CHAPTER 32 Endoscopic Release of the First Dorsal Extensor Tendon Compartment
Rationale
One might ask if arthroscopic first dorsal compartment release is a triumph of technology over reason, given that the open release is usually considered straightforward and effective. However, upon closer examination there are several reasons to consider this approach. First, although traditional open release gives generally good results complications (due to the approach) do occur and are most likely underreported. One study by Harvey et al. documented scar adherence to the underlying tendon in 2 of 20 surgical patients, 1 minor wound infection, and temporary parathesias of the radial sensory nerve in 3 patients.1 Another study by Arons et al. documents in 16 consecutive patients 14 complications, including 2 neuromas, 3 adhesions, 1 tendon subluxation, and 3 hypertrophic painful scars.2 A study by Ta et al. documented a 5% recurrence rate, 2% sensory nerve injury, and 2% with severe scar tenderness out of 43 patients.3 There have been other case reports of palmar subluxation of the tendon following operative release.4 Clearly, although an open release of the first extensor compartment is perceived as a safe, simple, and effective surgical procedure when examined closely there is room for improvement.
Surgical Technique
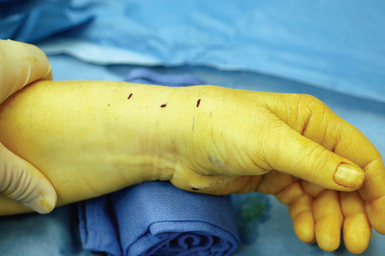
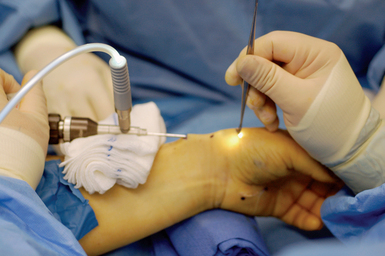
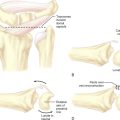
The patient is placed supine. The arm is elevated and exsanguinated, and a tourniquet inflated. The wrist is placed over a towel roll in a neutral position. A distal portal is established by making a 5-mm superficial transverse incision just distal to the thumb carpometacarpal (CMC) joint. The incision is placed in line with the first dorsal compartment at the insertion of the abductor pollicis longus (APL) tendon, 2 to 3 cm distal to the end of the radial styloid (Figure 32.1). Blunt dissection with a small hemostat is used to clear the overlying subcutaneous tissue from the fascia enveloping the thumb CMC joint, which is then elevated off the tendons of the first dorsal compartment with a small right-angle retractor.
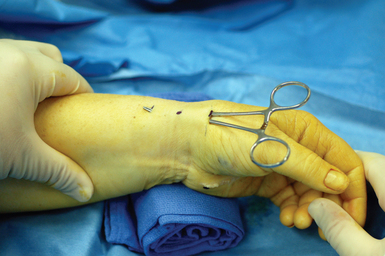
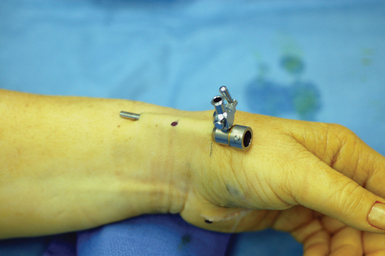
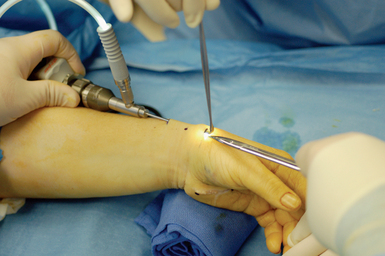
A narrow and long hemostat snap is next used to bluntly create a working space between the skin and subcutaneous tissue (Figure 32.2). A small-joint arthroscopic cannula and trocar are inserted above the fibrous fascial sheath of the first dorsal compartment, proximal to the radial styloid and the extensor tendon retinaculum. A proximal portal is established by making a second 5-mm transverse incision over the trocar tip approximately 4 to 6 cm proximal to the radial styloid (Figure 32.3). The trocar is removed and a 2.7-mm 30-degree angled scope is inserted into the cannula through the proximal portal (Figure 32.4).
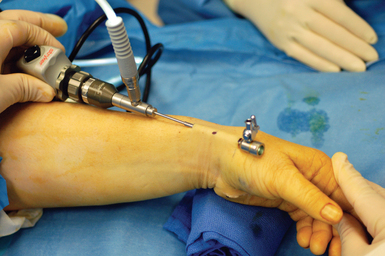
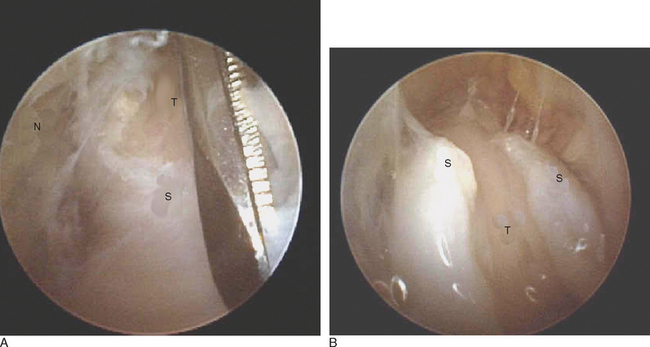
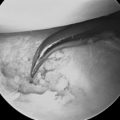
The scope is fully inserted into the cannula until the tip of the scope is visible through the distal portal. The cannula is then removed. A small right-angled retractor is placed in the proximal portal to maintain a working space, and a dry endoscopic inspection of the fascia overlying the first dorsal compartment is performed starting distally over the CMC joint (Figure 32.5). The superficial radial nerve (SRN) is easily identified in the subcutaneous tissue above as it sweeps downward, crossing the fascia below (Figure 32.6). Long thin cardiovascular Mueller scissors are introduced into the distal portal and used to bluntly and carefully dissect the overlying subcutaneous tissue from the fascia (Figure 32.7).
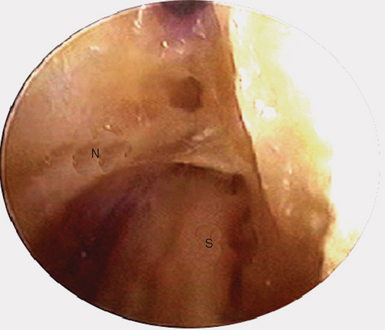
FIGURE 32.6 Endoscopic visualization of superficial radial nerve (N) and first dorsal compartment sheath (S) below.
This is a key step because we hypothesize that there are small neurofibrils extending from the major branches of the SRN down to the underlying fascia, which are divided off the fascia and swept aside. We believe this procedure serves as a neurectomy, separating these terminal branches from their sensory receptors on the fascia. Although there are some corroborating anatomical studies published, much of this needs further substantiation. One might suggest that the open procedure also provides for some subcutaneous dissection that has an element of neurectomy. However, an open procedure is addressing only a centimeter or so around the sheath. In the endoscopic procedure, we are providing a subcutaneous dissection/neurectomy of 6 to 8 cm. We believe that this step may be responsible for the immediate and profound relief in symptoms.
Next, the fascia of the first dorsal compartment is incised—starting proximal to the radial styloid and moving distally. The tendon slips of the APL and extensor pollicis brevis (EPB) are identified (Figure 32.8a and b). The EPB is often in a separate compartment and must be released. This tendon can be easily identified by stabilizing the first metacarpal and manually flexing and extending the metacarpal phalangeal joint. Through the endoscope, the EPB tendon can be visualized gliding proximally and distally (whereas the APL tendons remain stationary). As necessary, the camera and working instruments can be switched to evaluate the adequacy of the release or to improve access to different parts of the sheath. The wound is irrigated, the skin is closed with nylon suture, and a sterile dressing and volar spica splint are applied. The tourniquet is deflated. The typical tourniquet time is approximately 10 minutes.
The technique achieves two goals by addressing two possible sources of pain. The first goal is to perform a neurectomy of the small SRN branches to the first dorsal extensor compartment. Lin et al. demonstrated that the ligaments of the dorsal wrist capsule have an extensive array of sensory nerve endings.5 We surmise that a similar arrangement may exist in the first extensor compartment and may help explain the severe pain that occurs in De Quervain’s from the mechanical constriction of the thumb extensor tendons.
Berger and Weinstein have shown that partial wrist denervation by ablation of the terminal portions of the anterior and posterior interosseous nerves (which supply pro prioceptive fibers to the wrist capsule) can be an effective treatment for a variety of chronic unreconstructable pathologies.6,7 Our surgical technique for De Quervain’s may provide pain relief through a similar denervation of the first extensor compartment. Additional support for the neurectomy hypothesis is found in the pattern of referred pain from the APL. It has been shown to resemble the C6, 7, and 8 dermatomes. This parallels the superficial radial sensory nerve distribution, and is very similar to the radiation of pain experienced in de Quervain’s tenosynovitis.8
The second goal is the reversal of increase in friction, which results in a restriction of tendon gliding. This is accomplished by release of the unyielding fascial compartment overlying the thumb extensor tendons. This fascial release facilitates tendon gliding by decreasing friction, allowing for a gradual reduction in tendon irritation. Over time, swelling decreases and the tissues recover. We postulate that the sheath will likely be eventually reinnervated, but only after the tendon pathology has resolved—which breaks the cycle of local nerve irritation.
1 Harvey FJ, Harvey PM, Horsely MW. De Quervain’s disease: Surgical or nonsurgical treatment. J Hand Surg. 1990;15A:83-87.
2 Arons MS. De Quervain’s release in working women: A report of failure, complications and associated diagnoses. J Hand Surg. 1987;12A:540-544.
3 Ta KT, Eidelmen D, Thomson JG. Patient satisfaction and outcomes of surgery for de Quervain’s tenosynovitis. J Hand Surg. 1999;24A:1071-1077.
4 White GM, Weiland AJ. Symptomatic palmar tendon subluxation after surgical release for de Quervain’s disease. J Hand Surg. 1984;9A:704-706.
5 Lin YT, Berger RA, Berger EJ, Tomita K, Jew JY, Yang C, et al. Nerve endings of the wrist joint: A preliminary report of the dorsal radiocarpal ligament. J Orthop Res. 2006;24(6):1225-1230.
6 Berger RA. Partial denervation of the wrist: A new approach. Tech Hand Up Extrem Surg. 1998;2(1):25-35.
7 Weinstein LP, Berger RA. Analgesic benefit, functional outcome, and patient satisfaction after partial wrist denervation. J Hand Surg [Am]. 2002;27(5):833-839.
8 Hwang M, Kang YK, Shin JY, Kim DH. Referred pain pattern of the abductor pollicis longus muscle. Am J Phys Med Rehabil. 2005;84(8):593-597.