CHAPTER 11 Embryonic induction and cell division
EMBRYONIC INDUCTION AND CELL DIVISION
Tissue interactions
There are two types of cell and tissue interaction, namely, permissive and instructive.
An instructive (directive) interaction (induction) changes the cell type of the responding tissue, so that the cell population becomes restricted. Wessells (1977) proposed four general principles in most instructive interactions:
1 In the presence of tissue A, responding tissue B develops in a certain way.
2 In the absence of tissue A, responding tissue B does not develop in that way.
3 In the absence of tissue A, but in the presence of tissue C, tissue B does not develop in that way.
4 In the presence of tissue A, a tissue D, which would normally develop differently, is changed to develop like tissue B.
Principles 1–4 are exemplified during induction of the lens vesicle by the optic cup (p. 699). An example of principle 4 is the experimental association of chicken flank ectoderm with mouse mammary mesenchyme, which results in the morphogenesis of mammary gland-like structures: chickens do not normally develop mammary glands.
Tissue interactions continue into adult life and are probably responsible for maintaining the functional heterogeneity of adult tissues and organs. This is exemplified by the complex tissue heterogeneity, with sharply compartmentalized boundaries, that occurs in the oral cavity. The junctions between the mucosa of the vestibule and the lip, and between the vermilion border and the facial skin, are distinct boundaries of specific epithelial and mesenchymal differentiation, and are almost certainly maintained by continuing epithelial–mesenchymal interactions in adult life. Perturbation of these interactions throughout the body may underlie a wide variety of adult diseases, including susceptibility to cancer and proliferative disorders.
Signalling between embryonic cells and tissues
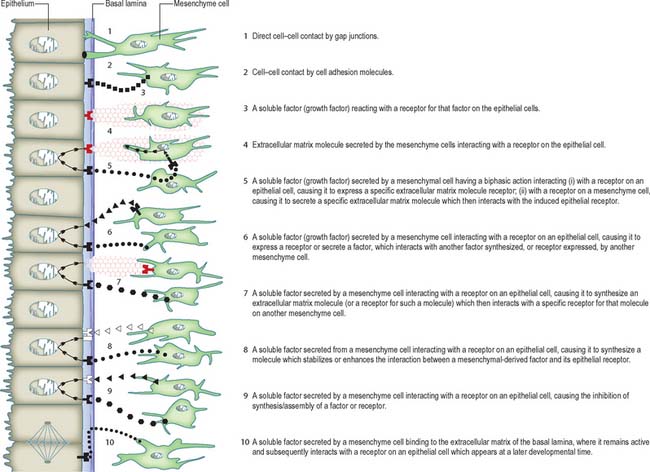
Cellular interactions may be signalled by four principal mechanisms: direct cell–cell contact; cell adhesion molecules and their receptors; extracellular matrix molecules and their receptors; growth factors and their receptors. Many of these mechanisms interact, and it is likely that combinations of them are involved in development. Figure 11.1 illustrates diagrammatically some ways by which mesenchymal cells could signal to epithelial cells. An additional set of identical mechanisms could operate for epithelial-to-mesenchymal cell signalling. Clearly, the complexity of these mechanisms will increase in reciprocal interactions; moreover, a single molecule may have different effects on epithelial and mesenchymal cells.
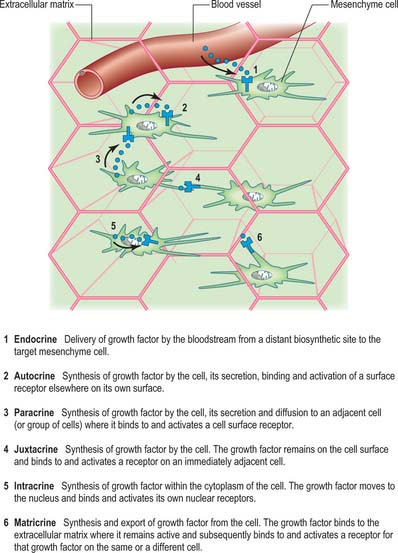
Growth factors are distinguished from extracellular matrix molecules. They can be delivered to, and act upon, cells in a variety of ways, namely endocrine, autocrine, paracrine, intracrine, juxtacrine and matricrine (Fig. 11.2). Many growth factors are secreted in a latent form, e.g. associated with a propeptide (latency-associated peptide) in the case of transforming growth factor β, or attached to a binding protein, in the case of insulin-like growth factors.
MORPHOGENESIS AND PATTERN FORMATION
The most obvious examples of morphogenesis are the large migrations that occur during gastrulation; local examples include branching morphogenesis, which occurs e.g. in the developing lungs and kidneys, and in most glandular organs. The development of branches from a tubular duct occur over a period of time. An interaction between the proliferating epithelium of the duct and its surrounding mesenchyme and extracellular matrix results in a series of clefts that produce a characteristic branching pattern (Fig. 11.3). During tubular and acinar development, hyaluronidase secreted by the underlying mesenchymal cells breaks down the basal lamina produced by the epithelial cells; this increases epithelial mitoses locally and results in an expanding acinus. Cleft formation is initiated by the mesenchyme, which produces collagen III fibrils within putative clefts. (If the collagen is removed, no clefts develop, whereas if excess collagen is not removed, supernumerary clefts appear.) The collagen acts to protect the basal lamina from the effects of the hyaluronidase, which means that the overlying epithelia have a locally reduced rate of mitosis. The region of rapid mitoses at the tip of the acinus is therefore split into two, and two branches develop from this point.
Pattern formation concerns the processes whereby the individual members of a mass of cells, initially apparently homogeneous, follow a number of different avenues of differentiation which are precisely related to each other in an orderly manner in space and time. The patterns embraced by the term apply not only to regions of regular geometric order, e.g. the crystalline lens, but also to asymmetric structures such as the limb. For such a process to occur, individual cells must be informed of their position within the embryo, and utilize that information for appropriate differentiation. Patterning of regions is seen in: the progress zone and zone of polarizing activity within the limbs; the fates of the medial and lateral and later the cranial and caudal halves of the somites; the neural crest mesenchyme within the pharyngeal arches. For details of patterning in vertebrate development, see Tickle (2003).
Hox genes in development
Homeobox genes are believed to be responsible, at least in part, for the evolutionary origin of the embryonic body plan (Robert 2001). Experimental study of transgenic animals in which the homeobox genes have been knocked out provide some evidence of their function: however, because developmental processes permit significant recovery from insult, some of the outcomes cannot be directly interpreted as demonstrating the effect of such gene loss.
Experimental approaches to embryology
One of the most exciting techniques to provide information on cell movements and fates during development is the use of chimeric embryos. Small portions of an embryo are excised and replaced with similar portions of an embryo from a different species at the same stage and the resulting development is then studied. This technique has been particularly effective using chick and quail embryos, because the nucleolus is especially prominent in all quail cells, whereas it is not prominent in chick cells, which means that quail cells may be easily identified within a chick embryo after chimeric transplantation (Le Douarin 1969). The technique has also confirmed the reciprocity of tissue interaction between the embryonic species, a phenomenon that had previously been illustrated, for a limited period, in co-cultures of embryonic avian and mammalian tissues. Somite development and vertebral formation have been studied in mouse–chick chimeras (Fontaine-Pérus 2000). The production in vitro, of human-animal chimeric cell lines is providing new ways of studying cellular pathways, as is the introduction of human artificial chromosome vectors into animal cells to study their interaction.
Fontaine-Pérus J. Mouse-chick chimera: an experimental system for study of somite development. Curr Top Dev Biol. 2000;48:269-300.
Le Douarin NM. Particularités du noyau interphasique chez la Caille japonaise (Coturnix coturnix japonica). Utilisation de ces particularités comme ‘marquage biologique’ dans les recherches sur les interactions tissulaires et les migrations cellulaires au cours de l’ontogenèse. Bull Biol Fr Belg. 1969;103:435-452.
Robert JS. Interpreting the homeobox: metaphors of gene action and activation in development and evolution. Evol Dev. 2001;3:287-295.
Tickle C. Patterning in Vertebrate Development. Oxford: Oxford University Press, 2003.
Wessells NK. Tissue Interaction and Development. Menlo Park CA: Benjamin, 1977.