Emergency Anaesthesia
PREOPERATIVE ASSESSMENT
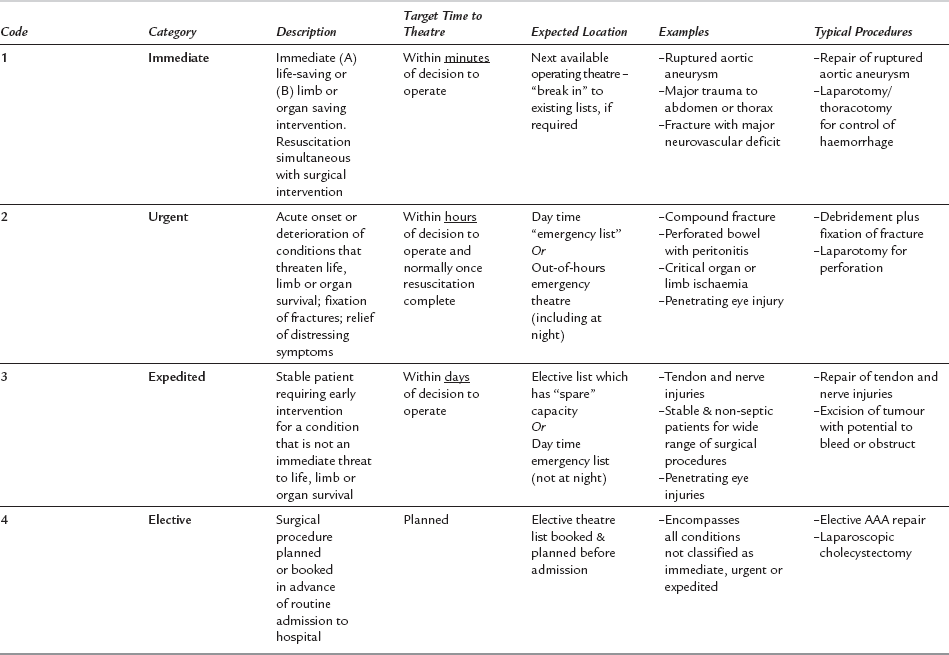
The likely surgical diagnosis, and the extent and urgency of the proposed surgery must be discussed with surgical and medical colleagues preoperatively. The urgency for surgery is most helpfully conveyed using a recognized classification system, such as the one created by the National Confidential Enquiry into Patient Outcome and Death (NCEPOD) (Table 37.1). The nature and urgency of the planned surgery dictate the extent of preoperative preparation and anaesthetic technique. They also influence plans for postoperative care, which may include transfer to a HDU/ICU facility.
During the preoperative visit a past medical and drug history is elicited. In particular, the patient’s degree of cardiorespiratory reserve should be established, even if there is no formal diagnosis of cardiovascular or respiratory disease. The presence and severity of symptoms suggestive of reduced reserve such as angina, productive cough, orthopnoea or paroxysmal nocturnal dyspnoea should be sought. The patient’s functional capacity is of useful prognostic value and can be simply quantified in terms of metabolic equivalents (METs). 1 MET is a unit of resting oxygen consumption and appropriate questioning can allow an estimate of the patient’s maximal oxygen consumption capacity (VO2 max) (Table 37.2 and Ch 18 [Table 18.1]). A patient who is unable to perform activity at 4 METs or more is at increased risk of perioperative cardiac complications.
TABLE 37.2
| MET Score | Approximate Level of Activity |
| 1 | Dress, walk indoors |
| 2 | Light housework, slow walk |
| 4 | Climb one flight of stairs |
| 6 | Moderate sport eg golf or dancing |
| 10 | Strenuous sport or exercise |
Preoperative evaluation of the airway is always important. The standard clinical tests of airway assessment should be used (see Ch 21: The practical conduct of anaesthesia) and any previous anaesthetic charts consulted if available. A history of difficult intubation is of considerable significance; however, a past record of easy tracheal intubation does not guarantee future success. In emergency anaesthesia, airway difficulties may be caused by the patient’s usual anatomy, but also surgical pathology such as dental abscesses, trauma and bleeding or haematoma. If a rapid-sequence induction is contemplated, then contingency plans are required for management of the patient in the event of failure to intubate the trachea. If a high degree of difficulty in tracheal intubation is anticipated then an awake technique may be necessary.
Assessment of Circulating Volume
Intravascular Volume Deficit
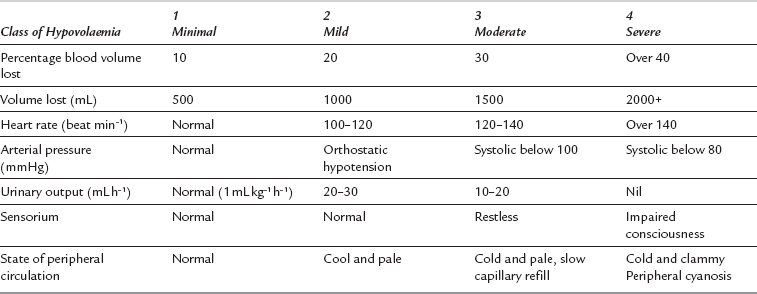
Blood loss may be assessed using the patient’s history and any measured losses, but more commonly the anaesthetist has to rely on clinical evaluation of the patient’s current cardiovascular status. Profound circulatory shock with hypotension, poor peripheral perfusion, oliguria and altered cerebration is easy to recognize. However, a more careful assessment is needed to recognise the early manifestations of haemorrhage, such as tachycardia and cutaneous vasoconstriction. Useful indices include heart rate, arterial pressure (especially pulse pressure), the state of the peripheral circulation, central venous pressure and urine output. Table 37.3 describes approximate correlations among these clinical indices and the extent of haemorrhage, but it should be stressed that these refer to the ‘ideal’ patient. In young, healthy adults, arterial pressure may be an unreliable guide to volume status because compensatory mechanisms can prevent a measurable decrease in arterial pressure until more than 30% of the patient’s blood volume has been lost. In such patients, attention should be directed to pulse rate, skin circulation and a narrowing pulse pressure. Tachycardia in the presence of a normal arterial pressure should never automatically be attributed to pain or anxiety if there is a clinical history consistent with the potential for intravascular volume loss. In elderly patients with widespread arterial disease, limited cardiac reserve and a rigid vascular tree (fixed total peripheral resistance), signs of severe hypovolaemia may become evident when blood volume has been reduced by as little as 15%. However, as baroreceptor sensitivity decreases with age, elderly patients may exhibit less tachycardia for any degree of volume depletion.
Extracellular Volume Deficit
Assessment of extracellular fluid volume deficit is difficult. Guidance may be obtained from the nature of the surgical condition, the duration of impaired fluid intake and the presence and severity of symptoms associated with abnormal losses (e.g. vomiting). At the time of the earliest radiological evidence of intestinal obstruction, there may be 1500 mL of fluid sequestered in the bowel lumen. If the obstruction is well established and vomiting has occurred, the extracellular fluid deficit may exceed 3000 mL. Table 37.4 describes some of the clinical features seen with varying degrees of severity of extracellular fluid losses. It is clear that considerable losses must occur before clinical signs are apparent, and that these signs are often subjective in more minor degrees of extracellular fluid deficit.
TABLE 37.4
Indices of Extent of Loss of Extracellular Fluid
| Percentage Body Weight Lost as Water | mL of Fluid Lost per 70 kg | Signs and Symptoms |
| Over 4% (mild) | Over 2500 | Thirst, reduced skin elasticity, decreased intraocular pressure, dry tongue, reduced sweating |
| Over 6% (mild) | Over 4200 | As above, plus orthostatic hypotension, reduced filling of peripheral veins, oliguria, low CVP, apathy, haemoconcentration |
| Over 8% (moderate) | Over 5600 | As above, plus hypotension, thready pulse with cool peripheries |
| 10–15% (severe) | 7000–10 500 | Coma, shock followed by death |
Once the extent of blood volume or extracellular fluid volume deficit has been estimated, deficits should be corrected with the appropriate intravenous fluid. The overall priority is to maintain adequate tissue perfusion and oxygenation, therefore correction of intravascular deficit takes precedence – hypovolaemia due to blood loss should be treated with either a balanced crystalloid solution (such as Hartmann’s solution) or a suitable colloid until packed red cells are available (see Ch 12: Fluid, electrolyte and acid–base balance). Resuscitation is usually guided by clinical indices of circulating volume status and organ perfusion. Central venous pressure (CVP) measurement has often been used to guide fluid therapy but CVP has limitations when used to predict intravascular volume status and responsiveness to infused fluids. High-risk surgical patients may benefit from the use of (non-invasive) cardiac output measuring devices to direct fluid resuscitation towards predetermined goals for cardiac output and systemic oxygen delivery.
THE FULL STOMACH
Gastric Emptying
Gastric emptying results from peristaltic waves sweeping from cardia to pylorus at a rate of approximately three per minute, although temporary inhibition of gastric motility follows recent ingestion of a meal. The gastric emptying of clear fluids is an exponential process, i.e. the rate of emptying at any given time is proportional to the volume of liquid in the stomach. The half-time for this process is about 20 min, so less than 2% of ingested clear fluid remains in the stomach at 2 h. The gastric emptying of solids is roughly linear, i.e. occurs at a constant rate, and usually begins about 30 min after ingestion of a meal. The rate of emptying varies depending on the composition of food ingested. Typically, about 50% of food reaches the duodenum within 2 h although meals high in fat content may take considerably longer. The rate of gastric emptying is also significantly delayed if the mixture reaching the duodenum is very acidic or hypertonic (the inhibitory enterogastric reflex), but both the nervous and humoral elements of this regulating mechanism are still poorly understood. Many pathological conditions reduce gastric emptying (Table 37.5). In the absence of any of these factors, it is reasonably safe to assume that the stomach is empty provided that solids have not been ingested within the preceding 6 h, or clear fluids consumed in the preceding 2 h, and provided that normal peristalsis is occurring. This is the usual case for elective surgical patients. However, in emergency surgery it may be necessary to induce anaesthesia urgently before an adequate period of starvation occurs. In addition, the patient’s surgical condition is often accompanied by delayed gastric emptying or abnormalities of peristalsis. In these circumstances, even if the usual period of fasting has been observed it cannot be assumed that the patient’s stomach is empty.
TABLE 37.5
Situations in Which Vomiting or Regurgitation May Occur
Full stomach
With absent or abnormal peristalsis
Peritonitis of any cause
Postoperative ileus
Metabolic ileus: hypokalaemia, uraemia, diabetic ketoacidosis
Drug-induced ileus: anticholinergics, those with anticholinergic side-effects
With obstructed peristalsis
Small or large bowel obstruction
Gastric carcinoma
Pyloric stenosis
With delayed gastric emptying
Diabetic autonomic neuropathy
Fear, pain or anxiety
Late pregnancy
Opioids
Head injury
Other causes
Hiatus hernia
Oesophageal strictures – benign or malignant
Pharyngeal pouch
TECHNIQUES OF ANAESTHESIA
Phase I – Preparation
 Although not completely effective, insertion of a nasogastric tube to decompress the stomach and to provide a low-pressure vent for regurgitation may be helpful. Aspiration through the tube may be useful if gastric contents are liquid, as in bowel obstruction, but is less effective when contents are solid. Cricoid pressure is still effective at reducing regurgitation even with a nasogastric tube in situ.
Although not completely effective, insertion of a nasogastric tube to decompress the stomach and to provide a low-pressure vent for regurgitation may be helpful. Aspiration through the tube may be useful if gastric contents are liquid, as in bowel obstruction, but is less effective when contents are solid. Cricoid pressure is still effective at reducing regurgitation even with a nasogastric tube in situ.
 Clear oral antacids (e.g. sodium citrate) may be used to raise the pH of gastric contents immediately before induction. However, this also increases gastric volume. Particulate antacids should not be used, as they may be very damaging to the airway if aspirated. The preoperative administration of H2-receptor antagonists consistently raises gastric pH and may reduce the chance of chemical pulmonary injury occurring in the event of inhalation. Although this is standard practice in obstetric anaesthesia, few anaesthetists employ these measures for emergency general surgery. The regimens available are described in Chapter 35.
Clear oral antacids (e.g. sodium citrate) may be used to raise the pH of gastric contents immediately before induction. However, this also increases gastric volume. Particulate antacids should not be used, as they may be very damaging to the airway if aspirated. The preoperative administration of H2-receptor antagonists consistently raises gastric pH and may reduce the chance of chemical pulmonary injury occurring in the event of inhalation. Although this is standard practice in obstetric anaesthesia, few anaesthetists employ these measures for emergency general surgery. The regimens available are described in Chapter 35.
Phase II – Induction
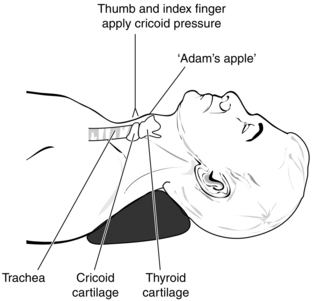
Heart rate, arterial pressure (and, when appropriate, central venous pressure) and ECG are monitored before induction of anaesthesia, and a skilled assistant is in position at the patient’s side to perform Sellick’s manoeuvre (cricoid pressure). It is important that the assistant can identify the cricoid cartilage, as compression of the thyroid cartilage distorts laryngeal anatomy and may render tracheal intubation very difficult. To perform Sellick’s manoeuvre correctly, the thumb and forefinger press the cricoid cartilage firmly in a posterior direction with a force of 20–40 N, thus compressing the oesophagus between the cricoid cartilage and the vertebral column. Because the cricoid cartilage forms a complete ring, the tracheal lumen is not distorted (Fig. 37.1).
Awake Intubation
Although blind nasal intubation is a valuable skill, the introduction of the narrow-gauge fibreoptic intubating laryngoscope has replaced it as the technique of choice in those patients who are likely to develop unrelievable airway obstruction when loss of consciousness occurs (e.g. trismus from dental abscess) or who are known or suspected to pose difficulties with intubation. Such endoscopic tracheal intubations may be performed via either the nasal route (more commonly used) or the oral route (see Ch 22: Management of the difficult airway). Before embarking on awake fibreoptic nasal intubation, the nasopharynx and, to a greater or lesser extent, the upper airway must be rendered insensitive, so that the patient can tolerate the introduction of a tracheal tube (see Ch 43: Complications during anaesthesia).
Phase III – Maintenance of Anaesthesia
When the neuromuscular blocker has been injected, the tracheal tube is connected to a mechanical ventilator and minute volume adjusted to produce normocapnia. Ventilators are now increasingly sophisticated and incorporate a choice of ventilation modes. The choice is usually between pressure controlled or volume controlled ventilation. It can be difficult to predict ventilator requirements, but initial settings should aim to produce a minute volume of 75–100 mL kg –1 min –1 with a tidal volume of 6–8 mL kg –1. The inspiratory flow rate should be adjusted to minimize peak airway pressure, and the capnograph waveform and pressure volume loops should be inspected regularly to guide the further adjustment of ventilator settings. Maintenance of core temperature is a very important aspect of intraoperative management – core temperature should be monitored throughout the procedure and hypothermia avoided whenever possible (see Ch 43: Complications during anaesthesia).
The use of supplemental doses of analgesic and neuromuscular blocking drugs is described in Chapters 5 (Analgesic drugs) and 6 (Muscle function and neuromuscular blockade). The trainee should be familiar with the pharmacokinetics and pharmacodynamics of all drugs used and be aware that these may change during emergency anaesthesia, when acute circulatory changes or impaired organ function often occur.
Fluid Management
During emergency intra-abdominal surgery, there may be large blood and fluid losses, which exceed the patient’s maintenance fluid replacement. Hartmann’s solution (compound sodium lactate) is still the preferred i.v. fluid during surgery. More sophisticated methods of determining intravascular volume status and cardiac output are increasingly used. Although much of the work in this field has been performed in patients undergoing elective surgery, there are a few examples of fluid optimization using cardiac output monitoring proving beneficial in emergency patients (Sinclair et al 1997). Methods of determining cardiac output are covered in Chapter 16 (Clinical measurement and monitoring).
The requirement for blood transfusion varies in different groups of patients. In general the threshold for transfusion of blood (or more commonly packed red blood cells), the ‘transfusion trigger’, is a haemoglobin concentration 8 g dL –1 (Carless et al 2010). A higher transfusion trigger is often used for certain patient populations, for example 10 g dL –1 in patients with ischaemic heart disease, though the evidence for this is not established. Near-patient-testing devices, sampling from arterial or venous catheters are invaluable aids to guide transfusion during surgery.
Phase IV – Reversal and Emergence
The adequacy of reversal of paralysis may be determined by observing the patient’s ability to sustain a head lift for 5 s and sustain a firm grip without fade, although clinical signs can be misleading. Preferably, a nerve stimulator is used to define reversal of neuromuscular transmission (see Ch 6: Muscle function and neuromuscular blockade).
Phase V – Postoperative Management
Prophylactic Postoperative IPPV
Continuation of IPPV should be considered electively in several circumstances, some of which are listed in Table 37.6. There should be close cooperation between intensive care colleagues, surgeons and anaesthetists when the decision is made to continue ventilation.
TABLE 37.6
Indications for Postoperative Ventilation
Prolonged shock/hypoperfusion state of any cause
Massive sepsis (faecal peritonitis, cholangitis, septicaemia)
Severe ischaemic heart disease
Extreme obesity
Overt gastric acid aspiration
Severe pulmonary disease
Emergency Laparotomy in the Older Patient
 Is it likely that the patient will die with or without surgery? If the answer is yes, then surgery is not indicated unless it is likely that it will contribute to the physical comfort of the patient during the dying process, i.e. contribute to a ‘good death’.
Is it likely that the patient will die with or without surgery? If the answer is yes, then surgery is not indicated unless it is likely that it will contribute to the physical comfort of the patient during the dying process, i.e. contribute to a ‘good death’.
 Is it likely that the patient would survive a laparotomy if the underlying cause were found to be curable/treatable (e.g. perforated duodenal ulcer, appendicitis)? If the answer is yes, then surgery is indicated and agreement on the appropriate level and duration of postoperative organ support must be reached.
Is it likely that the patient would survive a laparotomy if the underlying cause were found to be curable/treatable (e.g. perforated duodenal ulcer, appendicitis)? If the answer is yes, then surgery is indicated and agreement on the appropriate level and duration of postoperative organ support must be reached.
 If, having embarked on surgery, the underlying cause is found to be treatable but not curable (e.g. perforated carcinoma with metastases, gangrenous bowel), would radical surgery be appropriate, given the patients overall state? If not, aggressive postoperative intensive care is not indicated. If yes, then what would be an appropriate level and duration of postoperative support? These questions need to be answered by experienced clinicians.
If, having embarked on surgery, the underlying cause is found to be treatable but not curable (e.g. perforated carcinoma with metastases, gangrenous bowel), would radical surgery be appropriate, given the patients overall state? If not, aggressive postoperative intensive care is not indicated. If yes, then what would be an appropriate level and duration of postoperative support? These questions need to be answered by experienced clinicians.
THE ANAESTHETIST AND MAJOR TRAUMA
Effective management of major trauma requires:
1. Rapid primary survey. Recognition and treatment of any immediately life-threatening injuries, such as airway obstruction, tension/open pneumothorax, massive bleeding (chest, abdomen, pelvis, long bones or external), cardiac tamponade or intracranial injury.
2. Damage control resuscitation. Control of haemorrhage, achieving adequate tissue perfusion, haemostatic resuscitation and damage control surgery.
3. On-going, repeated examination to identify threats to life or limb and all associated injuries.
Primary Survey/Damage Control Resuscitation
Airway/Breathing
In general, airway assessment reveals one of three clinical scenarios:
 Patient is conscious, alert, talking. Give high-flow oxygen via face mask. There is no need for immediate airway intervention and a full clinical evaluation can be done. Persisting signs of shock and/or the diagnosis of serious underlying injuries might be an indication for planned endotracheal intubation and mechanical ventilation
Patient is conscious, alert, talking. Give high-flow oxygen via face mask. There is no need for immediate airway intervention and a full clinical evaluation can be done. Persisting signs of shock and/or the diagnosis of serious underlying injuries might be an indication for planned endotracheal intubation and mechanical ventilation
 Patient has a reduced conscious level but some degree of airway control and gag reflex still present. If the patient is maintaining the airway and breathing adequately then there is no need for immediate intervention. Endotracheal intubation will be necessary but a clinical evaluation can be done whilst equipment is being readied.
Patient has a reduced conscious level but some degree of airway control and gag reflex still present. If the patient is maintaining the airway and breathing adequately then there is no need for immediate intervention. Endotracheal intubation will be necessary but a clinical evaluation can be done whilst equipment is being readied.
 Patient has a reduced conscious level, gag reflex absent. If the patient is unable to maintain the airway or is breathing poorly tracheal intubation and artificial ventilation should be carried out at once.
Patient has a reduced conscious level, gag reflex absent. If the patient is unable to maintain the airway or is breathing poorly tracheal intubation and artificial ventilation should be carried out at once.
Circulation
 Adequate oxygen delivery to the tissues;
Adequate oxygen delivery to the tissues;
 Stabilisation and/or correction of metabolic derangements;
Stabilisation and/or correction of metabolic derangements;
 Correction of acute coagulopathy of trauma and prevention of iatrogenic coagulopathy.
Correction of acute coagulopathy of trauma and prevention of iatrogenic coagulopathy.
 Application of a pelvic binder
Application of a pelvic binder
 The binder should be placed around the trochanters not the iliac crests. The aim is to close the pelvis, not compress it.
The binder should be placed around the trochanters not the iliac crests. The aim is to close the pelvis, not compress it.
 If the binder was applied pre-hospital leave it in place, check it is positioned correctly, and x-ray.
If the binder was applied pre-hospital leave it in place, check it is positioned correctly, and x-ray.
Damage Control Surgery
 Systolic blood pressure > 90 mmHg: urgent trauma CT
Systolic blood pressure > 90 mmHg: urgent trauma CT
 Systolic blood pressure 70-90 mmHg: senior decision making; if choose CT MUST be accompanied by trauma team
Systolic blood pressure 70-90 mmHg: senior decision making; if choose CT MUST be accompanied by trauma team
 Systolic blood pressure < 70 mmHg and not responding: operating theatre
Systolic blood pressure < 70 mmHg and not responding: operating theatre
The aims of Damage Control Surgery are:
 control haemorrhage: packs, clamps, ligation, splinting of fractures
control haemorrhage: packs, clamps, ligation, splinting of fractures
 decompression of at risk compartments: head, heart, limbs, abdomen
decompression of at risk compartments: head, heart, limbs, abdomen
 minimise contamination: debridement of fractures and wounds, closure or resection of hollow viscus injuries.
minimise contamination: debridement of fractures and wounds, closure or resection of hollow viscus injuries.
 Normal coagulation: fibrinogen > 1.5 g dL– 1; normal APTT and PT.
Normal coagulation: fibrinogen > 1.5 g dL– 1; normal APTT and PT.
 Normal or improving lactate – a marker of adequacy of resuscitation
Normal or improving lactate – a marker of adequacy of resuscitation
It is often necessary to induce anaesthesia in a hypovolaemic patient; this requires meticulous attention to fluid and drug management. A controlled rapid-sequence induction using thiopental, propofol or ketamine should be performed, but with extreme care over the dose of induction agent. Often very small doses of ketamine (0.3–0.7 mg kg–1) suffice. The use of etomidate in trauma is still contentious due to its metabolic effects. The depressant effects of i.v. induction agents are exaggerated because the proportion of the cardiac output going to the heart and brain is increased. In addition, the rate of redistribution and/or metabolism is decreased as a result of reduced blood flow to muscle, liver and kidneys and thus blood concentrations remain increased for longer periods in comparison with healthy patients. Ketamine can be used in patients with a significant head injury as the benefits of maintained arterial blood pressure outweigh any concerns about cerebrometabolic effects. Figure 37.2 illustrates monitoring often used for the management of major trauma. Particular care needs to be taken in the presence of hypovolaemia and severe traumatic brain injury as hypotension is associated with a doubling of mortality.
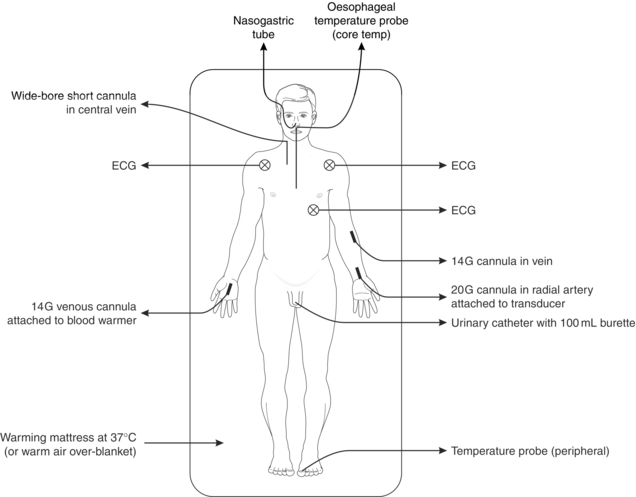
FIGURE 37.2 Commonly used monitoring and resuscitation attachments in management of a patient with multiple injuries. Pulse oximetry and capnography are also used during anaesthesia.
When surgical bleeding has been controlled, the patient’s cardiovascular status should improve, but if hypotension persists despite apparently adequate fluid administration, other causes of haemorrhage should be sought (Table 37.7). It is important that the anaesthetist assesses the patient regularly during prolonged anaesthesia to exclude these latent complications of major trauma.
TABLE 37.7
Causes of Persistent Hypotension
Surgical or medical (check platelets and clotting screen)
Continued overt bleeding
Continued concealed bleeding – chest, abdomen, retroperitoneal space, pelvis, soft tissues of each thigh
Pump failure – haemothorax, pneumothorax, tamponade, myocardial contusion
Metabolic problem – acidaemia (only correct pH less than 7.1), hypothermia (largely preventable), hypocalcaemia
American College of Surgeons. Advanced trauma life support program for doctors, sixth ed. Chicago: American College of Surgeons; 1997.
Carless, P.A., Henry, D.A., Carson, J.L. Transfusion thresholds and other strategies for guiding allogenic red blood cell transfusion (Review). The Cochrane Collaboration, 2010. 2010. Cochrane Database Syst. Rev. CD002042.
Funk, D.J., Moretti, E.W., Gan, T.J. Minimally invasive cardiac output monitoring in the perioperative setting. Anesth. Analg. 2009;108:887–897.
Marik, P.E., Baram, M., Vahid, B. Does central venous pressure predict fluid responsiveness?; a systematic review of the literature and the tale of seven mares. Chest. 2008;134:172–178.
Moran, C.G., Forward, D. The early management of patients with multiple injuries : an evidence-based, practical guide for the orthopaedic surgeon. J. Bone Joint Surg. Br. 2012;94-B:446–453.
National Confidential Enquiry into Patient Outcome, Death (NCEPOD). An age old problem: ‘A review of the care received by elderly patients undergoing surgery’, 2010. http://www.ncepod.org.uk/2010report3/downloads/EESE_fullReport.pdf
Sinclair, S., James, S., Singer, M. Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: randomised controlled trial. BMJ. 1997;315:909–912.
Stainsby, D., MacLennan, S., Hamilton, P.J. Management of massive blood loss: a template guideline. Br. J. Anaesth. 1997;85:487–496.