Chapter 53 Emerging Endoluminal Bariatric Techniques
Introduction
Obesity is the pandemic of the 21st century and is associated with considerable morbidity and mortality.1 Management of obesity depends on body mass index (BMI) and the presence of comorbidities, including heart disease, diabetes, hypertension, dyslipidemia, osteoarthritis, and sleep apnea.2 Approximately 1.6 billion adults are overweight; at least 400 million adults are obese. The World Health Organization further projects that by 2015, approximately 2.3 billion adults will be overweight, and more than 700 million will be obese. Previously considered a problem only in high-income countries, overweight and obesity are now dramatically increasing in low-income and middle-income countries, particularly in urban settings.3
The only effective therapy at the present time for morbid obesity, as defined by BMI of 40 kg/m2 or more or by BMI of 35 kg/m2 or more in the presence of comorbidities, is surgery.4 Bariatric surgery has been shown to be effective in the long-term and significantly reduces the risk of mortality associated with morbid obesity. In the United States, the indications for bariatric surgery increased by 80% during the period 1998–2004,5 and in terms of health care demand, morbid obesity clearly represents the pandemic of the 21st century.
Bariatric surgery for morbidly obese patients has shown significant clinical benefits. It induces and maintains satisfactory weight loss while decreasing comorbidities in the patient associated with overweight.6 Efficacy varies with the type of procedure, which can be divided into restrictive (lap band, vertical gastroplasty, sleeve gastrectomy), malabsorptive (biliopancreatic diversion), or a combination of both (gastric bypass). The first and second types of operations are the most frequently performed sometimes as an optional two-step procedure starting with gastric restriction. Although very effective, laparoscopic and surgical bariatric procedures have complication rates of 3% to 20% and mortality rates of 1%.7 Cardiopulmonary events and anastomotic leaks are the major sources of severe morbidities.
Endoscopic Options for Endoluminal Primary Treatment of Obesity
Intragastric Balloon
The use of an intragastric device to induce weight loss in obese patients was first described in 1982.8 Since then, numerous intragastric balloons have been in use worldwide, and several have been withdrawn from the market. The BioEnterics Intragastric Balloon (BIB; Allergan, Irvine, CA) has a spherical shape and larger capacity than earlier models and has been the most extensively studied device. Among the more recently improved minimally invasive procedures, the intragastric balloon has been one temporary nonsurgical option that can promote weight loss in obese patients by partially filling their stomach and inducing a sense of early satiety.9,10 One of the major drawbacks of balloon implantation is weight regain after balloon removal. Two more recent studies help clinicians to understand better what can be expected from balloon implantation.
In the first study, Mathus-Vliegen and Tytgat11 included patients who had participated in a randomized controlled trial comparing a balloon with a sham for a 3-month period in an additional trial including 9 months of balloon treatment and follow-up for 1 year after removal. The authors excluded eight patients who had not met the weight loss goal during the first 3 months (five patients) or who did not tolerate the balloon (three patients). Although there was no difference between the sham and balloon during the first 3 months, after 1 year of balloon treatment, a mean weight loss of 21.3 kg (17.1%) was achieved in all patients; 12.6 kg (9.9%) was maintained at the end of the second balloon-free year. Overall, 47% of patients sustained a 10% weight loss at the end of 2 years of follow-up. Although this study could not show an independent benefit of balloon treatment beyond diet, exercise, and behavioral therapy in the first treatment, balloon treatment for 1 year, in the patients who tolerated the treatment, resulted in substantial weight loss, a significant part of which was maintained during the first year after removal of the balloon.
The second study looked at the long-term outcome after treatment with an intragastric balloon for 6 months, with no structured weight maintenance program after balloon removal. After BIB placement, 100 consecutive morbidly obese individuals were prospectively followed; 97 patients completed the final follow-up at a mean of 4.8 years. After 6 months, 63% of patients had more than 10% baseline weight loss, whereas there were only 28% at final follow-up. At that time, 35 patients had undergone bariatric surgery, and 34 patients had no significant weight change from baseline.12
These studies confirm further that balloon implantation may be helpful for long-term weight loss in a few patients. It is a potential option for patients who are unwilling to undergo bariatric surgery or are not candidates for bariatric surgery. Balloon implantation could also be used as a temporary measure in superobese patients to induce weight loss and decrease the risk of complications associated with further bariatric surgery.13
Gastric Restriction
Endoluminal Vertical Gastroplasty Using EndoCinch
The EndoCinch suturing system (C.R. Bard, Murray Hill, NJ) was initially designed for endoscopic treatment of gastroesophageal reflux disease. This system allows the placement of a series of stitches in the lower esophagus to create a pleat in the sphincter. This pleat alters the gateway between the stomach and the esophagus and potentially prevents acid from flowing out of the stomach. Although associated with encouraging early results, use of the EndoCinch for the treatment of gastroesophageal reflux disease has been called into question because of the lack of retention of plications in the long-term.14
Fogel and colleagues15 first described the use of this technology for the treatment of obesity in 64 patients. Their technique comprised the deployment of seven sutures in a continuous and cross-linked design from the proximal fundus to the distal body. The result of the treatment is suggested to be a significant decrease in distensibility of the stomach. The procedure was performed as an ambulatory procedure, and of the 59 patients followed for 12 months, the percentage excess weight loss reported was 21% at 1 month and 58% at 12 months. Only a few patients (n = 14) underwent repeated endoscopy in the follow-up period. In 11 patients, the suture line was reported as completely or partially intact. A randomized controlled trial is ongoing in the United States to investigate this technique further. It is hoped that this trial will also provide long-term data that are relevant both clinically and anatomically.
Transoral Gastroplasty
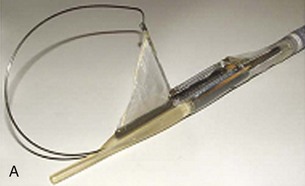
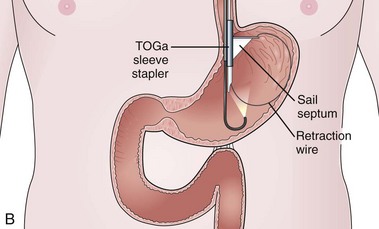
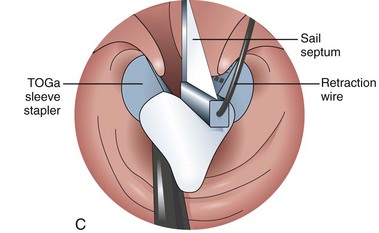
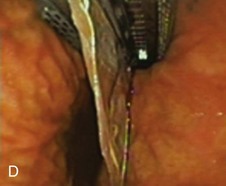
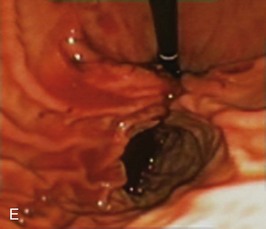
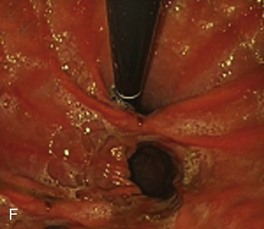
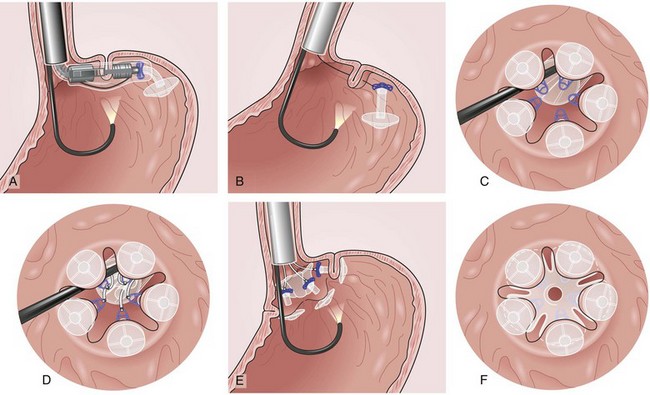
The system consists of the TOGa Sleeve Stapler, a flexible 18-mm diameter shaft device, which rides over a guidewire for introduction. It is specifically designed for the procedure and accommodates a standard endoscope up to 8.6 mm in diameter and creates full-thickness plications of the anterior and posterior walls of the stomach, which are acquired using vacuum pots located parallel to the staple line (Fig. 53.1). The stapling allows a serosa-to-serosa apposition and is performed via two successive applications of staple lines of 5 cm each.
In the first evaluation, this procedure was completed by the placement of restriction in the distal part of the pouch, using a dedicated stapling device also based on vacuum acquisition of the tissues. Only two series in humans have been published so far as full articles. The first series16 comprised 21 patients with a mean BMI of 43.3 kg/m2. The mean excess weight loss was 16%, 23%, and 25% at 1 month, 3 months, and 6 months after treatment. No severe adverse events were reported; endoscopy at 6 months showed gaps between the two staple lines in 13 of 21 patients. After this first evaluation, the technique was improved, especially concerning the successive application of the two staple lines, and the device was modified. A second human pilot series17 was subsequently reported in 11 patients, showing better results with mean excess weight loss of 19%, 34%, and 46% at 1 month, 3 months, and 6 months. A 1-year follow-up on a larger multicenter European study has confirmed these data.17a Although the TOGa technique is performed under general anesthesia, recovery is very fast, and the procedure could be performed within 30 to 45 minutes on an outpatient basis, suggesting that this technique might have become a high ideal first step for bariatric treatment strategy. A multicentric randomized sham-controlled study was performed in 11 centers comparing the active and sham procedure (2 : 1 ratio) in a series of more than 200 patients. This randomized controlled trial showed significant but modest differences between the two groups and therefore the FDA required additional data. In shortage of financing, the company (Satiety Inc., Palo Alto) closed and sold its assets.
Duodenojejunal Bypass Sleeve
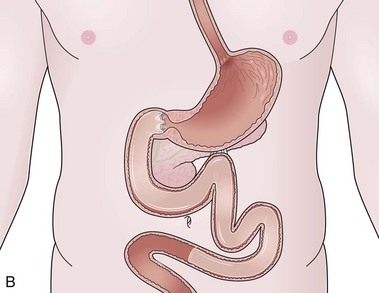
The bypass technique has to be put in line with the development of metabolic surgery and particularly with the clinical observation that bypassing the proximal bowel improves type 2 diabetes and induces weight loss, a feature that partly explains the greater improvement of diabetes observed in patients undergoing a laparoscopic RYGB for the same weight loss compared with a pure restrictive procedure.19 The first strictly endoluminal device used to bypass the proximal small intestine is the duodenojejunal bypass sleeve (DJBS), also called the EndoBarrier (GI Dynamics Inc., Watertown, MA). It is composed of a self-expanding implant that is placed in the duodenum and has antimigration features. It is attached to a 60-cm plastic sleeve that extends into the proximal jejunum and works by creating a physical barrier between food that has been ingested and the intestinal wall and biliopancreatic secretions (Fig. 53.2). The device is left in place for a maximum of 3 to 6 months and is removed with a dedicated and relatively easy system.
Three trials showed the potential benefit of the DJBS. In the first trial,20 the DJBS was successfully deployed in 12 patients in less than 30 minutes (and required approximately 40 minutes to be removed). With the exception of pain prompting early removal, it was associated with a significant decrease in hemoglobin A1c compared with a control group. A second multicenter trial from Chile showed a weight loss significantly greater in 24 patients treated with the EndoBarrier system at 12 weeks compared with the diet control group in a preoperative setting.21 The last available study is a sham-controlled trial with a 12-week study design comprising 24 patients treated and 13 patients undergoing the sham procedure. Excess weight loss (11.9% vs. 2.7%) and loss of more than 10% excess weight (62% of patients vs. 17% of patients) were in favor of the DJBS. However, seven subjects terminated the study earlier because of gastrointestinal bleeding (three patients) or device intolerance (pain or vomiting or both, four patients), showing that some technologic developments are still needed.22 Although these techniques have disadvantages of requiring the implantation of a foreign body and the limited duration of indwell, they warrant further technical improvement and clinical investigation because they could be techniques that have the most impact on comorbidities and might be of particular interest in diabetic obese patients.19 Other implantable devices, potentially mimicking more closely RYGB, are in development (Valentx Inc., Carpinteria, CA), but no clinical data are currently available.
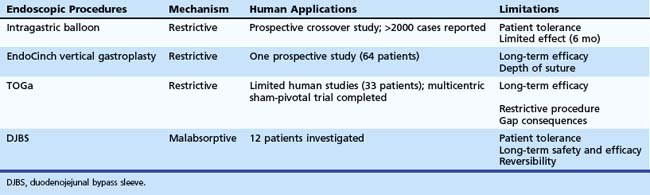
Table 53.1 summarizes current techniques of bariatric endotherapy for which clinical data are available. Further data are likely to become available, and the role of endotherapy in the management of obese patients undergoing bariatric surgery is likely to increase, rendering necessary the integration of endotherapy into the armamentarium of multidisciplinary treatment of these patients.
Transoral Endoscopic Restrictive Implant
If the DJBS may be seen as the endoscopic counterpart of RYGB, and the TOGa may be seen as the endoscopic counterpart of sleeve gastrectomy or vertical banded gastroplasty, a more recently reported endoscopic implantable device (Transoral Endoscopic Restrictive Implants System [TERIS]; Barosense, Redwood City, CA) may be seen as an endoscopic equivalent to gastric banding (Fig. 53.3). A phase I pilot trial has been reported.23 Successful placement has been achieved in 12 of 13 patients with complications (including perforation) in 3 of them. This system is technically demanding (22-mm insertion tube, median procedural time 142 minutes), but an excess weight loss of 28% was observed at 3 months, suggesting that with technical improvements, it could become another option in the endoscopic armamentarium. This procedure is associated with the placement of an implant, which means that it offers the potential advantage of being removable (the drawback being, similar to other implants, the long-term durability of the procedure, which can be shown only by long-term follow-up).
Endoscopy for Managing Complications after Bariatric Surgery
Stomal ulceration usually occurs within the first 3 months after the procedure with a decreased incidence thereafter. Although the exact cause is unclear, management consists of proton pump inhibitor therapy, liquid sucralfate, eradication of Helicobacter pylori if present, and elimination of ulcerogenic medications.24 Ulcers may also occur as a result of foreign body reaction to nonabsorbable suture. In this case, endoscopic removal of this foreign material may induce the resolution of the ulcer and symptoms.25
Stomal stenosis represents a different problem and may occur at the gastrojejunal anastomosis of RYGB or at the level of the gastric band in restrictive procedure. The treatment consists of dilation, which must be performed cautiously, usually at a diameter of 15 mm, which represents a good balance between efficacy and risk of complication.26
Gastric band erosion represents a more difficult problem that is traditionally approached by surgical revision. This latter can be difficult, however, and may compromise further bariatric surgery if needed. The need to avoid potential gastrostomies and disruption of blood supply is an argument for endoscopic management of these complications. Blero and coworkers27 reported their experience in removing partially migrated lap bands or vertical banded gastroplasty rings by using a technique combining the placement of self-expandable plastic stents for a limited duration to induce the more complete migration of the band and a technique of section of the foreign bodies to allow their endoscopic removal. Using this original technique, these authors were able to remove all but one partially migrated band in their first series. With the past proliferation of vertical banded gastroplasty and the associated complication of decompensated proximal pouch owing to stenosis at the level of the band and the current increase of the use of adjustable gastric banding, this less invasive approach could provide an elegant way of managing this complication in the setting of a multidisciplinary team.
Anastomotic leaks are probably the most severe complications occurring after RYGB and sleeve gastrectomies. The use of sealants and sclerosing agents has been described in small series and in attempts to close staple line dehiscence. Use of endoclips has also been reported in a small group of patients with unequal results. The largest experience has been reported in treating post–bariatric surgery gastrocutaneous leaks in two more recent series using self-expandable metal stents. Salinas and colleagues28 reported a series of 17 patients with 16 successes and 3 complications (mucosal tears and migration to the colon). Eisendrath and associates29 reported on 21 patients who underwent endoscopic treatment of persisting large anastomotic leaks before considering redo surgery. They described a technique of successive placement of metal stents for closure of the fistula followed by placement, after 2 to 4 months, of a plastic stent, which induces by pressure a necrosis of the hyperplasia and allows the removal of the metal stent. Eisendrath and associates29 reported an 80% success rate in these difficult patients. This experience has been expanded to more than 70 patients with similar encouraging results that are expected to be reported soon.
Revisional Endoscopy after Bariatric Surgery
Staple line disruption resulting in gastrogastric fistula is a classic cause of weight regain in patients. Besides the classic surgical reintervention, endoscopic suturing and fibrin glue application have been successfully reported in small series.30 Endoscopic suturing (EndoCinch) or volume reduction by dedicated devices (StomaphyX; EndoGastric Solutions, Redmond, WA) has also been reported in patients having an enlarged pouch or anastomosis after RYGB.31,32 Although reported series are uncontrolled, they have documented significant weight loss at 6- to 12-month follow-up. Endoscopic interventions are potential options for patients who are at high risk of complications and often desperate after failure of bariatric surgery.
1 World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation on obesity. WHO/NUT/NCD/98.1. WHO Technical Support Series. Geneva: WHO. 1998.
2 World Health Organization 2005 data. Available at http://www.who.int/ncd_surveillance Accessed September 16, 2006
3 Glenny AM, O’Meara S, Melville A, et al. The treatment and prevention of obesity: A systematic review of the literature. Int J Obes Relat Metab Disord. 1997;21:715-737.
4 Magnusson M, Freedman J, Jonas E, et al. Five-year results of laparoscopic vertical banded gastroplasty in the treatment of massive obesity. Obes Surg. 2002;12:826-830.
5 Zhao Y, Encinosa W, Agency for Health Care Research and Quality (AHRQ): Bariatric surgery utilization and outcomes in 1998 and 2004. Statistical Brief # 23, January 2007.
6 Paxton J, Matthews J. The cost effectiveness of laparoscopic versus open gastric bypass surgery. Obes Surg. 2005;15:24-34.
7 Rosenthal RJ, Szomstein S, Kennedy CI, et al. Laparoscopic surgery for morbid obesity: 1,001 consecutive bariatric operations performed at The Bariatric Institute, Cleveland Clinic Florida. Obes Surg. 2006;16:119-124.
8 Nieben OG, Harboe H. Intragastric balloon as an artificial bezoar for treatment of obesity. Lancet. 1982;1:198-199.
9 Evans JD, Scott MH. Intragastric balloon in the treatment of patients with morbid obesity. Br J Surg. 2001;88:1245-1248.
10 Genco A, Bruni T, Doldi SB, et al. Bioenterics intragastric balloon: The Italian experience with 2515 patients. Obes Surg. 2005;15:1161-1164.
11 Mathus-Vliegen EM, Tytgat GN. Intragastric balloon for treatment-resistant obesity: Safety, tolerance, and efficacy of 1-year balloon treatment followed by a 1-year balloon-free follow-up. Gastrointest Endosc. 2005;61:19-27.
12 Dastis NS, François E, Deviere J, et al. Intragastric balloon for weight loss: Results in 100 individuals followed for at least 2.5 years. Endoscopy. 2009;41:575-580.
13 Spyropoulos C, Katsakoulis E, Mead N, et al. Intragastric balloon for high-risk super obese patients: A prospective analysis of efficacy. Surg Obes Relat Dis. 2007;3:78-83.
14 Schwartz MP, Wellink H, Gooszen HG, et al. Endoscopic gastroplication for the treatment of gastroesophageal reflux disease: A randomized sham controlled trial. Gut. 2007;56:20-28.
15 Fogel R, De Fogel J, Bonilla Y, et al. Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest Endosc. 2008;68:51-58.
16 Devière J, Ojeda Valdes G, Cuevas Herrera L, et al. Safety, feasibility and weight loss after transoral gastroplasty: First human multicenter study. Surg Endosc. 2008;22:589-598.
17 Moreno C, Closset J, Dugardeyn S, et al. Transoral gastroplasty is safe, feasible, and induces significant weight loss in morbidly obese patients: Results of the second human pilot study. Endoscopy. 2008;40:406-413.
17a Familiari P, Costamanga G, Blero D, et al: Transoral gastroplasty for morbid obesity: A multicentre trial with a 1-year outcome. Gastrointest Endosc, in press.
18 Closset J, Germanova D, Loi P, et al. Laparoscopic gastric bypass as a revision procedure after transoral gastroplasty. Obes Surg. 2011;21:1-4.
19 Rubino F, Cohen RV, Schiavon CA, et al. Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22–34 kg/m2: A report of 2 cases. Surg Obes Relat Dis. 2007;3:195-197.
20 Rodriguez-Grunert L, Galvao Neto MP, Alamo M, et al. First human experience with endoscopically delivered and retrieved duodenal-jejunal bypass sleeve. Surg Obes Relat Dis. 2008;4:55-59.
21 Tarnoff M, Rodriguez L, Escalona A, et al. Open label, prospective, randomized controlled trial of an endoscopic duodenal-jejunal bypass sleeve versus low calorie diet for pre-operative weight loss in bariatric surgery. Surg Endosc. 2009;23:650-656.
22 Gersin KS, Rothstein RI, Rosenthal RJ, et al. Open-label, sham-controlled trial of an endoscopic duodenojejunal bypass liner for preoperative weight loss in bariatric surgery candidates. Gastrointest Endosc. 2010;71:976-982.
23 de Jong K, Mathus-Vliegen EMH, Veldhuyzen EAML, et al. Short-term safety and efficacy of the trans-oral endoscopic restrictive implant system for the treatment of obesity. Gastrointest Endosc. 2010;72:497-504.
24 Rasmussen JJ, Fuller W, Ali MR. Marginal ulceration after laparoscopic gastric bypass: An analysis of predisposing factors in 260 patients. Surg Endosc. 2007;21:1090-1094.
25 Frezza EE, Herbert H, Ford R, et al. Endoscopic suture removal at gastrojejunal anastomosis after Roux-en-Y gastric bypass to prevent marginal ulceration. Surg Obes Relat Dis. 2007;3:619-622.
26 Peifer KJ, Shiels AJ, Azar R, et al. Successful endoscopic management of gastrojejunal anastomosis stricture after Roux-en-Y gastric bypass. Gastrointest Endosc. 2007;66:248-252.
27 Blero D, Eisendrath P, Vandermeeren A, et al. Endoscopic removal of dysfunction rings or bands after restrictive bariatric procedures. Gastrointest Endosc. 2010;71:468-474.
28 Salinas A, Baptista A, Santiago E, et al. Self expandable metal stent to treat gastric leaks. Surg Obes Relat Dis. 2006;2:570-572.
29 Eisendrath P, Cremer M, Impens J, et al. Endotherapy including temporary stenting of fistula of the upper gastrointestinal tract after laparoscopic bariatric surgery. Endoscopy. 2007;39:625-630.
30 Obstein KL, Thompson CC. Endoscopy after bariatric surgery. Gastrointest Endosc. 2009;70:1161-1166.
31 Thompson CC, Slattery J, Bundga ME, et al. Peroral endoscopic reduction of dilated gastro-jejunal anastomosis after Roux-en-Y gastric bypass: A possible new option for patients with weight regain. Surg Endosc. 2006;20:1744-1748.
32 Mikami D, Needleman B, Narula V, et al. Natural orifice surgery: Initial US experience utilizing the StomaphyX device to reduce gastric pouches after Roux-en-Y gastric bypass. Surg Endosc. 2010;24:223-228.