CHAPTER 34 Endoscopic Carpal Tunnel Release: Chow Technique
Sir James Paget first described median nerve compression after a distal radius fracture in 1854.1,2 In 1880, James Putman, a neurologist from Boston, reported similar symptoms in a group of his patients.3 The first formal description of surgical decompression for treatment of this condition was reported in 1933; this was followed by Phalen’s classic article in 1950.4 Since then, open carpal tunnel release has been established as the gold standard for surgical treatment of carpal tunnel syndrome.
The first two reports in the literature on the topic of endoscopic carpal tunnel release (ECTR), written separately by Chow and by Okutsu and colleagues, were published in the March issue of Arthroscopy in 1989.5,6 In the following year, Chow presented a paper describing his clinical results after ECTR in 149 cases at the 1990 Arthroscopy Association of North America (AANA) Annual Meeting in Orlando, Florida. In the fall of 1990, Agee and associates7 reported to the American Society for Surgery of the Hand Annual Meeting in Toronto, Canada, the clinical results of a multicenter study of the use of Agee’s technique for endoscopic release of the carpal ligament. Since the publication of these three endoscopic carpal ligament release techniques, there has been increasing interest and much debate among surgeons regarding the safety and efficacy of the endoscopic procedures compared with the open technique. Several modifications and variations of the three original ideas have been made since their initial demonstration.
ANATOMY
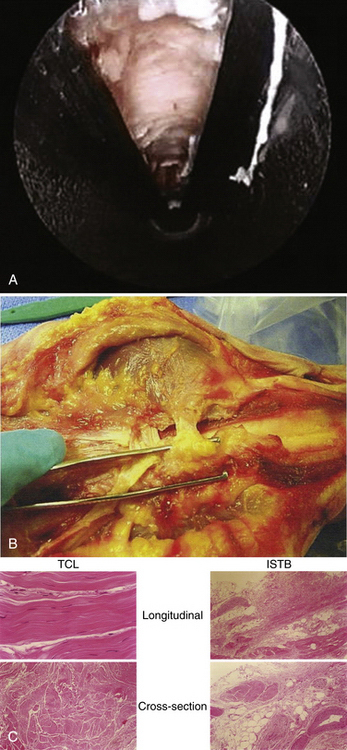
Superficial to the transverse carpal ligament lies a sling of vascular and nervous connective tissue, subcutaneous fat, and densely innervated palmar dermis and epidermis. This sling of soft tissue is an interthenar soft tissue band (ISTB) (Fig. 34-1A and B) that bridges the thenar and hypothenar musculature. It is a histologically distinct structure (see Fig. 34-1C) that lies volar to the transverse carpal ligament. The ISTB and the palmaris brevis muscle should be preserved. The ISTB prevents bowstringing of the flexor tendons after surgery, thereby maintaining their strength during contraction.8–10 It is the goal of ECTR to preserve this natural anatomy while selectively releasing only the transverse carpal ligament.
Carpal tunnel syndrome is a compressive neuropathy of the median nerve at the wrist. It can result from any condition that increases intra–carpal tunnel pressure, such as irritation and swelling of any of the nine flexor tendons and their tenosynovium, edema of the median nerve, anatomic abnormality or scarring of any of the bordering structures of the carpal tunnel, anomalous lumbrical anatomy, an intra–carpal tunnel mass (e.g., a deep ganglion cyst), or stiffening and contracture of the transverse carpal ligament itself.
PATIENT EVALUATION
History and Physical Examination
Carpal tunnel syndrome accounts for approximately 463,673 carpal tunnel releases performed annually in the United States.11 Patients who have developed this syndrome usually present with a history of characteristic symptoms, such as nocturnal pain, paresthesias, and numbness in the median nerve distribution distal to the wrist, and thenar muscular weakness. The physician should be aware of the patient’s general health condition and family history. Congenital diseases or anomalies, connective tissue diseases, systemic and metabolic disorders, and previous injury to the distal forearm or wrist should be taken into consideration.
The physical examination is critical for accurate diagnosis. In an acute case, there is tenderness along the carpal canal area. Light percussion over the median nerve at the wrist produces a sensation of an electric current that radiates to the median nerve distribution; this is known as Tinel’s sign. Phalen’s sign is evoked by holding the wrists at maximum flexion with the dorsal aspects of the hands in full contact (i.e., reverse praying position). This position narrows the carpal canal; if it reproduces the paresthesias in the fingers within 60 seconds, the sign is considered positive. As the pathologic condition advances, less time is necessary to evoke a response. Other indicators include the monofilament test, two-point discrimination, the reverse Phalen’s test, and the tourniquet test. In the late stages, with thenar muscle atrophy, the examiner can observe the muscle wasting in the thenar area.12 Muscle weakness is tested subjectively by resisted palmar abduction of the thumb against the examiner’s index finger and comparison of one hand with the other.
Diagnostic Tests and Imaging
Electromyography and nerve conduction velocity tests aid in the detection of carpal tunnel syndrome. Indications for surgery should not be decided or altered according to the results of nerve conduction velocity tests, especially when the results are normal but the patient has the clinical signs and symptoms of the syndrome.13–15 A delay in the distal latency of the median nerve of 7.0 msec or longer represents significant compression of the median nerve; if such a delay is present, surgery should be considered immediately. The most important aspects in diagnosing carpal tunnel syndrome are the history and the physical examination results. Electrodiagnostic studies of the median nerve are used adjunctively to confirm the diagnosis and perhaps to suggest how the patient will respond to surgery.
Wrist radiography can rule out the possibility of congenital or acquired bone or joint deformity, abnormality, or pathology. Previously sustained fractures of the distal forearm and wrist should be taken into account. A malunited fracture of the distal radius, previously performed surgery in the wrist area, or a hypoplastic or aplastic hook of the hamate16 can produce difficulties for the surgeon during placement of and operation through the slotted cannula. Standard anteroposterior and lateral views of the distal forearm and wrist and a tunnel view of the wrist are required. If a more extensive study is indicated, magnetic resonance imaging (MRI), computed tomography (CT), ultrasound, bone scanning, or arthrography of the wrist may be necessary.17,18
TREATMENT
Open Versus Arthroscopic Management
The indications for the open surgical release of transverse carpal tunnel ligament have been well established, and in most cases they apply to ECTR. The advantages of endoscopic over open carpal tunnel release include no hypertrophic scar or scar tenderness, no pillar pain, less compromise of pinch or grip strength, and an earlier return to work and daily activities. However, ECTR can become a dangerous procedure if it is performed by an inexperienced surgeon.19–21 Significant intraoperative complications have been reported throughout the United States,22–24 raising questions about the value of ECTR. However, ECTR can be performed safely by experienced surgeons. Despite its steep learning curve, the results of ECTR can be satisfying for the patient and the surgeon.25 As the knowledge, technique, and instrumentation have evolved, the safety of ECTR has improved.
Arthroscopic Technique
The original technique was described by Chow as a transbursal approach to the carpal tunnel requiring penetration of the ulnar bursa.5,26 After a multicenter study,27,28 the original technique was modified in an attempt to decrease the complications and improve the learning curve. Conversion to an extrabursal technique made the surgical procedure much easier and safer because it offered better visualization of the proximal transverse carpal ligament.29–31 The following paragraphs describe the extrabursal, dual-portal technique.
Operating Room Setup
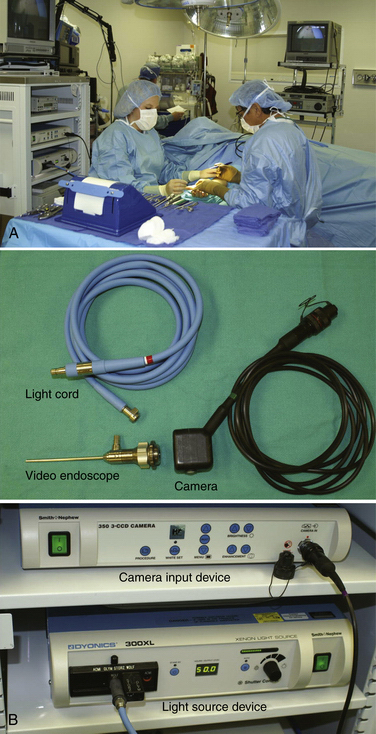
The patient is placed in a supine position, and a hand table is used. Two video monitors are preferred, although some surgeons can manage the procedure with only one. One of the two monitors should face the surgeon, and the other should face the assistant. The surgeon sits on the ulnar side of the patient, and the assistant faces the surgeon (Fig. 34-2A). The arthroscopic equipment consists of a short, 4.0-mm, 30-degree video endoscope. The light post is on the same side as the direction of view, the camera apparatus, the light cord, the camera input device, and the light source (see Fig. 34-2B). Optional equipment includes a digital video recorder and a video printer for the printing of any captured images. Water pump and shaver equipment are not used.
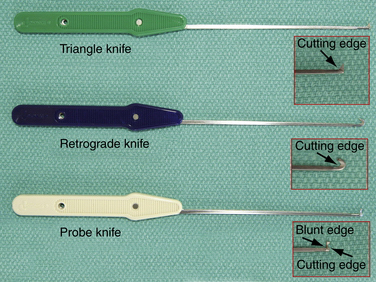
A standard handset should be available. Specific instrumentation for the procedure, designed by Chow, includes an ECTRA System Kit and an ECTRA Disposable Kit (Smith & Nephew Endoscopy, Andover, MA). The ECTRA System Kit includes a video endoscope, slotted cannula, dissecting obturator, curved blunt dissector, palmar arch suppressor, probe, retractors, and hand holder (Fig. 34-3). The dissecting obturator is attached with a detachable handle that can also take some other types of obturators included in the kit (e.g., conical, boat-nose obturator), but the latter are not used routinely. The ECTRA Disposable Kit includes a probe knife, triangle knife, retrograde knife, hand pad, and swabs (Fig. 34-4). These knives allow the surgeon to determine the direction and depth of the cut.
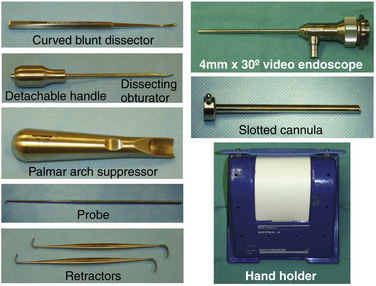
FIGURE 34-3 Instrumentation included in the ECTRA System Kit (Smith & Nephew Endoscopy, Andover, MA).
Standard preparations and draping are performed as usual, without the application of a tourniquet. Before local anesthesia is introduced, a skin marker is used to map landmarks for the entry and exit portals.
Anesthesia
Local anesthesia combined with intravenous medication is recommended for the procedure, because it allows the patient and the surgeon to communicate. An alert patient can inform the surgeon during the procedure about any abnormal sensation in the hand, which can indicate a potential problem caused by a variance of nerve structure in the wrist and palm region.32–38 Usually, when the patient first comes into the operating room, 100 μg of fentanyl citrate (Sublimaze, Baxter Healthcare Corporation, Westlake Village, CA) is given intravenously. This is a narcotic analgesic medication with an onset of 7 to 8 minutes and a peak action of approximately 30 minutes. Usually, the surgical time does not exceed 10 minutes. A 1% solution of Xylocaine (Astra, Westboro, MA) without epinephrine is injected, approximately 2 to 3 mL at the entry portal and 6 to 7 mL at the exit portal (because of the higher degree of sensitivity of the skin on the palmar region). Special care is taken to limit the injection to the skin and subcutaneous tissue and to avoid affecting the nerve by penetrating deeply.
Positioning the Entry Portal
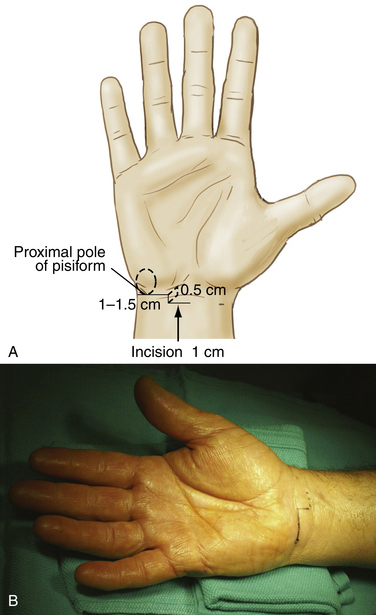
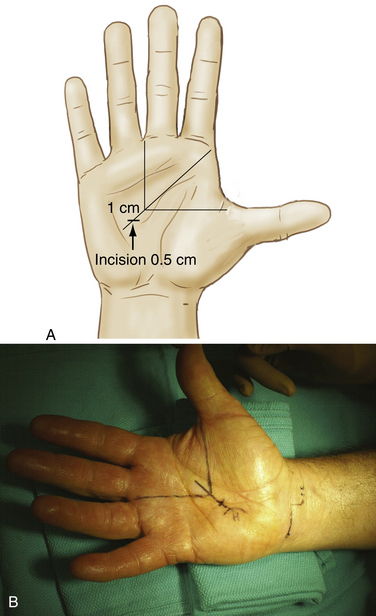
The proximal end of the pisiform bone is palpated on the volar surface of the wrist within the flexor carpi ulnaris tendon at the distal wrist flexor crease and is marked with a small circle. A line from the proximal pole of the pisiform is drawn radially and is approximately 1.0 to 1.5 cm long. From that point, a second, 0.5-cm line is drawn proximally. A third dotted line, approximately 1.0 cm long, is drawn radially from the proximal end of the second line to create the entry portal (Fig. 34-5). If the palmaris longus muscle is present, the center of the entry portal should be located at the ulnar border of its tendon, almost at the level of the proximal wrist flexor crease. Average dimensions of these lines vary slightly, depending on the overall size of the hand.
Positioning the Exit Portal
The patient’s thumb is placed in full abduction. A line is drawn across the palm from the distal border of the thumb to the approximate center of the palm, perpendicular to the long axis of the forearm. A second line is drawn from the third web space, parallel to the long axis of the forearm, to meet the first line. These two lines should form a right angle. A third line is drawn, bisecting this angle and extending approximately 1.0 cm proximally from its vertex, which establishes the site of incision for the exit portal (Fig. 34-6). The surgeon should be able to palpate the hook of the hamate. The exit portal should fall into the soft spot at the center of the palm and should line up with the ring finger, just slightly radial to the hook of the hamate.
Creation of Portals and Placement of the Cannula
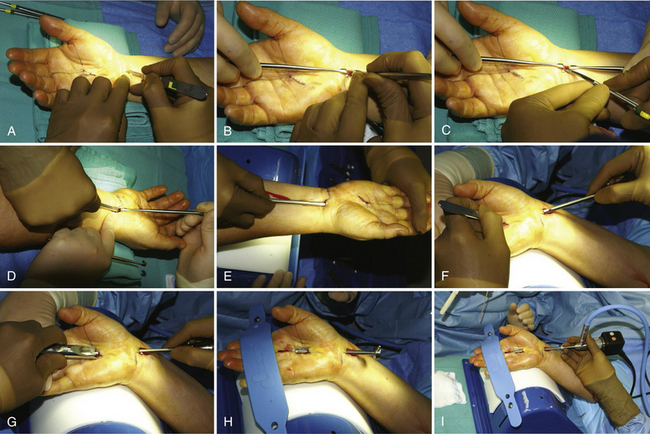
The procedure begins with the creation of the entry portal. A transverse incision of approximately 1.0 cm (Fig. 34-7A) is made at the marked entry portal site, extending just through the skin. Subcutaneous tissue is bluntly dissected off the volar forearm fascia with the use of a hemostat and is retracted with the retractors. Care must be taken to avoid damage to the small subcutaneous blood vessels.
A knife is used to make a small, longitudinal opening of the antebrachial fascia that is extended distally with the use of a Stephen’s tenotomy scissors (see Fig. 34-7B and C). If the palmaris longus muscle is present, the longitudinal cut should be along the ulnar border of palmaris longus tendon. Care should be taken, because sometimes there are two layers of fascia that must be cut. Retractors are passed just beneath the fascia, with one of them lifting the skin distally to create a vacuum that separates the transverse carpal ligament from the ulnar bursa. A blunt, curved dissector is gently slipped into the carpal tunnel, just under the transverse carpal ligament. A gentle “pop” of resistance should be felt as the blunt dissector punches through the bursal sheath at about midway past the transverse carpal ligament. Maneuvering the dissector back and forth should produce a type of washboard feeling because of the rough undersurface of the carpal ligament. The curved dissector is then removed.
A dissecting obturator and slotted cannula assembly unit can be guided into the space vacated by the curved dissector. The slotted cannula assembly is advanced into the carpal tunnel on the underside of the transverse carpal ligament to the level of the hook of the hamate, staying to the ulnar side of the carpal tunnel (see Fig. 34-7D). With the tip of this unit touching the hook of the hamate, the surgeon gently picks up and hyperextends the hand. The hand and cannula assembly are now moved as a unit (see Fig. 34-7E) and placed on the hand holder with the wrist and fingers in full hyperextension. The cannula assembly is advanced along the undersurface of the carpal ligament, while the assistant keeps the hand on the hand holder, until the tip of the cannula assembly can be easily palpated in the palm area where the mark for the exit portal was previously made.
A small, transverse or oblique incision is made just over the palpable cannula assembly tip, cutting only the skin (see Fig. 34-7F). The palmar skin and soft tissue are depressed with the palmar arch suppressor, and the cannula assembly is pushed into the receptacle of the palmar arch suppressor to exit through the distal portal (see Fig. 34-7G). The obturator is then removed from the cannula, which should lie just below the transverse carpal ligament, and the hyperextended hand is strapped onto the hand holder (see Fig. 34-7H and I). Hyperextension of the wrist brings the superficial palmar arch to a level lower than the exiting point of the slotted cannula assembly, thereby protecting it from injury. The creation of two portals is essential, because they stabilize the slotted cannula while it passes through the portals, ensuring the reproducibility of the technique. The slotted portion of the cannula allows a safe cutting zone, whereas delicate structures such as the median nerve and flexor tendons are protected by the walls of the cannula.
Endoscopic Examination
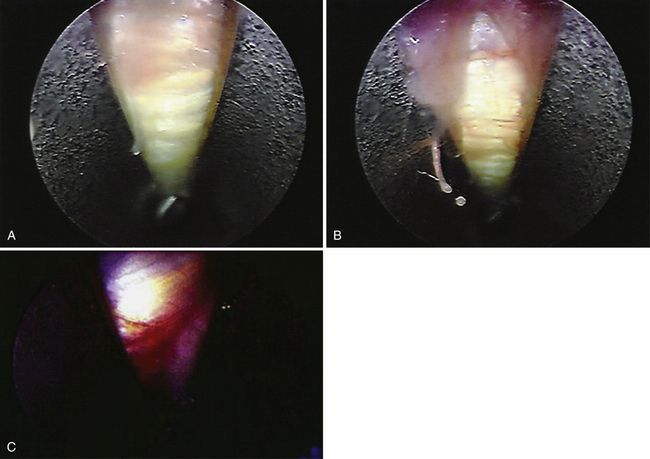
The video endoscope is inserted into the slotted cannula at the proximal portal. The camera and scope should rest comfortably in the first web space of the surgeon’s hand. A cotton swab can be inserted into the tube from the distal portal to clean the lens while focus is adjusted to the best visualization. A blunt probe is inserted to palpate the undersurface of the transverse carpal ligament proximally to distally; if a thin bursal membrane is seen above the cannula’s slotted opening, it is carefully dissected with the probe to gain access to the ligament, which has an ivory-white appearance and fibers that run transversely (Fig. 34-8A and B). If the median nerve is present in the field (see Fig. 34-8C), the patient will feel sharp pain radiating to the fingers when the nerve is probed; this response should alert the surgeon to the presence of the nerve. If abundant soft tissue is identified in the opening of the cannula, the procedure should not be performed. The slotted cannula may need to be reinserted to ensure better visualization; however, to avoid irreversible damage, surgery should not be carried out if tendons or other important structures are entrapped between the slotted cannula and the undersurface of the carpal ligament (see Fig. 34-8D).
Technique for Release of the Transverse Carpal Ligament
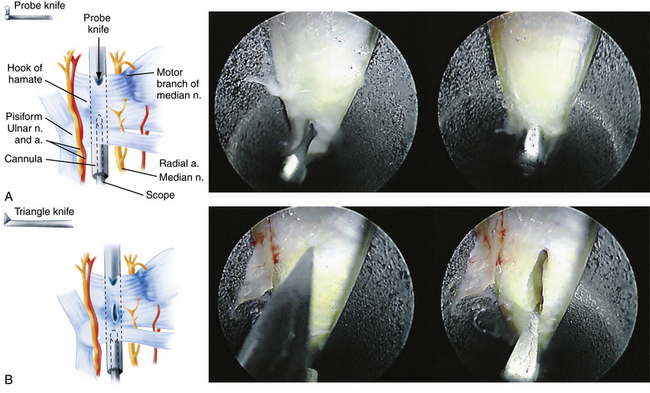
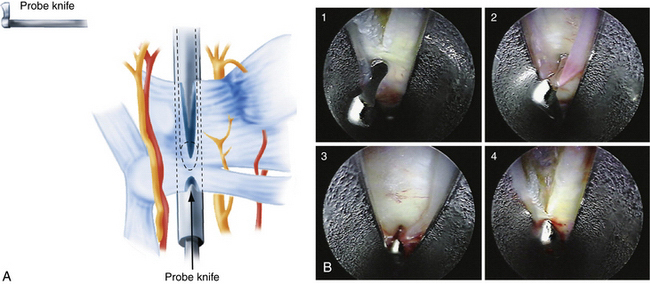
With the scope in the proximal portal and the probe in the distal portal, the distal border of the transverse carpal ligament is identified. The probe knife, which permits forward cutting only, is inserted into the distal portal. The blunt edge of the knife can be used to probe proximally to distally along the ligament. The cutting edge is then used to release the distal border of the ligament by drawing the knife distally to proximally (Fig. 34-9A). Anything beyond the distal border of the carpal ligament should not be excised. The scope is withdrawn proximally about 1 cm, and the triangle knife is used to make a small upward cut in the midsection of the ligament (see Fig. 34-9B). The retrograde knife is then inserted through the distal portal, and its blunt tip is gently positioned at the incision made by the triangle knife (Fig. 34-10). The proximal cutting edge of the retrograde knife is drawn distally, making an incision that joins the previous two cuts, thereby completing the release of the distal portion of transverse carpal ligament (see Fig. 34-10).
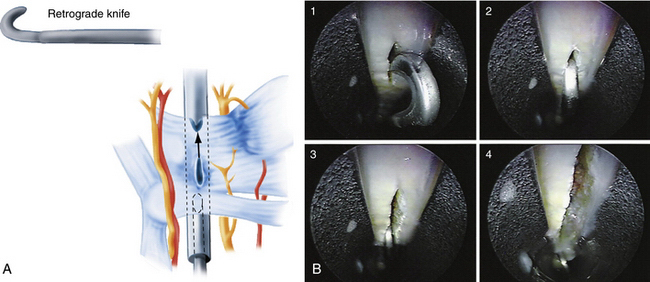
FIGURE 34-10 A and B, The retrograde knife is placed in the incision made by the triangle knife (B1, B2). It is drawn distally to make an incision that joins the previous two cuts (B3, B4).
The scope is removed from the proximal opening and inserted into the distal opening of the slotted cannula. The camera view on the screen then forms a mirror effect, with the previous ulnar side now being the radial side. By moving the scope proximally and distally, the previous distal cut is identified. The probe knife is inserted into the proximal portal and is drawn toward the level of the previous distal cut with its blunt tip touching the underside of the transverse carpal ligament, just before the beginning of the distal cut (Fig. 34-11). From that point, the blunt edge of the knife is used to retract the thick bursal membrane, which sheaths the proximal portion of the carpal ligament, distally to proximally along the ligament’s undersurface (see Fig. 34-11). After the cutting edge of the knife has engaged the proximal border of the ligament, the knife is advanced distally to make an incision that joins the previous cut, accomplishing the release of the transverse carpal ligament (see Fig. 34-11). This is a slight modification of the technique in which the retrograde knife is used to complete the release of the ligament.31,39 The thick bursal membrane contains small vessels, and it should be preserved to avoid bleeding into the carpal canal. The slotted cannula is gently rotated about a few degrees, clockwise and counterclockwise sequentially, enabling the surgeon to view the edges of the transected carpal ligament. If additional fibers remain, the triangle knife, or any other knife that feels appropriate, can be used to release these fibers until the surgeon is satisfied.
Only one suture is required for closure of each portal. Immediately after the procedure, the surgeon should clinically examine the patient while still in a sterilized environment. If there is any dysfunction indicating intraoperative damage to the median nerve or tendons, exposure and exploration of the carpal tunnel can be performed at that time.
PEARLS& PITFALLS
PEARLS
PITFALLS
COMPLICATIONS
Several complications after ECTR by the Chow technique have been reported in the literature. Nagle and colleagues28 performed a multicenter, prospective review study on a total of 640 cases. The initial transbursal technique was used in 110 cases, and the modified extrabursal technique was used in 530 cases. An overall (perioperative and late) complication rate of 11% was found with the transbursal technique, compared with 2.2% with the extrabursal technique. In 21 (3.3%) of the 640 cases, perioperative complications occurred. Fourteen of these 21 cases involved neurapraxia, all of which resolved without sequelae, and no nerves were lacerated or transected. One laceration of the superficial flexor tendon of the ring and small fingers, four incomplete releases, and one case with hematoma, and one laceration of the superior palmar arch were reported. Late complications included three cases of reflex sympathetic dystrophy (0.5%). This complication resolved in all cases without the use of sympathetic nerve blocks. The study authors concluded that ECTR performed by the extrabursal, dual-portal technique reliably decompresses the carpal tunnel and can be effectively performed with low perioperative and late complication rates.
Malek and Chow,24 in a national study of the complications of 10,246 procedures in 9562 patients treated with the dual-portal Chow technique, found a complication rate of 2.3% (240 cases with complications were reported). Of these, there were 154 nerve-related complications (i.e., median or ulnar nerve neurapraxias, lacerations, or transections), 38 complications related to blood vessels, 15 tendon injuries, 18 incomplete releases of the transverse carpal ligament, and 6 cases of reflex sympathetic dystrophy. The remaining 9 cases were listed as miscellaneous complications, including hematoma and superficial wound infection. Most of the intraoperative nerve injuries occurred in cases in which general or regional anesthesia was used.
The reported complication rates of ECTR compare favorably with published series of open carpal tunnel release. Complications of the latter include incomplete ligament release, nerve injuries, palmar hematomas, bowstringing of the flexor tendons, adhesions between nerves and tendons, reflex sympathetic dystrophy, deep wound infections, scar tenderness, pillar pain, tendon lacerations, and vascular injuries.40–48 The damage to the surrounding anatomic structures that occurs during open or endoscopic carpal tunnel surgery usually requires a second surgical procedure to be repaired.
OUTCOMES
Chow has encountered the anatomic variant of an extreme ulnar transligamental motor branch of the median nerve in 24 cases (0.55%), or approximately 1 of every 200 patients (Fig. 34-12). This variant is easily visualized and accommodated only by ECTR. This anatomic variant is thought to account for the higher rate of nerve injury and nerve-related complications reported for the open procedure.
CONCLUSIONS
Data gathered from the experience of past 21 years strongly indicate that, because the normal anatomic structures of the hand (especially the ISTB) are preserved, clinical results of ECTR are better than those of the standard open procedure. ECTR with the Chow dual-portal technique is a reliable method of treating carpal tunnel syndrome and can be performed safely by a well-trained surgeon. Although debate among surgeons still exists, endoscopic release of the transverse carpal ligament has become established as an accepted minimally invasive surgical technique.
1. Pfeffer GB, Gelberman RH, Boyes JH, Rydevik B. The history of carpal tunnel syndrome. J Hand Surg Br. 1988;13:28.
2. Paget J. Lectures on Surgical Pathology delivered at the Royal College of Surgeons of England, 2nd American ed. Philadelphia, PA: Lindsay & Blakiston; 1860.
3. Putman JJ. A series of paresthesia, mainly of the hand, of periodical recurrence, and possibly of vaso-motor origin. Arch Med (New York). 1880;4:147-162.
4. Phalen GS, Gardner WJ, Lalonde AA. Neuropathy of the median nerve due to compression beneath the transverse carpal ligament. J Bone Joint Surg Am. 1950;32:109-112.
5. Chow JCY. Endoscopic release of the carpal ligament: a new technique for carpal tunnel syndrome. Arthroscopy. 1989;5:19-24.
6. Okutsu I, Nonomiya S, Takatori Y, Ugawa Y. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5:11.
7. Agee JM, Tortsua RD, Palmer CA, Berry C. Endoscopic release of the carpal tunnel: a prospective randomized multicenter study, Paper presented at the 45th Annual Meeting of the American Society for Surgery of the Hand, Toronto, Canada; September 24-27, 1990, 1990.
8. Viegas S, Pollard A, Kaminski K. Carpal arch alteration and related clinical status after endoscopic carpal tunnel release. J Hand Surg Am. 1992;17:1012-1016.
9. Garcia-Elias M, Sanches-Freijo J, Salo J, et al. Dynamic changes of the transverse carpal arch during flexion-extension of the wrist: effects of sectioning the transverse carpal ligament. J Hand Surg Am. 1992;17:1017-1019.
10. Richman JA, Gelberman RH, Rydevik BL, et al. Carpal tunnel syndrome: morphologic changes after release of transverse carpal ligament. J Hand Surg Am. 1989;14:852-857.
11. Duncan KH, Lewis RC, Foreman KA, Nordyke MD. Treatment of carpal tunnel syndrome by members of the American Society for Surgery of the Hand: results of a questionnaire. J Hand Surg Am. 1987;12:384-391.
12. Phalen GS. The carpal tunnel syndrome: clinical evaluation of 598 hands. Clin Orthop. 1972;83:29-40.
13. Grundberg AB. Carpal tunnel decompression in spite of normal electromyography. J Hand Surg Am. 1983;8:348-349.
14. Jackson DA, Clifford JC. Electrodiagnosis of mild carpal tunnel syndrome. Arch Phys Med Rehabil. 1989;71:199-204.
15. Cioni R, Passero S, Paradiso C, et al. Diagnostic specificity of sensory and motor nerve conduction variables in early detection of carpal tunnel syndrome. J Neurol. 1989;236:208-213.
16. Chow JC, Weiss MA, Gu Y. Anatomic variations of the hook of hamate and the relationship to carpal tunnel syndrome. J Hand Surg Am. 2005;30:1242-1247.
17. Murphy RX, Chernofsky MA, Osborne MA, Wolson AH. Magnetic resonance imaging in the evaluation of persistent carpal tunnel syndrome. J Hand Surg Am. 1993;18:113-120.
18. Richman JG, Gelberman RH, Rydevik B, Gylys-Morin V. Carpal tunnel volume determination by magnetic imaging 3-D reconstruction. J Hand Surg Am. 1987;12:712.
19. Rotman MB, Manske PR. Anatomical relationships of an endoscopic carpal tunnel device to surrounding structures. J Hand Surg Am. 1993;18:442-450.
20. Seiler JGIII, Barnes K, Gelberman RH, Chalidapong P. Endoscopic carpal tunnel release: an anatomic study of the two-incision method in human cadavers. J Hand Surg Am. 1992;17:996-1002.
21. Schwartz JT, Waters PM, Simmons BP. Endoscopic carpal tunnel release: a cadaveric study. Arthroscopy. 1993;9:209-213.
22. Chow JCY, Malek M, Nagle D. Complications of endoscopic release of the carpal ligament using the Chow technique, Paper presented at the 47th Annual Meeting of the American Society for Surgery of the Hand, Phoenix, AZ; November 11-24, 1992, 1992.
23. Chow JCY, Malek MM. Complications of endoscopic release of the carpal ligament using the Chow technique, Paper presented at the 60th Annual Meeting of the American Academy of Orthopaedic Surgeons, San Francisco, CA; February 18-23, 1993, 1993.
24. Malek MM, Chow JCY. National study of the complications of over 10,000 cases of endoscopic carpal tunnel release, Paper presented at the 61st Annual Meeting of the American Academy of Orthopaedic Surgeons, New Orleans, LA; February 24–March 1, 1994, 1994.
25. Chow JC, Hantes ME. Endoscopic carpal tunnel release: thirteen years’ experience with the Chow technique. J Hand Surg. 2002;27:1011-1018.
26. Chow JCY. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical results. Arthroscopy. 1990;6:288-296.
27. Nagle DJ, Fischer T, Hastings H, et al. A multicenter prospective study of 640 endoscopic carpal tunnel releases using the Chow extrabursal technique, Paper presented at the 47th Annual Meeting of the American Society for Surgery of the Hand,Phoenix, AZ; November 11-14, 1992, 1992.
28. Nagle D, Fischer T, Harris G, et al. A multi-center prospective review of 640 endoscopic carpal tunnel releases using the Chow technique. Arthroscopy. 1996;12:139-143.
29. Chow JCY. The Chow technique of endoscopic release of the carpal ligament for carpal tunnel syndrome: four years of clinical results. Arthroscopy. 1993;9:301-314.
30. Chow JCY. Endoscopic carpal tunnel release. Clin Sports Med. 1996;15:769-784.
31. Chow JCY. Endoscopic carpal tunnel release. In: Chow JCY, editor. Advanced Arthroscopy. New York, NY: Springer-Verlag; 2001:271-286.
32. Mannerfelt L, Hybbinette CH. Important anomaly of the thenar motor branch of the median nerve. Bull Hosp Joint Dis. 1972;33:15.
33. Caffee HH. Anomalous thenar muscle and median nerve: a case report. J Hand Surg. 1979;4:446.
34. Ogden J. An unusual branch of the median nerve. J Bone Joint Surg Am. 1972;54:1779-1781.
35. Papathanassiou BT. A variant of the motor branch of the median nerve in the hand. J Bone Joint Surg Br. 1968;50:156.
36. Lanz U. Anatomical variations of the median nerve in the carpal tunnel. J Hand Surg Am. 1977;2:44.
37. Johnson RK, Shrewsbury MM. Anatomical course of the thenar branch of the median nerve, usually in a separate tunnel through the transverse carpal ligament. J Bone Joint Surg Am. 1970;52:269.
38. Seradge H, Seradge E. Median innervated hypothenar muscle: anomalous branch of median nerve in the carpal tunnel. J Hand Surg Am. 1990;15:356-359.
39. Chow JCY. Carpal tunnel release. In: McGinty JB, editor. Operative Arthroscopy. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:798-818.
40. Das SK, Brown HG. In search of complications in carpal tunnel decompression. Hand. 1976;8:243-249.
41. MacDonald RI, Lictman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg. 1978;3:70-76.
42. Lilly CJ, Magnell TD. Severance of the thenar branch of the median nerve as a complication of carpal tunnel release. J Hand Surg Am. 1985;10:399-402.
43. Louis DS, Green TL, Noellert RC. Complications of carpal tunnel surgery. J Neurol. 1985;62:352-355.
44. Kessler FB. Complications of the management of carpal tunnel syndrome. Hand Clin. 1986;2:401-406.
45. Gartsman GM, Kovach JC, Crouch CC, et al. Carpal arch alteration after carpal tunnel release. J Hand Surg Am. 1986;11:372-374.
46. Terrino AL, Belskey MR, Feldon PG, et al. Injury to the deep motor branch of the ulnar nerve during carpal tunnel release. J Hand Surg Am. 1993;18:1038-1040.
47. May JW, Rosen H. Division of the sensory ramus communicans between the ulnar median nerves: a complication following carpal tunnel release. J Bone Surg Am. 1981;63:836.
48. Brown RA, Gelberman RH, Seiler JGIII, et al. Carpal tunnel release: a prospective randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75:1265-1275.