Embryology, Anatomy, Normal Findings, and Imaging Techniques
Embryology of the Eye
Development of the eye originates from neuroectoderm, surface ectoderm, and neural crest cells.1–3 The neuroectodermal layer gives rise to the retina, iris, and optic nerve, the surface ectoderm gives rise to the lens, and the neural crest cells are responsible for vascular structures, sclera, choroid, and mesenchymal tissue from which the adnexal structures, bony orbit, fat, and nerve sheaths arise.4,5
The prime regulatory gene for human eye development is PAX6, a member of the PAX (paired box) family of transcription factors. This gene initially is expressed in a band contained in the anterior neural ridge of the neural plate before the process of neurulation.6 A single eye field is present at this stage, which then separates into two primordial optic structures under the direction of sonic hedgehog (SHH), which belongs to a family of vertebrate genes involved with encoding inductive signals during embryogenesis and is part of a vast signaling network that affects development of many tissues and organs.6
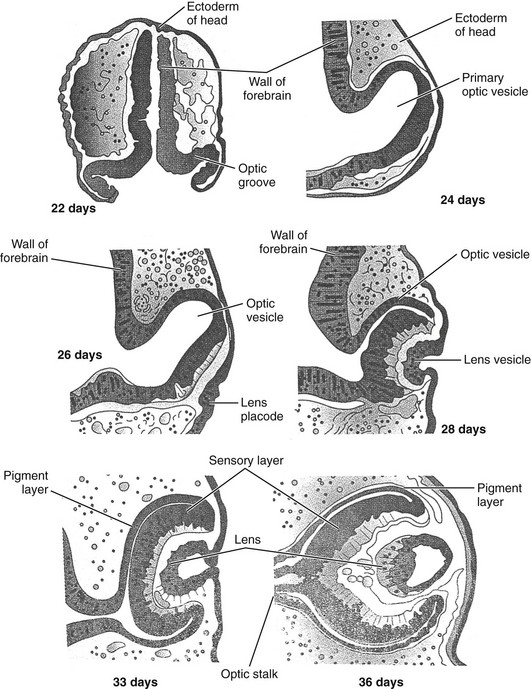
The earliest sign of eye development is the appearance in a 22-day-old embryo of a pair of shallow grooves evaginating on either side of the prosencephalon or forebrain.6 The grooves form the optic vesicles in contact with surface ectoderm. Invagination of the optic vesicle leads to formation of a double-walled optic cup. This cup has two layers that become the retina, separated by the intraretinal space. The outer layer contains pigment granules that appear during the fifth week of development. The inner layer has two parts. The pars optica retinae occupies the posterior four fifths and contains cells that ultimately differentiate into the rod and cone cells responsible for light reception. The pars ceca retinae, within the anterior fifth of the inner layer, divides into the pars iridica retinae, which will form the inner layer of the iris, and the pars ciliaris retinae, which will be involved with formation of the ciliary body. The central retinal artery results from the residuum of the embryonic hyaloid artery, which regresses by 4 months of gestation. The lips of the choroid fissure fuse during the seventh week; the mouth of the optic cup becomes the future pupil.
Elongation of surface ectoderm cells in contact with the optic vesicle results in formation of the lens placode, which develops into the lens vesicle. The lens vesicle becomes located in the mouth of the optic cup (Fig. 4-1). Cells located in the posterior wall of the vesicle elongate, forming long fibers that fill the lumen of the vesicle, reaching the anterior wall of the lens vesicle by the seventh week of development. The vascular capsule is a mesenchymal condensation that covers the lens. The hyaloid artery supplies the lens, forming a plexus on its posterior surface.7
The hyaloid artery and its branches are a transient vascular system that nourishes structures of the eye, subsequently involuting by the 35th week of gestation.5
The choroid and sclera are formed from mesenchyme surrounding the optic cup. The ciliary body and ciliary processes are formed from the anterior portion of the choroid.7
The optic cup and brain are connected by the optic stalk. The choroid fissure is a groove on the ventral surface of the optic stalk, wherein lie the hyaloid vessels. The fissure closes at 7 weeks’ gestation, during which time a narrow tunnel is formed inside the optic stalk. The inner and outer walls of the stalk fuse, becoming the optic nerve. The center contains a portion of the hyaloid artery, the central artery of the retina. The exterior of the optic nerve is covered by a continuation of the choroid and sclera, along with the pia, arachnoid, and dura.6
Development of the eye continues into postnatal life. The fovea centralis of the retina becomes differentiated by 4 months after birth. The cones remain poorly developed until 4 months after birth.8 The infant globe and orbit reach 80% of adult size during the first few years of life, with full size reached by approximately 13 years of age.
Normal Anatomy of the Orbit and Eye
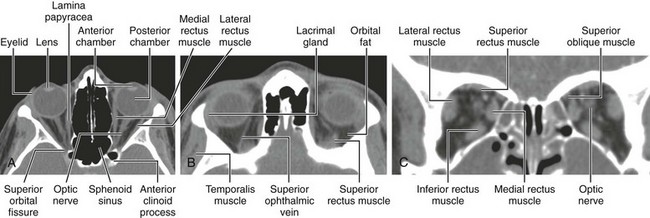
The orbits are cone-shaped cavities containing the globes, extraocular muscles, blood vessels, nerves, retrobulbar fat, and lacrimal glands. Each orbit is bounded by the floor of the anterior cranial fossa superiorly, the maxillary sinus inferiorly, the ethmoid sinus medially, and the temporal bone and middle cranial fossae anterolaterally and posterolaterally (Figs. 4-2 and 4-3).
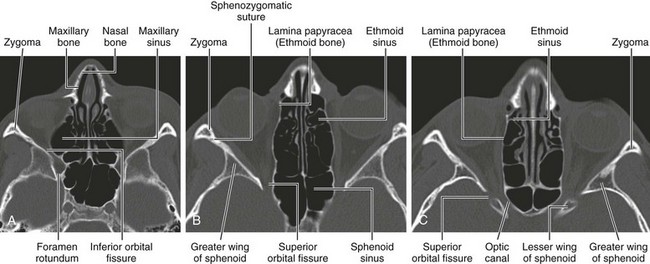
Figure 4-2 Bony orbit.
Axial noncontrast computed tomography images from inferior to superior location.
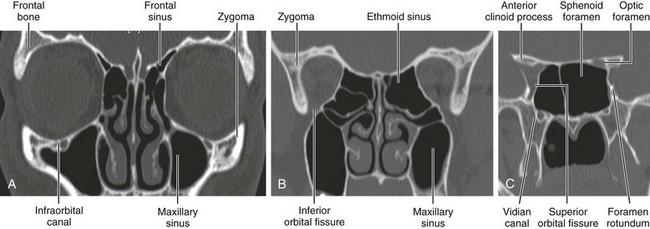
Figure 4-3 Bony orbit.
Coronal noncontrast computed tomography images from anterior to posterior location.
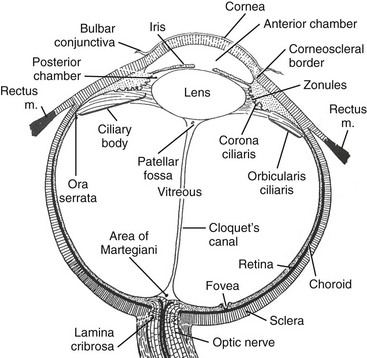
The globe occupies about 20% of the orbital cavity. It is divided by the lens into an anterior chamber filled with aqueous humor and a posterior chamber containing vitreous humor found between the lens and retina. The globe contains the retina, uveal layer, and sclera. The sclera is covered anteriorly by the conjunctiva, continuous with the mucous membrane of the eyelid (Fig. 4-4). Tenon fascia separates the globe and extraocular muscle insertions from the orbital fat.
Optic nerve sheath diameter in children as measured on magnetic resonance imaging (MRI) is 3.1 mm in the 0- to 3-year age group, 3.41 mm between 3 and 6 years, 3.55 mm between 6 and 12 years, and 3.56 mm in the 12- to 18-year age group.9
Imaging Techniques
Ultrasound
Ultrasound is extremely beneficial in infants and children because it does not use ionizing radiation, it is widely available, and the need for sedation is rare. The primary indications are disease or conditions of the anterior chamber preventing funduscopic examination of the remainder of the eye, such as hyphema and trauma, non-neoplastic leukokoria, assessment of vascular integrity of the retina or an underlying lesion, and follow-up of neoplastic causes of leukokoria after treatment.10 Secondary indications include further elucidation of intraconal and extraconal lesions and assessment of lacrimal masses.7 Ultrasound is less helpful in showing osseous abnormalities of the orbit.
The procedure is performed with the patient supine. Initially a small amount of gel is placed over the upper eyelid. Images are obtained using a high-resolution (7.0 to 10.0 MHz) linear-array transducer with color Doppler capability.10 Power output must be decreased to avoid mechanical or thermal injury. Scanning is usually performed in the axial plane; however, other planes also may be used to evaluate the entire globe10 (Fig. 4-5, A).
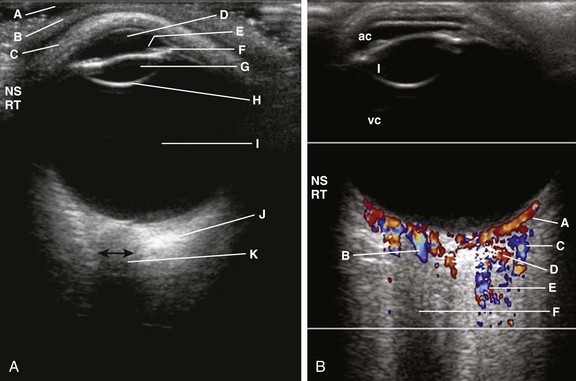
Figure 4-5 Normal ophthalmic axial ultrasound of a teenager.
A, Using a broadband, high-resolution linear array transducer (5-17 MHz extended-frequency range), sterile gel (A) is placed on the skin (B) over the upper eyelid (C), which remains shut throughout the examination. The anechoic anterior chamber (D), the iris (E), the ciliary apparatus (F), the anechoic lens (G) with the posterior reflective echo (H), the anechoic vitreous chamber (I), and the hypoechoic optic nerve (J) are demonstrated and the optic nerve width (double-ended black arrow) can be measured. B, Normal color Doppler examination of the eye in the same patient. Color Doppler imaging shows very good flow in the choroidal vessels (A), central retinal artery (B), lacrimal vein (C) and artery (D), as well as in the ophthalmic artery (E). The anterior chamber (ac), lens (l), and vitreous chambers (vc) are again noted. Different parts of the eye can be examined by having the patient move the eye as instructed. (Courtesy Faridali Ramji, MD.)
Color Doppler imaging is an important component of the ultrasound examination because it permits demonstration of the ophthalmic artery and vein, the central artery of the retina, the retinal vein, ciliary and lacrimal arteries and accompanying veins, and the superior ophthalmic vein (Fig. 4-5, B).10
Computed Tomography Scan
High-resolution, thin-section scans with a slice thickness of 2 to 3 mm are obtained in the axial planes with subsequent coronal and sagittal reformatted images, which eliminate further radiation to the patient (Fig. 4-6, A, B, and C). The axial plane is usually parallel to the canthomeatal line. Thinner images, especially in the coronal plane, may be obtained when indicated, such as visualization of the orbital floor for a blow-out fracture. Direct coronal images may be obtained in either the supine or the prone position.
Magnetic Resonance Imaging
MRI is of great value for showing soft tissue abnormalities of the orbit. Although the lack of ionizing radiation is appealing in infants and children, MRI requires a longer scan time than other modalities, and it is not helpful in detecting calcifications. Orbital MRI requires high-resolution imaging with thin sections (Fig. 4-7). MRI of the brain often is performed concomitantly.
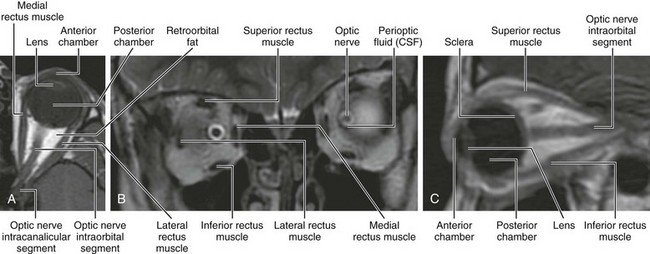
Figure 4-7 Magnetic resonance imaging of the orbit.
A, Axial T1-weighted image. B, Coronal T2-weighted image. C, Sagittal oblique T1-weighted image. CSF, Cerebrospinal fluid.
Contrast-enhanced MRI of the orbit and brain, using a head coil, is recommended for the evaluation of suspected retinoblastoma, possible subarachnoid seeding of retinoblastoma, and bilateral retinoblastoma. Early detection is made possible for optic nerve involvement, orbital spread, and asymptomatic pineoblastoma and suprasellar tumors.11
Chiasmatic lesions, such as gliomas, are studied in a manner similar to that of other supratentorial brain tumors. The fast three-point Dixon technique, which combines with FSE acquisition, has been used to look at the retrobulbar space within the orbit.12 However, the Dixon method is rarely used because it requires a minimum of two data acquisitions and is as susceptible to static field inhomogeneities as is fat saturation.13
Bilaniuk, LT, Farber, M. Imaging of developmental anomalies of the eye and the orbit. Am J Neuroradiol. 1992;13:793–803.
Mafee, MF, Karimi, A, Shah, J, et al. Anatomy and pathology of the eye: role of MR imaging and CT. Neuroimaging Clin N Am. 2005;15:23–47.
Ramji, FG, Slovis, TJ, Baker, JD. Orbital sonography in children. Pediatr Radiol. 1996;26:245–258.
References
1. Ozanics, V, Jakobiec, FA. Prenatal development of the eye and its adnexa. In: Jakobiec FA, ed. Ocular anatomy, embryology and teratology. Philadelphia: Harper and Row, 1982.
2. Smith, CG, Gallie, BLK, Morin, JD. Normal and abnormal development of the eye. In: Cawford JS, Morin JD, eds. The eye in childhood. New York: Grune and Stratton, 1983.
3. Torczynski, E. Normal development of the eye and orbit before birth: the development of the eye. In: Isenberg SJ, ed. The eye in infancy. Chicago: Year Book Medical, 1989.
4. Bilaniuk, LT, Farber, M. Imaging of developmental anomalies of the eye and the orbit. Am J Neuroradiol. 1992;13:793–803.
5. Tortori-Donato, P, Rossi, A, Biancheri, R. The orbit. In: Totori-Donato P, Rossi A, eds. Pediatric neuroradiology. Heidelberg: Springer-Verlag, 2005.
6. Sadler, TW. Eye. In Sadler TW, ed.: Langman’s medical embryology, 12th ed, Philadelphia: Lippincott Williams & Wilkins, 2012.
7. Mafee, MF. Eye and orbit. In Mafee MF, Valvassori GE, Becker M, eds.: Imaging of the head and neck, 2nd ed, New York: Thieme, 2005.
8. Hoar, RM. Embryology of the eye. Environment Health Perspect. 1982;44:31–34.
9. Shofty, B, Ben-Sira, L, Constantini, S, et al. Optic nerve sheath diameter on MR imaging: establishment of norms and comparison of pediatric patients with idiopathic intracranial hypertension with healthy controls. AJNR Am J Neuroradiol. 2012;33:366–369.
10. Ramji, FG, Slovis, TJ, Baker, JD. Orbital sonography in children. Pediatr Radiol. 1996;26:245–258.
11. Kaufman, IM, Mafee, ME, Song, CD. Retinoblastoma and simulations lesion: role of CT, MR imaging, and use of GD-DTPA contrast enhancement. Radiol Clin North Am. 1998;36:1101–1117.
12. Dixon, WT. Simple proton spectroscopic imaging. Radiology. 1984;153:189–194.
13. Delfaut, EM, Beltran, J, Johnson, G, et al. Fat suppression in MR imaging: techniques and pitfalls. Radiographics. 1999;19:373–382.