Chapter 34 Endoscopic Management of Upper Gastrointestinal Familial Adenomatous Polyposis Syndrome and Ampullary Tumors
Introduction
Classic familial adenomatous polyposis (FAP) is an autosomal dominant condition caused by mutation in the adenomatous polyposis coli (APC) gene that results in profuse adenomatous polyposis of the gastrointestinal (GI) tract, most marked in the colon. Attenuated FAP is associated with a smaller number of polyps in the colon and upper GI tract. The MUTYH mutation causes a recessively inherited polyposis condition, MUTYH-associated polyposis, which is characterized by a slightly increased risk of developing colon cancer and adenomas in both the upper and the lower GI tract. Patients with FAP and its variants generally undergo prophylactic colectomy during adolescence or early adult life. After colectomy, the proximal duodenum is the most common site of malignancy in FAP subjects.1 The periampullary region is especially prone to adenomatous change, presumably related partly to the trophic effects of bile on the mucosa.2–5 Duodenal adenomas develop in 40% to 100% of FAP patients. Less commonly, periampullary adenomas are sporadic (arising in non-FAP patients).
Familial Adenomatous Polyposis Syndrome
FAP is an autosomal dominant condition with virtually complete penetrance, affecting approximately 1 in 8000 in the United States.6 Mutation of the APC gene on the long arm of chromosome 5 is responsible for most cases of FAP.7 The condition is classically characterized by the development of hundreds to thousands of adenomatous polyps in the colon with the inevitable progression of one or more of these adenomas to carcinoma. It is increasingly apparent, however, that an attenuated form of FAP exists. These patients generally present with fewer colorectal polyps developing later in life compared with classic FAP and often distributed more proximally in the colon. These patients develop upper GI disease, and the upper GI findings may be more marked in some patients than the findings in the colon.8,9 Mutations in particular regions of the APC gene account for the attenuated forms of FAP.8,10,11
Extracolonic disease is common in classic FAP but varies in severity from family to family and between individuals within families. At one extreme is Gardner’s syndrome, characterized by GI adenomatous polyps with other benign neoplasms, such as desmoid tumors, osteomas, and fibromas.12 Gardner’s syndrome also results from germline APC mutations and is best regarded as part of the spectrum of FAP. Turcot’s syndrome is characterized by central nervous system tumors, often glioblastomas or medulloblastomas,12 and colonic polyposis. The inheritance of this disorder has been difficult to determine12 because the association of central nervous system tumors and polyposis may arise through germline mutation of more than one gene.13 Germline APC mutations have been identified in some patients with Turcot’s syndrome, particularly patients with cerebellar medulloblastomas and profuse colonic polyposis.13 For the purposes of this chapter, the term FAP incorporates Gardner’s syndrome and cases of Turcot’s syndrome attributable to APC mutations.
The colonic manifestations of FAP are cured by colectomy (although a risk of rectal cancer remains after ileorectal anastomosis or in any residual rectal mucosa after restorative proctocolectomy). After colectomy, extracolonic manifestations of FAP assume greater importance. The entire upper GI tract seems to be at risk of malignancy in FAP.1 The duodenum is the most common site of malignancy in patients after colectomy. Duodenal cancer develops in 4.5% to 8.5% of these patients.14,15 Adenomas and carcinomas have also been encountered in the distal ileal segment and within ileoanal pouches 5 to 10 years after proctocolectomy.16
Gastric polyps are a common finding in FAP. Multiple 3- to 5-mm fundic polypoid lesions are seen in at least 50% of patients (Fig. 34.1).17 These fundic gland or fundic cystic gland polyps are hamartomatous lesions and were previously regarded as having no malignant potential. Histologic examination of fundic gland polyps reveals cystic dilation of fundic glands with generally no epithelial dysplasia.18 However, Bianchi and colleagues19 reported a series of 75 FAP patients undergoing upper endoscopic screening for adenomas. Fundic gland polyps, detected in 88% of patients and sampled by mucosal biopsy, showed dysplasia in 41% (3% with high-grade dysplasia). On multivariate analysis, the investigators found larger fundic gland polyps (>1 cm), higher Spigelman stage (Table 34.1), and antral gastritis were associated with fundic gland dysplasia. Fundic gland polyps with size greater than 1 cm had almost 16 times greater odds for dysplasia compared with polyps 1 to 4 mm in size. Acid suppressive medication use was found to be associated with a marked decrease in dysplastic fundic gland polyps.
Similar findings were noted in a series of 24 pediatric patients undergoing screening endoscopy with 51% showing fundic gland polyps with dysplasia in 42%.20 The investigators found that APC mutations between codons 1225 and 1694 may be associated with more aggressive gastroduodenal involvement in FAP. Several case reports also describe gastric adenocarcinoma arising from fundic gland polyps in patients with FAP and attenuated FAP.21–24 Although a smaller series (n = 29)21 revealed polyps (predominantly fundic gland) in 35% of patients in which all were hyperplastic without dysplasia, it is important to perform a thorough examination of the fundus to obtain a biopsy specimen or remove large fundic gland polyps (>1 cm or enlarging lesions on serial surveillance examinations) or polyps with atypical endoscopic appearances.
Gastric adenomatous polyps (predominantly in the gastric antrum) have been reported in 5% of patients with FAP in a British cohort1 and 25% in a Japanese series.25 The risk of gastric malignancy has been estimated to be 3.4 times that of controls.26 However, this report was from Japan, where the epidemiology of gastric malignancy differs from that of Western societies. In a British study of 1255 subjects with FAP and mean follow-up of 22 years, only 7 developed gastric malignancy.1 Nevertheless, just as adenomas are treated in other parts of the GI tract, if screening upper endoscopy is performed to assess for duodenal adenomas, inspection and biopsy or removal of suspected adenomas in the stomach should also be planned. Surveillance strategies of the upper GI tract for FAP patients are discussed subsequently.
Incidence of Sporadic and Familial Adenomatous Polyposis–Related Periampullary Adenomas
An understanding of the natural history of duodenal neoplasia in patients with FAP is essential to the development of surveillance strategies and decisions regarding management in this condition. Periampullary tumors represent 5% of GI tumors and 36% of resectable pancreaticoduodenal tumors.27 A periampullary adenoma is an uncommon lesion in clinical practice, although not as rare as previously thought. An early review at the Mayo Clinic by Baggenstoss28 found 25 of these lesions in 4000 consecutive autopsies (0.62%), suggesting that the lesion may be subclinical. A review of the case notes in this study suggested that only 6 (24%) of these 25 lesions might have been symptomatic.
Asymptomatic adenomatous change of the ampulla is extremely common in FAP patients, occurring in a high proportion of patients with one series showing adenoma in all of the patients studied.29 The incidence of FAP-related duodenal and periampullary adenomas depends on the diligence of screening (see section on Diagnosis later). A review of the Johns Hopkins FAP registry indicated that the relative risk of duodenal adenocarcinoma in FAP compared with the general population was 330, and the relative risk of ampullary cancer was 123.30 However, the combined absolute risk of duodenal cancer in FAP patients was only 1 per 1698 years. Because follow-up was incomplete and most cancers occurred later in life, the risk of malignancy may be underestimated. A study from the United Kingdom reported development of malignancy in 3 of 70 patients followed over 40 months.31 Although adenomatous change in the duodenum may be almost universal in FAP, only a few patients apparently develop cancer. Several studies have indicated that the median age at onset of periampullary malignancy complicating FAP is in the 6th decade.15,30,32 The literature on this subject often has inadequately differentiated sporadic from FAP-related periampullary adenomas.
Pathogenesis of Periampullary Adenoma
As an autosomal dominant condition, all nucleated cells in FAP patients contain one normal and one abnormal APC gene (a germline mutation). In the colon, a somatic mutation in the previously normal (wild-type) APC allele is generally an early event in carcinogenesis. Accumulation of other somatic mutations (in genes such as TP53 and K-ras) drives the progression toward malignancy.33 The situation with respect to periampullary malignancy seems to be similar except that somatic APC mutations may be relatively less frequent and K-ras mutations relatively more frequent.34 Another study has shown TP53 mutations associated with high-grade malignant change in periampullary tumors.27 There may be other familial factors, possibly unidentified modifier genes, that influence the development of periampullary adenomas in FAP kindreds, partly explaining the familial segregation of periampullary disease observed within FAP families.32 This segregation was independent of the specific APC mutation of the kindred.
Histology
Most periampullary lesions are tubular or tubulovillous adenomas that arise from the intestinal-type epithelium of the ampulla.35 Foci of severe dysplasia or frank malignancy may be found within a lesion.36 Other neoplasms of the ampulla are far less common; these include benign lesions (adenomyoma, leiomyoma, lipoma, lymphangioma, hemangioma, carcinoid, and paraganglioma) and primary and metastatic malignancies (lymphoma, melanoma, and metastatic small cell carcinoma).37 A retrospective study by Bleau and Gostout29 supported the temporal progression of periampullary adenomas to carcinoma, with diagnosis of adenoma at a mean age of 39, high-grade dysplasia at age 47, and malignancy at age 54.
Clinical Presentation
Lesions of the periampullary area may be asymptomatic or manifest relatively early with symptoms of pancreaticobiliary origin. Clinical presentation is usually a consequence of obstruction, resulting in abdominal pain, nausea, vomiting, cholangitis, or jaundice37 or, less commonly, GI bleeding, iron deficiency anemia, or acute pancreatitis.38 Courvoisier’s sign is occasionally present, suggesting advanced disease.39 Biochemical evidence of biliary obstruction is common in symptomatic patients.40 The diagnosis is usually unsuspected before visualization of the ampulla, with most patients thought to have pancreatic malignancy, chronic pancreatitis, or choledocholithiasis.
Endoscopic Management of Periampullary Adenoma
Endoscopic management of periampullary adenomas can be separated into diagnosis, surveillance, and therapy. Colonic involvement in FAP can be asymptomatic, patients with FAP may have no family history (new mutations), and colonic manifestations of the disease may be delayed in attenuated forms of FAP.33 For these reasons, all patients with apparently “sporadic” periampullary adenomas should undergo colonoscopy.
Diagnosis
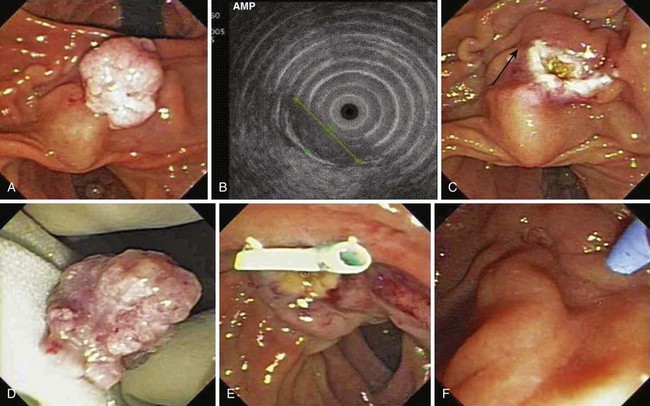
In patients with FAP, diagnosis of upper GI and particularly periampullary adenomas requires proficiency in side-viewing duodenoscopy because forward-viewing endoscopy alone may miss 50% of gross lesions visible with the side-viewer.29,41 Patients with FAP may have adenomas beyond the ampulla that are not seen with standard endoscopy. At least at index screening, it may be appropriate in FAP patients to include extended duodenoscopy with either a pediatric colonoscope or a push enteroscope. Biopsy specimens of the ampulla are necessary to detect early adenomatous change in light of the frondlike appearance of many normal papillae. Care should be taken to avoid the expected location of the pancreatic orifice (5 o’clock position) to reduce the risk of biopsy-related pancreatitis, and multiple biopsy specimens (at least six) are needed for adequate sampling. Nonetheless, ampullary biopsy carries a small risk of necrotizing pancreatitis and is not mandatory in an asymptomatic patient with an essentially normal-appearing ampulla. For larger lesions, mucosal biopsy alone may not sufficiently exclude invasive malignancy.42 Additional cross-sectional imaging and endoscopic ultrasound (EUS) is often used to evaluate for invasive disease.
A small series of patients undergoing high-resolution endoscopy with chromoendoscopy suggested an improved yield of adenoma detection in periampullary and duodenal polyposis. Dekker and associates43 evaluated 43 patients with FAP who underwent screening endoscopy using high-resolution endoscopes. They found that the additional use of indigo carmine chromoendoscopy increased the detection of adenomas from a median of 16 adenomas per patient to 21 adenomas per patient (P = .02) and enhanced margin detection in 51%. The addition of chromoendoscopy resulted in upgrading of the Spigelman stage (see Table 34.1) in 12% of patients (P = .03). Itoi and colleagues44 found that the use of either indigo carmine or narrow band imaging was superior to standard white light examination in 13 of 14 cases. In this study, visualization was categorized as difficult, fair, or excellent. Narrow band imaging had significantly higher proportion of “excellent” visualization of the tumor margin than indigo carmine chromoendoscopy (P = .012).
Because of the poor sensitivity of endoscopic biopsies in detecting malignant change, we routinely employ EUS to assess for depth of invasion and adjacent biliary or pancreatic involvement. Because we generally avoid diagnostic cholangiopancreatography, we pursue endoscopic retrograde cholangiopancreatography (ERCP) to assess for biliary or pancreatic ductal extension only when endoscopic ampullectomy is being considered during the same session. Although limitations of EUS include compression of the affected tissues and inability to “water-fill” the second portion of the duodenum adequately to assess for depth of invasion consistently, it has shown utility in the TNM (primary tumor, regional nodes, metastases) staging of periampullary malignancy with staging accuracy of 84%.42,45–48
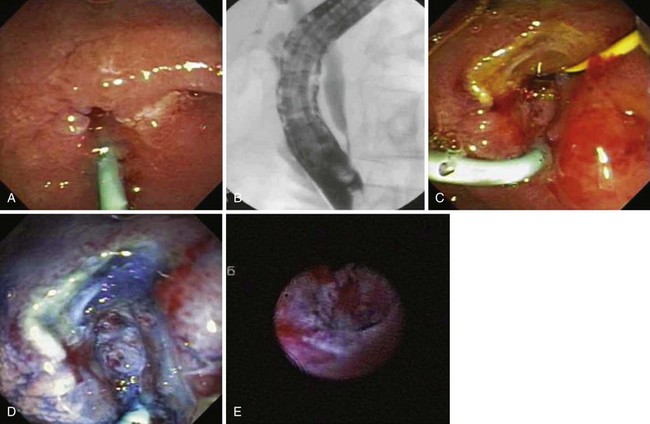
Ito and coworkers49 evaluated the role of EUS and intraductal ultrasound (IDUS) in preoperative or preendoscopic resection of ampullary neoplasms (adenomas and adenocarcinomas) and compared it with pathologic staging. The overall T staging accuracy of EUS was 63% (62% for pT1, 45% for pT2, and 88% for pT3-4) and of IDUS was 78% (86% for pT1 lesions, 64% for pT2 lesions, and 75% for pT3-4 lesions). Accuracy for determining intraductal biliary or pancreatic extension was similar between EUS and biliary IDUS (88% to 90%). We have also selectively employed cholangioscopy to assess for intraductal extension (Fig. 34.2).
For ampullary adenocarcinoma staging, comparative studies using EUS, computed tomography (CT), and magnetic resonance imaging (MRI) showed inconsistently higher accuracy of T staging by EUS but a surprising lack of difference in nodal staging.50,51 In a study by Kahaleh and associates,52 only EUS stage and a lack of a “lifting” sign were found to be predictive of malignancy (variables analyzed included gender, age, size of lesion, EUS T1 vs. T2 stage, and lifting) on univariate analysis, and only lifting was found to be a statistically significant predictor on multivariate analysis. Overall, these studies suggest that EUS and IDUS may be useful for assessing ampullary lesions by determining the extent of local invasion or intraductal involvement before consideration of endoscopic resection. However, the disadvantage of IDUS is the need for performing ERCP, which is unlikely necessary if invasive disease is identified by EUS or noninvasive imaging such as magnetic resonance cholangiopancreatography.
Surveillance
In 1950, Halsted and colleagues53 advocated upper GI surveillance of FAP patients. Given the risk of progression to malignancy, concerns regarding residual adenomatous tissue after ablation or resection, and the ongoing proliferative nature of these lesions, surveillance seems justified, although no studies have shown improved survival as a result. An ideal regimen for surveillance of these lesions is yet to be determined. As discussed previously, virtually all patients with FAP eventually have at least microscopic involvement of the ampulla, and most have multiple tiny adenomas spread over the proximal duodenum.
The optimal time interval for surveillance in FAP patients remains to be determined. Spigelman and coworkers54 retrospectively developed a scoring system to determine which patients are most likely to progress to malignancy and warrant more intense surveillance (see Table 34.1). Some authors have recommended surveillance intervals based on the Spigelman scoring system: 5 years for Spigelman stage 0–1, 3 years for stage 2, 1 to 2 years for stage 3, and consideration for surgery for stage 4.55 In addition to the Spigelman stage, other authors have modified this surveillance regimen based on the presence of any dysplasia.19 Endoscopic surveillance should also include examination of the papilla with a side-viewing duodenoscope. Video capsule endoscopy for Spigelman stages 3 and 4 has also been suggested, but push enteroscopy during surveillance of the ampulla could be performed at the same endoscopic session.
Therapy
Endoscopic therapy for ampullary adenomas is a minimally invasive alternative to major abdominal surgery. The ideal technique for endoscopic therapy for periampullary adenomas has not been established. Excision has the advantage of submitting en bloc tissue for histologic examination followed by tissue ablation of residual adenoma at the conclusion of the initial endoscopic session and at follow-up examinations. Preliminary data from our series of 50 patients comprising FAP patients and patients with sporadic ampullary adenoma suggest that a positive resection margin or suspected residual adenoma treated predominantly with argon plasma coagulation at the index ampullectomy session does not preclude adenoma-free follow-up, owing mostly to vigilant surveillance and ablation techniques.56
Snare Excision
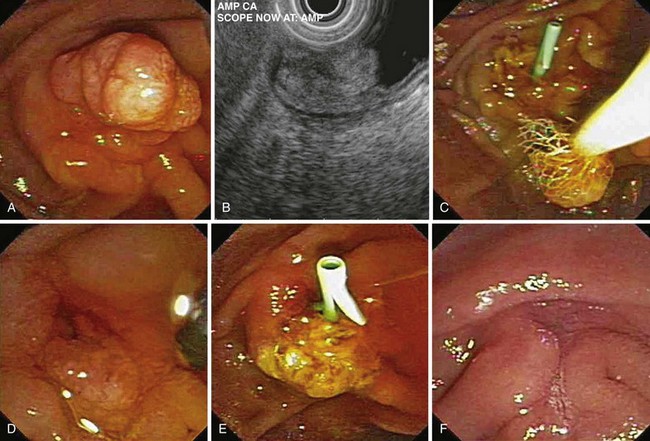
Snare excision removes the tumor en bloc (see Fig. 34.1). This technique has an advantage over piecemeal resection or preresection sphincterotomy by limiting cautery effect during histologic interpretation and potentially achieving a more accurate assessment of margin status. The procedure may be preceded by submucosal lift to raise the tumor. We add methylene blue to the solution, which enhances margin detection. Preresection cholangiopancreatography assists in assessing intraductal extension and provides a road map for pancreatic duct orifice identification and access after en bloc resection.
We prefer to perform postresection biliary and pancreatic sphincterotomies potentially to reduce subsequent orifice stenoses followed by prophylactic pancreatic and, when necessary, biliary stent placement. A concern with en bloc resection is the inability to identify the pancreatic orifice after ampullectomy. Methylene blue mixed in the contrast solution during pancreatography may assist in pancreatic orifice detection after resection. Other investigators have used indigo carmine staining to locate the pancreatic orifice.57,58 Obtaining initial pancreatic duct access, advancement of a snare over an intraductal guidewire followed by en bloc resection has been described to permit prophylactic stent placement.59 Whether this technique reduces pancreatitis complications compared with a conventional en bloc resection technique requires further study.
Piecemeal Resection
After cholangiopancreatography and dual biliary and pancreatic sphincterotomy (or pancreatic sphincterotomy alone), pancreatic stent insertion is performed. For smaller adenomas, extension of the sphincterotomies to the upper margin of the adenoma or to normal mucosa is attempted. Tissue is raised with a submucosal “lift,” and the adenoma is snared piecemeal. A potential concern with this technique is residual adenoma. Caution is required when using cautery techniques, such as argon plasma coagulation, to ablate residual tissue around the pancreatic orifice. During endoscopic follow-up, a guidewire left within the pancreatic duct during side-by-side cautery ablation in this area may permit ablation and preserve access for prophylactic stent placement (personal observation). Given its relatively shallow depth of injury, argon plasma coagulation may be an attractive method for destroying residual tissue.60
Efficacy
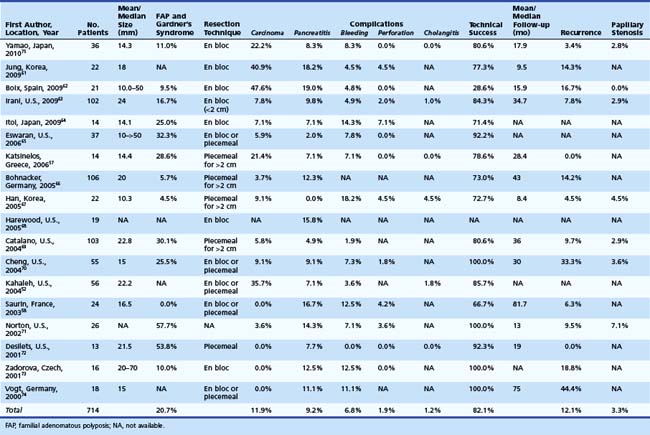
We reviewed the medical literature on endoscopic snare resection of the ampulla. We searched PubMed for a combination of key words: “ampullary adenoma,” “ampullary tumor,” “ampullary neoplasm,” “ampullectomy,” “endoscopic resection,” and “papillectomy.” Only published series with at least 10 subjects were included. Overall percentages are tabulated from the studies in which specific numbers are reported. In the last decade (2000–2009), 18 published series were found comprising 704 patients undergoing endoscopic snare resection of ampullary tumors.52,57,58,61–75 Table 34.2 summarizes these studies. The median number of patients in these studies was 23. Resected lesions ranged in size from 5 to 70 mm. When reported, patients with FAP or Gardner’s syndrome constituted approximately 21% of study subjects. Adenocarcinoma found within resection specimens ranged from 0% to 47%62 in these individual studies. This large variation largely depends on patient selection.
Thermal therapies (e.g., argon plasma coagulation, monopolar coagulation, snare tip cautery, or neodymium:yttrium-aluminum-garnet laser) have been reported as adjunctive therapy during the initial ampullectomy session. Thermal therapies were used in all but 1 of 14 evaluable studies.57 Catalano and coworkers69 reported a trend toward higher recurrence rates when index ampullectomy was performed without adjunctive ablative therapy. These methods are frequently used for retreatment of the ampullectomy site at follow-up.
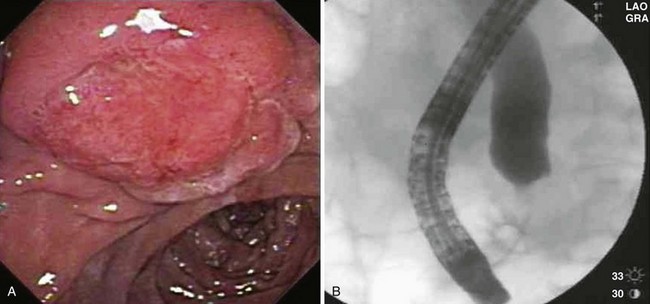
Patient selection for endoscopic resection is necessary to optimize results. Generally, endoscopic snare resection was not attempted for distal biliary or pancreatic duct involvement; larger size (>3 cm); circumferential involvement of the duodenum; lateral extension; duodenal infiltration; endoscopic features of malignancy (spontaneous bleeding, friability, ulceration, depressed areas, erythema, or induration; Fig. 34.3); nonlifting after submucosal injection; histologic diagnosis of malignancy; coagulopathy; or significant comorbid conditions precluding a safe procedure. High-grade dysplasia without frank malignancy has not been considered a contraindication to endoscopic resection in most studies.
In one of the largest series to date, Irani and colleagues63 evaluated 168 patients with ampullary lesions; 112 adenomas and 38 adenocarcinomas were found (18 nonadenomatous lesions were diagnosed and excluded from the analysis). Of patients, 41 (27.3%) were referred to surgery without ampullectomy. Of surgically treated patients, 20 patients had malignant mucosal biopsy specimens, 9 malignancies were identified at surgical resection, and 12 were large adenomas (of which two-thirds had high-grade dysplasia). Of the 150 patients with adenomatous lesions, 102 underwent endoscopic resection of the ampullary lesion. On univariate analysis, size smaller than 2 cm, younger age, absence of dilated ducts, and lack of lateral extension were factors affecting success of resection. On multivariate analysis, only size smaller than 2 cm and absence of dilated ducts were predictive of technically successful endoscopic resection.
With respect to resection technique, most studies have attempted en bloc removal of ampullary neoplasia for lesions smaller than 2 cm, whereas larger lesions often required piecemeal removal. In four studies that specifically described en bloc resection, success was achieved in 71.5% overall (range 54.5% to 94.4%).58,61,70,75 In a study specifically describing piecemeal resection, Desilets and associates72 described preampullectomy pancreatic sphincterotomy or stent placement and biliary sphincterotomy before resection in 13 patients (Fig. 34.4). After a mean of 2.7 procedures (mean follow-up 19 months), 92% were disease-free. One patient developed mild pancreatitis.
Overall, of the studies reviewed, technical success of endoscopic resection of ampullary lesions was approximately 84% (range 28% to 100%) in individual studies depending on inclusion criteria and definitions of success. The inclusion criteria ranged from assessment following the index procedure alone to multiple treatments including adjunctive thermal ablative therapy to a negative resection margin alone. At follow-up (range 16 to 81 months), approximately 73% of patients were adenoma-free after endoscopic resection and adjunctive ablation. Patient selection was another key variable in the reported wide range for success among studies. Boix and associates62 reported only 28.6% endoscopic success related mostly to inclusion of a high proportion of patients with carcinoma (10 of 21 [47.6%]) and intraductal extension (all of which failed endoscopic resection).
Endoscopic Complications
Complications of endoscopic snare resection of ampullary lesions have been classified as early or late. Early complications include bleeding, perforation, pancreatitis, and cholangitis. Late complications include papillary orifice stenoses. In the studies reviewed, bleeding has been reported in approximately 6% of patients, ranging from 0% to 18% requiring endoscopic hemostasis or transfusions. Perforation has been reported in approximately 2% (range 0% to 7%) of patients. Cholangitis has been reported in approximately 1% (range 0% to 4.5%) of patients. Only one mortality has been reported in the 18 studies.52
The most frequent complication with snare ampullectomy is acute pancreatitis. The studies reviewed reported pancreatitis in approximately 9% (range 0% to 19%) of patients. The only reported death from the reviewed ampullectomy series was due to complications of severe pancreatitis.52 Although many series have reported routine pancreatic stent placement with ampullectomy, there is only one study to date that prospectively evaluated the effect of pancreatic stent placement on postresection pancreatitis. Harewood and colleagues68 randomly assigned 19 consecutive patients undergoing en bloc resection of the ampulla to either pancreatic stent placement after ampullectomy or no pancreatic stent. Of 19 patients, 10 received a 5-Fr pancreatic stent after snare resection. Postresection pancreatitis occurred in three patients, all in the nonstent group (P = .02), with a median hospitalization of 2 days (range 1 to 6 days). Although our experience suggests that stent placement does not prevent pancreatitis, it is likely that it may help prevent severe pancreatitis. We prefer to place stents with an internal flange (one internal and two external or one internal with a full external pigtail) to reduce the risk of premature migration or dislodgment in the setting of a postprocedural bleeding event.
Surgical Therapy
Winter and associates76 reported 450 patients who underwent surgical treatment. Of these patients, 77% had ampullary adenocarcinoma (41.1% of which had T2 disease). Almost 97% of the patients underwent pancreaticoduodenectomy (96.1% with R0 resection), and approximately 3% underwent surgical ampullectomy. Metastasis to regional lymph nodes (N1) was noted in 54.5%. Morbidity after surgery was reported in 52.2% for pancreaticoduodenectomy and 33.3% for ampullectomy (P = .15). The most common complications with pancreaticoduodenectomy included pancreatic leak (20.7%), delayed gastric emptying (16.0%), and wound infection (11.1%). Postoperative mortality was 2.1%.
Yoon and coworkers77 reported their results for ampullectomy for early ampullary cancer. In their study, 98 patients underwent conventional Whipple’s pancreaticoduodenectomy, 100 patients underwent pylorus-preserving pancreaticoduodenectomy, and 3 patients underwent pancreatic head resection with segmental duodenectomy. R0 resection was achieved in 95.5% of patients. Two deaths occurred (1%) from bleeding and sepsis. Postsurgical complications were experienced in 33.8%, including pancreatic fistula (9.5%), wound infection (6.5%), delayed gastric emptying (5.5%), bleeding (5.5%), intraabdominal abscess, biliary fistula, pneumonia, pleural effusion, and ileus. Lymph node metastasis in this select surgical series was high (33.3%).
Surgical ampullectomy is not a new technique, having been reported for ampullary lesions by Halsted in 1899 and used as a less invasive surgical alternative to pancreaticoduodenectomy.40,42,78–80 Transduodenal resection may be inadequate therapy in many patients. Recurrence of benign adenomas has been reported in 25% to 33%.40,42,80 FAP patients are a particularly difficult treatment group. Recurrent duodenal adenomas after transduodenal resection (mean recurrence in 13 months) led one group to conclude that this is inadequate therapy for these patients.81 The potential for desmoid formation after surgery is another factor favoring the use of nonsurgical (i.e., endoscopic) techniques.
Grobmyer and colleagues82 reported on their experience of surgical ampullectomy for 29 patients with preoperative adenoma (n = 26) and carcinoid (n = 3). However, 4 (13.7%) of the 29 patients were found to have carcinoma on surgical pathology and subsequently required pancreaticoduodenectomy. Morbidity was experienced by 45% and included wound infection, pneumonia, pancreatitis, pancreatic or biliary fistula, abdominal abscess, delayed gastric emptying, Clostridium difficile colitis, and deep vein thrombosis. There was one death (3.4%). Recurrence of adenoma was noted in 8%.
Pharmacologic Treatment
A randomized controlled study showed that sulindac slows the progression of polyps in the colon of patients with FAP syndrome.83 Similar findings have been reported with the use of the cyclooxygenase-2 (COX-2)–specific drug celecoxib.84 A randomized placebo-controlled trial showed that celecoxib was an effective chemoprotective agent against sporadic colorectal adenomas85; however, it was associated with significant cardiovascular events. There is less compelling evidence for the use of nonsteroidal antiinflammatory drugs for progression of duodenal disease. The St. Mark’s group randomly assigned 24 patients with advanced duodenal disease to sulindac, 200 mg twice daily, or placebo.86 After 6 months of treatment, there was a reduction in epithelial proliferation in the sulindac group but no significant regression of large polyps. However, blinded review of videotapes showed significant regression of small polyps (<2 mm) compared with the placebo group.
This evidence supports the hypothesis that sulindac may also have an effect on polyp proliferation in the duodenum; however, it is unknown whether this would translate into a clinically significant benefit. The FAP Study Group randomly assigned 83 patients to one of two celecoxib groups (100 mg twice daily or 400 mg twice daily) or placebo.87 Blinded, shuffled review of surveillance endoscopy by five panelists found a significant reduction in duodenal polyposis after 6 months of treatment with celecoxib, 400 mg twice daily. The cardiovascular toxicity of COX-2 inhibitors is well publicized, however, and use of these agents as chemopreventive agents must be cautiously considered.
1 Spigelman AD, Williams CB, Talbot IC, et al. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1988;2:783-785.
2 Takano S, Matsushima M, Ertuk E, et al. Early induction of rat colonic ornithine decarboxylase activity by n-methyl-n-nitro-n-nitrosoguanidine or bile salts. Cancer Res. 1981;41:624-628.
3 Spigelman AD, Scates DK, Venitt S, et al. DNA adducts, detected by 32P-postlabelling, in the foregut of patients with familial adenomatous polyposis and in unaffected controls. Carcinogenesis. 1991;12:1727-1732.
4 Deschner EE, Raicht RF. Influence of bile on kinetic behavior of colonic epithelial cells of the rat. Digestion. 1979;19:322-327.
5 Spigelman AD, Crofton-Sleigh C, Venitt S, et al. Mutagenicity of bile and duodenal adenomas in familial adenomatous polyposis. Br J Surg. 1990;77:878-881.
6 Powell SM, Petersen GM, Krush AJ, et al. Molecular diagnosis of familial adenomatous polyposis. N Engl J Med. 1993;328:1982-1987.
7 Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis gene. Cell. 1991;66:589-600.
8 Lynch HT, Smyrk T, McGinn T, et al. Attenuated familial adenomatous polyposis (AFAP): A phenotypically and genotypically distinctive variant of FAP. Cancer. 1995;76:2427-2433.
9 Leggett BA, Young JP, Biden K, et al. Severe upper gastrointestinal polyposis associated with sparse colonic polyposis in a familial adenomatous polyposis family with an APC mutation at codon 1520. Gut. 1997;41:518-521.
10 Soravia C, Bapat B, Cohen Z. Familial adenomatous polyposis (FAP) and hereditary nonpolyposis colorectal cancer (HNPCC): A review of clinical, genetic and therapeutic aspects. Schweiz Med Wochenschr. 1997;127:682-790.
11 Soravia C, Berk T, Madlensky L, et al. Genotype-phenotype correlations in attenuated adenomatous polyposis coli. Am J Hum Genet. 1998;62:1290-1301.
12 Boland CR, Kim YS. Gastrointestinal polyp syndromes. In: Sleisenger M, Fordtran J, editors. Gastrointestinal disease, Vol. 2. Philadelphia: Saunders; 1993:1430-1448.
13 Paraf F, Jothy S, Van Meir EG. Brain tumor-polyposis syndromes: Two genetic diseases? J Clin Oncol. 1997;15:2744-2758.
14 Arvantis ML, Jagelman DG, Fazio VW, et al. Mortality in patients with familial adenomatous polyposis. Dis Colon Rectum. 1990;33:639-642.
15 Jagelman DG, DeCosse JJ, Bussey HJ. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet. 1988;1:1149-1151.
16 Geller A, Wang KK, Batts KP, et al. Ileostomy pouch polyposis in a patient with familial adenomatous polyposis. Gastrointest Endosc. 1995;41:377.
17 Sarre RG, Frost AG, Jagelman DG, et al. Gastric and duodenal polyps in familial adenomatous polyposis: A prospective study of the nature and prevalence of upper gastrointestinal polyps. Gut. 1987;28:306-314.
18 Debinski HS, Spigelman AD, Hatfield A, et al. Upper intestinal surveillance in familial adenomatous polyposis. Eur J Cancer. 1995;31A:1149-1153.
19 Bianchi LK, Burke CA, Bennett AE, et al. Fundic gland polyp dysplasia is common in familiar adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6:180-185.
20 Attard TM, Cuffari C, Tajouri T, et al. Multicenter experience with upper gastrointestinal polyps in pediatric patients with familiar adenomatous polyposis. Am J Gastroenterol. 2004;99:681-686.
21 Cordero-Fernandez C, Garzon-Benavides M, Pizarro-Moreno A, et al. Gastroduodenal involvement in patients with familial adenomatous polyposis: Prospective study of the nature and evolution of polyps: Evaluation of the treatment and surveillance methods applied. Eur J Gastroenterol Hepatol. 2009;21:1161-1167.
22 Garrean S, Hering J, Saied A, et al. Gastric adenocarcinoma arising from fundic gland polyps in a patient with familiar adenomatous polyposis syndrome. Am Surg. 2008;74:79-83.
23 Hofgartner WT, Thorp M, Ramus MW, et al. Gastric adenocarcinoma associated with fundic gland polyps in a patient with attenuated familial adenomatous polyposis. Am J Gastroenterol. 1999;94:2275-2281.
24 Zwick A, Munir M, Ryan CK, et al. Gastric adenocarcinoma and dysplasia in fundic gland polyps of a patient with attenuated adenomatous polyposis coli. Gastroenterology. 1997;113:659-663.
25 Sawada T, Muto T. Familial adenomatous polyposis: Should patients undergo surveillance of the upper gastrointestinal tract? Endoscopy. 1995;27:6-11.
26 Iwama T, Mishima Y, Utsonomiya J. The impact of familial adenomatous polyposis on the tumorigenesis and mortality in several organs. Ann Surg. 1993;217:100-108.
27 Scarpa A, Capelli P, Zamboni G, et al. Neoplasia of the ampulla of Vater: Ki-ras and p53 mutations. Am J Pathol. 1993;142:1163-1172.
28 Baggenstoss AH. Major duodenal papilla: Variations of pathologic interest and lesions of the mucosa. Arch Pathol. 1938;26:853-868.
29 Bleau BL, Gostout CJ. Endoscopic treatment of ampullary adenomas in familial adenomatous polyposis. J Clin Gastroenterol. 1996;22:237-241.
30 Offerhaus GJ, Giardiello FM, Krush AJ, et al. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992;102:1980-1982.
31 Nugent KP, Spigelman AD, Williams CB, et al. Surveillance of duodenal polyps in familial adenomatous polyposis: Progress report. J R Soc Med. 1994;87:704-706.
32 Sanabria JR, Croxford R, Berk TC, et al. Familial segregation in the occurrence and severity of peri-ampullary neoplasms in familial adenomatous polyposis. Am J Surg. 1996;171:136-140.
33 Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim Biophys Acta. 1997;1332:F127-F147.
34 Gallinger S, Vivona AA, Odze RD, et al. Somatic APC and K-ras codon 12 mutations in peri-ampullary adenomas and carcinomas from familial adenomatous polyposis patients. Oncogene. 1995;10:1875-1878.
35 Noda Y, Watanabe H, Iida M, et al. Histologic follow-up of ampullary adenomas in patients with familial adenomatosis coli. Cancer. 1992;70:1847-1856.
36 Yamaguchi K, Enjoji M. Adenoma of the ampulla of Vater: Putative precancerous lesion. Gut. 1991;32:1558-1561.
37 Sobol S, Cooperman AM. Villous adenoma of the ampulla of Vater: An unusual cause of biliary colic and obstructive jaundice. Gastroenterology. 1978;75:107-109.
38 Guzzardo G, Kleinman MS, Krackov JH, et al. Recurrent acute pancreatitis caused by ampullary villous adenoma. J Clin Gastroenterol. 1990;12:200-202.
39 Ponchon T, Berger F, Chavaillon A, et al. Contribution of endoscopy to diagnosis and treatment of tumors of the ampulla of Vater. Cancer. 1989;64:161-167.
40 Alstrup N, Burcharth F, Hauge C, et al. Transduodenal excision of tumours of the ampulla of Vater. Eur J Surg. 1996;162:961-967.
41 Church JM, McGannon E, Hull-Boiner S, et al. Gastroduodenal polyps in patients with familial adenomatous polyposis. Dis Colon Rectum. 1992;35:1170-1173.
42 Cahen DL, Fockens P, De Wit LT, et al. Local resection or pancreaticoduodenectomy for villous adenoma of the ampulla of Vater diagnosed before operation. Br J Surg. 1997;84:948-951.
43 Dekker E, Boparai KS, Poley JW, et al. High resolution endoscopy and the additional value of chromoendoscopy in the evaluation of duodenal adenomatosis in patients with familial adenomatous polyposis. Endoscopy. 2009;41:666-669.
44 Itoi T, Tsuji S, Sofuni A, et al. A novel approach emphasizing preoperative margin enhancement of tumor of the major duodenal papilla with narrow-band imaging in comparison to indigo carmine chromoendoscopy (with videos). Gastrointest Endosc. 2008;69:136-141.
45 Tio TL, Tytgat GN, Cikot RJ, et al. Ampullopancreatic carcinoma: Preoperative TNM classification with endosonography. Radiology. 1990;175:455-461.
46 Tio TL, Mulder CJ, Eggink WF. Endosonography in staging early carcinoma of the ampulla of Vater. Gastroenterology. 1992;102:1392-1395.
47 Tio TL, Sie LH, Kallimanis G, et al. Staging of ampullary and pancreatic carcinoma: Comparison between endosonography and surgery. Gastrointest Endosc. 1996;44:706-713.
48 Rosch T, Braig C, Gain T, Feuerbach S, et al. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography: Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology. 1992;102:188-199.
49 Ito K, Fujita N, Noda Y, et al. Preoperative evaluation of ampullary neoplasm with EUS and transpapillary intraductal US: A prospective and histopathologically controlled study. Gastrointest Endosc. 2007;66:740-747.
50 Artifon EL, Couto DJr, Sakai P, et al. Prospective evaluation of EUS versus CT scan for staging of ampullary cancer. Gastrointest Endosc. 2009;70:290-296.
51 Chen CH, Yang CC, Yeh YH, et al. Reappraisal of endosonography of ampullary tumors: Correlation with transabdominal sonography, CT, and MRI. J Clin Ultrasound. 2008;37:18-25.
52 Kahaleh M, Shami VM, Brock A, et al. Factors predictive of malignancy and endoscopic resectability in ampullary neoplasia. Am J Gastroenterol. 2004;99:2335-2339.
53 Halsted JA, Harris EJ, Bartlett MK. Involvement of the stomach in familial polyposis of the gastrointestinal tract. Gastroenterology. 1950;15:763-770.
54 Spigelman AD, Williams CB, Talbot IC, et al. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783-785.
55 Vasen HF, Moslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut. 2008;57:704-713.
56 Meier CB, Mummadi RR, Brauer BC, et al. Does positive resection margin at index endoscopic ampullectomy predict residual or recurrent adenoma? Gastrointest Endosc. 2010;71:AB308.
57 Katsinelos P, Paroutoglou G, Kountouras J, et al. Safety and long-term follow-up of endoscopic snare excision of ampullary adenomas. Surg Endosc. 2006;20:608-613.
58 Saurin JC, Chavaillon A, Napoleon B, et al. Long-term follow-up of patients with endoscopic treatment of sporadic adenomas of the papilla of Vater. Endoscopy. 2003;35:402-406.
59 Moon JH, Cha SW, Cho YD, et al. Wire-guided endoscopic snare papillectomy for tumors of the major duodenal papilla. Gastrointest Endosc. 2005;61:461-466.
60 Norton ID, Wang L, Levine SA, et al. In vivo characterization of colonic thermal injury by the argon plasma coagulator. Gastrointest Endosc. 2002;55:631-636.
61 Jung MK, Cho CM, Park SY, et al. Endoscopic resection of ampullary neoplasms: A single-center experience. Surg Endosc. 2009;23:2568-2574.
62 Boix J, Lorenzo-Zuniga V, Moreno de Vega V, et al. Endoscopic resection of ampullary tumors: 12-year review of 21 cases. Surg Endosc. 2009;23:45-49.
63 Irani S, Arai A, Ayub K, et al. Papillectomy for ampullary neoplasm: Results of a single referral center over a 10-year period. Gastrointest Endosc. 2009;70:923-932.
64 Itoi T, Tsuji S, Sofuni A, et al. A novel approach emphasizing preoperative margin enhancement of tumor of the major duodenal papilla with narrow-band imaging in comparison to indigo carmine chromoendoscopy. Gastrointest Endosc. 2009;69:136-141.
65 Eswaran SL, Sanders M, Bernadino KP, et al. Success and complications of endoscopic removal of giant duodenal and ampullary polyps: A comparative series. Gastrointest Endosc. 2006;64:925-932.
66 Bohnacker S, Seitz U, Nguyen D, et al. Endoscopic resection of benign tumors of the duodenal papilla without and with intraductal growth. Gastrointest Endosc. 2005;62:551-560.
67 Han J, Lee SK, Park DH, et al. Treatment outcome after endoscopic papillectomy of tumors of the major duodenal papilla. Korean J Gastroenterol. 2005;46:110-119.
68 Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367-370.
69 Catalano MF, Linder JD, Chak A, et al. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225-232.
70 Cheng CL, Sherman S, Fogel EV, et al. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2002;60:757-764.
71 Norton ID, Gostout CJ, Baron TH, et al. Safety and outcome of endoscopic snare excision of the major duodenal papilla. Gastrointest Endosc. 2002;56:239-243.
72 Desilets DJ, Dy RM, Ku PM, et al. Endoscopic management of tumors of the major duodenal papilla: Refined techniques to improve outcome and avoid complications. Gastrointest Endosc. 2001;54:202-208.
73 Zadorova Z, Dvorak M, Hajer J. Endoscopic therapy of benign tumors of the papilla of Vater. Endoscopy. 2001;33:345-347.
74 Vogt M, Jakobs R, Benz C, et al. Endoscopic therapy of adenomas of the papilla of Vater: A retrospective analysis with long-term follow-up. Dig Endosc. 2000;32:339-345.
75 Yamao T, Isomoto H, Kohno S, et al. Endoscopic snare papillectomy with biliary and pancreatic stent placement for tumors of the major duodenal papilla. Surg Endosc. 2010;24:119-124.
76 Winter JM, Cameron JL, Olino K, et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: Implications for surgical strategy and long-term prognosis. J Gastrointest Surg. 2010;14:379-387.
77 Yoon YS, Kim SW, Park SJ, et al. Clinicopathologic analysis of early ampullary cancers with a focus on the feasibility of ampullectomy. Ann Surg. 2005;242:92-100.
78 Knox RA, Kingston RD. Carcinoma of the ampulla of Vater. Br J Surg. 1986;73:72-73.
79 Asbun HJ, Rossi RL, Munson JL. Local resection for ampullary tumors: Is there a place for it? Arch Surg. 1993;128:515-520.
80 Farouk M, Niotis M, Branum GD, et al. Indications for and the technique of local resection of tumors of the papilla of Vater. Arch Surg. 1991;126:650-652.
81 Penna C, Phillips RK, Tiret E, et al. Surgical polypectomy of duodenal adenomas in familial adenomatous polyposis: Experience of two European centres. Br J Surg. 1993;80:1027-1029.
82 Grobmyer SR, Stasik CN, Draganov P, et al. Contemporary results with ampullectomy for 29 “benign” neoplasms of the ampulla. J Am Coll Surg. 2008;206:466-471.
83 Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313-1316.
84 Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946-1952.
85 Bertagnolli MM, Eagle CJ, Redson M, et al. APC Study Investigators. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873-884.
86 Debinski HS, Trojan J, Nugent KP, et al. Effect of sulindac on small polyps in familial adenomatous polyposis. Lancet. 1995;345:855-856.
87 Phillips RK, Wallace MH, Lynch PM, et al. A randomized, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut. 2002;50:857-860.