices have been produced but can be placed only over 0.018-inch guidewires. An alternative to using these brushes is to insert a catheter over the previously placed guidewire, withdraw the guidewire, and place a brush with a long spring-tip nose (Geenen brush, Wilson-Cook Medical) (see Fig. 40.1B). By slightly withdrawing the catheter while leaving the brush above the stricture, the tissue to be sampled can be accessed. Brushing now would not lose position above the stricture because the long nose maintains position. The principal drawback of the technique is the loss of cells when the brush is withdrawn from the catheter after the catheter is readvanced above the stricture to maintain access.19 If the entire assembly is withdrawn, reinsertion of the guidewire is required, which is an uncomfortable, and occasionally unsuccessful, challenge.
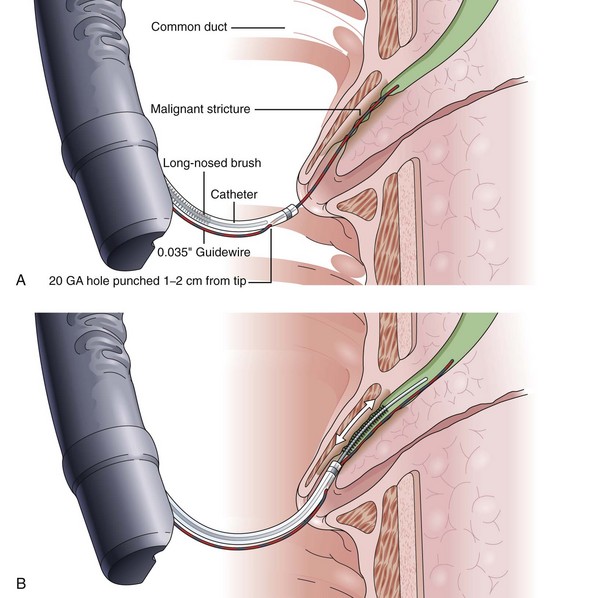
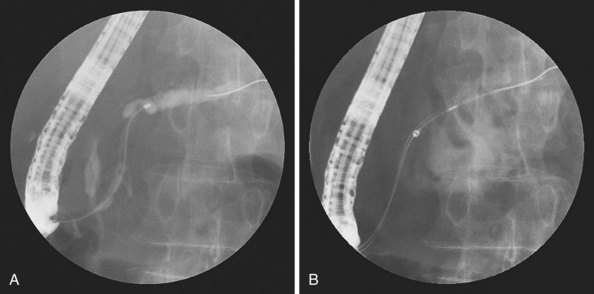
Another alternative is to create a “monorail” brushing device by piercing a catheter with a sharp 20-gauge needle 1 to 2 cm from its tip and passing the guidewire through its end hole and out this newly produced side hole. The spring-tipped brush is positioned just behind this, and the assembled device can be more easily passed into the duct (Fig. 40.2). Once above the stricture, the catheter is passed beyond the tip of the guidewire. The brush is free to advance beyond the tip of the catheter, exposing the bristles for specimen collection and leaving the guidewire in place above the stricture. The principal advantage is that the brush can be pulled into the end of the catheter, and both components can be withdrawn to minimize cellular loss (Fig. 40.3).20
Published yields of ERCP brush cytology devices vary widely for reasons that can only be speculated. Generally, series that have a higher proportion of pancreatic adenocarcinomas and, perhaps, earlier smaller tumors have a much lower yield of positive results compared with series with more cholangiocarcinomas. Published overall sensitivities using these devices range from 8% to 57%.8,21–26 As discussed subsequently, many of these series are also flawed by including patients with “suspicious for malignancy” reports as positive results. The probable pathologic explanation for these varied yields relates to the observation that the interiors of malignant strictures are composed of benign epithelium compressed by surrounding neoplastic tissue, with the exception of cholangiocarcinoma of the major bile ducts. This fact explains the low yield of simple bile aspiration for cytology because few, if any, malignant cells are in contact with the bile flow as previously discussed. When the stricture is traumatized by dilation, removing the benign epithelium, the yield of aspirating bile increases.27
The type of brush bristles, the overall brush length, and the amount of time spent brushing all affect yield. Rabinovitz and colleagues28 used three separate brushes at each ERCP and repeated the procedure with three new brushes when suspicious strictures were initially negative. Positive yield continued to increase until diagnoses were eventually made by brushing alone in 62% of their patients. Two more recent ERCP brushing studies have been performed in an attempt to increase yield. In a large number of patients, a newer long cytology brush with stiff angulated bristles was compared with the standard length brushes described previously. The true-positive yields were uniformly disappointing—only 27% and 30%—and no advantage was observed with the new brush.29 The second study compared brushing with a more traumatic technique of inserting a grasping basket through the suspicious stricture. Of 50 malignant strictures, the basket technique had a near doubling of yield to 80% compared with a brush yield of 48% (P = .018). The unexpected high yield of the brushing suggests some selection bias, and this technique requires further study.30 Additional improvements of techniques and equipment for brush cytology are needed to improve yield. Alternatively, additional techniques and devices can be used, as reviewed in this chapter.
Fine Needle Aspiration Cytology
Chiba needle aspiration cytology was pioneered in Japan in the 1950s using 22-gauge long percutaneous needles. Still the standard for most radiologically guided biopsies, Chiba needle aspiration has proved exceedingly safe and is widely applied. However, Warshaw31 reported a high rate of recovery of intraperitoneal malignant cells in patients undergoing attempted resection for pancreatic cancer who had undergone CT-guided percutaneous transabdominal Chiba needle biopsy 24 to 48 hours preoperatively. This apparent strong potential for intraperitoneal seeding has caused most physicians to seek an alternative to the Chiba technique in patients who might undergo subsequent surgery. In light of these concerns, some centers formally advocate proceeding with surgical exploration without any attempts at tissue sampling, if the clinical situation is sufficiently suspicious.32 As discussed earlier, the prospect of autoimmune pancreatitis should be considered.
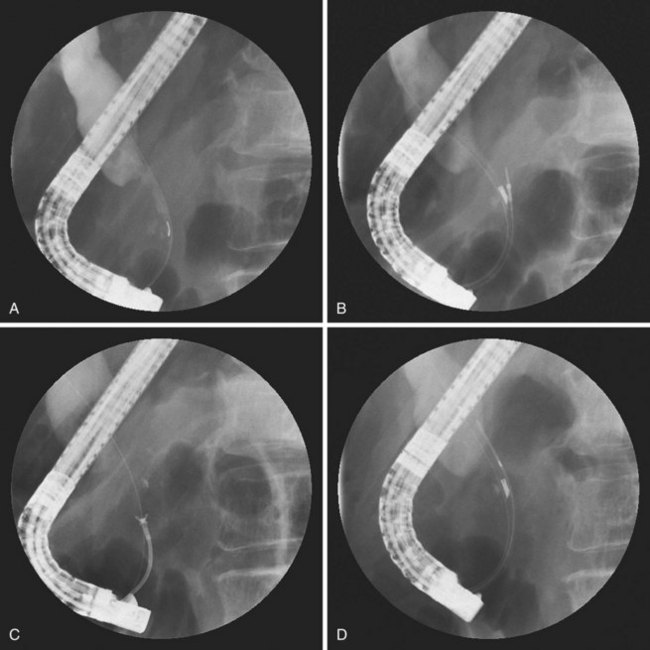
Endoscopic needle biopsy of pancreatic head masses was pioneered in 1977 by Tsuchiya and coworkers33 using straight 22-gauge needles directed at bulges seen compressing the central duodenal wall. This situation represents only a small minority of such patients, but endoscopic needle biopsy remains a viable tissue sampling technique throughout the upper gastrointestinal tract for submucosal tumors.34 Intraductal FNA during ERCP required the development of a specifically designed endoscopic accessory device. Howell and colleagues26 reported on such a device after developing a ball-tipped catheter with a retractable 22-gauge Chiba-type biopsy needle (HBAN-22; Wilson-Cook Medical). The needle extends 7 mm beyond the ball tip when the catheter is placed within the duct and permits deeper sampling than afforded by brushing (Fig. 40.4). In contradistinction, intraductal FNA cytology during ERCP traverses only tissue to be resected en bloc. There can be no contamination of the peritoneal cavity including the lesser sac behind the stomach. The technique requires sphincterotomy, however, and proves to be technically challenging.
The initial high yield of 62% (positive and suspicious samples) has not been reproduced in more recent series. The true-positive sensitivity has been reported to be 27% to 30% of cases in three series.24,35,36 Nevertheless, FNA may add to the total yield when added to other techniques, as discussed later in this chapter. No complication of this technique has been reported to date.
Forceps Biopsy
The technique of forceps biopsy involves insertion of the device to the lower edge of the stricture. Using fluoroscopy, an accurate biopsy specimen can be obtained from the lower edge of the apparent tumor. Several passes of the forceps are required to produce an optimal yield. Reporting on their experience, Ponchon and coworkers13 suggested a minimum of three forceps bites.
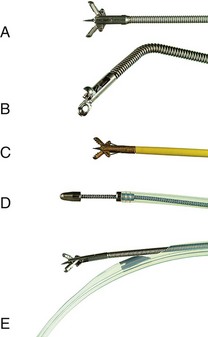
For the technique to be practical, specialized forceps were developed. Several devices have been marketed to permit easier insertion, including two devices that are purported not to need sphincterotomy (Fig. 40.5). Easier to insert but still unguided, pediatric forceps of 5-Fr to 6-Fr work reasonably well but provide small specimens. Disposable 6-Fr pediatric forceps are now available, although they are relatively expensive (see Fig. 40.5D). Full-sized angled forceps (Maxum Carr-Locke forceps; Wilson-Cook Medical) designed to permit cannulation without sphincterotomy were also reported to be successful in a small series of patients (see Fig. 40.5B).37 This larger cup forceps has a premade angled tip but is fairly large and, in my experience, is unsuitable for cannulation except after sphincterotomy. No data on postprocedural complications have been published. A device has been marketed to enable forceps placement over a guidewire. As previously discussed, the guidewire is generally placed early in therapeutic ERCP to ensure that the major goal of biliary stent placement is successful. It is logical to use the in-place guidewire for subsequent tissue sampling.
The guidewire-based device currently in use, developed by the author, is the Howell biliary introducer (HBI; Wilson-Cook Medical) (see Fig. 40.5E). The 10-Fr device goes over a 0.035-inch or smaller guidewire while permitting the passage of a specially designed reusable 5-Fr long forceps. Multiple passes of the forceps and other sampling devices can be quickly accomplished once the introducer is in position. At the present time, I advocate using this forceps as part of a multimodality sampling sequence. Introducing this device, its uses, and results are discussed later in this chapter because it is intended to facilitate the introduction of multiple sampling devices to maximize yield while maintaining guidewire position.
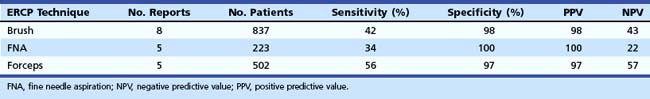
In a review published in two consecutive issues of Gastrointestinal Endoscopy, the journal of the American Society of Gastrointestinal Endoscopy (ASGE), de Bellis and colleagues38 tabulated all reports in the literature for the three major techniques including forceps biopsy and ERCP tissue sampling since 1989 (Table 40.1). Complications of forceps biopsy have been reported but seem to be rare. Among the 502 patients tabulated in Table 40.1, major bleeding requiring transfusion in one cancer patient was reported,39 and a significant perforation of a benign stricture required surgery in one additional patient.22 The perforation may have been caused by the use of a large cup forceps and repeated biopsy sampling of the same location. Pediatric forceps produce a smaller specimen, but we have experienced no complications in more than 200 cases using them to date. Rare but serious complications have not been noted using endobiliary brushing or ERCP-directed FNA. A review of the devices for endoscopic tissue sampling has been published by the ASGE Technology Assessment Committee.40
Combining Multiple Sampling Techniques
With the disappointing yields of single technique sampling as presented previously, endoscopists began to report their experience of combining techniques during the same ERCP procedure. This logical approach parallels standard endoscopic practice in which brush cytology and forceps biopsy are usually combined during both upper and lower procedures. Although this approach takes more time than a single technique, improved yields have made the combined approach the preferred sequence in many, particularly academic, centers. Using standard brushes and nonguidewire forceps, Ponchon and colleagues13 reported improved combined yields for diagnosing cancer at ERCP. Although brushing had a sensitivity of 43% and forceps biopsy had a sensitivity of 30%, their combined yield was increased to 63% (a 20% overall gain). A more comprehensive approach was studied by the Indiana University group. Researchers attempted to perform all three techniques of brush, FNA, and forceps sampling and submitted withdrawn indwelling stents for cytology when present. This demanding approach resulted in positive diagnosis in 82% of patients at a single ERCP, the highest success yet reported.24 When the investigators analyzed their results, each technique contributed to making the diagnosis in at least some patients. In other words, many patients had only one of the techniques positive and the other two or three techniques were negative or equivocal.
Despite this report and the logic of this approach, the technique of triple sampling has not become a standard practice during ERCP. Several explanations can be advanced. The first and probably most important reason was previously discussed: Triple sampling is technically difficult, time-consuming, and ancillary to the main goal of the therapeutic procedure. The second reason may be a clinical bias that pancreatic cancers and advanced biliary cancers are hopeless diseases, and conservative palliation by stent placement is all that is required. The case for tissue diagnosis beyond selection for chemotherapy or radiation therapy has been poorly made. A more recent proposed algorithm from a major surgical hospital suggests that patients with appropriate images suggestive of resectable pancreatic cancer need to undergo surgical exploration only.32 Finally, the advent and availability of EUS-guided FNA cytology have caused many authors to advocate a separate EUS procedure before ERCP is done to place a stent. Although this approach may shorten the ERCP, have a higher yield with multiple needle passes, and permit more confident placement of an initial metal stent, an additional invasive procedure is required. In most patients, a role remains for ERCP tissue sampling, especially because EUS seems to be less valuable for staging in the setting of newer 64-slice multidetection dynamic contrast CT scanning.
To enhance the ability of the average endoscopist to perform triple sampling with a minimum of time, expense, and risk, my colleagues and I assisted in the development of a multiuse biliary introducer (HBI; Wilson-Cook), mentioned previously. A goal was to permit maximum sampling at various depths to increase the chance of detecting all three types of malignancy and to do so without requiring a sphincterotomy if so desired (Fig. 40.6). The details of the device and our initial procedural sequence, techniques, and yields were reported in 1996.41 An overall sensitivity of 69%, despite a large proportion of small early pancreatic cancers, suggested the potential of the device. The HBI introducer is a double-lumen 10-Fr tapered dilator that contains a 0.035-inch channel for a standard ERCP guidewire and a 6-Fr large channel for the introduction of endoscopic accessories. The larger channel exits 3 cm below the tip and is fronted by a metal angled ramp that deflects the advance of devices at a 30-degree angle to assist in device positioning. The purpose of the ramp is to direct sampling away from the axis of the biliary stricture and into the deeper tissues where malignant tissue diagnosis is more likely to be made.
The needle and forceps exit to obtain a biopsy specimen at this 30-degree angle into the lower edge of the stricture. With fluoroscopic guidance, repeated biopsy at the same point may also help to obtain a deep sample. Care should be taken when using this repeated biopsy technique when a clear mass is not present on CT scan before ERCP to minimize the risk of perforation. To date, no perforation has been reported using the 22-gauge needle or the small 5-Fr or 6-Fr biopsy forceps. We advise against ever obtaining a biopsy specimen of the bile duct above a stricture because the bile duct is thinned by obstructive dilation, and a bile leak may occur. Finally, the HBI and the previously mentioned devices serve to remove benign epithelium before introducing the specially designed cytology brush (HBIB; Wilson-Cook Medical). This brush has extra-long, extra-stiff bristles and is mounted on a stiff braided-wire shaft resulting in an aggressive device (see Fig. 40.1D).
We have standardized the technique of triple sampling using the HBI. After placement of a guidewire through the stricture, the preloaded HBI containing the specially designed 22-gauge needle is passed as a unit into the stricture (Fig. 40.7). It is important that the retracted needle within its ball-tipped 5-Fr catheter be kept just inside the angled port. As this unit is passed over the prepositioned guidewire (see Fig. 40.7A), the elevator of the duodenoscope is relaxed to prevent damage to the needle. Once positioned under the stricture, the ball tip is advanced and locked, and the needle is thrust forward into the tumor (see Fig. 40.7B). The stylet is removed from the needle, 10 mL of syringe vacuum suction is applied, and the needle is thrust in and out using a standard Chiba technique. A second thrust can be made without withdrawing the needle, but we generally perform a single FNA. The specimen is expressed into a cytologic transport medium (Cyto-Rich; Roche, Elon College, NC) that lyses red blood cells and fixes tissue for cell block. This approach shortens procedure time and avoids improper slide preparation or cellular loss.
The second device is always the 5-Fr HBIN forceps. This forceps can be passed through the HBI and over the elevator, if the angle of entry into the duct is not too sharp (see Fig. 40.7C). If resistance is felt, moving the HBI back into the endoscope permits the forceps to advance to the angled port, and the HBI and forceps can be advanced again as a unit, to ensure easy forceps placement. We attempt to open the forceps below the stricture, but often the distal duct is too small to accommodate this action. The HBI containing the forceps can be advanced above the stricture to permit the forceps to be advanced into the dilated proximal duct and opened. The HBI is pulled down leaving only the narrow tip in the stricture. The open forceps can be pulled through the stricture while open and closed on the appropriate lower stricture edge. Care should be exercised never to obtain a biopsy specimen in the duct above the stricture. Occasionally, the hard nature of pancreatic tumors does not permit easy severing of tissue, despite firm biting force. Diagnostic pieces can still be obtained after closing the cups but pushing the HBI forward, which then often shears off the desired sample. We have generally used three bites as a standard, but more biopsies increase yield, as discussed later in the chapter.
Finally, the specially designed brush (HBIB) is introduced while the angled port is advanced above the stricture. When the brush is advanced into the duct, the HBI is pulled back into the stricture so that the wide 10-Fr portion just in front of the angled port lies inside the stricture. The spring-nosed brush is vigorously moved to and fro keeping the nose always just above the upper aspect of the stricture (see Fig. 40.7D). The brush is very tight but abrades the encasing tumor and obtains a deep sample. After 60 to 120 seconds of brushing, the brush is pulled back just into the 6-Fr channel and left there while the entire HBI and brush is removed leaving the guidewire in place. Cellular loss is prevented by not withdrawing the brush through the HBI. The brush is simply cut off and dropped into the transport medium to shorten the procedure time and permit slide preparation in the cytology department.
After some initial experience, this sequence of triple sampling using the HBI takes 12 to 15 minutes and does not interfere with planned stent insertion. Our initial experience reported a positive and suspicious yield of 69% using the previously described technique in consecutive patients.35 This sequence showed the value of using all three devices because patients often had only one device sample positive with the others being read as suspicious or falsely negative. For the purposes of the initial report, we considered “suspicious for adenocarcinoma” as positive (45% positive and 24% suspicious, for a total of 69%); in the appropriate study setting of a documented mass, this resulted in maintaining a specificity of 100%. A comparative trial of the HBI device against standard brushing was reported more recently.42 For the purpose of their study, the authors considered any “positive,” “suspicious,” or “atypical or suggestive of malignancy” to be true-positive samples. The authors used only the HBIN 22-gauge needle and HBI brush and reported an 85% yield compared with a sensitivity of 57% for brushing alone. Presumably, if the HBI forceps had also been used, yield would have been greater.
For the diagnosis of malignancy, this method of reporting “suspicious” and “atypical or suggestive of malignancy” results has been generally done in reports of ERCP tissue sampling, but a higher standard must be demanded. We have modified our technique of HBI sampling to attempt to increase the true-positive yield. We take six biopsy specimens and brush for 2 full minutes; this prolongs tissue sampling by about 6 minutes. In our 2003 report, we considered any result but true-positive to be negative.43 The true-positive yield increased from 45% to 71.4%. The greatest increase of positive samples proved to be with the extra forceps biopsies. In this series of 35 consecutive patients, 9 cases had only forceps positive samples, 3 had only FNA positive samples, and 1 had a brush positive sample alone. No complications were noted.
Other Methods of Endoscopic Retrograde Cholangiopancreatography Tissue Sampling
Numerous less productive or controversial techniques of tissue acquisition have been reported and warrant review. Leung and coworkers44 originally reported that examining indwelling plastic biliary stents on their removal may produce a positive cytologic specimen when the diagnosis had not been established at the initial ERCP placement. Since 1989, only one series38 has approached the initial 70% yield of Leung and coworkers. Most centers report only 11% to 44% positive specimens.8,18,45 The most recent report of stent cytology included withdrawn pancreatic stents and stents from biliary strictures.46 The true-positive yield from pancreatic stents was 25% compared with only 11% from biliary stents. In addition, the investigators agreed with other authors that the technique had limited clinical value because of the long delay in diagnosis when positive results were obtained.
Another approach to tissue acquisition at ERCP has been to attempt to collect specimens from the pancreatic duct. Collection of pancreatic juice has been advocated by a few authors.47,48 The technique involves deep insertion of a standard ERCP catheter and aspiration of juice below a malignant-appearing stricture. Yields may increase to greater than 50% with the infusion of secretin. This approach has not become popular, perhaps because of its complexity and concern for inducing pancreatitis. Pugliese and coworkers49 concluded that pancreatic juice collection did not add to positive diagnosis when pancreatic duct strictures were directly sampled by brushing.
Brushing in the pancreatic duct has been reported since 1979.50 More recent reports emphasize that yields increase very little when a biliary stricture is also present and can be sampled.51–53 Most concerning has been the report of postprocedural pancreatitis after pancreatic ductal stricture brushing. Vandervoort and colleagues54 noted a 21.5% pancreatitis rate after such procedures in both benign and malignant cases but noted a marked decrease in risk if pancreatic temporary plastic stents were placed. Other authors have also advocated stent placement after brushing, but all studies do not outline the eventual management and outcome of these temporary stents. The utility of stents is likely outweighed by subsequent procedures for pancreatic stent removal and delayed stent obstruction with resulting pancreatitis or sepsis, and other approaches to tissue sampling are favored. At the present time, we sample from the pancreatic duct only when a pancreatic stent is clinically warranted to manage obstructing pancreatitis, fistula, or upstream pseudocyst. We prefer to use the monorail technique for brushing as outlined previously so that the guidewire, which was placed before tissue sampling, can remain in position (Fig. 40.8).
Specimen Handling and Analysis
Improper handling of collected specimens remains a problem in many endoscopy units. A major cause of uninterpretable smears is air-drying artifact that can occur rapidly after creation of appropriate thin smear.10 Thick smears and specimens with excessive blood are other significant problems.55 Slide preparation requires the time and attention of ERCP team members during a busy and often complex procedure. As outlined in the section outlining the technique of triple sampling with the HBI device, until more recently, we preferred to deposit all collected specimens into transport media rather than preparing any smears or slides in the ERCP suite. Available transport media include 95% ethanol or commercially prepared solutions such as CytoLyt (Cytyc Corporation, Boxborough, MA) or CytoRich (UtoCyte, Burlington, NC). Papanicolaou-stained spun smears and, when applicable, hematoxylin and eosin–stained cell block sections are prepared for cytologic evaluation. In our institution, we prefer CytoLyt solution, which lyses red blood cells and minimizes obscuring debris, with smears prepared via the ThinPrep method (Cytyc Corporation). We are currently studying a technique of intraprocedural cytologic diagnosis using small forceps biopsies, which is discussed subsequently in the section on future trends.
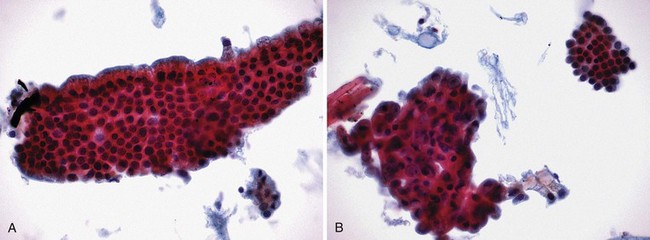
Interpretation of specimens should follow accepted cytologic criteria to be clinically useful. Several such schemes exist with each accepting frankly positive (Fig. 40.9A) and negative (Fig. 40.9B) features. Intermediate cytologic abnormalities present on the slides may lead to interpretations such as “atypical,” in which mild cellular abnormalities are usually associated with inflammation and reparative changes, and “suspicious,” in which there are rare cells exhibiting cytologic features of malignancy but they are present in insufficient numbers to render a definitive diagnosis of malignancy. As previously stated, “suspicious for malignancy” should contain adequate features to make the diagnosis highly likely especially when interpreted in the appropriate clinical setting. In our institution, “suspicious for adenocarcinoma” is equivalent to a true-positive when the patient has a malignant-appearing distal biliary stricture at ERCP and a definite mass on CT scan. “Suspicious” is inadequate in interpreting samples from biliary strictures in patients with primary sclerosing cholangitis or postbiliary irradiation resulting in significant false-positive samples.
Ponshon and coworkers13 reported four false-positive ERCP brush cytology specimens among three patients with primary sclerosing cholangitis. A larger series noted only 80% specificity in this setting.56 The findings of “cellular atypia” should include criteria that make the diagnosis of malignancy unlikely and demand further attempts at confirmation. We interpret all atypia findings as negative, understanding that many turned out to be falsely negative. Finally, because of the inherent difficulties in ERCP tissue sampling, negative results can never be accepted as definitive.11 Sampling problems are often due to the relative hardness of pancreaticobiliary adenocarcinomas that may greatly resist needle puncture and forceps sampling. These desmoplastic tumors can be relatively hypocellular resulting in inadequate numbers of cells for interpretation. A small fraction of adenocarcinomas are so well differentiated they can be diagnosed histologically only in the setting of an excisional biopsy to permit the recognition of invasion; this is also true of lymphoma, which does occasionally produce biliary obstruction. As previously discussed, metastatic lesions obstructing the biliary tree are relatively deep and cannot be readily accessed at ERCP from within the ductal stricture except occasionally by intraductal FNA.
All of these factors result in a low negative predictive value in all series reporting techniques of tissue sampling. This low negative predictive value should not discourage endoscopists from developing a preferred sequence of tissue sampling at ERCP because specificity in all reports is generally 100% for true-positive samples. A false-negative result leads to additional invasive tests, procedures, or surgery1—the same result as when no effort is made. Using the outlined techniques of endobiliary sampling during ERCP, patients receive benefit at a minimum of expense and risk.
Future Trends
Flow cytometry was one of the first ancillary techniques reported with the hope that cytologically negative specimens might reveal DNA aneuploidy sufficient to advance the diagnosis of cancer. Early reports revealed the problem of poor specificity most likely resulting from the mixed population of inflammatory cells and debris.57 This problem may be resolved with a technique of digital image analysis, but a large prospective study has not been produced yet.58
The K-ras mutation has been identified as often present in pancreaticobiliary cancers. Several polymerase chain reaction–based studies to detect K-ras mutations in ERCP-collected specimens seemed useful with positive yields increasing by up to 30%.59 However, as with flow cytometry, specificities proved to be poor when patients with chronic pancreatitis were included. Two studies reported finding K-ras mutations in 25% and 36% of chronic pancreatitis patients with subsequently proven benign strictures.49,60 This potential for false-positive results precludes this technique from use in clinical decision making. Other research techniques—screening for loss of genetic heterozygosity and immunostaining for the TP53 gene—have been preliminarily examined but do not have sufficient data to support their use. These techniques are likely to receive further attention because they are being actively researched for possible use in colon cancer stool specimen screening.61 Additional tumor markers found in ERCP brush specimens have been reported to increase diagnostic sensitivity.
Minichromosome maintenance replication proteins seem to be good markers for dysplasia. Their dysregulation in the setting of biliary strictures were found in more than 75% of tested specimens compared with only 20% positive cytology brush specimens. Specificity was good but less than the 100% required in clinical practice.62 DNA methylation alterations have been used as a marker for malignant neoplasia and have been studied more recently in ERCP brushing samples. Abnormalities were found in nearly 75% of malignant cases but also in 13.69% of benign cases. Further study is ongoing to improve specificity.63 Additional advanced molecular markers are being examined, again to attempt to increase the diagnostic accuracy from otherwise low-yield ERCP brushing samples. Fluorescent in situ hybridization, digital image analysis, and quantitative nuclear morphometry individually and in combination all hold promise in this regard.64–67
All these efforts would be unnecessary if adequate tissue collection at ERCP could permit accurate histologic or cytologic diagnosis in greater than 90% of patients while maintaining the expected 100% specificity. This need is witnessing an advance in equipment that may have an impact on tissue sampling at ERCP. Newer, small endoscopes of 10-Fr, termed baby scopes, have permitted direct examination of the biliary tree and, with the smallest 7-Fr baby scopes, pancreatic duct lesions. A 3-Fr biopsy forceps and a long cytology brush can be inserted through the 1.0-mm accessory channel of the 10-Fr baby scope to permit directed biopsy. Efforts are under way to permit accurate visual diagnosis of malignancy using rigid and reproducible criteria.68 Narrow band imaging and other advanced optical technology have been made available more recently in these tiny endoscopes with encouraging prospects. Development of a 10-Fr disposable intraductal fiberoptic endoscope (Spyglass; Boston Scientific) with a 1.2-mm biopsy channel and a 1-mm biopsy forceps has enabled visually directed forceps biopsies to be performed.69 Initial reports of this technique emphasize accuracy of tissue collection and yields of 70% or more. Series are small, and cases are highly selected, but the procedure seems to be safe. Nevertheless, the cost is substantial compared with “blind” forceps biopsy. Efforts are being made to enable direct cholangioscopy with standard small-caliber front-viewing videoendoscopes for direct visualization and biopsy using larger capacity forceps.70
Regarding advances in techniques, a major advantage of EUS-guided FNA has been immediate diagnosis using a cytopathologist in the endoscopy suite to guide the number and adequacy of needle passes. ERCP-guided FNA has the disadvantages of technical difficulty, more shallow depth of puncture, and limited number of passes before needing to employ a new needle. A new technique developed in our unit using a new cytologic preparation at ERCP may permit direct intraprocedural diagnosis similar to the sequence used at EUS.71 Intraoperative margins during brain tumor resection have been sampled by preparing quick fixed smears on dry slides. Termed squash prep, this technique evolved because frozen sections cannot be done of fat-rich brain tissue. Paralleling this established technique, our group has prepared small 5-Fr or 6-Fr forceps biopsy specimens by vigorously smashing them between two glass slides to attempt to create a monolayer. These specimens are immediately stained by rapid Papanicolaou and read. Preliminary experience suggests this approach adds only an additional 10 to 20 minutes to the ERCP procedure and produces a definitive positive diagnosis in greater than 90% of pancreaticobiliary cancers; this high yield is directed by the cytopathologist who can request additional specimens. We generally halt at 10 specimens, in favor of collecting additional biopsy specimens for histology and a final one or two ERCP-guided FNA passes. This new approach at tissue sampling adds little time and little cost and avoids delay in tissue diagnosis in most patients. Immediate tissue diagnosis avoids additional efforts at biopsy such as EUS-guided FNA or CT-guided FNA and often shortens hospital stay.71 Other centers need to confirm these preliminary experiences, but if confirmed, this technique holds promise to be widely applicable and available especially in medical facilities where EUS is not readily available.
Intraductal EUS has been used through biliary strictures to this same end. However, specificity is much less than that of directed tissue sampling, and as such this technique cannot substitute. One report of comparing EUS with tissue sampling and intraductal EUS in 30 patients suggested that intraductal EUS increased accuracy from 68% to 90%.72 However, the need for accurate tissue determination of the type of malignancy required further efforts at tissue diagnosis. The ERCP tissue diagnosis specificity was 100%. Optical coherence tomography may be adaptable to be used over a guidewire during ERCP and provide a histologic quality image for pathologic interpretation.73 So-called optical biopsy has been proposed using confocal laser scanning microscopy without biopsy. Initial reports have a disappointing specificity, but study is ongoing.74 A more promising approach to increase the yield and specificity of ERCP tissue sampling lies in developing better devices to collect larger specimens more successfully. The more recent introduction of a cutting needle for EUS-guided biopsy is such an example.75
Summary
Tissue sampling at ERCP remains an underused technique that when properly used is safe, effective, fast, and cost-effective. Multimodality sampling and collection of multiple specimens increase positive yield. The consequence of not attempting ERCP sampling commits the patient to other expensive and often uncomfortable biopsy attempts if a tissue diagnosis is to be established. Based on the literature at the present time, rapid collection of multiple specimens for cytology and histology is possible during a single therapeutic ERCP and can be recommended. Intraprocedural diagnosis by forceps cytology is newly described and may be a simpler, cost-effective technique.71
1 Howell DA, Mazzaglia P, Sheth S, et al. Clinical value of tissue sampling at ERCP [abstract]. Gastrointest Endosc. 2002;55:AB196.
2 Bain VG, Abraham N, Jhangri GS, et al. Prospective study of biliary strictures to determine the predictors of malignancy. Can J Gastroenterol. 2000;14:397-402.
3 Kelly SM, Sanowski RA, Foutch PG, et al. A prospective comparison of anoscopy and fiberendoscopy in detecting anal lesions. J Clin Gastroenterol. 1986;8:658-660.
4 Desa LA, Akosa AB, Lazzara S, et al. Cytodiagnosis in the management of extrahepatic biliary stricture. Gut. 1991;32:1188-1191.
5 Davidson B, Varsamidakis N, Dooley J, et al. Value of exfoliative cytology for investigating bile duct strictures. Gut. 1992;33:1408-1411.
6 Kurzawinski TR, Deery A, Dooley JS, et al. A prospective study of biliary cytology in 100 patients with bile duct strictures. Hepatology. 1993;18:1399-1403.
7 Sugiyama M, Atomi Y, Wada N, et al. Endoscopic transpapillary bile duct biopsy without sphincterotomy for diagnosing biliary strictures: A prospective comparative study with bile and brush cytology. Am J Gastroenterol. 1996;91:465-467.
8 Mansfield JC, Griffin SM, Wadehra V, et al. A prospective evaluation of cytology from biliary strictures. Gut. 1997;40:671-677.
9 Ross WA, Wasan SM, Evans DB, et al. Combined EUS with FNA and ERCP for the evaluation of patients with obstructive jaundice from presumed pancreatic malignancy. Gastrointest Endosc. 2008;68:461-466.
10 Kocjan G, Smith AN. Bile duct brushings cytology: Potential pitfalls in diagnosis. Diagn Cytopathol. 1997;16:358-363.
11 Logrono R, Kurtycz DF, Molina CP, et al. Analysis of false-negative diagnoses on endoscopic brush cytology of biliary and pancreatic duct strictures: The experience at 2 university hospitals. Arch Pathol Lab Med. 2000;124:387-392.
12 Eloubeidi MA, Chen VK, Jhala NC, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209-213.
13 Ponchon T, Gagnon P, Berger F, et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: Results of a prospective study. Gastrointest Endosc. 1995;42:565-572.
14 Renshaw AA, Madge R, Jiroutek M, et al. Bile duct brushing cytology: Statistical analysis of proposed diagnostic criteria. Am J Clin Pathol. 1998;110:635-640.
15 Bardales RH, Stanley MW, Simpson DD, et al. Diagnostic value of brush cytology in the diagnosis of duodenal, biliary, and ampullary neoplasms. Am J Clin Pathol. 1998;109:540-548.
16 Ryu JK, Chung JB, Park SW, et al. Review of 67 patients with autoimmune pancreatitis in Korea: A multicenter nationwide study. Pancreas. 2008;37:377-385.
17 Alexander S, Bourke MJ, Williams SJ, et al. Diagnosis of autoimmune pancreatitis with intraductal biliary biopsy and treatment of stricture with serial placement of multiple biliary stents. Gastrointest Endosc. 2008;68:396-399.
18 Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386-1391.
19 Baron TH, Lee JG, Wax TD, et al. An in vitro, randomized, prospective study to maximize cellular yield during bile duct brush cytology. Gastrointest Endosc. 1994;40(2 Pt 1):146-149.
20 Foutch PG, Harlan JR, Kerr D, et al. Wire-guided brush cytology: A new endoscopic method for diagnosis of bile duct cancer. Gastrointest Endosc. 1989;35:243-247.
21 Lee JG, Leung JW, Baillie J, et al. Benign, dysplastic, or malignant–making sense of endoscopic bile duct brush cytology: Results in 149 consecutive patients. Am J Gastroenterol. 1995;90:722-726.
22 Pugliese V, Conio M, Nicolò G, et al. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: A prospective study. Gastrointest Endosc. 1995;42:520-526.
23 Glasbrenner B. Prospective evaluation of brush cytology of biliary strictures during endoscopic strictures: A review of 406 cases. J Clin Pathol. 2001;54:449-455.
24 Jailwala J, Fogel EL, Sherman S, et al. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc. 2000;51(4 Pt 1):383-390.
25 Macken E, Drijkoningen M, Van Aken E, et al. Brush cytology of ductal strictures during ERCP. Acta Gastroenterol Belg. 2000;63:254-259.
26 Howell DA, Beveridge RP, Bosco J, et al. Endoscopic needle aspiration biopsy at ERCP in the diagnosis of biliary strictures. Gastrointest Endosc. 1992;38:531-535.
27 Glasbrenner B, Ardan M, Boeck W, et al. Prospective evaluation of brush cytology of biliary strictures during endoscopic retrograde cholangiopancreatography. Endoscopy. 1999;31:712-717.
28 Rabinovitz M, Zajko AB, Hassanein T, et al. Diagnostic value of brush cytology in the diagnosis of bile duct carcinoma: A study in 65 patients with bile duct strictures. Hepatology. 1990;12(4 Pt 1):747-752.
29 Fogel EL, de Bellis M, McHenry L, et al. Effectiveness of a new long cytology brush in the evaluation of malignant biliary obstruction: A prospective study. Gastrointest Endosc. 2006;63:71-77.
30 Dumonceau JM, Macias Gomez C, Casco C, et al. Grasp or brush for biliary sampling at endoscopic retrograde cholangiography? A blinded randomized controlled trial. Am J Gastroenterol. 2008;103:333-340.
31 Warshaw AL. Implications of peritoneal cytology for staging of early pancreatic cancer. Am J Surg. 1991;161:26-30.
32 Farnell MB, Nagorney DM, Sarr MG. The Mayo Clinic approach to the surgical treatment of adenocarcinoma of the pancreas. Surg Clin North Am. 2001;81:611-623.
33 Tsuchiya R, Henmi T, Kondo N, et al. Endoscopic aspiration biopsy of the pancreas. Gastroenterology. 1977;73:1050-1052.
34 Kochhar R, Rajwanshi A, Malik AK, et al. Endoscopic fine needle aspiration biopsy of gastroesophageal malignancies. Gastrointest Endosc. 1988;34:321-323.
35 Lo SK. A prospective blinded evaluation of all ERCP sampling methods on biliary strictures. Gastrointest Endosc. 1996;43:386A.
36 Farrell RJ, Jain AK, Brandwein SL, et al. The combination of stricture dilation, endoscopic needle aspiration, and biliary brushings significantly improves diagnostic yield from malignant bile duct strictures. Gastrointest Endosc. 2001;54:587-594.
37 Vandervoort J. Use of a new angled forceps to biopsy pancreatic and biliary strictures. Gastrointest Endosc. 1997;45:AB41.
38 de Bellis M, Sherman S, Fogel EL, et al. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 2). Gastrointest Endosc. 2002;56:720-730.
39 Schoefl R, Haefner M, Wrba F, et al. Forceps biopsy and brush cytology during endoscopic retrograde cholangiopancreatography for the diagnosis of biliary stenoses. Scand J Gastroenterol. 1997;32:363-368.
40 Technology Assessment CommitteeBarkun A, Liu J, Carpenter S, et al. Update on endoscopic tissue sampling devices. Gastrointest Endosc. 2006;63:741-745.
41 Howell DA, Parsons WG, Jones MA, et al. Complete tissue sampling of biliary strictures at ERCP using a new device. Gastrointest Endosc. 1996;43:498-502.
42 Wiersema M. Difficulties encountered when using ERCP-based tissue sampling techniques to establish a diagnosis. Gastrointest Endosc. 2002;56:463-464.
43 Howell DA. What is the true yield of tissue sampling at ERCP? Gastrointest Endosc. 2002;55:AB170.
44 Leung JW, Sung JY, Chung SC, et al. Endoscopic scraping biopsy of malignant biliary strictures. Gastrointest Endosc. 1989;35:65-66.
45 Pescatore P, Heubner C, Heine M, et al. The value of histological analysis of occluded biliary endoprostheses. Endoscopy. 1995;27:597-600.
46 Simsir A, Greenebaum E, Stevens PD, et al. Biliary stent replacement cytology. Diagn Cytopathol. 1997;16:233-237.
47 Devereaux BM, Fogel EL, Bucksot L, et al. Clinical utility of stent cytology for the diagnosis of pancreaticobiliary neoplasms. Am J Gastroenterol. 2003;98:1028-1031.
48 Nakaizumi A, Tatsuta M, Uehara H, et al. Cytologic examination of pure pancreatic juice in the diagnosis of pancreatic carcinoma: The endoscopic retrograde intraductal catheter aspiration cytologic technique. Cancer. 1992;70:2610-2614.
49 Pugliese V, Pujic N, Saccomanno S, et al. Pancreatic intraductal sampling during ERCP in patients with chronic pancreatitis and pancreatic cancer: Cytologic studies and k-ras-2 codon 12 molecular analysis in 47 cases. Gastrointest Endosc. 2001;54:595-599.
50 Osnes M, Serck-Hanssen A, Kristensen O, et al. Endoscopic retrograde brush cytology in patients with primary and secondary malignancies of the pancreas. Gut. 1979;20:279-284.
51 Nakaizumi A, Tatsuta M, Uehara H, et al. Effectiveness of the cytologic examination of pure pancreatic juice in the diagnosis of early neoplasia of the pancreas. Cancer. 1995;76:750-757.
52 McGuire DE, Venu RP, Brown RD, et al. Brush cytology for pancreatic carcinoma: An analysis of factors influencing results. Gastrointest Endosc. 1996;44:300-304.
53 Ferrari Junior AP, Lichtenstein DR, Slivka A, et al. Brush cytology during ERCP for the diagnosis of biliary and pancreatic malignancies. Gastrointest Endosc. 1994;40(2 Pt 1):140-145.
54 Vandervoort J, Soetikno RM, Montes H, et al. Accuracy and complication rate of brush cytology from bile duct versus pancreatic duct. Gastrointest Endosc. 1999;49(3 Pt 1):322-327.
55 Layfield LJ, Wax TD, Lee JG, et al. Accuracy and morphologic aspects of pancreatic and biliary duct brushings. Acta Cytol. 1995;39:11-18.
56 Lindberg B, Arnelo U, Bergquist A, et al. Diagnosis of biliary strictures in conjunction with endoscopic retrograde cholangiopancreaticography, with special reference to patients with primary sclerosing cholangitis. Endoscopy. 2002;34:909-916.
57 Ryan ME, Baldauf MC. Comparison of flow cytometry for DNA content and brush cytology for detection of malignancy in pancreaticobiliary strictures. Gastrointest Endosc. 1994;40(2 Pt 1):133-139.
58 Rumalla A, Baron TH, Leontovich O, et al. Improved diagnostic yield of endoscopic biliary brush cytology by digital image analysis. Mayo Clin Proc. 2001;76:29-33.
59 Sturm PD, Rauws EA, Hruban RH, et al. Clinical value of K-ras codon 12 analysis and endobiliary brush cytology for the diagnosis of malignant extrahepatic bile duct stenosis. Clin Cancer Res. 1999;5:629-635.
60 Iwao T, Hanada K, Tsuchida A, et al. The establishment of a preoperative diagnosis of pancreatic carcinoma using cell specimens from pancreatic duct brushing with special attention to p53 mutations. Cancer. 1998;82:1487-1494.
61 Ahlquist DA, Skoletsky JE, Boynton KA, et al. Colorectal cancer screening by detection of altered human DNA in stool: Feasibility of a multitarget assay panel. Gastroenterology. 2000;119:1219-1227.
62 Ayaru L, Stoeber K, Webster GJ, et al. Diagnosis of pancreaticobiliary malignancy by detection of minichromosome maintenance protein 5 in bile aspirates. Br J Cancer. 2008;98:1548-1554.
63 Parsi MA, Li A, Li CP, et al. DNA methylation alterations in endoscopic retrograde cholangiopancreatography brush samples of patients with suspected pancreaticobiliary disease. Clin Gastroenterol Hepatol. 2008;6:1270-1278.
64 Barr Fritcher EG, Kipp BR, Slezak JM, et al. Correlating routine cytology, quantitative nuclear morphometry by digital image analysis, and genetic alterations by fluorescence in situ hybridization to assess the sensitivity of cytology for detecting pancreatobiliary tract malignancy. Am J Clin Pathol. 2007;128:272-279.
65 Fritcher EG, Kipp BR, Halling KC, et al. A multivariable model using advanced cytologic methods for the evaluation of indeterminate pancreatobiliary strictures. Gastroenterology. 2009;136:2180-2186.
66 Levy MJ, Baron TH, Clayton AC, et al. Prospective evaluation of advanced molecular markers and imaging techniques in patients with indeterminate bile duct strictures. Am J Gastroenterol. 2008;103:1263-1273.
67 Moreno Luna LE, Kipp B, Halling KC, et al. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064-1072.
68 Telford JJ, Carr-Locke DL. The role of ERCP and pancreatoscopy in cystic and intraductal tumors. Gastrointest Endosc Clin N Am. 2002;12:747-757.
69 Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: A clinical feasibility study (with video). Gastrointest Endosc. 2007;65:832-841.
70 Larghi A, Waxman I. Endoscopic direct cholangioscopy by using an ultra-slim upper endoscope: A feasibility study. Gastrointest Endosc. 2006;63:853-857.
71 Howell DA. Immediate tissue diagnosis during ERCP using a new simple forceps biopsy cytologic preparation: Technique, yield and outcome. Gastrointest Endosc. 2007;65:AB235.
72 Vazquez-Sequeiros E, Baron TH, Clain JE, et al. Evaluation of indeterminate bile duct strictures by intraductal US. Gastrointest Endosc. 2002;56:372-379.
73 Van Dam J. Novel methods of enhanced endoscopic imaging. Gut. 2003;52(Suppl 4):iv12-iv16.
74 Papaxoinis K, Patsouris E, Athanassiadou P, et al. Contribution of nuclear morphometry by confocal laser scanning microscopy to the diagnosis of malignant bile duct strictures. Acta Cytol. 2009;53:137-143.
75 DeWitt J, LeBlanc J, McHenry L, et al. Endoscopic ultrasound-guided fine needle aspiration cytology of solid liver lesions: A large single-center experience. Am J Gastroenterol. 2003;98:1976-1981.