Chapter 14 Emerging Indications and Other Applications of Spinal Cord Stimulation
 Motor, vasoactive, genitourinary, and even cognitive effects of spinal cord stimulation have been extensively explored since the modality was introduced half a century ago.
Motor, vasoactive, genitourinary, and even cognitive effects of spinal cord stimulation have been extensively explored since the modality was introduced half a century ago.Introduction
However, in addition to the beneficial effect on chronic pain, SCS has been used successfully in many other conditions. Indications for clinical and laboratory SCS applications other than pain may be divided into several large categories (Box 14-1):
 Vasoactive SCS effects that are used in treatment of peripheral vascular disease and coronary ischemia
Vasoactive SCS effects that are used in treatment of peripheral vascular disease and coronary ischemia Genitourinary applications ranging from incontinence control in paraplegics to augmentation of female sexual function in anorgasmia
Genitourinary applications ranging from incontinence control in paraplegics to augmentation of female sexual function in anorgasmia More esoteric indications such as impaired consciousness caused by various cerebral pathologies, management of autonomic hyperreflexia, prevention and treatment of cerebral arterial vasospasm, and improvement in tissue perfusion in brain tumors aimed at increased radiosensitivity and chemosensitivity
More esoteric indications such as impaired consciousness caused by various cerebral pathologies, management of autonomic hyperreflexia, prevention and treatment of cerebral arterial vasospasm, and improvement in tissue perfusion in brain tumors aimed at increased radiosensitivity and chemosensitivityMotor Control
Beneficial effects of SCS on spasticity were discovered early; multiple reports in the 1970s documented usefulness of SCS in improvement of spasticity. Objective evaluation of stretch and H reflexes was used to support clinical results,1 and the most responsive cause of spasticity was dysfunction of the spinal cord as a result of injury or demyelination.2 Developed as an alternative to destructive interventions,3,4 SCS was used in many clinical centers throughout Europe, Asia, and America with impressive long-term results.5–8 In addition to patients with spinal cord injuries, SCS was tried in patients with multiple sclerosis, poststroke hemiparesis, dystonia, and cerebral palsy. Animal experiments were used to confirm clinical observations and to find an explanation for the SCS effect and putative mechanism of SCS action in these circumstances.9
Although the initial impression suggested that spasticity of cerebral origin does not respond to SCS,2 subsequent studies showed sustained benefits of SCS in patients with poststroke weakness,10,11 dystonia,12 and posthypoxic encephalopathy.13 The general enthusiasm was lowered by reports indicating a lack of clinical long-term effectiveness14,15 or cost-effectiveness of SCS in spasticity,16 but the main reason for almost complete abandonment of this once popular SCS indication was introduction of intrathecal baclofen administration.17 However, in countries where intrathecal baclofen is not available because of regulatory barriers, SCS remains a useful tool for treatment of otherwise refractory spasticity through nondestructive intervention.18,19
In addition to suppression of spasticity in symptomatic patients, SCS may be effective in recovery of motor function in paraplegic patients. A study of 10 patients with complete motor spinal cord injury indicated that epidural SCS at the lumbosacral spinal cord level recruited leg muscles in a segmental-selective way, generating integrated motor behavior of sustained extension and rhythmic flexion and extension movements.20 In the case of an incomplete spinal cord injury, a wheelchair-dependent patient was able to walk with a walker essentially in effortless manner after prolonged SCS. The superiority of gait assisted by SCS was particularly impressive in ambulation at longer distances.21
The latest surge of interest to SCS in treatment of motor disorders came from an experimental study showing improvement in locomotion in an experimental model of Parkinson disease (PD).22 The improvement in mobility and restoration of normal patterns of neuronal activity were observed with dorsal column stimulation in both the acute PD model of pharmacologically dopamine-depleted mice and the chronic PD model of hydroxydopamine lesioned rats.22
Vasoactive Applications of Spinal Cord Stimulation
With the primary intent of pain relief, early SCS implanters noticed that in addition to paresthesias and/or sense of vibration, patients described a sensation of warmth in their extremities; along with this subjective sensation there may have been objective vasodilation and blood flow augmentation. As early as 1976, multiple groups described changes in peripheral blood flow in response to SCS, laying a foundation for subsequent widespread clinical applications.23,24
This consistent and reproducible effect on autonomic functions became the basis of SCS application for blood flow augmentation and ischemic pain relief in treatment of vascular disorders such as peripheral arterial occlusive disease,25 coronary ischemia/intractable angina,26 and vasospastic disease in extremities.27
Genitourinary Effects of Spinal Cord Stimulation
Conus medullaris SCS for micturition control in a paraplegic patient was first performed in 1970; this approach was later used in a group of 10 other paraplegic patients with long-lasting symptomatic improvement.28 Improved bladder control was one of the major, results of SCS in a group of 24 patients with upper motor neuron disease, including multiple sclerosis, traumatic spinal cord injury, and neurodegenerative conditions,29 and another group of 11 patients with multiple sclerosis.30
When SCS was implanted specifically to treat neurogenic bladder, most patients developed complete or almost complete normalization of urination with relief of bladder spasticity, marked increase of bladder capacity, and reduction or abolition of postvoid residual urine volume.31 The same group of authors noticed no changes in bladder striatal activity or detrusor reflexes in patients who underwent SCS for pain treatment and had intact bladder function.31
The urodynamic changes do not occur in all patients undergoing SCS. In a study of patients with spinal cord injury who underwent SCS implantation for control of spasticity, less than 20% (6 of 33) were found to have changes in lower urinary tract function.32
In addition to bladder function normalization, SCS appeared to facilitate normalization of bowel regimen and morning erections in a group of patients with posttraumatic paraplegia.3
In a somewhat unconventional approach, SCS was used to treat female orgasmic dysfunction.33 In this series of 11 patients, a single percutaneous SCS electrode was used to produce pleasurable genital stimulation and subsequent orgasm. In 91% of subjects, SCS resulted in increased lubrication, greater frequency in sexual activity, and overall satisfaction. An orgasmic capacity returned in 80% of patients with secondary anorgasmia while using SCS, but anorgasmia returned once the device was removed. Despite pleasurable paresthesias in the genital area, none of the patients with primary anorgasmia (those who never had an orgasm) experienced orgasm during the study, making the researchers speculate on whether the underlying difficulty that prevented orgasm from occurring throughout the patient’s life could not be overcome with SCS application. At the same time a possibility of a longer stimulation period (longer than 9 days) resolving primary anorgasmia was also brought up.33
Other Areas of Spinal Cord Stimulation Application
Impaired Consciousness
Anecdotal experience exists with use of SCS for treatment of impaired consciousness. Out of eight patients with severe brain dysfunction resulting from head injury, vasospasm, or tumor resection, two regained consciousness and speech after 1 to 2 months of cervical SCS.34 The patients were implanted with a four-contact paddle electrode at the C2-C4 level, and the stimulation was delivered twice a day for 4 hours. The authors concluded that SCS may accelerate the natural course of recovery in patients after brain injury.
In the treatment of a vegetative state, 8 out of 23 patients who underwent SCS exhibited symptomatic improvement, and 7 of these were able to follow verbal orders.35 It was noted that onset of improvement varied from the first few weeks to as long as 10 to 12 months after SCS initiation. There was significant improvement in cerebral blood flow (CBF) associated with SCS in some of the patients, but this phenomenon did not correlate with clinical improvement.
As to the mechanism of symptomatic improvement, positron emission tomography revealed changes in glucose consumption in two patients with prolonged posttraumatic unconsciousness.36 The patient who improved clinically had higher glucose uptake in the brainstem, hypothalamic, thalamic, and certain cortical regions, whereas the other patient whose consciousness did not improve had no or minimal changes in glucose uptake.
SCS was investigated as an early-stage intervention in patients with hypoxic encephalopathy.37 An SCS electrode was inserted, and therapy was started within a month after a hypoxic event in 12 patients ranging in age from 7 to 72. The improvement was observed in 58% of patients within 2 weeks after start of SCS. Although there was an improvement in ability to communicate with others and express emotions, disturbances of writing, picture drawing, and calculation were not improved by stimulation.
In the most recent update on this topic, it appears that, based on clinical experience with more than 200 patients treated with SCS for impaired consciousness, indications for surgery may include young age, history of brain trauma, evidence of brain atrophy with no other major lesions, and CBF values of 20 mL/100 g/min or higher.38 It appears that, of 15 patients who satisfied all criteria for surgery, 12 improved with SCS, and 7 of these improved significantly, thereby indicating that SCS was effective in 80% of this selected patient group.38
Another direction recently explored in the literature involves a combination of cervical SCS and hyperbaric oxygenation (HBO) in 12 patients whose coma lasted more than 3 months.39 Six patients (50%) emerged from coma as a result of combined treatment and regained consciousness. SCS was delivered through four-contact paddle electrodes, and the stimulation regimen was set as 15 minutes on/15 minutes off for a duration of 14 hours during the daytime. However, it is unclear whether SCS or HBO was responsible for symptomatic improvement since every patient who emerged from his or her vegetative state did so within the first 6 months of treatment, during or soon after the period when both SCS and HBO were administered, and there were no additional dramatic improvements when SCS was used alone.40
Autonomic Hyperreflexia
Autonomic hyperreflexia, a frequent and difficult-to-manage symptom of spinal cord injury, was significantly reduced or eliminated in four of five patients implanted with SCS.41
Spinal Cord Stimulation and Cerebral Blood Flow
Although mechanism of vasoregulation appears different between cerebral and peripheral or coronary circulations, the ability of SCS to augment peripheral and coronary blood flow was tested in regard to CBF in the mid-1980s. Similar to other fields of SCS use, human experience preceded animal studies. In 1985 Hosobuchi42 found that SCS at upper cervical levels can increase CBF. The same result was not found with stimulation of thoracic levels. Later, the same author tested cervical SCS for three patients with symptomatic cerebral ischemia (one with anterior and two with posterior circulation occlusion); although positive results were obtained, further studies were suggested to confirm its clinical application.43
Multiple animal experiments in rats, cats, rabbits, and dogs44–52 have shown augmentation of CBF with cervical SCS. Level of stimulation seemed to have direct effect on the blood flow, with stimulation of upper levels (C1-C3) generating higher flow values.
Using a cat model, a group from Japan showed that CBF augmentation with cervical SCS is no longer observed after sectioning of the dorsal columns at the cervicomedullary junction.44 Based on this, the authors postulated that CBF is increased from cervical SCS mainly through a central pathway. Later, similar results were obtained using a rat model by a group of American researchers.48 They also showed lack of changes in CBF after resection of superior cervical ganglion while using SCS.
Researchers from Italy demonstrated that SCS can increase, decrease, or have no effect in CBF.53 The difference correlated mainly with the stimulated level of the spinal cord. Thoracic stimulation had low effect and sometimes even decreased CBF. Cervical stimulation more frequently produced CBF augmentation (61%). In another study the same group found that vasoconstriction of carotid arteries with sympathetic trunk stimulation was attenuated by cervical SCS.54 In this experiment they used rabbit models to observe CBF changes with SCS alone, sympathetic trunk stimulation alone, and simultaneous spinal cord and sympathetic trunk stimulation.
The hypothetic treatment for cerebral vasospasm after subarachnoid hemorrhage (SAH) with SCS has been tried in different animal models. Increased blood flow was found in rats with SAH and SCS compared to control groups.46 Similarly, prevention of early vasospasm was described in rabbits treated with SCS after induced SAH.55 Recently the vasodilation effect of SCS was shown in the basilar artery of rats 5 days after induction of SAH. Radiotracer studies, laser Doppler flowmetry, and histological photomicrographs were used to prove these changes in the delayed spasm.51
Based on the literature data suggesting several possible mechanisms for SCS action in the prevention and treatment of SAH-related vasospasm, we hypothesized that stimulation at different levels of the cervical spinal cord results in different clinical effects.56 In theory, stimulation of the lower cervical spinal cord may allow one to prevent vasospasm by acting through modulation of sympathetic activity, essentially constituting a functional, temporary sympathectomy and preventing cerebral arteries from vasoconstriction after SAH. But once the vasospasm is present, the patient may receive additional benefit and possibly improve clinical outcome by CBF augmentation and treatment of the vasospasm by stimulation of the upper cervical spinal cord, possibly acting through more central, medullary mechanisms that are responsible for immediate vasospasm after SAH and for subsequent vasodilation needed for vasospasm treatment.56
A pioneering study related to the use of SCS for cerebral vasospasm in humans was performed in the late 1990s in Japan.57 Ten SAH patients with a secured cerebral aneurysm were implanted with percutaneous quadripolar epidural cervical leads. The stimulation was continuous and started on day 5 (±1) after bleeding for 10 to 15 days. CBF was measured with Xenon computed tomography; it was significantly increased in the distribution of the middle cerebral artery with SCS. Four patients presented with angiographic vasospasm, and three were reported with clinical vasospasm. One patient died, and the overall outcome was good or excellent in seven. No major adverse effect was attributed to the use of SCS. The data analysis correlated an increase in CBF with SCS.
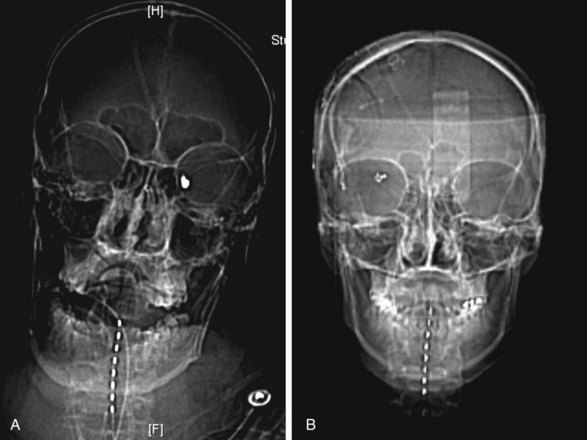
To prove the concept, we recently performed a prospective safety/feasibility study of cervical SCS in the prevention/treatment of cerebral vasospasm after aneurysmal SAH. In our study 12 patients were implanted with percutaneous eight-contact SCS electrodes immediately on completion of the aneurysm-securing procedure, either clipping or coiling, while the patient was still under general anesthesia (Fig. 14-1). By the study protocol SCS had to be initiated the following morning and within 72 hours after SAH and then administered continuously for 14 consecutive days. We found that cervical SCS was safe and feasible since there were no complications related to the electrode insertion or the stimulation itself. One patient died during the study from unrelated causes, and two electrodes were pulled out prematurely. Angiographic vasospasm was observed in 6 of 12 patients, and clinical vasospasm in 2 out of 12. Both incidences were smaller than predicted based on Fisher and Hunt and Hess grades, although this incidence reduction did not reach statistical significance. There were no long-term side effects of SCS during 1-year follow up. Subsequent data analysis indicated that preventive effects of cervical SCS on vasospasm may correlate with stimulated level.58
In addition to acute ischemia from cerebral vasospasm, SCS has been shown to increase CBF in chronic ischemic conditions. The results were encouraging in the patients with chronic vascular occlusion,43 and in a case of old cerebral infarction, SCS resulted in a dramatic increase of blood flow velocities measured by transcranial Doppler.59
Spinal Cord Stimulation and Brain Tumors
In a novel application of SCS, at cervical level it was shown to increase local blood flow in patients with brain tumors.60 This phenomenon was then used in a clinical series of 23 patients with high-grade malignant brain tumors.61 Based on the known association between hypoxia and low perfusion in malignant neoplasms and resistance to radiotherapy and the significant increase in tumor radiosensitivity with increased local tissue oxygenation, the researchers postulated that, with its augmentation of CBF and ability to increase glucose metabolism, SCS may improve treatment outcome in high-grade gliomas. Although clinical outcome data are still pending, the preliminary results were described as promising, and the blood flow and glucose metabolism have been consistently higher in patients with high-grade gliomas undergoing continuous cervical SCS.61 In this patient group a single four-contact percutaneous SCS electrode was placed over the dorsal surface of the spinal cord at the C2-C4 level; the stimulation was delivered at amplitude of 3 V or less, producing mild paresthesias in upper extremities.
Spinal Cord Stimulation and Radiation-Induced Brain Injury
Since hypoxia and impaired tissue perfusion are hallmarks for the radiation-induced brain injury, cervical SCS was used to improve glucose metabolism in a prospective series of eight patients.62 As the glucose metabolism increased by about 40% as the result of stimulation, the authors noted a decrease in corticosteroid requirements in patients without concurrent tumor. These results may offer a new avenue for treatment of radiation-induced brain injury, perhaps decreasing or eliminating the need in radical surgical interventions for this frustrating and hard-to-manage pathological condition.
1 Siegfried J, et al. Electrical spinal cord stimulation for spastic movement disorders. Appl Neurophysiol. 1978;41:134-141.
2 Siegfried J. Treatment of spasticity by dorsal cord stimulation. Int Rehabil Med. 1980;2:31-34.
3 Richardson RR, et al. Percutaneous epidural neurostimulation in modulation of paraplegic spastic: six case reports. Acta Neurochir (Wien). 1979;49:235-243.
4 Barolat G. Surgical management of spasticity and spasms in spinal cord injury: an overview. J Am Paraplegia Soc. 1988;11:9-13.
5 Reynolds AF, Oakley JC. High frequency cervical epidural stimulation for spasticity. Appl Neurophysiol. 1982;45:93-97.
6 Koulousakis A, Buchhaas U, Nittner K. Application of SCS for movement disorders and spasticity. Acta Neurochir (Wien). 1987;39(suppl):112-116.
7 Barolat G, Myklebust JB, Wenninger W. Effects of spinal cord stimulation on spasticity and spasms secondary to myelopathy. Appl Neurophysiol. 1988;51(1):29-44.
8 Kanaka TS, Kumar MM. Neural stimulation for spinal spasticity. Paraplegia. 1990;28:399-405.
9 Maiman DJ, Mykleburst JB, Barolat-Romana G. Spinal cord stimulation for amelioration of spasticity: experimental results. Neurosurgery. 21, 1987. 331-313
10 Nakamura S, Tsubokawa T. Evaluation of spinal cord stimulation for postapoplectic spastic hemiplegia. Neurosurgery. 1985;17:253-259.
11 Cioni B, Meglio M. Spinal cord stimulation improves motor performances in hemiplegics: clinical and neurophysiological study. Acta Neurochir (Wien). 1987;39(suppl):103-105.
12 Goetz CG, Penn RD, Tanner CM. Efficacy of cervical cord stimulation in dystonia. Adv Neurol. 1988;50:645-649.
13 Terao T, et al. Therapeutic effect of spinal cord stimulation for a patient suffering spasticity after hypoxia of the brain. No Shinkei Geka. 2004;32:613-618.
14 Gottlieb GL, et al. Evaluation of cervical stimulation for chronic treatment of spasticity. Neurology. 1985;35:699-704.
15 Hugenholtz H, et al. Cervical spinal cord stimulation for spasticity in cerebral palsy. Neurosurgery. 1988;22:707-714. 1988;
16 Midha M, Schmitt JK. Epidural spinal cord stimulation for the control of spasticity in spinal cord injury patients lacks long-term efficacy and is not cost-effective. Spinal Cord. 1998;36:190-192.
17 Lazorthes Y, et al. The surgical management of spasticity. Eur J Neurol. 2002;9(suppl 1):35-41. discussion 53-61
18 Shabalov VA, et al. The use of chronic epidural electrostimulation of the spinal cord in children with spastic diplegia—a type of infantile cerebral palsy. Zh Vopr Neirokhir Im N N Burdenko. 2000;3:2-6.
19 Shabalov VA, Dekopov AV, Troshina EM. Preliminary results of treatment for spastic forms of infantile cerebral paralysis by chronic epidural neurostimulation of lumbar enlargement. Zh Vopr Neirokhir Im N N Burdenko. 2006;3:10-13.
20 Minassian K, et al. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord. 2004;42:401-416.
21 Herman R, et al. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord. 2002;40:65-68.
22 Fuentes R, et al. Spinal cord stimulation restores locomotion in animal models of Parkinson’s disease. Science. 2009;323:1578-1582.
23 Dooley DM, Kasprak M. Modification of blood flow to the extremities by electrical stimulation of the nervous system. South Med J. 1976;69:1309-1311.
24 Cook AW, et al. Vascular disease of extremities: electric stimulation of spinal cord and posterior roots. NY State J Med. 1976;76:366-368.
25 Vincenzo S, Kyventidis T. Epidural spinal cord stimulation in lower limb ischemia. Acta Neurochir. 2007;97(suppl 1):253-258.
26 Hautvast RW, et al. Effect of spinal cord stimulation on myocardial blood flow assessed by positron emission tomography in patients with refractory angina pectoris. Am J Cardiol. 1996;77:462-467.
27 Robaina FJ, et al. Spinal cord stimulation for relief of chronic pain in vasospastic disorders of the upper limbs. Neurosurgery. 1989;24:63-67.
28 Nashold BSJr, et al. Electrical stimulation of the conus medullaris in the paraplegic: a 5-year review. Appl Neurophysiol. 1977;40:192-207.
29 Campos RJ, et al. Clinical evaluation of the effect of spinal cord stimulation on motor performance in patients with upper motor neuron lesions. Appl Neurophysiol. 1981;44:141-151.
30 Read DJ, Matthews WB, Higson RH. The effect of spinal cord stimulation on function in patients with multiple sclerosis. Brain. 1980;103:803-833.
31 Meglio M, et al. Epidural spinal cord stimulation for the treatment of neurogenic bladder. Acta Neurochir (Wien). 1980;54:191-199.
32 Katz PG, et al. Effect of implanted epidural stimulator on lower urinary tract function in spinal-cord-injured patients. Eur Urol. 1991;20:103-106.
33 Meloy TS, Southern JP. Neurally augmented sexual function in human females: a preliminary investigation. Neuromodulation. 2006;9:34-40.
34 Matsui T, et al. Beneficial effects of cervical spinal cord stimulation (cSCS) on patients with impaired consciousness: a preliminary report. Pacing Clin Electrophysiol. 1989;12:718-725.
35 Kanno T, et al. Effects of dorsal column spinal cord stimulation on reversibility of neuronal function: experience of treatment for vegetative states. Pacing Clin Electrophysiol. 1989;12:733-738.
36 Yamaguchi N, et al. Effects of cervical spinal cord stimulation in glucose consumption in patients with post traumatic prolonged unconsciousness. Neurol Med Chir (Tokyo). 1995;35:797-803.
37 Fujii M, et al. Spinal cord stimulation in an early stage for unresponsive patients with hypoxic encephalopathy. No Shinkei Geka. 1998;26:315-321.
38 Morita I, Keith MW, Kanno T. Dorsal column stimulation for persistent vegetative state. Acta Neurochir. 2007;97(1):455-459. (suppl)
39 Liu JT, et al. Neuromodulation on cervical spinal cord combined with hyperbaric oxygen in comatose patients—a preliminary report. Surg Neurol. 2009;72(S2):28-34.
40 Slavin KV. Commentary to Liu et al (ref 39). Surg Neurol. 2009;72(S2):34-35.
41 Richardson RR, Cerullo LJ, Meyer PR. Autonomic hyper-reflexia modulated by percutaneous epidural neurostimulation: a preliminary report. Neurosurgery. 1979;4:517-520.
42 Hosobuchi Y. Electrical stimulation of the cervical spinal cord increases cerebral blood flow in humans. Appl Neurophysiol. 1985;48:372-376.
43 Hosobuchi Y. Treatment of cerebral ischemia with electrical stimulation of the cervical spinal cord. Pacing Clin Electrophysiol. 1991;14:122-126.
44 Isono M, et al. Effect of spinal cord stimulation on cerebral blood flow in cats. Stereotact Funct Neurosurg. 1995;64:40-46.
45 Sagher O, Huang DL. Effects of cervical spinal cord stimulation on cerebral blood flow in the rat. J Neurosurg. 2000;93(suppl 1):71-76.
46 Ebel H, et al. High cervical spinal cord stimulation (CSCS) increases regional cerebral blood flow after induced subarachnoid haemorrhage in rats. Minim Invasive Neurosurg. 2001;44:167-171.
47 Patel S, Huang DL, Sagher O. Sympathetic mechanisms in cerebral blood flow alterations induced by spinal cord stimulation. J Neurosurg. 2003;99:754-761.
48 Patel S, Huang DL, Sagher O. Evidence for a central pathway in the cerebrovascular effects of spinal cord stimulation. Neurosurgery. 2004;55:201-206.
49 Gurelik M, et al. Cervical spinal cord stimulation improves neurological dysfunction induced by cerebral vasospasm. Neuroscience. 134, 2005. 827-332
50 Karadağ Ö, et al. Cervical spinal cord stimulation increases cerebral cortical blood flow in an experimental vasospasm model. Acta Neurochir (Wien). 2005;147:79-84.
51 Lee JY, et al. Effect of electrical stimulation of the cervical spinal cord on blood flow following subarachnoid hemorrhage. J Neurosurg. 2008;109:1148-1154.
52 Yang X, et al. Roles of dorsal column pathway and transient receptor potential vanilloid type 1 in augmentation of cerebral blood flow by upper cervical spinal cord stimulation in rats. Neuroscience. 2008;152:950-958.
53 Visocchi M. Spinal cord stimulation and cerebral haemodynamics. Acta Neurochir. 2006;99(suppl):111-116. 2006
54 Visocchi M, et al. Spinal cord stimulation and cerebral blood flow: an experimental study. Stereotact Funct Neurosurg. 1994;62:186-190.
55 Visocchi M, et al. Spinal cord stimulation and early experimental cerebral spasm: the “functional monitoring” and the “preventing effect.”. Acta Neurochir (Wien). 2001;143:177-185.
56 Goellner E, Slavin KV. Cervical spinal cord stimulation may prevent cerebral vasospasm by modulating sympathetic activity of the superior cervical ganglion at lower cervical spinal level. Med Hypotheses. 2009;73:410-413.
57 Takanashi Y, Shinonaga M. Spinal cord stimulation for cerebral vasospasm as prophylaxis. Neurol Med Chir (Tokyo). 2000;40:352-356.
58 Slavin KV, et al. Cervical spinal cord stimulation for prevention of cerebral vasospasm in aneurysmal subarachnoid haemorrhage: preliminary results of first North American study. J Cerebr Blood Flow Metabolism. 2009;29:S308.
59 Visocchi M, et al. Increase of cerebral blood flow and improvement of brain motor control following spinal cord stimulation in ischemic spastic hemiparesis. Stereotact Funct Neurosurg. 1994;62:103-107.
60 Clavo B, et al. Increased locoregional blood flow in brain tumors after cervical spinal cord stimulation. J Neurosurg. 2003;98:1263-1270.
61 Robaina F, Clavo B. The role of spinal cord stimulation in the management of patients with brain tumors. Acta Neurochir. 2007;97(suppl 1):445-453.
62 Clavo B, et al. Modification of glucose metabolism in radiation-induced brain injury areas using cervical spinal cord stimulation. Acta Neurochir (Wien). 2009;151:1419-1425.