Chapter 4 Embryo and fetus
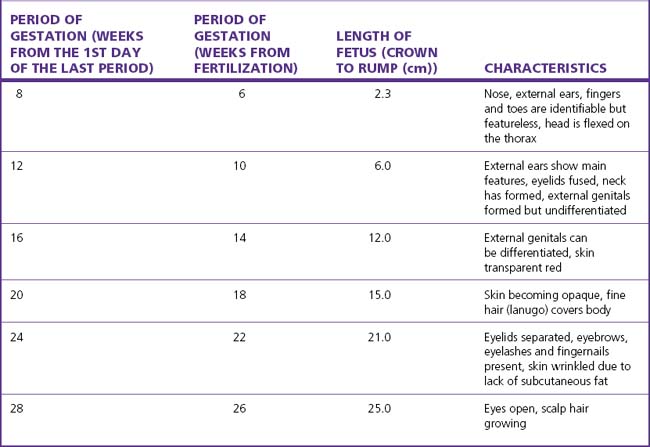
By the seventh week after fertilization most of the organs have formed and the embryo becomes a fetus. The early growth of the fetus is shown in Table 4.1.
NUTRITION
From the 30th gestational week the fetal liver becomes increasingly efficient and converts glucose into glycogen, which is stored in the fetal heart muscle, the skeletal muscle and the placenta. Should fetal hypoxia occur the fetus is able to obtain energy from the heart muscle and placenta for anaerobic glycolysis (see Ch. 20).
As the placenta clears the blood of bilirubin and other metabolic products that require a transferase activity, the fetal (and neonatal) liver is deficient in certain transferases. The result is that unless the deficiencies are corrected in the early neonatal period, bilirubin may accumulate in the neonate’s blood, which is of some consequence in haemolytic disease of the newborn (see p. 129–130).
CARDIOVASCULAR SYSTEM
The circulatory pattern of the fetus is shown in Figure 4.1. It should be noted that over 50% of the cardiac output passes through the umbilical arteries to perfuse the placenta. The cardiac output increases to term, at which time about 200 mL/kg per minute is usual. The heart rate lies between 110 and 150 bpm to maintain this output. The fetal blood pressure also increases through the pregnancy and, after the 36th week, has a mean of 75 mmHg systolic, 55 mmHg diastolic.
MUSCULAR SYSTEM
Almost weightless in its amniotic capsule, the fetus makes movements from an early age. As the pregnancy advances the fetal movements become stronger, and occur more often. Bouts of activity are followed by periods when the fetus seems to be sleeping. These movements strengthen the fetal muscles and a count of them gives an indication of fetal wellbeing (see p. 153).