Chapter 19 Endometriosis
OVERVIEW AND AETIOLOGY
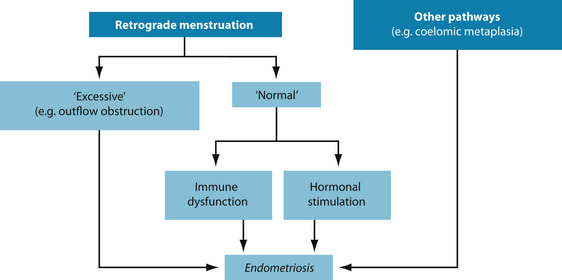
Endometriosis is the abnormal growth of endometrial tissue in areas other than the wall of the uterus.1 The exact cause of endometriosis is unknown although a number of theories do exist. It is one of the more common causes of infertility in Western societies. However, sufferers may experience combinations of many underlying causes and no person is identical in their symptoms or causes. Theories in naturopathic medicine include the following (see Figure 19.1):
RISK FACTORS
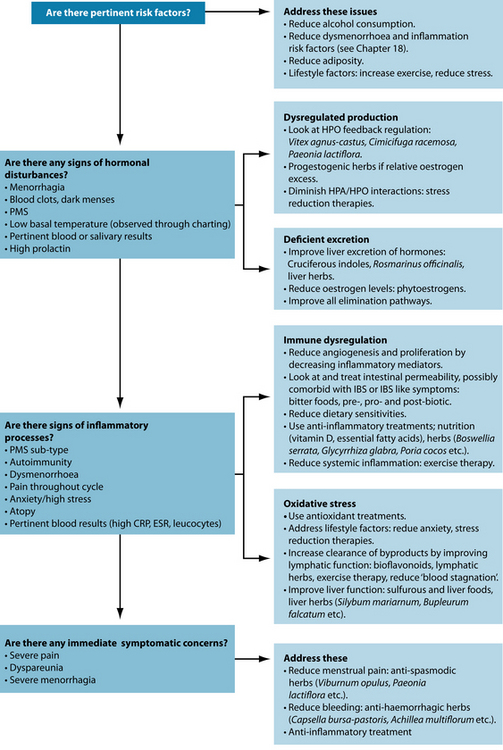
Several risk factors need to be addressed in the patient with endometriosis. Although often hormonal or inflammatory in nature, removal of these risk factors may be enough to significantly reduce symptoms. Lack of exercise can increase levels of oestrogen and inflammatory mediators and reduce oestrogen excretion.9 However, strenuous physical activity during menstruation may increase risk. Epidemiological data also suggest that positive correlations of symptoms and occurrence are seen with increased cigarette smoking; increased carbohydrate, alcohol and coffee intake; stress; and low body mass index.10 Aromatase found in adipose tissue may also increase the formation of oestrogen.11 Therefore weight loss may be indicated in some patients.
CONVENTIONAL TREATMENT
Conventional medical treatment aims to reduce the symptoms of endometriosis and improve fertility. This can be done surgically (most often laparoscopic to remove tissue, although occasionally hysterectomy is required) or medically. Medical interventions focus predominantly on reduction of excessive oestrogen levels. These include those androgenic in nature, such as danazol or progesterone supplementation; inducing hypo-oestrogenic states by decreasing FSH and LH through the use of gonadotrophin-releasing hormone agonists (GnRH agonists); continuous hormonal contraception to stop bleeding; or aromatase inhibitors to block the formation of oestrogens, particularly in adipose tissue.1
KEY TREATMENT PROTOCOLS
Oestrogen modulation
Hypothalamic–pituitary–ovarian axis modulation
Oestrogen levels in the body may be affected by disruptions of the hypothalamic–pituitary–ovarian (HPO) axis. The anterior pituitary releases FSH (follicle stimulating hormone) and LH (luteinising
AROMATASE
Aromatase is an enzyme of the cytochrome P450 superfamily the function of which is to aromatise androgens, thereby producing oestrogens. Normally, it is found in the ovaries, and to a much lesser extent in the skin and fat. Aromatase is not present in the normal endometrium but is expressed aberrantly in endometriosis.2,12–14
Prostaglandin E2 (PGE2) was found to be the most potent known inducer of aromatase activity in endometrial cells.15,16 Inflammation may not only increase aromatase, but also make endometrial tissue more sensitive to its effects.17 Factors known to increase aromatase activity are hyperinsulinaemia, increased adiposity, obesity and ageing.18
Aromatase activity may be decreased by increased consumption of dietary phyto-oestrogens19 in addition to reduction in adiposity and inflammation.
hormone), which encourages oestrogen release from growing ovarian follicles. Ordinarily feedback loops regulate hormone release from the HPO axis, but in some reproductive disorders this may be disrupted. Herbal medicines such as Vitex agnus-castus20 and Cimicifuga racemosa21 may help restore proper functioning of the HPO axis through direct and indirect means. Exercise has been shown to both reduce oestrogen production and increase oestrogen excretion10 (see Chapter 18 on premenstrual syndrome and dysmenorrhoea).
Oestrogen-like compounds and oestrogen receptor activity
Many compounds—both natural and synthetic—may mimic endogenous sex hormones.4,22,23 Several chemicals in current industrial use may interfere with the body’s hormone responses. Compounds such as dioxins, polychlorinated biphenyls (PCBs) and bisphenols (found in pesticides, petrochemicals and plastics) may bind to and activate endogenous oestrogen receptor sites. However, unlike natural hormones these xenoestrogens (literally ‘foreign oestrogens’) may exert effects many times more potent than endogenous oestrogens.24 Phytoestrogens (literally ‘plant oestrogens’) also bind to and activate these oestrogen receptor sites although they are often much less powerful than regular oestrogen and therefore act as oestrogen modulators by preventing the more powerful compounds—endogenous hormones and the xenoestrogens—binding in excess oestrogen conditions but binding to empty sites in oestrogen-deficient conditions.25 A compound exhibiting this activity is known as a selective oestrogen receptor modulator (SORM)—similar in effect to the pharmaceutical compound tamoxifen. The isoflavones (such as genistein and dadzein) from soy products, lignans from lentils and flaxseed, coumestans from Trifolium pratense and flavonoids found in a variety of sources are examples of phytoestrogens. Sources of phytoestrogens are listed in the box below. Although most research has focused on phytoestrogenic compounds from soy products, most dietary consumption of these compounds in Western diets occurs from lignans.26 Different soy products may also vary in their phytoestrogenic content: soybeans, tofu and tempeh are good sources while soy milk is generally not. Studies suggest that Cimicifuga racemosa may contain negligible amounts of phytoestrogenic compounds while still exerting strong oestrogen modulating ability.13 This is thought to be related more to its effects on luteinising hormone. V. agnus-castus has also shown significant competitive binding to oestrogen receptors in vitro.27
Table 19.1 Relative phytoestrogenic content (μg/100 g) of commonly used therapeutic supplements2,14,15,33,34
| Trifolium pratense | 1,767,000 |
| Flaxseed (crushed) | 546,000 |
| Soybeans | 103,920 |
| Tofu | 27,150 |
| Sesame seed | 8,008 |
| Flax bread | 7,540 |
| Multigrain bread | 4,798 |
| Pumpkin | 3,870 |
| Chickpeas | 3,600 |
| Lentils | 3,370 |
| Soy milk | 2,457 |
The generalisation that all phytoestrogens are inherently weaker than endogenous oestrogen is not correct. The herbs T. pratense and Humulus lupulus actually exert stronger activity in the body than endogenous oestrogen. This may make them therapeutically useful in oestrogen-deficient conditions—those associated with menopause, for example—but may potentially exacerbate symptoms of oestrogen-dependent disorders and render their use inappropriate in high doses in conditions such as endometriosis.28,29 It is also prudent to avoid herbs known to promote oestrogenic symptoms, such as Chamaelirium luteum and Dioscorea villosa. Studies suggest that long-term treatment with high-dose phytoestrogenic compounds (in excess of 150 mg of soy isoflavones daily for 5 years) can lead to endometrial hyperplasia.30 This suggests a role for lower doses associated with modified dietary intake for long-term management. Cruciferous indoles, in addition to their activity on oestrogen excretion and conversion, may also directly inhibit stimulation of oestrogen receptors by oestrogen or oestrogen-like compounds31,32 though the particular mechanism is unknown at this time.
Oestrogen excretion
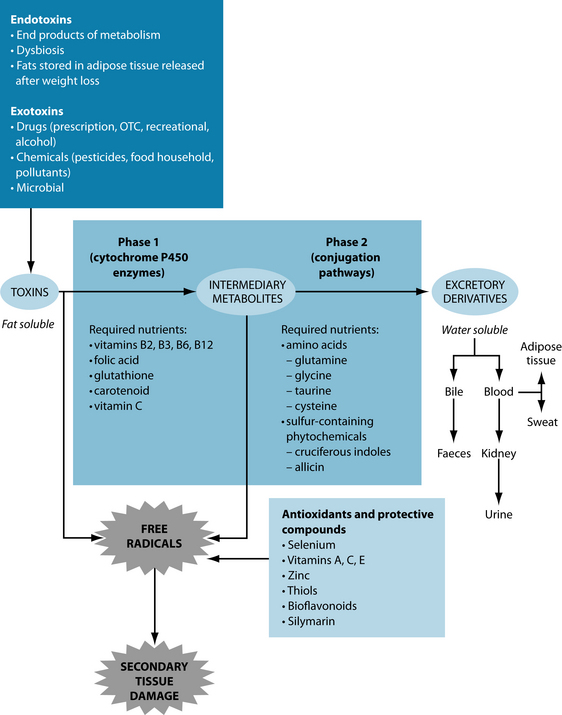
Inadequate oestrogen excretion may result in excess circulating oestrogens. The main route of elimination of excess oestrogens is the liver. The major pathways of elimination are the phase II liver pathways glucoronidation, sulphation and methylation.35 These pathways bind the used hormones with a water-soluble substance, which can then be eliminated through bile and eventually faeces.
If toxins overload the system, these pathways can become congested. Cruciferous indoles, such as indole-3-carbinol (I3C) and di-indolyl-methane (DIM), found in brussels sprouts, broccoli, cabbage, garlic and other ‘sulfurous’ vegetables,36 are
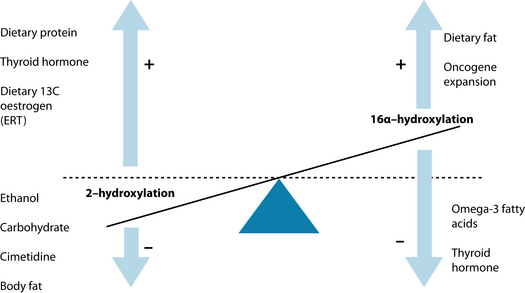
particularly useful for the oestrogen-specific pathways as they induce enzyme reactions that assist with detoxification and conversion of 17β-estradiol to less active forms (2-hydroxyestrone as opposed to 16α-hydroxyestrone).37–43 While these trials are largely based on direct supplementation (of 300–400 mg/day of I3C or 100 mg/day of DIM) studies suggest food supplementation may also be effective.36,39,44 Herbs such as Silybum marianum and Bupleurum falcatum can also improve liver enzyme activity in regards to oestrogen clearance.45 Figure 19.2 shows examples of supplements, foods and herbs useful in improving liver function. Rosmarinus officinalis has been found to directly increase hepatic metabolism of oestrogens and reduce their uterotropic action in animal studies.46 Vitamin B complexes may increase the inactivation of oestrone in the body.47 Other useful treatment tools are listed in Table 19.2.
Table 19.2 Detoxification enzyme reactions involving common naturopathic medicines
| PHASE | INDUCE | INHIBIT |
|---|---|---|
| I∗ | Cruciferous vegetables51 | Legumes52 |
| Garlic53 | Grapefruit juice54 | |
| Smoking55 | Starfruit juice56 | |
| Thymus vulgare57 | Taraxacum officinale58 | |
| Adhatoda vasica59 | Mentha pieperita58 | |
| Charcoaled meat products | Matricaria recutita58 | |
| Niacin | Humulus lupulus60 | |
| High protein diets | Glycyrrhiza glabra61,62 | |
| Hypericum perforatum63 | Rosmarinus officinalis64 | |
| Withania somnifera65 | ||
| Echinacea spp.66 | ||
| Chlorophyll36 | ||
| Berberine-containing herbs67,68 | ||
| Schisandra chinensis69 | ||
| II∗ | Curcumin70,71 | Low protein status |
| Vaccinum spp.72 | Zinc deficiency | |
| Green tea73,74 | B12 deficiency | |
| Cruciferous vegetables75–77 | Folic acid deficiency | |
| Taraxacum officinale58 | ||
| Humulus lupus60,78 | ||
| Glycyrrhiza glabra61 | ||
| Rosmarinus officinalis64,79 | ||
| Thymus vulgare57 | ||
| Adhatoda vasica59 | ||
| Withania somnifera65 | ||
| Flavonoids36 | ||
| Schisandra chinensis80 |
∗ For simplification purposes, phases I and II have not been split into their components.
Phase I liver detoxification processes convert oestrogens to either 2-hydroxyoestrone (2OH oestrone), 16- or 4-hydroxyoestrone. 2OH oestrone is a ‘cancer-protective’ metabolite (oestrogen antagonist) and the latter two are ‘pro-carcinogenic’ (oestrogen agonists).48 Each of the enzymes involved are subject to genetic polymorphisms that are measurable in more complex cases. Other factors can also affect this oestrogen conversion (see Figure 19.3).48
The liver is not the only organ associated with oestrogen excretion. The entero-hepatic circulatory system will recycle sex hormones if intestinal transit time is sufficiently slow. If there is not enough fibre in the diet, the oestrogens will be recirculated before they are excreted. Increased fibre consumption has been associated with lower oestrogen metabolites.34 Fibre is also required to increase dioxin, PCB and other oestrogen-like molecules from the body in animal models.49,50
Supporting liver function
Healthy detoxification consists of both appropriate phase I and phase II detoxification. Phase I is a stage in which lipid soluble substances are transformed into intermediate substances via the cytochrome P450 set of enzymes.33 In many instances this may render substances even more toxic or otherwise reactive than previously.33 Therefore appropriate phase II detoxification processes need to be supported. Phase II processes make the intermediate substances from phase I detoxification water-soluble by conjugating them with amino acids like glucoronic acid, glutathione and glycine or undergoing processes such as methylation, sulphation, acetylation and sulphoxidation.33 These substances can then be excreted through the stool, sweat or urine (and to lesser extent lungs)—elimination pathways, which also need to be appropriately encouraged in detoxification.
Many studies are either in vitro or animal in vivo and therefore clinical significance often remains unknown. It should also be noted that there often exists a lack of in vivo–in vitro correlation with respect to studies on interaction of phase I interactions. For example, while Silybum marianum has documented in vitro evidence of induction of cytochrome p450 enzymes, in vivo evidence does not seem to suggest a significant effect.81–83 Further studies have also demonstrated that co-administration of S. marianum with other medications does not reduce levels of that medication, again suggesting no clinically significant interaction.84–87 The induction of CYP enzymes by H. perforatum also seems to bear little clinical significance when compared to in vitro results.88,89 However, quality issues need to be considered as evidence suggests that specific compounds in naturopathic medicines that vary from product to product may be responsible for this induction—for example, levels of hyperforin may be responsible for induction in H. perforatum products and these levels can differ significantly in different products.88,90 However, a number of factors can belie in vitro pharmacokinetic suggestion in human physiology, including not just individual differences in people themselves, but also significant differences in different versions of the ‘same’ naturopathic products. Therefore caution should still be observed. It should also be noted that in a clinical setting induction or inhibition of phase I or phase II enzymes, and by extension possible interaction with other medications, does not necessarily preclude use of these naturopathic medicines. Rather it implies that these factors should be taken into appropriate consideration when prescribing them and that the patient’s use of concomitant medicines should be routinely monitored (see the drug–CAM interactions table in Appendix 1).
In some instances dietary inclusion may be more beneficial than supplementation. Glutathione—a key nutrient in phase II metabolism of toxins—is best obtained from food sources, as many supplements may have limited bioavailability.36 Diets low in protein may predispose patients to lowered liver detoxification, as key amino acids are involved in these liver processes.91 Increasing crude protein intake will also help to improve availability of amino acid precursors to conjugation.
Detoxification
Modulation of intestinal microflora with probiotics and synbiotics (including dietary intervention with yoghurt) has also been demonstrated to improve the liver’s general function and detoxification ability in humans, most probably through the reduction of endotoxin load or ammonia production and resorption in the intestine.92–95
Exercise will also promote hepatic biotransformation processes.96,97 Fasting is also known to enhance the detoxification process,98 in part because the main source of energy is hydrolysed fatty acid tissue from adipose tissue stores, where many toxins are stored.99 However, due prudence needs to be displayed, as fasting may liberate toxins faster than they can be eliminated (because in part the adequate substrates from phase II detoxification may be missing or compromised) and may potentially endanger the patient.
Hydrotherapy is thought to increase filtration through the liver by encouraging blood circulation, in addition to aiding excretion through sweating.100 Sauna therapy has also been used to encourage elimination through the skin, with substantial elimination and significant clinical improvement thought possible through this mechanism.101,102 Sauna exposure of 5–15 minutes per day is safe and effective in enhancing detoxification, though caution is advised in patients with recent myocardial infarction or other serious cardiovascular complications, and patients need to be advised that eliminating toxins through the skin may initially irritate (though ultimately improve) conditions such as atopic dermatitis.103 Many nutrients—particularly trace elements such as zinc, copper, iron and chromium as well as electrolytes—may be lost through sweating and may need monitoring or replacement.
An Ayurvedic herbal formula consisting of Capparis spinosa, Cichorium intybus, Solanum nigrum, Terminalia arjuna, Cassia occidentalis, Achillea millefolium and Tamarix gallica has been found to stimulate liver detoxification in addition to exerting hepatoprotective properties.104
Some herbs have a history of use for detoxification and are used in traditional herbal medicine for their role in supporting liver function. Herbs traditionally labelled hepatics or ‘liver’ herbs including S. marianum, Cynara scolymus, Bupleurum falcatum, Schisandra chinensis, Peamus boldo and Taraxacum officinale) have also been used to detoxify wastes, including excessive hormones, and may also be considered.105–107
Detoxification may be of assistance to people with chronic, though not life-threatening, diseases. One study investigated the use of a nutrient supplement specifically targeted at supporting phase I and phase II detoxification mechanisms in 84 patients for 10 weeks, and found significant improvement in symptoms in the intervention group.108 The same product exhibited similar results in another trial as well as a 23% increase in liver detoxification as measured by caffeine-clearance tests.109 Other small or uncontrolled trials have also demonstrated improvement in nutritional and psychological symptoms, as well as excretion of toxic markers such as PCBs, following a detoxification regimen using high dose niacin, individualised vitamin and mineral supplementation and polyunsaturated oils combined with physical exercise and sauna therapy.110–112 Other more generalised integrative detoxification (that have not relied purely on high-level supplementation) programs have also shown improvements.113
Detoxification is also an area in which unproven and often ineffective remedies are aggressively marketed, both to practitioners and to patients, implying careful consideration before their use in clinical practice.114 Therefore, while detoxification regimens may provide a clinically valuable adjuvant to treatment, naturopathic practitioners should be sure to focus on more relevant primary treatment aims in the clinical setting. More extreme detoxification methods can be every dangerous and should be avoided. While it is true that many traditional methods may fall into this category, it should also be acknowledged that these traditions were borne of a time when environmental toxic burdens were far lower and would have resulted in fewer side effects and lower risk.
Inflammation
Inflammatory cytokines
Endometriosis is a chronic inflammatory disease, characterised by altered function of immune-related cells, an increased number of activated macrophages and their secreted products, such as growth factors, cytokines and angiogenic factors in the peritoneal environment.6,118–125 The presence of inflammatory mediators may actually encourage endometrial tissue ordinarily found in retrograde menstruation to adhere to tissue.5
ENDOMETRIOSIS AND IBS
Endometriosis and irritable bowel syndrome exhibit markedly similar symptoms and one is, in fact, quite commonly misdiagnosed for the other.115,17 IBS is often associated with marked increases in inflammatory mediated cytokines IL-6, IL-10 and TNF-α. These inflammatory mediators are associated with altered bowel bacteria.116 These same cytokines are implicated in encouraging endometrial tissue to adhere to other tissue when in excess amounts in the peritoneal fluid. This is thought to be in some part due to the migration from these cells in dysbiosis (see the ‘leaky gut’ theory in Chapter 3 on irritable bowel syndrome). Positive correlations have been observed between dysbiosis and endometriosis in rhesus monkeys,117 and successful treatment of IBS with probiotics has been demonstrated to improve endometriosis outcomes.17
IL-6, IL-8 and TNF-α appear to be most associated with this phenomenon though others may also play a role. These specific cytokines are also linked to irritable bowel syndrome (IBS) and intestinal dysbiosis (see the box above). One theory is that these cytokines migrate to nearby areas, and in this case encourage endometrial tissue to adhere. High oestrogen levels may exacerbate this role by modulating immune response on macrophages and monocytes through their functional receptors.126 Higher oestrogen levels have also been associated with increased intestinal permeability and promotion of gram-negative intestinal bacteria.127 Probiotics, increasing fibre (and in particular the immune regulating fibres such as FOS), anti-inflammatory foods such as fish oils, garlic and ginger, and herbs such as Viburnum opulus and Boswellia serrata can also reduce inflammation in endometriosis.128 Even subclinical inflammation has been associated with progression of endometriosis indicating that even mild anti-inflammatory approaches may prove clinically useful.129
Angiogenesis and apoptosis
Excessive endometrial angiogenesis is proposed as an important mechanism in the pathogenesis of endometriosis and is thought to play an underlying role in the proliferation seen in the condition.130 Nuclear factor-kappa B (NF-кB) is thought to play an integral role in this increased proliferation. In endometriosis cells NF-кB appears to be continuously activated, and suppression of NF-кB activity by NF-кB inhibitors or proteasome inhibitors suppresses proliferation of endometrial cells in vitro.131 Various inflammatory mediators, growth factors and oxidative stress are thought to be responsible for this activation. Curcumin has been demonstrated to reduce angiogenesis and proliferation and induce apoptosis in endometrial cells in vitro and inactivates NF-кB.36,132 Other therapeutic agents specifically indicated to reduce angiogenesis and proliferation—although they have not been specifically studied in endometriosis—include vitamin D and foods rich in flavonoids, cruciferous indoles and resveratrol (see the box below).36,128 Correction of exacerbating factors, particularly inflammatory mediators, may also reduce proliferation.
VITAMIN D IN ENDOMETRIOSIS
Vitamin D analogues, such as danazol, have long been used in the treatment of endometriosis. However, vitamin D itself, and the vitamin D system, is thought to play a role in the immune complex changes observed in endometriosis.136 The active D3 form has been described as a potent regulator of cell growth and differentiation in endometriosis.137 Variations in vitamin D binding protein (DBP) have also been found in patients with endometriosis.138 It is thought that DBP may influence inflammatory mediators in the body. Only 5% of DBP is actually bound to vitamin D and its metabolites. The remainder has several important functions, including conversion to a powerful macrophage activating factor involved in increasing inflammatory processes in the body. Vitamin D is also associated with increased HDL cholesterol levels,139 which are associated with reductions in symptoms of endometriosis.140
An experimental treatment protocol using aromatase inhibitors given concomitantly with supplemental vitamin D has achieved promising results in the treatment of endometriosis.141 Research has also demonstrated reduced lesion weight in endometriosis cells with vitamin D.142
Some evidence suggests that endometriotic tissue may not have enhanced proliferative abilities, but rather reduced apoptosis.133–135 Therapeutic interventions that may increase apoptosis, such as Curcuma longa, Scutellaria baicalensis, zinc, selenium and foods rich in a broad range of various phytochemicals (including flavonoids, cruciferous indoles and isothiocynates) may be useful in the treatment of endometriosis.36,128
Prostaglandin regulation
Oestrogen is also reported to increase PGE2 formation by stimulating cyclooxygenase type 2 (COX-2) enzymes in endometrial stromal cells,143,144 thereby producing a positive feedback loop for continuous local production of oestrogen and prostaglandins; this favours the proliferative and inflammatory characteristics of endometriosis.
Animal studies have found reduced production levels of inflammatory prostaglandins PGE2 and PGF2α, and decreased endometrial implant diameter in those animals treated with eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) of marine origin.145 In vitro studies have also found that omega-3 fatty acids found in fish oils reduced survival rates of endometrial cells when compared to omega-6 (n-6), mixed polyunsaturated fats (PUFA) or control groups.146 Epidemiological data of fish oil consumption in women with endometriosis have shown reduced symptoms with increased consumption.147 Increased consumption of other inflammatory polyunsaturated fats, particularly of the omega-6 series, was also linked with an increase in symptoms.148 Exercise, increasing water consumption and eliminating food allergies will also reduce inflammatory mediators.
Endometrial tissue damage
Adhesions
Adhesions increase when women have endometriosis. Vitamin E has been specifically demonstrated to reduce adhesion formation.149–151 This action is thought to be primarily through reduction of series 2 prostaglandins and better removal of pelvic debris by white blood cells.152 Although unpublished, Italian research has demonstrated a reduction in adhesion weight when treated with vitamin D compounds.144,153 Zinc, Calendula officinalis and vitamin C are also traditionally used.
Surgery
It is accepted that in specific patients endometriosis can spread directly. The risk of endometrial implantation is increased by surgery, particularly surgery to remove endometrial tissue.3 This is due to the increase of inflammatory mediators to the area.6 Reducing inflammation and encouraging appropriate healing responses can reduce the risk of recurring endometrial growths after surgery.
dysmenorrhoea, endometriosis and fibroids (see Chapter 18 on dysmenorrhoea), in particular the emperor herbs Cinnamomum zeylanicum, Poria cocos and Paeonia lactiflora.156 Chinese exercise therapy (qi gong) referral may also be appropriate in this condition.
INTEGRATIVE MEDICAL CONSIDERATIONS
Traditional Chinese medicine
Acupuncture has documented success in relieving the pain of dysmenorrhoea. In traditional Chinese medicine endometriosis is seen as a disorder of Qi, Blood and Liver stagnation, an approach that has had documented success in the literature.154,155 Gui Zhi Fu Ling Wan is indicated to move blood, transform stagnation and remove masses—particularly in lower abdominal areas. Its modern integrated naturopathic application extends to
Oral contraceptive pills
Hormone supplementation is commonly used in conventional medicine to reduce endometriosis symptoms. Women taking contraceptive agents therapeutically may become deficient in vitamins B2, B3, B5, B12 and folate and may experience raised levels of vitamin A.128 Hormone use may reduce the effects of Vitex agnus-castus and increase the hypertensive side effects associated with Glycyrrhiza glabra. Any treatment that aims to increase liver function will reduce the effectiveness of the hormonal drugs.128
Example treatment
Herbal formula
| Vitex agnus-castus 1:2 | 10 mL |
| Cimicifuga racemosa 1:2 | 20 mL |
| Achillea millefolium 1:2 | 20 mL |
| Viburnum opulus 1:2 | 30 mL |
| Glycyrrhiza glabra 1:2 | 20 mL |
At the second consultation the patient had adhered to the dietary changes and followed all recommendations. She had begun qi gong classes and started walking 20 minutes every day. She had no further
abdominal pain or digestive discomfort. Her bleeding had reduced noticeably since the previous cycle. She was advised to continue dietary and lifestyle prescriptions, along with the herbal formula until review in 1 month.
At the third consult, the patient had slipped a little on her diet, but to her surprise this had led to only minor symptoms returning. At this stage it was suggested that the patient move towards a tablet form of Vitex agnus-castus—to be continued for a period of 4 months before review at a dose of two 500 mg tablets, twice daily (at which stage it was discontinued). The patient at this time had vastly improved menstrual symptoms though she still had significant dark clotting in her menses and occasional stabbing pain—though to nowhere near the extent previously experienced. A Chinese herbal formula for blood stasis was prescribed: cinnamon and hoelen formula (containing Poria cocos, Cinnamomum zeylanicum and Paeonia lactiflora) at a dose of 10 g per day. The patient returned after 3 months with a ‘normal’ menstrual cycle. Nervous support and preconception care were the new foci of her treatment.
Expected outcomes and follow-up protocols
Pain reduction is often the most commonly sought treatment. This is thought to be due to underlying excessive cytokine levels157 and will be addressed in due course, though immediate symptomatic relief for dysmenorrhoea (through, for example, the supplementation of Viburnum opulus in the 3 days before the period) and menorrhagia (through supplementation of Capsella bursa-pastoris or Achillea millefolium) can occur in the first cycle after treatment. Concomitant with these symptomatic treatments, longer-term aims of normalising underlying menstrual function should be undertaken. Noticeable improvement in general should occur by the second cycle, providing dietary and lifestyle changes are also addressed. This should be especially noticeable in terms of abdominal pain, bloating and concomitant gastrointestinal symptoms. Women with larger or more ingrained endometrial growths will require oestrogen modulating and anti-inflammatory treatment for some months to reduce and remove well-established endometrial growths, particularly those in ‘enclosed’ or ‘removed’ areas—such as those in areas not commonly associated with menstrual flow (for example, the intestines and kidneys). Patients should be notified that this could take up to 12 months in difficult cases, and that results are extremely variable between patients.
KEY POINTS
Fjerbaek A., Knudsen U.B. Endometriosis, dysmenorrhea and diet—what is the evidence? Eur J Obstet Gynecol Reprod Biol. 2007;132(2):140-147.
Guidice L.C., Kao L.C. Endometriosis. Lancet. 2004;364(9447):1789-1799.
Halis G., Arici A. Endometriosis and inflammation in infertility. Ann N Y Acad Sci. 2004;1034:300-315.
Hudson T. Women’s encyclopedia of natural medicine. New York: Mcgraw-Hill; 2008.
Trickey R. Women, hormones and the menstrual cycle. Sydney: Allen & Unwin; 2003.
1. Edmonds K., editor. Obstetrics and gynaecology. London: Blackwell, 2007.
2. Mazur W., Adlercreutz H. Naturally occurring estrogens in food. Pure Applied Chem. 1998;70:1759-1776.
3. Guidice L., Kao L. Endometriosis. Lancet. 2004;364(9447):1789-1799.
4. Rier S. The potential role of exposure to environmental toxicants in the pathophysiology of endometriosis. Ann N Y Acad Sci. 2002;955:201-212.
5. Halme J., et al. Peritoneal macrophages from patients with endometriosis release growth factor activity in vitro. J Clin Endocrinol Metab. 1988;66:1044-1049.
6. Leibovic D., et al. Immunobiology of endometriosis. Fertil Steril. 2001;75:1-10.
7. Schrodt G., et al. Endometriosis of the male urinary system: a case report. J Urol. 1980;124(5):722-723.
8. Martin J., Hauck A. Endometriosis in the male American surgeon. Am Surg. 1985;51(7):426-430.
9. Westerlind K., Williams N. Effect of energy deficiency on estrogen metabolism in premenopausal women. Med Sci Sports Exerc. 2007;39(7):1090-1097.
10. Missmer S., et al. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric and lifestyle factors. Am J Epidemiol. 2004;160(8):784-796.
11. Wake D., et al. Increased aromatase expression in human subcutaneous adipose tissue in obesity. Endocrine Abstracts. 2004;7:60.
12. Adlercreutz H. Epidemiology of phytoestrogens. Ballieres Clin Endocrin Metabol. 1998;12:605-623.
13. Petterson H., Kiessling K. Liquid chromatographic determination of the plant estrogens coumestrol and isoflavones in animal feed. J Assoc Off Anal Chem. 1984;67(3):503-506.
14. Bulun S., et al. Role of aromatase in endometrial disease. J Steroid Biochem Mol Biol. 2001;79:19-25.
15. Lea R., Whorwell P. Irritable bowel syndrome or endometriosis or both? Eur J Gastroenterol Hepatol. 2003;15(10):1131-1133.
16. Noble L., et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600-606.
17. Bukulmez O., et al. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology. 2008;149(3):1190-1204.
18. Simpson E., et al. Aromatase—a brief overview. Annu Rev Physiol. 2002;64:93-127.
19. Brooks J., Thompson L. Mammalian lignans and genistein decrease the activities of aromatase and 17beta-hydroxysteroid dehydrogenase in MCF-7 cells. J Steroid Biochem Mol Biol. 2005;94(5):461-467.
20. Wuttke W., et al. Chaste tree (Vitex agnus-castus) pharmacology and clinical indications. Phytomedicine. 2003;10(4):348-357.
21. Seidlova-Wuttke D. Evidence for selective estrogen receptor modulator activity in a black cohosh (Cimicifuga racemosa) extract: comparison with estradiol-17 beta. Eur J Endocrinol. 2003;149:351-362.
22. Birnbaum L., Cummings A. Dioxins and endometriosis: a plausible hypothesis. Environ Health Perspect. 2002;110(1):15-21.
23. Foster W., Agarwal S. Environmental contaminants and dietary factors in endometriosis. Ann N Y Acad Sci. 2002;955:213-229.
24. Tsutsumi O. Assessment of human contamination of estrogenic endocrine-disrupting chemicals and their risk for human reproduction. J Steroid Biochem Mol Biol. 2005;93(2–5):325-330.
25. Wang L. Mammalian phytoestrogens: enterodiol and enterolactone. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777(1–2):289-309.
26. Valsta L., et al. Phyto-oestrogen database of foods and average intake in Finland. Br J Nutr. 2003;89:S31-S38.
27. Liu J. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J Agric Food Chem. 2001;49(5):2472-2479.
28. Beck V., et al. Comparison of hormonal activity (estrogen, androgen and progestin) of standardized plant extracts for large scale use in hormone replacement therapy. J Steroid Biochem Mol Biol. 2003;84(2–3):259-268.
29. Zava D., et al. Estrogen and progestin bioactivity of foods, herbs, and spices. Proc Soc Exp Biol Med. 1998;217(3):369-378.
30. Unfer V., et al. Endometrial effects of long-term treatment with phytoestrogens: a randomized, double-blind, placebo-controlled study. Fertil Steril. 2004;82:145-148.
31. Ashok B., et al. Abrogation of oestrogen-mediated cellular and biochemical effects by indole-3-carbinol. Nutr Cancer. 2001;41(1–2):180-187.
32. Meng Q., et al. Indole-3-carbinol is a negative regulator of estrogen receptor-alpha signalling in human tumour cells. J Nutr. 2000;130(12):2927-2931.
33. Sowers M., et al. Selected diet and lifestyle factors are associated with estrogen metabolites in a multiracial/ethnic population of women. J Nutr. 2006;136(6):1588-1595.
34. Thompson L., et al. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans and coumestan. Nutr Cancer. 2006;54(2):184-201.
35. Voet D. Biochemistry, 3rd edn. New York: Wiley & Sons; 2004.
36. Higdon J. An evidence-based approach to dietary phytochemicals. Stuttgart: Thieme; 2007.
37. Bradlow H., et al. Long-term responses of women to indole-3-carbinol or a high fibre diet. Cancer Epidemiol Biomarkers Prev. 1994;3(7):591-595.
38. Dalessandri K., et al. Pilot study: effect of 3,3’-diindolyl-methane supplements on urinary hormone metabolites in postmenopausal women with a history of early stage breast cancer. Nutr Cancer. 2004;50(2):161-167.
39. McAlindon T., et al. Indole-3-carbinol in women with SLE: effect on estrogen metabolism and disease activity. Lupus. 2001;10(11):779-783.
40. Michnovicz J., Bradlow H. Altered oestrogen metabolism and excretion in humans following consumption of indole-3-carbinol. Nutr Cancer. 1991;16:59-66.
41. Michnovicz J., et al. Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Natl Cancer Inst. 1997;89(10):718-723.
42. Michnovicz J. Increased estrogen 2-hydroxylation in obese women using oral indole-3-carbinol. Int J Obes Relat Metab Disord. 1998;22(3):227-229.
43. Wong G., et al. Dose-ranging study of indole-3-carbinol for breast cancer prevention. J Cell Biochem Suppl. 1997;28–29(Supp 1):S111-S116.
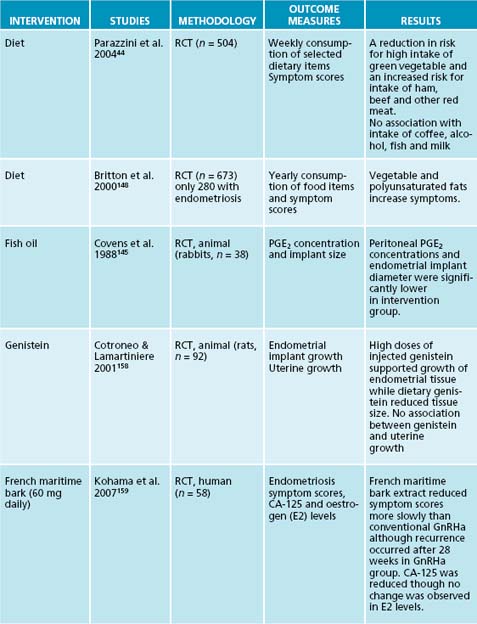
44. Parazzini F., et al. Selected food intake and risk of endometriosis. Human Reprod. 2004;19(8):1755-1759.
45. Morazzoni P., Bombardelli E. Silybum marianum (Carduus Marianus). Fitoterapia. 1995;66:3-42.
46. Zhu B., et al. Dietary administration of an extract from rosemary leaves enhances the liver microsomal metabolism of endogenous estrogens and decreases their uterotropic action in CD-1 mice. Carcinogenesis. 1998;19(10):1821-1827.
47. Zondek B., Finkerlstein M. Effect of vitamin B complex on inactivation of estrone in vivo and in vitro. Science. 1947;105(2723):259-260.
48. Sepkovic D., Bradlow H. Estrogen hydroxylation—the good and the bad. Ann N Y Acad Sci. 2009;1155:57-67.
49. Aozasa O., et al. Enhancement in fecal excretion of dioxin isomer in mice by several dietary fibers. Chemosphere. 2001;45(2):195-200.
50. Kimura Y., et al. Some dietary fibers increase elimination of orally administered polychlorinated biphenyls but not that of retinol in mice. J Nutr. 2004;134:135-142.
51. Wattenberg L. Studies on polycyclic hydrocarbon hydroxylases of the intestine possibly related to cancer: effects of diet on benzpyrene hydroxylase activity. Cancer. 1971;28:99-102.
52. Cardador-Martínez A., et al. Relationship among antimutagenic, antioxidant and enzymatic activities of methanolic extract from common beans (Phaseolus vulgaris L). Plant Foods Hum Nutr. 2006;61(4):161-168.
53. Brady J., et al. Modulation of rat hepatic microsomal monooxygenase activities and cytotoxicity by diallyl sulfide. Toxicol Appl Pharmacol. 1991;108:342-354.
54. Uno T., Yasui-Furukori N. Effect of grapefruit juice in relation to human pharmacokinetic study. Curr Clin Pharmacol. 2006;1(2):157-161.
55. Kroon L. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64(18):1917-1921.
56. Zhang J., et al. Inhibition of human liver cytochrome P450 by star fruit juice. J Pharm Pharm Sci. 2007;10(4):496-503.
57. Sasaki K., et al. Thyme (Thymus vulgaris L.) leaves and its constituents increase the activities of xenobiotic-metabolizing enzymes in mouse liver. J Med Food. 2005;8(2):184-189.
58. Maliakal P., Wanwimolruk S. Effect of herbal teas on hepatic drug metabolizing enzymes in rats. J Pharm Pharmacol. 2001;53(10):1323-1329.
59. Singh R., et al. Modulatory influence of Adhatoda vesica (Justicia adhatoda) leaf extract on the enzymes of xenobiotic metabolism, antioxidant status and lipid peroxidation in mice. Mol Cell Biochem. 2000;213(1–2):99-109.
60. Stevens J., Page J. Xanthohumol and related prenylflavonoids from hops and beer: to your good health!. Phytochemistry. 2004;65(10):1317-1330.
61. Chan H., et al. Inhibition of glycyrrhizic acid on aflatoxin B1-induced cytotoxicity in hepatoma cells. Toxicoloy. 2003;188(2–3):211-217.
62. Jeong H., et al. Hepatoprotective effects of 18beta-glycyrrhetinic acid on carbon tetrachloride-induced liver injury: inhibition of cytochrome P450 2E1 expression. Pharmacol Res. 2002;46(3):221-227.
63. Whitten D., et al. The effect of St John’s wort extracts on CYP3A: a systematic review of prospective clinical trials. Br J Clin Pharmacol. 2006;62(5):512-516.
64. Offord E., et al. Mechanisms involved in the chemoprotective effects of rosemary extract studied in human liver and bronchial cells. Cancer Lett. 1997;114(1–2):275-281.
65. Padmavathi B., et al. Roots of Withania somnifera inhibit forestomach and skin carcinogenesis in mice. Evid Based Complement Alternat Med. 2005;2(1):99-105.
66. Raner G., et al. Effects of herbal products and their constituents on human cytochrome P450(2E1) activity. Food Chem Toxicol. 2007;45(12):2359-2365.
67. Xin H., et al. The effects of berberine on the pharmacokinetics of cyclosporin A in healthy volunteers. Methods Find Exp Clin Pharmacol. 2006;28(1):25-29.
68. Imanshahidi M., Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res. 2008;22(8):999-1012.
69. Iwata H., et al. Identification and characterization of potent CYP3A4 inhibitors in Schisandra fruit extract. Drug Metab Dispos. 2004;32(12):1351-1358.
70. Dinkova-Kostova A., Talalay P. Relation of structure of curcumin analogs to their potencies as inducers of phase 2 detoxification enzymes. Carcinogenesis. 1999;20:911-914.
71. Iqbal M., et al. Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: possible role in protection against chemical carcinogenesis and toxicity. Pharmacol Toxicol. 2003;92(1):33-38.
72. Bomser J., et al. In vitro anticancer activity of fruit extracts from Vaccinium species. Planta Med. 1996;62:212-216.
73. Khan S., et al. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: possible role in cancer chemoprevention. Cancer Res. 1992;52:4050-4052.
74. Stoner G., Mukhtar H. Polyphenols as cancer chemopreventive agents. J Cell Biochem. 1995;22(Suppl):169-180.
75. Zhang Y., et al. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89:2399-2403.
76. Uda Y., et al. Induction of the anticarcinogenic marker enzyme, quinone reductase, in murine hepatoma cells in vitro by flavonoids. Cancer Lett. 1997;120:213-216.
77. Nho C., Jeffery E. The synergistic upregulation of phase II detoxification enzymes by glucosinolate breakdown products in cruciferous vegetables. Toxicol Appl Pharmacol. 2001;174(2):146-152.
78. Dietz B., et al. Xanthohumol isolated from Humulus lupulus inhibits menadione-induced DNA damage through induction of quinone reductase. Chem Res Toxicol. 2005;18(8):1296-1305.
79. Sotelo-Félix J., et al. Evaluation of the effectiveness of Rosmarinus officinalis (Lamiaceae) in the alleviation of carbon tetrachloride-induced acute hepatotoxicity in the rat. J Ethnopharmacol. 2002;81(2):145-154.
80. Lee S., et al. Induction of the phase II detoxification enzyme NQO1 in hepatocarcinoma cells by lignans from the fruit of schisandra chinensis through nuclear accumulation of Nrf2. Planta Med. 2009. In press
81. Gurley B., et al. In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, and saw palmetto. Clin Pharmacol Ther. 2004;76(5):428-440.
82. Gurley B., et al. Clinical assessment of CYP2D6-mediated herb-drug interactions in humans: effects of milk thistle, black cohosh, goldenseal, kava kava, St John’s wort, and echinacea. Mol Nutr Food Res. 2008;52(7):755-763.
83. Gurley B., et al. Effect of milk thistle (Silybum marianum) and black cohosh (Cimicifuga racemosa) supplementation on digoxin pharmacokinetics in humans. Drug Metab Dispos. 2006;34(1):69-74.
84. DiCenzo R., et al. Coadministration of milk thistle and indinavir in healthy subjects. Pharmacotherapy. 2003;23(7):866-870.
85. Piscitelli S., et al. Effect of milk thistle on the pharmacokinetics of indinavir in healthy volunteers. Pharmacotherapy. 2002;22(5):551-556.
86. Mills E., et al. Milk thistle and indinavir: a randomized controlled pharmacokinetics study and meta-analysis. Eur J Clin Pharmacol. 2005;61(1):1-7.
87. Doehmer J., et al. Assessment of drug-drug interaction for silymarin. Toxicol. Vitro. 2008;22(3):610-617.
88. Mueller S., et al. No clinically relevant CYP3A induction after St John’s wort with low hyperforin content in healthy volunteers. Eur J Clin Pharmacol. 2009;65(1):81-87.
89. Will-Shahab L., et al. St John’s wort extract (Ze 117) does not alter the pharmacokinetics of a low-dose oral contraceptive. Eur J Clin Pharmacol. 2009;65(3):287-294.
90. Mueller S., et al. The extent of induction of CYP3A by St. John’s wort varies among products and is linked to hyperforin dose. Eur J Clin Pharmacol. 2006;62(1):29-36.
91. Soeters P., et al. Amino acid adequacy in pathophysiological states. J Nutr. 2004;134(6 Suppl):1575S-1582S.
92. Liu Q., et al. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39(5):1441-1449.
93. Bajaj J., et al. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol. 2008;103(7):1707-1715.
94. Sharma P., et al. An open-label randomized controlled trial of lactulose and probiotics in the treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2008;20(6):506-511.
95. Sheth A., Garcia-Tsao G. Probiotics and liver disease. J Clin Gastroenterol. 2008;42(Supp 2):80-84.
96. Duncan K., et al. Running exercise may reduce risk for lung and liver cancer by inducing activity of antioxidant and phase II enzymes. Cancer Lett. 1997;116(2):151-158.
97. Yiamouyiannis C., et al. Chronic physical activity: hepatic hypertrophy and increased total biotransformation enzyme activity. Biochem Pharmacol. 1992;44(1):121-127.
98. Imamura M., Tung T. A trial of fasting cure for PCB-poisoned patients in Taiwan. Prog Clin Biol Res. 1984;137:147-153.
99. Müllerová D., Kopecký J. White adipose tissue: storage and effector site for environmental pollutants. Physiol Res. 2007;56(4):375-381.
100. Stiefelhagen P. Functional disorders call for total therapy—Kneipp’s hydrotherapy instead of psychopharmaceuticals. MMW Fortschr Med. 2005;147(18):4-8.
101. Krop J. Chemical sensitivity after intoxication at work with solvents: response to sauna therapy. J Altern Complement Med. 1998;4(1):77-86.
102. Chambaz A., et al. Urinary caffeine after coffee consumption and heat dehydration. Int J Sports Med. 2001;22(5):366-372.
103. Hannuksela M., Ellahham S. Benefits and risks of sauna bathing. Am J Med. 2001;110(2):118-126.
104. Huseini H., et al. The efficacy of Liv-52 on liver cirrhotic patients: a randomized, double-blind, placebo-controlled first approach. Phytomedicine. 2005;12(9):619-624.
105. Scientific Committee of the British Herbal Medical Association. British herbal pharmacopoeia, 1st edn. Bournemouth: British Herbal Medicine Association; 1983.
106. Blumenthal M., et al, editors. Herbal medicine: expanded Commission E monographs (English translation). Austin: Integrative Medicine Communications, 2000.
107. Mills S., Bone K. Principles and practice of phytotherapy. Edinburgh: Churchill Livingstone; 2000.
108. Bland J., et al. A medical food-supplemented detoxification program in the management of chronic health problems. Altern Ther Health Med. 1995;1(5):62-71.
109. MacIntosh A., Ball K. The effects of a short program of detoxification in disease-free individuals. Altern Ther Health Med. 2000;6(4):70-75.
110. Schnare D., et al. Evaluation of a detoxification regimen for fat stored xenobiotics. Med Hypotheses. 1982;9(3):265-282.
111. Tretjak Z., et al. PCB reduction and clinical improvement by detoxification: an unexploited approach? Hum Exp Toxicol. 1990;9(4):235-244.
112. Kilburn K., et al. Neurobehavioral dysfunction in firemen exposed to polycholorinated biphenyls (PCBs): possible improvement after detoxification. Arch Environ Health. 1989;44(6):345-350.
113. Rea W., et al. Reduction of chemical sensitivity by means of heat depuration, physical therapy and nutritional supplementation in a controlled environment. J Nutr Environ Med. 1996;6:141-148.
114. Cohen M. Detox: science or sales pitch? Aust Fam Physician. 2007;30(12):1009-1010.
115. Kumar D. Irritable bowel syndrome, chronic pelvic inflammatory disease and endometriosis. Eur J Gastroenterol Hepatol. 2004;16(12):1251-1252.
116. Tamboli C., et al. Dysbiosis in inflammatory bowel disease. Gut. 2004;53(1):1-4.
117. Bailey M., Coe C. Endometriosis is associated with an altered profile of intestinal microflora in female rhesus monkeys. Human Reprod. 2002;17(7):1704-1708.
118. Braun D., et al. Spontaneous and induced synthesis of cytokines by peripheral blood monocytes in patients with endometriosis. Fertil Steril. 1996;65:1125-1129.
119. Bullimore D. Endometriosis is sustained by tumour necrosis factor-alpha. Med Hypotheses. 2003;60:84-88.
120. Gurgan T., et al. Serum and peritoneal fluid levels of IGF I and II and insulinlike growth binding protein-3 in endometriosis. J Repro Med. 1999;44:450-454.
121. Kim J., et al. Insulin-like growth factors (IGFs), IGF-binding proteins (IGFBPs), and IGFBP-3 protease activity in the peritoneal fluid of patients with and without endometriosis. Fertil Steril. 2000;73:996-1000.
122. Koninckx P., et al. Endometriotic disease: the role of peritoneal fluid. Hum Reprod Update. 1998;4:741-751.
123. Punnonen J., et al. Increased levels of interleukin-6 and interleukin-10 in the peritoneal fluid of patients with endometriosis. Am J Obstetr Gynecol. 1996;174:1522-1526.
124. Rana N., et al. Basal and stimulated secretion of cytokines by peritoneal macrophages in women with endometriosis. Fertil Steril. 1996;65:925-930.
125. Richter O., et al. TNF-alpha secretion by peritoneal macrophages in endometriosis. Zentralbl Gynakol. 1998;120:332-336.
126. Capellino S., et al. Role of estrogens in inflammatory response: expression of estrogen receptors in peritoneal fluid macrophages from endometriosis. Ann N Y Acad Sci. 2006;1069:263-267.
127. Enomoto N., et al. Role of Kuppfer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15(Suppl):D20-D25.
128. Braun L., Cohen M. Herbs and natural supplements: an evidence based guide. Melbourne: Churchill Livingstone; 2007.
129. Agic A., et al. Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest. 2006;62:139-147.
130. Healy D.L., et al. Angiogenesis: a new theory for endometriosis. Human Reproduction Update. 1998;4(5):736-740.
131. Guo S. Nuclear factor-кB (NF-кB): an unsuspected major culprit in the pathogenesis of endometriosis that is still at large? Gynecol Obstet Inv. 2007;63(2):71-97.
132. Wieser F., et al. Curcumin suppresses angiogenesis, cell proliferation and induces apoptosis in an in vitro model of endometriosis [Poster]. Fertil Steril. 2007;88:S204-S205.
133. Beliard A., et al. Reduction of apoptosis and proliferation in endometriosis. Fertil Steril. 2004;82:80-85.
134. Gebel H., et al. Spontaneous apoptosis of endometrial tissue is impaired in women with endometriosis. Fertil Steril. 1998;69:1042-1047.
135. Scotti S., et al. Reduced proliferation and cell adhesion in endometriosis. Mol Hum Reprod. 2000;6:610-617.
136. Vigano P., et al. Cycling and early pregnant endometrium as a site of regulated expression of the vitamin D system. J Mol Endocrinol. 2006;36(3):415-424.
137. Agic A., et al. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod Sci. 2007;14(5):486-497.
138. Ferroro S., et al. Vitamin D binding protein in endometriosis. J Soc Gynecol Investig. 2005;12(4):272-277.
139. Moyad M. The potential benefits of dietary and/or supplemental calcium and vitamin D. Urol Oncol. 2003;21(5):384-391.
140. Choktanasiri W., et al. Long-acting triptorelin for the treatment of endometriosis. Int J Gynecol Obstet. 1996;54(3):237-243.
141. Ailawadi R., et al. Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate: a pilot study. Fertil Steril. 2004;81(2):290-296.
142. Panina P. Use of vitamin D compounds to treat endometriosis. Bioxil SpA. Italy. 2006.
143. Tamura M., et al. Estrogen up-regulates cyclooxygenase-2 via estrogen receptor in human uterine microvascular endothelial cells. Fertil Steril. 2004;81(5):1351-1356.
144. Kluft C., et al. Pro-inflammatory effects of oestrogens during use of oral contraceptives and hormone replacement treatment. Vascul Pharmacol. 2002;39(3):149-154.
145. Covens A., et al. The effect of dietary supplementation with fish oil fatty acids on surgically induced endometriosis in the rabbits. Fertil Steril. 1988;49(4):698-703.
146. Gazvani M., et al. High n-3:n-6 fatty acids rations in culture medium reduce endometrial-cell survival in combined endometrial gland and stromal cell cultures from women with and without endometriosis. Fertil Steril. 2001;76(4):717-722.
147. Fjerbaek A., Knudsen U. Endometriosis, dysmenorrhea and diet—what is the evidence? Eur J Obstet Gynecol Reprod Biol. 2007;132(2):140-147.
148. Britton J., et al. Diet and benign ovarian tumours (United States). Cancer Causes Control. 2000;11(5):389-401.
149. Hemedah O., et al. Prevention of peritoneal adhesions by administration of sodium carboxymethyl cellulose and oral vitamin E. Surgery. 1993;114(5):907-910.
150. Kalferentzos F., et al. Prevention of peritoneal adhesion formation in mice by vitamin E. J R Coll Surg Edinb. 1987;32(5):288-290.
151. Kagoma P., et al. The effect of vitamin E on experimentally induced peritoneal adhesions in mice. Arch Surg. 1985;120(8):949-951.
152. Meydani M. Vitamin E. Lancet. 1995;345(8943):170-175.
153. Perez-Fernandez R., et al. Vitamin D, Pit-1, GH, and PRL: possible roles in breast cancer development. Curr Med Chem. 2007;14(29):3051-3058.
154. Li J., et al. [Clinical observation on treatment of endometriosis by tonifying qi and promoting blood circulation to remove stasis and purgation principle] [in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1999;19(9):533-535.
155. Wang R., Zhou L. [Clinical observation on treatment of endometriosis with principle of activating blood circulation to remove stasis]. [in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24(3):258-259.
156. Maciocia G. Obstetrics and gynecology in Chinese medicine. Edinburgh: Churchill Livingstone; 1997.
157. Thomson J., Redwine D. Chronic pelvic pain associated with autoimmunity and systemic and peritoneal inflammation and treatment with immune modification. J Reprod Med. 2005;50:745-758.
158. Cotroneo M., Lamartiniere S. Pharmacologic, but not dietary genistein supports endometriosis in a rat model. Toxicol Sci. 2001;61(1):68-75.
159. Kohama T., et al. Effect of French maritime pine bark extract on endometriosis as compared with leuprorelin acetate. J Reprod Med. 2007;52(8):703-708.