Chapter 12 Endobronchial and Endoesophageal Ultrasound Techniques
Transbronchial Needle Aspiration
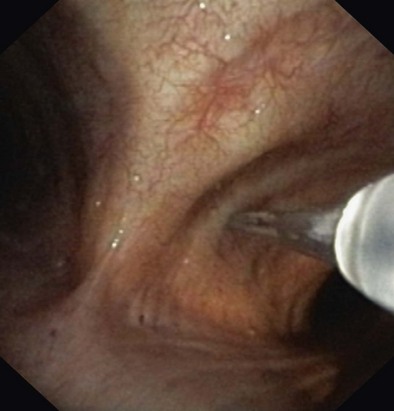
Merely a curiosity at its inception, flexible bronchoscopy has emerged as an essential diagnostic and therapeutic modality in the management of a variety of lung diseases. The addition of transbronchial needle aspiration (TBNA) not only improved bronchoscopy’s diagnostic yield but further extended the role of this modality in the evaluation of mediastinal disease, and in the diagnosis and staging of bronchogenic carcinoma. The first description of sampling mediastinal lymph nodes through the tracheal carina using a rigid bronchoscope was by Schieppati. In 1978, Wang and associates demonstrated that it was feasible to sample paratracheal nodes using TBNA. Subsequent publications highlighted the use of the technique in the diagnosis of endobronchial (Figure 12-1) and peripheral lesions and the ability of TBNA to provide a diagnosis even in the absence of endobronchial disease.
Endobronchial Ultrasound Technique
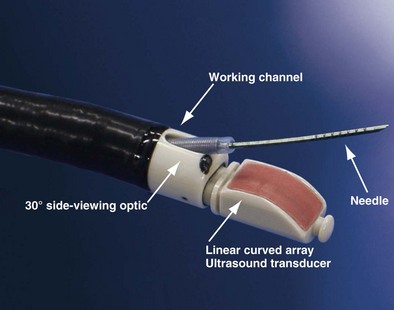
The integration of ultrasound technology and flexible fiberoptic bronchoscopy enables imaging of lymph nodes, lesions, and vessels located beyond the tracheobronchial mucosa. Developed in 2002, the EBUS bronchoscope looks similar to a normal bronchovideoscope (Figure 12-2) but is 6.9 mm wide and has a 2-mm instrument channel and a 30-degree side viewing optic. Furthermore, a curved linear array ultrasound transducer sits on the distal end and can be used either with direct contact to the mucosal surface or with an inflatable balloon that can be attached at the tip. This setup produces a conventional endoscopic picture side by side with the ultrasound view. Ultrasound scanning is performed at a frequency of 7.5 to 12 MHz, with tissue penetration of 20 to 50 mm. An ultrasound processor generates the ultrasound image.
Procedure
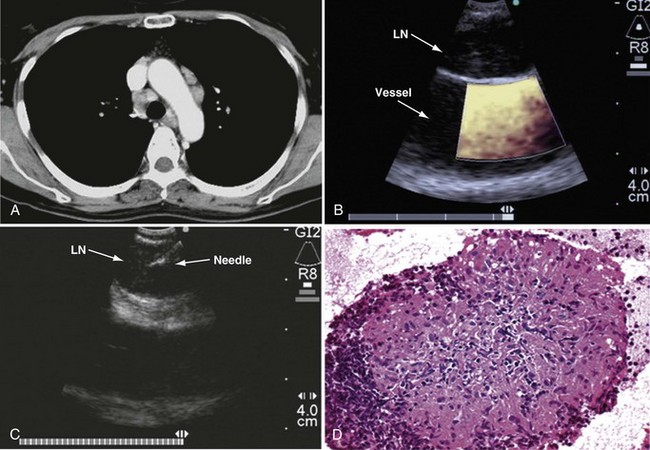
The actual TBNA is performed using direct transducer contact with the wall of the trachea or bronchus. When a lesion is outlined, a 21 or 22 gauge needle can be advanced through the working channel, and lymph nodes can be punctured under real-time ultrasound visualization. The needle is encased in an internal sheath in order to avoid contamination during biopsy. At the same time, color Doppler can be used to identify surrounding vascular structures. Once the target lymph node or mass has been clearly identified with EBUS, the needle is inserted under real-time ultrasound guidance and then placed within the lesion (Figures 12-3 and 12-4). Suction is applied with a syringe, and the needle is moved back and forth to achieve multiple punctures. The stylet of the needle is left in place on the first puncture to minimize bronchial cell contamination; once the needle tip is inside the target tissue, the stylet is removed. We stab the target 10 to 15 times without suction and apply suction only for the last two or three stabbing motions. Before retraction of the needle into the needle sheath, suction must be removed to minimize sample loss into the syringe. The specimen is then air-flushed onto a slide, and the needle is flushed with heparin-saline solution to avoid clotting; the same procedure is repeated three times at every lymph node station.
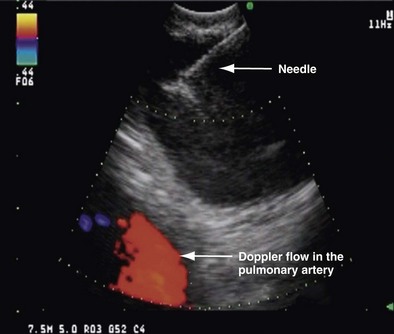
Figure 12-3 Endobronchial ultrasound–transbronchial needle aspiration (EBUS-TBNA) of a lymph node in position 4R.
Endoesophageal Ultrasound Technique
Procedure
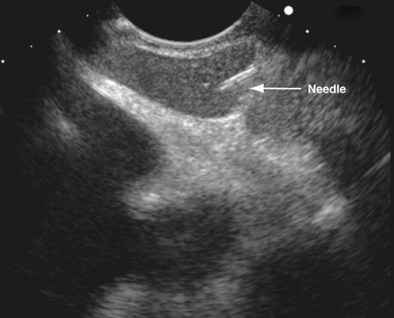
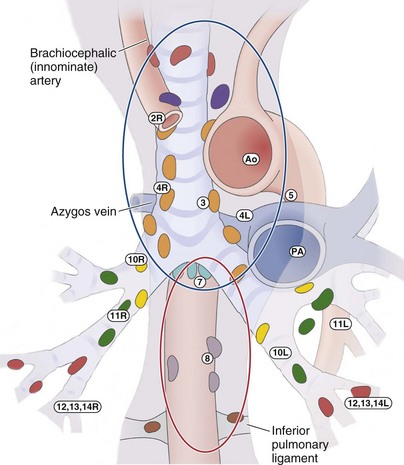
The linear EUS bronchoscope has the same basic architecture as that of the EBUS scope and uses a scanner frequency of between 5 and 10 MHz. The penetrating ultrasound depth can be up to 8 cm. Needles used for biopsy are 19 or 21 gauge, again equipped with a stylet. The procedure usually is performed on an outpatient basis and takes approximately 30 minutes. As with EBUS, the puncture of lymph nodes is performed under real-time ultrasound guidance (Figure 12-5). However, EUS-FNA has limited access, because only lymph node stations 2L, 4L, 7, 8, and 9 are accessible through a transesophageal approach. Lymph node station 5 is not routinely accessible by EUS, and transvascular FNA may be required to sample this tissue.
Combining Endobronchial and Endoesophageal Ultrasound Techniques
For tissue sampling of mediastinal lymph nodes after conventional TBNA, our own preference is for minimally invasive methods such as EBUS-TBNA and EUS-FNA over more invasive procedures such as mediastinoscopy and VATS. EUS-FNA and EBUS-TBNA have been shown to avoid the need for mediastinoscopy to a large extent (Figure 12-6). EBUS-TBNA and EUS-FNA have a complementary reach in analyzing mediastinal nodes: EBUS has access to the paratracheal, subcarinal, and hilar regions, and EUS, to the lower mediastinum and aortopulmonary window.
Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA. 2010;304:2245–2252.
Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer. 2009;45:1389–1396.
Herth FJ. Mediastinal staging—the role of endobronchial and endo-oesophageal sonographic guided needle aspiration. Lung Cancer. 2004;45(Suppl 2):S63–S67.
Herth FJ, Eberhardt R, Krasnik M, Ernst A. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest. 2008;133:887–891.
Herth FJ, Krasnik M, Kahn N, et al. Combined endoesophageal-endobronchial ultrasound-guided, fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest. 2010;138:790–794.
Holty JE, Kuschner WG, Gould MK. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-analysis. Thorax. 2005;60:949–955.
Hwangbo B, Lee GK, Lee HS, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest. 2010;138:795–802.
Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: a systematic review and metaanalysis. Chest. 2007;131:539–548.
Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:178S–201S.
Varadarajulu S, Eloubeidi M. Can endoscopic ultrasonography-guided fine-needle aspiration predict response to chemoradiation in non-small cell lung cancer? A pilot study. Respiration. 2006;73:213–220.
Vilmann P, Herth F, Krasnik M. State of the art lecture: mediastinal EUS. Endoscopy. 2006;38(Suppl 1):S84–S87.
Wang KP, Terry P, Marsh B. Bronchoscopic needle aspiration biopsy of paratracheal tumors. Am Rev Respir Dis. 1978;118:17–21.
Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. 2004;126:122–128.