Chapter 55 Endoscopic Endonasal Approaches to the Skull Base and Paranasal Sinuses
 Videos corresponding to this chapter are available online at www.expertconsult.com.
Videos corresponding to this chapter are available online at www.expertconsult.com.
INTRODUCTION/BACKGROUND
Approaches to the skull base via the paranasal sinuses were introduced in the late 19th and early 20th century. Tumors of the sellar region were first approached through various incisions in the forehead to gain access to the sphenoid sinus via the ethmoid sinuses. Cushing introduced what became the standard for transsphenoidal surgery in 1910 when he first used a sublabial incision to gain access to the sinuses.1 coincidentally, on the exact same day, June 4, Oscar Hirsch used an endonasal approach to gain access to the sphenoid sinus. This would eventually replace the sublabial approach, but Cushing’s influence delayed this for decades.
This transsphenoidal approach to the sella turcica was conceptually unchanged for approximately 70 years. However, the operation and its outcomes were changed greatly during this period by advances in technology which provided improved visualization, intraoperative image-guidance and instrumentation. Dott, Guiot and Hardy added pneumoencephalocisternography, radiofluoroscopic guidance, and the operative microscope. All of this revolutionized the approach and improved outcomes. A similar change in technology began in the 1980s with the development of early intraoperative CT and MR image-guidance systems and the introduction of the endoscope by Carrau.2,3 At the same time, otolaryngologists were developing and refining functional endoscopic sinus surgery (FESS).4–9 It was the collaboration between otolaryngologists and neurosurgeons that led to the expansion of the standard transsphenoidal approach to include the rest of the anterior skull base and beyond.
EXPANDED ENDONASAL APPROACHES: ANATOMIC MODULES
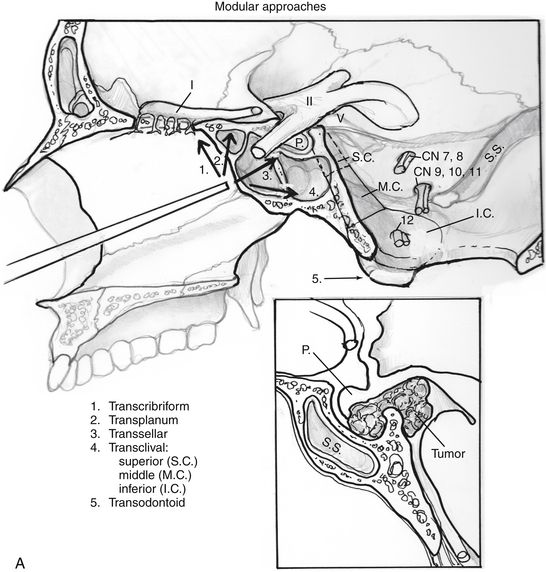
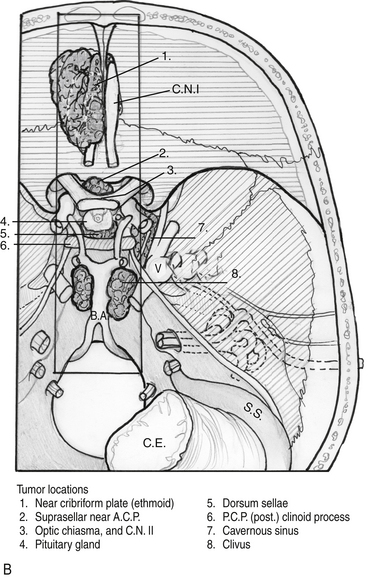
Endoscopic endonasal approaches (EEAs) can be divided into the sagittal or rostral-caudal plane (between the carotids) and coronal or paramedian plane (lateral to the carotid). The sagittal plane can be divided from rostral to caudal, anterior to posterior into the transfrontal, transcribriform, transplanum/transtuberculum, transsellar, transclival and transodontoid approaches (Fig. 55-1). The coronal plane is somewhat more complex in that the lateral expanded approaches vary based upon which fossa is involved (Fig. 55-2). The anterior fossa, lateral approaches consist of the supra- and transorbital approaches. The middle fossa is the most complex and is broken into five “transpterygoid” anatomic zones of approach: medial pterygoid (petrous apex), petroclival junction, quadrangular space or Meckel’s cave, superior cavernous sinus, and the infratemporal fossa (Fig. 55-3). These are all critically dependent upon their relationship with the internal carotid artery (ICA). Finally, the posterior fossa or inferior expanded approaches consist of the transcondylar and parapharyngeal space approaches.
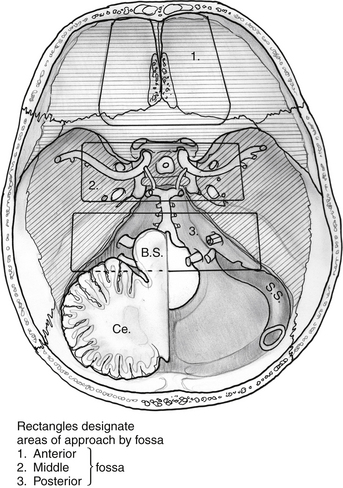
Figure 55-2 Superior view of skull base with general regions of approach by fossa. 1) anterior, 2) middle, 3) posterior.
Sagittal Plane
Transcribriform Approach
A complete sphenoethmoidectomy is performed on each side and the nasofrontal recesses are visualized. If needed, exophytic tumor within the nasal cavity can be debulked. The superior nasal septum is transected from the crista galli to the sphenoid rostrum as needed. As mentioned above, a transfrontal approach can be performed to establish an anterior margin. The transcribriform approach provides direct access to the vascular supply of tumors such as olfactory groove meningiomas, allowing early devascularization. The anterior and posterior ethmoidal arteries should be identified early and cauterized or clip ligated. The lateral bony margins are drilled to form “gutters” or osteotomies for the length of the tumor or planned resection as needed. If necessary, the lamina papyracea can be removed as well to allow retraction on the periorbita, thereby displacing the orbital contents for even more lateral access. In fact, the lateral access can extend all the way to the midorbital line at the level of the superior rectus muscle. After the lateral margins are drilled, the planum or tuberculum can be drilled as needed for a posterior margin. Even if necessary for tumor resection, it may be safest to leave the bone of the optic canals intact as long as possible. Finally, the dura is dissected from the crista galli to allow its removal. The remaining bone of the cribriform plate can now be dissected free (Fig. 55-4). If necessary for purely extradural lesions, the dura should be left intact. This may not be possible over the olfactory filaments, which can be cauterized to minimize CSF leakage, but should nonetheless undergo formal repair (see below). Obviously, olfaction (when present) is sacrificed if a bilateral approach is undertaken.
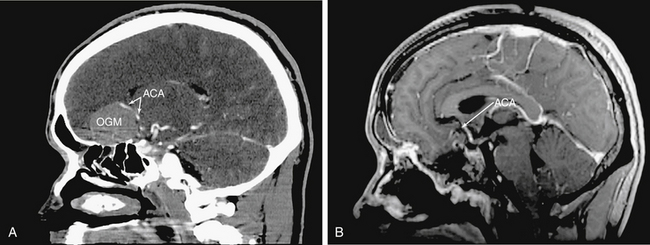
Intradural tumor resection must be performed with caution, especially when approaching the interhemispheric fissure, as there are frequently anterior cerebral artery branches or even the anterior communicating artery on the surface of the tumor (Fig. 55-5). An endonasal ultrasonic aspirator or two suctions can be used depending upon tumor consistency and proximity of involved structures. Microsurgical concepts of preserving arachnoid planes when possible and sharp dissection of critical structures is maintained throughout. All of this may require the use of angled scopes (45° or 70°).
Transplanum/Transtuberculum Approach
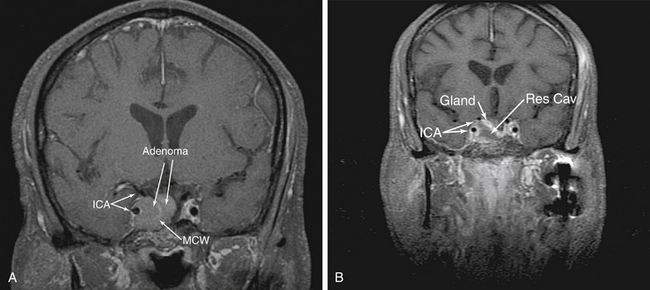
Also described as an “extended” approach, the transplanum approach was the first expansion of a traditional transsphenoidal approach.10–13 This approach provides a natural corridor for many suprasellar tumors such as craniopharyngiomas, tuberculum meningiomas and large pituitary adenomas. It may also be used for biopsy or resection of infundibular lesions such as metastases or hypophysitis. This approach is often an integral addition to transsellar and transcribriform approaches.
A transsphenoidal exposure (see transsellar approach below) is augmented by a posterior ethmoidectomy. The posterior ethmoidal arteries are a good landmark for anterior extent to preserve olfaction while providing adequate access. The optic canals are obviously critical to identify in order to avoid damage to the nerves. Whenever drilling over or near the optic canals, it is important to constantly irrigate to avoid heat transmission from the drill to the nerves. After the sella is exposed, the planum can be drilled and thinned. This can require an angled endoscope for adequate visualization depending upon the slope of the anterior skull base. Though somewhat counterintuitive, the planum is most easily removed in an anterior to posterior direction after the bone has been adequately thinned, exposing dura at the anterior extent and laterally. The lateral margins are actually the optic nerves which form the sides of a trapezoid which include the anterior planum and tuberculum sellae. Often there is no need to expose the optic nerves. However, in many tuberculum meningiomas, there is extension of disease into the medial optic canals (Fig. 55-6). This disease is not easily accessed via a craniotomy and requires release and retraction of an often already compromised optic nerve. In these cases, the bone overlying the tumor in the optic canal should be thinned (“blue-lined”) with a drill and carefully removed with a dissector.
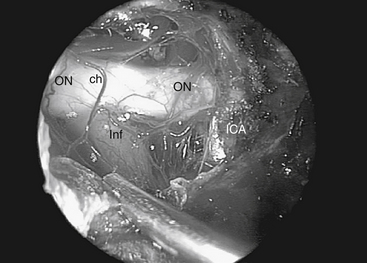
When approaching suprasellar lesions, the superior intercavernous sinus (SIS) can often merely be retracted inferiorly rather than being transected. If, however, it must be traversed, it is useful to open the dura of the sella below the SIS in the midline as well as the dura of the planum above the SIS. This provides access on both sides of the sinus to perform a controlled ligation, either with a bipolar or clips. The dura can be reduced bilaterally with bipolar coagulation, using care to not damage the optic nerves. It is also important not to coagulate the superior hypophyseal artery as this can lead to hypopituitarism. Once the dura is opened, tumor resection proceeds carefully, identifying critical structures systematically. First, one supraclinoid internal carotid artery (ICA) is identified. Resection proceeds until the ipsilateral optic nerve is identified, followed by the chiasm, then the contralateral optic nerve and supraclinoid ICA (Fig. 55-7). In order to gain direct and unencumbered access to the paraclinoid ICA it is imperative to remove the medial optico-carotid recess (mOCR). This represents the lateral extension of the tuberculum and the pneumatization of the middle clinoid.14 The mOCR is the key anatomic landmark for this module.
Transsellar Approach
Transsellar approaches are well known for access to pituitary adenomas as well as the myriad of sellar pathologies which exist, such as Rathke’s cleft cysts (RCCs), arachnoid cysts and rare craniopharyngiomas. In addition, by utilizing a pituitary transposition, retroinfundibular lesions, such as granular cell tumors and some craniopharyngiomas, can be easily accessed while potentially preserving pituitary function. Endoscopic approaches to the sella are different from standard microscopic approaches in that they both provide more lateral access and require more exposure. Indeed, tumor which extends into the medial cavernous sinus can be accessed effectively using an EEA (Fig. 55-8). The bone overlying the cavernous ICA which guards the medial cavernous sinus must be carefully thinned and removed to allow some displacement of the ICA. However, it is intimate and direct visualization which allows successful resection of tumor in the cavernous sinus. Lateral sphenoidal exposure will be covered in detail in the coronal plane section. Greater exposure is required because room must be made for instruments to work around the endoscope while maintaining visualization. As a result, the sphenoid must be opened completely to work unencumbered in the sella. For example, lesions such as RCCs which are commonly in the pars intermedia can be approached from below, under the anterior gland (“subsellar approach”) rather than through it. This requires drilling the floor of the sphenoid sinus flush with the clival recess to allow this inferior access and visualization that the endoscope provides. The guiding anatomic principle for this module is complete bony removal and exposure of the sella from “blue to blue”; that is, laterally from cavernous sinus to cavernous sinus and from SIS superiorly to the inferior intercavernous sinus (IIS) inferiorly.
Superior Clivus
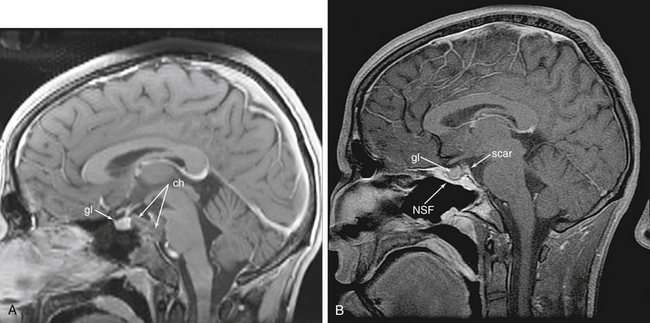
The pituitary transposition is a newly described technique for access to lesions directly behind the pituitary gland or infundibulum, such as retroinfundibular craniopharyngiomas, granular cell tumors and chordomas.15 In addition, clival lesions, such as “chondroid” tumors (e.g. chordomas and chondrosarcomas) often involve this portion of the clivus (Fig. 55-9). While conceptually simple, this is technically demanding. The pituitary gland is dissected free from the sella and lifted to allow access to the space behind it. However, there are many bands of fibrous connective tissue which anchor the gland in the sella, mostly along the lateral walls to the cavernous sinus. These bands must be sharply dissected to free and preserve the gland. There is often some venous bleeding during this portion of the dissection and a two-surgeon, three of four-hand technique becomes critical. The inferior hypophyseal arteries may need to be sacrificed. In our experience, as long as the superior hypophyseal arteries (SHAs) are preserved, gland function will be preserved. After all of the lateral connective bands are released, the aperture in the sellar dura through which the stalk enters should be opened to allow release of the stalk and complete freedom for transposition. This should be done carefully with a fine, pistol-grip scissor with visualization of the SHAs. At this point, the gland can be finally raised from the sella and held superiorly with fibrin glue. This gives direct access to the dorsum sellae and posterior clinoids which can then be drilled and removed for access to the interpeduncular and basilar cisterns. There is more brisk venous bleeding during this drilling, as the clival venous plexus extends into the clinoids and dorsum sellae. This tight space, surrounded by critical structures with often brisk bleeding, requires an experienced team for a safe and controlled approach. In our opinion, this may be the most complex EEA procedure.
Middle Clivus
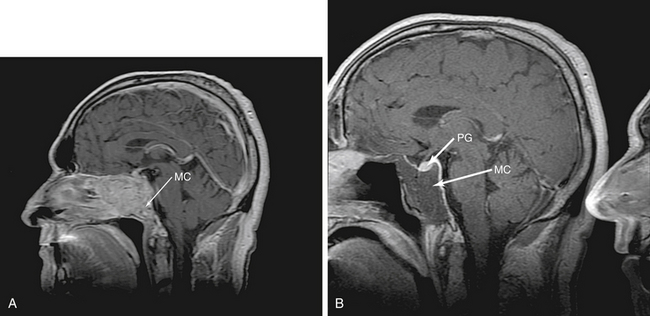
This approach accesses the majority of the clivus. Tumors such as chordomas and chondrosarcomas can often be relatively easily accessed endonasally (Fig. 55-10). In addition, we have used EEAs to address large portions of petroclival meningiomas, neurenteric cysts and a clival arteriovenous malformation (AVM). As with any approach it is necessary to understand the anatomic structures involved. The paraclival (vertical portion of the cavernous ICA) creates the lateral boundaries for this approach. It is therefore essential to stay medial to the pterygoid base, beneath which runs the paraclival ICA. At times, it is necessary to work behind the paraclival ICA to completely resect clival tumors. In addition, the abducens nerve is always a concern in this area and its course must be understood. The abducens nerve emerges at the level of the vertebrobasilar junction (VBJ), making preferable to open dura or tumor as caudal as possible. Abducens nerve monitoring plus slow, careful dissection toward the superior end of the clivus is critical. In addition, tumors such as petroclival meningiomas, which often have their origin posterior to the VIth nerve can displace the nerve anteriorly, even pressing it against the dura. Careful opening of only the dura when approaching these tumors can prevent inadvertent injury.
Transodontoid Approach
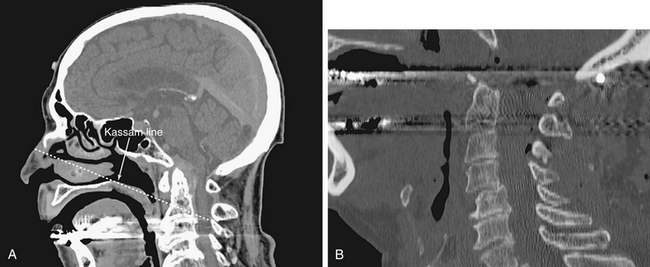
The most common pathology treated in this location is basilar invagination/pannus associated with longstanding degenerative arthritis. Additional pathology includes craniocervical meningiomas and chordomas. The anatomic landmarks for this module are the floor of the sphenoid above and the soft/hard palate below. Laterally, the region is bounded by the Eustachian tubes (ETs) that guard the parapharyngeal ICAs (internal carotid arteries). Inferior extent of endonasal resection can be determined preoperatively by using the ‘Kassam line’, which is drawn from the tip of the bony nasal bridge to the posterior hard palate and extended to the pathology in the depth as needed (Fig. 55-11). The nasopharyngeal mucosa and the longus capitus muscles are resected to expose the basion, arch of C1 and base of the odontoid process. The arch of C1 is removed and the dens resected using an 18 cm high-speed drill. If the lesion extends intradurally, the circular sinus is packed and the dura opened. Intradural resection requires respecting the lateral plane of the vertebral arteries as all of the lower cranial nerves are located laterally at this level.
Coronal Plane
Anterior Fossa
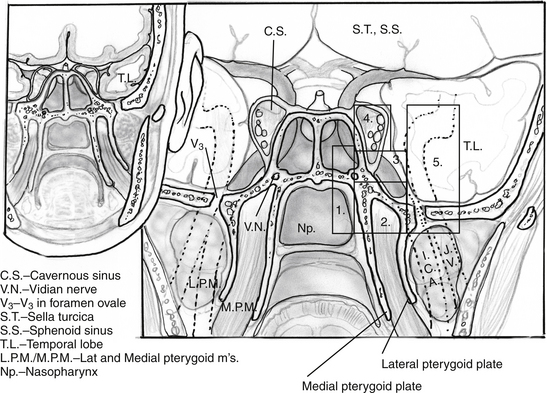
Transpterygoid Approach
II Petroclival Junction
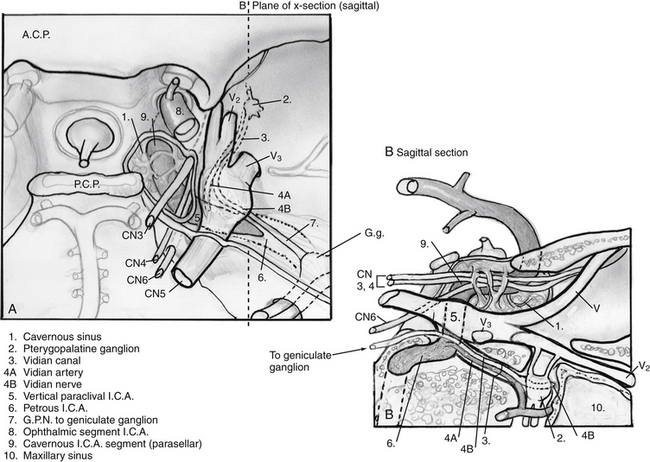
1. An extension of the petrous apex approach, this zone is usually involved with similar tumors. In addition, debulking of petroclival meningiomas can be achieved via this approach, often in combination with a clival approach. Occasionally, lateralization of the ICA is necessary to increase exposure when there is minimal medial expansion of the lesion. In such cases, the vidian canal is carefully drilled and followed to the 2nd (anterior) genu of the ICA, starting inferomedially to prevent carotid injury. After the bone overlying the paraclival (vertical cavernous) carotid artery is thinned, it can be carefully removed, allowing the artery to be displaced laterally without compressing it on an edge of bone. The relationship between this portion of the carotid artery and the vidian canal16,17 is critical to understand even if a transpterygoid approach is not needed (Fig. 55-12). In addition, the oblique course of the abducens nerve, running from the vertebrobasilar junction (VBJ) to Dorello’s canal, just lateral to the superior-most aspect of the paraclival ICA, to the cavernous sinus, is critical to fully understand in order to prevent injury during these transpterygoid approaches.
III Quadrangular Space (Meckel’s Cave)
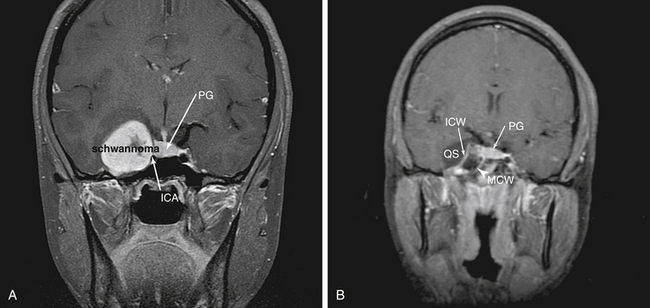
The cranial nerves which are contained in the cavernous sinus are crowded into the superolateral cavernous sinus. This allows relatively safe access to the medial cavernous sinus via the previously described transsellar route. Tumor in Meckel’s cave can also be relatively safely accessed, but much greater care must be taken to avoid ophthalmoplegia. Tumors such as pituitary adenomas rarely are found in this location. However, tumors such as adenoid cystic carcinoma and other nasopharyngeal carcinomas can extend via neural spread along trigeminal branches (usually the maxillary [V2] or mandibular [V3]) directly into this space. In addition, this space provides good endonasal access to schwannomas of the cranial nerves in the cavernous sinus (Fig. 55-13). The “quadrangular space” (Fig 55-2) is an area of the skull base which contains the Gasserian ganglion and is bounded inferiorly by the petrous ICA, medially by first the vertical segment of the cavernous ICA (also know as the paraclival ICA), laterally by the maxillary branch of the trigeminal nerve (V2) and superiorly by the abducens nerve (VI). The abducens nerve runs directly under the ophthalmic branch (V1) of the trigeminal nerve and can be easily damaged. Therefore, the ideal way to avoid this is to not cross the superior plane of V2. Again, the vidian canal is the key landmark for accessing this area as it leads to the anterior genu of the ICA which forms the inferomedial corner of the quadrangle. Unless there is wide pneumatization of the lateral sphenoid sinus, bone between the vidian canal and V2 must be removed carefully after the inferomedial aspect of the vidian canal is exposed as described above.
Infratemporal Fossa
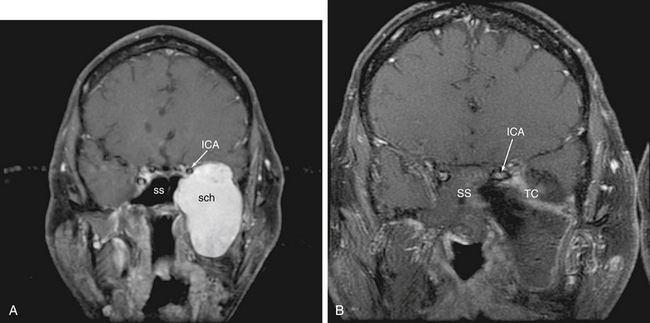
Medial temporal fossa lesions are usually an extension of tumors such as medial sphenoid wing, petroclival meningiomas, and schwannomas as well as nasopharyngeal carcinomas which are addressed as an extension of the approach chosen for these tumors. There are only rare tumors which occur in this region in isolation. However, we have addressed neurenteric cysts and schwannomas in this area effectively via an EEA (Fig 55-14). Often, the inferior tail of the cavernous sinus dura as it ensheathes the trigeminal ganglion and its branches must be transgressed. This is approached initially via a quadrangular region approach. Care should be taken upon opening dura to determine if the ganglion is medial (and therefore between the surgeon and the tumor) or lateral (and therefore displaced away from the operative trajectory) to the lesion. If the ganglion is medial, a lateral approach such as an anterior transpetrosal could be considered. However, the fibers of the trigeminal nerve may be thinned and splayed thus allowing one to work between them to at least biopsy the lesion. Tumors which displace the nerves and/or ganglion laterally are ideally suited for an endonasal approach which will provide direct access. A complete understanding of the course of the parapharyngeal, petrous and cavernous ICA is a necessity both for injury avoidance as well as control.
Lower Infratemporal Fossa/Parapharyngeal Space
2. Perhaps the most challenging endonasal approach is the most inferior and lateral, the approach to the infratemporal fossa. Filled with muscle and soft tissue interspersed with large vessels including the ICA and IMA (internal maxillary artery) and lacking in clearly defined anatomical landmarks, this region is challenging to navigate. As with the other EEAs, control of the ICA is critical. Tumors such as schwannomas, nasopharyngeal carcinomas, JPAs, paragangliomas meningiomas, and lesions such as meningoencephaloceles can all involve this area. Once again, the majority of the work is done through a medial maxillectomy. The Eustachian tube must be transected and cartilage of the tarus which blends with foramen lacerum very carefully resected. The IMA branches are identified, coagulated or clip ligated. These can also be coiled using endovascular techniques preoperatively. The parapharyngeal ICA is identified following removal of the pterygoid plates. The lateral pterygoid plate points to the ICA in the depth. Careful, blunt dissection allows identification of the fat pad which protects the ICA. Once the parapharyngeal ICA and petrous bone are identified, resection can proceed. This exposure can be carried further laterally to the level of the posterior ICA canal, providing direct access to the jugular foramen.18
RECONSTRUCTION
Initial rates using techniques developed for simple CSF leak repair were as high as 58% for certain tumor types (craniopharyngiomas).19 This prompted us to pursue the development of vascularized reconstructive techniques. As a result, the pedicled, vascularized nasal septal mucosal flap (NSF) was adopted for reconstruction.20 Rates since the adoption of this flap have been reduced to 5.4% (in press), well within the range of traditional approaches. This has been a critical component of the development of EEA and has allowed it to become a feasible approach for many tumors of the skull base. This flap is durable, effective and has even proven to be reusable in reoperations, an extremely valuable characteristic.
There are patients in whom nasal septal mucosa is not available, either due to prior surgery, radiation therapy, other septal pathology, or tumor involvement. There are also options for vascularized flaps other than the nasal septal flap in those patients who need it . We have used turbinate flaps for repair of small defects in close proximity to a turbinate.21 We have also used a vascularized temporoparietal fascial flap (TPFF) which can be tunneled through the pterygomaxillary fissure via a small lateral canthal incision using a percutaneous tracheostomy tube.22 The advent of these vascularized reconstruction techniques has been critical for endoscopic endonasal skull base surgery.
1. Rosegay H. Cushing’s legacy to transsphenoidal surgery. J Neurosurg. 1981;54:448-454.
2. Carrau R.L., Jho H.D., Ko Y. Transnasal-transsphenoidal endoscopic surgery of the pituitary gland. Laryngoscope. 1996;106:914-918.
3. Jho H.D., Carrau R.L., Ko Y., et al. Endoscopic pituitary surgery: Early experience. J Neurosurg. 1996;84:744.
4. Friedrich J.P., Terrier G. Chirurgie endoscopique de la sinusite par voie endonasale. Med Hyg. 1983;41:3722-3726.
5. Kennedy D.W., Zinreich S.J., Rosenbaum A.E., et al. Functional endoscopic sinus surgery: theory and diagnostic evaluation. Arch Otolaryngol. 1985;111:576-582.
6. Jankowski R., Auque J., Simon C., et al. Endoscopic pituitary tumor surgery. Laryngoscope. 1992;102:198-202.
7. Messerklinger W. Endoscopy of the Nose. Baltimore: Urban & Schwarzenberg; 1978.
8. Wigand M.E. Transnasale endoscopishe chirurgie der nasennebenhohlen bei chronischer sinusitis. HNO. 1981;29:215-221.
9. Stammberger H. Endoscopic endonasal surgery for mycotic and chronic recurring sinusitis. Ann Otol Rhinol Laryngol. 1985;119(Suppl):1-11.
10. Weiss M.H. Transnasal transsphenoidal approach. In: Apuzzo M.L.J., editor. Surgery of the Third Ventricle. Baltimore: Williams & Wilkins; 1987:476-494.
11. Mason R.B., Nieman L.K., Doppman J.L., et al. Selective excision of adenomas originating in or extending into the pituitary stalk with preservation of pituitary function. J Neurosurg. 1997;87:343-351.
12. Kouri J.G., Chen M.Y., Watson J.C., et al. Resection of suprasellar tumors by using a modified transsphenoidal approach. Report of four cases. J Neurosurg. 2000;92:1028-1035.
13. Kaptain G.J., Vincent D.A., Sheehan J.P., et al. Transsphenoidal approaches for the extracapsular resection of midline suprasellar and anterior cranial base lesions. Neurosurgery. 2001;49:94-100. discussion 100-101
14. Rhoton A.L.Jr. The supratentorial cranial space: microsurgical anatomy and surgical approaches. Neurosurgery. 2002;51:S1-385.
15. Kassam A.B., Prevedello D.M., Thomas A., Gardner P., Mintz A., Snyderman C., Carrau R. Endoscopic endonasal pituitary transposition for transdorsum sellae approach to the interpeduncular cistern. Neurosurgery. 62(ONS. Suppl. 2008;1):ONS57-74.
16. Kassam A.B., Vescan A.D., Carrau R.L., Prevedello D.M., Gardner P., Mintz A.H., Snyderman C.H., Rhoton A.L.Jr. Expanded endonasal approach: vidian canal as a landmark to the petrous internal carotid artery. J Neurosurg. 2008;108:177-183.
17. Vescan A.D., Snyderman C.H., Carrau R.L., Mintz A., Gardner P., Branstetter B. 4th, Kassam AB: Vidian canal: analysis and relationship to the internal carotid artery. Laryngoscope. 2007;117:1338-1342.
18. Kassam A.B., Snyderman C., Carrau R., Gardner P., Hirsch B., Mintz A. Endoscopic, expanded endonasal approach to the jugular foramen. Operative Techniques in Neurosurgery. 2005;8(1):35-41.
19. Gardner P., Kassam A., Snyderman C., Carrau R., Mintz A., Grahovac S., Stefko S.T. Outcomes following endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas: a case series. J Neurosurg, in press. 2008.
20. Hadad G., Bassagasteguy L., Carrau R.L., Mataza J.C., Kassam A., Snyderman C.H., Mintz A. A novel reconstructive technique following endoscopic expanded endonasal approaches: Vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1881-1885.
21. Fortes F.S., Carrau R.L., Snyderman C.H., Prevedello D., Vescan A., Mintz A., Gardner P., Kassam A.B. The posterior pedicle inferior turbinate flap: a new vascularized flap for skull base reconstruction. Laryngoscope. 2007;117(8):1329-1332.
22. Fortes F.S., Carrau R.L., Snyderman C.H., Kassam A., Prevedello D., Vescan A., Mintz A., Gardner P. Transpterygoid transposition of a temporoparietal fascia flap: a new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope. 2007;117:970-976.