Chapter 56 Emerging Intramural and Transmural Endoscopy
![]() Video related to this chapter’s topics: Endocytoscope observation of the muscularis propria in the stomach with SEMF technique
Video related to this chapter’s topics: Endocytoscope observation of the muscularis propria in the stomach with SEMF technique
Introduction
Limitless possibilities exist for the endoscope to serve as a sophisticated diagnostic probe and a platform for endoluminal therapeutic interventions. Flexible gastrointestinal (GI) endoscopy has achieved major accomplishments so far, which include inspection of the total digestive canal, detection of grossly invisible minute mucosal abnormalities, reliable hemostasis for GI bleeding, pancreaticobiliary intervention, ultrasound staging of neoplasms, and radical excision of mucosal malignancies. Despite these practical technologic accomplishments, the working space for flexible endoscopy has been confined to the gut lumen. The gut wall has always been the great barrier for flexible endoscopy, instilling a tradition of respect to maintain its integrity and limiting intervention. Both gastroenterologists and surgeons are now exploring the new frontier through the gut wall to achieve incisionless surgery.1 More recent research has approached the gut wall as an opportunity and showed that an artificially created intramural space could become a working space for novel endoscopic interventions.2,3
Techniques for Creation of a Submucosal Working Space: Submucosal Endoscopy with Mucosal Safety Valve Technique
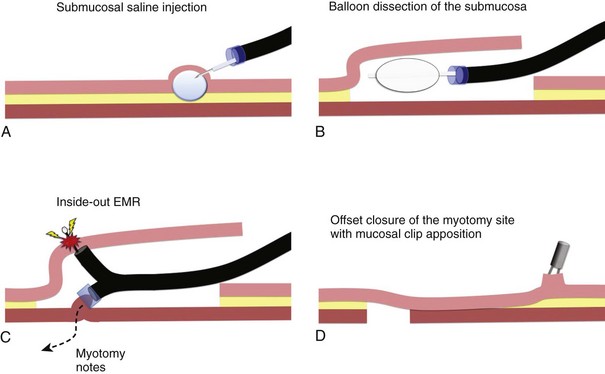
The submucosal endoscopy with mucosal flap (SEMF) “safety valve” technique represents a global concept of using a submucosal space as a practical working space for endoscopic intervention.4 Using the SEMF technique, major technical challenges to standard endoscopic intervention may be overcome by using the endoscope and surgical devices inserted into the intramural space—formally the submucosa. It was hypothesized that the technique may offer safe (offset) access into the peritoneal cavity for natural orifice transluminal endoscopic surgery (NOTES) by using the submucosal space as a tunneled portal and the isolated free overlying mucosa as a protective sealant flap to minimize the potential for contamination of the extraluminal anatomic cavity (Fig. 56.1).3 Also, mucosal resection could be safely performed in an “inside-out” manner, directing intervention toward the lumen with a greatly reduced risk of perforation, regardless of the size for a mucosal based lesion.5
In the original SEMF technique,6 the submucosal layer was mechanically dissected by a combination of high-pressure carbon dioxide (CO2) and balloon dissection. The procedure was initiated by a submucosal injection of a small amount of saline to identify the submucosal tissue plane (see Fig. 56.1A). Millisecond bursts of high-pressure CO2 (100 psi on average) were injected into the bleb to create a gas-filled giant submucosal bleb and gas-dissected submucosa. This gas dissection technique allows instant isolation of a large area of the mucosa as desired, greater than 10 cm in diameter. An injection of 1.5% hydroxypropyl methylcellulose into this dissected plane prevents gas escape and maintains the large bleb. A mucosal incision (10 mm) made with a needle-knife at one margin of the combination bleb and fluid cushion allows entry below the mucosa. With persistent submucosal tissue stranding present, balloon dissection using a 15-mm biliary retrieval balloon can be performed to disrupt the tissue strands to transform the submucosa into an intramural free space (see Fig. 56.1B). Instead of the combination of gas and balloon dissections, blunt dissection with a grasping forceps also could be used for the mechanical disruption of the submucosal layer. In contrast to the SEMF technique and long preceding it, Japanese endoscopists have used repetitive time-consuming needle-knife dissection directed into the submucosal plane using one of a series of needle-knives specially designed for endoscopic submucosal dissection (ESD) technique.
Chemically Assisted Submucosal Dissection Technique
The original gas dissection SEMF technique was developed with the intent of rapidly creating an open submucosal space. This technique can be slow because of tedious balloon dissection in the setting of a resilient submucosa. The large mucosal flap can be susceptible to necrosis because of inadvertent mechanical trauma and, perhaps, microvascular ischemia. To preserve blood supply of the free mucosal flap, it is necessary to identify an optimal width and length of the submucosal tunnel or space matched to the specific intervention. In an effort to minimize mechanical trauma to the mucosal flap, we have discovered mesna (C2H5NaO3S2) as a practical pretreatment of the submucosa before the blunt dissection. Mesna can chemically soften the submucosa by dissolving disulfide bonds in connective tissues that play an important role in the folding and stability of proteins.7 Mesna is clinically used as a mucolytic agent for patients with respiratory disorders and is better known in oncology as a uroprotective drug to prevent ifosfamide-induced and cyclophosphamide-induced hemorrhagic cystitis. Drug safety has been shown from these clinical experiences.
The concept of chemically assisted surgical dissection with mesna was initially introduced to assist blunt dissection of the facial planes in the field of otorhinolaryngology. The benefits of mesna have been confirmed in other surgical interventions. In the chemically assisted SEMF technique, 4 to 8 mL of 10% mesna solution is injected into the saline submucosal bleb before initiating balloon dissection. A randomized porcine study using this method showed that a longitudinal submucosal tunnel greater than 5 cm could be created within 10 minutes in porcine stomachs with significantly less tissue resistance to insertion of a balloon catheter and subsequent mechanical balloon dissection. Clinical experience has subsequently been obtained using mesna to facilitate excision of colon polyps and gastric neoplasms.8 The preliminary clinical experiences in the stomach and colon support the benefits of mesna in “softening” the submucosa.
Intramural Endoscopy (Submucosal Endoscopic Procedures)
In Vivo Histologic Imaging of the Muscularis Propria
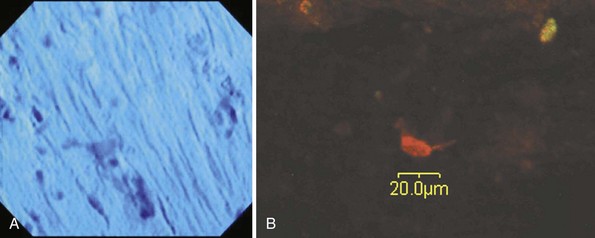
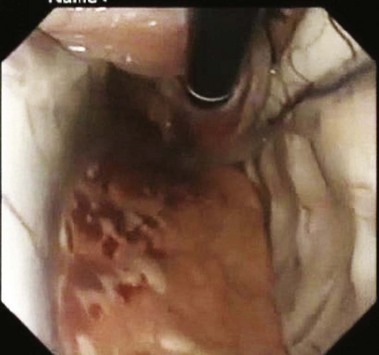
We have so far applied two imaging technologies for muscle layer imaging in an animal study using porcine models,9 both of which have been newly introduced into flexible endoscopy in a clinical setting. These imaging technologies are endocytoscopy (EndoCytoscope, Olympus, Tokyo, Japan), which is a catheter-style ultrahigh-magnifying endoscopy with optical magnification greater than 1000× and confocal microscopy (Fig. 56.2). The chemically assisted SEMF technique was used to access the muscularis propria in esophageal and gastric locations. Nissl stains and two types of neuronal molecular stains were topically applied onto the exposed muscularis propria within the SEMF submucosal space. The muscular layer was imaged in vivo 2 minutes after the muscular staining with the endocytoscope inserted into the SEMF submucosal space. A sample of the muscularis propria was stained with molecular-based stains and evaluated, ex vivo after necropsy by confocal microscopy. Endocytoscopy revealed cellular microstructures resembling spindle-shaped smooth muscle cells comparable to bench microscopy (Fig. 56.3A). Confocal microscopy performed after necropsy showed that the in vivo topical application of neuronal molecular stains successfully stained the muscularis and highlighted neuronlike cells (Fig. 56.3B).
Submucosal Endoscopic Myotomy
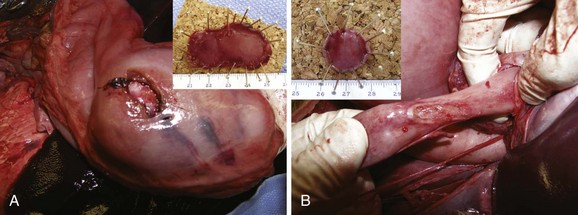
In addition to muscularis propria imaging, full-thickness sampling of the muscularis propria has been achieved with the SEMF technique combined with the cap endoscopic mucosal resection (EMR) technique to obtain the muscle sample from within the SEMF submucosal space. The suction and snare myotomy with the cap EMR technique provides a myotomy specimen greater than 2 cm in size. This technique is theoretically safer than direct electrosurgical resection of a muscle sample because it minimizes the risk of collateral coagulation injury to surrounding organs by blind insertion of a cutting tool. The technical feasibility of this technique has been confirmed in the stomach and esophagus by a series of porcine animal studies (Fig. 56.4).10,11 The free overlying mucosa over the myotomy has been sufficiently durable to maintain the integrity of the gut wall. We anticipated that this method for a full-thickness myotomy would be desirable for histologic and etiologic analysis of GI motility disorders, for safe access for NOTES procedures, and as an alternative to the Heller myotomy in the treatment of achalasia.
Pasricha and the Apollo group12 showed that selective subtotal needle-knife incision of only the circular layer of the muscularis propria from within the submucosal working space reduced lower esophageal sphincter pressure in a porcine model. The efficacy and safety of the SEMF-based myotomy technique was subsequently confirmed by a Japanese group in patients with achalasia.13 In this experience, repetitive electrosurgical dissection with a needle-knife was used to create a working space for the myotomy in the lower esophagus. Although successful, this dissection method would be intuitively less appealing from a safety perspective compared with blunt dissection.
Submucosal Endoscopy with Mucosal Resection
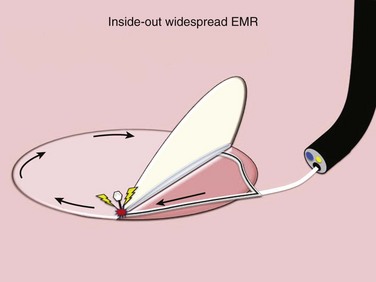
In Japan and other Asian countries with a high incidence of gastric cancer, a widespread EMR technique, ESD, has become front-line treatment for mucosal cancers associated with a negligible risk of lymph node metastasis. In ESD, tedious repetitive needle-knife dissection is used to isolate the mucosal lesion from the deeper gastric wall layers. Although the concept of ESD providing en bloc excision of malignancy is appealing, the electrosurgical dissection is overwhelmingly labor-intensive and time-consuming and is associated with significant risk for perforation and bleeding. It is anticipated that transformation of the submucosa into a working space by SEMF could be applied to the undermining of mucosally based disease offering a safer and more expedient alternative for the isolation and resection of diseased mucosa, redirecting the most hazardous aspect of tissue resection, electrosurgical cutting, toward the lumen.14 The Apollo Group has developed a unique tool set designed to perform submucosal endoscopy with mucosal resection (SEMR), an inside-out widespread EMR, using uniquely designed, highly compliant balloons to undermine targeted diseased mucosa safely and accommodate a simple en bloc excision of the freed-up area of diseased mucosa (Fig. 56.5).
Transmural Procedures with Submucosal Endoscopy with Mucosal Flap Approach
Transgastric Cholecystectomy via a Cephalad Submucosal Tunnel
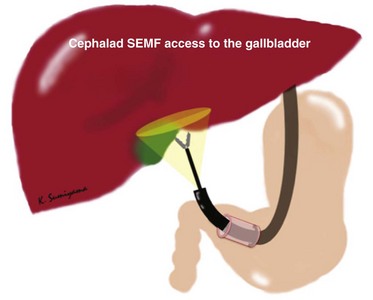
The organs in the lower half of the peritoneal cavity and pelvis are easily accessible regardless of any anterior gastrostomy technique. Conversely, there are technical challenges to performing surgical procedures in the upper abdominal cavity (e.g., cholecystectomy) using the currently designed flexible endoscope from a standard anterior gastrostomy. To access the organs located higher than the level of the stomach, retroflexion of the endoscope is mandatory. This access is restrictive because of the potential target organ distance and the angle of approach after exiting from a typical anterior gastrostomy. The gallbladder may not be consistently identified. Pai and colleagues15 reported that the gallbladder was visualized in only 55.6% of cases in their porcine model study. Using the SEMF technique from a retroflexed position, the submucosal tunnel can be directed cephalad and can effectively create a biologic endoscope and instrument guide into the upper abdominal cavity.16 This cephalad access using the SEMF technique allowed visualization of the gallbladder in all eight attempts in our experiments with NOTES unassisted transgastric cholecystectomy using the porcine model (Fig. 56.6).
Transesophageal Thoracic Natural Orifice Transluminal Endoscopic Surgery
The transesophageal SEMF technique may provide the most appealing platform to allow safe access to important thoracic organs including the cardiovascular system.11,17 For transesophageal SEMF, a long submucosal tunnel (8 to 10 cm) is longitudinally created with a 15-mm biliary occlusion balloon after preliminary CO2 gas dissection, water dissection, or a “softening” mesna submucosal cushion over this length. Because the esophageal mucosa is thin, the mucosal entry site can be securely and simply closed by clip application. To access the thoracic cavity safely via the esophagus, a myotomy must be located in the distal esophagus because of the position of critical organs within the thoracic cavity. The muscularis propria is snare resected by cap EMR providing access to the posterior mediastinum (Fig. 56.7 and see Fig. 56.4), which contains the descending thoracic aorta, the esophagus, the azygos vein, and the autonomic ganglia and nerves. These structures can be easily visualized with a SEMF transesophageal approach. The pleural cavity can be accessed through a pleural incision using a hook knife (Hook knife; Olympus, Tokyo, Japan) inserted through the endoscope retroflexed at the level of transesophageal entry.
We have accessed the epicardium using the transesophageal SEMF technique and successfully created a pericardial window. This approach has allowed endoscopic contact and coagulation of the epicardial surface testing the technical feasibility of cardiac interventions. After visual identification of the heart within the pleural cavity, our method included a pericardial puncture (2 to 3 mm) created with the hook knife followed by creation of a pericardial window (approximately 3 cm in length) with an insulated-tip needle-knife (IT knife; Olympus) (see Fig. 56.5). A point coagulation was performed on the exposed epicardium through the pericardial window with a heat probe (Olympus), with two to four pulses of 30 J, and a hook knife, with a monopolar coagulation current (30 W) while monitoring the cardiac rhythm. There were no rhythm disturbances. After a period of survival, no gross contamination or signs of contamination in the thoracic cavity were identified on necropsy. The pericardial space was normal in appearance. The epicardial coagulation sites were healing, without exudative ulceration.
Transgastric Peritoneoscopy for Preoperative Staging of Pancreatic Cancer
SEMF transgastric access to the peritoneal cavity has been clinically evaluated as a less invasive alternative to diagnostic peritoneoscopy for preoperative staging of pancreatic cancer.18 The clinical procedure used a cephalad submucosal tunnel within the anterior gastric wall. The gastric access site was identified by transillumination and finger palpation over the anterior abdominal wall. A 3-cm long submucosal tunnel was created using an ESD technique. A small needle-knife incision of the seromuscular layer was made at the distal end of the tunnel and enlarged with a 15-mm dilation balloon for endoscopic access to the peritoneal cavity. Peritoneal exploration and biopsy were performed. The gastrostomy was closed with mucosal clip apposition of the tunnel entry site. This experience with five transgastric peritoneoscopies has been published.19 The SEMF-based transgastric peritoneoscopy was negative in all cases, followed by radical surgical excision of the primary tumor. There were no procedure-related complications. Secure closure of the gastrostomy without leakage and the absence of peritoneal contamination were confirmed during the open portion of the operation.
1 ASGE, SAGES. ASGE/SAGES Working Group on Natural Orifice Transluminal Endoscopic Surgery White Paper October 2005. Gastrointest Endosc. 2006;3:199-203.
2 Sumiyama K, Gostout CJ. Techniques for transgastric access to the peritoneal cavity. Gastrointest Endosc Clin N Am. 2008;8:235-244.
3 Sumiyama K, Tajiri H, Gostout CJ. Submucosal endoscopy with mucosal flap safety valve (SEMF) technique: A safe access method into the peritoneal cavity and mediastinum. Minim Invasive Ther Allied Technol. 2008;7:365-369.
4 Sumiyama K, Gostout CJ, Rajan E, et al. Submucosal endoscopy with mucosal flap safety valve. Gastrointest Endosc. 2007;65:688-694.
5 Sumiyama K, Gostout CJ. Novel techniques and instrumentation for EMR, ESD, and full-thickness endoscopic luminal resection. Gastrointest Endosc Clin N Am. 2007;17:471-485.
6 Sumiyama K, Gostout CJ, Rajan E, et al. Submucosal endoscopy with mucosal flap safety valve. Gastrointest Endosc. 2007;65:688-694.
7 Sumiyama K, Gostout CJ, Rajan E, et al. Chemically assisted endoscopic mechanical submucosal dissection by using mesna. Gastrointest Endosc. 2008;67:534-538.
8 Sumiyama K, Tajiri H, Gostout CJ, et al: Chemically assisted submucosal injection facilitates endoscopic submucosal dissection of gastric neoplasms. Endoscopy 42:627–632, 2010.
9 Sumiyama K, Tajiri H, Kato F, et al. Pilot study for in vivo cellular imaging of the muscularis propria and ex vivo molecular imaging of myenteric neurons (with video). Gastrointest Endosc. 2009;69:1129-1134.
10 Sumiyama K, Gostout CJ, Rajan E, et al. Submucosal endoscopy with mucosal flap safety valve. Gastrointest Endosc. 2007;65:688-694.
11 Sumiyama K, Gostout CJ, Rajan E, et al. Transesophageal mediastinoscopy by submucosal endoscopy with mucosal flap safety valve technique. Gastrointest Endosc. 2007;65:679-683.
12 Pasricha PJ, Hawari R, Ahmed I, et al. Submucosal endoscopic esophageal myotomy: A novel experimental approach for the treatment of achalasia. Endoscopy. 2007;39:761-764.
13 Inoue H, Minami H, Satodate H, et al. First clinical experience of submucosal endoscopic esophageal myotomy for esophageal achalasia with no skin incision. Gastrointest Endosc. 2009;69:AB122.
14 Sumiyama K, Gostout CJ. Novel techniques and instrumentation for EMR, ESD, and full-thickness endoscopic luminal resection. Gastrointest Endosc Clin N Am. 2007;17:471-485.
15 Pai RD, Fong DG, Bundga ME, et al. Transcolonic endoscopic cholecystectomy: A NOTES survival study in a porcine model (with video). Gastrointest Endosc. 2006;64:428-434.
16 Sumiyama K, Gostout CJ, Rajan E, et al. Transgastric cholecystectomy: transgastric accessibility to the gallbladder improved with the SEMF method and a novel multibending therapeutic endoscope. Gastrointest Endosc. 2007;65:1028-1034.
17 Sumiyama K, Gostout CJ, Rajan E, et al. Pilot study of transesophageal endoscopic epicardial coagulation by submucosal endoscopy with the mucosal flap safety valve technique (with videos). Gastrointest Endosc. 2008;67:497-501.
18 Kitano S, Yasuda K, Shibata K, et al. Natural orifice transluminal endoscopic surgery for preoperative staging in a pancreatic cancer patient. Digest Endosc. 2008;20:198-202.
19 Yasuda K, Yoshizumi F, Kawaguchi K. Current status and future perspectives of NOTE in Japan. Endoscopia Digestiva. 2009;21(6):969-973. In Japanese with English abstract