Chapter 1 Embryology and Developmental Anatomy of the Elbow
Introduction
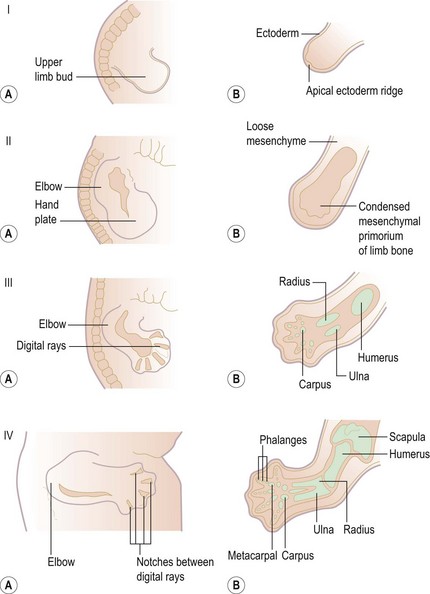
Development of the upper limb (Fig. 1.1) can best be understood as a series of ectodermal and mesodermal interactions controlled by a complex system of signalling during embryogenesis. The development starts as a limb bud in the ventrolateral wall of the embryo on the ‘Wolff crest’ 28 days after fertilization. Differentiation of the limb buds occur between the 5th and 8th week.1–4 The formation of limb buds is induced by the adjacent somites. The bones are formed as mesenchymal condensations that first chondrify then ossify. Limb muscles are formed from myogenic precursor cells that originate in mesodermal somites. The peripheral nerves arise from the neural crest. They migrate and extend their axons later in response to trophic cues, such as ephrins, produced by the muscles and connective tissues.5,6
Elbow joint development
| Structure | Origin |
|---|---|
| Bone | Mesenchymal condensations that chondrify then ossify |
| Muscle | Myogenic precursor cells |
| Peripheral nerves | Neural crest |
Prenatal development
The ectodermal covering at the site of each putative limb thickens to form the apical ectodermal ridge (AER). This together with the somatopleuric mesenchyme is called the progress zone. The orientation and progression of limb development are controlled by the progress zone. The differentiation occurs in a proximodistal, craniocaudal and dorsoventral axis. The proximodistal axis is regulated by the progress zone, which is controlled by factors including fibroblast growth factor (FGF), bone morphogenic protein (BMP) and Hox genes.7
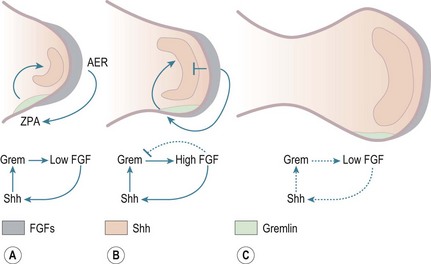
Craniocaudal polarization is regulated by a small population of mesenchymal cells on the postaxial border of the limb bud termed the zone of polarizing activity, which produces the peptide, sonic hedgehog (Shh).8 BMPs and Gremlin, a secreted inhibitor of BMPs, also participate in this pathway; the BMPs produced throughout the limb mesenchyme and in the AER inhibit production of FGFs by the AER.5 A key role of Shh is to activate expression of the BMP antagonist Gremlin, thereby preventing this inhibition of FGF expression (Fig. 1.2). The dorsoventral axis (radio-ulnar axis in upper limb) is controlled by the ectoderm of the limb.
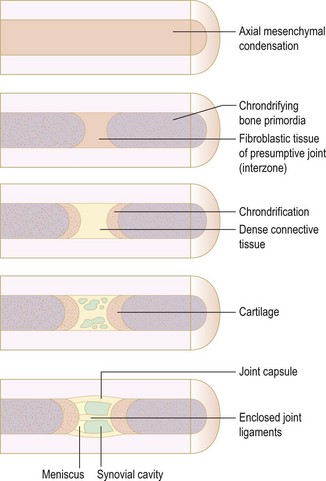
While the exact mechanism of chondrogenesis is unclear, the Hox genes, BMP, Sox9, Indian hedgehog (Ihh) and parathyroid hormone-related protein (PTHrP) play an important role.9,10 The elbow joint develops from mesenchymal interzones.11 The mesenchymal interzone between the chondrifying bone ends differentiates into fibroblastic tissue (Fig. 1.3). This further differentiates into three layers. The outer layers form the cartilage layer. Vacuoles appear within the central layer, which coalesces to form the synovial cavity. The capsule is formed from the mesenchymal sheath surrounding the entire interzones.
Also by this stage muscles and tendons are well developed and have obtained a nerve supply from the developing limb bud (Fig. 1.4).
Postnatal development
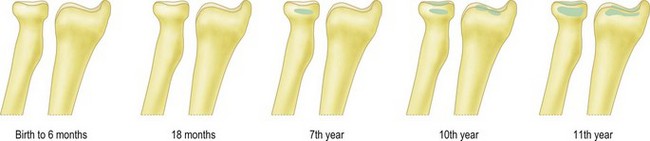
The distal humerus ossifies by four ossific centres: the capitellum and lateral part of trochlea, the medial epicondyle, the medial two-thirds of the trochlea and the lateral epicondyle (Fig. 1.5). The distal humerus is cartilaginous at birth. This is important as trauma to the distal humerus in the first 6 months of life can displace the whole of the distal humerus, which can be difficult to diagnose by plain radiographs. In addition, it is essential to be aware of the sequence in which the ossific centres appear at the elbow in order to appropriately recognize and manage paediatric elbow injuries.
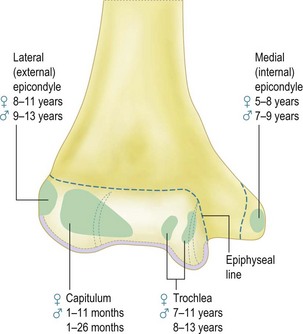
Figure 1.5 The time of appearance of the ossific centres at the lower end of the humerus.
Redrawn from Stanley D and Kay NRM (eds). Surgery of the elbow. Hodder Arnold 1998 (fig 1.7, p 5).
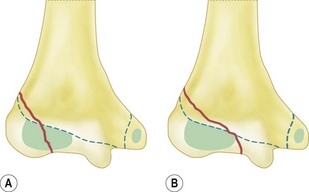
The ossific centre for the capitellum and the lateral one-third of the trochlea appears first. This occurs early in females at between the 1st and 11th month and between 1st and the 26th month in males. The narrower medial portion of the lozenge-shaped epiphysis forms the lateral part of the trochlea, while the lateral portion is closely associated with the lateral epicondyle. This anatomical arrangement is called the lateral condylar epiphysis. If, therefore, the capitellar epiphysis is displaced due to trauma, it almost invariably displaces as one piece including the lateral epicondyle, irrespective of whether or not the lateral epicondyle epiphysis is ossified. This arrangement of the capitellar ossific centre explains the classification of lateral condyle fracture as described by Milch. Milch type 1 is the same as Salter–Harris type IV and Milch type 2 as Salter–Harris type II (Fig. 1.6). The capitellar ossification centre fuses with the trochlear and the lateral epicondylar epiphyses at puberty and to the humerus shaft at 14–16 years.
The ossification centre of the head of the radius appears at 4 years in females and at 5 or 6 years in males. It is entirely intracapsular and is liable to develop avascular necrosis (AVN). It fuses with the radial shaft at 14 and 17 years of life. The ossification centre of the tip of olecranon appears, usually in two parts, at the 9th year of life in females and the 11th year in males. It fuses with the rest of the shaft between the ages of 14 and 16 (Fig. 1.7).
Ossification at the elbow
| Site | Appear | Fuse |
|---|---|---|
| Capitellar and lateral third of trochlea | Female 1–11 months | 14–16 years |
| Male 1–26 months | ||
| Radial head | Female 4 years | 14–17 years |
| Male 5–6 years | ||
| Medial epicondyle | Female 5–8 years | 20 years |
| Male 7–9 years | ||
| Medial two-thirds trochlea | Female 7–11 years | 14–16 years |
| Male 8–13 years | ||
| Olecranon | Female 9 years | 14–16 years |
| Male 11 years | ||
| Lateral epicondyle | Female 8–11 years | To capitellum at 6 months |
| Male 9–11 years | To humerus at puberty |
Knowledge of the normal epiphyseal ossification times is essential in the diagnosis of paediatric elbow injuries. The order of appearance of the centres can be remembered using the useful mnemonic CRITOE (Capitellum, Radial head, Internal (medial) epicondyle, Trochlea, Olecranon, External (lateral) epicondyle).When doubt exists it is appropriate to undertake radiographs of the contralateral elbow. Abnormalities of development and their management will be dealt with in Chapter 15.
1 Lewis WH. The development of the arm in Man. Am J Anat. 1902;1:145-184.
2 O’Rahilly R, Gardner E. The timing and sequence of events in the development of the limbs in the human embryo. Anat Embryol. 1975;147:1-23.
3 Gray DJ, Gardner E. Prenatal development of the human elbow joint. Am J Anat. 1951;88:429-469.
4 Anderson H. Histochemical studies of the histiogenesis of the human elbow joint. Acta Anat. 1962;51:50-68.
5 Lyons K, Ezaki M. Molecular regulation of limb growth. J Bone Joint Surg Am. 2009;91:47-52.
6 Jacob J, Hacker A, Guthrie S. Mechanisms and molecules in motor neuron specification and axon pathfinding. Bioessays. 2001;23:582-595.
7 Salsi V, Vigano MA, Cocchiarella F, et al. Hoxd13 binds in vivo and regulates the expression of genes acting in key pathways for early limb and skeletal patterning. Dev Biol. 2008;317:497-507.
8 Riddle RD, Johnson RL, Laufer E, et al. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401-1416.
9 Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332-336.
10 Verheyden JM, Sun X. An Fgf/Gremlin inhibitory feedback loop triggers termination of limb bud outgrowth. Nature. 2008;454:638-641.
11 Larsen WJ. Essentials of human embryology. Edinburgh: Churchill Livingstone; 1998.