Chapter 5 Endocrinology of Pregnancy and Parturition
 Hormones
Hormones
PEPTIDE HORMONES
STEROID HORMONES
Progesterone
The fetus inactivates progesterone by transformation to corticosteroids or by hydroxylation or conjugation to inert excretory products. However, the placenta can convert these inert materials back to progesterone. Steroid biochemical pathways are shown in Figure 5-1.
Estrogens
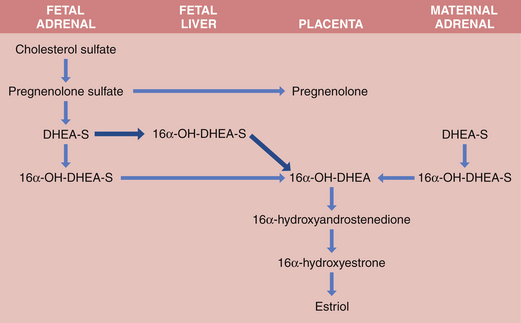
Both fetus and placenta are involved in the biosynthesis of estrone, estradiol, and estriol. Cholesterol is converted to pregnenolone and pregnenolone sulfate in the placenta. These precursors are converted to DHEA-S largely in the fetal, and to a lesser extent the maternal, adrenals. The DHEA-S is further metabolized by the placenta to estrone (E1) and, through testosterone, to estradiol (E2). Estriol (E3), the most abundant estrogen in human pregnancy, is synthesized in the placenta from 16α-hydroxy-DHEA-S, which is produced in the fetal liver from adrenal DHEA-S. Placental sulfatase is required to deconjugate 16α-hydroxy-DHEA-S before conversion to E3 (Figure 5-2). Steroid sulfatase activity in the placenta is high except in rare cases of sulfatase deficiency.
Androgens
During pregnancy, androgens originate mainly in the fetal zone of the fetal adrenal cortex. Androgen secretion is stimulated by ACTH and hCG, the latter being effective primarily in the first half of pregnancy, when it is present in high concentration. The fetal adrenal favors production of DHEA over testosterone and androstenedione. Fetal androgens enter the umbilical and placental circulation and serve as precursors for estradiol and estriol (see Figure 5-1).
Glucocorticoids
Cortisol is derived from circulating cholesterol (see Figure 5-1). Maternal plasma cortisol concentrations rise throughout pregnancy, and the diurnal rhythm of cortisol secretion persists. The plasma level of transcortin rises in pregnancy, probably stimulated by estrogen, and the plasma-free cortisol concentration doubles.
Both the fetal adrenal and the placenta participate in cortisol metabolism. The fetal adrenal is stimulated by ACTH, originating from the fetal pituitary, to produce both cortisol and DHEA-S. In contrast to DHEA-S, which is produced in the fetal zone, cortisol originates in the definitive zone (see Figure 5-1). Toward the end of pregnancy cortisol promotes differentiation of type II alveolar cells and the biosynthesis and release of surfactant into the alveoli. Surfactant decreases the force required to inflate the lungs. Insufficiency of surfactant leads to respiratory distress in the premature infant, which can cause death. Cortisol also plays an important role in the activation of labor, increasing the release of placental CRH and prostaglandins.
OTHER HORMONES AND TRANSMITTERS
Prostaglandins and Leukotrienes
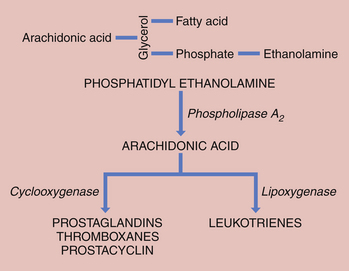
Prostaglandins are thought to play a major role in the initiation and control of labor. Prostaglandin synthesis begins with the formation of arachidonic acid, an obligatory precursor of the prostaglandins of the “2” series (i.e., PGE2, PGF2α). Arachidonic acid is stored in esterified form as glycerophospholipid in the trophoblastic membranes. The initial step is the hydrolysis of glycerophospholipids, which is catalyzed by phospholipase A2 or C. Phospholipase A2 preferentially acts on chorionic phosphatidyl ethanolamine to release arachidonic acid (Figure 5-3). Free arachidonic acid does not accumulate. Labor appears to be accompanied by a cascade of events in the chorion, amnion, and decidua that releases arachidonic acid from its stored form and converts it to active prostaglandins. 17β-Estradiol stimulates several enzymes active in the synthesis of prostaglandins from arachidonic acid.
An additional pathway for arachidonic acid metabolism is the conversion of arachidonic acid to leukotrienes (see Figure 5-3). Both prostaglandins and leukotrienes induce decidualization, which means that they initiate changes in the endometrium to facilitate implantation of the fertilized ovum.
 Parturition
Parturition
BIOCHEMICAL BASIS OF CONTRACTION
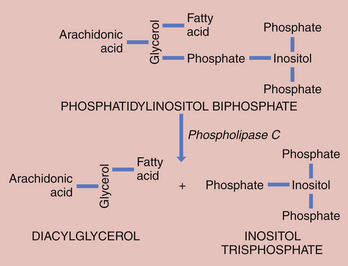
The binding of oxytocin and prostaglandins to their respective receptors activates phospholipase C, which hydrolyzes phosphatidylinositol bisphosphate, a lipid present in the cell membrane, to inositol trisphosphate and diacylglycerol (Figure 5-4). Inositol trisphosphate induces release of calcium from the sarcoplasmic reticulum, an intracellular calcium storage area. The resulting high intracellular free calcium concentration enables the myofibrils of the myometrium to contract. Subsequently, the calcium is pumped back into the sarcoplasmic reticulum with the help of ATP, and more calcium may enter from the extracellular fluid through both voltage-operated and receptor-operated channels that open briefly.
HORMONAL CONTROL OF GESTATIONAL LENGTH AND INITIATION OF LABOR
Animal Models
Most studies have been conducted in the sheep, where the fetus appears to control the onset of labor. The fetal hypothalamus stimulates the fetal pituitary to secrete ACTH, which brings about a surge of cortisol from the fetal adrenal. The cortisol surge induces the placental enzyme 17α-hydroxylase and formation of androgens, which are estrogen precursors (see Figure 5-1), simultaneously decreasing progesterone formation. The rise in the estrogen-to-progesterone ratio leads to (1) greater secretion of prostaglandins; (2) formation of myometrial gap junctions, which provide areas of low resistance to current flow and increase coordinated uterine contractions; (3) cervical ripening; and (4) the onset of labor. Administered ACTH, glucocorticoids, or dexamethasone can also initiate parturition. Removal of the fetal pituitary or adrenal, both of which are required for the cortisol surge, results in prolonged pregnancy.
Behrman R.E., Butler A.S., editors. Preterm birth: Causes, consequences, and prevention. Institute of Medicine: Committee on Understanding Premature Birth and Assuring Healthy Outcomes, Board on Health Sciences Policy. Washington, DC: National Academies Press, 2007.
Challis J.R.G. Mechanism of parturition and preterm labor. Obstet Gynecol Surv. 2000;55:650-660.
Jeyabalan A., Shroff S.G., Novak J., Conrad K.P. The vascular actions of relaxin. Adv Exp Med Biol. 2007;612:65-87.
Norwitz E.R., Robinson J.N., Challis J.R.G. The control of labor. N Engl J Med. 1999;341:660-666.
Vidaeff A.C., Ramin S.M. Potential biochemical events associated with initiation of labor. Curr Med Chem. 2008;15:614-619.

 Fetoplacental Unit
Fetoplacental Unit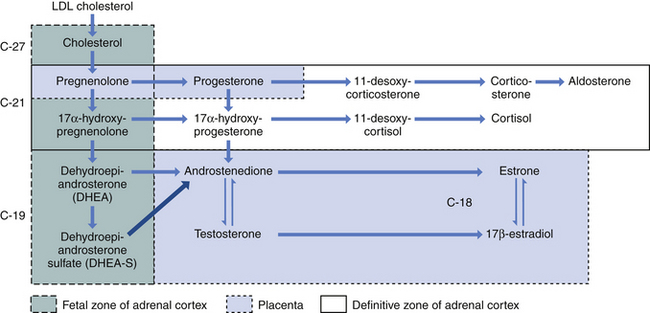
 Changes in Maternal Metabolism
Changes in Maternal Metabolism