Chapter 6 Electrosurgery in Therapeutic Endoscopy
Introduction
Endoscopic electrosurgery involves the use of electrical current to achieve a desired tissue effect (cutting, tissue ablation, desiccation, or a combination of these). Electrical energy to produce heating and tissue effect has been a part of endoscopy since the early 1970s.1,2 Common indications include biliary sphincterotomy, polypectomy, hemostasis and ablation of vascular lesions such as arteriovenous malformations, radiation proctopathy, and other forms of vascular ectasia. Other forms of nonelectrosurgical thermal effect may be achieved without the direct use of electrical current, such as laser photoablation and heater probe. Incorrect use of electrical equipment may contribute to poor patient outcomes, such as postpolypectomy serositis; colonic perforation; and biliary sphincterotomy–associated complications such as acute pancreatitis, hemorrhage, and duodenal perforation.
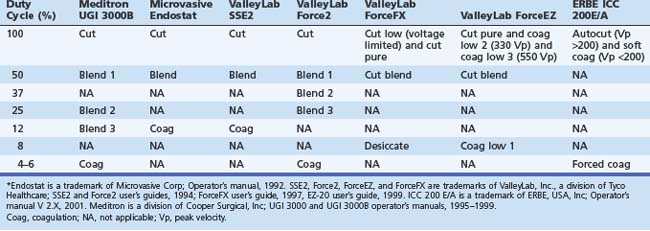
From the clinician’s point of view, this field has been hampered by problems with nomenclature (Table 6.1). First, there is often lack of uniformity in terms used by different manufacturers (e.g., blend may have quite different characteristics from one manufacturer’s generator to another). Second, the terms themselves may be misleading (e.g., a low duty-cycle waveform may be called pure coag but be capable of cutting tissue; this situation leads to statements such as “I only cut with coag”) Lastly, some terms in common usage are manufacturer specific (e.g., Endocut refers specifically to an ERBE [Marietta, GA] generator output). Usually a clinician becomes adept with the particular generator that he or she uses, and most modern generators have storable or preset parameters for common scenarios such as polypectomy and sphincterotomy. Problems may occur, however, when the clinician is confronted with a different generator (most likely to happen in an unusual and stressful environment, such as an emergency procedure in the operating room or emergency department or first list at a new appointment).
Electricity and Tissue
Early pioneers of electrosurgical devices discovered that applying an electrical current to biologic tissue might produce different effects. The first effect was electrolytic. Charged molecules in the tissue flowed toward the opposite poles of an electrode if the current applied was direct or alternated slowly. Alternating the current more rapidly eliminated the electrolytic effect and produced heating at a cellular level. However, a current alternating at less than 100 kHz resulted in undesired neuromuscular effects (the result of applying household current of 60 Hz is well known). Alternating at very high frequency (300 kHz) eliminates neuromuscular effects but retains the desired cellular heating effects. This thermal effect is the basis for all electrosurgery.3
Endoscopic Thermal Modalities
Bipolar (Multipolar) Electrosurgery
Bipolar (multipolar) electrosurgery uses technology similar to the monopolar circuit, but both electrodes are on the tip of the instrument. Current flows through a relatively small area of tissue, and there is no need for a return electrode on the patient’s skin. The common uses for this technology are coagulation of arteriovenous malformations and ulcer hemostasis. Bipolar (or multipolar) probes have been shown to be effective devices for hemostasis for bleeding peptic ulcers. Optimal results seem to be obtained by using forceful tamponade, large probes, low power settings (15 to 25 W) and prolonged contact times (10 seconds).4,5 This technique uses relatively low power outputs (approximately 16 W) with limited depth of injury.6 Bipolar snares, biopsy forceps, and sphincterotomes have been developed but are not widely used. A new, important use of bipolar current is radiofrequency ablation catheters employed to ablate Barrett’s esophagus. In this situation, the current moves from one adjacent electrode on a balloon catheter to another. This energy passes via the mucosa and is sufficient to obliterate the mucosa, while being superficial enough to prevent significant submucosal damage, minimizing stricture formation.
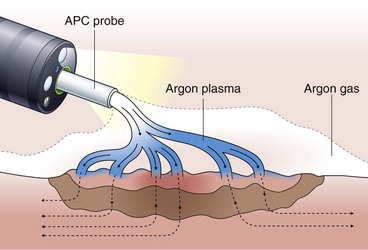
Argon Plasma Coagulation
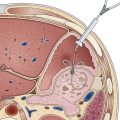
Argon plasma coagulation (APC) is a newer technique in flexible endoscopy with many potential applications, including tumor debulking,7 ablation of vascular malformations,8 and coagulation of peptic ulcer bleeding sites.9 It also has a role in “tidying up” residual adenoma after piecemeal polypectomy, although overuse in this setting suggests poor polypectomy technique. APC is a unique means of energy delivery (Fig. 6.1). Argon gas flows from the catheter tip and provides a medium for current flow from the catheter tip to an adjacent mucosal surface (and via the patient to a remote return pad). APC is a form of monopolar electrocoagulation, unique in its noncontact nature. It can be used effectively either tangential or perpendicular to the mucosal surface. Side-firing catheters are available, but the standard end-firing catheter can be used for tangential and perpendicular applications.
High current density at the mucosal surface leads to tissue effect. At high power output (e.g., 75 to 90 W), APC results in tissue debulking. At lower energy levels (e.g., 30 to 45 W), tissue desiccates with limited depth of injury.10 It has been theorized that as tissue desiccates, its electrical resistance increases, resulting in electrical arcing to adjacent, nondesiccated tissue. This mechanism could act to limit depth of injury. It has been shown, however, that APC can cause transmural injury at high power outputs,10 and there have been reports of perforation using this device.11,12 Nonetheless, the relative ease of use, nontouch technique, and relatively shallow depth of injury make this a versatile and useful instrument, especially in thin parts of the bowel such as small intestine and right colon.
Heater Probe
The heater probe unit (Olympus Corporation, Melville, NY) comprises a power source, catheter, and irrigation system. At the tip of the catheter is a heating coil within a polytef (Teflon) cap. This energy delivery is unique because it supplies a predetermined amount of energy (e.g., 30 J) to the cap without an electrical circuit flowing through tissue. Because this is a direct transfer of heat, it is an example of electrocautery, not electrosurgery. The main indications are ulcer hemostasis and coagulation of angiodysplastic lesions. Heater probe results in ulcer hemostasis in 80% to 90% of bleeding lesions.13 The energy and probe size are inadequate for tumor ablation indications, and this method cannot produce any electrosurgical cutting effect.
Principles of Electrosurgery
Tissue effect is determined by the rapidity of tissue heating. Rapid heating causes intracellular boiling and explosion of cells resulting in a cleavage plane (incision).14 In contrast, slow heating results in protein denaturization and desiccation or “coagulation” of tissue.15 Understanding the different characteristics of tissue heating under different circumstances is essential to understanding electrosurgery.
Variables in Electrosurgery
Many variables in electrosurgery can influence tissue effect.
Power Output
Two formulas, including Ohm’s law, elegantly relate all of the electrosurgical variables:
Power and voltage are also directly related (e.g., P = V2/I).
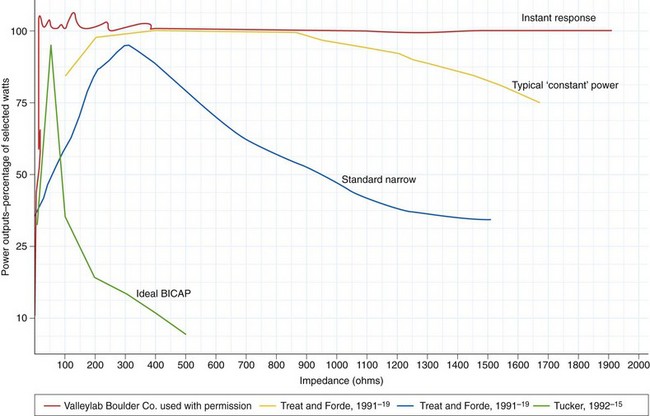
These formulas illustrate that there is a relationship between power output and tissue resistance. As the resistance (or impedance) in the tissue increases (as it desiccates), power falls off unless either current or voltage is increased. Manufacturers addressed this problem by developing microprocessor-controlled generators that could measure resistance in the circuit and adjust output automatically (within certain set parameters) to maintain the desired tissue effect.16 Some power outputs are constant over a wide range of resistance; when this is graphically represented, it results in the so-called flat or wide power curve (Fig. 6.2). The initiation of an incision is problematic for generators, especially if the electrode is pressed firmly against the tissue surface because this presents a large surface area with low current density and low tissue impedance. The generator must supply particularly high power output to initiate the formation of microelectric arcs and commencement of the cutting effect. This initial power output is often greater than the output needed to continue the incision. Microprocessors can recognize this situation and transiently provide a high power output to initiate cutting.
Voltage
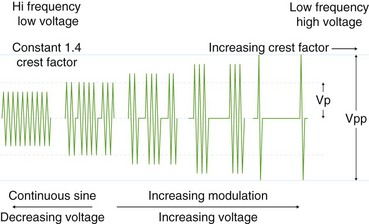
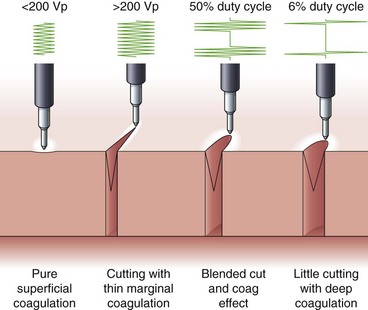
Voltage refers to the potential difference between the two electrodes and defines the force making electrons move from one point in the circuit to another. Using the analogy of a waterfall, with current being the water flowing, voltage is analogous to the height of the waterfall. As one can see from the aforementioned formulas, voltage is an integral determinant of circuit behavior (and tissue effect). To complete a circuit, sufficient voltage must exist across the device-patient interface for microelectric arcs to occur between patient and tissue. Generally, a peak of at least 200 V is required. At greater than 500 V, tissue tends to char. Manufacturers have manipulated voltage in an attempt to create specific effects. In particular, increasing the voltage for a given continuous sinusoidal output (i.e., pure cut) varies the depth of coagulation adjacent to the cut; this has been termed cut effect 1, cut effect 2, and so forth (Figs. 6.3 and 6.4).
Duty Cycle
Current flows for only a fraction of each second that the activating pedal is depressed. This fraction or percentage of time current flows is the duty cycle. A pure-cut mode delivers current during most or all of the activation period, whereas blended mode delivers current in an interrupted fashion (e.g., 25% to 50%). A pure coag mode may have a duty cycle of 6% to 10% (see Fig. 6.4). This mode gives tissue time to dehydrate (rather than rapid heating causing an explosive tissue cleavage seen in cutting mode). Some new generators have an alternating cut and coagulation mode (e.g., 50 msec of pure cut followed by 700 msec of coagulation). This mode results in a characteristic “staccato” type of incision. The aim of this type of output is to slow the rate of incision and prevent uncontrolled (“zipper”) cut17; whether this translates to improved patient outcome is yet to be determined.
Current Density
Blood Flow
Blood flow may help dissipate heat at the site of the tissue. Similarly, submucosal saline injection may act as a “heat sink” and help dissipate energy and prevent transmural injury during polypectomy.19 Saline is widely used in clinical practice to reduce the risk of transmural injury, but it has not been proven to increase safety.
Electrical Hazards
Cardiac Pacemakers and Implanted Defibrillators
Guidelines for endoscopic electrosurgery in the presence of cardiac pacemakers and implanted defibrillators have been published by the American Society for Gastrointestinal Endoscopy (ASGE).20 Electrosurgical units should be used with caution in patients with these devices. Although there have been no reports of serious adverse events in endoscopic applications, monopolar electrosurgical energy in urology and general surgery has been reported to cause pacemaker dysfunction, especially in older pacemakers. In short, the electrical current flowing through the body could be misinterpreted by an implanted defibrillator as a tachyarrhythmia, leading to an unnecessary shock, or could interfere with pacemaker sensing. General principles for the use of electrosurgery in the presence of a permanent pacemaker are as follows:
Deep brain electrode stimulation is increasingly used for refractory Parkinson’s disease. Although no adverse effects with electrosurgery have been reported, manufacturers and neurologists are very reluctant to allow patients to have any monopolar electrosurgery. There is a single case report of severe lancinating pain experienced by a patient with one of these devices while undergoing dermatologic surgery with a monopolar instrument.21
Endoscopic Electrosurgical Techniques
Biliary and Pancreatic Sphincterotomy
Cutting may be performed with either pure-cut (100% duty cycle) or blended (approximately 50% duty cycle) current. Use of pure-cut current results in a more rapid incision with less edema of surrounding tissues but less hemostasis. Several studies have shown reduced risk of pancreatitis in patients having pure-cut sphincterotomy compared with coag current.22,23 As would be expected with less coagulation, increased mild hemorrhage is seen, but this does not translate into clinically significant bleeding.22,23 However, because of the potential for less control of a rapid incision with pure-cut current, great care must be taken with this current output. As mentioned earlier, new generators automatically switch between cut and coag settings or interrupted pulses of cut current alone during the course of the incision. As with colonic polypectomy, excessive tissue desiccation should be avoided because this may lead to stalling of the incision.
Complications of Sphincterotomy—Link with Electrosurgery
Pancreatitis
Acute pancreatitis is the most common complication of endoscopic sphincterotomy, occurring in at least 5% of cases.24,25 Development of pancreatitis may be partly a function of iatrogenic trauma to the periampullary region. This trauma results in edema and obstruction to pancreatic flow. Post-ERCP pancreatitis may be reduced by the use of pure-cut current instead of the usual blended current.22
Hemorrhage
Hemorrhage during endoscopic sphincterotomy is due in part to inadequate coagulation effect of tissue during the incision. Mild oozing at the sphincterotomy site at the time of endoscopic sphincterotomy is common, settles spontaneously, and is of no clinical significance. Minor bleeding at the time of sphincterotomy may be more common with pure-cut technique.22 Significant hemorrhage occurs in 1% to 3% of patients26 with an associated mortality of less than 1%.26–28 This hemorrhage is due to incision of a significant vessel, which is partly “bad luck” and sometimes due to poor orientation of the incision, cutting into a diverticulum or overcutting the incision. Arterial bleeding is not prevented by one electrosurgical output versus another. It is believed, however, that a half-incised vessel (the ends of which cannot retract) bleeds much more than a fully cut one, implying that cutting a little more may be useful in this situation.
Perforation
Duodenal perforation during endoscopic sphincterotomy is usually the result of a poorly aligned or too long incision beyond the boundaries of the intramural common bile duct. Clinically significant perforation occurs in less than 1% of sphincterotomies.24,29 The risk of perforation may be 8% in patients with a small papilla and patients with papillary stenosis.30 Asymptomatic perforation may be more common, possibly occurring in 15% of sphincterotomies.31 Duodenal perforation may occur during a rapid, uncontrolled cut of the sphincter (“zipper cut”). The occurrence of this type of rapid incision is a function of current delivery to the tissue and operator experience.
Polypectomy
Polypectomy is most commonly used to remove colonic polyps, either in one piece or piecemeal. Polypectomy and other thermal ablative techniques such as hot biopsy and ablation have the potential to cause transmural damage, resulting in either serosal inflammation (postpolypectomy syndrome) or perforation. Perforation occurs in 0.1% to 0.8% of colonoscopic polypectomies.32,33 Serositis without perforation occurs in 1% of polypectomies32; it manifests 6 hours to 5 days after the procedure with pain, fever, and leukocytosis. The right side of the colon is particularly at risk because of its thinner wall.
Immediate hemorrhage occurs in about 1% of polypectomies, and delayed bleeding may occur in 2% of polypectomies.34 Significant hemorrhage is much more likely when cutting through a thick stalk. Delayed bleeding may occur any time up to 2 weeks after the procedure. From the preceding discussion, it can be seen that sphincterotomy and polypectomy complications may be related partly to either an overrapid, poorly controlled incision or an overdesiccated, poorly progressed incision. Modern generators use bursts of cut alternating with coagulation to progress the incision in a predictable fashion. Microprocessor-controlled feedback varies generator output in response to changes in tissue resistance (i.e., increasing desiccation) to prevent stalling of the incision.
Osmotic sugar preparations (e.g., mannitol) should be avoided because gut fermentation generating hydrogen could cause explosion during colonic electrosurgery. This risk has been greatly reduced with the introduction of nonfermentable preparations. Nonetheless, to reduce risk of explosion further, preparation should be optimized to reduce fecal residue and colonic gas (i.e., hydrogen and methane). Explosion has also been reported with the use of APC of the rectum after enema preparation.35–37 If cautery must be performed on an unprepared colon, effort to exchange the gas in the colon with repeated insufflation and suction should be undertaken. Carbon dioxide (CO2) insufflation, mainly used for patient comfort, may reduce the risk of gas ignition further.
Hot Biopsy Forceps
HBF have been a popular means of removing diminutive polyps for many years.38 HBF employs monopolar circuitry. Blended or coagulation current should be used, at a relatively low setting and applied only until blanching of the tissue occurs (1 to 2 seconds). The bowel should be deflated before power application, and care must be taken not to touch other parts of the bowel wall with the cups while coagulating. Postpolypectomy syndrome, perforation, and significant bleeding all have been reported after removal of diminutive polyps using HBF.38 This technique is relatively poor at removing all polyp tissue.39
Cold Snare Technique
Chopping polyps off without using any thermal energy has been advocated for polyps less than 5 mm.40,41 The technique is fast, but, more importantly, it does not leave a significant thermal ulcer, which may take 2 weeks to heal. This technique has particular appeal for patients requiring anticoagulation after the procedure.
1 Shinya H, Wolff WI. Polypectomy via the fibreoptic colonoscope. N Engl J Med. 1973;288:328-332.
2 Blackwood WD, Silvis E. Gastroscopic electrosurgery. Gastroenterology. 1971;61:305-314.
3 Tucker RD. Principles of electrosurgery. In: Sivak MV, editor. Gastroenterologic endoscopy. 2 ed. Philadelphia: Saunders; 2000:125-135.
4 Jutabha R, Jensen DM, Machicado G, et al. Randomized controlled studies of injection gold probes compared with monotherapies for hemostasis of bleeding canine gastric ulcers. Gastrointest Endosco. 1998;48:598-605.
5 Bianco MA, Rotondano G, Marmo R, et al. Combined epinephrine and bipolar probe coagulation vs. bipolar probe coagulation alone for bleeding peptic ulcer: a randomized, controlled trial. Gastrointest Endosc. 2004;60:910-915.
6 Norton ID, Wang L, Levine SA, et al. Submucosal saline injection limits the depth of colonic thermal injury. Gastrointest Endosc. 2002;56:95-100.
7 Zlatanic J, Waye JD, Kim PS, et al. Large sessile colonic adenomas: use of argon plasma coagulator to supplement piecemeal snare polypectomy. Gastrointest Endosc. 1999;49:731-735.
8 Roman S, Saurin JC, Dumortier J, et al. Tolerance and efficacy of argon plasma coagulation for controlling bleeding in patients with typical and atypical manifestations of watermelon stomach. Endoscopy. 2003;35:1024-1028.
9 Cipolletta L, Bianco MA, Rotondano G, et al. Prospective comparison of argon plasma coagulator and heater probe in the endoscopic treatment of major peptic ulcer bleeding. Gastrointest Endosc. 1998;48:191-195.
10 Norton ID, Wang L, Levine S, et al. In vivo characterization of colonic thermal injury caused by argon plasma coagulation. Gastrointest Endosc. 2002;55:631-636.
11 Hoyer N, Thouet R, Zellweger U. Massive pneumoperitoneum after endoscopic argon plasma coagulation. Endoscopy. 1998;30:S44-S45.
12 Tan AC, Schellekens PP, Wahab P, et al. Pneumatosis intestinalis, retroperitonealis, and thoracalis after argon plasma coagulation. Endoscopy. 1995;27:698-699.
13 Lin HJ, Tsai YT, Lee SD, et al. Heater probe therapy for severe haemorrhage from a peptic ulcer with a visible vessel. Endoscopy. 1988;20:131-133.
14 Tucker RD, Platz CE, Landas SK. Histologic characteristics of electrosurgical injuries. J Am Assoc Gynecol Laparosc. 1997;4:201-206.
15 Barlow DE. Endoscopic applications of electrosurgery: a review of basic principles. Gastrointest Endosc. 1982;28:73-76.
16 Tucker RD, Hudrlik TR, Silvis SE, et al. Automated impedance: a case study in microprocessor programming. Comput Biol Med. 1981;11(3):153-160.
17 Norton ID, Petersen BT, Bosco J, et al. A randomized trial of endoscopic biliary sphincterotomy using pure-cut versus combined cut and coagulation waveforms. Clin Gastroenterol Hepatol. 2005;3:1029-1033.
18 Electrosurgical devices. Health Dev. 1997;26:400-415.
19 Waye JD. New methods of polypectomy. Gastrointest Endosc Clin N Am. 1997;7:413-422.
20 Technology Assessment Committee A: Electrocautery use in patients with implanted cardiac devices. ASGE Policy Manual. American Society of Gastroenterologists, 1993.
21 Weaver J, Kim SJ, Lee MH, et al. Cutaneous electrosurgery in a patient with a deep brain stimulator. Dermatol Surg. 1999;25:415-417.
22 Elta GH, Barnett JL, Wille RT, et al. Pure cut electrocautery current for sphincterotomy causes less post-procedure pancreatitis than blended current. Gastrointest Endosc. 1998;47:149-153.
23 Stefanidis G, Karamanolis G, Viazis N, et al. A comparative study of postendoscopic sphincterotomy complications with various types of electrosurgical current in patients with choledocholithiasis. Gastrointest Endosc. 2003;57:192-197.
24 Freeman ML. Complications of endoscopic biliary sphincterotomy: a review. Endoscopy. 1997;29:288-297.
25 Gottlieb K, Sherman S. ERCP and biliary endoscopic sphincterotomy-induced pancreatitis. Gastrointest Endosc Clin North Am. 1998;8:87-114.
26 Sherman S, Uzer MF, Lehman GA. Wire-guided sphincterotomy. Am J Gastroenterol. 1994;89:2125-2129.
27 Ferrari AP, Slivka A, Lichtenstein DR: Factors affecting RCP complications: looking backwards and forwards. 10th World Congress of Gastroenterology, 1994, A1866.
28 Hawes RH, Sherman S. Choledocholithiasis. In: Haubrick WS, Schaffner F, Berk JE, editors. Bochus’ Gastroenterology. Philadelphia: Saunders; 1995:2745.
29 Foutch PG, Harlan JR, Hoefer M. Endoscopic therapy for patients with a post-operative biliary leak. Gastrointest Endosc. 1993;39:416-421.
30 Leese T, Neoptolemos JP, Carr-Locke DL. Successes, failures, early complications and their management following endoscopic sphincterotomy: results in 394 consecutive patients from a single centre. Br J Surg. 1985;72:215-219.
31 de Vries JH, Duijm LE, Dekker W, et al. CT before and after ERCP: detection of pancreatic pseudotumor, asymptomatic retroperitoneal perforation and duodenal diverticulum. Gastrointest Endosc. 1997;45:231-235.
32 Waye JD, Lewis BS, Yessayan S. Colonoscopy: a prospective report of complications. J Clin Gastroenterol. 1992;15:347-351.
33 Nivatvongs S. Complications in colonoscopic polypectomy: lessons to learn from an experience with 1576 polyps. Am Surg. 1988;54:61-63.
34 Sorbi D, Norton I, Conio M, et al. Postpolypectomy lower GI bleeding: descriptive analysis. Gastrointest Endosc. 2000;51:690-696.
35 Manner H, Plum N, Pech O, et al. Colon explosion during argon plasma coagulation. Gastrointest Endosc. 2008;67:1123-1127.
36 Nurnberg D, Pannwitz H, Burkhardt KD, et al. Gas explosion caused by argon plasma coagulation of colonic angiodysplasias. Endoscopy. 2007;39(Suppl 1):E182.
37 Seth AK, Kapoor N, Puri P. Colonic explosion with use of argon plasma coagulation for radiation proctitis. Indian J Gastroenterol. 2009;28:118-119.
38 Gilbert DA, DiMarino AJ, Jensen DM, et al. Status evaluation: hot biopsy forceps. American Society for Gastrointestinal Endoscopy. Technology Assessment Committee. Gastrointest Endosc. 1992;38:753-756.
39 Peluso F, Goldner F. Follow-up of hot biopsy forceps treatment of diminutive colonic polyps. Gastrointest Endosc. 1991;37:604-606.
40 Tappero G, Gaia E, De Giuli P, et al. Cold snare excision of small colorectal polyps. Gastrointest Endosc. 1992;38:310-313.
41 Uno Y, Obara K, Zheng P, et al. Cold snare excision is a safe method for diminutive colorectal polyps. Tohoku J Exp Med. 1997;183:243-249.