Endoscopic and surgical treatment of early gastric cancer
Introduction
Definition of early gastric cancer
Early gastric cancer (EGC) is defined as a tumour that is limited to the mucosal or submucosal layers (T1 cancer) and by definition absence of invasion into the proper muscle layer. This is irrespective of the presence of lymph node metastasis.1
Risk and development of early gastric cancer
The incidence of gastric cancer has steadily declined over many decades, yet it remains worldwide one of the most common malignancies. Most gastric cancers arise as a result of lifelong colonisation with Helicobacter pylori, inducing chronic active gastritis. An abundance of research over the past 20 years has yielded endoscopic and non-invasive methods to recognise both this infection and the various stages of the cascade leading from chronic gastritis via atrophic gastritis, intestinal metaplasia and dysplasia to early and advanced gastric cancer. Cohort studies with these methods have revealed the cancer risks associated with each premalignant condition, and showed that these risks increase with each subsequent stage of the cascade.2 These advances have led to common identification of patients with dysplasia and early cancer of the stomach, a development which likely will be further enhanced by the recent introduction of a guideline for surveillance of patients with intestinal metaplasia and dysplasia of the stomach,3 in particular when present in both antrum and corpus.4 This chapter deals with the management of early gastric cancer.
Classification of early gastric cancer
Early gastric cancer usually arises in a mucosa that has undergone atrophic and metaplastic changes. These are recognisable with modern high-definition endoscopic equipment, in particular when combined with additional image enhancement techniques such as narrow-band imaging (NBI). Against this background, most EGCs amenable for endoscopic resection can be recognised by experienced endoscopists.5 Complete staging and classification of EGCs is usually done in three steps. The first step is a pre-interventional endoscopic staging determining depth of tumour infiltration (with or without endoscopic ultrasonography) combined with histopathological sampling. The second step is the actual endoscopic resection, where the most valuable information is gathered from the submucosal lifting properties of the lesions. The third step is the final histopathological staging of the resected lesion.
Endoscopic appearance
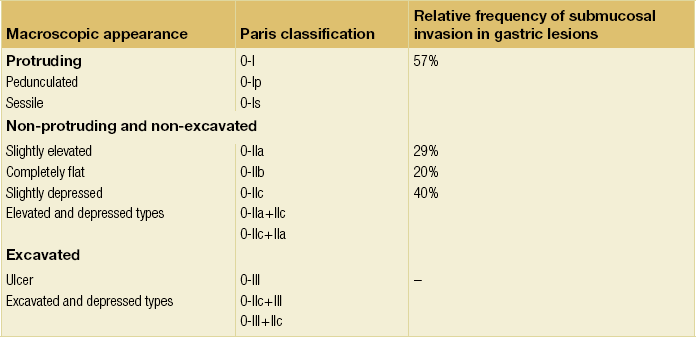
Experienced endoscopists performing endoscopic mucosal resections (EMRs) or endoscopic submucosal dissections (ESDs) are trained to recognise and delineate early neoplasia, which includes assessment of invasion depth and thus the endoscopic resectability of the lesion. EGCs are classified by their endoscopic appearance according to the Paris classification (Fig. 8.1).1 Superficial lesions are classified as either protruding (Paris 0-I), elevated (0-IIa), depressed (0-IIc) or excavated and often ulcerated lesions (0-III). Lesions that have both elevated and depressed components are classified into two groups: depressed lesions in which most of the surface is depressed and there is elevation in a portion of the peripheral ring are classified as 0-IIc + IIa, while elevated lesions with a central depression encircled by the elevated ring at the periphery are called 0-IIa + IIc. The combined patterns of excavation and depression are called 0-III + IIc or 0-IIc + III, depending on the relative surface area of the ulcer and of the depressed area. The classification helps to predict the extent of invasion into the submucosal layer and thus the choice between endoscopic or surgical treatment (Table 8.1). For instance, true protruding lesions in the stomach demonstrate a 57% relative frequency of submucosal invasion, whereas non-protruding, non-excavated lesions demonstrate submucosal invasion in frequencies between 20% and 40%. In excavated lesions, often the proper muscle layer is already involved. Although the exact percentage of involvement of the proper muscle layer is not reported in the literature, this number comes close to 100% and the Paris 0-III excavated types of lesions are usually not resectable by endoscopic means.
Additional characteristics that predict submucosal or deeper invasion are larger tumour size (> 30 mm), presence of discoloration (remarkable redness) and ulceration.5 Lesions that are confined to the mucosa tend to move over the peristaltic waves, whereas peristaltic waves seem to curve around tumours that have invaded the proper muscle layer. The latter provides a strong argument against endoscopic resection.
In most EMR and ESD techniques, submucosal injection of fluid is used to lift the early cancer from the proper muscle layer. This method has three major benefits: (i) it provides information on invasion depth and thus endoscopic resectability; (ii) it facilitates endoscopic resection; and (iii) it provides a safety fluid cushion for resecting the superficial lesion without damaging the deeper layers when using snares, knives or electrocautery.6
The amount of lifting provides information on the invasion depth of the lesion. Mucosal or superficial submucosal lesions usually demonstrate complete lifting (m–sm1), whereas the lesions that infiltrate into the deeper submucosal layers often lift incompletely (sm2–sm3). A non-lifting sign most often represents invasion deeper than sm3.6
Lymph node metastasis
Endoscopic staging before resection does provide useful information on the prediction of lymph node metastasis. Differentiation grade can be assessed through simple biopsy. A large retrospective study from Japan assessed the occurrence of lymph node metastasis in 5265 patients who had undergone gastrectomy with lymph node dissection for early gastric cancer.7 All specimens were reassessed for macroscopic appearance, size, ulceration, invasion depth and extent of submucosal invasion, differentiation grade and lymphovascular involvement.
The results are summarised in Table 8.2 and define the criteria for EMR and ESD.7,8
Endosonography
The role of endosonography remains debatable in EGCs. Endosonography lacks sufficient diagnostic accuracy in discriminating T1 from T2 lesions. The combined results of large recent studies show a diagnostic accuracy for T3 and T4 tumours of 88–100%, with 64–85% accuracy for T2 lesions and 75–82% accuracy for T1 tumours.9,10 T1 lesions are often overstaged, probably because of submucosal fibrosis, connective tissue hyperplasia or ulceration. Diagnostic accuracy can be improved using mini-probes; however, complete assessment of larger lesions is difficult and bears the risk of underestimating the deepest invasion when parts of the lesion are missed and subsequently not assessed.11 Many endoscopists rely, for these reasons, on endoscopic assessment alone followed by histopathological assessment of the resected specimen. A German study demonstrated a similar diagnostic accuracy of 83.4% and 79.6% using either high-resolution endoscopy or endosonography, respectively, in assessing infiltration depth in early neoplasia in the oesophagus.12 The authors believe the same applies for early neoplasia in the stomach.
Revised Vienna classification
Large discrepancies between Western and Japanese pathologists in the diagnostic criteria for adenoma, dysplasia and carcinoma in the gastrointestinal tract have led to considerable problems in the comparison between Western and Japanese data. This led to a consensus meeting in 1998 in Vienna, ultimately resulting in the Vienna classification of gastrointestinal epithelial neoplasia and the revised Vienna classification in 2002.13 This classification allows not only for a more universal nomenclature of gastrointestinal epithelial neoplasia but also corresponds more properly with clinical management. The revised Vienna classification is shown in Table 8.3.
Table 8.3
The revised Vienna classification of gastrointestinal epithelial neoplasia
| Category | Diagnosis | Clinical management |
| 1 | Negative for neoplasia | Optional follow-up |
| 2 | Indefinite for neoplasia | Endoscopic follow-up |
| 3 | Mucosal low-grade neoplasia | Endoscopic resection or follow-up |
| Low-grade dysplasia | ||
| Low-grade adenoma | ||
| 4 | Mucosal high grade neoplasia | Endoscopic or local surgical resection |
| 4.1 | High-grade adenoma/dysplasia | |
| 4.2 | Non-invasive carcinoma (carcinoma in situ) | |
| 4.3 | Suspicious for invasive carcinoma | |
| 4.4 | Intramucosal carcinoma | |
| 5 | Submucosal invasion by carcinoma | Surgical resection |
Endoscopic treatment
The basic principle of what is nowadays termed endoscopic mucosal resection (EMR) is the removal of a mucosal lesion by resecting it from its deeper layers using a snare instrument. This method does not allow for lesions larger than 2 cm to be removed en bloc.14 Larger lesions can be removed by EMR, but only in a piecemeal fashion. The technique is fundamentally different from ESD, where the submucosal layer is carefully and stepwise dissected. Using EMR, early neoplasia is often lifted from the proper muscle layer before resection using different solutions of saline for submucosal injection.15–17 The lesions can then be sucked into a cap that is placed at the tip of the endoscope with a snare preloaded on to the rim of the cap. After sucking the lesion into the cap, the snare is pulled. The content of the snare is then resected using high-frequency current. An alternative resection method that is frequently used is the so-called ‘band-and-cut’ method. A lesion is sucked into a modified multiband ligator. By ligating the mucosa bearing the lesion, a pseudopolyp is created that can be resected using a snare. This multiple band mucosectomy allows for larger segments of neoplasia to be completely resected.18 This method is increasingly used in larger Barrett’s segments but is also applicable in the cardia and the antrum of the stomach.
Endoscopic submucosal dissection
Endoscopic submucosal dissection (ESD) was originally developed in Japan for the local treatment of superficial EGC limited to the mucosal layer or with a minimal invasion of the submucosal layer. The main goal of submucosal dissection is to retrieve the lesion en bloc for histopathological staging and to minimise the chance for local recurrence. ESD is performed in several steps. First, the lesion is delineated by placing circumferential dots using electrocautery around the lesion with a few millimetres of free margin. The lesion is then lifted from the proper muscle layer by submucosal injection in the same fashion as in EMR.15–17 The solutions used are often stained with either indigo carmine or methylene blue. This dye will colour the submucosal layer and facilitate recognition of the separate layers and blood vessels. After lifting the lesion a circumferential incision of the mucosal layer is made, placing the markers just inside the circumferential cut. From this point onwards the submucosal layer is carefully dissected from the muscle layer, often with additional submucosal injections. In Fig. 8.2 an example of a Paris 0-IIa + IIc is shown, which is subsequently removed by ESD. Different speciality knives have been developed for ESD. A breakthrough in ESD was the development of the insulated tip (IT) knife in 1996.14 This is a speciality endoscopic knife with an insulated tip. An insulated small ceramic sphere is mounted on the top of a high-frequency needle knife, allowing safe and easy incision and separation of the mucosal and submucosal layers. Subsequently, the design of the original IT knife has been further adapted leading, for example, to the IT-2 knife, the hook knife, the flex knife, the triangular tip (TT) knife and the hybrid knife, the latter combining radiofrequency with a water-jet application.19 This novel technique combines radiofrequency with a distance-dependent water-jet application, allowing for easier and safer submucosal lifting and cutting in ESD.20,21
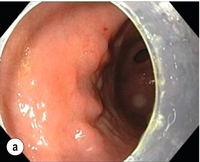
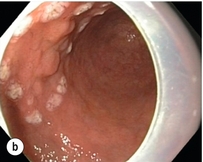
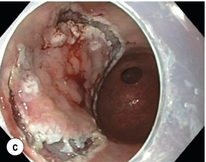
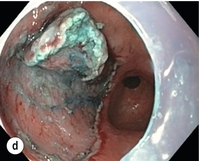
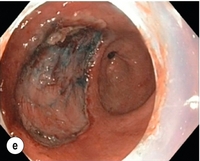
Figure 8.2 Early gastric cancer removed by ESD. (a) A Paris 0-IIc + IIa early gastric cancer located in the antrum. (b–e) Markings are placed around the lesion (b) and after submucosal lifting and circumferential incision (c), the lesion is stepwise dissected (d) and finally fully removed (e). Histopathological assessment demonstrated a well-differentiated adenocarcinoma confined to the mucosal layer with a maximum extent into the muscularis mucosae and tumour-free resection margins.
Complete resections
The aim of both EMR and ESD is to strive for complete resection of the neoplastic lesion.
Routinely, after endoscopic resection, the resected specimens are stretched and pinned on cork or paraffin and sent for pathological assessment. This final staging step by the pathologist should provide the necessary information on (i) quantitative criteria (lateral margins, deeper margins, maximum extent of submucosal invasion) and (ii) qualitative criteria (differentiation grade, lymphovascular involvement), which correspond with the risk of lymph node metastasis.7,8 This ultimate information is pivotal for further management. It mandates additional surgery or allows for a surveillance policy. Because of an approximately 14% chance of metachronous gastric cancer over a 5-year period23 and possible remnant neoplastic tissue, surveillance is usually carried out after endoscopic resection at 6-month intervals during the first year, followed by annual surveillance thereafter. Early detection of metachronous neoplasia can be treated by repeated endoscopic resection.
Complications of endoscopic resections
Most bleeding complications occur during the procedures and can be dealt with instantly. Superficial bleeding vessels are identified and treated either by using coagulation, adrenaline injections or clips. Delayed bleeding also occurs and might necessitate subsequent re-intervention. A large prospective study found no risk factors for bleeding besides the presence of a gastric malignancy itself.24
In expert hands, perforations occur during EMR and ESD in about 0.2% and 1–4% of cases, respectively. A recent European study reported a 20% perforation rate after ESD at a tertiary referral centre.25 This study clearly demonstrates a difference in expertise in some Western referral centres compared to Japanese and Korean expert centres. This is mostly due to a much lower incidence of EGCs in the Western world, resulting in an insufficient exposure to this type of pathology and resection techniques. Recently, a panel of European experts has attempted to set standards for quality criteria for ESD in European countries.26 Perforations can lead to pneumoperitoneum and in severe cases to generalised peritonitis. Usually, perforations are recognised immediately during the procedure and endoscopic management is possible and frequently adequate.27 Closure with clips is a safe treatment option together with nasogastric drainage and fasting.28 Recently, in a small case series the use of the over-the-scope clip (OTSC) was described for closure of perforations in a clinical setting.29 The OTSC device is a promising novel technique with a reported closure success rate of 92%.
Surgical resection
As described in Chapter 7, the type of gastrectomy and extent of lymphadenectomy for EGC depend on the location of the tumour. In general, a total gastrectomy is performed for tumours in the middle and upper third of the stomach and a distal gastrectomy for tumours in the distal third. Unfortunately, these extensive resections are associated with substantial postoperative short- and long-term complications and impaired quality of life. The low recurrence rates and high 5-year survival rates of EGC have led to the development of less extensive resections with a potentially improved postoperative quality of life and a comparable excellent long-term survival. In Japan and Korea proximal gastrectomy for EGC in the upper third of the stomach and pylorus-preserving gastrectomy (PPG) for EGC in the middle portion of the stomach are well-accepted procedures. Segmental or local gastric resections with or without lymphadenectomy have been described for gastric tumours, but still have to be considered experimental.
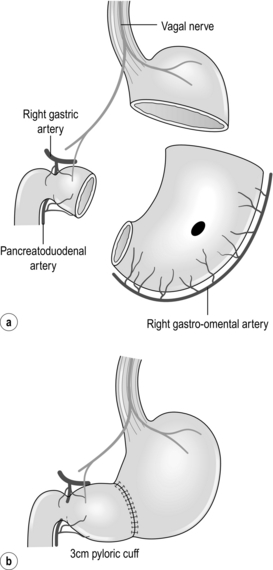
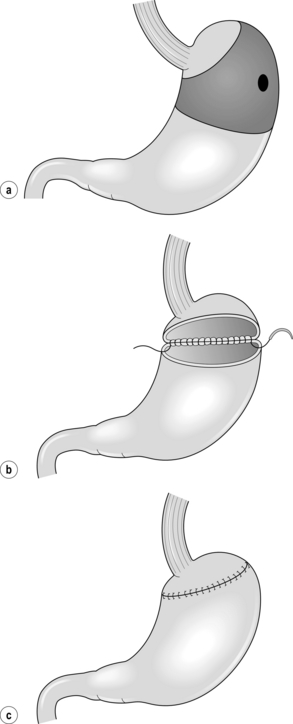
Proximal gastrectomy
Total gastrectomy with D1 or D2 lymphadenectomy is still the standard treatment for tumours in the upper third of the stomach. This type of resection is associated with the postgastrectomy syndrome, including dumping, epigastric pain, diarrhoea, hypoglycaemia, malnutrition and anaemia. As nodal metastases in the distal gastric lymph nodes (stations 5 and 6) are rare in EGC of the proximal stomach, less extensive resections have been proposed. Proximal gastrectomy (Fig. 8.3a) was developed as an alternative to total gastrectomy in order to reduce the long-term postoperative problems without compromising the oncological outcome. In proximal gastrectomy the proximal half of the stomach is resected, including a D1 or D1 + lymphadenectomy.30 Several techniques for surgical reconstruction after proximal gastrectomy have been described, including reconstruction by jejunal interposition and a gastric tube (Fig. 8.3b,c), but the optimal method remains controversial.
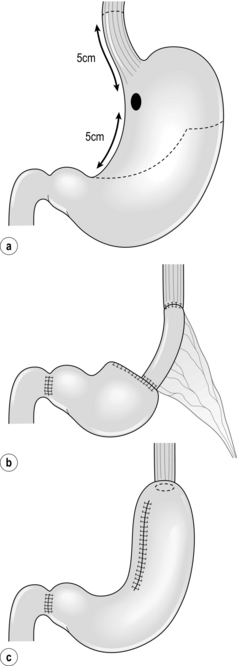
Figure 8.3 Proximal gastrectomy (a), with reconstruction by jejunal interposition (b) or gastric tube (c).
As could be expected due to the relatively high incidence of EGC in the East, most studies on proximal gastrectomy are from Japan and Korea,31–35 while data from Western countries are scarce.36 Several studies have shown that proximal gastrectomy for upper EGC is a safe procedure with comparable 5-year survival rates.31–34,36 However, conflicting data have been reported on the long-term complications and quality of life. Some studies showed an improved clinical outcome after proximal gastrectomy with oesophagogastrostomy or jejunal interposition, while others report a markedly higher complication rate including anastomotic stenosis and reflux oesophagitis.32–35 Therefore, further evaluation on this type of gastric resection for proximal EGC is needed. As yet, randomised data are not available.
Pylorus-preserving gastrectomy
PPG is a function-preserving procedure initially described for the treatment of peptic ulcer disease37 (Fig. 8.4). For patients with EGC, favourable functional results have been reported with PPG compared to conventional distal gastrectomy. PPG is feasible for early tumours in the middle third of the stomach with the distal border at least 4 cm proximal to the pylorus. In PPG a distal gastrectomy is performed with preservation of the pylorus and both the right gastric artery and the pyloric branch of the vagal nerve, followed by a stomach-to-stomach anastomosis.38 As a result, it is assumed that the pyloric function remains intact, preventing gastrointestinal symptoms such as dumping syndrome, epigastric fullness, reflux oesophagitis, bile regurgitation and cholelithiasis.39 In contrast to conventional distal or total gastrectomy, the suprapyloric (second-tier) lymph nodes are not resected in PPG. In EGC this is probably justified as 80–90% of EGCs are negative for lymph node metastases and the vast majority of positive lymph nodes are located in the first-tier lymph nodes only. Retrospective data of a Japanese database of 3646 T1 tumours located in the middle third of the stomach showed only 0.2% metastases to the pyloric lymph nodes.40 High 5-year survival rates up to 98% are reported after PPG with modified D2 lymph node dissection in patients with clinically diagnosed mucosal or submucosal gastric cancer (cT1) without lymph node metastases (cN0).41 Similar to studies on proximal gastrectomy, most data on PPG are derived from studies done in Japan and Korea. In Western countries PPG is still considered investigational.
Local (or wedge) segmental resection
Local or wedge resection involves removal of only the tumour including the nearby lymph nodes and primary closure of the stomach (Fig. 8.5). In a segmental resection a limited segment between the lesser and the greater curvature of the stomach is resected including the nearby lymph nodes and a formal gastro-gastrostomy is performed (Fig. 8.6). Local and segmental gastric resections for (early) gastric cancer have the obvious potential advantage of a better functional postoperative outcome, because digestive and reservoir functions of the stomach are mostly preserved. Several small studies show superior results on nutritional status and the occurrence of dumping and reflux oesophagitis after local or segmental resection, compared to a total or distal gastrectomy.42–45 These procedures can be safely performed in EGC with favourable features that cannot be resected by EMR or ESD for technical reasons. As mentioned before, the likelihood of lymph node metastases in these patients is very low and local or segmental resection with a limited lymphadenectomy seems justified.
Minimally invasive surgery
Some smaller studies from Europe and the USA showed similar results.49,50 Thus, laparoscopic gastric resection for EGC is a technically safe procedure and seems to be justified for selected patients. However, results from RCTs will have to prove the oncological safety of laparoscopic gastrectomy.
Recently, natural orifice transluminal endoscopic surgery (NOTES) has been proposed as a new technique for the treatment of EGC. One study has reported the feasibility of a combined NOTES and laparoscopic resection of EGC in 14 patients.51 Further studies have to define whether there is a role for NOTES in the treatment of EGC.
Lymphadenectomy
In gastric cancer, lymph node status is one of the crucial prognostic factors,52 and the type of resection and the extent of lymphadenectomy are largely based on the likelihood of lymph node metastases to first- and second-tier lymph nodes. However, in parallel with many other tumours it is still debatable whether (extended) lymphadenectomy in gastric cancer really leads to improved survival.53
As mentioned at the beginning of this chapter, mucosal and submucosal tumours with favourable features (Table 8.2)8 can be safely treated by EMR or ESD. This is based on the fact that these tumours have a very low risk of lymphatic dissemination. EGCs with unfavourable tumour characteristics harbour a high risk of lymph node metastases and are not amenable for endoscopic treatment. For these patients a gastric resection with lymphadenectomy is indicated. As discussed in Chapter 7, the extent of lymph node dissection in gastric cancer remains controversial. The discussion is mainly focused on the adequacy of a limited D1 lymphadenectomy versus the necessity of extended D2 or even D3 lymphadenectomy. In general, surgeons in Western countries tend to perform a more limited lymphadenectomy (D1), while surgeons in Japan and Korea perform a more extended lymphadenectomy (D2). The rationale for extended lymphadenectomy is improved staging, enhanced locoregional control and improved survival. The clearance of possible metastatic nodes in the region outside the perigastric nodes is presumed to have an impact on overall survival rates.
In Taiwan an RCT was performed comparing D1 with D3 lymphadenectomy; in the extended arm a level 3 lymph node dissection (hepatoduodenal ligament, retropancreatic region and superior mesenteric vein lymph nodes) and an optional hemipancreatosplenectomy were performed. This trial demonstrated an overall survival benefit in favour of a D3 lymphadenectomy, with 5-year overall survival rates of 59.5% versus 53.6%.57 These data suggest that extended (D2) lymphadenectomy can result in survival benefit for patients with advanced gastric cancer.
The optimal extent of lymph node dissection for EGC remains controversial. The incidence of lymph node metastases for mucosal EGC is only 2–4%, but increases to up to 45% in mucosal tumours with unfavourable features such as depressed or ulcerated lesions, tumours larger than 30 mm in size, tumours with undifferentiated histological type or with lymphovascular involvement.7,58 Most patients with mucosal EGC are safely treated by EMR or ESD without lymphadenectomy (Table 8.2). In submucosal EGC lymph node metastases are found in approximately 20%, with a wide range from 10% to 64%.59–61 In addition, approximately 5% of lymph node metastases from submucosal EGC are located in second-tier lymph nodes (mainly node stations 7, 8a and 9).62,63 Because of the relatively high risk of lymph node metastases in submucosal EGC and mucosal EGC with unfavourable features, a lymphadenectomy is generally performed in these patients. However, there is no consensus on the required extent of the lymphadenectomy. The Japanese Gastric Cancer Association (JGCA) recommends D2 lymphadenectomy for patients with EGC clinically positive node(s) (cN +),30 but this guideline lacks clear evidence.
For patients without suspicion of positive lymph nodes (cN0), currently no evidence exists that D2 dissection can improve survival rates in EGC. Considering the fact that in EGC the rate of lymph node metastases is relatively low and, when positive, generally confined to the first-tier lymph nodes, D2 resection can probably be omitted in EGC. Therefore, in Europe and the USA D1 resection is advocated for these patients, and also the JGCA recommends a D1 resection for all EGCs that are clinically node negative and do not meet the criteria for EMR or ESD. In Japan and Korea an additional so-called D1 + lymphadenectomy is advocated for submucosal EGC with unfavourable features (poorly differentiated, size > 1.5 cm) based on the 5% risk of second-tier lymph nodes.30 D1 + lymphadenectomy includes a D1 lymphadenectomy plus a selective dissection of second-tier lymph nodes dependent on the location of the tumour (nodes 8a, 9 and 11p in total or proximal gastrectomy; 8a and 9 in distal or pylorus-preserving gastrectomy). However, no randomised controlled studies have been conducted that prove the usefulness of these selective additional lymphadenectomies.
Sentinel node biopsy
Therefore, SLNB in EGC has been a focus for research in the past 10 years. Many different techniques and protocols have been employed. In most study protocols a double tracer method is preferred using a radioactive colloid, e.g. technetium-99 m, in combination with a dye agent such as isosulfan blue, patent blue violet or indocyanine green. The day before surgery the radioactive colloid is injected endoscopically in four quadrants into the submucosal layer at or in the vicinity of the primary lesion. The dye is injected intraoperatively in the same manner. By this means the sentinel node can be located by both a gamma probe for detection of a radioactive sentinel node and by visualisation of a blue or green coloured sentinel node.64 Most studies show increased rates of successful identification of sentinel lymph nodes by combining both techniques.65,66
Various studies have shown the technical feasibility of SLNB in EGC. However, the accuracy of SLNB in gastric cancer remains to be determined. The difficulty of the SLNB technique in gastric cancer is that, unlike the single-course lymphatic flow in breast cancer, the lymphatic flow of the stomach is multidirectional, which can result in multiple sentinel lymph nodes located in different first-tier lymph node stations (greater and lesser curvature), but also in second-tier stations.67,68
However, although it has been suggested that SLNB in gastric cancer is a useful tool for individualising the extent of lymphadenectomy in patients,70 these figures are currently too low to justify the introduction of SLNB as a standard procedure in EGC.
References
1. , The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003; 58:S3–43. 14652541
2. de Vries, A.C., van Grieken, N.C., Looman, C.W., et al, Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology 2008; 134:945–952. 18395075
3. Dinis-Ribeiro, M., Areia, M., de Vries, A.C., et al, Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012; 44:74–94. 22198778
4. Capelle, L.G., de Vries, A.C., Haringsma, J., et al, The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc 2010; 71:1150–1158. 20381801
5. Abe, S., Oda, I., Shimazu, T., et al, Depth-predicting score for differentiated early gastric cancer. Gastric Cancer 2011; 14:35–40. 21327924
6. Kato, H., Haga, S., Endo, S., et al, Lifting of lesions during endoscopic mucosal resection (EMR) of early colorectal cancer: implications for the assessment of resectability. Endoscopy 2001; 33:568–573. 11473326
7. Gotoda, T., Yanagisawa, A., Sasako, M., et al, Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225. 11984739
8. Gotoda, T., Yamamoto, H., Soetikno, R.M., Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol 2006; 41:929–942. 17096062 Based on histopathological assessment of 5265 gastrectomy specimens with formal lymph node dissections, it has been demonstrated that differentiated EGCs less than 30 mm in size and undifferentiated lesions without ulceration and less than 20 mm in size have a negligible risk of lymph node metastases.
9. Puli, S.R., Batapati Krishna Reddy, J., Bechtold, M.L., et al, How good is endoscopic ultrasound for TNM staging of gastric cancers? A meta-analysis and systematic review. World J Gastroenterol 2008; 14:4011–4019. 18609685
10. Mouri, R., Yoshida, S., Tanaka, S., et al, Usefulness of endoscopic ultrasonography in determining the depth of invasion and indication for endoscopic treatment of early gastric cancer. J Clin Gastroenterol 2009; 43:318–322. 19077733
11. Okada, K., Fujisaki, J., Kasuga, A., et al, Endoscopic ultrasonography is valuable for identifying early gastric cancers meeting expanded-indication criteria for endoscopic submucosal dissection. Surg Endosc 2011; 25:841–848. 20734082
12. May, A., Gunter, E., Roth, F., et al, Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective, and blinded trial. Gut 2004; 53:634–640. 15082579
13. Dixon, M.F., Gastrointestinal epithelial neoplasia: Vienna revisited. Gut 2002; 51:130–131. 12077106
14. Ono, H., Kondo, H., Gotoda, T., et al, Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001; 48:225–229. 11156645
15. Hirao, M., Masuda, K., Asanuma, T., et al, Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline–epinephrine. Gastrointest Endosc 1988; 34:264–269. 3391382
16. Inoue, H., Endo, M., Endoscopic esophageal mucosal resection using a transparent tube. Surg Endosc 1990; 4:198–201. 2291159
17. Tada, M., Murakami, A., Karita, M., et al, Endoscopic resection of early gastric cancer. Endoscopy 1993; 25:445–450. 8261986
18. Alvarez Herrero, L., Pouw, R.E., van Vilsteren, F.G., et al, Safety and efficacy of multiband mucosectomy in 1060 resections in Barrett’s esophagus. Endoscopy 2011; 43:177–183. 21365511
19. Yahagi, N., Neuhaus, H., Schumacher, B., et al, Comparison of standard endoscopic submucosal dissection (ESD) versus an optimized ESD technique for the colon: an animal study. Endoscopy 2009; 41:340–345. 19340739
20. Isomoto, H., Nishiyama, H., Yamaguchi, N., et al, Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 2009; 41:679–683. 19670135
21. Tanaka, S., Oka, S., Kaneko, I., et al, Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc 2007; 66:100–107. 17591481
22. Nakamoto, S., Sakai, Y., Kasanuki, J., et al, Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy 2009; 41:746–750. 19681023 Compared to EMR, ESD allows for higher en bloc resection rates (94.3% vs. 53.8%, P < 0.001) and overall 5-year recurrence-free rates (100% vs. 82.5%, P < 0.001) for EGCs larger than 10 mm in size.
23. Nasu, J., Doi, T., Endo, H., et al, Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy 2005; 37:990–993. 16189772
24. Jang, J.S., Choi, S.R., Graham, D.Y., et al, Risk factors for immediate and delayed bleeding associated with endoscopic submucosal dissection of gastric neoplastic lesions. Scand J Gastroenterol 2009; 44:1370–1376. 19891589
25. Coda, S., Trentino, P., Antonellis, F., et al, A Western single-center experience with endoscopic submucosal dissection for early gastrointestinal cancers. Gastric Cancer 2010; 13:258–263. 21128062
26. Deprez, P.H., Bergman, J.J., Meisner, S., et al, Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy 2010; 42:853–858. 20623442
27. Minami, S., Gotoda, T., Ono, H., et al, Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc 2006; 63:596–601. 16564858
28. Jeon, S.W., Jung, M.K., Kim, S.K., et al, Clinical outcomes for perforations during endoscopic submucosal dissection in patients with gastric lesions. Surg Endosc 2010; 24:911–916. 19789921
29. Manta, R., Manno, M., Bertani, H., et al, Endoscopic treatment of gastrointestinal fistulas using an over-the-scope clip (OTSC) device: case series from a tertiary referral center. Endoscopy 2011; 43:545–548. 21409741
30. Japanese Gastric Cancer Association, Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123. 21573742
31. Kaibara, N., Nishimura, O., Nishidoi, H., et al, Proximal gastrectomy as the surgical procedure of choice for upper gastric carcinoma. J Surg Oncol 1987; 36:110–112. 3657174
32. Katai, H., Sano, T., Fukagawa, T., et al, Prospective study of proximal gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg 2003; 90:850–853. 12854112
33. Shiraishi, N., Adachi, Y., Kitano, S., et al, Clinical outcome of proximal versus total gastrectomy for proximal gastric cancer. World J Surg 2002; 26:1150–1154. 12209245
34. An, J.Y., Youn, H.G., Choi, M.G., et al, The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg 2008; 196:587–591. 18519129
35. Kondoh, Y., Okamoto, Y., Morita, M., et al, Clinical outcome of proximal gastrectomy in patients with early gastric cancer in the upper third of the stomach. Tokai J Exp Clin Med 2007; 32:48–53. 21319057
36. Harrison, L.E., Karpeh, M.S., Brennan, M.F., Total gastrectomy is not necessary for proximal gastric cancer. Surgery 1998; 123:127–130. 9481396
37. Maki, T., Shiratori, T., Hatafuku, T., et al, Pylorus-preserving gastrectomy as an improved operation for gastric ulcer. Surgery 1967; 61:838–845. 5338114
38. Hiki, N., Kaminishi, M., Pylorus-preserving gastrectomy in gastric cancer surgery – open and laparoscopic approaches. Langenbeck’s Arch Surg 2005; 390:442–447. 16096761
39. Shibata, C., Shiiba, K.I., Funayama, Y., et al, Outcomes after pylorus-preserving gastrectomy for early gastric cancer: a prospective multicenter trial. World J Surg 2004; 28:857–861. 15593456
40. Nakajima, T., Yamaguchi, T. Gastric cancer data base in Cancer Institute, Japan, 1946–2004. Tokyo: Kanehara; 2006.
41. Hiki, N., Sano, T., Fukunaga, T., et al, Survival benefit of pylorus-preserving gastrectomy in early gastric cancer. J Am Coll Surg 2009; 209:297–301. 19717032
42. Seto, Y., Nagawa, H., Muto, Y., et al, Preliminary report on local resection with lymphadenectomy for early gastric cancer. Br J Surg 1999; 86:526–528. 10215830
43. Seto, Y., Yamaguchi, H., Shimoyama, S., et al, Results of local resection with regional lymphadenectomy for early gastric cancer. Am J Surg 2001; 182:498–501. 11754858
44. Ohwada, S., Nakamura, S., Ogawa, T., et al, Segmental gastrectomy for early cancer in the mid-stomach. Hepatogastroenterology 1999; 46:1229–1233. 10370697
45. Shinohara, T., Ohyama, S., Muto, T., et al, Clinical outcome of high segmental gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg 2006; 93:975–980. 16739101
46. Chen, X.Z., Hu, J.K., Yang, K., et al, Short-term evaluation of laparoscopy-assisted distal gastrectomy for predictive early gastric cancer: a meta-analysis of randomized controlled trials. Surg Laparosc Endosc Percutan Tech 2009; 19:277–284. 19692873
47. Kitano, S., Shiraishi, N., Uyama, I., Japanese Laparoscopic Surgery Study Group, A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg 2007; 245:68–72. 17197967
48. Lee, S.W., Nomura, E., Bouras, G., et al, Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg 2010; 211:33–40. 20610246 This meta-analysis of six randomised controlled trials and two large retrospective studies showed that laparoscopic gastric resection for EGC is a technically safe procedure.
49. Huscher, C.G., Mingoli, A., Sgarzini, G., et al, Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 2005; 241:232–237. 15650632
50. Strong, V.E., Devaud, N., Allen, P.J., et al, Laparoscopic versus open subtotal gastrectomy for adenocarcinoma: a case–control study. Ann Surg Oncol 2009; 16:1507–1513. 19347407
51. Cho, W.Y., Kim, Y.J., Cho, J.Y., et al, Hybrid natural orifice transluminal endoscopic surgery: endoscopic full-thickness resection of early gastric cancer and laparoscopic regional lymph node dissection – 14 human cases. Endoscopy 2011; 43:134–139. 21108175
52. Saka, M., Katai, H., Fukagawa, T., et al, Recurrence in early gastric cancer with lymph node metastasis. Gastric Cancer 2008; 11:214–218. 19132483
53. Cady, B., Lymph node metastases. Indicators, but not governors of survival. Arch Surg 1984; 119:1067–1072. 6383272
54. Bonenkamp, J.J., Hermans, J., Sasako, M., et al, Extended lymph-node dissection for gastric cancer. N Engl J Med 1999; 340:908–914. 10089184
55. Cuschieri, A., Weeden, S., Fielding, J., et al, Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999; 79:1522–1530. 10188901
56. Hartgrink, H.H., van de Velde, C.J., Putter, H., et al, Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch Gastric Cancer Group trial. J Clin Oncol 2004; 22:2069–2077. 15082726 These two large randomised studies compared D1 and D2 resection for gastric cancer. Both studies show no survival benefit or decreased relapse rate, while morbidity and mortality were increased. For EGC currently no evidence exists that D2 resection can improve survival rates.
57. Wu, C.W., Hsiung, C.A., Lo, S.S., et al, Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol 2006; 7:309–315. 16574546
58. Hirasawa, T., Gotoda, T., Miyata, S., et al, Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152. 19890694
59. An, J.Y., Baik, Y.H., Choi, M.G., et al, Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg 2007; 246:749–753. 17968165
60. Hayes, N., Karat, D., Scott, D.J., et al, Radical lymphadenectomy in the management of early gastric cancer. Br J Surg 1996; 83:1421–1423. 8944462
61. Popiela, T., Kulig, J., Kolodziejczyk, P., Polish Gastric Cancer Study Group, Long-term results of surgery for early gastric cancer. Br J Surg 2002; 89:1035–1042. 12153632
62. Nakamura, K., Morisaki, T., Sugitani, A., et al, An early gastric carcinoma treatment strategy based on analysis of lymph node metastasis. Cancer 1999; 85:1500–1505. 10193939
63. Kunisaki, C., Shimada, H., Nomura, M., et al, Appropriate lymph node dissection for early gastric cancer based on lymph node metastases. Surgery 2001; 129:153–157. 11174707
64. Kitagawa, Y., Saikawa, Y., Takeuchi, H., et al, Sentinel node navigation in early stage gastric cancer – updated data and current status. Scand J Surg 2006; 95:256–259. 17249274
65. Lee, J.H., Ryu, K.W., Kim, C.G., et al, Sentinel node biopsy using dye and isotope double tracers in early gastric cancer. Ann Surg Oncol 2006; 13:1168–1174. 16924376
66. Park do, J., Kim, H.H., Park, Y.S., et al, Simultaneous indocyanine green and (99m)Tc-antimony sulfur colloid-guided laparoscopic sentinel basin dissection for gastric cancer. Ann Surg Oncol 2011; 18:160–165. 20652640
67. Kelder, W., Nimura, H., Takahashi, N., et al, Sentinel node mapping with indocyanine green (ICG) and infrared ray detection in early gastric cancer: an accurate method that enables a limited lymphadenectomy. Eur J Surg Oncol 2010; 36:552–558. 20452171
68. Kitagawa, Y., Fujii, H., Kumai, K., et al, Recent advances in sentinel node navigation for gastric cancer: a paradigm shift of surgical management. J Surg Oncol 2005; 90:147–152. 15895450
69. Wang, Z., Dong, Z.Y., Chen, J.Q., et al, Diagnostic value of sentinel lymph node biopsy in gastric cancer: a meta-analysis. Ann Surg Oncol. 2012;19(5):1541–1550. 22048632 A meta-analysis of 38 studies on SLNB for gastric cancer reports a sensitivity of only 76.9% and a false-negative rate of 23.1%. Currently, the reliability of SLNB for gastric cancer is too low for clinical practice.
70. Ichikura, T., Chochi, K., Sugasawa, H., et al, Individualized surgery for early gastric cancer guided by sentinel node biopsy. Surgery 2006; 139:501–507. 16627059