86 Emergent Valvular Disorders
In the critical care setting, there are two distinct presentations of valvular heart disease: acute valve dysfunction resulting in acute heart failure and chronic valve disease with decompensation due to increased metabolic demands (Table 86-1).1 Valve regurgitation is the most common type of acute valve dysfunction. Valve stenosis, with rare exceptions, is a chronic slowly progressive disease. However, in patients with asymptomatic chronic valve stenosis, acute deterioration can occur if there is a superimposed hemodynamic burden. For example, patients with previously asymptomatic mitral stenosis may present with pulmonary edema in the setting of a systemic infection. Another example is the elderly adult with asymptomatic aortic stenosis who presents with cardiogenic shock in the setting of an acute gastrointestinal bleed. Prosthetic valve dysfunction also can present emergently, particularly mechanical valve thrombosis.
TABLE 86-1 Causes of Acute Valve Dysfunction
| Mitral regurgitation | Myxomatous disease with flail leaflet |
| Spontaneous chordal rupture | |
| Endocarditis | |
| Acute myocardial infarction: Papillary muscle rupture Regional wall motion abnormality LV dilation and systolic dysfunction |
|
| Aortic regurgitation | Endocarditis |
| Spontaneous rupture of a congenital fenestration | |
| Aortic dissection | |
| Tricuspid regurgitation | Endocarditis |
| Penetrating chest trauma | |
| Blunt chest wall trauma | |
| Prosthetic valves | Endocarditis |
| Valve thrombosis | |
| Paravalvular dehiscence | |
| Leaflet tear |
 Mitral Regurgitation
Mitral Regurgitation
Etiology
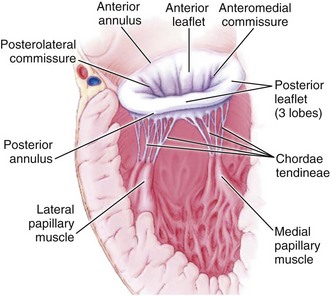
Mitral regurgitation may be caused by disease or distortion of any component of the mitral valve apparatus—the mitral annulus, leaflets, chordae, and papillary muscles—as well as by alterations in left ventricular (LV) geometry or systolic function (Figure 86-1).2 Primary causes of chronic mitral regurgitation include myxomatous valve leaflets (mitral valve prolapse) and rheumatic disease. Chronic secondary mitral regurgitation may be due to dilated cardiomyopathy or to coronary artery disease with regional or global LV dysfunction.
Acute mitral regurgitation also may be due to involvement of the valve leaflets or the left ventricle. Patients with myxomatous mitral valve disease may develop acute regurgitation due to spontaneous chordal rupture.3 Bacterial endocarditis results in acute mitral regurgitation due to destruction of valve tissue, often with leaflet perforation. Moderate to severe mitral regurgitation due to papillary muscle involvement or regional myocardial dysfunction complicates 12% of acute myocardial infarctions and is associated with an increased risk of heart failure or death.4
Clinical Presentation
Although patients with chronic mitral regurgitation may be asymptomatic for many years, the regurgitant lesions impose a volume load on the left ventricle, because an increased total stroke volume is needed to maintain a normal forward cardiac output. Left ventricular volume overload results in progressive LV dilation and may lead to an irreversible decline in ventricular contractility, even in the absence of clinical symptoms. Evaluation of ventricular contractility is problematic in patients with mitral regurgitation, given that measures of ventricular performance are affected by preload and afterload.5 However, based on outcomes after mitral valve surgery, the empirical parameters of ventricular end-systolic dimension and ejection fraction can be used to optimize the timing of surgical intervention. Thus, patients with moderate to severe chronic regurgitation undergo periodic echocardiography, with valve repair or replacement recommended when the end-systolic dimension is ≥ 40 mm and the ejection fraction is ≤ 60%.6
Chronic mitral regurgitation usually is well tolerated even when there is a superimposed hemodynamic load such as systemic infection, pregnancy, or trauma. However, mitral regurgitant severity may acutely worsen by at least two mechanisms. An increase in afterload, for example with a hypertensive crisis, may increase regurgitant severity due to an increased driving pressure from the left ventricle to the left atrium. Alteration in LV geometry, for example with ventricular dilation due to decompensated heart failure, may change the orientation of the papillary muscles such that leaflet closure is impaired, resulting in a larger regurgitant orifice area.7 In this situation, a vicious cycle may ensue where LV dilation worsens mitral regurgitant severity, which increases LV dilation, and so forth.
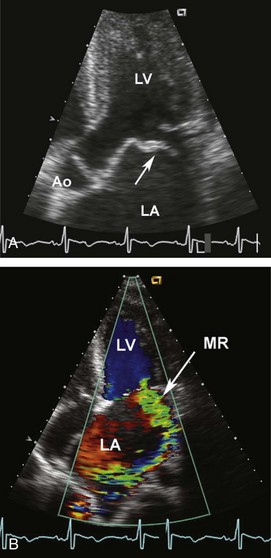
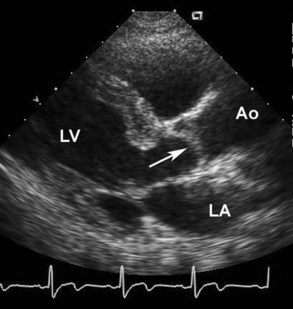
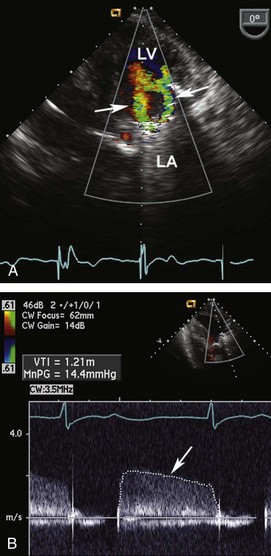
Acute mitral regurgitation presents with acute pulmonary edema and is a surgical emergency (Figures 86-2 and 86-3). Mitral chordal rupture results in the acute presentation of heart failure, often in patients who were unaware of a diagnosis of mitral valve prolapse. Patients with mitral valve perforation due to endocarditis present with pulmonary edema superimposed on signs and symptoms of endocarditis. Papillary muscle rupture or dysfunction after MI usually presents several days after acute MI; in some cases, the initial presentation is acute pulmonary edema, with the MI being clinically silent.
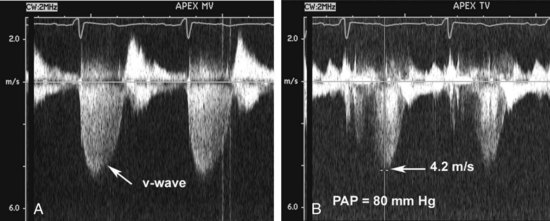
Figure 86-3 In the same patient as Figure 86-2, severe mitral regurgitation was recorded with continuous wave Doppler ultrasound (A). The rapid rise in left atrial pressure due to the regurgitant jet entering the left atrium results in a rapid decline in Doppler velocity in late systole—the Doppler equivalent of the v-wave seen on a pulmonary wedge pressure tracing. The continuous-wave Doppler recording of maximum tricuspid regurgitant jet velocity (B) of 4.2 m/sec indicates a right ventricular–to–right atrial pressure difference of 70 mm Hg. The patient’s right atrial pressure was estimated to be 10 mm Hg, based on size and respiratory variation in the inferior vena cava, so the estimated pulmonary systolic pressure is 80 mm Hg.
Diagnosis
A high level of clinical suspicion is needed to make the diagnosis of acute mitral regurgitation (Table 86-2). Acute pulmonary edema often obscures the signs and symptoms of the underlying disease process. The classical finding is a holosystolic murmur at the apex, radiating to the axilla. Although there is some correlation between the loudness of the murmur and regurgitant severity with chronic regurgitation, the murmur may be soft with acute severe mitral regurgitation. In patients with severe mitral regurgitation after MI, a murmur cannot be appreciated at all in up to 50% of patients.
| Physical examination | Unreliable |
| Consider valve dysfunction in all patients with pulmonary edema | |
| Echocardiography (transthoracic) | Accurate diagnosis of etiology of disease |
| Quantitation of severity of stenosis or regurgitation | |
| Measurement of ventricular ejection fraction | |
| Estimation of pulmonary pressures | |
| Transesophageal echocardiography | Sensitive for detection of valvular vegetations |
| Detection of paravalvular abscess | |
| Essential for prosthetic mitral valve dysfunction | |
| Useful for prosthetic aortic valve dysfunction | |
| Right heart catheterization | Not reliable for diagnosis of valve disease |
| May be helpful for optimizing loading conditions | |
| Chest computed tomography | Sensitive and specific for diagnosis of aortic dissection |
| Angiography | Used when coronary angiography is needed |
Management
In patients with chronic mitral regurgitation and heart failure, management is directed at treating the process leading to decompensation and optimizing loading conditions (Table 86-3). For example, in a patient with a systemic infection, treatment of the infection, control of fever and tachycardia, and invasive monitoring to optimize preload and afterload are utilized. Medical therapy typically includes afterload reduction with nitroprusside or other vasodilators and preload reduction with diuretics.8,9 The goal is to support the patient through the period of decompensation. Typically, hemodynamics return to the baseline compensated state after the acute illness.
In contrast, acute severe mitral regurgitation is a surgical emergency. Mortality is extremely high without restoration of valve competence; even with prompt valve surgery, 30-day mortality is 23%.10 Medical stabilization should occur concurrently with consultation by a cardiac surgeon. Acutely, placement of an intraaortic balloon pump (IABP) provides optimal afterload reduction while improving diastolic coronary blood flow.
The timing of surgery for endocarditis depends on the disease course in that individual, but most centers now advocate early surgical intervention in the patient with heart failure or severe valve regurgitation to prevent progressive valve damage and paravalvular abscess formation.11,12 In a large prospective multicenter study, early surgery was associated with a lower mortality than medical therapy (12% versus 21%).13 Valve repair is preferred but may not be possible, depending on the extent of tissue destruction. Early surgery is particularly beneficial in patients with paravalvular complications or systemic embolization.14
In patients with acute ischemic mitral regurgitation, treatment depends on the exact etiology of valve dysfunction.15 In patients with acute mitral regurgitation due to a regional wall-motion abnormality, myocardial function may improve after percutaneous revascularization.16 In these patients, use of an IABP and medical therapy may be advantageous during the acute episode, with weaning of therapy as myocardial function improves.
Mitral regurgitation due to partial or complete papillary muscle rupture requires surgical intervention. Although the risk of surgery is high, with an operative mortality rate of about 50%, outcome is even worse with medical therapy, with a mortality of 75% at 24 hours and 95% within 2 weeks after complete papillary muscle rupture.17 With the use of echocardiography, partial papillary muscle rupture can be recognized; prognosis in these patients depends on the extent of myocardial damage and severity of mitral regurgitation.18 With partial papillary muscle rupture, some surgeons prefer to stabilize the patient and delay surgery for 6 to 8 weeks after MI to avoid operating on the necrotic myocardial tissue. However, many patients cannot be stabilized, so acute intervention must be considered. Again, valve repair is preferred, but myocardial necrosis may necessitate valve replacement. Risk factors for surgery include older age, female gender, and poor LV systolic function. In some patients, the risk of surgical intervention may be so high as to be futile.
 Aortic Regurgitation
Aortic Regurgitation
Etiology
Chronic aortic regurgitation most often is due to a congenital bicuspid valve, rheumatic valve disease, or aortic root dilation. There are numerous causes of aortic root dilation, including hypertension, cystic medial necrosis, Marfan syndrome, and a bicuspid aortic valve.19 The most common causes of acute aortic regurgitation are endocarditis, rupture of a congenital fenestration, and acute aortic dissection.1 Endocarditis results in aortic regurgitation by destruction of the valve leaflet tissue, with a high percentage of cases also having paravalvular abscess formation. Aortic dissection results in acute aortic regurgitation either due to enlargement of the aortic annulus or to extension of the dissection into the valve region, resulting in a flail aortic valve leaflet.
Diagnosis
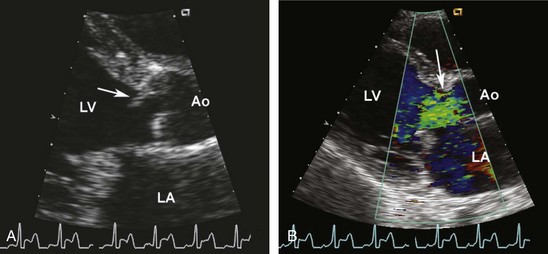
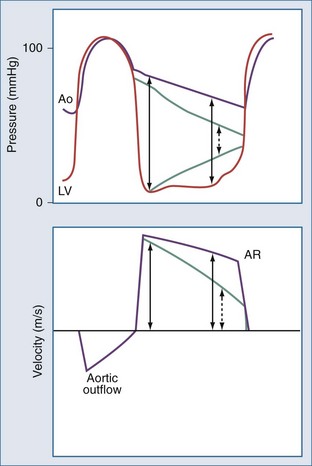
The clinical diagnosis of acute aortic regurgitation differs markedly from chronic aortic regurgitation (Figure 86-4). In contrast to the high-pitched diastolic decrescendo murmur of chronic aortic regurgitation, there is a “to-and-fro” murmur across the aortic valve which many clinicians fail to recognize as indicating aortic regurgitation. The pulse pressure is narrow due to the low forward stroke volume, and peripheral signs of aortic regurgitation are not seen. As with acute mitral regurgitation, the physical examination findings often are subtle, so a high index of suspicion and prompt echocardiography are needed to make this diagnosis.
(From Otto CM. Textbook of clinical echocardiography. 4th ed. Philadelphia: Saunders; 2009, p. 303.)
Acute aortic regurgitation should be considered in the patient with signs or symptoms of endocarditis, in patients with a personal or family history of aortic root disease, and in those with a presentation consistent with acute aortic dissection.20
Echocardiography allows imaging of the aortic valve and root and determination of the severity of aortic regurgitation based on a combination of two-dimensional (2D) imaging and pulsed, continuous-wave, and color Doppler modalities21 (Figures 86-5, 86-6, and 86-7). The continuous-wave Doppler curve shows a steep diastolic slope corresponding to the rapid equalization of diastolic pressure in the aorta and left ventricle. With severe acute regurgitation, there is no pressure gradient at end-diastole, so cuff diastolic blood pressure is equal to LV end-diastolic pressure. Echocardiography also allows accurate assessment of LV size and systolic function. When the differential diagnosis includes aortic dissection, transthoracic echocardiography is inadequate to exclude this possibility. Instead, TEE or computed tomography (CT) images should be obtained.
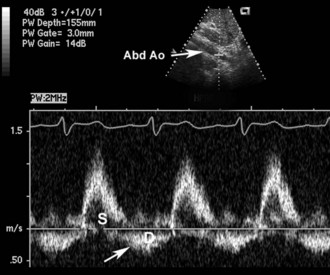
Figure 86-6 Same patient as Figure 86-5. Pulsed Doppler flow in the proximal abdominal aorta (Ao) shows normal forward flow in systole (S), with abnormal reversed flow in diastole (D) that extends throughout diastole. This finding is highly specific for severe aortic regurgitation and can be helpful in the acute setting.
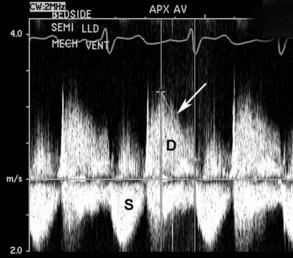
Figure 86-7 Same patient as Figure 86-5. Continuous-wave Doppler recording of flow across the aortic valve shows an increased antegrade velocity in systole (S) consistent with a high transaortic stroke volume. In diastole (D), a dense signal of retrograde flow is seen, with a steep deceleration slope (arrow) consistent with equalization of pressures between the aorta and left ventricle in diastole.
Management
Acute aortic regurgitation is a surgical emergency.1 Preoperative management is supportive, with ventilatory support and invasive hemodynamic monitoring. While the diagnosis is being made, therapy may include the use of diuretics, inotropic agents, and nitroprusside or other vasodilators in an attempt to stabilize hemodynamics.1,9 However, an IABP is contraindicated, as inflation of the balloon in the descending thoracic aorta in diastole will increase the amount of backflow across the aortic valve.
 Mitral Stenosis
Mitral Stenosis
Etiology and Clinical Presentation
Mitral stenosis is nearly always due to rheumatic disease, with only rare cases of calcific mitral stenosis seen in the elderly. Rheumatic mitral stenosis is a slowly progressive disease with an insidious decline in exercise tolerance and symptom onset over many years.22 However, in the asymptomatic patient with compensated moderate or severe mitral stenosis, acute decompensation can occur in the setting of increased systemic hemodynamic demands. Because mitral stenosis is more common in women (80% of cases) and occurs during the reproductive years, the most common emergency presentation of mitral stenosis is a pregnant woman with heart failure. Many of these patients are unaware of underlying valve disease and are initially diagnosed during pregnancy. The clinical presentation may also be due to or exacerbated by the onset of atrial fibrillation.
Diagnosis
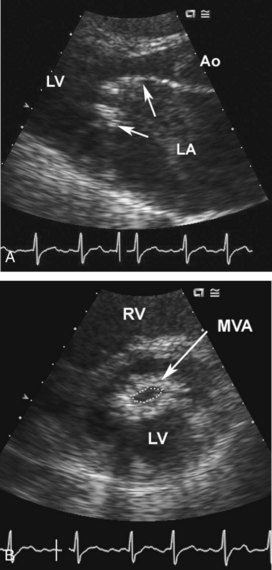
The apical diastolic rumble and opening snap of mitral stenosis is challenging to appreciate even in a quiet room with optimal patient positioning and frequently is inaudible in the ICU setting. However, the diagnosis is easily made by transthoracic echocardiography, with the mitral leaflet showing the characteristic findings of rheumatic disease: commissural fusion, chordal shortening and fusion, and restriction of the diastolic opening of the leaflets (Figure 86-8).23 Mitral stenosis severity can be quantitated by calculation of valve area by 2D planimetry or by the Doppler pressure half-time method, with moderate to severe stenosis defined as a valve area less than 1.5 cm2 (Figure 86-9). Transthoracic echocardiography also provides information on LV size and systolic function, left atrial size, pulmonary pressures, and any associated valve lesions. If evaluation for left atrial thrombus is needed, TEE has a sensitivity of only 60% compared to a sensitivity of nearly 100% from the transthoracic approach.
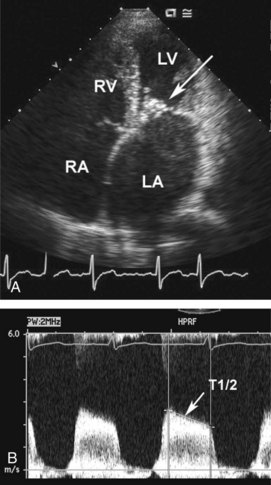
Figure 86-9 Same patient as Figure 86-10. Apical four-chamber view (A) shows severe left atrial enlargement due to mitral obstruction, with thickened valve leaflets (arrow). Haziness in left atrium is due to stasis of blood flow, with spontaneous contrast on echocardiography. Continuous-wave Doppler recording of flow across mitral valve shows increased velocity corresponding to transvalvular pressure gradient. Pressure half-time (T1/2) can be used to accurately calculate mitral valve area (0.7 cm2).
Management
Most patients with mitral stenosis and acute decompensation can be managed conservatively with treatment of the superimposed illness.9 Efforts should be directed towards decreasing overall metabolic demand and increasing oxygen delivery by controlling fever, maintaining a normal hemoglobin level, and providing supplemental oxygen. If atrial fibrillation is present, rate control is essential, preferably with conversion back to sinus rhythm. Even when sinus rhythm is present, beta-blockers may improve ventricular diastolic filling by prolonging the duration of diastole as heart rate is decreased.24 Invasive hemodynamic monitoring and ventilatory support may be needed when severe heart failure is present.
In patients who do not respond to conservative therapy, emergency intervention should be considered. The optimal intervention is percutaneous balloon mitral valvotomy (PBMV), which typically results in an increase in mitral valve area to more than 1.5 cm2.25–27 PBMV can be safely performed even during pregnancy.28–30 Patients with a left atrial thrombus, coexisting moderate to severe mitral regurgitation, or heavily calcified and deformed mitral valves are not candidates for PBMV; in theses patients, surgical mitral valve replacement may be needed.
 Aortic Stenosis
Aortic Stenosis
Etiology and Clinical Presentation
Valvular aortic stenosis in adults is most often due to calcification of a normal trileaflet or congenital bicuspid valve (Figure 86-10). Rheumatic aortic stenosis is less common and is invariably accompanied by mitral valve involvement. In younger adults, congenital aortic stenosis may be encountered; some of these patients have restenosis after prior commissurotomy in childhood.
Like mitral stenosis, aortic valve stenosis is a chronic, slowly progressive disease that presents acutely only in patients who have not been receiving regular medical care.31–33 As in mitral stenosis, acute decompensation may occur with a superimposed systemic condition. Young women with congenital aortic stenosis may present with angina or heart failure during pregnancy. In older adults, asymptomatic patients with moderate to severe valve obstruction may present with heart failure in the setting of pneumonia, anemia, or other condition with increased metabolic demands.
Diagnosis
The classic physical examination findings for aortic stenosis include a delayed and decreased carotid upstroke, a narrow pulse pressure, a single second heart sound (S2), and a systolic ejection murmur at the aortic region that radiates to the carotids. However, while a grade 4 murmur (palpable thrill) with a single S2 and diminished carotids is specific for severe stenosis, these findings are very insensitive for the diagnosis.34 Particularly when the patient is decompensated, the murmur may be soft, and carotid upstrokes may be altered by coexisting vascular disease or loading conditions.
Echocardiography provides reliable evaluation of aortic stenosis severity based on the maximum velocity through the narrowed orifice and valve area, calculated with the continuity equation (Figure 86-11). Disease severity is a continuum, and velocities may be relatively low despite severe stenosis when cardiac output in reduced. In general, stenosis can be graded as severe (valve area <1.0 cm2 or jet velocity >4 m/sec), moderate (valve area 1.0-1.5 cm2 or jet velocity 3-4 m/sec) or mild (valve area >1.5 cm2 or jet velocity <3 m/sec). Echocardiography also allows evaluation of ventricular systolic and diastolic function and any associated valve disease.23
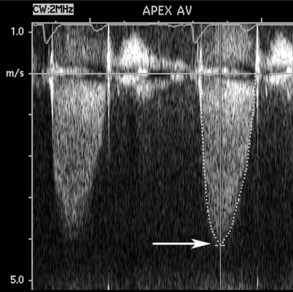
Figure 86-11 Continuous-wave Doppler examination of the aortic valve (same patient as Figure 86-8) demonstrates a high-velocity signal consistent with severe aortic stenosis. The maximum velocity of 4.2 m/sec corresponds to a maximum transaortic pressure gradient of 69 mm Hg and a mean gradient of 41 mm Hg. Valve area, calculated by the continuity equation, was 0.8 cm2.
Management
As with mitral stenosis, most patients with decompensated aortic stenosis can be managed conservatively by (1) treating the underlying disease process that led to decompensation and (2) restoring the patient’s normal loading conditions. However, in the patient who has denied symptoms or has not been receiving medical care, the first presentation of aortic stenosis may be syncope or pulmonary edema. In these patients, aortic stenosis is the cause of decompensation, as evidenced by very severe valve obstruction, often with a low ejection fraction. Treatment is urgent aortic valve replacement. Some centers advocate the use of balloon aortic valvuloplasty in these patients, but the magnitude and duration of benefit is limited. Cautious use of nitroprusside may improve hemodynamics prior to valve replacement in severe decompensated aortic stenosis if mean arterial pressure is above 60 mm Hg,35,36 and some patients can be managed with careful diuresis. However, in unstable patients who are surgical candidates, it is more prudent to proceed promptly to valve replacement. Transcatheter aortic valve implantation is currently in clinical trials for high-risk patients with severe aortic stenosis and may become an option for acutely ill patients in the future.37
 Right-Sided Valve Disease
Right-Sided Valve Disease
Pulmonic valve disease is nearly always congenital in origin, with a chronic disease course. Tricuspid valve stenosis is rare and usually accompanies rheumatic mitral valve disease. Tricuspid valve endocarditis often results in acute severe regurgitation; pulmonic valve endocarditis is rare. Cases of acute traumatic disruption of the tricuspid valve with blunt chest trauma have been described, although myocardial contusion or thoracic aorta disruption is more common.38,39 Acute severe tricuspid regurgitation results in a low forward cardiac output and signs of an elevated right atrial pressure.
 Prosthetic Valves
Prosthetic Valves
Mechanical Valves
Prosthetic mechanical heart valves are very durable, with complications most often due to valve thrombosis or paravalvular regurgitation.40 Valve thrombosis occurs in the setting of inadequate anticoagulation and may result in functional valve stenosis if movement of the valve occluder is restricted, or valve regurgitation if the clot prevents full closure of the valve. The clinical presentation of valve thrombosis is similar to that of native valve stenosis or regurgitation. Again, echocardiography provides key information on the presence and severity of valve dysfunction (Figure 86-12).41 TEE is especially important with mitral prosthetic valves; the valve itself blocks ultrasound penetration from a transthoracic approach.
Treatment of prosthetic valve thrombosis is controversial. When only a small thrombus and mild hemodynamic compromise are present, conservative therapy with full-dose intravenous anticoagulation for several days may be adequate. With severe hemodynamic compromise, surgical intervention with repeat valve replacement may be necessary, although operative mortality is reported to be high, ranging from 17% to 40%.6 Systemic thrombolytic therapy can restore valve function in about 80% of patients but is associated with death in 20%, systemic embolism due to fragmentation of the valve thrombosis in 16%, and the need for emergency surgery in 20%.6 The duration of thrombolytic therapy is based on the resolution of Doppler echocardiographic evidence of resolution of thrombus and valve dysfunction (see Figure 86-12). Current guidelines recommend emergency operation for left-sided valve thrombosis and severe symptoms or a large clot burden, except in patients with excessively high surgical risk. Fibrinolytic therapy is reasonable for right-sided valve thrombosis, for left-sided thrombosis with mild obstruction or a small clot burden, and for patients who are not surgical candidates.6,42,43
Paravalvular regurgitation early after valve replacement may be related to suture dehiscence at a site of annular calcification. Paravalvular regurgitation may be associated with hemolytic anemia, which can be treated conservatively if mild but may require reoperation if severe recurrent anemia is present. The new onset of paravalvular regurgitation should prompt careful evaluation for endocarditis (see Chapter 87).
Tissue Valves
Tissue valves are subject to degeneration of the leaflets, with superimposed calcification that may result in stenosis or regurgitation. Usually this is a slowly progressive process with presentation 10 to 15 years after valve implantation.44 As with native valve disease, acute decompensation may occur in patients with chronic prosthetic valve dysfunction if there is a superimposed hemodynamic stress.
Stout KK, Verrier ED. Acute valvular regurgitation. Circulation. 2009;119:3232-3241.
Prendergast BD, Tornos P. Surgery for infective endocarditis: who and when? Circulation. 2010;121:1141-1152.
Lalani T, Cabell CH, Benjamin DK, et al. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation. 2010;121:1005-1013.
Chandrashekhar Y, Westaby S, Narula J. Mitral stenosis. Lancet. 2009;374:1271-1283.
Sun JC, Davidson MJ, Lamy A, Eikelboom JW. Antithrombotic management of patients with prosthetic heart valves: current evidence and future trends. Lancet. 2009;374:565-576.
1 Stout KK, Verrier ED. Acute valvular regurgitation. Circulation. 2009 June 30;119(25):3232-3241.
2 Otto CM. Clinical practice. Evaluation and management of chronic mitral regurgitation. N Engl J Med. 2001 September 6;345(10):740-746.
3 Zuppiroli A, Rinaldi M, Kramer Fox R, Favilli S, Roman MJ, Devereux RB. Natural history of mitral valve prolapse. Am J Cardiol. 1995;75(15):1028-1032.
4 Bursi F, Enriquez-Sarano M, Nkomo VT, et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation. 2005 January 25;111(3):295-301.
5 Nishimura RA, Schaff HV. Mitral regurgitation: timing of surgery. In Otto CM, Bonow RO, editors: Valvular Heart Disease: A Companion to Braunwald’s Heart Disease, 3rd ed, Philadelphia: Saunders/Elsevier, 2009.
6 Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006 August 1;48(3):e1-148.
7 Rosario LB, Stevenson LW, Solomon SD, Lee RT, Reimold SC. The mechanism of decrease in dynamic mitral regurgitation during heart failure treatment: importance of reduction in the regurgitant orifice size. J Am Coll Cardiol. 1998 December;32(7):1819-1824.
8 Levine HJ, Gaasch WH. Vasoactive drugs in chronic regurgitant lesions of the mitral and aortic valves. J Am Coll Cardiol. 1996;28:1083-1091.
9 Boon NA, Bloomfield P. The medical management of valvar heart disease. Heart. 2002 April;87(4):395-400.
10 Lorusso R, Gelsomino S, De CG, et al. Mitral valve surgery in emergency for severe acute regurgitation: analysis of postoperative results from a multicentre study. Eur J Cardiothorac Surg. 2008 April;33(4):573-582.
11 Feringa HH, Shaw LJ, Poldermans D, et al. Mitral valve repair and replacement in endocarditis: a systematic review of literature. Ann Thorac Surg. 2007 February;83(2):564-570.
12 Prendergast BD, Tornos P. Surgery for infective endocarditis: who and when? Circulation. 2010 March 9;121(9):1141-1152.
13 Lalani T, Cabell CH, Benjamin DK, et al. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation. 2010 March 2;121(8):1005-1013.
14 Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Eur Heart J. 2009 October;30(19):2369-2413.
15 Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation. 2005 August 2;112(5):745-758.
16 Picard MH, Davidoff R, Sleeper LA, et al. Echocardiographic predictors of survival and response to early revascularization in cardiogenic shock. Circulation. 2003 January 21;107(2):279-284.
17 Tcheng JE, Jackman JDJr, Nelson CL, et al. Outcome of patients sustaining acute ischemic mitral regurgitation during myocardial infarction. Ann Intern Med. 1992;117(1):18-24.
18 Manning WJ, Waksmonski CA, Boyle NG. Papillary muscle rupture complicating inferior myocardial infarction: identification with transesophageal echocardiography. Am Heart J. 1995;129(1):191-193.
19 Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation. 2002 August 20;106(8):900-904.
20 Roberts WC, Ko JM, Moore TR, Jones WHIII. Causes of pure aortic regurgitation in patients having isolated aortic valve replacement at a single US tertiary hospital (1993 to 2005). Circulation. 2006 August 1;114(5):422-429.
21 Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003 July;16(7):777-802.
22 Chandrashekhar Y, Westaby S, Narula J. Mitral stenosis. Lancet. 2009 October 10;374(9697):1271-1283.
23 Otto CM. Textbook of Clinical Echocardiography, 4th ed. Elsevier Saunders; 2009.
24 Carabello BA. Modern management of mitral stenosis. Circulation. 2005 July 19;112(3):432-437.
25 Turi ZG, Reyes VP, Raju BS, et al. Percutaneous balloon versus surgical closed commissurotomy for mitral stenosis. A prospective, randomized trial. Circulation. 1991;83(4):1179-1185.
26 Reyes VP, Raju BS, Wynne J, et al. Percutaneous balloon valvuloplasty compared with open surgical commissurotomy for mitral stenosis. N Engl J Med. 1994;331(15):961-967.
27 Ben Farhat M, Ayari M, Maatouk F, et al. Percutaneous balloon versus surgical closed and open mitral commissurotomy: seven-year follow-up results of a randomized trial. Circulation. 1998 January 27;97(3):245-250.
28 de Souza JA, Martinez EEJr, Ambrose JA, et al. Percutaneous balloon mitral valvuloplasty in comparison with open mitral valve commissurotomy for mitral stenosis during pregnancy. J Am Coll Cardiol. 2001 March 1;37(3):900-903.
29 Sivadasanpillai H, Srinivasan A, Sivasubramoniam S, et al. Long-term outcome of patients undergoing balloon mitral valvotomy in pregnancy. Am J Cardiol. 2005 June 15;95(12):1504-1506.
30 Abouzied AM, Al Abbady M, Al Gendy MF, et al. Percutaneous balloon mitral commissurotomy during pregnancy. Angiology. 2001 March;52(3):205-209.
31 Otto CM. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol. 2006 June 6;47(11):2141-2151.
32 Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe asymptomatic aortic valve stenosis. N Eng J Med. 2000;343:611-617.
33 Carabello BA. Clinical practice. Aortic stenosis. N Engl J Med. 2002 February 28;346(9):677-682.
34 Munt B, Legget ME, Kraft CD, Miyake-Hull CY, Fujioka M, Otto CM. Physical examination in valvular aortic stenosis: correlation with stenosis severity and prediction of clinical outcome. Am Heart J. 1999 February;137(2):298-306.
35 Khot UN, Novaro GM, Popovic ZB, et al. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med. 2003 May 1;348(18):1756-1763.
36 Zile MR, Gaasch WH. Heart failure in aortic stenosis–improving diagnosis and treatment. N Engl J Med. 2003 May 1;348(18):1735-1736.
37 Zajarias A, Cribier AG. Outcomes and safety of percutaneous aortic valve replacement. J Am Coll Cardiol. 2009 May 19;53(20):1829-1836.
38 Elie MC. Blunt cardiac injury. Mt Sinai J Med. 2006 March;73(2):542-552.
39 Bruce CJ, Connolly HM. Right-sided valve disease deserves a little more respect. Circulation. 2009 May 26;119(20):2726-2734.
40 Vongpatanasin W, Hillis LD, Lange RA. Prosthetic heart valves. New Eng J Med. 1996;335:407-416.
41 Zabalgiotia M. Echocardiographic recognition and quantitation of prosthetic valve dysfunction. In: Otto CM, editor. The Clinical Practice of Echocardiography. 3rd ed. Philadelphia: Saunders/Elsevier; 2007:577-604.
42 Lengyel M, Fuster V, Keltai M, et al. Guidelines for management of left-sided prosthetic valve thrombosis: a role for thrombolytic therapy. Consensus Conference on Prosthetic Valve Thrombosis. J Am Coll Cardiol. 1997 November 15;30(6):1521-1526.
43 Sun JC, Davidson MJ, Lamy A, Eikelboom JW. Antithrombotic management of patients with prosthetic heart valves: current evidence and future trends. Lancet. 2009 August 15;374(9689):565-576.
44 Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000 October;36(4):1152-1158.