22 Emergency Presentations
After reading this chapter, you should be able to:
• describe the uniqueness of the emergency care environment
• outline the development of Australasian triage models
• discuss the process of initial patient assessment and triage nursing practice
• integrate emergency nursing principles and practice in initial patient care
• describe the various roles of extended nursing practice in the emergency setting
• describe the principles and practice of patient preparation for retrievals or transfers
• discuss the principles for the management of disaster victims in the emergency department
• discuss the initial nursing management of common presentations to the ED, including chest pain, abdominal pain, neurological, respiratory, poisoning, envenomation, submersion and heat illness.
Introduction
Emergency nursing practice covers an enormous range of clinical presentations. As the focus of this book is critical care, this chapter discusses conditions at the critical end of the practice spectrum. Please read in conjunction with Chapters 23 and 24, which describe the management of additional common presentations to the Emergency Department (ED): trauma and resuscitation emergencies, respectively.
Emergency nursing practice is the holistic care of individuals of all ages who present with perceived or actual physical and/or emotional alterations. These presentations are often undiagnosed and require a range of prompt symptomatic and definitive interventions. Emergency clinical practice is usually unscheduled, episodic and acute in its nature, and is therefore unlike any other type of nursing in the demands it places on nursing staff.1,2 In many instances the emergency nurse is the first healthcare professional to be in contact with an acutely ill or injured patient. Patient presentations include a full range of acuity across the spectrum of possible illnesses, injuries and ages.
Background
Emergency nursing is unique, in that it involves the care of patients with health problems that are often undiagnosed on presentation but are perceived as sufficiently acute by the individual to warrant seeking emergency care in the hospital setting. As patients present with signs and symptoms rather than medical diagnoses, refined assessment skills are paramount. Many skills required by emergency nurses are based on a broad foundation of knowledge that serves as a guide in collecting information, making observations and evaluating data, and to sort and analyse relevant information.2–6 This foundation enables an emergency nurse to communicate appropriately with other members of the healthcare team, and to implement appropriate independent and collaborative nursing interventions. Assessment is an important element of emergency care; other chapters provide detailed information on the evaluation of critically ill patients.
Emergency nurses are specialists in acute episodic nursing care, and their knowledge, skills and expertise encompass almost all other nursing specialty areas. Emergency nurses therefore possess a unique body of knowledge and skillsets to manage a wide variety of presentations across all age groups; this includes familiarity with general physical and emotional requirements of each age group as these relate to their presenting health needs.2–4 ED nurses work cooperatively with prehospital emergency personnel, doctors and other healthcare personnel and agencies in the community to provide patient care.2,3,5 Roles in the ED include triage, direct patient care, expediting patient flow, implementing medical orders, providing emotional support during crises, documenting care, and arranging for ongoing care, admission to the hospital, transfer to another healthcare facility, or discharge into the community.5,6
Triage
Central to the unique functions of an ED nurse is the role of triage; perhaps the one clinical skill that distinguishes an emergency nurse from other specialist nurses. Triage literally means ‘to sieve or sort’, and is the first step in any patient’s management on presentation to an ED.1–3
History of Triage
Triage was first described in 1797 during the Napoleonic wars by Surgeon Marshall Larrey, Napoleon’s chief medical officer,7 who introduced a system of sorting casualties that presented to the field dressing stations. His aims were military rather than medical, however, so the highest priority were given to soldiers who had minor wounds and could be returned quickly to the battle lines with minimal treatment.1,8
The documented use of triage was limited until World War I, when the term was used to describe a physical area where sorting of casualties was conducted, rather than a description of the sorting or triage process itself.8 Triage continued to develop into a formalised assessment process, with subsequent adoption for initial categorising of patient urgency and acuity within most civilian EDs.1,7,8
Development of Triage Processes in Australia and New Zealand
Australia is a world leader in the development of emergency triage and patient classification systems. In the late 1960s patients presenting to ‘casualty’ departments in Australia were not always triaged,1,2,4,5 with many EDs using random models of care; ambulance presentations were given priority and the ‘walking wounded’ seen in order of arrival. In the mid-1970s, staff at Box Hill Hospital in Melbourne developed a five-tiered system that included a time-based scale and different colours on the medical record to indicate priority.1,7,8 Subsequent modification and refinement led to the Ipswich Scale in the 1970s–80s.1,2,4,5,7 These early triage systems reinforced the concepts developed by Larrey, and established a process for patients’ presentations to be seen in order of clinical priority rather than time of attendance. In the 1990s the impact of community expectations and national health policy led to further enhancements of triage systems in Australia, and the Ipswich triage scale was adapted into the national triage scale (NTS). The NTS was subsequently tested and demonstrated to have the essential characteristics of utility, reliability and validity.1,2,4,5,7,9–11 In 1993, the NTS was adopted by the Australasian College for Emergency Medicine (ACEM) in its triage policy,7 and subsequently renamed the Australasian triage scale (ATS) as it was implemented in most EDs in Australia and New Zealand (see Table 22.1).4
| Code | Descriptor | Treatment acuity |
|---|---|---|
| 1 | Resuscitation | Immediately |
| 2 | Emergency | Within 10 minutes |
| 3 | Urgent | Within 30 minutes |
| 4 | Semiurgent | Within 1 hour |
| 5 | Non-urgent | Within 2 hours |
The ATS is now a world-leading, reliable and valid triage classification system for emergency patients, with demonstrated predictive properties for severity of illness, mortality and the need for admission.5,7,9–11 When properly applied, presenting patients should receive the same triage score no matter which ED they present to.5,9,11
The Process of Triage
All patients presenting to an ED are triaged on arrival by a suitably experienced and trained registered nurse.2,10 This assessment represents the first clinical contact and the commencement of care in the department. The ideal features of a triage area are: a well-signposted location close to the patient entrance; ability to conduct examination and primary treatment of patients in privacy; a close physical relationship with acute treatment and resuscitation areas; and appropriate resources including an examination table, thermometer, a sphygmomanometer, stethoscope, glucometer and pulse oximetry.2,4,10
As the first clinician in the ED to interview the patient, the triage nurse gathers and documents information from the patient, family and friends, or prehospital emergency personnel. Professional maturity is required to manage the stress inherent in dealing with an acutely ill patient and family members (under significant stress themselves), while rapidly making informed judgement on priorities of care for a wide range of clinical problems.10
The triage nurse receives and records information about the patient’s reason for presentation to the ED, beginning with a clear statement of the complaint in the patient’s own words, followed by historical information and related relevant details, such as time of onset, duration of symptom/s, and what aggravates or relieves the symptom/s. A brief, focused physical assessment including vital signs may be undertaken to identify the urgency and severity of the condition, and may be collected as part of the triage process to inform decision making.5,10,11 Triage assessment generally should be no longer than 2–5 minutes, balancing between speed and thoroughness.11 From the information collected, the triage nurse determines the need for immediate or delayed care,1–3 and assigns the patient a 1–5 ATS category in response to the statement: This patient should wait for medical assessment and treatment no longer than ….11
Patients with acute conditions that threaten life or limb receive the highest priority while those with minor illness or injury are assigned a lower priority. It may not be possible to categorise the patient correctly in all instances, but it is better to allocate priority on a conservative basis and err on the side of a potentially more serious problem.3,8,10,11 Importantly, a triage allocation is dynamic and can be altered at any time.5–8 If a patient’s condition changes while waiting medical assessment/treatment, or if additional relevant information becomes available that impacts on the patient’s urgency the patient should be re-triaged to a category that reflects the determined urgency.11,12 Frequent, ongoing observation and assessment of patients is therefore routine practice following the initial triage assessment.
The premise for a triage decision is that utilisation of valuable healthcare resources provide the greatest benefit for the neediest, and that persons in need of urgent attention always receive that care.1,2,4,5,11,12 Triage encompasses the entire body of emergency nursing practice, and nurses complete a comprehensive triage education program prior to commencing this role. A formal national triage training resource has been developed that provides the essential education components to promote consistency in application of the ATS.11,12
Triage Categories
After triage assessment is undertaken on arrival, patients are allocated one of five triage categories using the Australasian triage scale (ATS) (see Table 22.2). Prompt assessment of airway, breathing, circulation and disability remains the cornerstone of patient assessment in any clinical context, including triage.
TABLE 22.2 Australasian triage scale (ATS) category characteristics5,11
| ATS code | Typical description |
|---|---|
| 1 | Immediately Life-Threatening (or imminent risk of deterioration) Patients are critically ill, and require immediate transfer to a resuscitation area for initial resuscitation, with no delay at triage.3,5,11,12 The majority will arrive by ambulance, and will be suffering: |
Patients ‘at high risk’ of critical deterioration or have very severe pain from any cause. Assessment and treatment needs to commence within 10 minutes for:3,5,11,12
• chest pain or other symptoms suggestive of myocardial ischaemia, pulmonary embolism or aortic dissection
• important time-critical treatment (e.g. thrombolysis, antidote)
• severe abdominal pain or other symptoms suggestive of ruptured aortic aneurysm
• severe dyspnoea from any cause
• altered levels of consciousness
• acute hemiparesis / dysphasia
• fever, rash, headache, suggestive of sepsis or meningitis
• severe skeletal trauma such as femoral fractures or limb dislocations
• very severe pain from any cause (practice mandates the relief of pain or distress within 10 minutes)
Patients have significant illness or injury and should have assessment and treatment commenced within 30 minutes of presentation. Typical patients include those with:3,5,11,12
• moderately severe pain from any cause (e.g. abdominal pain, acute headache, renal colic), but not suggestive of critical illness; practice mandates relief of severe discomfort or distress within 30 minutes
• symptoms of significant infections (e.g. lung, renal)
• moderate injury (e.g. Colles’ fracture, severe laceration without active haemorrhage)
The patient’s condition may deteriorate, or adverse outcome may result, if assessment and treatment is not commenced within 1 hour of arrival. Patients have moderate symptoms, symptoms of prolonged duration, or acute symptoms of low-risk preexisting conditions, including:3,5,11,12
• minor acute trauma (e.g. sprained ankle)
• minor head injury, no loss of consciousness
• earache or other mildly painful conditions
• practice mandates relief of discomfort or distress within one hour
• there is a potential for adverse outcome if time-critical treatment is not commenced within one hour
• likely to require complex work-up and consultation and/or inpatient management.
The patient’s condition is minor or chronic; acute symptoms of minor illness, symptoms of chronic disease or with a duration of greater than 1 week. Symptoms or clinical outcome will not be significantly affected if assessment and treatment are delayed up to 2 hours from arrival. Examples include:3,5,11,12
Triage Assessment
Patient assessment at triage has three major components: quick, systematic and dynamic. Speed of assessment is required in life-threatening situations, with the focus on airway, breathing, circulation and disability (A,B,C,D), and a quick decision on what level of intervention is required. A systematic approach to assessment is used for all patients in all circumstances, to ensure reproducibility. Finally, the triage assessment must be dynamic, in that several aspects can be undertaken at once, and acknowledging that a patient’s condition can change rapidly after initial assessment. Various assessment models are available, but fundamentally they all include components of observation, history-taking, primary survey and secondary survey.1–3,4,6,11,12
Patient History/interview
The triage interview provides the basis for data gathering and clinical decision making regarding patient acuity. After an introduction, the triage nurse asks person-specific open-ended questions. Use of close-ended questions or summative statements enables clarification and confirmation of information received, and to check understanding by the patient.12 Privacy is important to ensure that the patient is comfortable in answering questions of a personal nature. Most EDs need to balance providing an area that is private and accessible, yet safe for staff to work in relative isolation.
A large component of the triage assessment may be based on subjective data, which are then compared and combined with the objective data obtained through the senses of smell, sight, hearing and touch to determine a triage category: pulse, blood pressure, respiratory rate and characteristics, oxygen saturation, capillary return, temperature, blood glucose level. One aspect of the history that is difficult to quantify is intuition. This is that ‘sixth sense, or gut feeling’ that tells us that something not yet detectable is wrong with the patient. This unexplained sense is difficult to outline or apply scientific research models to, but it has an important role to play in patient assessment and should be acknowledged when something ‘doesn’t feel right’.6,11,12
Primary Survey
While taking a patient history, the triage nurse also simultaneously conducts a primary survey. As noted earlier, airway, breathing, circulation and neurological function (deficit) is observed. If any major problem is observed, the interview is ceased and the patient is transferred immediately to the acute treatment or resuscitation area.8
Secondary Survey and Physical Examination
A secondary survey, involving a concise, systematic physical examination, is conducted after the patient history and primary survey have been completed. The equipment used includes a thermometer, stethoscope, oxygen saturation monitor and sphygmomanometer, in combination with clinical skills. This examination is not comprehensive but focuses on the presenting complaint while avoiding tunnel vision and wrong conclusions.3,12 Remember that the patient may not be able to lie down or be exposed for an examination in the triage area, and may be distressed. The triage process should reflect a system of rapid assessment that is reproducible and adaptable to a variety of presentations.
Approaches to Triage Assessment
A range of approaches to nursing assessment is applicable to triage assessments (see Table 22.3).8 Body systems approach enables systematic examination of each body system to discover abnormalities (i.e. central nervous system, cardiovascular system, respiratory system, gastrointestinal system, etc.).6,11,12 See also the relevant ‘system-based’ chapters.
| Mnemonic | Components |
|---|---|
| SOAPIE |
Triage Assessment of Specific Patient Groups
Mental Health Presentations
Patients with psychiatric problems presenting to an ED should be triaged, assessed and treated as for other presenting patients, with particular attention to appropriate initial medical assessment and management.6,11,12 Resources outlining specific mental health triage category descriptors are readily available, and relate specific aspects of mental health presentation with clinical urgency and triage categories (see Table 22.4), including an outline of suggested responses, such as patient placement requirements based on the level of risk and urgency.6,11–14
| ATS | Observation | Action |
|---|---|---|
| 1. Immediate | Severe behavioural disorder with immediate threat of dangerous violence to self or others |
Paediatric Presentations
Children presenting to the ED are assessed and assigned a triage category as for adults, although vital differences in paediatric anatomy, physiology and clinical presentations should be considered (see Chapter 25). The reliance of information from parents or primary carers and their capacity to identify deviations from normal is important, particularly in supporting recognition of often subtle indicators of serious illness in infants and young children. Paediatric triage resources are available to assist in identifying physiological alterations and applying the ATS based upon identified physiological discriminators.12 Other important points to consider include:
• children may suffer rapid decompensation due to limited physiological reserves; a short time is a long time in the life of a child, and may develop serious illness in a much shorter time than for an adult.11,12,15
• children are less able to tolerate pain in either physical or psychological terms.11,12,15
• it is difficult to rationalise long waiting periods with a child or parent of a sick child. The longer they wait, the more difficult an examination becomes.11,12,15
• parents are much less tolerant of waiting times for their sick child than they would be for themselves.11,12,15
Extended Roles
Contemporary roles in many EDs have expanded to include clinical roles and functions that have emerged as a result of reengineering work practice processes in response to an increasing number of emergency presentations, and to improve performance in patient flow, waiting times, length of stay and patient satisfaction.16–18 This expanded scope of practice includes advanced clinical skills performed using agreed protocols and accreditation supported by additional education and regular periods of performance review. The role has become known as an advanced clinical nurse (ACN) or advanced practice nurse (APN),17 involving, but not limited to, the following advanced clinical skills:16,18
• venipuncture and cannulation
• administration of nurse-initiated narcotic analgesia and other medications.
Nurse-Initiated X-Rays
Nurse-initiated radiology ordering enables investigations of extremities, joints such as hips and shoulders, the chest and abdomen according to clinical protocols that list inclusion and exclusion criteria19 based on findings from the ACN’s history-taking and clinical examination. The inclusion criteria reflect well-established clinical indicators. While nurse-initiated radiology ordering is often undertaken as an extended triage nurse function, it can be performed by any accredited nurse. The use of nurse-initiated radiology, especially in association with extremity injuries, is safe and accurate, reducing both waiting time and department transit time and improving both patient and staff satisfaction.17,19–21
Nurse-Initiated Analgesia
Although pain is a common complaint in the majority of patients presenting to the ED,22,23 management has previously been insufficient, especially in relation to the timeliness, adequacy and appropriateness of analgesia administered,22,23 and resulting in poor patient satisfaction.22 To address these findings, many EDs developed nurse-initiated analgesia protocols, standing orders or pathways. Nurse-initiated analgesia protocols enable designated emergency nurses to implement analgesia regimens prior to assessment by a medical officer. These protocols are locally derived and note patient inclusion and exclusion criteria for managing mild, moderate or severe pain in both adult and paediatric patients, and often include administration of an antiemetic.22,23 A numerical pain rating scale or a visual analogue scale is used to direct the type and route of analgesia administration. Severe pain protocols outline incremental intravenous narcotic administration, including incremental and total maximum administration dosages. After administration of the initial dose, the administering nurse gives subsequent incremental doses in response to a reevaluation of the patient’s pain score and vital signs (pulse, blood pressure and respiratory rate). Protocols directed towards moderate and minor pain may include either single or incremental IV analgesia or oral analgesia. Nurse-initiated analgesia protocols have also been found to be safe and effective and to shorten the time ED patients wait for analgesia,23,24 which should assist in improving patient outcomes and satisfaction.
Clinical Initiative Nurse
The clinical initiative nurse (CIN) is a specific advanced practice role introduced to primarily provide care for waiting-room patients awaiting medical officer assessment. The role was initially introduced into levels 5 and 6 metropolitan and several large rural EDs in New South Wales, to manage and reduce ED waiting times and associated patient distress, improve ‘time seen’ rates, patient service satisfaction and patient outcomes (key performance indicators). These and similar roles are now being implemented nationally.25 The role includes initiation of treatment for lower-acuity waiting-room patients, following advanced practice protocols. The treatment provided by the CIN includes ordering of radiology and/or pathology investigations, administration of oral analgesia, review and reassessment of waiting patients (particularly those who have waited longer than their triage benchmark time), and providing information and education to waiting patients and carers regarding waiting times, ED processes and patient education. The role acts as an adjunct to the triage role, and maintains a close working relationship with the triage nurse.25–27 The CIN role has contributed to timely access to interventions, investigations and care for waiting patients, increases autonomous practice, independent decision making and enhances patient advocacy. The role also provides opportunity for the clinical and professional development of emergency nurses.26
Nurse Practitioner
Introduction of the NP has been complicated by existing nomenclature relating to advanced practice roles in nursing, with titles such as advanced specialist, clinical nurse consultant, clinical nurse specialist and advanced practice nurse used interchangeably and at times problematically in the literature,28,29 including internationally.30 Consensus is gradually emerging that the NP role is evolving and developing globally as the most significant of the advanced practice roles in modern health care.29
1. Extended practice: The scope of practice of the NP is subject to different practice privileges that are protected by legislation, and occur outside the scope of practice for a registered nurse. These extended practice privileges mean that the NP functions in a grey area that incorporates some of both medical and nursing activities.28–31
2. Autonomous practice: The NP engages in clinical practice with significant clinical autonomy and accountability, including responsibility for the complete episode of care. This autonomy means that the NP works in a multidisciplinary team in a clinical partnership role to optimise patient outcomes.28–31
3. Nursing model: Practice is firmly located in a nursing model, and an extensive, but evolving, body of literature relating to the NP role and practice.28–31
National and international experience has demonstrated a specific service that is highly regarded32–35 and in demand.36,37 The NP service provides care to many underserviced groups such as the homeless,38 women and children, the elderly,39 rural and remote communities36,40 and specialist services in acute care areas.41 Nurse practitioners are effective in managing common acute illnesses and injuries and stable chronic conditions,39 and provide an emphasis on health promotion and assessment and disease prevention.42
The Australian experience has demonstrated that pressure on EDs can be relieved when NPs manage lower priority cases. Waiting times and overall length of ED stay are significantly reduced when NPs manage triage category 3–5 presentations such as sprains and superficial wounds.37
Retrievals and Transport of Critically Ill Patients
The care of an acutely ill patient often includes transport, either within a hospital to undergo tests and procedures or between hospitals to receive a higher level of care or to access a hospital bed. The movement of critically ill patients places the patient at a higher risk of complications during the transport period,50–53 because of condition changes, inadequate available equipment to or support from other clinicians, or the physical environment in the transport vehicle. For this reason the standard of care during any transport must be equivalent to, or better than that at the referring clinical area.43,44 Safe transport of patients therefore requires adequate planning and stabilisation from a team of staff with appropriate skills and experience. This section focuses on the movement of critically ill patients by nurses, doctors and/or paramedics between hospitals.45,46
Retrievals
Australasia has a variety of retrieval or transport models, although most retrieval teams comprise doctors, nurses and paramedics with specialised training in critical care. The skills of the escort personnel need to match the acuity of the patient, so that they can respond to most clinical problems.47,48 Retrieval team staff therefore need to deliver high-level critical care equal to the standard of the receiving centre, but need to be familiar with the challenges associated with working outside the hospital environment. Standards for the transport of critically ill patients have been established by the College of Critical Care Medicine (CICM) and the Australian College of Emergency Medicine (ACEM).48
When transporting an unstable patient it is essential that a minimum of two people focusing solely on the clinical care aspects of the patient are present, in addition to other staff transporting the patient and equipment. The transport team leader is usually a medical officer with advanced training in critical care medicine, or for the transport of critical but stable patients, a registered nurse with critical care experience. The skillset includes advanced cardiac life support, arrhythmia interpretation and treatment and emergency airway management.48
Preparing a Patient for Interhospital Transport
Adequate and considered preparation of the transport of a critically ill patient from one hospital area to another should be appropriately planned and not compromised by undue haste. While strong evidence to support a ‘scoop and run’ approach to patients in the field exists, this principle does not apply to interhospital or intrahospital transport of a critically ill patient. Appropriate evaluation and stabilisation is required to ensure patient safety during transport, including assessment of ABCs and suitable IV access.48
If potential airway compromise is suspected, careful consideration should be given to an elective intubation rather than an emergency airway intervention in a moving vehicle or a radiology department. A laryngeal mask airway is not an acceptable method of airway management for critically ill patients undergoing transport, because of the associated problems of movement.48 A nasogastric or orogastric tube is inserted in all patients requiring mechanical ventilation.
Fluid resuscitation and inotropic support are initiated prior to transporting the patient. Planning for the trip needs to include adequate reserves of blood or other IV fluid for use during transport. If the patient is combative or uncooperative, the use of sedative and/or neuromuscular blocking agents and analgesia may be indicated.51–53 A syringe pump with battery power is the most appropriate method for delivering medications for sedation and pain relief. A Foley catheter is inserted for transports of extended duration and all unconscious patients.52–54
The patient’s medical records and relevant information such as laboratory and radiology findings are copied for the receiving facility, and other documentation includes initial medical evaluation, and medical officer to medical officer communication, with the names of the accepting doctors and the receiving hospital.44,47
Patient Monitoring During Transport
• equipment for airway management, sized appropriately transported with each patient (check for operation before transport)
• portable oxygen source of adequate volume to provide for the projected timeframe, with a 30-minute reserve
• a self-inflating bag and mask of appropriate size
• handheld spirometer for tidal volume measurement
• available high-pressure suction
• basic resuscitation drugs, and supplemental medications, such as sedatives and narcotic analgesics (considered in each specific case)
• a transport monitor, displaying ECG and heart rate, oxygen saturation, end-tidal CO2, and as many invasive channels as required for pressure measurements. The monitor should have a capacity for storing and reproducing patient bedside data and printouts during transport.48
Monitoring equipment should be selected for its reliable operation under transport conditions, as monitoring can be difficult during transport; the effects of motion, noise and vibration can make even simple clinical observations (e.g. chest auscultation or palpation) difficult, if not impossible.49 As transport of mechanically-ventilated patients is associated with risk,29,56,58,59 consistent ventilation and oxygenation should be a goal; transport ventilators provide more constant ventilation than manual ventilation. An appropriate transport ventilator provides full ventilatory support, monitors airway pressure with a disconnect alarm, and should have adequate battery and gas supply for the duration of transport.47
Adverse events during transport of critically ill patients fall into two categories:46,48 (1) equipment dysfunction, such as ECG lead disconnection, loss of battery power, loss of IV access, accidental extubation, occlusion of the endotracheal tube, or exhaustion of oxygen supply (at least one team member should be proficient in operating and troubleshooting all equipment); and (2) physiological deteriorations related to the critical illness.
Multiple Patient Triage/Disaster
Disaster triage is a process designed to provide the greatest benefit to multiple patients when treatment resources and facilities are limited. Disaster triage systems differ from the routine triage system used within the ED (e.g. the ATS); system care is focused on those victims who may survive with proper therapy, rather than on those who have no chance of survival, or who will live without treatment. The system was first devised during war as a method of managing large numbers of battlefield casualties. Today it is applicable for treating multiple victims of illness or injury outside and within the hospital setting. Variations exist between states and countries regarding disaster victim triage classifications. It is therefore important to be familiar with local plans and policy.11,12
Triage of mass victims may be necessary in common situations, like vehicle collisions with multiple occupants, as well as large-scale disasters, such as earthquakes, floods, public transport incidents or explosions. The principles of triage vary little, though the methods used to communicate triage information and to match victims with available resources may differ. Triage at the scene of a major incident or disaster is commenced by the first qualified person to arrive (i.e. the one with the most medical training). This person is initially responsible for performing immediate primary surveys on all victims and to determine and communicate the numbers and types of resources needed to provide initial care and transport.8
In Australia and New Zealand, disaster systems have up to five triage categories (depending on jurisdictional and local protocols). To provide the best level of care and ensure the highest number of survivors, those who are mortally injured but alive may be given a low treatment priority, though this will almost certainly ensure their death. These decisions are therefore best made by an experienced doctor. In a situation with a large number of casualties, one or more doctors should be present at the site to lead the triage effort. Further, it is not within the scope of practice of non-physician emergency personnel to pronounce a patient dead, but properly trained ambulance or rescue personnel can recognise the signs of death for the purposes of triage until doctors can formally declare death.50,51
Emergency Department Response to an External Disaster: Receiving Patients
Disasters may produce mass victims on a scale that means routine processes and practices in the ED and hospital will be overwhelmed. The ED response to an external disaster forms part of the overall hospital response, outlined in a hospital disaster plan. These plans are reviewed regularly for currency, and practised for preparedness. The following aspects form part of the ED’s planning and response to receiving patients from an external disaster.51,52
Department Preparation
If the disaster site is close to the hospital, a significant number of disaster victims will self-evacuate from the site and arrive at the hospital without any prehospital triage, treatment or decontamination before any formal notification has been received. In this instance the ED will need to declare the incident and commence the notification process required.48 The ED may be quickly overwhelmed with arriving patients; the closest local medical facility may receive up to 50–80% of the disaster victims within 90 minutes of the incident.52 On notification of a disaster response a number of key positions should be allocated (medical coordinator, nursing coordinator, triage nurse, medical triage officer). These personnel are senior staff with specific disaster training and knowledge of the hospital’s disaster plan.48,51 Nursing and medical coordinators are responsible for allocating staff to specific duties; all designated roles are outlined on action cards available for staff to read prior to commencing their roles.52
The capacity of the ED to accommodate a large influx of patients needs to be maximised. Patients currently in the department are reviewed for a decision to admit. Patients requiring admission are transferred out of the Department to a suitable location in the hospital. Patients suitable for discharge or referral to their local medical officer, including patients with minor complaints currently waiting, should be discharged or referred to community resources. A small number of patients may need to remain in the ED, and their care will need to be prioritised in conjunction with arriving disaster victims.50–52
Areas of the department are designated to accommodate the expected severity of the victims (e.g. resuscitation room for priority 1 patients, observation areas for priority 2). Walking wounded casualties with relatively minor injuries and who are unlikely to require admission to hospital are best accommodated in a treatment area outside the ED, as this cuts congestion and increases the capacity for more significantly-injured victims to be managed.51
Additional staff members are notified from the current staff lists to participate in the disaster management. Staff members are allocated to teams to manage designated bed spaces within designated treatment areas. Additional staff from outside the ED may be deployed to assist; these staff should be teamed with routine ED staff, because of the latter’s familiarity with the layout and location of equipment and other resources. It is important to recognise the need to replace staff to avoid fatigue, especially in incidents of a protracted nature. Therefore, not all staff should be called in initially. Where possible, staff that work together on a daily basis should work in teams during the disaster period.51,52
Triage and Reception
Routine, day-to-day triage and reception processes will be ineffective when receiving large numbers of disaster victims. A registration process for disaster victims generally involves collecting minimal personal information from the patients, where possible, and the allocation of a prepared disaster hospital number used for identification and ordering investigations.8 Triage assessments will often be undertaken by both a medical officer and a nurse, and the process will be brief and focused. Most victims will have been allocated a triage tag in the field, but are reevaluated for any changes, as their condition may have deteriorated. Triage assessment is based on observations of the nature and extent of the victims’ injuries. Patients present in the ED prior to disaster notification are considered part of the disaster event and triaged in the same manner.4,7,8,52
Treatment
Treatment provided during a disaster will not reflect routine practices; priorities focus on resuscitation, identification of serious injuries, identification of patients requiring urgent surgery and stabilisation of patients for transfer out of the ED. The best overall outcome during a disaster are achieved when the routine principles of resuscitation and management are adapted to reflect the resources available.51,52
Respiratory Presentations
Patients with respiratory dysfunctions are a common presentation to the ED and are seen across all age groups. Respiratory symptoms can be associated with a broad range of underlying pathologies. This section will discuss the initial assessment and treatment of several common respiratory diseases seen in the ED. Chapter 14 provides more detailed information regarding respiratory diseases.
Presenting Symptoms and Incidence
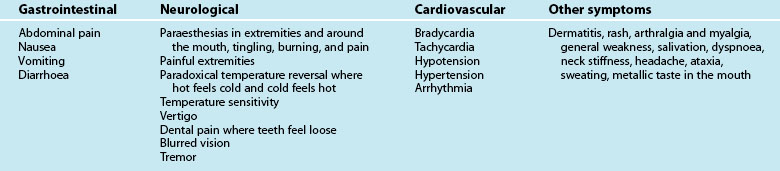
Patients presenting with respiratory complaints can display a range of symptoms (see Box 22.1), and these may vary based on the patient’s age, the underlying cause of the symptoms and severity.
Box 22.1
Signs and symptoms commonly associated with respiratory presentations
• Dyspnoea (painful or difficulty breathing)
• Alteration in respiratory rate: tachypnoea/bradypnoea
• Alterations in respiratory depth or pattern
• Intercostal and/or subcostal recession
• Inability to speak in full sentences
• Stridor (upper airway respiratory disorders)
Shortness of breath (SOB) or dyspnoea is a frequent complaint for patients presenting to the ED. Respiratory presentations are not isolated to any one specific patient population or age group and are encountered in patients across the lifespan. While dyspnoea is commonly associated with respiratory conditions such as asthma, pneumonia, chronic obstructive pulmonary disease (COPD) and cardiac conditions, it has multiple aetiologies and related to disease in almost any organ system. A complaint of SOB is a significant symptom and is commonly associated with the need for hospital admission.53–55
Assessment, Monitoring and Diagnostics
On arrival, patients with respiratory complaints are assessed quickly using the ABC approach to determine any potential life-threatening disturbance that requires immediate medical assessment and/or resuscitative intervention. Initial assessment includes a thorough history focused on the presenting complaints. A detailed history often identifies the underlying process; however a high index of suspicion should be maintained for other potential causes during initial assessment.53,54 History focuses on the nature of symptoms, the timing of onset of symptoms, associated features, the possibility of trauma or aspiration and past medical history (particularly the presence of chronic respiratory conditions). During physical examination, the patient assumes a position of comfort while inspection of the chest is undertaken, followed by auscultation, palpation and percussion (see Chapter 13 for more detail).
Patients with significant respiratory symptoms are best managed in an acute monitored bed or resuscitation area of the department. An initial set of observations including heart rate, respiratory rate, blood pressure, temperature and oxygen saturation is supported by continuous monitoring heart rate and oxygen saturation. Pulse oximetry plays an important role in the monitoring of the patient with a respiratory complaint, as recognition of hypoxaemia is significantly improved when it is used.56
IV access enables collection of venous blood samples for full blood count (FBC) and urea, electrolytes, creatinine (UEC) where clinically indicated. A chest X-ray (CXR) is ordered in most instances, and interpreted in relation to the clinical history and other examination findings.55 Spirometry or peak flow measurements enable assessment of peak expiratory flow rate (PEFR), forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1), to determine the nature and severity of the underlying respiratory condition. These tests are however effort- and technique-dependent and may not be able to be performed by a patient who is acutely SOB.55 An arterial blood gas (ABG) is often indicated in patients with a significant respiratory presentation, and provides information on oxygenation, ventilation and acid–base status.55
Oxygen therapy is commenced early for a patient presenting with signs of acute respiratory compromise, including those with chronic obstructive pulmonary disease (COPD); importantly, patients with acute hypoxia require oxygen. Any potential detrimental effects are uncommon, and concentration and time dependent with a slow onset; this allows for monitoring (pulse oximetry, ABG analysis) and clinical review.55,57
Candidate Diagnoses and Management
Asthma
Asthma is a very common patient presentation to Australasian EDs. Over 2.2 million Australians have asthma, with 16% of children and 12% of adults affected by the condition.58–61 Asthma is a chronic inflammatory disease of the airways with many cells and cellular elements playing a role (mast cells, eosinophils, T lymphocytes, macrophages, neutrophils and epithelial cells). Inflammatory changes cause recurrent episodes of wheezing, breathlessness, chest tightness and coughing associated with widespread reversible airflow obstruction of the airways. This airflow obstruction or excessive narrowing results from smooth muscle contraction and swelling of the airway wall due to smooth muscle hypertrophy, inflammatory changes, oedema, goblet cell and mucous gland hyperplasia and mucus hypersecretion.61
Normally, airways widen during inspiration and narrow in expiration. In asthma, the above responses combine to severely narrow or close the lumen of the bronchial passages during expiration, with altered ventilation and air trapping.58–60 The causes of asthma are related to many factors, including allergy,58 infection (increased reaction to bronchoconstrictors such as histamine),58,59 irritants (e.g. noxious gases, fumes, dusts, dust mites, powders), or heredity (although the exact role or importance of any hereditary tendency is difficult to assess).59
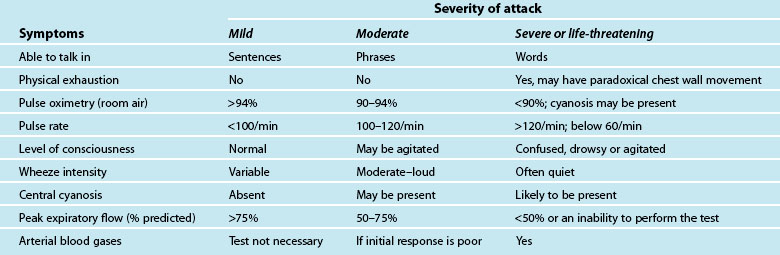
A patient usually has a history of previous asthma attacks. Often, an acute episode follows a period of exercise or exposure to a noxious substance, or a known allergen.58,60 The onset of the asthma may be characterised by vague sensations in the neck or pharynx, tightness in the chest with breathlessness, loose but non-productive cough with difficulty in raising sputum, difficulty breathing, particularly on expiration, with increasing severity as the episode continues; apprehension and tachypnoea may follow as the patient becomes hypoxic, with audible wheezing.58,60 The characteristics and initial assessment of acute mild, moderate and severe/life threatening asthma in adults and associated clinical management guidelines are outlined in Table 22.5.61,62
Be alert to the high-risk patient whose ability to ventilate is impaired: this is a life-threatening condition. These patients will exhibit an inability to talk, central cyanosis, tachycardia, use of respiratory accessory muscles, a silent chest on auscultation, and a history of previous intubation for asthma.55,57–59 See Chapters 14 and 15 for ongoing management.
Acute Respiratory Failure
Acute respiratory failure occurs when the lungs provide insufficient gas exchange to meet the body’s need for O2 consumption, CO2 elimination, or both. Acute respiratory failure results from a number of causes63 (see Chapter 14). When alveolar ventilation decreases, arterial O2 tension falls and CO2 rises. This rise in arterial CO2 produces increased serum carbonic acid and pH falls, resulting in respiratory acidosis.63 If uncorrected, low arterial O2 combines with low cardiac output to produce diminished tissue perfusion and tissue hypoxia. Anaerobic metabolism results, increasing lactic acid and worsening the acidosis caused by CO2 retention. Other symptoms develop involving the central nervous and cardiovascular systems.59,60,63 ABGs confirm the diagnosis, with hypercarbia (PaCO2 >45 mmHg and hypoxaemia (PaO2 <80 mmHg), and a low pH evident. A CXR identifies the specific lung disease.63
Clinical management focuses on correction of hypercapnia, treatment of hypoxaemia, correction of acidosis, and identification and correction of the specific cause63 (see Chapter 14). For a spontaneously breathing patient, administer oxygen by ventilation mask (24%) or nasal cannula. Adjust oxygen therapy according to ABG findings at 15–20-minute intervals to achieve a PaO2 of 85–90 mmHg. For a patient with inadequate respiratory effort, non-invasive ventilation may be instituted. In an apnoeic situation, initiate ventilatory assistance with bag–mask ventilation prior to endotracheal intubation, then commence mechanical ventilation (see Chapter 15).
Pneumonia
Pneumonia is an acute inflammation of lung tissue caused by a variety of viral and bacterial organisms, fungi and parasites.64–66 Pneumonia can occur in previously healthy patients, but more often it is associated with conditions that impair the body’s defence mechanisms.64,66 Predominant symptoms are a combination of cough, chest pain (usually pleuritic), dyspnoea, fever (with or without chills), and mucoid, purulent or bloody sputum, with an abrupt or gradual onset.64 Physical examination demonstrates tachypnoea, fever, tachycardia, possible cyanosis, diminished respiratory excursion due to pleuritic pain, end-respiratory crackles or rales on auscultation with bronchial breathing over areas of consolidation64,66 (see Chapter 13).
A CXR may reveal varying infiltrates: interstitial, segmental or lobar; or may initially be clear until later in the illness and following adequate rehydration.55 Venous blood samples will identify a raised white cell count and/or leucocytosis. Blood cultures and sputum cultures assist in identifying the causative organism. ABGs usually identify the degree of impaired gas exchange;57 hypoxaemia and hypocarbia may be present.66
Initial treatment involves administration of oxygen therapy via face-mask, evaluated frequently in response to ABG results and pulse oximetry.57 Treatment will also require IV fluid therapy to ensure adequate hydration, and administration of antibiotics orally or parentally in accordance with antibiotic guidelines. Ventilatory support may be required in some cases; in spontaneously breathing patients non invasive ventilation (NIV) via a face mask should be used before invasive ventilation. Mechanical ventilation is not normally required unless there is underlying cardiopulmonary disease.57,64,66
Chest Pain Presentations
Description of Presenting Symptoms and Incidence
The incidents of acute chest pain presentations appear to be increasing as patients are more aware of the importance of early treatment for myocardial infarction due to public awareness campaigns.67 Up to 7% of all ED presentations are for complaints of chest pain.68 The pain or discomfort is often described in variety of ways; as pressure, a weight on the chest, tightness, constriction about the throat, or an aching feeling. The pain may also be described in less typical terms such as epigastric pain, indigestion, stabbing pain, pleuritic or sharp.69,70 Onset is usually gradual, reaching a peak over 2–3 minutes and last for several minutes or longer.68,69 Pain may be mild to severe, and can be associated with physical exertion or emotional stress and may subside with rest, or be unprovoked and may wake the patient from sleeping. Pain may also radiate to an arm, to both arms, to the neck, jaw or back.67,69,70 A patient may have a number of associated symptoms including: shortness of breath, nausea, vomiting, weakness, dizziness, anxiety, feeling of impending doom, palpitations and diaphoresis.69,71 Up to 9% of patients diagnosed with an acute coronary syndrome (ACS) may present with a number of these associated symptoms but without chest pain; these patients tend to be elderly, female or diabetic.69,70,72
Assessment, Monitoring and Diagnostics
Any patient presenting with a complaint of chest pain requires urgent assessment (within 10 minutes of arrival to the ED). A patient with evidence of a disturbance to airway, breathing or circulation requires close monitoring, immediate medical assessment and resuscitative interventions. Initial assessment includes a 12-lead ECG and evaluation of the pain using the PQRST mnemonic shown in Table 22.3. The ECG should be rapidly evaluated for presence of ST segment elevation or a new left bundle branch block (LBBB) suggestive of an acute myocardial infarction (AMI), as treatment for AMI is time critical. If the initial ECG is nondiagnostic and symptoms persist, continue repeat ECGs at 15 minute intervals.68
Continuous cardiac monitoring is commenced to identify any life-threatening arrhythmias, along with supplemental oxygen to improve PaO2 and increase oxygen availability especially in the presence of myocardial ischaemia. An IV cannula is inserted and routine venous blood samples are collected for cardiac enzymes: troponin T or troponin I. A physical examination may identify non-cardiac causes of the pain or complications associated with cardiac related conditions;69,73 a number of significant abdominal complaints may present with chest pain as a feature.68,73 A CXR may also identify any potential causes for the patient’s pain.
Candidate Diagnoses and Management
Common cardiovascular diagnoses presenting to the ED include ACS and thoracic aneurysm.
Acute Coronary Syndrome
Chest pain of cardiac origin results from reduced or obstructed coronary blood flow, commonly by atherosclerosis, but also coronary artery spasm or an embolism.73–75 Acute coronary syndrome (ACS) collectively describes unstable angina and acute myocardial infarction (AMI). Angina (stable or unstable) is pain but no damage to myocardial cells. A time-critical obstruction results in death or necrosis of a segment of myocardial cell resulting in an acute myocardial infarction (AMI).
Coronary heart disease is the largest single cause of death and the most common cause of sudden death in Australia and New Zealand.76 It is the leading cause of premature death and disability in both countries, although death rates have fallen since the 1960s. Over half of all coronary heart disease deaths were from AMI.76 ACS is the most common life-threatening condition seen in the ED and therefore represents an important area of clinical practice.58,73,77 Chapters 9 and 10 provide additional information about presentations of cardiac dysfunction, including the pathophysiology, clinical manifestations and treatment.
Initial management focuses on rapid identification of patients with AMI and their suitability for reperfusion therapy. Reperfusion therapy involves either thrombolysis or percutaneous coronary intervention (PCI) (angioplasty ± stent). PCI is usually only available to patients in larger centres with cardiac catheter facilities. Management in the ED includes oxygen therapy, administration of aspirin 300 mg (if not already administered by prehospital personnel) and pain relief (commonly IV morphine in small incremental doses, and nitrates initially sublingual route). If pain persists despite IV morphine, IV nitrates may be indicated.73 Patient and family reassurance, information and emotional support is required to allay anxiety and further stress.
Patients without initial evidence of AMI are stratified into high-, intermediate- and low-risk groups based on the significance and duration of pain, ECG findings, past history, cardiovascular disease risk factors and cardiac enzyme results. Specific treatment is guided by the associated risk pathway78 (see Chapter 10).
Thoracic Aortic Dissection
A tear in the intimal layer of the aortic wall results in a thoracic aortic dissection (TAD): blood passes through the tear; separates the intima from the vessel media or adventitia resulting in a false channel; and shear forces lead to dissection as blood flows through the false channel.69 Identification of this life threatening condition is important as patients often require immediate surgery; TAD is most common in men aged 50–70 years with a history of hypertension, while other risk factors include Marfan’s disease, other connective tissue disorders, cocaine or ecstasy use, pregnancy and aortic valve replacement.69,72 TAD presents with acute and sudden onset of severe pain (often described as sharp, tearing or ripping in nature)69,72 which is maximal at symptom onset. Pain is usually located in the midline, may be present in the back but rarely radiates. Pulse deficits or blood pressure differences (>20 mmHg) between the arms may be evident. CXR will be abnormal in 80–90% of cases; a widened mediastinum is present in 50% of cases.69,79 Diagnosis is confirmed by contrast CT. Management is aimed at aggressive control of blood pressure and pulse with sodium nitroprusside and beta blockers, relief of pain with narcotic analgesia and referral and/or transport to cardiothoracic services for definitive surgical intervention.79
Abdominal Symptom Presentations
Acute abdominal pain is a common complaint, accounting for 5–8% of all presentations to the ED.80,81A specific cause for the presenting abdominal pain will not be found in 30–40% of patients of all ages;80 for children a diagnosis of non-specific abdominal pain accounts for up to 60% of cases.82 About 20% of adult patients presenting will require surgical intervention and/or hospital admission.82,83
Common causes in the elderly include biliary tract disease (25%), diverticular disease (10%), bowel obstruction (10%) or malignancy (13%).84 Elderly patients are more likely to have catastrophic illnesses rarely seen in younger patients, including mesenteric ischaemia, leaking or ruptured abdominal aortic aneurysm and myocardial infarction.80,81,83 Up to a third require surgical intervention,82 while 15% will not have a cause for their abdominal pain found.84 Presentations by elderly patients are often complicated by a delay in seeking medical attention, atypical presentations, associated medical conditions, medications and cognitive function.
Assessment, Monitoring and Diagnostics
Patients presenting with abdominal pain are assessed quickly for any disturbance to airway, breathing or circulation requiring close monitoring, immediate medical assessment and/or resuscitative interventions. Abnormal vital signs are suggestive of clinically significant abdominal pain.83 A thorough history includes location and timing of onset, quantity, quality and radiation of pain, associated symptoms, previous history and general state of health. A complaint-specific history and physical examination is performed for a differential diagnosis.80,81,83 Physical assessment includes visual inspection of the abdomen with the patient in a supine position, followed by auscultation, then gentle palpation and percussion of all four quadrants of the abdomen, working towards the area of reported pain85 (see Chapter 19 for more details). While location of the pain is important, it can be misleading, as various pathological processes can localise to different areas of the abdomen (see Figure 22.1).86 An ECG is considered to rule out myocardial ischaemia or infarction, as some cardiac patients may present with upper abdominal pain as the predominant symptom (see previous section). Myocardial ischaemia may also be caused by the physiological stress of the intra-abdominal pathology.80
Administration of a narcotic analgesia in acute abdominal pain does not hinder assessment or obscure abdominal findings, nor cause increased morbidity or mortality, and may allow for a better abdominal examination.87 Incremental doses of a narcotic minimise pain but not palpation tenderness. Analgesics enable relaxation of the patient’s abdominal muscles and decrease anxiety, potentially improving information obtained from the physical examination.87
Venous blood samples are collected for full blood count (FBC), urea, electrolytes, creatinine (UEC), and amylase and lipase.85A dipstick urinalysis can suggest specific disease (e.g. leucocytes and/or blood with urinary tract infection; haematuria with renal colic), within the context of other clinical findings and formal microscopy.85 Women of child-bearing age with abdominal pain provide the challenge of a broader range of potential causative pathologies, although history and physical examination are unreliable in determining pregnancy.85 If pregnancy or a pregnancy-related disorder is possible, a urine beta-human chorionic gonadotrophin (hCG) test is performed. Test sensitivity is extremely high; a positive finding occurs within a few days of conception, and accuracy is comparable to blood sampling. An ectopic pregnancy may be missed if pregnancy is not considered; an ectopic pregnancy is extremely unlikely if the hCG result is negative.85
Candidate Diagnoses and Management
Abdominal Aortic Aneurysm
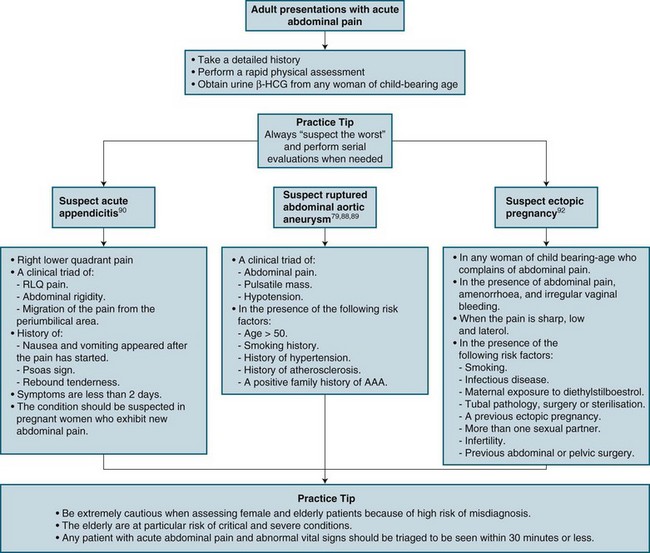
Abdominal aortic aneurysm (AAA) is a common cause of death in all patients over the age of 65 years and is responsible for 0.8% of all deaths.82,88,89 The traditional presentation is acute pain in the back, flank, or abdomen, with hypotension and a palpable abdominal mass in the older patient.88 Missed diagnoses primarily occur because physical examination is frequently unreliable.88 Many patients with dissecting AAA are misdiagnosed with renal colic, because of haematuria present, no palpable pulsatile mass and flank pain.88,89 Other common misdiagnoses include diverticulitis, gastrointestinal haemorrhage, acute myocardial infarction and musculoskeletal back pain.88 Abdominal aortic aneurysms are surgically repaired more than any other type of aneurysm. A ruptured AAA is fatal unless a patient receives immediate resuscitation and surgical intervention.88,89
Appendicitis
Appendicitis is the most common acute abdominal pain presentation that requires a surgical intervention. Diagnosis is based on clinical assessment as there is no specific test available to confirm diagnosis.90 Appendicitis can mimic almost all acute abdominal pain presentations, and is frequently misdiagnosed as gastroenteritis during the initial ED visit, or pelvic inflammatory disease or urinary tract infection.85 Whilst a well-studied disease, appendicitis continues to be a difficult ED diagnosis because of varied presentations. Women of childbearing age with appendicitis are commonly misdiagnosed due to anatomical changes due to their pregnancy. Treatment includes management of pain related symptoms and provision of intravenous hydration.90 Definitive treatment is surgical removal of the appendix.90
Bowel Obstruction
A bowel obstruction commonly results from impaired peristaltic movement, hernias, adhesions and neoplasms.91 Presentation includes poorly-localised colicky pain that increases in intensity and location, with subsequent abdominal swelling and vomiting of faecal fluid.91 Management includes both conservative options (management of symptoms, placement of a naso-gastric tube and replacement of intravenous fluids) and surgical therapy for neoplasms or hernias.91
Ectopic Pregnancy
An ectopic pregnancy is implantation outside the uterus; most commonly in the fallopian tubes. Ectopic pregnancies occur at a rate of about 11 : 1000 diagnosed pregnancies.92 Management is guided by the patient’s haemodynamic state: stable patients with no tubular ectopic may be managed with observation and drugs such as methotrexate; haemodynamically-unstable patients will require resuscitation and surgical intervention.92
Acute Stroke
Cerebrovascular disease is very prevalent in developed countries; the third-largest cause of death in Australia93 accounting for about 40,000 strokes (acute cerebrovascular accident [CVA]), with 73% of these initial strokes. The two general stroke classifications are:
• Ischaemic: are precipitated by disrupted blood flow to an area of the brain as a result of arterial occlusion. Acute ischaemic stroke presentations are now referred to as a ‘brain attack’, to promote early presentation for access to time-critical treatments,94,95 and because the pathophysiology and current treatment of acute (ischaemic) stroke mimics that of acute myocardial infarction (‘heart attack’). From an ED perspective, serious long-term disability can be minimised if ischaemic stroke is recognised and treated promptly; that is, within 3 hours of symptom onset.96,97
• Haemorrhagic strokes are caused by rupture of a blood vessel, which produces bleeding into the brain parenchyma. (Chapter 17 details the pathophysiological processes).
For patients diagnosed with a stroke, 30% will die in the first year after their stroke, most (15–20%) within the first 30 days. Of the 70% who survive, 35% will remain permanently disabled 1 year after a stroke, 10% of whom require care in a nursing home or other long-term facility.98,99
Assessment, Monitoring and Diagnostics
Symptoms of stroke are a common patient presentation to the ED; presenting signs vary from profound alterations in level of consciousness and limb hemiplegia to mild symptoms affecting speech, cognition or coordination. Symptoms may include confusion, dizziness, ataxia, visual disturbances, dysphasia or receptive and expressive aphasia, dysphagia, weakness, numbness or tingling of the face, arm or leg (usually unilateral).97,98,100 As many disorders resemble a stroke presentation, emergency clinicians must quickly determine if another condition is responsible for the patient’s neurological deficits (e.g. post-ictal phase following seizures, migraine with neurological deficits, hypoglycaemia or hyperglycaemia, systemic infections, brain tumours, hyponatraemia, hepatic encephalopathy).93,97
The focus of initial assessment is A, B, C, D (see Chapter 24). Of note, for airway assessment, stroke symptoms include altered muscle function, affecting swallowing and speech functions. A patient with a GCS score of 9 or less may require intubation to protect and secure the airway.99,101 The patient’s breathing pattern should be assessed and continually monitored. Hypertension is common, with the increase improving any cerebral ischaemia so this should not be lowered unless dangerously high or contraindicated.94 Hypotension or dehydration decreases cerebral blood flow and perfusion and should be corrected, although fluid replacement is instituted with caution.100 Vital signs are documented every 15 minutes during drug therapy to identify changes suggestive of internal bleeding. Maintaining blood pressure less than 185/110 mmHg during fibrinolytic infusion decreases the risk of intracerebral haemorrhage.95
A thorough assessment of neurological disability should be undertaken, including a GCS (see Chapter 16). An ECG is recorded to detect any abnormal rhythm such as atrial fibrillation (AF), which may be associated with stroke presentation.99 IV access is obtained to administer medications, and collect blood for electrolytes, haematology and coagulation studies. A blood sugar level test will rule out hypoglycaemia or hyperglycaemia as a cause of the presenting symptoms. Abnormal glucose levels adversely affect cerebral metabolism.99,94 After obvious alternative diagnoses are excluded, a brain CT scan determines whether a stroke is haemorrhagic or ischaemic in origin. While a new-onset ischaemic stroke may not be evident for up to 24 hours, blood in the cranial cavity will be apparent immediately. Patients with any sign of haemorrhage are excluded for fibrinolytic therapy.95
Management
Acute ischaemic stroke (‘brain attack’) management includes timely administration of a fibrinolytic agent in appropriately selected patients (see Box 22.2), which facilitates reperfusion, minimises tissue damage and reduces long-term stroke sequelae. Longer times between symptom onset and fibrinolytic infusion are associated with higher rates of morbidity and mortality.94,98,99,102 Early presentation is therefore essential for appropriate assessments and investigations (including CT scanning) and thrombolytic administration to fall within the narrow treatment window. This has seen the emergence of acute stroke units, with specialised teams dedicated to the rapid assessment and management of presentations (see Chapter 17).
Box 22.2
Criteria for administering fibrinolytic therapy in ischaemic stroke101
Inclusion criteria (all must be positive):
Exclusion criteria (all must be negative):
• Evidence of intracranial haemorrhage on non-contrast head CT
• Only minor or rapidly improving stroke symptoms
• High suspicion of subarachnoid haemorrhage, even with normal CT
• Known bleeding condition, including but not limited to platelets <100,000/mm3
• Patient received heparin within 48 hr and had an elevated aPTT
• Current use of oral anticoagulants (e.g. warfarin)
• Recent use of anticoagulant and elevated PT (>15 sec) or INR
• Intracranial surgery or serious head trauma, or previous stroke within 3 months
• Major surgery or serious trauma within 14 days
• History of intracranial haemorrhage, arteriovenous malformation, or aneurysm
• Witnessed seizure at stroke onset
Overdose and Poisoning
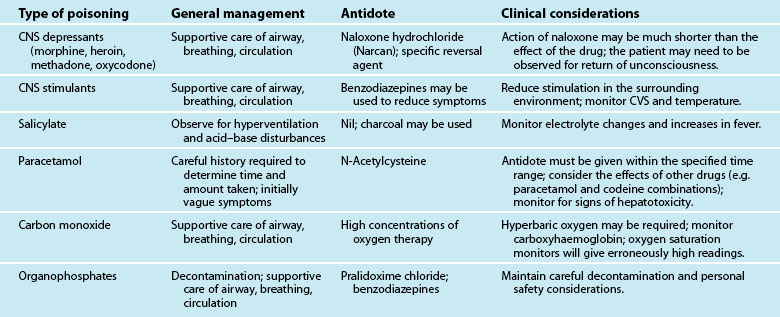
Poisoning is a common clinical presentation in Australia and New Zealand, accounting for 1–5% of admissions to public hospitals.103–105 Up to 25% of successful suicides are due to poisoning.105 Current clinical management with supportive and/or symptomatic control has resulted in death rates as low as 0.5% for overdose admissions to hospitals.105 New Zealand has a similar poisoning pattern to Australia but much higher rates of admission and a lower mortality rate than many countries.106 Common self-poisoning ED presentations include prescribed drugs, illicit drugs and ingestion of common dangerous substances (e.g. detergents, cleansers, psychotropic agents, analgesics, insecticides, paracetamol, aspirin).107
A range of artificial and naturally-occurring substances can produce acute poisonings. The toxicity of a substance depends on numerous factors, such as dose, route of exposure, and the victim’s preexisting conditions. Poisoning, whether intentional or unintentional, can occur at any time, and may involve single or multiple substances.107–109
The vast amount of knowledge required on all poisons prompted the development of poison control information centres to provide specific information and guidance for healthcare providers and the general public, on the management of a poisoned patient; to collect statistics on toxic substances; and to educate the public on the prevention or recognition of toxic exposures.108 Other initiatives to limit the incidence and severity of acute poisoning include the control of drugs, specific information on labels, the introduction of blister packs and enforced safety standards such as childproof caps.108–110
Assessment, Monitoring and Diagnostics
A poisoned patient may present with a wide range of clinical features – from no symptoms through to a life-threatening condition or the potential to deteriorate rapidly; patients should therefore always be assessed immediately. Triage decisions are based on the potential for rapid deterioration and the need for urgent intervention. Resuscitation may be necessary before any further definitive care can be commenced.107,109,110 Priorities include assessment and maintenance of an airway, adequate ventilation and circulation.104 Successful resuscitation may require removal of the toxin, counteraction of the poisoning by an antidote if available, and the treatment or support of symptoms.108–110
Note that many drugs such as paracetamol may have limited initial effects but serious, potentially fatal consequences if not treated in a timely manner.104,109,110 Once ascertained that a patient does not have an immediate, life-endangering problem, attention is directed towards a more thorough assessment and identification of the toxin involved. Accurate history is often the most significant aid in directing care. If a history is unobtainable or uncertain, there are several general guidelines available for dealing with a patient who has an altered mental state or consciousness level107,108,110 (see Table 22.6).
Poisoning should always be considered for a patient with a sudden-onset, acute illness. If there is a strong suspicion of poisoning, attempt to compare the patient’s presentation with the suspected toxin and the likelihood of exposure. Age and gender influence the types of presentation. Accidental poisonings are the most common cause of medical emergencies in the paediatric patient population. Childhood ingestions tend to be accidental and to involve a single substance. Boys are more likely to be the victims of poisoning than girls. Adult intentional poisonings occur more often with adults, and are more likely to involve multiple substances.105–107 Women attempt suicide with poisons more often than men, but men have a higher mortality rate.105–107 Poisonings in the aged population are often complicated by co-existing medical conditions, which may exaggerate the effects or impair the excretion of the substances involved.
Previous History
Patients with existing medical conditions often have multiple medications that could be either intentionally or unintentionally ingested. Use of multiple drugs may cause untoward reactions. A patient with a history of depression may attempt suicide with psychotropic drugs.105–107 A quick onset and acute illness or condition raises the level of suspicion of a poisoning, especially if there is no history of previous signs or symptoms that suggest another cause. If a patient presents with a history of poisoning, the benefits and risks of treatment should be considered and therapy given if there is any doubt.105,107
Suspected Toxin
Rescue personnel, family or friends should bring any container, plant product or suspected toxin with the patient to the hospital, as long as the substance presents no risk of contamination to the person retrieving it. If multiple plants are growing together, a sample of each should be included. A child’s play area should be inspected for possible sources of toxins.107,108
Time of Poisoning
History includes time of exposure, onset of symptoms and time since treatment began. If the toxin was ingested, determine the time since the last meal or alcohol consumption. Alcohol is the most common drug taken with other intentional self-poisonings, can potentiate a range of medication effects and increase the incidence of vomiting and potential aspiration.108,110,112 Poisonings in children tend to occur most often just prior to mealtimes, when they are hungry. Adults may take substances late in the evening, fall asleep and be found several hours later.104
Physical Assessment
A thorough assessment may provide clues with an unconscious, uncooperative or suspicious presentation. Assess for respiratory effort, skin colour, pupil size and reactivity, reflexes and general status. Auscultation of the lung fields, the apical pulse and bowel sounds provide a baseline for further assessment and clues to current problems. Check the blood pressure as often as necessary to determine cardiovascular stability. Percuss the thorax and abdomen to detect accumulations of fluid or air.108,111 Needle marks, pill fragments, uneaten leaves or berries, or drug paraphernalia assist in a diagnosis.108,111 The presence of pressure areas on the skin may indicate how long the patient has been unresponsive. Any odours are important to note; an oily-garlicky smell may be due to pesticides; other odours may indicate chronic medical disorders (e.g. fruity odour with diabetic ketoacidosis) or neglect of personal hygiene.112
Diagnostics
Toxicology screens include analysis of serum and urine to determine the presence and amount of a substance. Laboratory levels are helpful but are considered in relation to the nature of the substance and its rate of metabolism. Certain substances are sequestered in fatty tissues or bound to serum proteins, and may be present with a misleadingly low serum level.104 Serum electrolytes, non-electrolytes, osmolality, arterial blood gases and urine electrolytes are used to determine a patient’s overall status or response to therapy. Continuous cardiac monitoring supplemented with a 12-lead ECG or invasive monitoring devices may be required to guide symptomatic care.107,108,110
Management: Preventing Toxin Absorption
Initial and ongoing care of a victim follows three principles:104
1. preventing further absorption of the toxin
2. enhancing elimination of absorbed toxin from the body
3. preventing complications by providing symptomatic or specific treatments, including psychiatric management.
Ingested poisons are best removed while still in the upper gastrointestinal tract when possible. Emesis and gastric lavage were utilised in the past to empty the stomach, although a significant body of evidence now suggests that these approaches are relatively ineffective and effectiveness decreases rapidly after 1 hour.107,108,110 Both the patient and substance should be evaluated for appropriateness of gastric emptying.108 The patient’s consciousness level, gag reflex and ability to vomit while protecting the airway from aspiration is considered. Any central nervous system depressants are capable of obtunding the protective gag or cough reflex. If the ingested substance has a rapid onset of action (e.g. benzodiazepines), it is safer to avoid emetics because of the risk of a sudden fall in the level of consciousness.
Ingested Poisons
Evaluate the substance ingested to determine whether gastric emptying is appropriate. Physical properties of a drug may make it more responsive to a particular type of gastric emptying. For example, tricyclic antidepressants tend to reenter the stomach acid after absorption into the serum.113 Also consider the effects of substances on tissue. Corrosives, such as acids, alkalis and iron supplements, produce irritation and tissue breakdown when in contact with the skin or mucous membranes. Recognition is important, as therapy may cause further injury. Emesis could be contraindicated, and a lavage tube may traumatise injured tissue. Waiting for emesis also causes further delay in definitive treatment. Other substances have natural emetic qualities if taken in sufficient doses (e.g. hand soaps and liquid soap detergents).108
Evaluate other substances on an individual basis. Most petroleum distillates (e.g. furniture polish, cleaning fluids) present a greater hazard for chemical pneumonitis than a systemic intoxication.114 Even very small amounts can quickly disperse over the lung surface if accidentally introduced into the trachea. Avoid emesis or lavage when the chance of aspiration is high.114 There are situations, however, when the amount, character or additional chemicals present make it necessary to remove the ingested substance from the stomach.
Therapy can be based on the reported amount taken or time since ingestion. Time since ingestion is important to rule out the benefit of therapy, as the stomach tends to empty its contents after 1 hour unless the ingested substance slows gastric motility (e.g. narcotics slow peristalsis and may be found in the stomach several hours after ingestion).104 A patient may also under-report the dosage to avoid an obviously unpleasant experience. Although conservative management with observation is appropriate in certain situations, the risk of not treating might be greater in others.104 If a large number of tablets or pills are consumed at one time, they may clump together in the stomach and form a mass that is too large to pass out of the pylorus (e.g. aspirin).115
Once a substance enters the lower gastrointestinal tract, it can be absorbed into the mesenteric circulation. As absorption can vary according to substance, slow-release characteristics, rate of peristalsis and the presence of other substances, it is possible for a poison to be present in the bowel for an extended period of time. If intestinal motility can be stimulated or the toxin permanently bound until excretion, then further absorption is reduced.108
Activated charcoal is a refined product with an enormous surface area that binds to a large range of substances to enhance elimination, and is the most effective decontaminating agent currently available, when given early after ingestion.107,108,110 A solution of either water or sorbitol is mixed with 15–30 g of activated charcoal to form a thick, liquid slurry which is given to a compliant patient orally or through a nasogastric tube. It may be mixed with a cathartic, which reduces the time the substance or the charcoal is in contact with the bowel wall, although there is no evidence that this improves clinical outcome.116 Effectiveness can be improved through repeated administration of activated charcoal, ensuring that the entire drug is absorbed, and interrupting the drug reabsorption in the enterohepatic circulation.104 Cathartic agents such as sorbitol and polyethylene glycol reduce gastric transit time; in theory this limits absorption, although this has not significantly improved outcomes.103,104 Unfortunately, not all poisons that are ingested can be bound by charcoal (e.g. alcohol, heavy metals).107,108,110
Inhaled Poisons
A patient poisoned by inhalation of toxic gases or powders should be removed from the source as soon as it is safe to do so. Attempts to remove the substance, which is usually a vapour, gas or fine particulate matter, from the lungs are not normally useful.104 Staff involved in direct patient care should use contact precautions to reduce their own contamination risks with unknown substances. Clothing for many inhaled poisons may contain significant amounts of the poison and serve as a continuous source of the toxin. Contaminated linen and clothes should be removed carefully, sealed in a bag and destroyed.108,111
Contact Poisons
Contact poisons are dangerous because of their ability to enter the body via the skin or mucous membranes. All clothing and all of the toxic substance should be carefully removed, preferably with an irrigating and neutralising solution. Contact precautions to avoid direct skin contact and reduce the risk of self-contamination are used. Clothing may contain significant residual amounts of the poison and serve as a continuous source of the toxin. Contaminated linen and clothes should be sealed in a bag and destroyed.108,111
Management: Enhancing Toxin Elimination from the Blood
After a substance has entered the bloodstream, it is normally excreted from the body either in an unchanged form or after liver metabolism and detoxification. Various metabolic byproducts are eliminated in the bile and faeces or urine. Urinary excretion of substances can be enhanced by increasing the filtration process (i.e. forced diuresis: large volumes of IV solutions and/or diuretics), by inhibiting absorption in the renal tubules, or by stimulating the secretion of substances into the urine.108–110
Alkalinisation of Urine
Manipulation of the absorption or secretion process of a drug can be assisted by chemically altering the structure of some substances. All substances break down into ions at a specific pH for that substance. Altering the pH of urine with acidifying or alkalising drugs allows the poison to be forced into an ion state and then excreted in the urine. This ‘ion trapping’ process is effective only for substances that are primarily eliminated by the kidneys108–110 (e.g. salicylates, tricyclic antidepressants have increased excretion due to urinary alkalisation).104
Haemodialysis or Haemoperfusion
If a dangerous amount of a poison is present or if renal failure is evident, then haemodialysis or haemoperfusion may be used to promote excretion. Dialysis is effective in removing only substances that are reversibly bound to serum proteins, or not stored in body fat. This is a highly invasive approach and is normally reserved for life-threatening cases (see Chapter 18 for further discussion).107,108,110
Management: Preventing Complications and Specific Symptomatic Care
Supportive care is the key element in managing an acutely poisoned patient. Once a patient has either ingested or been exposed to many poisons, there are limited options other than to treat the symptoms as they present or become clinically significant (see Table 22.7).
Antidotes act to antagonise, compete with or override the effects of the poison, although few specific antidotes exist for toxins (see Table 22.8). In some cases, an absorbed toxin can be rendered benign by the use of an antidote (e.g. the interaction between naloxone and opiates).104 For chelating agents (desferrioxamine for iron poisoning), a non-toxic compound is formed and safely eliminated from the body.107,108,110 The effect of an antidote may be only temporary if it has a shorter half-life than the poison. Most antidotes are given either in a specific dose or as a response to dose rate.104
TABLE 22.8 Common emergency antidotes
| Poison | Antidote |
|---|---|
| Benzodiazepines | Flumazenil |
| Carbon monoxide | Oxygen |
| Insulin | Dextrose |
| Opioids | Naloxone |
| Paracetamol | N-Acetylcysteine |
| Organophosphates | Atropine and pralidoxime |
| Tricyclic antidepressants | Sodium bicarbonate |
A poisoning may be the physical manifestation of an emergency or crisis that requires emotional support. An underlying emotional conflict or mental health problem may exist, regardless of whether the poisoning was intentional or accidental. Psychological care is therefore an important component for all patients presenting with poisoning.107,108,110 Many facilities offer the services of a mental health worker while the patient is still in the ED. If the patient’s condition is stable and the poisoning has not altered their mental state, early psychological intervention is appropriate.
For adult patients, the desire for treatment is not as important as the manner in which treatment is received. Even though patients may initially refuse care, if approached in a non-threatening way and provided some form of control they will usually comply. If threatened with force or restraints, they are placed in a difficult position of either submitting to coercion or resisting therapy for self-protection. A paediatric patient may be too young either to fully understand or to effectively cooperate (see Chapter 26).
Central Nervous System Depressants
A large number of common medications are capable of depressing levels of consciousness, thought processes, or important regulatory centres in the central nervous system (CNS). Clinical findings can vary from class to class or within the same drug family, as physical effects are dependent on the chemical structure of the drug, dose, route of exposure and rate of metabolism. The chemical structure and/or purity of illicit drugs may also be affected by variations or deliberate aberrations in the manufacturing process.112,117–119 Drugs in this section include sedatives, hypnotics, tranquillisers and narcotics (see Table 22.9).
Assessment
The predominant observed effect is an altered level of CNS function.112,118,119 A spectrum of physical findings is possible with the selective action of the specific drug on inhibitory or excitatory centres of the brain; effects can vary from mild euphoria to convulsions, or mild sedation to coma, dependence, addiction and tolerance. Narcotics produce miosis (constriction of the pupil), and some patients experience nausea and vomiting due to stimulation of the chemoreceptor trigger zone in the medulla.112,118,119
A narcotic overdose is distinctive: a decreased respiratory rate and tidal volume, miosis, hypotension, and an altered level of consciousness.112,118,119 However, other factors may affect these findings:
• a decreased respiratory effort may produce hypercarbia, causing pupil dilation
• chronic narcotic users tend to have multiple problems associated with their drug use or lifestyle, which may modify findings
• a sufficiently high quantity of CNS depressant will depress vital regulatory centres in the brain
• altered respirations cause hypoventilation, stasis of secretions, and atelectasis; resultant hypoxia aggravates the sensorium and cerebral functioning119
• narcotics may produce idiopathic pulmonary oedema112,118,119
• CNS depressants may cause peripheral vasodilation, with a resultant hypotension and tachycardia
• arrhythmias may occur because of cardiac conduction effects or tissue hypoxia.112,118,119
Patients with an altered level of consciousness are at risk of injury from decreased sensory ability or prolonged immobilisation. Reddened areas over bony prominences or pressure points appear within a short time. Skin blisters indicate altered blood flow, usually due to excessive pressure. Actual skin breakdown can occur within 3 hours.112,118,119 If external pressure or altered circulation to an extremity continues for over 4 hours, compartment syndrome may develop.112,118,119
Effects of Multiple Drug Use
A patient who ingests a combination of drugs may experience toxicity because of additive or synergistic effects.112,118,119 Illicitly-produced drugs often have substances added (e.g. glucose powders, icing sugar, talcum powder) to dilute or ‘cut’ them, to increase the quantity of supply and profit for the supplier.117,118 Users may also intentionally inject other drugs (e.g. antihistamines, amphetamines, benzodiazepines) to modify or potentiate the effects of narcotics.112,118,119
Potential for Acute or Active Infections
The use of non-sterile solutions and equipment and the sharing of injection equipment significantly increases the risk of acute or active infections.112,118,119 Frequent exposure and a depressed immune response also predispose a patient to severe infections (e.g. hepatitis, osteomyelitis, infective bacterial endocarditis, encephalitis/meningitis).112,118,119
Central Nervous System Stimulants
CNS stimulants increase the activity of the reticular activating system, promoting alertness and affecting the medullary control centres for respiratory and cardiovascular function. Individuals using a CNS stimulant have an increased ability to perform muscular activity and a general sense of wellbeing. Many illegal stimulants are poorly manufactured, with no guarantee of purity or consistency in dosage. The possibility of overdose is therefore always present, producing profound CNS excitation.117,120,121 Commonly used stimulants include amphetamines, dextroamphetamine, methyphenidate, lysergic acid diethyamide (LSD), phencyclidine (PCP), caffeine, cocaine and methamphetamines.112,118,119,125
Assessment
Both psychological and physical symptoms are produced. A patient may demonstrate repetitive, non-purposeful movements, grind their teeth and appear suspicious or paranoid of others. Physiological stimulation causes an increase in metabolism, with flushing, diaphoresis, hyperpyrexia, mydriasis (excessive pupillary dilation) and vomiting evident. Dizziness, loss of coordination, chest pain, palpitations or abdominal cramps may also be present. During the acute phase of poisoning, severe intoxication and loss of rational mental functioning may lead individuals to behave irrationally and even attempt suicide. Anxiousness and a general state of tension may also lead the affected person to attempt to harm others.112,118,119 Death is possible from cardiovascular collapse or as a sequela to convulsions and acute drug toxicity.112,118,119
Management
If a patient has ingested the drug, emesis or lavage is of little value, and an individual risk–benefit assessment is required. Gastric emptying may precipitate more severe agitation with a concomitant rise in blood pressure, pulse rate and metabolism.112,118,119 Activated charcoal and cathartics may be administered to promote elimination. Note that there are no specific antidotes for CNS stimulants. Ongoing emergency management includes:
• support of vital functions112,118,119
• reduction of external stimulation by locating the patient in a quiet, non-threatening environment where a supportive person can attempt to calm and ‘talk the person down’ while observing for untoward reactions
• sedation when necessary, although it is not desirable to give more medications in a precarious situation; sedation may control seizures or keep the patient from self-harm.112,118,119
Amphetamines and Designer Drugs
Amphetamines and designer drugs have been drugs of abuse for a number of years. Originally, many were designed and introduced as anaesthetic agents, decongestants or for other legitimate purposes. Amphetamines are chemically related to the anaesthetic ketamine, with a similar CNS response.112,118,119 Most drugs in this group were discontinued or controlled because of the delirium and agitation experienced by patients who received them; paradoxically, these effects led to their popularity as recreational drugs.112,118,119 Amphetamines are synthetic sympathomimetic drugs, available in oral, intranasal or intravenous forms; crystalline rock forms such as ‘ice’ are smoked. Death may occur from overdose, self-mutilation or dangerous activities such as diving into shallow waters or walking on traffic-laden roads.
Assessment
Depending on the dose, route and time since exposure, a person exhibits characteristic behavioural and physical changes. With high-dose intoxication, the patient has pronounced CNS involvement: altered levels of consciousness, seizure activity, or a loss of protective gag, corneal and swallow reflexes. Nystagmus is a classic sign, along with hypertension and an elevated body temperature. A significant rise in arterial pressure presents a risk for intracerebral haemorrhage. One of the distinguishing features of amphetamines is their ability to produce coma without affecting respirations.112,119 The patient may be at risk of dehydration and renal failure if muscle breakdown has occurred. A high urine output should be maintained and serum urea and creatinine levels monitored to detect a decrease in renal function.112,118,119
Lower-dose intoxications do not produce unconsciousness but typically cause behavioural patterns that reflect depersonalisation and distorted perceptions of events or other people. The patient’s physical and mental responses may be dulled and slow, or their behaviour abusive and delusional. Intoxication is marked by paranoid thoughts, with the patient responding to therapeutic or friendly gestures with behaviours ranging from apprehension to aggressive hostility. To avoid stimulating the patient and intensifying their behaviour, use a quiet environment for initial assessment and treatment, although this is often difficult in the ED.112,118,119
Management
Gastric emptying is normally ineffective due to delays in seeking treatment. If a patient presents early, activated charcoal and cathartics are useful in preventing further absorption. Noises, sights and sounds provoke paranoid ideation and may present a risk to staff and other patients. ‘Talking down’ is usually not successful and probably only serves to exacerbate the situation. If the patient is demonstrating hostile or self-abusive behaviour, restraints may be needed to protect him/her and any others present. The use of physical restraints is not without danger, and they should never be used as a substitute for a more desirable environment. If the threat of danger or psychosis is significant, sedatives (diazepam, haloperidol) may be necessary to control the patient’s behaviour. Intravenous diazepam also controls frequent seizure activity.112,118,119
Salicylate Poisoning
Aspirin is the most common form of salicylate in the home and is found in many over-the-counter medications, such as combination analgesics122 and topical ointments. Aspirin may be ingested orally, absorbed through the rectal mucosa, or applied to the skin in topical preparations. Under normal circumstances, the kidneys serve as the principal organ of excretion. Aspirin was previously the most common poisoning in children,106,109,122 so legislation was implemented to limit the number of tablets per pack and to introduce packaging with childproof caps. In Australia, salicylate poisoning is now uncommon, accounting for only 0.3% of calls to poison information centres.106,122 The three common types of aspirin overdose are: accidental ingestion (more common in young children); intentional ingestion (more common in adults); and chronic toxicity (occurs in any age group).115,122
Assessment, Monitoring and Diagnostics
Intentional or accidental ingestion is straightforward, with a clear history of poisoning. Chronic toxicity is however often unrecognised. Individuals may not be aware of correct dosages, combine multiple drugs that contain aspirin, or may have impaired excretion due to dehydration. The symptoms of chronic aspirin overdose (i.e. dehydration, lethargy, fever) resemble the original problem being treated, and some people will continue treating themselves with aspirin for these symptoms. Chronic toxicity has a higher mortality than acute ingestion.115,122,123
Aspirin is problematic if ingested in amounts greater than 150 mg/kg; toxicity presents with tachypnoea, fever, tinnitus, disorientation, coma and convulsions due to systemic effects of aspirin.115,122,123 Acid–base disturbances arise from direct stimulation on the respiratory centre in the CNS; an increased rate and depth of respirations cause hypocarbia and respiratory alkalosis, with renal compensation by bicarbonate elimination. Salicylates, however, also alter metabolic processes, resulting in a metabolic acidosis. Blood gases can therefore reflect acidosis, alkalosis or a combination. Tinnitus (ringing in the ears) is a symptom of the effect on the 8th cranial (acoustic) nerve.115,122,123
Aspirin also interferes with cellular glucose uptake, causing initial hyperglycaemia. As cellular levels become depleted the patient demonstrates hypoglycaemic effects. Later, serum levels may be either normal or hypoglycaemic.115,122,123 Patients may be nauseated and vomit after ingestion, causing fluid and electrolyte imbalance.115,122,123 Aspirin use is also associated with local tissue irritation, gastrointestinal bleeding, and platelet dysfunction, increasing risk of bleeding. Concomitant use of anticoagulants therefore increases this risk.115,122,123
Management
Absorption can be reduced with activated charcoal, using repeat doses for patients with signs of ongoing absorption.115,122,123 Urine alkalisation and forced diuresis can significantly increase elimination, as salicylates are weak acids excreted by the kidneys.115,122,123 Haemodialysis is reserved for extreme cases with profound acidosis, high blood levels, persistent CNS symptoms or renal failure.115,122,123
As salicylates have no known specific antidote,115,122,123 supportive therapy includes prevention of dehydration with careful monitoring of fluid output and adequate fluid replacement, monitoring serum electrolytes for imbalance and replacement as needed. Evaluate ABGs to determine whether the patient continues to have metabolic effects from aspirin toxicity or is not responding to therapy. Control temperature elevations with external cooling methods if fever develops.
Paracetamol Poisoning
The incidence of paracetamol toxicity is associated with approximately half of all Australasian toxic ingestions, due in part to its common availability as an analgesic/antipyretic agent.109,124 The drug is absorbed in the stomach and small bowel, with 98% metabolised by the liver using one of two mechanisms: most by a pathway with breakdown into nontoxic byproducts; the second hepatic pathway usually metabolises about 4% of the drug, but the process has a toxic byproduct. The liver detoxifies this toxic byproduct by combining it with glutathione, a naturally-occurring substance. In an overdose or when the minor pathway has already been stimulated (e.g. concomitant barbiturate use), more paracetamol is metabolised by the secondary pathway and the toxic byproduct accumulates, quickly consuming the available glutathione, resulting in liver tissue destruction.109,124,126
Assessment, Monitoring and Diagnostics
The amount of paracetamol ingested is best determined from patient history, as serum levels (although helpful) can be easily distorted. A nomogram to plot measured levels against time postingestion is a relative indicator of toxicity. A relatively small dose of 200 mg/kg paracetamol is considered toxic, although hepatotoxicity occurs after ingestion of 140 mg/kg or 10 g in a single dose.109,124,126
Liver function (liver enzymes, serum bilirubin, protein) and coagulation tests (prothrombin time, partial thromboplastin time, platelets) identify the development of hepatic dysfunction or damage.109,124 The pattern of toxic damage occurs over a characteristic three-phase course:
1. First 24 hours: vague symptoms of nausea, vomiting, and malaise.
2. 24–48 hours: above symptoms subside with onset of right upper quadrant pain due to hepatic injury; urine output may decrease as paracetamol potentiates the effect of antidiuretic hormone; liver enzymes, bilirubin, proteins and clotting studies may be abnormal.
3. 60–72 hours: liver impairment becomes more obvious, with jaundice, coagulation defects, hypoglycaemia and hepatic encephalopathy; renal failure or cardiomyopathy may also occur; death from hepatic failure occurs in approximately 10% of severe overdoses.109,124–126
Management
Absorption can be reduced with activated charcoal when the patient presents to hospital early, however following periods of 2 hours postingestion activated charcoal is unlikely to be effective. Haemodialysis with a charcoal dialysate has been used to remove unchanged paracetamol from the liver, but this does not remove the toxic byproduct. Forced diuresis is also not effective, as minimal paracetamol (about 2%) is removed by the kidneys.126–128
Specific therapy is the use of an antidote, N-acetylcysteine, which is structurally similar to glutathione and binds to the toxic byproduct. When given within 24 hours of acute ingestion, N-acetylcysteine is effective in preventing hepatic damage.126–128
Carbon Monoxide Poisoning
Carbon monoxide (CO) is a gaseous byproduct of incomplete fuel combustion, and is present where there is a flame in a confined space with improper ventilation or air exchange. Levels of CO can accumulate rapidly, and the gas is dangerous as it is colourless, odourless, tasteless and non-irritating.129–131 Common sources of CO are faulty radiant heaters, kerosene lamps, cooking stoves, engine exhausts and fireplaces. Acute CO poisoning is the most common form of successful poisoning in the USA, UK and Australia.129–131
Assessment, Monitoring and Diagnostics
Haemoglobin has a 210–240 times greater affinity for CO than for oxygen, and shifts the oxygen–haemoglobin curve to the left (see Chapter 13). As CO displaces oxygen from red blood cells, the patient experiences hypoxaemia and hypoxia.132–134 Headache, nausea and vague pains are often experienced at onset of poisoning, with increasing tiredness and sleepiness, difficulty concentrating, and failure to recognise the onset of poisoning. With higher levels of inhalation, the patient may be tachypnoeic, tachycardiac and experience loss of consciousness. A characteristic red colour presents in the lips with skin flushing.132–134 The most important factors in determining CO poisoning are a history of exposure with an elevated blood carboxyhaemoglobin level.132–134
Management
As CO is an inhaled toxin, the patient should be removed from the contaminated environment to prevent further absorption and allowed to breathe fresh air until 100% oxygen can be administered. Although this may be ineffective because of the bond between CO and haemoglobin, high-flow high-concentration oxygen administration will reduce the half-life of CO.134 Hyperbaric oxygenation is used to treat severe cases of CO poisoning, as pressurised oxygen reduces the half-life of the carboxyhaemoglobin molecule and shortens the duration of effects. As hyperbaric resources are not available at every facility, treatment depends on carboxyhaemoglobin serum levels, time since exposure, transport time to the hyperbaric chamber and the clinical symptoms of the patient.132–134 Patients should be monitored for adverse effects of hypoxia, as they may have convulsions, cardiac arrhythmias and acid–base disturbances.
Corrosive Acids
Ingested corrosives produce immediate or late life-threatening complications. In general, acids dissolve tissue and destroy haemoglobin.135 Swallowing a strong acid can produce ulceration and perforation of oral and oesophageal mucosa, presenting a risk for haemorrhage and mediastinitis, and cardiac arrest as a result.135–137 The late sequelae of swallowing a corrosive substance involves mucosal scarring with constriction and mechanical obstruction of the oesophagus.
Assessment
Physical findings are site-specific and relate to the type of exposure – ingestion, inhalation or contact (see Table 22.10). Ingested acids present as burns to the mouth and pharynx. Patients who are able to vocalise complain of pain, gastric irritation with vomiting and haematemesis. Fumes from an ingested substance may cause pneumonitis. Contact with skin or the eyes is similar to other types of burns, with a sharply-defined blister or wound, inflammation, pain and ulceration. Hypotension and cardiovascular collapse are also possible when damage occurs to underlying vital structures.135–137
TABLE 22.10 Summary of assessment and management of acid and alkali exposure
| Nursing step | Corrosive acids or corrosive alkalis |
|---|---|
| Assessment |
Inhalation irritates respiratory tissues, producing direct damage, oedema and alterations in ventilation. Patients may initially experience coughing, choking, gasping for air and increased secretions. Evaluate for obvious tissue injury, impaired respiratory function, and subsequent effects of hypoxia and pulmonary oedema, which may occur up to 6–8 hours later.135,137Arterial blood gases, ventilation studies, serial chest X-rays and frequent physical assessments are used to monitor for changes.
Management
Contaminated clothing should be removed to prevent recontamination. Patients with external contamination should be washed thoroughly to remove any remaining surface material that may come into contact with treating staff. For acid contact with skin or eyes, begin immediate flushing with a non-reactive liquid and continue to do so for at least 15 minutes to guarantee complete removal. In most cases water will be the safest and best available liquid. Provide skin or eye protection with a sterile dressing.135
For ingested acids, emesis or lavage should not be attempted, as the substance will cause additional damage when ejected from the stomach. A gastric tube may also cause structural damage by penetrating or irritating friable tissues.135–137 Do not attempt to neutralise the acid, as this may result in a chemical reaction and generate heat as a byproduct, with potential further burning and damage.135–137 Suctioning of oral secretions should be done carefully and with as much visualisation of tissues as possible. A patient may be given water or milk to irrigate the upper gastrointestinal tract, although extreme care is required to ensure that the airway is adequately protected because of risk of aspiration.135
Corrosive Alkalis
Alkalis produce tissue destruction on contact by interacting with fats and proteins and producing necrotic tissue. Alkalis involved in toxic emergencies include many substances found around the house, such as ammonia (detergents, cleaning agents); cement and builder’s lime; low-phosphate detergents; sodium carbonate (dishwasher detergent); and sodium hypochlorite (laundry bleaches).137
Skin contact and ingestion are the most common types of injury from an alkali; ingestion is most immediately life-threatening. Erosion of the oesophagus and stomach occurs if ingested orally, and peritonitis or mediastinitis may develop as sequelae. Late effects are similar to those produced by acids. Oesophageal strictures due to scarring are common post-ingestion. About 25% of patients who ingest a strong alkali will die from the initial insult,137 while 98% will develop strictures.135–137
Assessment
The immediate response to ingestion is increased secretions, pain, vomiting or haemoptysis. Signs of perforation include fever, respiratory difficulty or peritonitis. Alkalis and skin contact produce a soap-like substance because of the interaction with tissue fats, giving a slimy, soapy feeling.135,137
Management
Induced vomiting or gastric lavage should not be attempted, as the alkalis will be neutralised by stomach acid, and lavage tubes may cause further tissue damage.135,137 External contact with alkalis requires copious irrigation at the point of contact; continue irrigation for at least 15 minutes; for the eye, irrigation can be for up to 30 minutes. Cover all wounds after irrigation with sterile dressings to reduce the risk of infection.
A patient is deemed ‘nil by mouth’ until inspection of the mouth and throat to determine the amount and extent of burns.135 An oesophagoscopy identifies the degree of injury and enables direct irrigation of any affected areas of mucosa.135 Alkalis that contain phosphates may produce a systemic hypocalcaemia, and IV calcium gluconate may be required. Continue to monitor for systemic effects of perforation or tissue injury.135
Petroleum Distillates
Petroleum distillates are common substances, and account for 7% of all poisonings.114 Typical petroleum products are benzene, fuel oils, petrol, kerosene, lacquer diluents, lubricating oil, mineral oil, naphthalene, paint thinners and petroleum spirits. Toxicity depends on: route of exposure (ingestion or aspiration); volatility (ease with which the substance evaporates); viscosity (density or thickness); amount ingested; and presence of other toxins.114
Products with a low viscosity are more likely to be aspirated and can quickly spread over the lung surface. Substances with low viscosity and high volatility (e.g. benzene, kerosene, turpentine) are toxic in doses as low as 1 mL/kg, with death from doses of 10–250 mL. Mortality is increased if an additional toxic substance is present, or if accidental aspiration occurs.114
Assessment
Aspiration causes a pneumonitis with low-grade fever, tachypnoea, coughing, choking, gagging and pulmonary oedema as a late effect.114,136 Immediately assess the patient’s respiratory tract for possible aspiration; coughing, cyanosis or hypoxia may indicate aspiration or chemical pneumonitis.137 As petroleum distillates are fat solvents and rapidly cross the lipid cell membrane, nerve tissue is especially sensitive to injury. A patient may exhibit local effects, such as depressed nerve conduction; or varied central effects, such as feelings of wellbeing, headache, tinnitus, dizziness, visual disturbances, through to respiratory depression, altered levels of consciousness, convulsions and coma.136
Management
In the awake and alert patient, the decision to treat is based on the physical properties of the substance, the likelihood of aspiration or other complications, and the amount consumed.136,137 When preventing absorption, carefully consider gastric emptying, as neither induced vomiting nor gastric lavage are recommended. If the patient is lethargic or unconscious, an endotracheal tube is placed for adequate airway protection,114,135–137 although this heightens the risk of aspiration as hydrocarbons adhere to the tube and increase the risk of chemical pneumonitis.114,135–137
Organophosphates
Organophosphates are a large and diverse group of chemicals used in domestic, industrial and agricultural settings (e.g. insecticides, herbicides).103,104,138 Organophosphates are absorbed through the skin, ingested or inhaled. Although most patients become symptomatic soon after ingestional exposure, the onset and duration of action depends on the nature and type of compound, the degree and route of exposure, the mode of action of the compound, lipid solubility, and rate of metabolic degradation.103,139,140 The primary effect of organophosphates is binding and inactivation of acetylcholinesterase (AChE), a neurotransmitter that metabolises acetylcholine (ACh).103,139,140
Mortality rates range from 3% to 25%, and are the most common mode of suicide in some developing countries (e.g. Sri Lanka and Fiji). In one Australian study, 36% of patients had suicidal intentions, compared with 65–75% in developing countries. Men aged 30–50 years were more likely to attempt suicide with organophosphates.141 Common complications include respiratory distress, seizures and aspiration pneumonia, with respiratory failure the most common cause of death.140
Assessment, Monitoring and Diagnostics
Clinical findings of organophosphates are divided into three broad categories:
1. Muscarinic effects; common manifestations are summarised by the mnemonic SLUDGE: Salivation, Lacrimation, Urination, Defecation, GI upset, pulmonary oEdema.103,104,138,142 Other symptoms include bradycardia, hypotension, bronchospasm, cough, abdominal pain, blurred vision, miosis and sweating.
2. Nicotinic effects: include muscle fasciculations, cramping, weakness and diaphragmatic failure. Autonomic effects include hypertension, tachycardia, pupillary dilation and pallor.
3. CNS effects: include anxiety, restlessness, confusion, ataxia, seizures, insomnia, dysarthria, tremors, coma and paralysis; three types of paralysis may present:103,104,138
Laboratory diagnosis is based on measurement of cholinesterase activity using either erythrocyte or plasma levels; erythrocyte cholinesterase is more accurate, but plasma cholinesterase is easier to test and is more widely available. Erythrocyte AChE is found in CNS grey matter, red blood cells, peripheral nerve and muscle. Plasma cholinesterase circulates in plasma and is found in CNS white matter, pancreas and heart.139–140 Levels of poisoning are categorized as mild (cholinesterase activity is reduced to 20–50% of normal; moderate (activity is 10–20% of normal); or severe (less than 10% of cholinesterase enzyme activity). Levels do not, however, always correlate with clinical illness.139
Management
Initial priorities are ABC, in concert with D (danger), as organophosphates also present considerable risk to staff caring for the patient, especially during the initial phases of management. All patients’ clothing should be removed and considered hazardous waste. Patient decontamination with soap and water is a priority, as soap with a high pH breaks down organophosphates.140,142 Staff should use personal protective equipment (PPE), such as neoprene or nitrile gloves, and gowns, when decontaminating patients. Charcoal cartridge masks for respiratory protection are used, although recent evidence suggests that the nosocomial risk may not be as significant as once thought.142
Intubation is commonly required after significant exposure due to respiratory distress from laryngospasm, bronchospasm or severe bronchorrhoea. Continuous cardiac monitoring and an ECG are used to identify bradycardias. Activated charcoal is used for gastric decontamination for patients who ingested organophosphate. The mainstay of treatment is atropine and pralidoxime, with a benzodiazepine used for seizure control.138,139,142Atropine blocks acetylcholine receptors and halts cholinergic stimulation. Large doses of atropine are usually required (1–2 g IV), and repeated if muscle weakness is not relieved or the signs of poisoning recur. Clearing of bronchial secretions is the endpoint of atropine administration, not pupil size or absolute dose.138,139,142 Pralidoxime hydrochloride reactivates acetylcholinesterase and is effective in restoring skeletal muscle function, but is less effective at reversing muscarinic signs. Over time, the bond between organophosphate and cholinesterase becomes permanent and the effectiveness of pralidoxime diminishes.142 The current recommendation is for administration within 48 hours of poisoning.142 Benzodiazepines are clinically indicated as the drug binds to specific receptor sites, potentiating the effects of gamma-aminobutyrate (GABA) and facilitating inhibitory transmitters for management of seizures.138,139,142
Chemical, Biological and Radiological (CBR) Events
Terrorist incidents and hoaxes involving toxic or infectious agents are frequent events, and there is now increased international attention paid to the potential risk of CBR attacks.143 While a nuclear weapon may be difficult for a terrorist group to obtain, there is evidence that groups have attempted to acquire nuclear materials.144,145 In addition, non-nuclear radioactive material may be easier to obtain and used in an explosive device (referred to as ‘dirty bombs’).144,145 Chemical agents or biological agents are also relatively easy to obtain, and pose a greater threat.143 The availability and the impact of chemical and biological threat materials are both relatively high, with potentially devastating impacts.143,146–149 As biological and chemical agents are dissimilar, each category is discussed separately, although there are common characteristics.
Chemical Agents
Chemical agents are super-toxic chemicals used to poison mass victims. The chemicals are similar to hazardous industrial chemicals, but hundreds of times more toxic. For example, while the Sarin attack on the Tokyo subway in 1995 killed 12 people, there were also 1039 injuries, and at least 4000 people with psychogenic symptoms.147 Sarin is approximately 60 times more toxic than methylisocyanate. To demonstrate this perspective: a leak of methylisocyanate from a factory in Bhopal, India in 1984 caused 200,000 people to be affected, 10,000 severely affected and 3300 deaths. Relatively small quantities of a military grade chemical agent could therefore have the same capability to produce large numbers of casualties (symptomatic and psychological).147,148
Biological Agents
Biological agents are living organisms or toxins with the capacity to cause disease in people, animals or crops. The toxins generally behave like chemical agents and serve the same function: to poison people.147,148 Biological agents are relatively inexpensive to produce and have the potential to be devastating in their effects. Organisms such as anthrax, plague and smallpox have been the agents of greatest concern from terrorists’ potential use.146 Biological agents have the longest history of use, having been available for centuries.148
Radiological Materials
Radiological materials pose both acute and long-term hazards to humans. Action is similar to some chemical agents: cellular damage. A major difference is that the radiological agents do not have to be inhaled or in skin contact to exert damage.145 Deployment of a nuclear weapon would be catastrophic; note evidence of events like Hiroshima and Chernobyl. While very different, both events produced immediate injury and the long-term effects of ionising radiation on large populations.146 Any radiological effects on human health from the 2011 tsunami in Japan and the subsequent damage to the Fukushima nuclear reactors remains unclear at the time of writing. The event of most risk is likely to be a ‘dirty bomb’ that combines conventional explosives with any available radioactive source.146
A CBR terrorism incident may or may not result in mass casualties and fatalities as intended. However, large numbers of psychological casualties are very likely and therefore, regardless of the effectiveness of the attack, and the number of people actually exposed to the agent, there will most likely be a mass casualty situation.146 The psychological implications of chemical and biological weapons may be worse than the physical ones. Chemical and biological weapons are weapons of terror; part of their purpose is to wreak destruction via psychological means by inducing fear, confusion, and uncertainty in everyday life.150 The long term social and psychological effects of an episode of chemical or biological attack, real or suspected, would therefore be as damaging as the acute effects, if not more so.
Envenomation
Venomous animals can be land-based or marine-based, and their distribution ranges from broad to very specific locations. Exposure of humans to venom produces a large and varied range of symptomatology, which often results in an emergency presentation. It is therefore important for critical care nurses to be familiar with the types of potentially venomous animals inhabiting the catchment area of their health setting. Be familiar with the presentation and management of specific envenomations, including antivenom availability. Contact the local poison information centre for advice from expert toxicologists (see Online resources). Common envenomations across Australia and New Zealand are described below.
Redback/Katipo Spider Bite
Description and incidence
The redback spider (Latrodectus hasseltii) is found throughout Australia but more commonly in temperate regions. Tasmania has the lowest incidence, while areas around Alice Springs, Perth and Brisbane are especially infested.151 The redback spider is easily identifiable by the presence of a red, orange or brownish stripe on its characteristic black, globular abdomen. The female is much larger than the male; generally only the female is considered dangerous. Juveniles are smaller, more variably coloured, and may lack any spots or stripes. Bites from both male and juvenile spiders may result in symptoms, although these tend to be less significant than bites from a female.152
The redback spider has also become established outside Australia, including in New Zealand and Japan.152,153 Although bites are rare, small populations of redback spiders have been reported in Central Otago (South Island) and New Plymouth (North Island) since the early 1980s.153 The only other venomous spider in New Zealand is the Katipo (Latrodectus katipo) from the same genus as the redback. The katipo has a black, rounded body, slender legs and a red stripe on the abdomen. Adult males and juveniles are black and white but are smaller than females. The black katipo is a shy and non-aggressive spider, found in coastal areas of New Zealand. They are found in much of the North Island and on the South Island as far south as Greymouth on the west coast and Dunedin on the east coast.154 Their habitat is generally warm, sandy beaches and dunes, although environmental changes have resulted in increasingly scarce sightings and bites are rare. Symptoms of katipo spider bite are similar to those of the redback spider and where indicated, redback antivenom is available to treat bites from both spiders in New Zealand.
A redback spider bite is a frequent cause for ED presentations and the most clinically significant spider bite in Australia.152,155 Most bites are minor, with either minimal or no symptoms and requiring no antivenom. In approximately 20% of cases, significant envenomation occurs and antivenom administration is generally indicated, although death is extremely unlikely in untreated cases.156 Redback antivenom is the most commonly administered antivenom in Australia.152
Clinical manifestations
Envenomation by a redback spider is known as latrodectism, as the venom contains excitatory neurotoxins that stimulate release of catecholamines from sympathetic nerves and acetylcholine from motor nerve endings.152,156 Signs and symptoms associated with a significant envenomation are distinctive, and diagnosis is by clinical findings; initially a minor sting at the bite site, where the spider may or may not have been seen. Over the first hour the bite becomes progressively painful to severe, spreading proximally with and involving swollen and tender local lymph nodes. Localised sweating at the bite site or limb or generalised sweating may appear, associated with hypertension and malaise. Pain eventually becomes generalised and may be expressed as chest, abdominal, head or neck pain suggestive of other acute conditions such as myocardial infarction.155
Progression of symptoms generally occurs in less than 6 hours but may take up to 24 hours, while people with minor untreated bites may experience symptoms for several weeks.152,156 Other less common signs and symptoms include local piloerection, nausea, vomiting, headache, fever, restlessness/insomnia, tachycardia, and neurological symptoms such as muscle weakness or twitching.152,157
Assessment
Patients presenting with pain from a bite who have the offending spider with them are straightforward in terms of initial assessment. Identification of the spider is confirmed and a history of the event obtained, including the time of the bite and any first aid initiated. A brief assessment of the bite site and the involved limb is undertaken, including the extent of pain, presence of sweating and painful tender lymph nodes, and a baseline set of vital signs. Patients are then placed in a suitable area for medical assessment and ongoing observation.157
Adult patients presenting with vague limb pain, or preverbal children who are ‘distressed’ and ‘cannot be settled’, may be unaware that they have been bitten by a redback. The pain may not have been felt at the time and no spider may have been seen. Thorough history-taking, physical assessment and knowledge of latrodectism’s effects enable detection of a suspected spider bite.157
Management
There is no recommended definitive first aid for a redback spider bite. Application of cold packs to the bite site and administration of simple analgesia (e.g. paracetamol) may assist with local pain relief. The use of a pressure immobilisation bandage is not necessary, as symptom progression is slow and not life-threatening,152,156–158 and will cause further pain only in the affected limb. Remove any pressure bandage that was applied during first aid after identification of the spider is confirmed.152
Presence of the above symptoms indicates systemic envenomation, requiring administration of redback spider antivenom.152,156 Prior to administration, the patient should be placed in a clinical area with readily available resuscitation equipment to treat any anaphylactic reaction, although this is rare. An IV cannula is inserted and adrenaline 1 : 1000 is prepared for the possiblity of anaphylaxis.
The initial dose of Red Back Spider Antivenom is two ampoules administered IM (500 units; approx 1.5 mL each ampoule), and symptoms should subside over the next 30–60 minutes. Complete resolution of symptoms requires no specific further treatment. If there has not been complete resolution of symptoms after 2 hours a further 2 doses of antinvenom are given. If after a further 2 hours there is incomplete resolution of symptoms or no discernable response after 4 ampoules of antivenom, expert advice should be sought via the local poison information centre. Patients who are symptom-free after 6 hours of observation or the administration of antivenom can be discharged home with instructions to represent should any symptoms return. Antivenom may be effective days after the bite (and possibly longer) however a larger amount of antivenom is usually required.152,156
IV administration has been advocated in severe cases or where there is poor response to IM administration.152,156 The manufacturer recommends that for life-threatening envenomation the IV route may be used after first diluting the antivenom to 1 : 10 with Hartmann’s solution and administered over 20 minutes.156,159 IV administration is safe with reactions uncommon (less than 5%).160 No significant benefit of IV administration over IM administration was demonstrated in a randomised controlled trial, so there is little evidence to justify one route of administration over another.160 Redback spider antivenom administration in various stages of pregnancy has not been associated with direct or indirect harmful effects to the fetus.152
Funnel-web Spider Bite
Description and incidence
Funnel-web spiders are the most venomous spiders to humans worldwide,157,159,161 and Australian funnel-web spiders (Atrax or Hadronyche genera) are found primarily along the east coast. The Sydney funnel-web spider (Atrax robustus) is found mainly within a 160 km radius of Sydney, while other species are found in eastern New South Wales and central and southern Queensland. The spider is large, black or dark brown, and approximately 3 cm long in the body. The cephalothorax is oval, smooth and shiny, and the eyes are closely grouped. The abdomen is similar in size to the thorax and is dull and hairy with spinnerets, that project noticeably behind the body. The legs are moderately long and are black or dark plum in colour. Male spiders have longer legs, smaller abdomens and are significantly more toxic than females.161
Clinical manifestations
Funnel-web spider bites are potentially rapidly lethal; however, only 10–20% of bites result in systemic envenomation, with the majority being minor and not requiring antivenom. The bite is extremely painful, and fang marks may be seen. Signs and symptoms of systemic envenomation may appear within 10 minutes, and include perioral tingling and tongue fasciculation; increased salivation, lacrimation, piloerection, sweating; nausea, vomiting, headache; hypertension, tachycardia; dyspnoea, pulmonary oedema; and irritability, decreased consciousness and coma.156,162 Regardless of the presence of symptoms, all possible funnel-web spider bites are managed as a medical emergency.156
Assessment
• airway compromise due to decreased level of consciousness requiring airway protection with an airway adjunct or endotracheal intubation
• breathing for respiratory compromise due to pulmonary oedema, requiring CPAP or intubation/ventilation with PEEP (see Chapter 13)
• circulatory compromise due to profound hypotension (although this is a late sign and hypertension is more commonly seen), requiring IV access and volume replacement. Circulatory compromise/failure may lead to cardiac arrest requiring cardiopulmonary resuscitation (see Chapter 24).
All patients require full monitoring with constant nursing observation. A patient with no signs of envenomation on arrival has a detailed history taken regarding the circumstances of the bite, the time, description of spider and any first aid undertaken. The patient is then regularly assessed for any symptoms suggesting systemic envenomation. After thorough medical assessment, if there are no signs of systemic envenomation, any first aid such as a pressure immobilisation bandage is removed and the patient observed for 6 hours.156 With no diagnostic test for funnel-web spider envenomation and no venom detection procedure available,156 clinical diagnosis is based on the history and symptoms.
Management
For signs of systemic envenomation, two ampoules of funnel-web spider antivenom is administered slowly IV over 15–20 minutes;161,162 premedication is not required,156 although the patient is observed closely for anaphylaxis. In severe envenomation associated with dyspnoea, pulmonary oedema or decreased LOC, the initial antivenom dose should be doubled to four ampoules.161,162 More antivenom may be required until all major symptoms have resolved (severe bites often require eight ampoules).156,161,162 The antivenom dose for children is the same as the adult dose.156,161,162 First aid measures such as a pressure immobilisation bandage can be removed after antivenom administration and the symptoms have stabilised; this may take several hours.156
Snake Bites
Description and incidence
The Australian continent is inhabited by a large number of snakes (over 140 recognised snakes from 30 different species; 25% of all known venomous snakes, and 40% of all dangerous front-fanged snakes).151,163 New Zealand has no known venomous terrestrial snakes.151 Australian venomous snakes are found in both rural areas and residential and metropolitan areas, especially when in close proximity to bushland and in periods of drought. Distribution is within known geographical areas, and nurses require familiarity with the venomous snakes that inhabit their locality of practice. The incidence of snakebite is estimated at 500–3000 each year, with approximately 200–500 cases requiring treatment with antivenom.164 There are on average 1–3 deaths per year, although this may be higher due to unrecognised snake bites.164
Clinical manifestations
The majority of snake bites do not result in significant envenomation.165 Bites are generally recognised by the patient at the time because of associated pain, although some bites are unrecognised. The bite site may show minimal to obvious signs of punctures or scratches, with accompanying swelling and bruising. Multiple bites are possible and are generally associated with major envenomation.156,165 Australian snake venoms contain a number of various toxins that are responsible for the systemic effects156,165,166 (see Table 22.11). Renal damage may occur as a consequence of myoglobinuria from severe rhabdomyolysis or haemoglobinuria associated with coagulopathies,165 leading to acute renal failure (see Chapter 18).164
TABLE 22.11 Characteristics and clinical manifestations of snake venom156,165,166
| Toxin | Effects | Signs and symptoms |
|---|---|---|
| Neurotoxin | Blocks transmission at the neuromuscular junctions, causing skeletal and respiratory muscle flaccid paralysis, either presynaptic and/or postsynaptic. |
• defibrination with low-fibrinogen, unclottable blood, but usually with a normal platelet count; or
• direct anticoagulation with normal fibrinogen and platelet count.
Both cause an elevated prothrombin ratio (INR).
Assessment
Patients presenting with snake bite(s) are allocated a high priority for assessment and treatment even if they appear well on arrival. Patients who present without effective first aid measures (the application of a pressure immobilisation bandage and splint) have these applied immediately.165 The pressure immobilisation bandage is applied with a broad (15 cm) crepe bandage, commencing over the bite site with the same pressure that would be used for a sprained ankle. The bandage is then extended to cover the whole limb, including fingers/toes, and the limb is splinted and immobilised.167 Correct application of the pressure bandage is important, as any benefit is lost with bandages that are too loose, not applied to the whole limb, or with no splinting or immobilistaion.167 Elasticised bandages are superior to crepe bandages in obtaining and maintaining adequate pressure.168 Do not wash the wound prior to applying the pressure immobilisation bandage, as swabbing of the bite site is used when performing venom detection. The patient should not mobilise, to minimise distribution of any injected venom. Once applied the pressure immobilisation bandage is not removed until the patient is in a hospital that is stocked with antivenom.164
A brief and focused history explores the time and circumstances of the bite, a description of the snake (colour, length), geographical location and the application of any first aid. The patient is assessed for general symptoms including headache, nausea, vomiting, abdominal pain, collapse, convulsions and anxiety (these alone do not indicate envenomation),164,165 as well as blurred or double vision, slurred speech, muscle weakness, respiratory distress, bleeding from the bite site or elsewhere, and pain and swelling at the bite site and associated lymph nodes.
All probable snake bites require observation for at least 12 hours, as some serious symptoms may be delayed.164,165 Assess for tachycardia, hypotension or hypertension, and a falling oxygen saturation, respiratory rate, forced vital capacity (FVC) or peak expiratory flow rate (PEFR), indicating respiratory muscle paralysis.165 Frequent neurological observations focus on identification of muscle weakness and paralysis; note any ptosis, diplopia, dysphagia, slurred speech, limb weakness or an altered level of consciousness. Insert an indwelling catheter for close monitoring of urine output and presence of any myoglobin.
To identify the likely snake involved and the correct antivenom required, a bedside snake venom detection kit (SVDK) is used at the bite site or with urine. A swab of the washings from the bite is collected by leaving the pressure immobilisation bandage on and creating a window over the bite site to expose the area. Testing takes about 25 minutes. If there are signs of systemic envenomation, urine can be used to perform the test; blood should be avoided, as it is unreliable. A positive result indicates that venom from a particular snake is present, but does not mean that systemic envenomation has occurred, while a negative result does not exclude systematic envenomation.163,165
Practice tip
Whole blood clotting time is performed by drawing 10 mL venous blood and placing in a glass test tube. If the blood has not clotted within 10 minutes, a coagulopathy is likely to exist, suggesting envenomation.166
In patients with known snake bite and systemic envenomation, antivenom administration is required if there is any degree of paralysis, significant coagulopathy, any myolysis (myoglobinuria or CK >500), or unconsciousness or convulsions. In an asymptomatic patient with normal pathology and a negative or positive SVDK, it is likely that envenomation has not occurred. In this case, the pressure immobilisation bandage is removed under close observation in a resuscitation area. The patient is fully reevaluated including repeat blood test, assessing coagulation parameters, within 1–2 hours after removal of the pressure bandage. If the patient’s condition remains unchanged, further observation and repeat blood tests at 6 and 12 hours are required. Patients with no evidence of envenomation after 12 hours may be discharged.163,167
Management
A patient with evidence of systemic envenomation requires antivenom administration; monovalent antivenom is used in preference to polyvalent antivenom when identity of the snake is known. Polyvalent antivenom is a mixture of all monovalent antivenoms, and is therefore used for severe envenomation where the identity of the snake is unknown and the patient’s condition does not allow time for a SVDK result, or where there is insufficient monovalent antivenom available.163,165 Expert advice from a poison information centre may assist in identifying the snake, based on known habitats and distribution as well as presenting symptoms.
Antivenom is always administered intravenously in a diluted strength of 1 : 10 (or less if volume is a concern) via an infusion. Administration is commenced slowly while observing for signs of any adverse reaction. The infusion rate can be increased if no reaction occurs, with the whole initial dose administered over 15–20 minutes. The dose will vary depending on the type of antivenom, type of snake and number of bites; the use of 4–6 ampoules is not uncommon in severe envenomation.156,165 Use of premedication before antivenom administration is controversial; at present the antivenom manufacturer does not recommend any premedication to reduce the chance of anaphylaxis. Regardless of whether a premedication is used, prepare to treat anaphylaxis.165,169
When the patient’s condition has stabilised after the initial dose of antivenom, the pressure immobilisation bandage is removed, with continuous close observation for any clinical deterioration caused by the release of venom contained by the pressure bandage. If deterioration is evident, further antivenom and reapplication of the pressure immobilisation bandage may be required.163 Patients without signs of deterioration still require ongoing observation in an HDU/ICU and repeat pathology (coagulation studies) at 3 and 6 hours post-antivenom administration. Ongoing observation and pathology studies will occur for at least 24 hours.165
In children, management for snake bite is similar, with antivenom dosages the same as for an adult. Dilution volume can be reduced (from 1 : 10 to 1 : 5) for children.163
Box Jellyfish Envenomation
Chironex fleckeri (box jellyfish) is one of the world’s most dangerous venomous animals.151 The jellyfish is a cubic (box-shaped) bell measuring 20–30 cm across and weighing up to 6 kg. Four groups of tentacles, with up to 15 tentacles in each group, can stretch up to 2 m and total length can exceed 60 m. Importantly, the animal is transparent in water and is therefore difficult to identify.170,171 The tentacles are covered with millions of stinging nematocysts, each a spring-loaded capsule that contains a penetrating thread which discharges venom. Threads are 1 mm in length and capable of penetrating the dermis of adult skin. The tentacles also produce a sticky substance that promotes adherence to a victim’s skin, causing some tentacles to be torn off and remain attached to the person, where the nematocysts remain active.151
Description and incidence
Most stings occur during the summer months (December, January) in the tropical waters of northern Australia, from Gladstone in Queensland around to Broome in Western Australia, on hot, calm and overcast days when the jellyfish moves from the open sea to chase prey in shallow water.151,170,171 The exact incidence of stings is difficult to determine, but they are common in children. One ED reported 23 confirmed C. fleckeri stings in a 12-month period.172 There have been at least 63 confirmed deaths from envenomation by Chironex fleckeri in the Indo-Pacific region.
Clinical manifestations
Most stings are minor, with clinically significant stings occurring from larger jellyfish. Stings generally occur on the lower half of the body, and are characterised by immediate and severe pain. Pain increases in severity and may cause victims, especially children, to become incoherent. While mechanisms of toxicity remain poorly understood, death is thought to occur from central respiratory failure, or cardiotoxicity leading to A–V conduction disturbances or paralysis of cardiac muscle. Victims may become unconscious before they can leave the water following envenomation, and death can occur within 5 minutes.170,171
The area of tentacle contact is seen as multiple linear lesions, purple or brown in colour. A pattern of transverse bars is usually seen along the lesions, along with an intense acute inflammatory response, initially as a prompt and massive appearance of wheals followed by oedema, erythema and vesicle formation, which can lead to partial- or full-thickness skin death.151,173
Management
Treatment focuses on appropriate first aid, administration of adequate pain relief, symptomatic management of cardiovascular and respiratory effects, and the administration of box jellyfish antivenom when indicated. First aid measures include liberal application of vinegar to the sting area for 30–60 seconds. Vinegar inactivates the undischarged nematocysts, so removal of any remaining tentacles should occur simultaneously to prevent further envenomation.151,173 Mild stings respond to the application of ice packs and simple oral analgesia, after the application of vinegar.151,172 Patients with moderate to severe pain require IV narcotic analgesia. For patients with continuing severe pain, antivenom is administered along with continued parenteral analgesia.171
Patients are observed for the development of cardiorespiratory symptoms, including arrhythmias. Management focuses on specific clinical effects, ranging from oxygen administration and IV fluid resuscitation through to intubation/mechanical ventilation or CPR.151,173 Antivenom is indicated in patients with cardiorespiratory instability, cardiac arrest or severe pain unrelieved by narcotic analgesia.171 Antivenom is carried by prehospital personnel, and administration may occur prior to ED presentation. A 20,000 unit ampoule of box jellyfish antivenom is diluted in 10 mL isotonic saline and administered IV over 5–10 minutes.172 The number of ampoules used varies with clinical status: at least one for cardiorespiratory instability; up to three for life-threatening situations with an inadequate response; and at least six for a cardiac arrest.151,173
While the application of a pressure immobilisation bandage to affected limbs after vinegar application was previously recommended as a first aid intervention, there is little current evidence supporting this in box jellyfish stings, and its application may promote additional venom release and therefore be potentially dangerous.171,174 Some animal research has suggested a role for magnesium sulfate in management for patients not responding to antivenom.175
Irukandji Envenomation
The Irukandji is a small marine jellyfish, with stinging tentacles capable of causing intense pain and catecholamine release.177
Description and incidence
Irukandji syndrome is a poorly-understood marine envenomation encountered in far northern and northwestern areas of Australia.178 Death is uncommon (two recorded deaths in Australia), attributed to cerebral haemorrhage and is associated with other comorbid conditions.179
Assessment
People stung by an Irukandji may have no symptoms initially, but may develop symptoms up to one hour after being stung. Irukandji syndrome produces clinical features of severe lower back pain, muscle cramps, raised blood pressure, pulse and respiratory compromise, vomiting and anxiety.177 A patient with suspected Irukandji envenomation is placed in an acute area with full monitoring available.
Management
The mainstays of patient management are pain control and symptom management. Application of vinegar as part of first aid is important, but due to delay in the presentation of symptoms following a sting this may be of limited value.178 Pain is severe, and opioid analgesia may be required; if requirements for opioids are very high, fentanyl is considered.177 There is anecdotal evidence that magnesium sulfate may have a role in the management of Irukandji syndrome not responsive to the above treatments, but this remains unproven.178
Ciguatera
Ciguatera is a type of seafood poisoning caused by the consumption of fish, especially certain tropical reef fish, that contain one or more naturally-occurring neurotoxins from the family of ciguatoxins. Ciguatera is reported as the most common form of seafood poisoning in the world,180 and is considered a mild non-fatal disease, with a world wide mortality rate ranging from 0.1–20%.181 Ciguatera as a tropical disease confined to latitudes 35°N–35°S is no longer tenable, as tropical fish are now marketed throughout the world and some species, like tuna, mackerel and dolphin fish, also migrate considerable distances. In Australia, there have been numerous outbreaks of ciguatera poisoning in Sydney and as far south as Melbourne.181,182
Ciguatera toxins (ciguatoxins) are among the deadliest poisons known, reportedly 1000 times more potent than arsenic.183 These heat-stable toxins originate from a microorganism that attaches to certain species of algae in tropical areas around the world; these toxins become altered after ingestion by progressively larger fish up the food chain.174,181
Clinical manifestations and diagnosis
Ciguatera poisoning typically presents as an acute gastrointestinal illness, followed by a neurological illness with classical symptoms of heat and cold reversal of sensation that may last for a few days after consumption of contaminated fish174 (see Table 22.12).
A patient may become sensitive to repeated exposure to ciguatoxins;174,181 additional exposure to poisoning from ciguatera may be more severe than the first episode. Importantly, patients exposed to ciguatera suffer recurrences following the consumption of seemingly innocuous foods (e.g. nuts, nut oils, caffeine, alcohol, or animal protein foods),147,181,183 with relapses months or years after the initial poisoning.183
Diagnosis is made on a patient’s history and clinical features: consumption of fish followed by an acute gastrointestinal and neurological illness. There is no conclusive diagnostic test for the presence of ciguatoxins.174,181