Chapter 28 Endoscopic Palliation of Malignant Dysphagia and Esophageal Fistulas
![]() Video related to this chapter’s topics: Esophageal Stent Placement
Video related to this chapter’s topics: Esophageal Stent Placement
Introduction
Annually, cancer of the esophagus and gastroesophageal junction (GEJ) is diagnosed worldwide in more than 500,000 patients, which makes it the eighth most common malignancy and sixth most common cause of cancer mortality.1 It is difficult to determine the true incidence because cancer of the GEJ is classified sometimes as gastric cancer and sometimes as esophageal cancer. In clinical practice, this distinction is unimportant because the curative and palliative options for treatment are the same for both adenocarcinoma of the esophagus and adenocarcinoma of the GEJ.
Overall, cancer of the esophagus and GEJ has a poor prognosis with a 5-year survival rate of less than 20% in the Western world.2 This poor prognosis is at least partly due to the fact that more than 50% of patients with carcinoma of the esophagus or GEJ already have inoperable disease at presentation.3 Most of these patients require palliative treatment to relieve progressive dysphagia or to treat associated problems such as the presence of a fistula.
Epidemiology
Squamous Cell Carcinoma
The incidence of squamous cell carcinoma (SCC) varies from country to country; also, it may occur more often in certain regions within a country. About two-thirds of new cases of SCC are detected in China (47%) and Central Asia (19%); this is known as the Central Asia Esophageal Cancer Belt. The incidence of SCC in this area ranges from 19 per 100,000 in Azerbaijan to 340 per 100,000 in northern China. Other areas of relatively high risk are southern and eastern Africa, south central Asia, and (in men only) Japan. The incidence of SCC in Western Europe and the United States is much lower (i.e., 53 to 86 per 100,000). In Western countries, SCC of the esophagus is mainly found in older people with the highest incidence between 50 and 70 years of age. Esophageal cancer is more common in men in most areas—the sex ratio is 7 : 1 in Eastern Europe—although in the high-risk areas of Asia and Africa, the sex ratio is much closer to unity.1 The distribution between men and women is 3 : 1 to 4 : 1.3
Adenocarcinoma
Until about 1970, more than 90% of esophageal cancers were SCCs. However, population-based studies have shown a large increase in the incidence of adenocarcinoma of the esophagus and GEJ over the last 30 years in North America and Western Europe, especially among white men but also in white women to a lesser degree.4,5 In men, the incidence of adenocarcinoma of the esophagus and GEJ has surpassed SCC.6 In the United States, the annual rates of esophageal adenocarcinoma per 100,000 population are 7.8% for white men and 6.5% for women, increasing from 0.7 during 1974–1976 to 3.2 during 1992–1994, an increase of more than 350%.5 The same trend, although occurring less rapidly, has been reported in other areas, including Australia, New Zealand, and Western Europe.7
It is generally believed that the increase in esophageal adenocarcinoma is related to an increase in the incidence of Barrett’s esophagus. In a report from The Netherlands, van Soestal and colleagues8 found that the incidence of new diagnoses of Barrett’s esophagus increased from 14.3 per 100,000 person-years in 1997 to 23.1 per 100,000 person-years in 2002. The number of upper gastrointestinal endoscopies decreased from 7.2 per 1000 person-years to 5.7 per 1000 person-years over the same time period. The rate of detection of Barrett’s esophagus increased over the same years from 1.4 to 42.7 (16.5 if only cases with histologic confirmation were included) per 1000 endoscopic procedures.
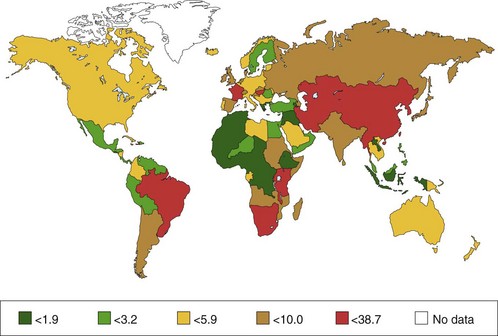
Multiple reports confirm that adenocarcinoma of the esophagus and GEJ occurs more frequently in white men. The distribution between men and women is 4 : 1. Most patients with esophageal adenocarcinoma are older individuals with a peak incidence around age 65 years.9 The worldwide distribution of esophageal cancer (for men) is shown in Fig. 28.1.
Pathogenesis
Squamous Cell Carcinoma
Smoking and Alcohol
The most important risk factors for SCC in Western Europe and the United States are smoking and alcohol intake. Risk of SCC is increased by a factor of 5 for moderate smokers and a factor of 10 for heavy smokers. It has been shown that alcohol intake and smoking are independent risk factors for the development of esophageal SCC.10
Food
In Hong Kong, a correlation has been established between the use of pickled vegetables and the development of SCC. This correlation was found to be caused by herbs that were used for these vegetables, which were often contaminated with toxic fungi.11
Other Factors
Prior radiation therapy has been associated with an increased risk of SCC. A study showed that patients who underwent radiation therapy for breast cancer more than 10 years ago had an increased risk of developing SCC in the esophagus.12
Hot drinks, particularly tea in certain areas in Asia, such as the Golestan province in northern Iran, are associated with an increased risk of developing SCC. The suggested mechanism is chronic irritation of the esophageal mucosa caused by the hot drinks.13
The role played by human papillomavirus (HPV) is unclear. In South Africa, where the incidence of SCC is high, HPV DNA was detected in more than 50% of cancers.14 In contrast, in the Netherlands, the presence of HPV in SCC is rare.15
Disorders Associated with Increased Risk of Squamous Cell Carcinoma
Achalasia
In a cohort study from Sweden, in which 1062 patients with achalasia were followed, the risk of SCC was increased by a factor of 16 after a follow-up of 9864 patient-years.16 Because most tumors were detected at an advanced stage, a curative resection was possible in only a few patients. Nonetheless, follow-up with endoscopic surveillance in patients with long-standing achalasia has been suggested. It needs to be determined whether this approach is cost-effective.
Caustic Ingestion
The incidence of esophageal SCC is increased by a factor of 1000 to 3000 in patients with a stricture in the esophagus caused by a caustic ingestion. The risk of developing a malignancy is probably highest after the ingestion of lye.17 The mean time between ingestion of a corrosive agent and the development of SCC is 30 to 40 years.
Head and Neck Cancer
SCC of the esophagus and the hypopharynx is associated with smoking and alcohol intake. Of patients with head and neck cancer, 1% to 8% also have esophageal cancer or develop it later on.18 The risk of esophageal cancer is increased by a factor of 3 to 10 in patients with head and neck cancer.
Adenocarcinoma
Gastroesophageal Reflux Disease
Lagergren and coworkers19 found a direct association between reflux and adenocarcinomas, rather than the presumed sequence of reflux disease leading to Barrett’s esophagus and this condition leading to adenocarcinoma. The esophageal adenocarcinoma risk was 7.7 times increased in individuals with heartburn and acid reflux occurring at least once a week. For people with severe symptoms for 20 years or longer, the risk was 43.5 times increased for esophageal adenocarcinoma but only 4.4 times increased for adenocarcinoma of the gastric cardia. There was no correlation with SCC.
Barrett’s Esophagus
Barrett’s esophagus is a disorder of the distal esophagus in which the squamous epithelium is replaced by metaplastic columnar epithelium. Barrett’s esophagus is a complication of long-standing gastroesophageal reflux disease.20 A causal relationship between Barrett’s esophagus and the development of esophageal adenocarcinoma has been established.
In older reports, the risk of esophageal adenocarcinoma in long-segment Barrett’s esophagus was 30 to 52 times greater than the normal population. Cancer was diagnosed at a median rate of about 1 per 100 patient-years of follow-up. These reports were often based on a short period of follow-up, however, with the possibility of including prevalent cancers as incidence cases, and may have overestimated the cancer risk. More recent reports with longer follow-up times found 1 cancer per 180 to 2200 patient-years of follow-up.21 The prevalence of Barrett’s esophagus in consecutive patients undergoing endoscopy for any clinical indication ranges from 0.3% to 2%.22 Several studies have shown that Barrett’s esophagus is a disorder of white patients and is mainly found in Western Europe. The distribution between men and women is 2.5 to 4 : 1.9
Clinical Features
Local effects of esophageal carcinoma include dysphagia, odynophagia, coughing, regurgitation, vomiting, or a vague discomfort in the back of the throat. At the time of diagnosis, tumor length is mostly more than 4 cm, and patients often already have 6 weeks to 4 months of dysphagia with accompanying substantial weight loss.23 Dysphagia is not diagnostic of an esophageal malignancy because nonmalignant diseases also manifest with dysphagia such as achalasia or peptic strictures caused by reflux esophagitis. In cases of rapidly progressive dysphagia and weight loss, however, the suspicion of a malignant tumor of the esophagus or GEJ is high. Dysphagia is a late symptom of an esophageal malignancy. Only when a mass lesion has come to a critical size does it impair the passage of food. At this time, the tumor has usually invaded the deeper layers of the esophageal wall, making the prognosis poor.
Patients with esophageal cancer may develop iron deficiency anemia. Bleeding from the tumor is usually a slow, occult process. Sometimes patients experience frank hemorrhage. Rarely, when the tumor invades the aorta or another major vessel, a patient may experience exsanguination, which is a frequent cause of death.23
Pathology
Squamous Cell Carcinoma
Of SCCs, 24% occur in the upper third, 47% occur in the middle third, and 29% occur in the lower third of the esophagus.24 It has been shown that SCC develops from low-grade or high-grade dysplasia to intraepithelial carcinoma and finally invasive esophageal carcinoma. Endoscopic follow-up in 327 Chinese patients with high-grade dysplasia showed that SCC was diagnosed at a median rate of 4 cases per 100 patient-years of follow-up.25 In the Western world, less than 10% of patients with SCC are diagnosed at an early stage.24
Adenocarcinoma
Adenocarcinomas of the esophagus and GEJ are located in the distal esophagus and proximal stomach. There is also clear evidence for a dysplasia-carcinoma sequence in Barrett’s esophagus, whereby Barrett’s esophagus without dysplasia progresses to low-grade dysplasia, high-grade dysplasia, and ultimately carcinoma.26 During a mean follow-up of 3 to 5.2 years, progression from Barrett’s esophagus without dysplasia to low-grade dysplasia occurred in 12% to 18% of patients, and progression from low-grade to high-grade dysplasia or adenocarcinoma occurred in 10% to 25% of patients.27,28 Progression from high-grade dysplasia to carcinoma occurs in 17% to 66% of patients over 0.75 to 9 years.29,30 The distribution of the grade of dysplasia in transversal studies of patients with Barrett’s esophagus is 80% no dysplasia, 18% low-grade dysplasia, and 2% high-grade dysplasia or adenocarcinoma.31,32
Given the dismal prognosis among patients with symptomatic esophageal cancer, guidelines from the American College of Gastroenterology33 recommend endoscopic surveillance of patients with Barrett’s esophagus in an attempt to prevent death from adenocarcinoma. Retrospective studies have found that patients whose esophageal adenocarcinoma was detected in a surveillance program presented at an earlier stage and had better 5-year survival rates than patients without surveillance who presented with cancer.34 A more recent study showed that less than 5% of patients who presented with esophageal adenocarcinoma actually underwent endoscopic surveillance.35
Esophageal cancer grows by intraesophageal spread, direct extension, and lymphatic and hematogenous metastases. The tumor typically invades adjacent structures, and lymph node metastases range from 40% to 70%. Because esophageal lymph node flow is bidirectional, sites of nodal metastases are many. Distant metastases, particularly to liver, lung, and bone, are present in 25% to 30% of patients at diagnosis.36
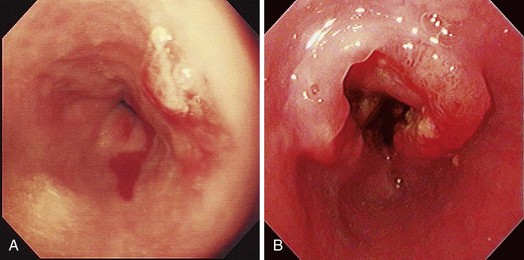
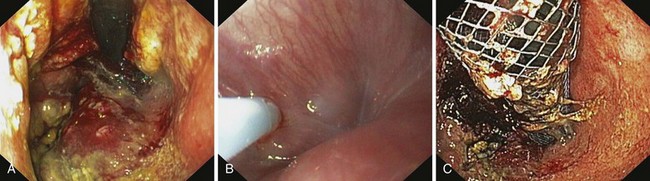
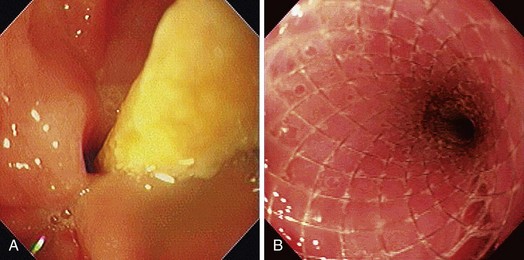
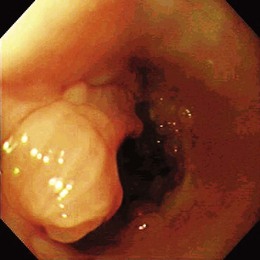
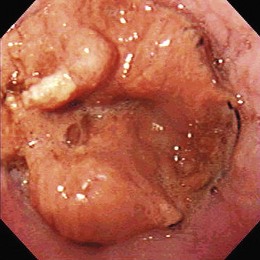
Early esophageal carcinomas are usually slightly elevated, coarse, or polypoid with denuded epithelium at endoscopy (Fig. 28.2A). The gross appearance of SCCs and adenocarcinomas is practically indistinguishable. Adenocarcinoma of the esophagus, especially in its early stage, can be distinguished from SCC by the presence of Barrett’s esophagus. If esophageal adenocarcinoma is advanced, however, it is often impossible to detect Barrett’s esophagus because the tumor has presumably overgrown its precursor. The macroscopic features of advanced esophageal cancers can be ulcerative, stenotic, polypoid, or a combination of these (Fig. 28.2B).
Treatment
The preferred treatment for esophageal cancer is surgical resection. Resection of the esophagus with a gastric pull-up or a colonic interposition is an invasive procedure, however, with significant morbidity and mortality.36 A discussion of the different surgical techniques, long-term results, and complications after surgery is beyond the scope of this chapter. In the past decade, endoscopic methods have been developed to remove early cancers in the esophagus nonsurgically. Indications and contraindications for endoscopic treatment of early esophageal cancer are discussed in Chapter 27.
Various palliative techniques are currently available (Table 28.1). The main options can be divided into nonendoscopic modalities, of which chemoradiation therapy is most commonly used, and endoscopic procedures, of which placement of a self-expanding metal stent to relieve obstruction resulting from a malignant stricture in the esophagus is the most frequently used technique. Some of the endoscopic procedures for palliation of malignant dysphagia are discussed.
| NONENDOSCOPIC TECHNIQUES |
Self-Expanding Metal Stents
Placement of a self-expanding metal or plastic stent is a frequently used method for palliation of malignant dysphagia. Since 1990, more than 130 studies have been published on the outcome of metal stent placement for palliation of malignant dysphagia and esophageal fistulas.37–39
Metal Stents versus Rigid Plastic Endoprosthetics
Several randomized trials have compared metal stents with prosthetic tubes.40–45 These studies have shown that placement of a metal stent is associated with fewer procedure-related complications than placement of a prosthetic tube.41,42,44,45 In one study, metal stents were also more effective in improving dysphagia.43 Studies on cost-effectiveness have shown that, despite the high initial purchase cost, metal stents were more cost-effective than prosthetic tubes because of a shorter hospital stay for procedures for stent-related complications.40,41,43,46
Covered versus Uncovered Metal Stents
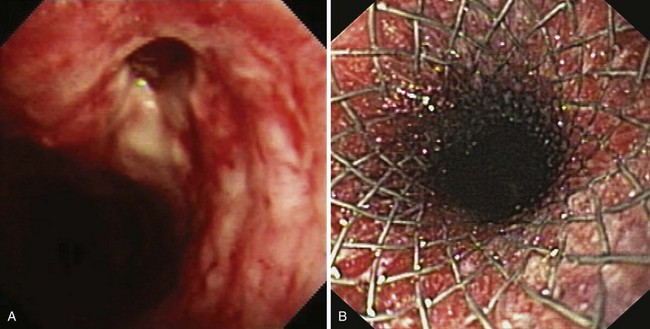
In a prospective randomized trial by Vakil and coworkers,47 covered and uncovered Ultraflex (Boston Scientific, Natick, MA) stents were compared in 62 patients with obstructing tumors at the GEJ (Figs. 28.3 and 28.4). Tumor ingrowth or overgrowth was significantly more common in the uncovered stent group (9 of 30 [30%]) than in the covered stent group (1 of 32 [3%]). Stent migration was not different between the two treatment groups (uncovered stent, 2 of 30 [7%], vs. covered stent, 4 of 32 [12%]). Covered stents apparently give better long-term palliation of malignant dysphagia than uncovered stents.
Currently Available Covered Metal Stents
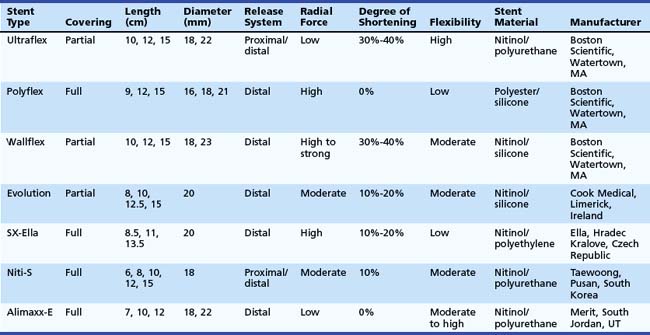
An ideal stent does not exist. However, all available covered metal stents meet some of these criteria (Table 28.2).
The Ultraflex stent consists of a knitted nitinol wire tube, and the covered version has a polyurethane layer that covers the midsection of the stent extending to within 1.5 cm of either end of the stent (see Figs. 28.3 and 28.4). The stent has a proximal flare with two sizes: 28 mm (distal diameter 23 mm) and 23 mm (distal diameter 18 mm). The Ultraflex stent has an easy-to-use delivery system of the stent and can be deployed gradually from the proximal to the distal end or vice versa. The degree of shortening after stent placement is 30% to 40%. The radial force of the Ultraflex stent is the lowest among currently available metal stents. Partial obstruction of the stent can occur in stents that are sharply angulated after passing across the GEJ.
The Polyflex stent (Boston Scientific) is a silicone device with an encapsulated monofilament braid made of polyester. The meshes are completely covered by a silicone layer with a smooth inner surface and a more structured outer surface (see Fig. 28.4). The edges of the monofilaments are protected with silicone to avoid impaction or tissue damage at the proximal and distal ends. The stent has a proximal flare of 25 mm, 23 mm, and 21 mm and body diameter of 21 mm, 18 mm, and 16 mm. It is available in three lengths: 9 cm, 12 cm, and 15 cm. The stent needs to be loaded in the introducer sheath before placement. This introduction device has a diameter of 14 mm, 13 mm, and 12 mm. This is the largest stent system compared with other systems and the system is rigid; investigators have suggested that the relatively high occurrence of perforations after Polyflex stent placement is at least partly due to these characteristics.48 In addition, the stent is less suitable for angulated strictures because the distal dilator is short. The inappropriate forced transmission of such an introduction sheath may complicate its passage across angulated strictures. Finally, because the stent has only a mesh on the outside and no other antimigration properties, it is associated with an increased risk of stent migration.48 The Polyflex stent is more frequently used for benign strictures in the esophagus than for malignant strictures.49
The Wallflex stent (Boston Scientific) consists of a wire braided construction and is partially covered with nitinol extending to within 1.5 cm of either end of the stent (see Fig. 28.4). The stent has a proximal and distal flare with two sizes: 28 mm (body diameter 23 mm) and 23 mm (body diameter 18 mm). The degree of shortening after Wallflex stent placement is considerable (i.e., 30% to 40%). The radial force of the Wallflex stent is one of the highest among currently available metal stents. Clinical experience is limited to one prospective follow-up study showing favorable results with the partially covered Wallflex stent.50
The Evolution stent (Cook, Limerick, Ireland) is constructed of a single woven nitinol wire. The stent has an internal and external silicone coating and uncoated flanges on both ends (see Fig. 28.4). The body diameter of the stent is 20 mm, and the flange diameter is 25 mm on both ends. The stent is available in four lengths: 8 cm, 10 cm, 12.5 cm, and 15 cm. The stent slightly foreshortens because of its design. A pistol-grip delivery system handle allows step-by-step stent deployment or recapturing. Initial studies have shown that this stent type is safe and effective in treating malignant esophageal strictures.51
The SX-Ella stent (Ella, Hradec Kralove, Czech Republic) is made of nitinol and a single braided wire. To decrease the risk of migration, the SX-Ella stent has a flip-flop type of antimigration ring that is circumferentially attached to the proximal stent portion (see Fig. 28.4). This ring functions as a circular hook that prevents migration; however, the ring is flexible and everts when the traction force is too strong. The stent flares to 25 mm at its proximal and distal ends with a body diameter of 20 mm. It is available in lengths of 85 mm, 110 mm, and 135 mm. Initial studies showed that the antimigration ring did not reduce stent migration, but the stent was associated with an increased risk of hemorrhage. In addition to hemorrhage, severe pain and fistula formation at the upper end of the SX-Ella stent were frequently observed. In normal circumstances, stents exert some pressure on the tumor and the normal mucosa of the esophagus to fixate the stent to the esophageal wall to reduce migration risk. In case of the SX-Ella stent, this effect may be more pronounced because of the pressure effect of the antimigration ring, particularly when it is flipping in and out for its antimigration effect.52
The Niti-S stent (Taewoong, Pusan, South Korea) has a double-layer configuration over its entire length, consisting of an inner polyurethane layer and an outer uncovered nitinol wire (see Fig. 28.4). This double layer was designed to reduce the risk of stent migration. The stent flares to 26 mm at its proximal and distal ends and has a body diameter of 18 mm. It is available in five lengths: 6 cm, 8 cm, 10 cm, 12 cm, and 15 cm. It has both a proximal and a distal release system.48,53
The Alimaxx-E stent (Merit, South Jordan, UT) is also made of nitinol, and it is fully covered with polyurethane to resist tissue ingrowth (see Fig. 28.4). This stent has a proximal flare of 27 mm and 23 mm, a luminal diameter of 22 mm and 18 mm, and a distal flare of 25 mm and 21 mm. The outward force of the stent is most pronounced at the body. The stent also has a pistol type of release system, similar to the Evolution stent (see earlier). In the initial versions of this stent, it could be introduced over a guidewire (Alimaxx-E GW system) but also under direct vision using a delivery system in which the delivery catheter fitted over a small-caliber endoscope (Alimaxx-E DV system). The latter system featured a window at the end of the introduction sheath to view stent deployment. The size of the introduction catheter of the Alimaxx-E DV was 30 Fr, whereas the size of the introduction catheter of the Alimaxx-E GW was 22 Fr. Because the DV system was difficult to manipulate owing to its size and stiffness, and perforations were noted with this system, this system was withdrawn from the market. Another issue was that the initial version of the Alimaxx-E stent had 20 antimigration struts to prevent stent migration; this was increased to 45 antimigration struts, however, because the migration rate was more than 30% with the original stent design.54
Comparison of Different Types of Metal Stents
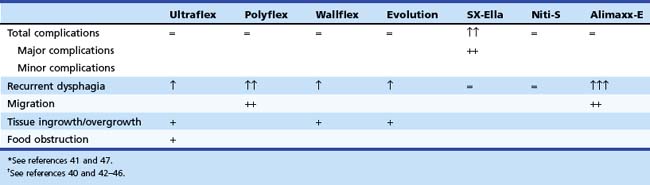
Four prospective randomized trials48,55–57 have compared the outcome of currently available stent designs of different types of metal stents. In one study, 101 patients with unresectable esophageal carcinoma were randomly assigned to placement of a Polyflex (n = 47) or a partially covered Ultraflex (n = 54) stent.55 Patients with GEJ malignancy were excluded. Placement was equally successful with both stent designs: A Polyflex stent was placed in 46 (98%) patients, and an Ultraflex stent was placed in 54 (100%) patients. There were no significant differences in dysphagia improvement between the two stent designs (after 1 week improvement by at least one grade in 100% of the Polyflex group and in 94% of the Ultraflex group). Major complications were observed in 48% of the Polyflex group and in 33% of the Ultraflex group. Intraprocedural perforation occurred in one Polyflex patient and one Ultraflex patient. Two Polyflex patients had postprocedural hemorrhage. Recurrent dysphagia occurred in 20 (44%) patients with a Polyflex stent and 18 (33%) patients with an Ultraflex stent because of tumor overgrowth, stent migration, hyperplastic granulomatous reaction, or food bolus impaction. Multivariate analysis showed a significantly higher complication rate with Polyflex stents than with Ultraflex stents (odds ratio 2.3, 95% confidence interval 1.2 to 4.4). Median survival was similar: 134 days with Polyflex stents and 122 days with Ultraflex stents. The authors concluded that palliation of dysphagia was not different between the two stents. Significantly more complications, especially late stent migration, were observed in the Polyflex group.
In another prospective study, 125 patients with dysphagia from inoperable carcinoma of the esophagus or gastric cardia were randomly assigned to placement of an Ultraflex stent (n = 42), Polyflex stent (n = 41), or Niti-S stent (n = 42).48 Stent placement was technically successful in all patients with an Ultraflex stent, in 34 of 41 (83%) patients with a Polyflex stent, and in 40 of 42 (95%) patients treated with a Niti-S stent (P = .008). The dysphagia score improved in all patients. There were no differences in major complications among the three stent types. Recurrent dysphagia, caused by tissue ingrowth or overgrowth, migration, or food obstruction, was significantly different between patients with an Ultraflex stent and patients with a Polyflex stent or Niti-S stent (22 [52%] vs. 15 [37%] vs. 13 [31%]; P = .03). Stent migration occurred more frequently with Polyflex stents, whereas tissue ingrowth or overgrowth was seen more frequently with partially covered Ultraflex stents and, to a lesser degree, Niti-S stents. No differences were found in survival (median survival Ultraflex stent, 132 days, vs. Polyflex stent, 102 days, vs. Niti-S stent, 159 days) among the three stent types. It was concluded that all three stents were safe and offered adequate palliation of dysphagia from esophageal or gastric cardia cancer. Nonetheless, Polyflex stents seemed the least preferable stent type in this patient group because placement of this device is technically demanding and associated with a high rate of stent migrations.
In a prospective trial, 100 patients were randomly assigned to one of three types of covered metal stents: the Ultraflex stent, the Flamingo Wallstent, and the Z-stent.56 There were no significant differences in dysphagia improvement and the occurrence of complications or recurrent dysphagia, although there was a trend toward more complications with the Z-stent (Ultraflex stent, 8 of 34 [24%]; Flamingo Wallstent, 6 of 33 [18%]; and Z-stent, 12 of 33 [36%]; P = .23). In another prospective trial, the Ultraflex stent and the Flamingo Wallstent were compared in patients with distal esophageal cancer.57 The two stent types were equally effective in the palliation of dysphagia in this patient group, and the complication rate associated with their use was comparable (Ultraflex stent, 7 of 31 [23%], and Flamingo Wallstent, 5 of 22 [23%]).
From these data, it can be concluded that there are only minor differences between the most commonly used stent types. The choice of stent in patients with a malignant stricture in the esophagus or gastric cardia should be determined by the location and the anatomy of the malignant stricture on the one hand and the specific characteristics of the stent on the other hand (Table 28.3; see Table 28.2). Based on the currently available results, caution is needed when using Polyflex stents (migration, robust introduction device), SX-Ella stents (complications owing to the antimigration ring), or Alimaxx-E stents (migration) for this indication.
Metal Stents for Tumors of the Gastroesophageal Junction
Because the incidence of adenocarcinoma of the distal esophagus is increasing rapidly,4,5 the deployment of metal stents across the GEJ is likely to increase. However, stent placement for tumors of the distal esophagus and GEJ constitutes a particular problem. Compared with stents placed for more proximally located esophageal tumors, these procedures provide inferior palliation and have higher complication rates.58 Migration is more likely with stents placed across the GEJ than with stents placed for more proximally located tumors because the distal part of the stent projects freely into the fundus of the stomach, and this part cannot fix itself to the wall.
How can migration of stents be prevented? Following are some considerations:
Table 28.4 Gastroesophageal Reflux (Symptoms) after Placement of an Antireflux Stent for Palliation of Malignant Dysphagia: Summary of Published Studies
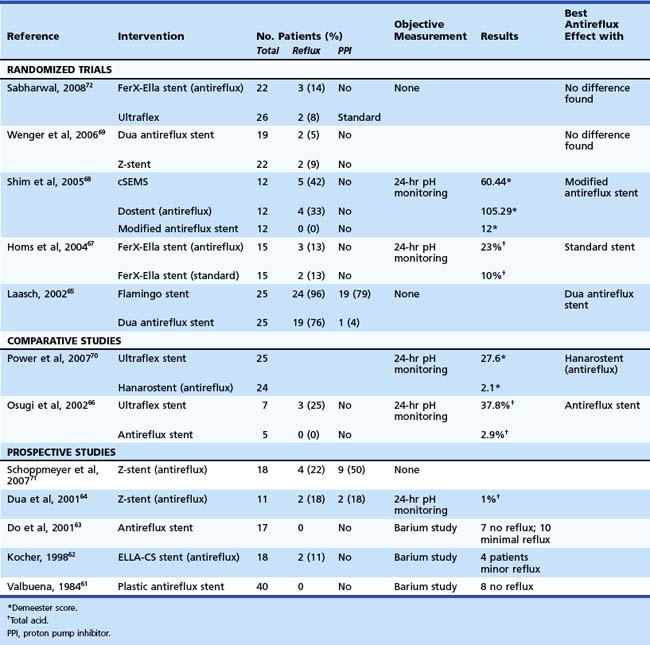
Five randomized trials comparing an antireflux stent with a standard stent have been published with conflicting results (see Table 28.4).65,67–69,72 In the only study in which the standard stent was combined with a PPI, no differences in reflux symptoms were noted. It still remains to be established whether stents with an antireflux mechanism are able to prevent the reflux of gastric fluid into the esophagus; this should be established in randomized trials measuring symptoms but also using objective measures such as pH/impedance measurement to demonstrate effectiveness.
Stent Placement Procedure
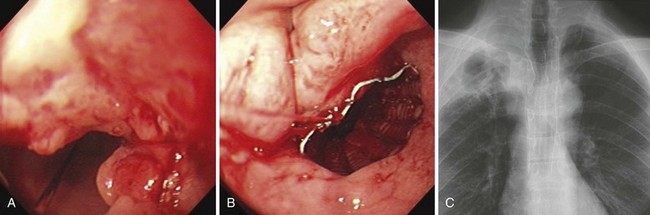
Placement of metal stents is usually done with the patient under sedation (see Vid![]() eo). The first step is to inspect the tumor for its characteristics (severity of stenosis, length, extension into the stomach) (Fig. 28.6A). When fluoroscopy is used, the proximal and distal margins of the stricture are demarcated endoscopically by skin markers, tissue clips, or intramucosal injection of a radiopaque contrast agent (Fig. 28.6B). Injection of the lipid-soluble contrast agent lipiodol results in a persistent mark. Accurate placement of the Ultraflex stent is also possible under endoscopic guidance without the aid of fluoroscopy. An external marker is applied at the level of the proximal radiopaque marker on the stent, allowing the stent to be placed under direct endoscopic visualization.73 In addition, some stents have markers on the introduction system that reliably aid in stent placement. The distance on the introduction catheter at the level of the incisors corresponds with the upper end of the stent. This technique is at present possible only with the Ultraflex stent and the SX-Ella stent. Finally, metal stents also can be placed under fluoroscopic guidance only, without the use of endoscopy.74
eo). The first step is to inspect the tumor for its characteristics (severity of stenosis, length, extension into the stomach) (Fig. 28.6A). When fluoroscopy is used, the proximal and distal margins of the stricture are demarcated endoscopically by skin markers, tissue clips, or intramucosal injection of a radiopaque contrast agent (Fig. 28.6B). Injection of the lipid-soluble contrast agent lipiodol results in a persistent mark. Accurate placement of the Ultraflex stent is also possible under endoscopic guidance without the aid of fluoroscopy. An external marker is applied at the level of the proximal radiopaque marker on the stent, allowing the stent to be placed under direct endoscopic visualization.73 In addition, some stents have markers on the introduction system that reliably aid in stent placement. The distance on the introduction catheter at the level of the incisors corresponds with the upper end of the stent. This technique is at present possible only with the Ultraflex stent and the SX-Ella stent. Finally, metal stents also can be placed under fluoroscopic guidance only, without the use of endoscopy.74
A premounted stent or a stent that has been advanced into the introduction system is advanced over the guidewire and deployed. It is reasonable to confirm endoscopically that the upper end of the stent is in place proximal to the upper tumor margin. However, the endoscope should be passed through the stent only if no friction with the endoscope is felt to avoid dislodgment of the stent (Fig. 28.6C).
Except for the Polyflex stent and the Alimaxx-E stent, all stents shorten to some extent. Both the Ultraflex stent and the Wallstent shorten during expansion (see Table 28.2), which must be taken into consideration when positioning the introduction system. To prevent migration of the stent on release from the introduction system, the system should not be advanced too far distally. An advantage of the Wallflex stent and the Evolution stent is that they can be recaptured (if not expanded >50%) by advancing the constraining sheath and repositioning the entire stent. The stent should be 2 to 4 cm longer than the stricture to allow for a 1- to 2-cm extension above and below the proximal and distal tumor margins.
Variations and Unusual Situations
Fistula Formation
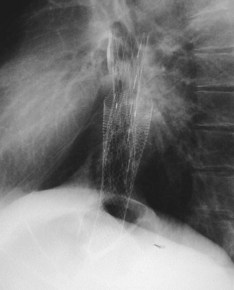
Progressive esophageal carcinoma can infiltrate into surrounding tissue with subsequent development of a fistula, most commonly between the esophagus and the respiratory tract (i.e., the trachea or bronchi) and occasionally between the aorta, the mediastinum, or pleura. In a series of 1943 patients with esophageal cancer, it was found that 5% of patients developed a fistula over time. In the same publication, it was reported that 0.2% of 5714 patients with bronchogenic carcinoma developed an esophagorespiratory fistula.75 Fistulas may also develop secondary to radiation or laser therapy or both. Finally, pressure necrosis caused by the proximal edge of a previously placed metal stent sporadically results in the development of a fistula. Treatment of a fistula should be expeditious because fistula formation is a life-threatening complication, which in the case of esophagorespiratory fistulas can result in serious pulmonary infections from aspiration pneumonia.
Esophagorespiratory Fistulas
A history of repeated coughing associated with drinking, eating, or both in combination with worsening dysphagia and dyspnea is highly suggestive of an esophagorespiratory fistula. The fistula can be diagnosed radiographically or endoscopically. Curative resection is usually impossible because of the concomitant presence of an advanced tumor stage; palliative surgery (including a combination of cervical esophagostomy and feeding gastrostomy or a bypass operation) is associated with a mortality rate of up to 50%.76
Until the early 1990s, cuffed prosthetic tubes were used to seal esophagorespiratory fistulas.77 These devices were effective in 60% to 90% of patients. However, cuffed endoprostheses were associated with a complication rate that was comparable to conventional prosthetic tubes. An additional disadvantage of these devices was that the cuff of the tube migrated through the lumen of the fistula into the bronchial lumen in 25% of patients, causing acute respiratory distress.78
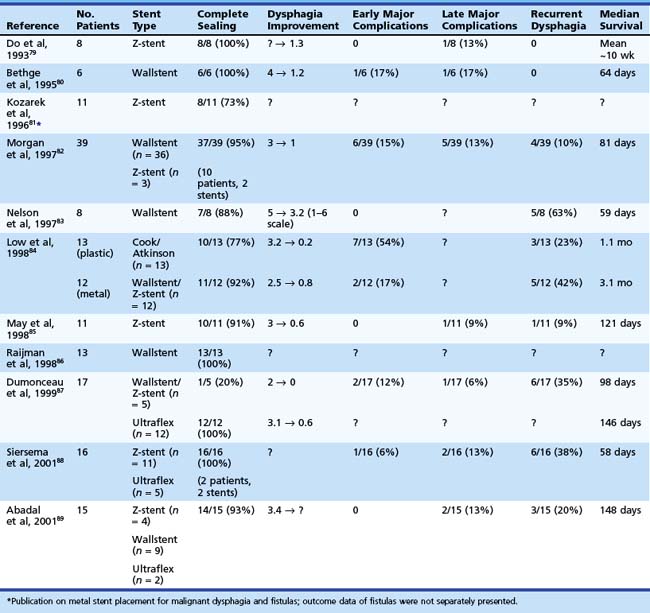
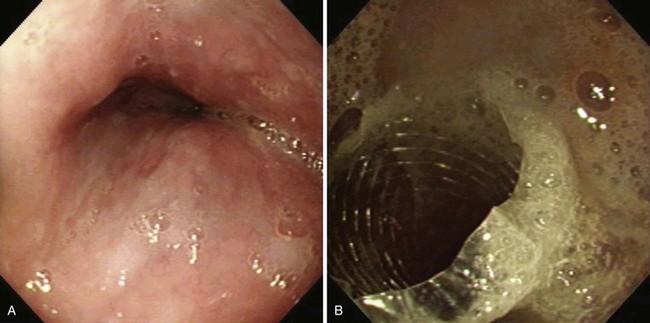
At the present time, endoscopic placement of a covered self-expanding metal stent is the treatment of choice for an esophagorespiratory fistula (Figs. 28.7 and 28.8). Several retrospective and prospective series have been published reporting the outcome of endoscopic placement of a covered metal stent for this indication (Table 28.5).79–89 In most of these publications, complete sealing of the fistula was established in more than 90% of patients with no clear difference between the presently available covered metal stents. Dysphagia scores improved significantly in most patients. The complication rate (early and late complications) ranged from 10% to 30%, whereas recurrent dysphagia was mainly the result of tumor overgrowth or stent migration. The median survival was poor and ranged from 35 to 148 days, which likely reflects the advanced tumor stage in most of these patients.
Parallel Stent Placement
In some patients with esophageal cancers that infiltrate into the trachea, dysphagia and dyspnea may develop simultaneously. In some cases, placement of a metal stent in the esophagus to seal a fistula can result in obstruction of the trachea and acute dyspnea. In these circumstances, it is important to consider the placement of a stent in the trachea or bronchi or both in combination with an esophageal stent. Tracheobronchial stents that are placed in the trachea used to be uncovered90 but presently at least partially or fully embed themselves in the mucosa of the respiratory tract.91 These stents are uncovered because this decreases the risk that the stent will migrate distally leading to acute respiratory distress. Another indication for parallel stent placement is a tracheoesophageal fistula near the upper esophageal sphincter. Esophageal stents alone are not always effective in sealing the fistula at this location. Placement of a covered stent in the proximal part of the trachea in addition to a proximal esophageal stent may be considered.79 Complications occur more commonly with parallel stent placement. Fatal complications such as perforation and bleeding have been described, caused by tissue necrosis resulting from the high radial force exercised by both stents.92
Proximal Esophageal Carcinoma
Endoscopic intubation has traditionally been contraindicated for malignancies within a few centimeters below the upper esophageal sphincter because of the risk of foreign body sensation, tracheal compression, and proximal migration of the prosthesis into the hypopharynx. However, positive results have been reported in studies on palliation of proximal lesions with metal stents.59.88,93–96 The results are summarized in Table 28.6. The largest study comes from our institution. We evaluated 104 patients with dysphagia from a malignant stricture close to the upper esophageal sphincter.96 Patients had primary esophageal carcinoma (n = 66) or recurrent cancer after gastric tube interposition (n = 38) within 8 cm distal of the upper esophageal sphincter. A tracheoesophageal fistula was also present in 24 (23%) patients. The mean distance from the upper esophageal sphincter to the upper tumor margin was 4.9 ± 2.6 cm and to the upper stent margin was 3.1 ± 2.3 cm.
Table 28.6 Placement of a Metal Stent for Proximal Esophageal Carcinoma: Summary of Published Studies
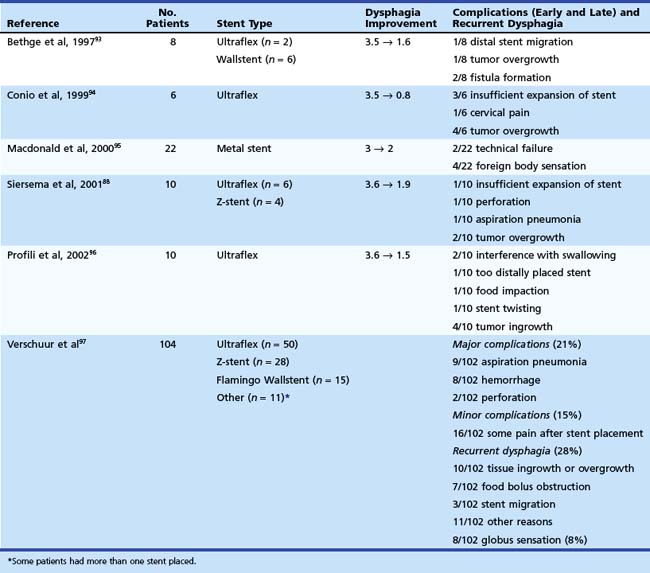
Stent characteristics are an important consideration in the treatment of cancers that are located in the cervical esophagus. First, in our opinion, metal stents should not shorten or minimally shorten to ensure exact placement just below the upper esophageal sphincter. Second, the stent should be covered to prevent tumor ingrowth and to seal any coexisting fistula. Finally, the stent should have a body diameter of 18 mm or less, and, most important, the stent should be flexible to avoid globus sensation and tracheal compression. Although such a stent is unavailable, the Ultraflex stent and the Alimaxx-E are probably the stent types that are most preferable in this situation (see Fig. 28.8). Other methods for the palliation of malignant strictures near the upper esophageal sphincter include radiation therapy with or without chemotherapy if the patient is fit enough to undergo a more intensive treatment or laser treatment. In case of failure of these treatments or a poor general condition of the patient, a nasoduodenal feeding tube and placement of a percutaneous endoscopic gastrostomy (PEG) are the safest options.
Extrinsic Compression
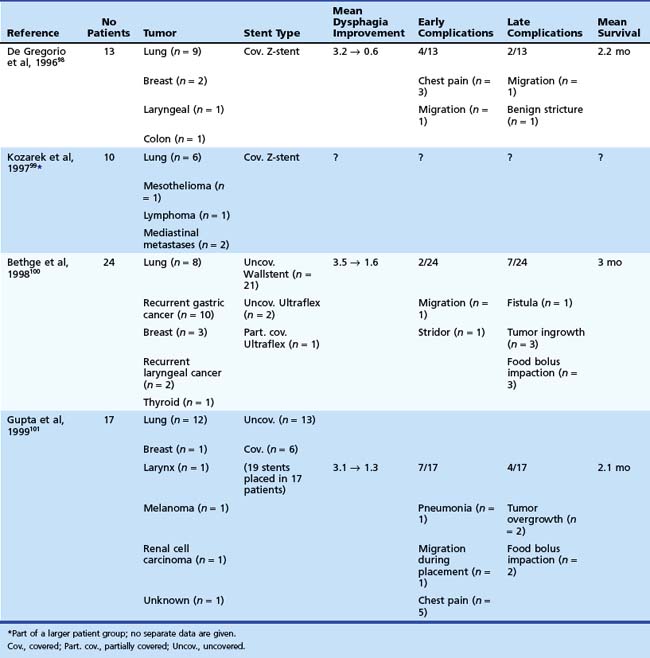
The experience with metal stents for palliation of dysphagia resulting from extrinsic compression is limited (Table 28.7).98–101 In a nonrandomized study, the safety and efficacy of stents for extrinsic compression (n = 24) were compared with stents for primary esophageal malignancies (n = 21).100 Dysphagia scores improved significantly in both groups; however, the improvement was significantly greater in patients with primary esophageal tumors (P = .012). The frequency of complications was comparable between both groups. Two other studies described the results of metal stent placement for extrinsic compression in 13 patients and 17 patients.98,101 These studies both concluded that dysphagia caused by extraesophageal malignancies can safely and effectively be treated with metal stents. In addition, there is no evidence that uncovered metal stents give better results than covered stents (Figs. 28.9 and 28.10). A characteristic of extrinsic compression is that the lesion is often irregular and noncircumferential. Because these tumors are often solid, stent deployment sometimes may take more than 24 to 48 hours. In addition, there is no tumor tissue in the esophageal lumen to fix the stent. Despite these unfavorable tumor characteristics, stent migration rate in these studies was comparable to that of stents for primary esophageal carcinoma. With regard to stent choice, there is a preference for flexible stent types, such as the Ultraflex stent and Alimaxx-E stent, which both tend to adjust to the asymmetric anatomy of the esophagus in case of extrinsic compression.
Table 28.7 Metal Stent Placement for Palliation of Dysphagia Caused by Extrinsic Compression of Extraesophageal Malignancies: Summary of Published Studies
Recurrent Dysphagia after Previous Surgery
Surgery is generally considered to offer the best chance for cure in patients with esophageal carcinoma; however, locoregional or systemic tumor recurrence occurs often in these patients. A few studies have been published in which metal stents were used to improve dysphagia resulting from tumor recurrence after esophagectomy.88,102–104 In our institution, 21 patients with recurrent tumor after esophagectomy were treated; the tumor was located in 10 patients in the proximal part of the gastric tube interposition (including the anastomosis) and in 11 patients in the midportion or distal portion of the interposition. In most of these patients, a large-diameter metal stent was used to cover the dilated lumen of the neo-esophagus effectively (see Fig. 28.10). Dysphagia improved from a mean of 3.2 (able to drink fluids only) to 1.5 (dysphagia for some solids), and median survival was 63 days. Major complications occurred in 4 of 21 (19%) patients, consisting of bleeding (n = 2), fistula formation (n = 1), and severe pain (n = 1). Recurrent dysphagia occurred in 8 of 21 (38%) patients and was due to tumor overgrowth.88 In case of recurrent cancer in a gastric tube interposition, it is preferred to use a large-diameter stent because the lumen above the stricture is often dilated owing to the gastric tube.
Dysphagia caused by recurrent tumor after partial or total gastrectomy presents specific problems because there is often complete luminal obstruction or sharp angulation of the luminal axis or both. We treated 10 patients with recurrent carcinoma after partial (n = 4) or total (n = 6) gastrectomy with a small-diameter Ultraflex stent or Z-stent. Dysphagia improved substantially, and median survival was 64 days. Complications occurred in 5 of 10 patients and consisted of perforation (n = 1), bleeding (n = 1), and pain (n = 3).88 Pain after stent placement generally is a problem following stent placement in a stenotic gastrojejunostomy. For that reason, we prefer stents with a low to moderate radial force (i.e., Ultraflex, Alimaxx-E, Niti-S, or Evolution). Although experience is limited, metal stent placement can be used for palliation of dysphagia from tumor recurrence after esophagectomy or gastrectomy.
Effect of Prior Radiation and Chemotherapy on Outcome of Stent Placement
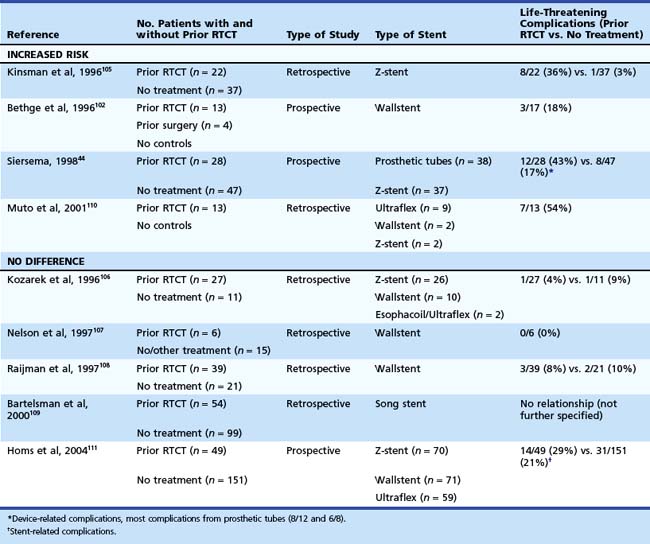
It has been suggested that prior radiation or chemotherapy or both increase the risk of complications after placement of a self-expanding metal stent in patients with inoperable cancer of the esophagus and GEJ; however, this relationship is controversial. Nine studies addressed this question.44,102,105–111 Four studies showed an increased risk for the development of complications after prior chemoradiation therapy,44,102,105–110 and five studies did not find such a relationship.106–109111 The results of these studies are summarized in Table 28.8. In a study with 200 prospectively followed patients from our institution, it was concluded that the incidence of complications and the outcome after placement of a self-expanding metal stent for carcinoma of the esophagus and GEJ were not affected by prior radiation or chemotherapy or both. Only retrosternal pain occurred more frequently in patients who had undergone prior chemoradiation.111 Stent placement in patients with prior radiation or chemotherapy or both is probably as safe as in patients who have not undergone prior treatment; however, patients should be informed that there is an increased risk of chest pain after stent placement.
Limitations and Success Rate
The technical success rate for placement of metal stents is close to 100%. Limitations to successful placement include severe pain during placement; extensive tumor growth in the stomach; failure of the stent to release from the introduction system, as can occur with Ultraflex stents; and immediate stent migration because the stent has been placed too deeply. Almost all patients experience improvement of dysphagia, and this is sustained unless and until a specific complication arises. The dysphagia grade usually improves from a mean of 3 (able to drink liquids only) to a mean of 1 (able to eat most solid foods), with no difference in effectiveness between the different available stent types (see Table 28.2), including the Ultraflex, the Wallstent, and the Z-stent. Some patients with advanced cancer at the distal esophagus or gastric cardia do not experience relief of dysphagia after technically successful stent placement because of other (unidentified) sites of intestinal obstruction, often peritoneal carcinomatosis, or gastric paresis resulting from neural involvement by the tumor. These patients usually require feeding through a nasoenteral tube or, preferably, a PEG.
Complications and Recurrent Dysphagia
Retrosternal Pain
Transient retrosternal pain is a frequently reported complication after stent placement, particularly after prior radiation or chemotherapy or both. Golder and coworkers112 recorded the daily opioid analgesic requirements of 52 patients from 3 days before until 7 days after stent placement. Of patients, 26 (50%) needed opioid analgesia for chest pain within 48 hours of the procedure compared with 11 (21.2%) before stent placement. This difference was statistically significant (P < .001). In other studies, figures ranging from 5% to 50% for chest pain after stent placement have been reported.* In our experience, mild retrosternal pain after stent placement can be treated effectively with acetaminophen or a nonsteroidal anti-inflammatory drug. Rarely, new opioid analgesics are indicated for a few days to a maximum of 14 days. Severe pain after stent placement occurs in 1% to 2% of patients. In these patients, removal of the stent is sometimes indicated to relieve the pain.
Gastroesophageal Reflux
Gastroesophageal reflux is a common problem, occurring in 10% to 20% of patients with distally located tumors where the distal end of the stent is placed through the lower esophageal sphincter.† The management of gastroesophageal reflux has already been discussed in the section on metal stents for tumors of the GEJ. As a preventive measure, PPIs are prescribed to many patients with a stent passing the lower esophageal sphincter. Metal stents with an antireflux mechanism have been developed to prevent gastroesophageal reflux. At the distal end of the stent, the cover of the stent is extended beyond the lower metal cage to form a windsock-type valve (see Fig. 28.5).
Stent Migration
Stent migration is a common complication with reported incidence rates ranging from 5% to 15%.* The most frequently used method for reintervention after stent migration is placement of a second stent. In certain cases, repositioning of a distally migrated stent is possible with the use of a forceps or a snare116 or by placing the endoscope in a retroflexed position.117,118 We do not recommend using the latter technique because esophageal perforation may occur. In addition, this method may result in damage to the endoscope.118 If repeated episodes of stent migration occur in the same patient, other palliative treatments, such as brachytherapy, photodynamic therapy (PDT), argon plasma coagulation therapy, cryoablation therapy, or laser therapy, need to be considered.
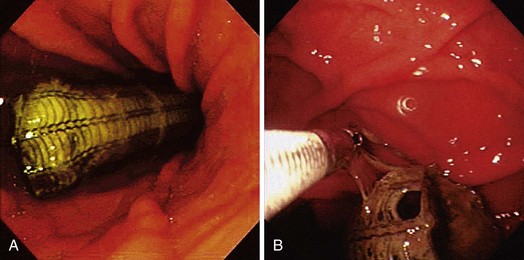
Based on our own experience and that of others,115 retrieval after migration is often not indicated because perforation or obstruction of the digestive tract is uncommon. If a migrated stent causes obstruction of the pylorus or symptoms of pain or if successful placement of a second stent is impossible, the stent should be removed. Several methods of stent retrieval have been described. In case of an Ultraflex stent with a lasso at the proximal end, this can be done by collapsing the stent with a grasping forceps using the lasso suture attached to the proximal flange of the stent (Fig. 28.11). The most frequently used described method is by decreasing the diameter of the stent with a polypectomy snare at 2 to 5 cm from the proximal end of the stent.109,120 Other investigators have used a biopsy forceps in combination with a snare, which requires passage of a double-channel therapeutic endoscope.121 Apart from a snare, one can use Endoloops, which may have a greater constriction force than a polypectomy snare.122
Tumor Ingrowth or Overgrowth
Tumor ingrowth or overgrowth is often the result of progression of the malignancy rather than a failure or a complication of the stent. It affects both ends of the stent at a similar rate and is seen in 10% to 20% of patients after a mean period of 2 to 4 months after stent placement (Fig. 28.12).* Tumor ingrowth or overgrowth can be reduced by inserting a stent that is, after expansion, approximately 2 to 4 cm longer than the malignant stricture to allow for a 1- to 2-cm extension above the proximal and below the distal end of the tumor.
The most frequently used method to treat tumor overgrowth is placement of a second stent. In addition, laser therapy or argon plasma coagulation can be used to debulk the tumor. In case of placement of a second stent, the stent is placed proximal or distal to the previously placed stent with a part of the second stent overlapping the primary stent (Fig. 28.13).
In our experience, another important cause of recurrent dysphagia is nonmalignant obstructive tissue, such as granulation tissue, reactive hyperplasia, and fibrosis at the proximal or distal end of the stent. The pathogenesis of nonmalignant tissue ingrowth or overgrowth has already been discussed in the section on metal stents for tumors of the GEJ, and nonmalignant tissue ingrowth or overgrowth is an unlikely event. Mayoral and colleagues60 reported this cause of recurrent dysphagia in more than 30% of their patients at a mean interval of 22 weeks after stent placement. We observed the development of this nonmalignant tissue in many patients undergoing endoscopy for reasons other than recurrent dysphagia. It was predominantly found at the proximal end of the stent but did not seem to cause dysphagia. It can also be treated by placing a second, partially overlapping stent.
Other Causes of Recurrent Dysphagia
Food bolus obstruction is common with reported rates of 5% to 15% (Fig. 28.14).* It can be treated successfully by endoscopic stent clearance. However, care should be taken to prevent the stent from migrating while doing this. Prevention of food bolus obstruction can be achieved by providing eating instruction, including chewing the food thoroughly and drinking carbonated drinks during and after a meal.
Laser Therapy
Thermal Therapy
Treatment of obstructing esophageal cancer with the high-power neodymium:yttrium-aluminum-garnet (Nd:YAG) laser was first described in 1982.123 Over the years, the procedure has become an accepted and effective method for palliation of malignant dysphagia. Early investigators used an anterograde technique; a retrograde approach with initial dilation is preferred at the present time.124 Tumors that are relatively short (<6 cm), nonangulated, exophytic, noncircumferential, and located in the midesophagus or distal esophagus are most amenable to laser ablation. Laser treatment is unsafe for submucosal tumors, tumors causing extrinsic compression, and angulated tumors, whereas circumferential tumors are vulnerable to stricture formation. In various studies, technical success was around 90%, and functional success was approximately 70%.125–129 Depending on the length of follow-up, recurrent dysphagia occurs in 40% to 60% of patients 4 to 10 weeks after initial treatment. Patients are usually reassessed at 4- to 6-week intervals. Complications include perforation, fistula formation, hemorrhage, and sepsis in 5% to 10% of patients.
Photodynamic Therapy
PDT involves the local destruction of tumor tissue by light of a specific wavelength activating a previously administered photosensitizer that is retained in malignant tissue. Porphyrin compounds, such as porfimer sodium (Photofrin), have been the most commonly used photosensitizers for the palliation of malignant dysphagia. As opposed to the thermal destruction induced by the Nd:YAG laser, the damage by PDT is initiated by a photochemical effect. PDT may be useful for long tumors, for tumors that are narrow or angulated, and for flat infiltrating tumors. The costs of PDT are high because of the high costs of a special laser unit and the costs of the photosensitizer Photofrin.130
The most frequent complication of PDT is prolonged skin photosensitivity. Patients must avoid direct sunlight for at least 6 weeks after treatment. Major complications, including perforation, fistula formation, and strictures, have been reported in 30% of patients. Other side effects include fever, chest pain, and pleural effusion, probably secondary to a transient, local inflammation, but these side effects are usually mild.131–135
Clinical experience with PDT for palliation of malignant dysphagia is limited to a few centers worldwide. One or two treatment sessions are usually required for an adequate tumor response. PDT is considered technically easier and less operator-dependent than Nd:YAG laser ablation. Two randomized trials compared PDT with Nd:YAG laser therapy.134,135 Lightdale and coworkers134 found equivalent improvements in dysphagia score with a trend toward an improved response with PDT in patients with tumors located in the upper third or lower third of the esophagus, in patients with long (>10 cm) tumors, and in patients who had prior chemotherapy or radiation therapy or both. Complications were similar in both treatment groups, but side effects (i.e., skin photosensitivity, nausea, and transient fever) occurred more often after PDT. Heier and colleagues135 found an improved performance status and a longer duration of response (84 days vs. 57 days) after PDT compared with laser therapy, with similar functional results and complication rates. Because of the high costs of the treatment, the side effects, and the necessity of repeated treatments every 8 weeks, PDT as a sole treatment is not an optimal treatment for palliation of malignant dysphagia.
Laser Therapy in Combination with Brachytherapy
It has been suggested that thermal laser therapy in combination with brachytherapy (intraluminal radiotherapy) should be able to increase the long-term effectiveness of laser therapy. First, laser therapy should be applied to reduce the tumor bulk, followed by a single treatment of brachytherapy with a maximum dose of 10 Gy, leading to a sustained effect of laser therapy with a better improvement of the dysphagia score and a reduction in the need for repeated treatment sessions. In four nonrandomized studies, laser therapy (Nd:YAG) plus brachytherapy was studied prospectively and proved to be safe and effective.136–139 Laser therapy plus brachytherapy was compared with laser therapy alone in two prospective, randomized trials in 39 patients and 22 patients.140,141 Both studies showed a prolonged dysphagia-free interval after the combination of laser therapy and brachytherapy; however, this did not result in a difference in survival between the two treatment groups.
Laser Therapy versus Stent Placement
Two nonrandomized studies142,143 and two randomized trials compared laser treatment with stent placement.129,144 Both nonrandomized trials concluded that laser therapy was a safer treatment than stent placement with a similar effectiveness. Dallal and coworkers129 compared laser treatment (mostly Nd:YAG) with metal stents in 65 patients with esophageal carcinoma in a randomized trial. Median survival was longer for the laser group, but relief of dysphagia was disappointing in both groups with similar complication rates and costs. Adam and colleagues144 randomly assigned 60 patients to laser therapy, uncovered stent placement, or covered stent placement and concluded that stent placement was more effective for the palliation of malignant dysphagia.
Other Endoscopic Methods
Dilation (Repeat)
Dilation can relieve dysphagia temporarily, but it provides palliation often for only a few days to 2 weeks. It is frequently used to allow access through the tumor for different forms of palliative treatments, such as metal stent placement or laser therapy. Dilation is a simple and cheap modality; however, complications, including perforation and hemorrhage, are common.145–147
Some authors advocate dilation of the malignant stricture gradually over several sessions before stent placement to reduce the risk of complications.42,147 The most commonly used dilators are polyvinyl wire-guided bougies, including tapered dilators (Savary), rubber dilators (Maloney), and through-the-scope hydrostatic balloons. There is as yet no study comparing these dilators in patients with malignant strictures. Because dilation as a sole therapy must be repeated at frequent intervals, it should be performed only in extremely ill patients with a very short life span.
Chemical Injection Therapy
Chemical injection therapy for the treatment of malignant dysphagia is an inexpensive alternative requiring no special equipment. Ethanol or polidocanol in aliquots of 0.5 to 1 mL is injected into the tumor, leading to tumor necrosis within several days after therapy. Exophytic tumors are most amenable to injection therapy, whereas firm and fibrotic tumors (after radiation therapy) prove difficult to inject. Studies on injection therapy for malignant dysphagia are limited.148–151 Dysphagia score improved from 3 (liquids only) to 1 (some difficulties with solid foods). Complications are rare; only fistula formation (n = 2), perforation (n = 1), and mediastinitis (n = 1) have been reported.149,150 Generally, two sessions were necessary to obtain a maximum effect, and retreatment was necessary at 4- to 5-week intervals. A comparative study in 34 patients between injection therapy with polidocanol and Nd:YAG laser therapy concluded that both techniques were safe and equally effectively for the palliation of malignant dysphagia.149
Nutritional Support
In case of failure of different palliative therapies or if other palliative modalities are technically impossible, nutritional support to maintain an adequate calorie intake should be considered. The overall condition and the prognosis of the patient are important factors to take into consideration before nutritional support is offered to the patient. Placement of a nasoenteral feeding tube is the easiest and least invasive feeding method; however, for patients with a longer life expectancy, placement of a PEG or percutaneous endoscopic jejunostomy is preferred. Rarely, central venous alimentation is indicated for maintaining or restoring an adequate nutritional status.152
Placement of a PEG using the classic pull method through a preexisting esophageal stent or in the presence of a malignant tumor can be problematic. In these cases, PEG placement without endoscopy using a nasogastric tube with gastric insufflation, fluoroscopic monitoring, and a direct percutaneous catheter insertion technique (push method) should be considered.153 Adler and coworkers154 described the results of nine patients undergoing classic PEG placement after stent placement and reported a good functional result and only one stent migration following PEG placement (11%).
Quality of Life
To evaluate the effectiveness of a palliative treatment modality, it is important not only to assess functional outcome and complications but also to assess the outcome from the perspective of patients, particularly quality of life. The aim of palliative treatment of esophageal cancer is to relieve dysphagia with minimal morbidity and mortality and maximum quality of life.155 Data on quality of life after palliative treatment for esophageal carcinoma are scarce. Validated measures that can be used to assess quality of life before and after treatment are the oncology-specific European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire156 and the esophageal carcinoma–specific EORTC OES-24 or OES-18 questionnaires.157
O’Hanlon and colleagues158 investigated quality of life at 6 and 16 weeks after treatment in 43 patients undergoing intubation or radiation therapy. Although the dysphagia score improved significantly after treatment, none of the other parameters assessed were significantly improved at 16 weeks after treatment. Barr and Krasner125 assessed quality of life after palliative Nd:YAG laser treatment for malignant dysphagia in 40 patients at monthly intervals. The linear-analogue self-assessment questionnaire, assessing 25 items on physical condition, psychological effects, and social interactions and a physician’s assessment using a quality-of-life index consisting of a structured interview on specific items were performed. The patient’s swallowing ability, the items on the linear-analogue self-assessment questionnaire, and the quality-of-life index all were improved at some time after laser therapy. Blazeby and colleagues159 assessed quality of life in 37 patients after palliative treatment (intubation [n = 30] or palliative radiation or chemotherapy [n = 7]) and compared this with patients undergoing surgery for esophageal cancer using the EORTC QLQ C-30 questionnaire at 3-month intervals. Patients with palliative treatment reported worse baseline quality-of-life scores compared with patients undergoing surgery. However, following palliative treatment, most aspects of quality of life from the EORTC QLQ-C30 were maintained until death.
Our group investigated generic and disease-specific health-related quality of life (HRQoL) after palliative treatment (i.e., placement of a covered Ultraflex stent [n = 108] or single-dose [12 Gy] brachytherapy [n = 101]).160 Longitudinal data on disease-specific (dysphagia score, EORTC OES-23, visual analogue pain scale) and generic (EORTC QLQ-C30, Euroqol [EQ]-5D) HRQoL were obtained by monthly home visits of a specially trained research nurse. Dysphagia improved more rapidly after stent placement than after brachytherapy, but long-term relief of dysphagia was better after brachytherapy. For generic HRQoL, there was an overall significant difference in favor of brachytherapy on four out of five functional scales of the EORTC QLQ-C30 (role, emotional, cognitive, and social). Generic HRQoL deteriorated over time on all functional scales of the EORTC QLQ C-30 and EQ-5D, in particular, physical and role functioning (on average −23 and −24 on a 100-point scale during 0.5 year of follow-up). This decline was more pronounced in the stent group. Major improvements were seen on the dysphagia and eating scales of the EORTC OES-23, in contrast to other scales of this disease-specific measure, which remained almost stable during follow-up. Reported levels of chest or abdominal pain remained stable during follow-up in both treatment groups, and general pain levels increased to a minor extent. It was concluded that the effects of single-dose brachytherapy on HRQoL compared favorably with the effects of stent placement for the palliation of esophageal cancer.
Future Trends
New types of stents are being developed and include biodegradable stents, stents with a radioactive coating, and drug-eluting stents. Biodegradable stents have been developed for benign stenoses9,161; however, a possible application could be the initial treatment of dysphagia in patients undergoing palliative chemotherapy. Because results of chemotherapy for this indication are improving,162 stent migration is more likely to occur in patients with a good response to chemotherapy.
Other developments for malignant esophageal strictures include the incorporation of beta-emitting agents163 and cytotoxic agents in esophageal stents, which may prevent recurrent tumor overgrowth at both ends of the stent.
1 Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000: The global picture. Eur J Cancer. 2001;37:4-66.
2 Pisani P, Parkin DM, Bray F, et al. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18-29.
3 Siersema PD. Esophageal cancer. Gastroenterol Clin North Am. 2008;37:943-964.
4 Pera M, Cameron AJ, Trastek VF, et al. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993;104:510-513.
5 Devesa SS, Blot WJ, Fraumeni JFJr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049-2053.
6 Bollschweiler E, Wolfgarten E, Gutschow C, et al. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92:549-555.
7 Botterweck AA, Schouten LJ, Volovics A, et al. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol. 2000;29:645-654.
8 van Soest EM, Dieleman JP, Siersema PD, et al. Increasing incidence of Barrett’s oesophagus in the general population. Gut. 2005;54:1062-1066.
9 van den Boogert J, van Hillegersberg R, Siersema PD, et al. Barrett’s oesophagus: Pathophysiology, diagnosis and management. Scand J Gastroenterol. 1998;33:449-453.
10 Blot WJ. Esophageal cancer trends and risk factors. Semin Oncol. 1994;21:403-410.
11 Cheng KK, Day NE, Duffy SW, et al. Pickled vegetables in the aetiology of oesophageal cancer in Hong Kong Chinese. Lancet. 1992;339:1314-1318.
12 Ahsan H, Neugut AI. Radiation therapy for breast cancer and increased risk for esophageal carcinoma. Ann Intern Med. 1998;128:114-117.
13 Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038-1049.
14 Cooper K, Taylor L, Govind S. Human papillomavirus DNA in oesophageal carcinomas in South Africa. J Pathol. 1995;175:271-277.
15 Kok TC, Nooter K, Tjong-A-Hung SP, et al. No evidence of known types of human papillomavirus in squamous cell cancer of the oesophagus in a low-risk area. Rotterdam Oesophageal Tumour Study Group. Eur J Cancer. 1997;33:1865-1868.
16 Leeuwenburgh I, Scholten P, Alderliesten J. Long-term esophageal cancer risk in patients with primary achalasia: A prospective study. Am J Gastroenterol. 2010;105:2144-2149.
17 Appelqvist P, Salmo M. Lye corrosion carcinoma of the esophagus. Cancer. 1980;45:2655-2658.
18 Cooper JS, Pajak TF, Rubin P, et al. Second malignancies in patients who have head and neck cancer: Incidence, effect on survival and implications based on the RTOG experience. Int J Radiat Oncol Biol Phys. 1989;17:449-456.
19 Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825-831.
20 Spechler SJ. Barrett’s esophagus. N Engl J Med. 2002;346:836-842.
21 van der Burgh A, Dees J, Hop WCJ, et al. Oesophageal cancer is an uncommon cause of death in patients with Barrett’s oesophagus. Gut. 1996;39:5-8.
22 Cameron AJ. Epidemiology of Barrett’s esophagus and adenocarcinoma. Dis Esophagus. 2002;15:106-108.
23 Moses FM. Squamous cell carcinoma of the esophagus: Natural history, incidence, etiology, and complications. Gastroenterol Clin North Am. 1991;20:703-716.
24 Miller C. Carcinoma of the thoracic oesophagus and cardia: A review of 405 cases. Br J Surg. 1962;49:507-522.
25 Shu YJ. Cytopathology of the esophagus: An overview of esophageal cytopathology in China. Acta Cytol. 1983;27:7-16.
26 Falk GW. Endoscopic surveillance of Barrett’s esophagus: Risk stratification and cancer risk. Gastrointest Endosc. 1999;49(Pt 2):S29-S34.
27 Hameeteman W, Tytgat GN, Houthoff HJ, et al. Barrett’s esophagus: Development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249-1256.
28 Miros M, Kerlin P, Walker N. Only patients with dysplasia progress to adenocarcinoma in Barrett’s oesophagus. Gut. 1991;32:1441-1446.
29 Weston AP, Sharma P, Topalovski M, et al. Long-term follow-up of Barrett’s high-grade dysplasia. Am J Gastroenterol. 2000;95:1888-1893.
30 Schnell TG, Sontag SJ, Chejfec G, et al. Long-term nonsurgical management of Barrett’s esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607-1619.
31 Katz D, Rothstein R, Schned A, et al. The development of dysplasia and adenocarcinoma during endoscopic surveillance of Barrett’s esophagus. Am J Gastroenterol. 1998;93:536-541.
32 O’Connor JB, Falk GW, Richter JE. The incidence of adenocarcinoma and dysplasia in Barrett’s esophagus: Report on the Cleveland Clinic Barrett’s Esophagus Registry. Am J Gastroenterol. 1999;94:2037-2042.
33 Sampliner RE, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol. 1998;93:1028-1032.
34 van Sandick JW, van Lanschot JJ, Kuiken BW, et al. Impact of endoscopic biopsy surveillance of Barrett’s oesophagus on pathological stage and clinical outcome of Barrett’s carcinoma. Gut. 1998;43:216-222.
35 Corley DA, Levin TR, Habel LA, et al. Surveillance and survival in Barrett’s adenocarcinomas: A population-based study. Gastroenterology. 2002;122:633-640.
36 Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662-1669.
37 Ell C, May A. Self-expanding metal stents for palliation of stenosing tumors of the esophagus and cardia: A critical review. Endoscopy. 1997;29:392-398.
38 Baron TH. Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med. 2001;344:1681-1687.
39 Siersema PD, Marcon N, Vakil N. Metal stents for tumors of the distal esophagus and gastric cardia. Endoscopy. 2003;35:79-85.
40 O’Donnell CA, Fullarton GM, Watt E, et al. Randomized clinical trial comparing self-expanding metallic stents with plastic endoprostheses in the palliation of oesophageal cancer. Br J Surg. 2002;89:985-992.
41 Knyrim K, Wagner HJ, Bethge N, et al. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med. 1993;329:1302-1307.
42 De Palma GD, di Matteo E, Romano G, et al. Plastic prosthesis versus expandable metal stents for palliation of inoperable esophageal thoracic carcinoma: A controlled prospective study. Gastrointest Endosc. 1996;43:478-482.
43 Roseveare CD, Patel P, Simmonds N, et al. Metal stents improve dysphagia, nutrition and survival in malignant oesophageal stenosis: A randomized controlled trial comparing modified Gianturco Z-stents with plastic Atkinson tubes. Eur J Gastroenterol Hepatol. 1998;10:653-657.
44 Siersema PD, Hop WC, Dees J, et al. Coated self-expanding metal stents versus latex prostheses for esophagogastric cancer with special reference to prior radiation and chemotherapy: A controlled, prospective study. Gastrointest Endosc. 1998;47:113-120.
45 Sanyika C, Corr P, Haffejee A. Palliative treatment of oesophageal carcinoma: Efficacy of plastic versus self-expandable stents. S Afr Med J. 1999;89:640-643.
46 Nicholson DA, Haycox A, Kay CL, et al. The cost effectiveness of metal oesophageal stenting in malignant disease compared with conventional therapy. Clin Radiol. 1999;54:212-215.
47 Vakil N, Morris AI, Marcon N, et al. A prospective, randomized, controlled trial of covered expandable metal stents in the palliation of malignant esophageal obstruction at the gastroesophageal junction. Am J Gastroenterol. 2001;96:1791-1796.
48 Verschuur EM, Repici A, Kuipers EJ, et al. New design esophageal stents for the palliation of dysphagia from esophageal or gastric cardia cancer: A randomized trial. Am J Gastroenterol. 2008;103:304-312.
49 Siersema PD. Stenting for benign esophageal strictures. Endoscopy. 2009;41:363-373.
50 van Boeckel PGA, Siersema PD, Sturgess R, et al. A new partially covered metal stent for palliation of malignant dysphagia: A prospective follow-up study. Gastrointest Endosc. 2010;72:1269-1273.
51 van Boeckel PGA, Repici A, Vleggaar FP, et al. A new metal stent with controlled release system (Evolution) for the palliation of malignant dysphagia: A prospective multicenter study. Gastrointest Endosc. 2010;71:455-460.
52 Uitdehaag MJ, Siersema PD, Spaander MCW, et al. A new fully covered stent with antimigration properties for the palliation of malignant dysphagia: A prospective cohort study. Gastrointest Endosc. 2010;71:600-605.
53 Kim ES, Jeon SW, Park SY, et al. Comparison of double-layered and covered Niti-S stents for palliation of malignant dysphagia. J Gastroenterol Hepatol. 2009;24:114-119.
54 Uitdehaag MJ, van Hooft JE, Verschuur EM, et al. A fully covered stent (Alimaxx-E) for the palliation of malignant dysphagia: A prospective follow-up study. Gastrointest Endosc. 2009;70:1082-1089.
55 Conio M, Repici A, Battaglia G, et al. A randomized prospective comparison of self-expandable plastic stents and partially covered self-expandable metal stents in the palliation of malignant esophageal dysphagia. Am J Gastroenterol. 2007;102:2667-2677.
56 Siersema PD, Hop WC, van Blankenstein M, et al. A comparison of 3 types of covered metal stents for the palliation of patients with dysphagia caused by esophagogastric carcinoma: A prospective, randomized study. Gastrointest Endosc. 2001;54:145-153.
57 Sabharwal T, Hamady MS, Chui S, et al. A randomized prospective comparison of the Flamingo Wallstent and Ultraflex stent for palliation of dysphagia associated with lower third oesophageal carcinoma. Gut. 2003;52:922-926.
58 Spinelli P, Cerrai FG, Ciuffi M, et al. Endoscopic stent placement for cancer of the lower esophagus and gastric cardia. Gastrointest Endosc. 1994;40:455-457.
59 Verschuur EML, Steyerberg EW, Kuipers EJ, et al. Effect of stent size on complications and recurrent dysphagia in patients with esophageal or gastric cardia cancer. Gastrointest Endosc. 2007;65:592-601.
60 Mayoral W, Fleischer D, Salcedo J, et al. Nonmalignant obstruction is a common problem with metal stents in the treatment of esophageal cancer. Gastrointest Endosc. 2000;51:556-559.
61 Valbuena J. Palliation of gastroesophageal carcinoma with endoscopic insertion of a new antireflux prosthesis. Gastrointest Endosc. 1984;30:241-243.
62 Kocher M, Dlouhy M, Neoral C, et al. Esophageal stent with antireflux valve for tumors involving the cardia: Work in progress. J Vasc Interv Radiol. 1998;9:1007-1010.
63 Do YS, Choo SW, Suh SW, et al. Malignant esophagogastric junction obstruction: Palliative treatment with an antireflux valve stent. J Vasc Interv Radiol. 2001;12:647-651.
64 Dua KS, Kozarek R, Kim J, et al. Self-expanding metal esophageal stent with anti-reflux mechanism. Gastrointest Endosc. 2001;53:603-613.
65 Laasch HU, Marriott A, Wilbraham L, et al. Effectiveness of open versus antireflux stents for palliation of distal esophageal carcinoma and prevention of symptomatic gastroesophageal reflux. Radiology. 2002;225:359-365.
66 Osugi H, Lee S, Higashino M, et al. Usefulness of self-expandable metallic stent with an antireflux mechanism as a palliation for malignant strictures at the gastroesophageal junction. Surg Endosc. 2002;16:1478-1482.
67 Homs MY, Wahab PJ, Kuipers EJ, et al. Esophageal stents with antireflux valve for tumors of the distal esophagus and gastric cardia: A randomized trial. Gastrointest Endosc. 2004;60:695-702.
68 Shim CS, Jung IS, Cheon YK, et al. Management of malignant stricture of the esophagogastric junction with a newly designed self-expanding metal stent with an antireflux mechanism. Endoscopy. 2005;37:335-339.
69 Wenger U, Johnsson E, Arnelo U, et al. An antireflux stent versus conventional stents for palliation of distal esophageal or cardia cancer: a randomized clinical study. Surg Endosc. 2006;20:1675-1680.
70 Power C, Byrne PJ, Lim K, et al. Superiority of anti-reflux stent compared with conventional stents in the palliative management of patients with cancer of the lower esophagus and esophago-gastric junction: Results of a randomized clinical trial. Dis Esophagus. 2007;20:466-470.
71 Schoppmeyer K, Golsong J, Schiefke I, et al. Antireflux stents for palliation of malignant esophagocardial stenosis. Dis Esophagus. 2007;20:89-93.
72 Sabharwal T, Gulati MS, Fotiadis N, et al. Randomised comparison of the FerX Ella antireflux stent and the ultraflex stent: proton pump inhibitor combination for prevention of post-stent reflux in patients with esophageal carcinoma involving the esophago-gastric junction. J Gastroenterol Hepatol. 2008;23:723-728.
73 Austin A, Khan Z, Cole AT, et al. Placement of self-expanding metallic stents without fluoroscopy. Gastrointest Endosc. 2001;54:157-159.
74 Martin DF. Endoscopy is superfluous during insertion of expandable metal stents in esophageal tumors. Gastrointest Endosc. 1997;46:98-99.
75 Martini N, Goodner JT, D’Angio GJ, et al. Tracheoesophageal fistula due to cancer. J Thorac Cardiovasc Surg. 1970;59:319-324.
76 Weigert N, Neuhaus H, Rosch T, et al. Treatment of esophagorespiratory fistulas with silicone-coated self-expanding metal stents. Gastrointest Endosc. 1995;41:490-496.
77 Hordijk ML, Dees J, van Blankenstein M. The management of malignant esophago-respiratory fistulas with a cuffed prosthesis. Endoscopy. 1990;22:241-244.
78 Rosch W, Keller C. The cuffed esophageal prosthesis: A life-threatening instrument. Endoscopy. 1995;27:214-215.
79 Do YS, Song HY, Lee BH, et al. Esophagorespiratory fistula associated with esophageal cancer: Treatment with a Gianturco stent tube. Radiology. 1993;187:673-677.
80 Bethge N, Sommer A, Vakil N. Treatment of esophageal fistulas with a new polyurethane-covered, self-expanding mesh stent: A prospective study. Am J Gastroenterol. 1995;90:2143-2146.
81 Kozarek RA, Raltz S, Brugge WR, et al. Prospective multicenter trial of esophageal Z-stent placement for malignant dysphagia and tracheoesophageal fistula. Gastrointest Endosc. 1996;44:562-567.
82 Morgan RA, Ellul JP, Denton ER, et al. Malignant esophageal fistulas and perforations: Management with plastic-covered metallic endoprostheses. Radiology. 1997;204:527-532.
83 Nelson DB, Axelrad AM, Fleischer DE, et al. Silicone-covered Wallstent prototypes for palliation of malignant esophageal obstruction and digestive-respiratory fistulas. Gastrointest Endosc. 1997;45:31-37.
84 Low DE, Kozarek RA. Comparison of conventional and wire mesh expandable prostheses and surgical bypass in patients with malignant esophagorespiratory fistulas. Ann Thorac Surg. 1998;65:919-923.
85 May A, Ell C. Palliative treatment of malignant esophagorespiratory fistulas with Gianturco-Z stents: A prospective clinical trial and review of the literature on covered metal stents. Am J Gastroenterol. 1998;93:532-535.
86 Raijman I, Siddique I, Ajani J, et al. Palliation of malignant dysphagia and fistulae with coated expandable metal stents: Experience with 101 patients. Gastrointest Endosc. 1998;48:172-179.
87 Dumonceau JM, Cremer M, Lalmand B, et al. Esophageal fistula sealing: Choice of stent, practical management, and cost. Gastrointest Endosc. 1999;49:70-78.
88 Siersema PD, Schrauwen SL, van Blankenstein M, et al. Self-expanding metal stents for complicated and recurrent esophagogastric cancer. Gastrointest Endosc. 2001;54:579-586.
89 Abadal JM, Echenagusia A, Simo G, et al. Treatment of malignant esophagorespiratory fistulas with covered stents. Abdom Imaging. 2001;26:565-569.
90 van den Bongard HJ, Boot H, Baas P, et al. The role of parallel stent insertion in patients with esophagorespiratory fistulas. Gastrointest Endosc. 2002;55:110-115.
91 Ellul JP, Morgan R, Gold D, et al. Parallel self-expanding covered metal stents in the trachea and oesophagus for the palliation of complex high tracheo-oesophageal fistula. Br J Surg. 1996;83:1767-1768.
92 Binkert CA, Petersen BD. Two fatal complications after parallel tracheal-esophageal stenting. Cardiovasc Intervent Radiol. 2002;25:144-147.
93 Bethge N, Sommer A, Vakil N. A prospective trial of self-expanding metal stents in the palliation of malignant esophageal strictures near the upper esophageal sphincter. Gastrointest Endosc. 1997;45:300-303.
94 Conio M, Caroli-Bosc F, Demarquay JF, et al. Self-expanding metal stents in the palliation of neoplasms of the cervical esophagus. Hepatogastroenterology. 1999;46:272-277.
95 Macdonald S, Edwards RD, Moss JG. Patient tolerance of cervical esophageal metallic stents. J Vasc Interv Radiol. 2000;11:891-898.
96 Profili S, Meloni GB, Feo CF, et al. Self-expandable metal stents in the management of cervical oesophageal and/or hypopharyngeal strictures. Clin Radiol. 2002;57:1028-1033.
97 Verschuur EM, Kuipers EJ, Siersema PD. Esophageal stents for malignant strictures close to the upper esophageal sphincter. Gastrointest Endosc. 2007;66:1082-1090.
98 De Gregorio BT, Kinsman K, Katon RM, et al. Treatment of esophageal obstruction from mediastinal compressive tumor with covered, self-expanding metallic Z-stents. Gastrointest Endosc. 1996;43:483-489.
99 Kozarek RA, Raltz S, Marcon N, et al. Use of the 25 mm flanged esophageal Z stent for malignant dysphagia: A prospective multicenter trial. Gastrointest Endosc. 1997;46:156-160.
100 Bethge N, Sommer A, Vakil N. Palliation of malignant esophageal obstruction due to intrinsic and extrinsic lesions with expandable metal stents. Am J Gastroenterol. 1998;93:1829-1832.
101 Gupta NK, Boylan CE, Razzaq R, et al. Self-expanding oesophageal metal stents for the palliation of dysphagia due to extrinsic compression. Eur Radiol. 1999;9:1893-1897.
102 Bethge N, Sommer A, von Kleist D, et al. A prospective trial of self-expanding metal stents in the palliation of malignant esophageal obstruction after failure of primary curative therapy. Gastrointest Endosc. 1996;44:283-286.
103 Law S, Tung PH, Chu KM, et al. Self-expanding metallic stents for palliation of recurrent malignant esophageal obstruction after subtotal esophagectomy for cancer. Gastrointest Endosc. 1999;50:427-436.
104 Lyburn I, Blazeby JM, Barham P, et al. Palliation of malignant gastric outlet obstruction after oesophagectomy by percutaneous transthoracic placement of an expanding metal stent. Clin Radiol. 2001;56:82-83.
105 Kinsman KJ, DeGregorio BT, Katon RM, et al. Prior radiation and chemotherapy increase the risk of life-threatening complications after insertion of metallic stents for esophagogastric malignancy. Gastrointest Endosc. 1996;43:196-203.
106 Kozarek RA, Ball TJ, Brandabur JJ, et al. Expandable versus conventional esophageal prostheses: Easier insertion may not preclude subsequent stent-related problems. Gastrointest Endosc. 1996;43:204-208.
107 Nelson DB, Axelrad AM, Fleischer DE, et al. Silicone-covered Wallstent prototypes for palliation of malignant esophageal obstruction and digestive-respiratory fistulas. Gastrointest Endosc. 1997;45:31-37.
108 Raijman I, Siddique I, Lynch P. Does chemoradiation therapy increase the incidence of complications with self-expanding coated stents in the management of malignant esophageal strictures? Am J Gastroenterol. 1997;92:2192-2196.
109 Bartelsman JF, Bruno MJ, Jensema AJ, et al. Palliation of patients with esophagogastric neoplasms by insertion of a covered expandable modified Gianturco-Z endoprosthesis: Experiences in 153 patients. Gastrointest Endosc. 2000;51:134-138.
110 Muto M, Ohtsu A, Miyata Y, et al. Self-expandable metallic stents for patients with recurrent esophageal carcinoma after failure of primary chemoradiotherapy. Jpn J Clin Oncol. 2001;31:270-274.
111 Homs MY, Hansen BE, van Blankenstein M, et al. Prior radiation and/or chemotherapy has no effect on the incidence of life-threatening complications and the long-term outcome of self-expanding metal stent placement for esophagogastric carcinoma. Eur J Gastroenterol Hepatol. 2004;16:163-170.
112 Golder M, Tekkis PP, Kennedy C, et al. Chest pain following oesophageal stenting for malignant dysphagia. Clin Radiol. 2001;56:202-205.
113 Cwikiel W, Tranberg KG, Cwikiel M, et al. Malignant dysphagia: Palliation with esophageal stents—long-term results in 100 patients. Radiology. 1998;207:513-518.
114 Christie NA, Buenaventura PO, Fernando HC, et al. Results of expandable metal stents for malignant esophageal obstruction in 100 patients: Short-term and long-term follow-up. Ann Thorac Surg. 2001;71:1797-1801.
115 De Palma GD, Iovino P, Catanzano C. Distally migrated esophageal self-expanding metal stents: Wait and see or remove? Gastrointest Endosc. 2001;53:96-98.
116 Raijman I, Marcon NE, Kandel G, et al. Repositioning of an esophageal stent after migration using a snare. Gastrointest Endosc. 1994;40:652.
117 Rosen C, Goldberg RI. Repositioning of a migrated esophageal stent using a retroflexed endoscope. Gastrointest Endosc. 1995;42:278-279.
118 Berkelhammer C, Roberts J, Steinecker G. Repositioning a migrated esophageal stent using a retroflexed endoscope: A note of caution. Gastrointest Endosc. 1996;44:632-634.
119 Noyer CM, Forohar F. A simple technique to remove migrated esophageal stents. Am J Gastroenterol. 1998;93:1595.
120 Rollhauser C, Fleischer DE. Late migration of a self-expandable metal stent and successful endoscopic management. Gastrointest Endosc. 1999;49:541-544.
121 Farkas PS, Farkas JD, Koenigs KP. An easier method to remove migrated esophageal Z-stents. Gastrointest Endosc. 1999;50:277-279.
122 Seitz U, Thonke F, Bohnacker S, et al. Endoscopic extraction of a covered esophageal Z-stent with the aid of Endoloops. Endoscopy. 1998;30:S91-S92.
123 Fleischer D, Kessler F, Haye O. Endoscopic Nd:YAG laser therapy for carcinoma of the esophagus: A new palliative approach. Am J Surg. 1982;143:280-283.
124 Pietrafitta JJ, Bowers GJ, Dwyer RM. Prograde versus retrograde endoscopic laser therapy for the treatment of malignant esophageal obstruction: A comparison of techniques. Lasers Surg Med. 1988;8:288-293.
125 Barr H, Krasner N. Prospective quality-of-life analysis after palliative photoablation for the treatment of malignant dysphagia. Cancer. 1991;68:1660-1664.
126 Mason RC, Bright N, McColl I. Palliation of malignant dysphagia with laser therapy: Predictability of results. Br J Surg. 1991;78:1346-1347.
127 Carter R, Smith JS, Anderson JR. Palliation of malignant dysphagia using the Nd:YAG laser. World J Surg. 1993;17:608-613.
128 Carazzone A, Bonavina L, Segalin A, et al. Endoscopic palliation of oesophageal cancer: Results of a prospective comparison of Nd:YAG laser and ethanol injection. Eur J Surg. 1999;165:351-356.
129 Dallal HJ, Smith GD, Grieve DC, et al. A randomized trial of thermal ablative therapy versus expandable metal stents in the palliative treatment of patients with esophageal carcinoma. Gastrointest Endosc. 2001;54:549-557.
130 Marcon NE. Photodynamic therapy and cancer of the esophagus. Semin Oncol. 1994;21:20-23.
131 Patrice T, Foultier MT, Yactayo S, et al. Endoscopic photodynamic therapy with hematoporphyrin derivative for primary treatment of gastrointestinal neoplasms in inoperable patients. Dig Dis Sci. 1990;35:545-552.
132 Luketich JD, Christie NA, Buenaventura PO, et al. Endoscopic photodynamic therapy for obstructing esophageal cancer: 77 cases over a 2-year period. Surg Endosc. 2000;14:653-657.
133 Moghissi K, Dixon K, Thorpe JA, et al. The role of photodynamic therapy (PDT) in inoperable oesophageal cancer. Eur J Cardiothorac Surg. 2000;17:95-100.
134 Lightdale CJ, Heier SK, Marcon NE, et al. Photodynamic therapy with porfimer sodium versus thermal ablation therapy with Nd:YAG laser for palliation of esophageal cancer: A multicenter randomized trial. Gastrointest Endosc. 1995;42:507-512.
135 Heier SK, Rothman KA, Heier LM, et al. Photodynamic therapy for obstructing esophageal cancer: Light dosimetry and randomized comparison with Nd:YAG laser therapy. Gastroenterology. 1995;109:63-72.
136 Bader M, Dittler HJ, Ultsch B, et al. Palliative treatment of malignant stenoses of the upper gastrointestinal tract using a combination of laser and afterloading therapy. Endoscopy. 1986;18:27-31.
137 Renwick P, Whitton V, Moghissi K. Combined endoscopic laser therapy and brachytherapy for palliation of oesophageal carcinoma: A pilot study. Gut. 1992;33:435-438.
138 Shmueli E, Srivastava E, Dawes PJ, et al. Combination of laser treatment and intraluminal radiotherapy for malignant dysphagia. Gut. 1996;38:803-805.
139 Spencer GM, Thorpe SM, Sargeant IR, et al. Laser and brachytherapy in the palliation of adenocarcinoma of the oesophagus and cardia. Gut. 1996;39:726-731.
140 Sander R, Hagenmueller F, Sander C, et al. Laser versus laser plus afterloading with iridium-192 in the palliative treatment of malignant stenosis of the esophagus: A prospective, randomized, and controlled study. Gastrointest Endosc. 1991;37:433-440.
141 Spencer GM, Thorpe SM, Blackman GM, et al. Laser augmented by brachytherapy versus laser alone in the palliation of adenocarcinoma of the oesophagus and cardia: A randomised study. Gut. 2002;50:224-227.
142 Gevers AM, Macken E, Hiele M, et al. A comparison of laser therapy, plastic stents, and expandable metal stents for palliation of malignant dysphagia in patients without a fistula. Gastrointest Endosc. 1998;48:383-388.
143 Sihvo EI, Pentikainen T, Luostarinen ME, et al. Inoperable adenocarcinoma of the oesophagogastric junction: A comparative clinical study of laser coagulation versus self-expanding metallic stents with special reference to cost analysis. Eur J Surg Oncol. 2002;28:711-715.
144 Adam A, Ellul J, Watkinson AF, et al. Palliation of inoperable esophageal carcinoma: A prospective randomized trial of laser therapy and stent placement. Radiology. 1997;202:344-348.
145 Moses FM, Peura DA, Wong RK, et al. Palliative dilation of esophageal carcinoma. Gastrointest Endosc. 1985;31:61-63.
146 Lundell L, Leth R, Lind T, et al. Palliative endoscopic dilatation in carcinoma of the esophagus and esophagogastric junction. Acta Chir Scand. 1989;155:179-184.
147 Parker CH, Peura DA. Palliative treatment of esophageal carcinoma using esophageal dilation and prosthesis. Gastroenterol Clin North Am. 1991;20:717-729.
148 Payne-James JJ, Spiller RC, Misiewicz JJ, et al. Use of ethanol-induced tumor necrosis to palliate dysphagia in patients with esophagogastric cancer. Gastrointest Endosc. 1990;36:43-46.
149 Angelini G, Pasini AF, Ederle A, et al. Nd:YAG laser versus polidocanol injection for palliation of esophageal malignancy: A prospective, randomized study. Gastrointest Endosc. 1991;37:607-610.
150 Chung SC, Leong HT, Choi CY, et al. Palliation of malignant oesophageal obstruction by endoscopic alcohol injection. Endoscopy. 1994;26:275-277.
151 Nwokolo CU, Payne-James JJ, Silk DB, et al. Palliation of malignant dysphagia by ethanol induced tumour necrosis. Gut. 1994;35:299-303.
152 Boyce HWJr. Palliation of dysphagia of esophageal cancer by endoscopic lumen restoration techniques. Cancer Control. 1999;6:73-83.
153 Willis JS, Oglesby JT. Percutaneous gastrostomy: Further experience. Radiology. 1985;154:71-75.
154 Adler DG, Baron TH, Geels W, et al. Placement of PEG tubes through previously placed self-expanding esophageal metal stents. Gastrointest Endosc. 2001;54:237-241.
155 Blazeby JM. Measurement of outcome. Surg Oncol. 2001;10:127-133.
156 Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376.
157 Blazeby JM, Alderson D, Winstone K, et al. Development of an EORTC questionnaire module to be used in quality of life assessment for patients with oesophageal cancer. The EORTC Quality of Life Study Group. Eur J Cancer. 1996;32A:1912-1917.
158 O’Hanlon DM, Harkin M, Karat D, et al. Quality-of-life assessment in patients undergoing treatment for oesophageal carcinoma. Br J Surg. 1995;82:1682-1685.
159 Blazeby JM, Farndon JR, Donovan J, et al. A prospective longitudinal study examining the quality of life of patients with esophageal carcinoma. Cancer. 2000;88:1781-1787.
160 Homs MYV, Essink-Bot ML, Borsboom GJJM, et al. Quality of life after palliative treatment for esophageal carcinoma—a prospective comparison between stent placement and single dose brachytherapy. Eur J Cancer. 2004;40:1862-1871.
161 Fry SW, Fleischer DE. Management of a refractory benign esophageal stricture with a new biodegradable stent. Gastrointest Endosc. 1997;45:179-182.
162 Polee MB, Verweij J, Siersema PD, et al. Phase I study of a weekly schedule of a fixed dose of cisplatin and escalating doses of paclitaxel in patients with advanced oesophageal cancer. Eur J Cancer. 2002;38:1495-1500.
163 Won JH, Lee JD, Wang HJ, et al. Self-expandable covered metallic esophageal stent impregnated with beta-emitting radionuclide: An experimental study in canine esophagus. Int J Radiat Oncol Biol Phys. 2002;53:1005-1013.