CHAPTER 31 Endoscopic Carpal Tunnel Release: The Chow Dual-Portal Technique
Rationale and Basic Science
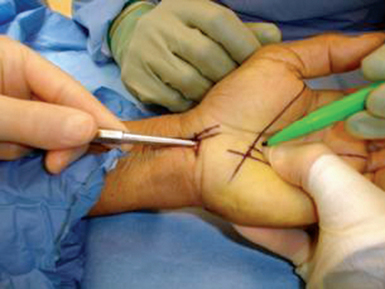
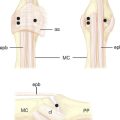
James C. Y. Chow published his dual-portal technique in 19891 and reported his clinical results in 1990.2 The rationale for developing an endoscopic carpal tunnel release is similar to that used in the development of other endoscopic techniques such as laparoscopic cholecystectomy and arthroscopy. A minimal incision approach to carpal tunnel release leads to a decrease in postoperative pain and an earlier return to daily activities. The dual-portal system has the advantage of creating a fixed space in which to operate. The cannula is fixed at the proximal and distal portals. The cannula creates an operative field into which instruments can be easily moved (Figure 31.1).
Technique
Anesthesia
IV regional anesthesia (Bier block) can also be used. It does not allow the instant feedback of local anesthesia, but once the tourniquet is released the patient’s neurological status returns quickly to preoperative levels such that a complete neuromuscular exam can be performed prior to breaking the sterile field. The Bier block eliminates the need to give fentanyl and midazolam in the doses needed during the “pure” local anesthesia as the patient does not feel the cannula pass into the carpal tunnel. Axillary block and general anesthesia have been used but eliminate the patient feedback noted with the local techniques.
Surgical technique
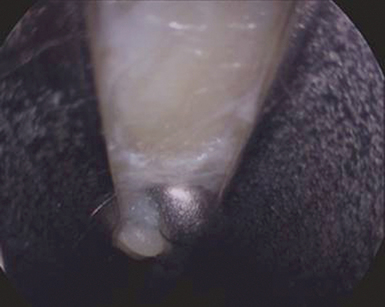
A “washboard” sensation is felt as the tip of the dissector passes over the ridges in the dorsal surface of the transverse carpal ligament (TCL). If this washboard sensation is not felt, the dissector is either palmar to the TCL or in Guyon’s canal. The distal edge of the transverse carpal ligament is able to be palpated using the curved dissector and the position of the distal edge of the transverse carpal ligament is marked on the overlying palmar skin (Figure 31.1).
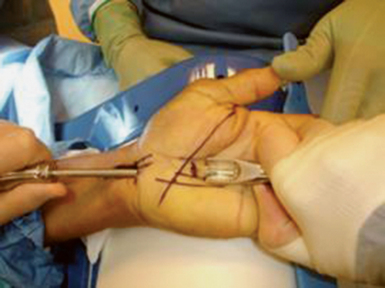
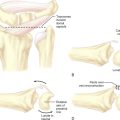
The curved dissector is removed and the slotted cannula/obturator is placed into the carpal tunnel. The operator’s hand is placed on the palmar aspect of the hand between the thenar and hypothenar eminences and the cannula system is lifted (the lift test). The examiner’s palpating fingers should not be able to feel the cannula. If the cannula is palpable, it is either palmar to the TCL or in Guyon’s canal and must be removed and repositioned. Once the cannula is safely placed, taking care to stay close to the hook of the hamate and keeping the cannula system parallel to the long axis of the arm, the wrist and fingers are extended and the hand is placed on the hand holder. The tip of the cannula system is palpated just distal to the distal edge of the TCL. The cannula system is then passed through the distal portal while pushing the superficial palmar arch dorsally with the palmar arch depressor (Figure 31.2).
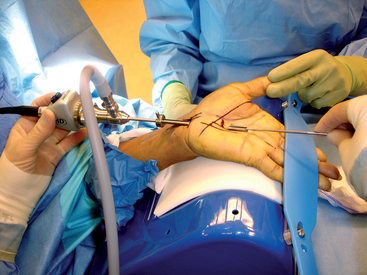
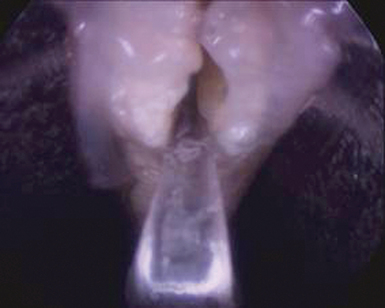
The distal portal is established approximately 4 to 5mm distal to the distal edge of the transverse carpal ligament along the long axis of the ring finger. This effectively allows the cannula system to pass between the common digital nerves of the third and fourth web spaces, distal and palmar to the superficial palmar arch and palmar to the communicating branch sensory branch between the median and ulnar nerves.3 Once the cannula system passes out through this distal portal, the system is fixed in position. The elastic band is placed across the fingers to hold the hand in position. The assistant extends the thumb, thus placing tension on the TCL (Figure 31.3).
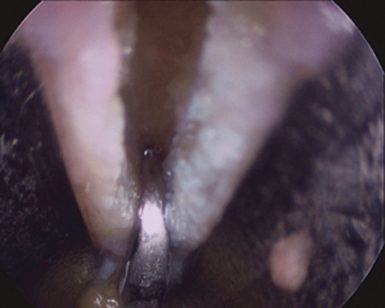
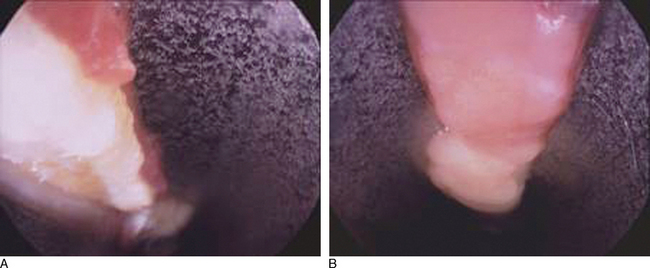
The endoscope is then passed into the proximal portal. A 30-degree 4-mm scope is used. Often, a layer of synovial-like tissue lining the dorsal aspect of the transverse carpal ligament must be cleared. A “nerve hook” or commercially available rasp can be used for this. It is of paramount importance the surgeon clearly identify the transverse carpal ligament and that there be no intervening soft tissues! The transverse carpal ligament has a very characteristic feel and morphology. Its transverse fibers are visible and palpable (the washboard feeling). Proximally, the transverse carpal ligament is whiter and distally is more yellowish in color. The distal edge of the transverse carpal ligament should be clearly identifiable. Just distal to the distal edge of the transverse carpal ligament, one will notice a fat pad falling into the cannula system (Figure 31.5).
The cannula system, when placed as described, is extrabursal; that is, it lies palmar to the flexor tendon bursa. The median nerve is also extrabursal and can occasionally be seen in the cannula system. If this is the case, the cannula system can be rotated radially 350 degrees. This will push the median nerve out of the cannula. If this does not clear the cannula system, it should be reinserted, and if clear visualization of the transverse carpal ligament without obstruction is not able to be achieved an open carpal tunnel release should be carried out.
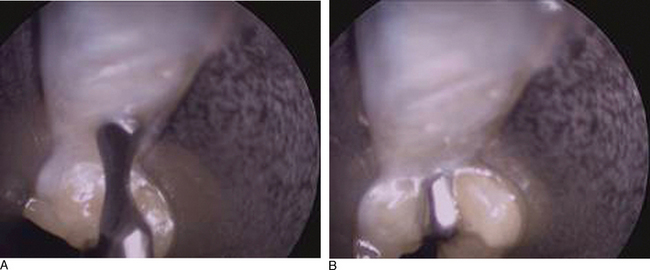
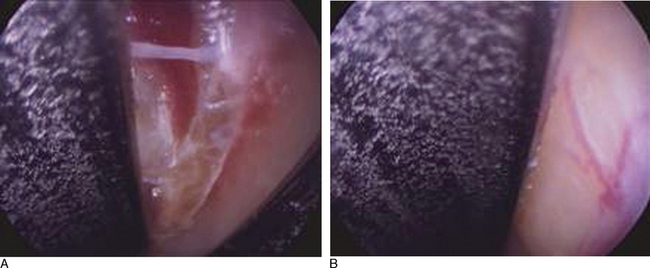
Once one is absolutely certain there is no obstruction and no intervening structures (such as the median nerve, flexor tendon, or superficial palmar arch), the probe blade is inserted. This is a blade designed to cut forward and is blunt except at its axilla. It resembles a meniscotome. The probe blade is passed into the cannula system under direct vision and then withdrawn from proximal to distal until it slips palmarly and its axilla engages the distal edge of the transverse carpal ligament (Figure 31.5a and b).
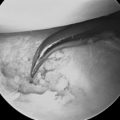
The probe blade is then removed and the triangular blade is inserted approximately 1.5 cm proximal to the distal edge of the transverse carpal ligament. An incision is made into the transverse carpal ligament. This incision is large enough to accept the retrograde blade, and deep enough to penetrate the full thickness of the TCL (Figure 31.6). The triangular blade should not be used to cut the distal edge of the TCL because it has a tendency to cut too deeply and cause more bleeding as it enters the palmar fascia and fat.
The triangular blade is then removed and the retrograde blade is passed into the cannula system and into the defect created by the triangular blade. The retrograde blade is then used to cut the rest of the distal edge of the transverse carpal ligament from proximal to distal (Figure 31.7). This technique is safe as long as the surgeon has full control of the retrograde blade. The surgeon should hold the handle of the retrograde knife in his/her palm with the small and ring fingers. The shank of the blade is held between the long finger and the thumb while the index finger controls the cut by pushing off the cannula system.
Once the release is complete, the proximal subfascial fat should cascade into the cannula system. In the rest of the cannula system, one should see the palmar fascia. The cut edges of the transverse carpal ligament will retract out of the field of view such that the cannula system must be rotated to see them (Figures 31.8 and 31.9). If this is not the case, the transverse carpal ligament has not been completely released and further release is needed. Once a complete release has been performed, the obturator is reinserted into the cannula and the cannula system is removed. A subcuticular stitch is used at the wrist, and a simple stitch at the palm, and a bulky dressing is applied. No splint is needed. The patient’s circulation, sensation, and motor function are then tested, and the patient is discharged from the operating room.
Results
We published our early results in the early 1990s.4,5 Since then, hundreds of studies have been published. These studies have confirmed the efficacy of ECTR. It is, however, beyond the scope of this chapter to present a detailed review of that literature. A relatively recent study by Trumble, Diao, Abrams, and Gilbert-Anderson is of note in that it compares ECTR to OCTR within the context of a randomized prospective study. The study attests to the fact that ECTR reliably and safely relieves patients of their carpal tunnel syndrome symptoms.6
The relative safety of ECTR has been looked at by Aaron Barr and his coauthors at our institutions. Dr. Barr and his colleagues performed a meta-analysis of the world’s literature, specifically looking at the complication rate of ECTR versus OCTR. They collected data on 15,787 endoscopic carpal tunnel releases and 5,669 open carpal tunnel releases. The complication rate (including nerve, tendon, and artery injury) was 0.49% for OCTR and 0.20% for ECTR. The frequency of major nerve injury was 0.11% for OCTR and 0.14% for ECTR.7 Their results compare well to those of Boeckstyns et al., who performed a similar meta-analysis of the outcomes of ECTR and OCTR.8 Jim Chow has looked at his long-term results in 2,402 patients (average follow-up of six years ten months) and has reported a recurrence rate of 0.5%.9
Summary
The first ECTR was reported 19 years ago by Okutsu.10 Some predicted endoscopic carpal tunnel release would be a passing fad. It would appear that endoscopic carpal tunnel release has withstood the test of time. Indeed, the Medicare data indicates that the frequency of endoscopic carpal tunnel release continues to increase.11
1 Chow JCY. Endoscopic release of the carpal ligament, a new technique for carpal tunnel syndrome. Arthroscopy. 1989;5:19-24.
2 Chow JCY. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22 month clinical result. Arthroscopy. 1990;6:288-296.
3 Meals RA, Shaner M. Variations in digital sensory patterns: A study of the ulnar nerve-median nerve palmar communicating branch. J Hand Surg [Am]. 1983;8:411-414.
4 Nagle DJ, Fischer TJ, Harris GD, Hastings HII, Osterman AL, Palmer AK, et al. A multicenter prospective review of 640 endoscopic Carpal tunnel releases using the transbursal and extrabursal Chow techniques. Arthroscopy. 1996;12(2):139-143.
5 Nagle D, Harris G, Foley M. Prospective review of 278 endoscopic carpal tunnel releases using the modified Chow technique. Arthroscopy. 1994;10(3):259-265.
6 Trumble TE, Diao E, Abrams RA, Gilbert-Anderson MM. Single-portal endoscopic carpal tunnel release compared with open release: A prospective, randomized trial. J Bone Joint Surg [Am]. 2002;84-A(7):1107-1115.
7 Aaron A, Bare LS, Benson CS, Williams JL, Visotsky D, Nagle J, Harder V. Complications of carpal tunnel surgery: An analysis of open and endoscopic techniques. Arthroscopy. 2006;22(9):919-924.
8 Boeckstyns ME, Sorensen AI. Does endoscopic carpal tunnel release have a higher rate of complications than open carpal tunnel release? An analysis of published series. J Hand Surg [Br]. 1999;24(1):9-15.
9 Chow JC, Hantes ME. Endoscopic carpal tunnel release: Thirteen years’ experience with the Chow technique. J Hand Surg [Am]. 2002;27(6):1011-1018.
10 Okutsu I, Ninomiya S, Natsuyama M, Takatori Y, Inanami H, Kuroshima N, et al. Subcutaneous operation and examination under the universal endoscope. Nippon Seikeigeka Gakkai Zasshi. 1987;61(5):491-498.