Electrophysiological testing and electrical stimulation in neurological rehabilitation
ALAIN CLAUDEL, PT, DPT, ECS, ROLANDO T. LAZARO, PT, PhD, DPT, GCS, GEORGE WOLFE, PT, PhD and JANET MARIE ADAMS, PT, MS, DPT
After reading this chapter the student or therapist will be able to:
1. Identify electrophysiological tests performed on clients with neurological disorders.
2. Describe the instrumentation and general procedures for electrophysiological testing.
3. Recognize normal and abnormal findings of various electrophysiological tests.
4. Recognize the differences in instrumentation, signal processing, and interpretation when performing electrophysiological testing versus kinesiological electromyographic testing.
5. Differentiate the basic mechanism underlying functional neuromuscular stimulation, electrical stimulation, and electromyographic biofeedback.
6. Describe the appropriate instrumentation, signal processing, and interpretation for kinesiological electromyographic testing.
7. Describe the indications and contraindication for the use of neuromuscular stimulation, electrical stimulation, and electromyographic biofeedback.
Electrophysiological testing
Electrophysiological tests are usually performed by neurologists, physiatrists, and PTs who have education, training, and experience in these procedures. Most PTs practicing in the area of clinical electrophysiology are board certified by the American Board of Physical Therapy Specialties.1 Some states (such as California) require additional licensing.
The general goal of electrophysiological testing is to answer the following questions:
The electrophysiological tests most commonly used are motor and sensory NCSs, including F-wave and H-reflex latency measurements; repetitive stimulation; somatosensory evoked potential (SSEP) tests; and needle EMG. Most often, a patient referred for electrophysiological testing will undergo at least two motor nerve conduction tests, at least two sensory conduction tests, and at least one limb needle EMG. The American Association of Neuromuscular and Electrodiagnostic Medicine (AANEM) has published evidence-based guidelines, which may be found on the AANEM website at www.aanem.org/Practice/Practice-Guidelines.aspx.2 A review of the client’s history, a relevant systems review, and a physical examination guide the examiner in the selection and sequencing of appropriate tests. In other words, muscle strength and tone, sensation, range of motion (ROM), neurological signs, and cognition are crucial in selecting and administering electrophysiological tests. Electrophysiological testing is considered by all authors on the subject as an extension of the clinical examination.3,4 It does not replace a careful history and physical examination of the patient. It does, however, establish the precise state of the nerves and muscles and can thus determine the location of a lesion more precisely than the clinical examination alone, particularly in cases of mild weakness or ill-defined sensory changes. In very clearly defined pathologies, electrophysiological tests are not necessary (except perhaps for medicolegal reasons). For example, in the case of a unilateral ankle dorsiflexion weakness coupled with a clearly defined L5 nerve root compression on magnetic resonance imaging (MRI) of the lumbar spine, the electrophysiological test may be of little added value. However, for a similar clinical presentation (foot drop) and no clear-cut imaging, the electrophysiological tests will differentiate between a peroneal palsy, a sciatic nerve neuropathy, a lumbosacral plexopathy, or an L5 radiculopathy.
Finally, evidence-based practice recommends that the practitioner have a good understanding of the implication of the sensitivity and specificity of each test to rule it in or out for a specific condition.5
Anatomical review
At the cellular level
A nerve is composed of axons covered with a sheath of myelin. Depolarization inside the axon is an “all-or-nothing” phenomenon in which an action potential moves along the surface of the cell membrane. This action potential is an electrical wave caused by a flow of ions across the cell membrane. A local current opens a sodium channel, allowing Na+ ions to rush inside the cell. The electrical resistance to this wave is inversely proportional to the diameter of the axon. Larger nerves conduct faster than smaller nerves. In order for efficiency as an organism to be achieved, nerve conduction must be fast. In complex organisms with billions of axons, increasing the nerve diameter is not a viable option; hence, the role of the myelin sheath. The myelin is produced by Schwann cells. These are special satellite cells that separate axons from the endoneural fluid. The myelin acts as a capacitor: the conduction “jumps” between gaps in the myelin called nodes of Ranvier. This saltatory conduction allows human nerves to be 50 times smaller but conduct four times faster than unmyelinated nerves.6 Consequently, recording and analyzing the conduction velocity of nerves primarily reflects on the state of the myelin. The amplitude of the response (if a supramaximal stimulation is delivered) is a reflection of the number of axons available to the stimulation.
The physiology of the nerve is such that when there is an injury to the axon, the portion of the axon distal to the injury will degenerate (wallerian degeneration). This is important because all muscles innervated by branches of the nerve distal to the lesion will show signs of denervation approximately 11 days after the lesion. Consequently, assessing a patient too early after a lesion may lead to false-negative results.6,7
At the anatomical level
The accurate performance and interpretation of the electrophysiological test—particularly the needle EMG—is significantly contingent on knowledge of the precise innervation of each muscle. As an example, an ulnar neuropathy at the elbow (UNE) clinically may be indistinguishable from a C8 radiculopathy. However, the astute clinician will remember that the cell body of the sensory nerve lies in the dorsal root ganglion, which is typically not involved in a radiculopathy. The therapist will also know that the abductor pollicis brevis is a C8- and median nerve–innervated muscle. Consequently, an ulnar nerve neuropathy is distinguishable from a C8 radiculopathy in that the ulnar sensory test will have decreased amplitude in the UNE and the abductor pollicis brevis will be denervated in the C8 radiculopathy. As a matter of fact, the clinician will keep in mind the innervation of each muscle while conducting the test. Electrophysiological testing is hence a dynamic process during which the choice of the next nerve to test or muscle to sample is predicated on the result of the previous test.6,7
As a result, the electrophysiological examination is not a single, stereotyped investigation but an evolutionary one during which several tests (nerve conduction, both sensory and motor, and EMG of several muscles) can be applied to a clinical presentation.3,8
Nerve conduction tests
A general overview of NCSs is presented to provide an understanding of their application and indications. Many excellent texts are available for details of the techniques.9–13
Nerve conduction velocity is faster in myelinated fibers because of saltatory conduction. Disorders involving peripheral demyelination can thus be differentiated from impairments primarily involving axonal degeneration. A mild localized compressive disorder (neurapraxia) may be distinguished from a more severe lesion in which the axons and surrounding connective tissue have been completely disrupted (neurotmesis).11,14 In the event that the findings of NCS and EMG are normal, the clinician may be able to rule out most conditions involving the PNS and look for central nervous system (CNS) or other pathology. Knowledge of the rationale for NCSs and EMG should help the therapist decide when the tests may be indicated and understand the reasoning behind reports of tests that have already been performed on clients.
Motor nerve conduction
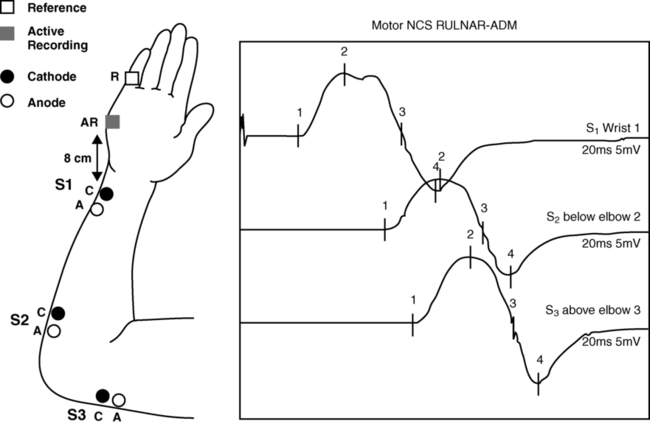
In motor NCSs the peripheral nerve is stimulated at various sites and the evoked electrical response is recorded from a distal muscle supplied by the nerve (a measure of orthodromic conduction). Surface electrodes are usually used for both stimulating and recording. An example of electrode configuration for a motor NCS is shown in Figure 33-1 (ulnar nerve study). The response represents the electrical activity of muscle fibers under the recording electrodes and is called the compound muscle action potential (CMAP). It is also called the M wave or M response. Measurements are taken of the latency (the time in milliseconds required for the impulse to travel from each stimulus site to the recording site) and the amplitude of the response in millivolts (mV). The shape and duration of the response are assessed, and motor nerve conduction velocity is calculated for each segment of interest by dividing the distance between stimulus sites (in millimeters) by the difference in latency measured at each respective site.
Velocities, latencies, and the shape and amplitude of the responses (Figure 33-2) are studied and compared with established normal values and often with values taken from tests of the uninvolved extremity (when possible). In infants and children, nerve conduction is slower than in adults and reaches adult values by age 4 years.13 Nerve conduction velocities gradually slow after age 60 years but generally remain within the outer limits of normal.11,13
Sensory nerve conduction
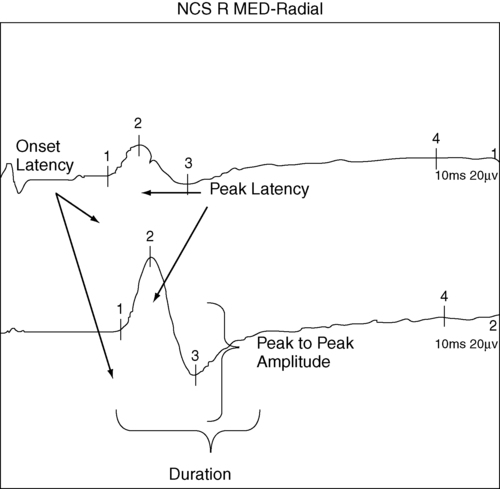
Sensory nerve conduction can be measured from many superficial sensory nerves, such as the superficial radial and sural nerves. It can also be measured from mixed motor and sensory nerves. The stimulus is applied over the nerve in question, and the recordings taken from electrodes placed over a distal sensory branch of the nerve. The recordings are called sensory nerve action potentials (SNAPs). An example of recording and stimulation sites is shown in Figure 33-3. Both orthodromic and antidromic conduction can be assessed. Response latencies and amplitudes are measured, and sensory nerve conduction velocities are calculated for each segment by dividing the distance between two adjacent stimulus and recording sites, or two stimulus sites, by the latency (conduction time) between these same sites. Sensory nerve responses are considerably smaller than motor responses. Their amplitudes are generally measured in microvolts (μV). Sensory recordings are more sensitive than motor recordings in cases of mixed sensory-motor neuropathies.
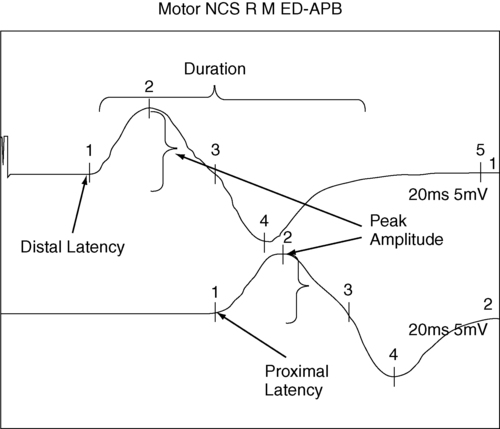
 Example of recording and stimulation sites.
Example of recording and stimulation sites.F-wave latency
When a motor nerve is stimulated in the periphery, both orthodromic (peripherally to the muscle) and antidromic (centrally toward the spinal cord) impulses are generated. A proportion of the antidromic impulses will, as it were, “bounce off” the axon hillock and return as a recurrent discharge along the same neurons to activate the muscle from which the recording is taken. This activity is termed the F wave (Figure 33-4), and it is observed as a small wave occurring after the M wave.11,13,14 No synapse is involved. Thus the F wave is not a reflex response, but rather only a measure of conduction along the motor neuron. Specific conditions of electropotential must exist at the soma-dendritic cell membrane to reactivate the efferent axon; therefore the occurrence of the F-wave response is inconsistent and variable in latency and waveform.15
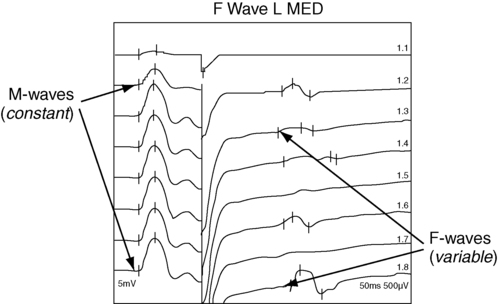
 Example of F-wave study.
Example of F-wave study.The F-wave latency can be useful in evaluating conduction in conditions usually involving the proximal portions of the peripheral neurons (e.g., radiculopathy, Guillain-Barré syndrome [refer to Chapter 17], or thoracic outlet syndrome [refer to Chapter 18]). Its value, however, has been questioned by some authors because of its variability. Normal values of F-wave latency are 22 to 34 ms in the upper extremity (stimulating at the wrist) and 40 to 58 ms in the lower extremity (stimulating at the ankle), depending on the height of the subject, with a bilateral difference in latency of no greater than 1 ms.16
H-reflex response
The H-reflex response latency (Figure 33-5) is a measure of the time for action potentials elicited by stimulating a nerve in the periphery to be propagated centrally over the Ia afferent neurons to the spinal cord, to be transmitted across the synapse to alpha motor neurons, and then to travel distally over these neurons to activate the muscle. The response therefore measures conduction in both the afferent and efferent neurons.11,13 It is also referred to as a “late” response (the other being the F wave).
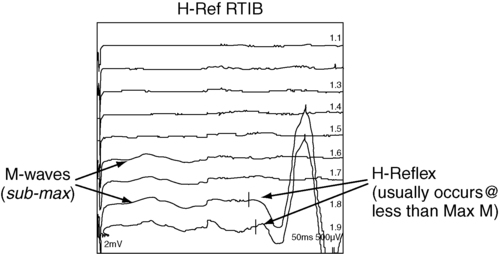
 Example of H-reflex study.
Example of H-reflex study.The H reflex is constant in latency and waveform, and it occurs with a stimulus usually below the threshold level required to elicit the M-wave response (Ia afferent fibers are larger in diameter than alpha motor neurons and thus more sensitive to electrical stimulation). This monosynaptic reflex response is most easily found by stimulating the tibial nerve at the popliteal area and recording from the soleus muscle. Braddom and Johnson17 reported a mean latency of 29.8 ms (±2.74 ms) for the tibial nerve in normal adults, and a bilateral difference of no more than 1.2 ms. The H-reflex latency is a valuable measure of conduction over the S1 nerve root in differentiating suspected proximal plexopathy and radiculopathy from a herniated disc or foraminal impingement. Sabbahi and Khalil18 have reported a technique for recording the H reflex from the flexor carpi radialis muscle when stimulating the median nerve. In normal human beings older than 1 year, the H reflex is usually seen only in the tibial, femoral, and median nerves. It can be elicited from several nerves in infants and in conditions of CNS dysfunction in adults.
Repetitive stimulation tests
The repetitive nerve stimulation (RNS) test is used to evaluate transmission at the neuromuscular junction (motor synapse) in patients with diffuse weakness. RNS tests are helpful in the differential diagnosis of disorders such as myasthenia gravis and Lambert-Eaton myasthenic syndrome (LEMS). One protocol uses a series of supramaximal electrical stimuli applied to a peripheral nerve at a distal site (e.g., median or ulnar nerve at the wrist) at a rate of three to five per second for five to seven responses. Changes in amplitude of the muscle response are assessed. Precise technical requirements are specified to prevent movement artifacts and other testing errors. Detailed descriptions of the RNS test can be found in other texts.11,19 Under normal conditions the amplitude does not change more than 10% from that of the initial response in a series of 10 stimuli recorded before and after resistive exercise. An amplitude decrease in the fifth or sixth response of more than 10% is considered abnormal and is compatible with a physiological defect at the postsynaptic receptor site of the neuromuscular junction, as in myasthenia gravis.
In another RNS protocol, stimuli are applied to a nerve, first at a slow rate, then at a faster rate, usually 10 to 20 per second for up to 10 seconds. Normally, the amplitude can decrease up to 40% from the initial amplitude. In some defects at the presynaptic site, the response may be lower than normal during a slow stimulation rate but show a significant amplitude increase at the higher rate. Increases in amplitude greater than 100% over the initial response are consistent with presynaptic neuromuscular junction defects such as seen in LEMS, which has a strong association with small-cell bronchogenic carcinoma, and in botulism. In 1957 Eaton and Lambert20 reported this phenomenon as a myasthenic syndrome.
Gilchrist and Sanders21 reported another protocol referred to as a double-step RNS test. This test measures amplitude before and after a temporarily induced ischemia of the extremity. They found the double-step RNS test to be slightly more sensitive than the routine RNS test, but only 60% as sensitive as the SFEMG technique. The RNS test is a good alternative test for neuromuscular transmission when the SFEMG is not available, but the examiner must meticulously adhere to technical details when conducting the test.
Blink reflex
 Trigeminal neuralgia (cranial nerve V) or Guillain-Barré: all responses delayed
Trigeminal neuralgia (cranial nerve V) or Guillain-Barré: all responses delayed
 Acoustic neuroma or Bell palsy (cranial nerve VII): ipsilateral responses delayed
Acoustic neuroma or Bell palsy (cranial nerve VII): ipsilateral responses delayed
Hence, the blink reflex is a nerve conduction test that assesses a portion of the CNS and has shown some utility in assisting in the diagnosis of multiple sclerosis (see Chapter 19) and Wallenberg syndrome.7
Clinical evoked potentials
Electrical potentials elicited by stimulation of nerves or sense organs in the periphery can be recorded from various sites as the impulses are transmitted centrally along the neuronal pathway and from the representative area of the brain.11,22–24 SSEP procedures are particularly useful in assessing the integrity of afferent pathways in the CNS. They are helpful in differentiating among lesions in areas such as the plexus, spinal cord, brain stem, thalamus, and cerebral cortex. Evoked potential tests have the advantage of providing data about the integrity of both peripheral and central neuronal pathways, including transmission across axodendritic synapses.
In visual evoked potential (VEP) procedures, visual stimuli such as variable light flashes of changing patterns are applied to one or both eyes under highly controlled conditions. The response is recorded from the scalp over the representative area of the cerebral cortex.11,23,24 The term pattern reversal evoked potentials (PREPs), a more descriptive term for these procedures, is recommended by the American Electroencephalographic Society.22 These tests and other VEP procedures are useful in assessing pathology of retinal photoreceptors, the optic nerve, and postchiasmal pathways. Abnormal conduction findings have been reported when VEP studies are used in demyelinating disorders such as multiple sclerosis and optic neuritis. The examiner may conclude that the patient is cortically blind because no response is recorded on the visual cortex. Although many causes for a stimulus not reaching the visual cortex are possible, the end result is considered blindness. If the cause for cortical inactivity is swelling or a neurochemical imbalance within a nuclear relay structure, once corrected, the individual may experience normal vision. A change in the reaction of the patient to the visual environment may reflect increased awareness and a change in coma scale rating. Similarly, just because an individual turns toward a light or visual stimulus does not mean an evoked potential reaches the visual cortex. Instead, the eyes as receptors and the visual tract to the brain stem may be intact even though a problem in the synaptic connections between or within the thalamus and visual cortex may exist.
Auditory evoked potential tests are used to evaluate neurological function of the cochlear division of the auditory nerve (eighth cranial nerve), central auditory pathways and synapses in the brain stem, and the receptor areas on the cerebral cortex.11,22–24 Brain stem auditory evoked potentials are frequently referred to as BAEPs. A series of high-intensity clicks is applied to auditory receptors in the ears through headphones, and several components of the response waveforms are recorded by using surface electrodes over the representative cortical areas. The BAEP is an effective test procedure for localizing and evaluating acoustic neuromas and other space-occupying lesions in the brain stem. This test is also used for assessment of brain damage in patients who are comatose as a result of traumatic brain injury (TBI). Robinson and Rudge25 recommend caution in using BAEP tests for this purpose because other factors, such as defective receptor organs, can cause abnormalities in BAEPs.
The evoked potential tests described in this chapter all require application of appropriate external stimuli that are rapidly repeated many times. The response is electronically averaged to sort out the desired signal from interference signals. The conduction times (latencies), waveform shape and amplitude, and sometimes conduction velocities are measured and compared with normal values. Absence of a response, increased latencies, decreased amplitudes, and slowing of conduction velocities are all abnormal findings. Normal values and details of techniques for the evoked potential tests are described elsewhere.11,22–24
Needle electromyography
Unlike NCSs, which use the electrical stimulation of the motor nerves to elicit muscle contraction, the needle EMG is used to record and analyze muscle activity at rest and during voluntary activation. It is particularly useful in identifying pathology of the lower motor neurons and of the muscle itself. EMG can also be used to identify abnormalities of motor neuron recruitment that are associated with certain disorders of the CNS especially when NCS findings are normal—as would be the case in radiculopathies. The primary recording studied is the insertional activity, along with activity at rest and the motor unit action potential (MUAP), which is produced by the depolarization of single motor units during voluntary or reflex activity. Spontaneous electrical activity of single muscle fibers at rest is termed fibrillation and is diagnostic of denervation. For recording of muscle activity, small-diameter needles are inserted within the muscles to be studied. Three electrodes are required: active (negative), reference (positive), and ground. The needles may be monopolar, requiring a second needle or surface electrode for reference, or bipolar, containing both the active and reference electrodes (usually concentric in cross section). The ground electrode is typically placed on the surface of the skin. Most commonly the needles used are disposable. The activity detected in the muscle is displayed on the video display terminal of a computer (and can be stored and printed later). It is simultaneously played through an audio amplifier. The electromyographer can often identify pathological conditions by the characteristic “sounds” of the electrical activity of the muscle. Many excellent resources are available for readers interested in details of the equipment and procedures for EMG.9–11,26,27 Details of contraindications and special precautions are described by Currier and colleagues.27
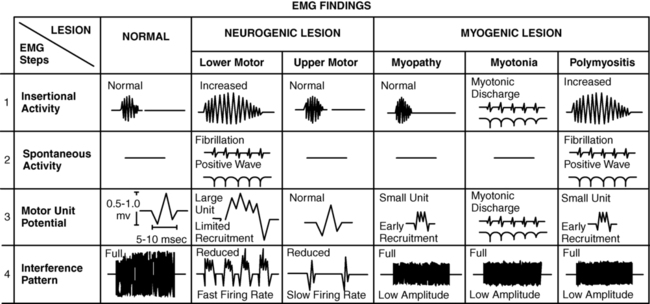
Needle EMG is sensitive in determining the state of the axons. When axons are interrupted, there is denervation of the muscle cell. The findings in denervation and partial denervation include increased insertional activity, fibrillation potentials or positive sharp waves at rest, and a reduced or absent interference pattern. CNS dysfunction can result in no resting potentials, but if motor control was impaired a decreased or abnormal interference pattern might be apparent because of difficulty in recruitment (Figure 33-6). Needle EMG also informs the clinician about muscle cell disorders such as myopathies. The primary finding in the case of a myopathy is fibrillation potentials and small-amplitude polyphasic MUAPs.
 Insertional activity: may be increased in acute denervation and myopathic processes
Insertional activity: may be increased in acute denervation and myopathic processes
 Spontaneous activity at rest: present (positive sharp waves and fibrillation potentials) in denervation
Spontaneous activity at rest: present (positive sharp waves and fibrillation potentials) in denervation
 Fasciculations: may be present in motor neuron disorders
Fasciculations: may be present in motor neuron disorders
• Amplitude: Low amplitude is seen in myopathy or nascent potential; large amplitude is a sign of chronicity.
• Phases: Normal MUAPs are biphasic or triphasic. Polyphasia is indicative of denervation-reinnervation.
• Recruitment pattern: A less-than-full recruitment is indicative of fewer motor units discharging, as can be seen in axon loss.
• Firing rate: If elevated, fewer motor units are contracting more often to provide the same tension in a denervated muscle.
Summary of clinical electroneuromyographic and nerve conduction studies
Instruments with computer-assisted analysis are now commonplace for studying electromyographic signals in great detail.11,28–30 Parameters of the waveform, including amplitude, duration, frequency spectrum, number of turns, or phase polarity reversals and area (the integral or total voltage of the waveform) can be automatically analyzed. The data are then compared electronically with predetermined patterns of electrical changes, which correlate with categories of neuromuscular disorders such as myelopathies and neuropathies.
The following is a summary of the more characteristic EMG and nerve conduction changes associated with selected groupings of neurological disorders. The intent is to assist in the understanding of reports of these studies and recognize changes that may be seen in sequential tests during the course of the disorders. The following is a simplified grouping of electrical changes; actual electrodiagnostic studies show considerably more detail and frequent variations of these findings.9–11,26,27
Electrical testing in CNS disorders typically shows normal motor and sensory nerve conduction. In the EMG, spontaneous activity is typically not seen, and individual motor units seen on muscle contraction usually have normal parameters. The recruitment pattern may show a slower-than-normal MUAP discharge frequency with an incomplete and irregular interference pattern. In the presence of tremor and other involuntary movements, bursts of MUAPs occur, consistent with the muscle contraction pattern. In cases involving the brain stem, the blink reflex may show abnormalities.7 The tests are important in differential diagnosis between a CNS and a PNS problem, but often they are not used when clinical examinations demonstrate the problem to be definitively in the CNS.
In myelopathies, which include upper and lower motor neuron disorders (e.g., amyotrophic lateral sclerosis [ALS], poliomyelitis, cervical spondylitis, and syringomyelia), motor and sensory nerve conduction is usually normal, although mild slowing may be present.11 The characteristic EMG changes, which usually appear in the more chronic stages of the disorders, are increased amplitude and duration of MUAPs because of the variable impulse conduction time in sprouting axon terminals. An increased number of polyphasic potentials with increased duration is usually found. Spontaneous activity is often seen, and on strong contraction fewer rapidly firing large MUAPs are recruited, resulting in a single-unit or partial interference pattern. In ALS, fasciculations and denervation potentials are typically found. The distribution of the EMG abnormalities determines the extent of the condition.
Peripheral neuropathies show a variety of electrical changes depending on the type and location of the pathology. In a proximal pathology (e.g., radiculopathy), motor and sensory nerve conduction generally remain normal, except F waves and H-reflex responses in specific spinal segments. If motor nerve roots are compromised, spontaneous activity and increased polyphasic potentials appear, and reduced recruitment of MUAPs results in an incomplete interference pattern. In more chronic stages MUAP amplitude and duration can be increased. As the lesion improves, spontaneous activity decreases and the recruitment patterns become more normal. If only sensory roots are injured, no EMG changes occur. Again, the distribution of the EMG abnormalities (all in one myotome) is pathognomonic, especially in the presence of denervation potentials in the corresponding paraspinal muscles.31
Generalized, systemic peripheral polyradiculoneuropathies can be divided into primarily demyelinating, primarily axon loss, or mixed axonal-demyelinating polyneuropathies. Some involve mostly sensory nerves (e.g., hereditary sensory neuropathy types I to IV, Sjögren syndrome, Friedreich ataxia), and others mostly motor nerves (e.g., chronic inflammatory demyelinating polyneuropathy [CIDP], lead neuropathy), but most involve both sensory and motor nerves. In the primarily demyelinating type, such as Guillain-Barré syndrome, motor and sensory nerve conduction and F waves become markedly slow. EMG changes usually do not occur, except for a reduced recruitment pattern consistent with weak muscle contraction or conduction block (when the demyelination is such that the impulse does not propagate). With primarily axonal polyneuropathies, such as uremic neuropathy, isoniazid or cisplatin toxicity, and lead poisoning, motor and sensory nerve conduction is mildly slowed or may remain normal. The duration and amplitude of the response, however, decrease. During advanced stages, many polyneuropathies develop both demyelinating and axonal pathology (e.g., diabetic neuropathy, which is by far the most commonly encountered polyneuropathy). On EMG, spontaneous activity is commonly seen. These electrical changes generally become more severe with worsening of the pathology, but they also improve if the pathology is reversed. From a patient management standpoint, remyelination occurs at a much more expedient pace than reinnervation.32–34
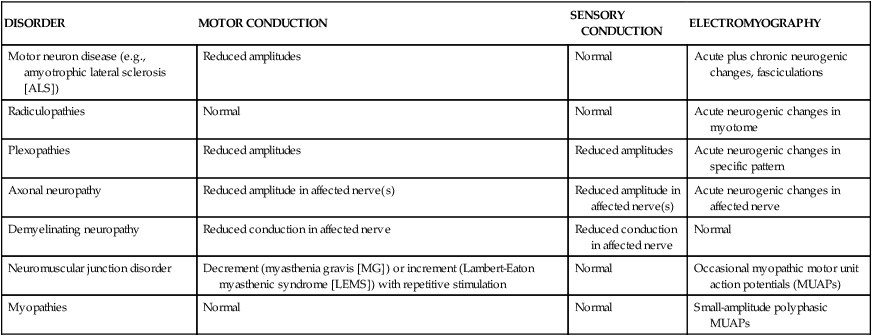
Table 33-1 provides a summary of typical findings.
TABLE 33-1 
| DISORDER | MOTOR CONDUCTION | SENSORY CONDUCTION | ELECTROMYOGRAPHY |
| Motor neuron disease (e.g., amyotrophic lateral sclerosis [ALS]) | Reduced amplitudes | Normal | Acute plus chronic neurogenic changes, fasciculations |
| Radiculopathies | Normal | Normal | Acute neurogenic changes in myotome |
| Plexopathies | Reduced amplitudes | Reduced amplitudes | Acute neurogenic changes in specific pattern |
| Axonal neuropathy | Reduced amplitude in affected nerve(s) | Reduced amplitude in affected nerve(s) | Acute neurogenic changes in affected nerve |
| Demyelinating neuropathy | Reduced conduction in affected nerve | Reduced conduction in affected nerve | Normal |
| Neuromuscular junction disorder | Decrement (myasthenia gravis [MG]) or increment (Lambert-Eaton myasthenic syndrome [LEMS]) with repetitive stimulation | Normal | Occasional myopathic motor unit action potentials (MUAPs) |
| Myopathies | Normal | Normal | Small-amplitude polyphasic MUAPs |
Single-fiber electromyography
During a carefully controlled minimal voluntary contraction, a 25-μm–diameter needle is inserted into the muscle, and several potentials from muscle fibers within the recording area are stored. Equipment with a trigger and delay line is necessary to “time lock” the tracings of the potentials. The slightly different conduction time or interpulse interval (IPI) required for impulses to be transmitted from a single motor neuron to each of its terminal endplates, cross the neuromuscular junction, and activate the muscle fiber is called jitter. This time difference is collected from several tracings and is converted into a mean consecutive time difference (MCD), which normally ranges from 5 to 55 ms. Values shorter or longer than this range are considered abnormal. The impulses from some axons to their muscle fibers may fail to be transmitted. This is referred to as blocking. Another capability of SFEMG is the measure of fiber density, that is, the average number of muscle fibers within the needle recording area. Fiber density is increased in reinnervation and also with certain myopathies because of axonal collateralization or splitting.28
Macroelectromyography
A variation of SFEMG uses a macroelectrode to record the majority of muscle fibers of a single motor unit as they are triggered by an initial potential, which is then time locked with all the other muscle fiber potentials recorded from a different part of the same or a nearby needle.11,.29,30,35,36 Two recording channels are used. Maximal amplitude of the potentials from several muscles has been reported by Stalberg.35 The findings are analyzed, along with findings of jitter, fiber density, and conventional EMG, to evaluate the status and prognosis of various neurological and neuromuscular disorders, such as motor neuron disease, peripheral nerve lesions, and myopathies.
In summary, the astute PT in the presence of a patient reporting weakness and/or numbness will refer that patient appropriately for electrophysiological testing based on the findings of a judicious clinical examination. In reading the report of an electrophysiological consultation, the PT will first correlate the results with the findings of the physical examination of the patient, determine whether the studies performed are complete (i.e., there are sufficient data to rule in the condition but also sufficient data to rule out other conditions), and correlate the findings with the conclusion. There is evidence that PTs performing NCSs and EMG tend to follow guidelines consistently.37
Kinesiological electromyography
Persons with neuromuscular disorders typically exhibit control errors, including the inability to initiate, execute, or terminate movement. Selective control may be absent or abnormal with errors in muscle timing, intensity, and sequencing. Spasticity or synergistic muscle action may impede smooth execution of tasks and prevent purposeful movement. With increased emphasis on evidence-based practice, KEMG can provide objective documentation of abnormal control and intervention outcomes and provide insight into optimizing strategies for improved functional performance. Many orthopedic surgeons rely on KEMG testing to supplement clinical evaluation in planning surgical interventions (muscle transfers and releases) in children with cerebral palsy,38 in patients with TBI,39 and in those who have had a stroke.40
KEMG interpretation depends on the examiner’s understanding of the instrumentation chosen for testing, including electrode selection, recording techniques, signal processing, and time and intensity normalization.41 Coupled with three-dimensional motion (infrared camera and motion capture) and force plate analysis, external moments are calculated that define internal force demands on muscles during functional activities such as walking. KEMG delineates the muscles that participate in meeting the internal force demand.
Recording instrumentation
KEMG can be performed by using surface or fine wire electrodes (intramuscular). Controversy exists about the choice of electrodes, with the selection dependent on the clinical or research question. If the examiner is interested in “muscle groups” (e.g., dorsiflexors, quadriceps) then surface electrodes are appropriate. Fine wire electrodes are optimal if activation of individual or deep muscles is desired (e.g., posterior tibialis, iliacus). Fine wire allows for specificity of muscle action required for surgical decisions related to muscle transfers, releases, or muscle lengthening.38,39,42
Needle or fine wire electrodes (indwelling or intramuscular).
A pair of 50-μg fine wire electrodes, also referred to as indwelling or intramuscular electrodes, are introduced through the skin and into the muscle with a 25-gauge hypodermic needle. The 50-μg, Teflon-coated wires are threaded through the needle’s core; 2 to 3 mm of the wire’s distal end are stripped of insulation and, once inserted, record adjacent motor unit activity. Before insertion the electrodes are sterilized. Inserted through the skin and into the muscle of interest, the barbed end “hooks” the muscle fibers when the needle is withdrawn. Accurate placement requires that the examiner have extensive knowledge of three-dimensional anatomy and excellent palpation skills. A maximal concentric voluntary contraction is elicited to anchor the wires into the muscle fibers, preventing displacement during subsequent contraction. This ensures sampling the same motor unit pool during subsequent tasks, trials, or conditions. Electrical stimulation is an essential testing element to verify electrode location. Wire electrodes allow a more precise definition of muscle timing (onset and cessation) by reducing the incidence of intramuscular crosstalk.43 Disadvantages include decreased reliability and insertional pain caused by skin penetration.44–46 In several states the examiner must possess specialized KEMG licensure to penetrate the skin with a needle.
Surface electrodes.
When the clinical question can be answered by using surface electrodes, electrical stimulation of motor points often defines optimal electrode placement over the muscle or group of muscles of interest. A maximal voluntary contraction (MVC) is elicited to confirm that optimal placement has been achieved. Standardizing electrode placement, size, interelectrode distance, and skin preparation enhances test-retest repeatability,47 with submaximal contractions more reliable than maximal contractions.48 Skin displacement under the recording site may introduce movement artifact, which can be minimized by securing the electrodes to the skin with tape. To improve interday reliability, electrode placement should be marked (with ink) and standardized electrodes used. When both recording electrodes are contained in the same housing, interelectrode distance is standardized and movement artifact attenuated. Advantages of using surface electrodes include improved reliability and the ease with which they can be applied without causing patient discomfort.44–46 Specialized licensure is not required.
Instrumentation for kinesiological electromyography acquisition
KEMG signal acquisition requires either a telemetry unit (FM modulation) or a hard-wired system that relies on a “cable” tethered to the subject to transmit signals from the electrode site to the receiver. The subject’s performance and nature of movement strategies performed may be altered by the cabling. Telemetry allows the subject unrestricted movement; KEMG signals are transmitted through the air from a small unit worn around the subject’s waist. The optimal characteristics of the receiver include a bandwidth frequency of 40 to 1000 Hz and an overall gain of 1000 Hz.41
Signal processing.
KEMG processing has become highly automated with the advent of high-speed computers and customized software. Once the signal has been acquired, it is stored digitally and processed by various computer programs. The “raw” signal is full wave rectified (all the negative values become positive), and a linear envelope is generated within a designated time interval. The area under the curve is mathematically integrated, and an average EMG profile is generated. Muscle-specific onset, cessation, and relative intensity are defined with a variety of software. According to a recent study, KEMG timing (onset and cessation) is optimally identified by using the intensity filtered average (IFA) and packet analysis (PAC) when compared with ensemble average (EAV).49 Despite a smaller recording volume with wires, Bogey and colleagues49 demonstrated no significant difference in signal amplitude when multiple insertion sites within the same muscle were compared.
Normalization.
Any acquired “raw” EMG signal needs to be referenced to a standard value. This is accomplished by dividing the raw EMG during a functional task such as walking by a reference value. The MVC serves this purpose. Subjects exert a maximal voluntary effort for each muscle that determines the maximal EMG activity possible. All subsequent efforts are compared with this maximal effort and expressed as a percentage of maximum (%MVC). In patients with neurological dysfunction who lack selective motor control, a maximal effort can be elicited in either an extensor or flexor synergy by using the upright motor control (UMC) test developed at Rancho Los Amigos National Rehabilitation Center, grading the effort as “weak,” “moderate,” or “strong” in synergy.50 Maximal efforts are elicited for 3 to 5 seconds, and the software determines the maximal activity for a 1-second interval. The muscles’ activation during a functional activity is subsequently expressed as a percentage of MVC.
Interpretation of kinesiological electromyography
Kinesiological electromyography and strength.
KEMG testing does not directly measure muscle strength, and the examiner should resist equating raw EMG signal amplitude directly with muscle force or torque output. Grading the strength (manual muscle testing [MMT] or UMC test) of each maximal effort must accompany the interpretation of the muscle participation during a functional task.51 For example, patients with postpolio syndrome (refer to Chapter 35) produce large-amplitude KEMG signals that often reflect the maximal exertion of a “weak” muscle (e.g., “MMT—Poor” or 2/5). Large-amplitude EMG signals represent activation of large motor units typical of reinnervation, not force output. Despite large-amplitude signals, the muscle is functionally weak. In other words, a 100% MVC normalized KEMG record for a muscle may represent the maximal effort of a “poor” or 2/5 muscle.
Muscle tone versus spasticity.
Therapists should resist making inferences about tone from KEMG testing. As previously stated, KEMG reflects the contractile activity of motor units. Muscle tone refers to the amount of resting tension in a muscle because of its viscoelastic properties. Because tone is not a function of motor unit activity, it cannot be measured with KEMG.41 In contrast, spasticity, defined as a hyperactive quick stretch response, can be recorded by KEMG because it reflects prolonged muscle activation (>0.1 s). Clonus has a distinct frequency characterized by a prolonged 5- to 8-Hz signal. Using signal duration in response to quick stretch, Cahan and colleagues52 identified significant decreases in spasticity in selected lower-extremity muscles in children with cerebral palsy after selective dorsal rhizotomy. In these children spasticity interfered with agonist activation during walking.
Electrical stimulation and electromyographic biofeedback
NMES and EMGBF are often used as tools in the management of neurological dysfunction. EMGBF can be used both alone and in conjunction with stimulation. The primary goal of use is improvement of function by improving voluntary motor control. To that end, strengthening and alteration of abnormal tone are also common goals of treatment. EMGBF is discussed later in this chapter. More detailed explication of treatment protocols is included in a variety of published work.53–64
Neuromuscular electrical stimulation
In NMES, muscle contraction is elicited by depolarization of the motor neurons. Electrodes may be placed over the muscle to be stimulated or over the motor nerve that controls the muscle. Firing order of neurons is a result of neuronal size, proximity of the electrical stimulus, and the intensity of stimulation.65 Muscle recruitment patterns triggered by electrical stimulation differ from those observed in normal muscle activation. In a voluntary muscle contraction, motor units fire asynchronously, with a larger proportion of type I, fatigue-resistant muscle fibers of the smaller motor units being recruited first. The order of muscle fiber firing occurs as a result of motor neuron size and the anatomy of synaptic connections.66 Conversely, an electrically stimulated muscle contraction elicits initial responses from larger motor units, which contain a greater number of fatigable, type II muscle fibers. The type II fibers are innervated by larger-diameter neurons that have a lower threshold for electrical stimulation than smaller neurons.67 A study of healthy subjects demonstrated recruitment of these higher-threshold motor units at relatively low NMES training levels. In voluntary exercise a much greater exercise intensity is required for activation of these larger motor units.67
Synchronous recruitment of muscle fibers is obtained with electrical stimulation. This does not occur with volitional activation. During a sustained volitional contraction motor units periodically “drop out” and then “drop in” to reduce fatigue. With NMES, once recruited the motor units will continue to fire until the stimulus is ended. This, coupled with the early recruitment of fatigable motor units, accounts for fatigue being a major problem in the use of NMES. It also is one reason functional activities performed under control by stimulation are much less smooth and balanced than when they are performed volitionally. At the same time, relatively low levels of NMES can recruit motor units that volitionally would be recruited only with maximal effort. This provides support for observed increases in strength with low NMES training intensities.68 Numerous potential benefits have been identified for NMES. Among them are improvement in ROM, edema reduction, treatment of disuse atrophy, and improvement of muscle recruitment for muscle reeducation.56
Parameters of stimulation
Waveform
NMES units use alternating currents. The waveform of the stimulus produced by most NMES units is either a symmetrical or an asymmetrical biphasic pulse. The two phases of each pulse continually alternate in direction between positive and negative polarity. Although an ideal waveform has not been identified, most studies have shown the symmetrical biphasic waveform to be more comfortable than either the asymmetrical biphasic or the monophasic waveform.53,69,70
Duration
Phase or pulse duration (also called pulse width) refers to the amount of time of a single pulse or phase. Stimulators with a pulse duration of 1 to 300 μs (0.3 ms) can be used to activate muscles with intact innervation. Waveforms of shorter durations require a greater current amplitude to produce a muscle contraction (because the current is on for a shorter period of time). They may be more comfortable but may not possess enough charge for good contraction levels. Longer-phase durations may be used but are less comfortable. In NMES units, pulse durations may be started at 100 μs, then increased to 200 to 300 μs if tolerated well by the patient.71,72 Denervated muscles require significantly longer phase durations (20 to 100 ms) because of the longer chronaxy of muscle cells compared with motor neurons.
Additional parameters: ramp time and on-off time ratio
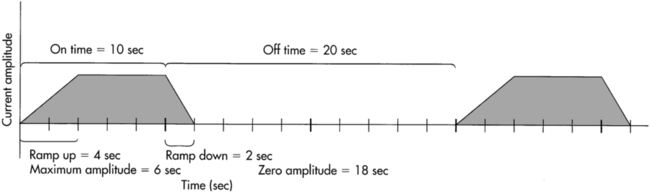
On time and off time.
NMES for facilitation of muscle contraction should be used to supplement exercise, and goals for stimulation should be consistent with the goals of the exercise program. To simulate isotonic or isometric muscle contractions, as in voluntary movement for exercise, the stimulator must have the capability of setting cycles of on and off times. Each period of muscle contraction is followed by a period of relaxation (Figure 33-7).2,56 In most cases a shorter on time than off time is desirable to avoid fatigue. For example, a 10-second on time may be followed by a 50-second off time in a cycle, resulting in an on-off ratio of 1:5. Packman-Braun73 investigated ratios of stimulation to rest time with NMES for wrist extension in a group of hemiplegic patients. Results supported the on-off time of 1:5 as being the most beneficial in training programs of 20 to 30 minutes because of the deleterious effects of fatigue with lower ratios (1:1, 1:2, 1:3, 1:4). If the goal is to reduce edema by providing a muscle pumping action, a ratio of 1:1 or 1:2 may be preferred,73 as the intent is to decrease the edema by continuous muscle pumping action. Lower ratios may be used when the goal is neuromuscular reeducation or endurance training.
Ramping.
Ramping is another modulation that can be set by the therapist. Ramp-up is the time it takes each train of pulses to increase amplitude or intensity sequentially from zero to maximum. Ramp-off is the period set at the end of the train of maximal intensity pulses to decrease sequentially from maximum to zero amplitude (see Figure 33-7). Ramp time can be adjusted so that the stimulation more nearly resembles a pattern of gradually contracting and relaxing muscles. For clients with hypertonicity or spasticity and a goal of facilitation and strengthening the antagonist muscle, a longer ramp-up time may avoid or minimize activation of the stretch reflex in the hyperactive agonist muscle.
Duty cycle.
The term duty cycle is sometimes confused with the on-off time ratio. Duty cycle is the percentage of time a series or train of pulses is on out of the total on and off time in a cycle.2,53 For example, if the train of pulses is on 10 seconds and off 30 seconds, the total cycle time is 40 seconds. The duty cycle would be 25% (10 seconds of the total 40-second cycle). The actual on time and off time of the pulses in a cycle is a more informative description than either the duty cycle or the on-off ratio.
Muscle reeducation
After an insult or injury affecting the CNS, problems with motor control frequently manifest. One of the common goals of therapy is to facilitate movement in the areas where control is lacking. If active movement is not present, NMES allows movement to occur by stimulation, which may be followed by resumption of active movement, possibly triggered by the sensory (visual and proprioceptive, among others) experience that accompanies the stimulation. When active movement is present but is weak or not well controlled, the therapist may choose to use NMES to supplement and strengthen the muscular contraction already present. Some evidence exists that NMES can increase activity in the somatosensory cortex and that the cortical activity is correlated with improvement in functional tasks.74 In the presence of hypertonicity, the muscles serving as antagonists to the spastic muscle may be targeted for NMES, not only to strengthen the antagonist but to inhibit the spastic muscle by reciprocal inhibition.
Functional electrical stimulation
The term functional electrical stimulation (FES) has been used casually to describe various applications of NMES. However, FES is defined by the Electrotherapy Standards Committee of the Section on Clinical Electrophysiology of the American Physical Therapy Association as the use of NMES (on innervated muscles) for orthotic substitution.2 Baker and Parker75 use the term to describe external control of innervated, paretic, or paralytic muscles “to achieve functional and purposeful movements.” Although NMES is generally considered to have therapeutic applications, such as increasing ROM, facilitation of muscle activation, and muscle strengthening, the key to application of FES is to enhance or facilitate functional control. It is used with clients with SCI, TBI, cerebrovascular accident (CVA), and other CNS dysfunction who have intact peripheral innervation.
An example of FES application is the electrical stimulation of the peroneal nerve to enhance ankle dorsiflexion during gait in patients with hemiplegia.76 Two of the more common applications of FES on the market include the WalkAide system (Innovative Neurotronics, Austin, Texas) and Bioness (Bioness, Valencia, California). In terms of neurological dysfunctions, research has been published that indicates efficacy of these devices and FES in general in improving functional performance in people with CVA,77–80 multiple sclerosis, TBI, SCI,81,82 and cerebral palsy.83 Moreover, numerous other uses of FES have been described, ranging from isolated motor control activities, such as decreasing shoulder subluxation and reducing scoliosis, to highly technical computerized gait and bicycling capabilities, sometimes referred to as computerized FES (CFES).84–92 The trigger that activates muscle contraction in synchrony with the functional activity can be manually initiated by the client, set within the stimulator to automatically trigger on and off cycles, or programmed into a complex computer system for bicycling or gait.
Stimulation is generally applied in short-duration pulses with a frequency sufficient to provide smooth, tetanizing muscle contractions and adjusted to cycle on and off, with adequate ramp functions, as indicated by the speed and time needed to synchronize the stimulation with the functional activity. The length of the intervention depends on the purpose and may vary from a few contractions during the functional activity, building to multiple 30-minute sessions working up to several hours, repeated daily or three to five times per week. With the more complex computerized systems used with clients with complete spinal cord lesions, electrically activated functional movements are the mechanism to achieve physiological and psychological benefits. In some situations, assisted function is also an important goal, although functional community ambulation is not yet a reality.88,90,93–100 Hooker and co-workers94 evaluated the physiological effects of use of FES-assisted leg cycling in SCI. Compared with resting levels, significant increases were found in cardiac output, heart rate, stroke volume, respiratory exchange rate, pulmonary ventilation, and other physiological phenomena. CFES for cycle ergometry and ambulation has also been shown to increase muscle mass, electrically induce muscle strength and endurance,89,101,102 increase circulation and aerobic capacity, decrease edema, and have a beneficial impact on self-image.90,102,103 Jacobs and colleagues100 compared the metabolic stress of FES-assisted standing versus frame-supported standing. Cardiorespiratory stress was significantly higher with FES, and the authors concluded that FES-assisted standing alone may provide a stress sufficient to meet minimal requirements for exercise conditioning.
The demonstrated benefits of FES clearly indicate that it is a valuable tool for supplementing functional activities. The practicality and cost of applications of the more complex computerized systems need further study, especially in terms of function in community activities. (Refer to Chapter 38 for additional discussion.)
Electromyographic biofeedback
Biofeedback is a general term used to describe the use of visual or auditory representation of physiological processes to allow an individual to modify those processes. EMGBF makes available to the client information regarding the electrical activity of muscle. EMGBF has several well documented applications, including alteration of physiological responses such as heart rate, temperature, and muscle tension.54 These applications may prove beneficial for clients with neurological dysfunction. An example would be relaxation to modify pain perception. The focus of this review is the use of EMGBF for improvement of active movement, which may include reduction of hypertonicity in addition to muscle reeducation. EMGBF units range from basic single-channel portable models to clinical units with multiple channels and multiple options for provision of feedback.
EMGBF may be used to assist a client in attaining greater levels of muscle activation in paretic muscle, decrease levels of muscle activation in spastic muscle, or attain a balance between agonist and antagonist muscle pairs.104 For most practicing clinicians, EMG levels are monitored through the use of surface electrodes. Monitoring of activation of deep muscles is often not feasible. Attention to size and specific electrode placement is critical to ensure feedback that will be useful. Smaller electrodes allow specific placement, although higher impedance will be encountered. Skin must be carefully prepared to take this into account.105 Because the EMG information recorded represents the sum of action potentials from motor units between the electrodes, large interelectrode distance will increase the area of muscle recorded. This may be desirable for large muscle groups or when minimal activity is present.
Smaller interelectrode distances are preferable if interference from “crosstalk” or “volume conduction” from muscles or motor units not part of the target group is a risk. Basmajian and Blumenstein105 provide an excellent review of electrode placements.
Reduction of hypertonicity
DeBacher106 described a progression of intervention with EMGBF designed to reduce spasticity. The program uses three stages of intervention: (1) relaxation of spastic muscles at rest even in the presence of distraction, mental effort, or use of muscles not targeted for EMGBF training; (2) inhibition of muscle activity during passive static and dynamic stretch of the spastic muscle, beginning with static stretch at the extremes of motion, then progressing to passive movement speed at a speed of 15 degrees per second; and (3) isometric contractions of the antagonist to the spastic muscle, with relaxation of the spastic muscle, progressing to prompt muscle contraction and relaxation of the spastic muscle, and grading of muscle contractions with movement for various force output requirements. Use of the technique in a small sample of young adults with cerebral palsy demonstrated improvement in resting levels of involuntary muscle activity.107 Improvements in function, however, were not demonstrated.
Inhibition of a spastic muscle alone may not be enough to improve function. Often the spastic muscle itself, once hypertonicity has diminished, is weak or has low functional tone. Weak antagonists to the affected muscle may contribute to the functional limitation. EMGBF to reinforce activity in the weak muscle may be done concurrently with its use to modify the tone in the agonist.108 EMGBF can be useful in helping a client decrease abnormal muscle activation, but persistence of control problems may be related to lack of force production, deficits in speed of muscle activation, and lack of reciprocal interaction of muscle groups.109
Muscle reeducation
Concurrent assessment of muscle activity (CAMA) is an application of EMGBF in which the therapist uses biofeedback as an adjunct in evaluation of client response to therapeutic exercise.110 In this procedure the therapist decides which muscle group(s) are desired for activation and adjusts the position of the client or the therapist intervention accordingly to get the correct responses. CAMA allows for the judgment of the effectiveness of a particular activity based on actual EMG responses rather than presumptions of what the intervention should cause. In a placebo-controlled study of hemiplegic patients, the addition of EMGBF to hand exercises based on the Brunnstrom approach resulted in significant improvement in active ROM in those using biofeedback compared with sham.111
Several authors suggest the use of biofeedback signals from homologous extremity muscles as a model for what the hemiplegic client needs to alter muscle activity in a particular function.112,113 This has been described as a “motor copy” and was compared with a more targeted training procedure. Indications showed that the motor copy resulted in better carryover in function than the comparison therapy in follow-up evaluations. A similar training study showed indications of benefits of the procedure, but the results were not statistically significant because of the small group size.112 At least two studies support patterning or copying EMG from other muscles as a potentially useful tool for individuals with C4-7 SCI.114,115 A meta-analysis that compared EMGBF with conventional physical therapy for upper-extremity function in individuals after stroke reviewed only six studies and concluded that neither approach was superior to the other.116
Feedback considerations
Experiments examining feedback frequency in the learning of motor tasks support the use of less than 100% relative frequency for the subject to learn the task. Feedback provided on every trial may improve performance but degrades learning in normal subjects.117 In a study of stroke patients attempting a pursuit tracking task, biofeedback was used for the experimental group (electrodes over the spastic biceps), whereas the control group performed the task without feedback.118 Posttests revealed that the use of continuous feedback had a negative transfer effect on learning of the movement task, suggesting that the experimental learners became dependent on the external feedback in performance of the task. The clinician must therefore carefully structure the use of external feedback so the client begins to develop a sense of muscle activation or relaxation that is present without the EMGBF apparatus. This may be accomplished by turning the screen away from the client and turning off the auditory signal as the patient progresses.
Integrating neuromuscular electrical stimulation and electromyographic biofeedback
The use of EMG-triggered NMES, in which NMES is initiated once the client has achieved a predetermined level of EMG activity in the targeted muscles, is an application that has been shown to have merit,119,120 although a more recent systematic review showed no statistically significant differences between EMG-NMES and usual care in improving upper-extremity function of the affected extremity in people who had had a stroke.121 Threshold levels of EMG activity could gradually be increased as the client gains the ability to activate muscles independently, with eventual discontinuance of the NMES as strength and active control allow. The success of this application has been shown in patients with hemiplegia in terms of increasing EMG activity and subsequent improvement in ROM and function in the involved arm and leg.119 In another study, patients who had had a stroke more than 1 year previously significantly improved wrist and finger extension strength and function after treatment with EMG-triggered stimulation compared with controls.120
A variation of this application is NMES triggered by positional feedback, such that NMES is initiated once the patient actively moves through a portion of the available ROM at a joint.122 The therapist may set the threshold angle in accordance with the patient’s goals and abilities. This method has been shown to be effective in improving wrist motion after stroke, although it was not as effective in altering control of the knee in a similar patient group.123
Applications
Many investigators have evaluated the use of NMES and EMGBF in clients who have had a stroke.108,124–126 FES has been used extensively with clients with SCI. Other populations that demonstrate neuromuscular impairment or dysfunction have not been as thoroughly studied. This may be because the heterogeneity of these groups may create difficulty in research design.
Upper-extremity management
Electromyographic biofeedback
EMGBF has been extensively studied, but success of the treatment is mixed, with difficulty in interpretation. A reduction in co-contraction has been observed,127 as well as improvement in several neuromuscular variables124,128,129; however, a lack of significant improvement in functional skill was noted. Given the challenge of improving upper-extremity functional ability after stroke, Wolf and Binder-Macleod129 suggested consideration of several key factors in predicting which clients may benefit from EMGBF: “Those patients who achieve the most substantial improvement in manipulative abilities initially possess voluntary finger extension; comparatively greater active ROM about the shoulder, elbow, and wrist; and comparatively less hyperactivity in muscles usually considered as major contributors to the typical flexor synergy.” Attention to the chronicity of motor dysfunction also appears critical in anticipating success with EMGBF, as clients 2 to 3 months after stroke demonstrated stronger functional gains after intervention with biofeedback compared with clients 4 to 5 months after stroke. A placebo-controlled study of EMGBF showed statistically significant improvement in active ROM of the hand in clients who received EMGBF in addition to exercise.111 A study by Dog˘an-Aslan130 and colleagues found improvements in the Ashworth scale, Brunnstrom stage, upper-extremity function test, wrist extension ROM, and surface EMG potentials in their subjects who were treated with EMGBF in conjunction with neurodevelopmental treatment (NDT) and conventional treatment in a population of subjects with hemiplegia secondary to stroke.
Neuromuscular electrical stimulation
FES for shoulder subluxation reduction appears beneficial for prevention of pain and subluxation, especially if used during the early stages of recovery. The functional benefits over the long term are not always clear, and cost-benefit ratios need to be considered. Baker and Parker,75 Faghri and colleagues,85 and Chantraine and colleagues131 have reported success in management of shoulder subluxation after stroke by using gradually increasing stimulation times that ultimately reached 6 to 7 hours per day. On-off ratios were typically 1:3.
Use of NMES after stroke to facilitate motor control and function has been studied, but many studies lack controls. The extensors of the fingers and wrist are typically the targeted muscle groups. de Kroon and colleagues61 reported a systematic review of literature that included six randomized controlled trials. A variety of stimulation parameters were used. Three studies tested subjects with acute conditions, and three tested subjects with chronic conditions. Outcome measures for motor control included the Fugl-Meyer Motor Assessment (four studies) and strength (two studies). Functional outcomes were reported by two studies, one using the Action Research Arm test and one using the Box and Block test. The authors concluded that there was a positive effect of stimulation on motor control. Only two studies reported functional outcomes, but both were positive. Of significance is the fact that only six studies met the criteria for review in terms of rigor.119,120,122,132–134
FES has also been investigated as an upper-extremity orthosis after hemiplegia, using movement of the uninvolved shoulder to trigger stimulation of elbow extension and hand opening.135 After an extensive training period, patients were able to demonstrate functional use of the involved hand for basic reach and grasp.
Systems with more than two channels of stimulation have proved difficult for patients to use.136 Popovic and colleagues137 reported two studies in which they used NMES to treat patients after stroke. They termed the intervention functional electrical therapy (FET). The stimulation was used during an exercise program composed of voluntary arm movements and opening and closing, holding and releasing objects with the stimulation serving as an electric prosthesis. Treatment was 30 minutes daily for 3 weeks in one study137 and 6 months in the other.138 Outcomes were reported as better than controls. Initially higher-functioning subjects benefited more than those who were rated as low functioning. Unfortunately, outcome measures in these studies were not standardized.
A study of use of daily NMES in the form of neuroprosthetic FES (NESS Handmaster) stimulation of hand and finger extensors in patients with subacute strokes (6 to 12 months after onset) was reported by Ring and Rosenthal.139 All subjects were receiving physical and occupational therapy three times a week. The stimulator was used at home. Those receiving stimulation had significantly greater improvements in spasticity, active ROM, and functional hand test scores compared with control subjects. The authors concluded that supplementation of outpatient rehabilitation with NMES improves upper-limb outcomes.139
Three-month interventions with EMG-triggered NMES, low-intensity NMES, proprioceptive neuromuscular facilitation (PNF) exercise, or no treatment were compared in a group of chronic stroke patients.140 At 3 and 9 months after treatment, Fugl-Meyer scores improved 18% for the PNF group, 25% for the patients receiving low-intensity NMES, and 42% for the group receiving EMG-triggered NMES. The control group did not change.
With the advent of the use of technology in neurological rehabilitation (see Chapter 38), an increasing number of investigations have been done to incorporate new technology with electrical stimulation to improve functional performance. Sayenko141 described the use of a video game–based training system combined with NMES in a person with chronic SCI. Results indicated improvement in the strength and endurance of the paralyzed lower extremities of the individual after the intervention.
Lower-extremity management
Electromyographic biofeedback
Several studies evaluating EMGBF for retraining lower-extremity control after stroke have focused on improvement of tibialis anterior control and reduction of gastrocnemius muscle activity. Results support increases in strength and ROM in ankle dorsiflexion with carryover into ambulation and maintenance of this improvement on follow-up evaluation.142–144
Wolf and Binder-Macleod124 examined a number of variables at the hip, knee, and ankle in a controlled group study of the effects of EMGBF. Subjects were assigned to one of four groups: lower-extremity EMGBF, upper-extremity EMGBF, general relaxation training, and no treatment. No significant changes were observed between experimental and control groups for EMG levels and ROM at the hip, but improvements were noted in knee and ankle active motion for the experimental group. Although subjects in the experimental group increased their gait speed, these changes were not significantly different from findings in the comparison groups.
Use of EMGBF with the intent of improving ambulation may require use of feedback during the task of ambulation instead of during static activity, as demonstrated by this study. Positional biofeedback regarding ankle position and traditional EMGBF were compared in a group of hemiplegic subjects.145 A computerized system provided audiovisual feedback during ambulation for both groups. Pretreatment and posttreatment measures of ankle motion, gait, and perceived exertion were conducted for the two treatment groups and a control group. The group receiving positional feedback increased walking speeds relative to the other groups, with improvements maintained at follow-up intervals of up to 3 months. The consideration of integrating feedback into functional ambulation bears further investigation.
Neuromuscular electrical stimulation
Peroneal nerve stimulation has been documented as an assistance for patients with hemiplegia to improve ambulation.76,146–148 Long-term stimulation with implanted electrodes has proved effective in improving gait patterns, but difficulties in achieving balanced dorsiflexion, infection, and equipment maintenance were drawbacks.148,149
Shorter-term use of peroneal nerve stimulation as an adjunct to traditional physical therapy may be considered. In a controlled study examining the use of 20 minutes of peroneal nerve stimulation six times per week for 4 weeks, the stimulated group demonstrated dorsiflexion recovery three times greater than the control group, as measured by an average of 10 maximal dorsiflexion contractions. These improvements were regardless of site of lesion, age, or time since lesion.146 Surface electrode stimulation is effective, and gait parameters can be improved with its use.150,151 If, however, a foot drop is the only major impediment to ambulation, lightweight plastic orthoses are a functional and much less expensive choice of intervention.
Multichannel electrical stimulation has been used in the management of ambulation in patients who have had a stroke.140,152–155 Although more effective than traditional gait training in some cases in terms of gait velocity and stride length, at follow-up evaluations 8 to 9 months after therapy the difference between groups had faded. However, the expense and availability of such systems make their use unlikely at this time.
NMES used for ankle dorsiflexion triggered by heel switch during gait and biofeedback to improve active recruitment of ankle dorsiflexors or relaxation of ankle plantar flexors has been studied in hemiplegic clients.156 Patients who received a combination of these interventions demonstrated significantly improved knee and ankle range parameters more rapidly than those using a single modality. This improvement was maintained over a 1-month period. Although all groups improved in gait cycle times, results in the combined intervention group were better. This may be attributable to the synergy of biofeedback and stimulation.51 Granat and colleagues157 also studied peroneal muscle stimulation effects on gait parameters after stroke. After intervention the subjects showed significant control of eversion on all surfaces. The Barthel Index score also improved after intervention. However, no improvement occurred when the patients were not using the stimulator.
Stroke (see chapter 23)
Wolf and Binder-Macleod124 examined client characteristics that are critical to success with biofeedback training for upper- and lower-extremity control after stroke. In a group of 52 clients with stroke, no significant relations between outcome and age, sex, number of EMGBF treatments, or side of hemiparesis were found. Lower-extremity treatment was associated with a greater probability of success, and this success did not seem related to chronicity of stroke sequelae. In contrast, success of upper-extremity treatment did appear to be related to length of time since onset of stroke, and poorer outcomes were noted if clients had received therapy to the involved arm for more than 1 year before EMGBF training. Improvements in elbow and shoulder function were obtained in this group of patients, but improvement in functional use of the hand was limited. Aphasia imposed a slight limitation to achieving improvement, but proprioceptive deficits were more significant in restricting functional gains. The role of client motivation in success with EMGBF training was emphasized. On follow-up over a 12-month period, the improvements made in the initial intervention were maintained in 33 of 34 clients evaluated.
A number of studies have been published discussing the muscle recruitment problems observed after CVA.108,125,126 Knowledge of these problems is a prerequisite for determination of the appropriate application of EMGBF and NMES. Delayed recruitment of the agonist and antagonist is a relatively consistent finding. Other findings include delayed termination of muscle activity once initiated,158 presence of co-contraction of agonist and antagonist muscles,125,126 lack of co-contraction,108 and maintenance of agonist muscle contractions.125 These reports emphasize the potential value of EMGBF in determining the best mode of intervention.
Jonsdottir and colleagues159 found that the application of EMGBF in a task-oriented manner, incorporating motor learning principles, resulted in improvements in gait velocity, stride length, and peak ankle power in a population of individuals with hemiparesis. Sander and colleagues160 found that NMES application to the tibialis anterior muscle for 30 minutes three times a week for 4 weeks was effective in improving strength of the affected lower extremity.
Lourencao and colleagues161 studied the effects of biofeedback with FES and occupational therapy on upper-extremity function of individuals with hemiplegia. Results indicated significant improvements in upper-extremity ROM and functional recovery with the addition of biofeedback in the treatment regimen.
Lastly, a systematic review was conducted by Woodford and Price162 on the use of EMGBF for recovery of motor function in individuals with stroke. The authors found evidence from a number of small studies that indicate that EMGBF resulted in improvement in gait, function, and muscle power compared with the usual physiotherapy interventions. The authors emphasized limitations in the results because of the small amount of evidence and problems with methodological designs and differing outcome measures.
Traumatic spinal cord injury (see chapter 16)
NMES has a variety of applications for clients who have sustained SCI. Muscle strengthening may occur for muscles innervated by segments just above a complete SCI, or a variety of strengthening applications may be appropriate in the case of incomplete SCI. EMGBF may be used to identify muscle activity in weak musculature, as a tool to judge improvement in muscle activation, and as a method of facilitating increased strength.163 Applications of EMGBF for individuals with SCI also include facilitation of unassisted ventilation in high-level quadriplegia164 and use of biofeedback for muscle reeducation with incomplete SCI in the acute stages when immobilization may be required.165
The use of NMES, EMGBF, and other physical therapy was examined in a group of clients with incomplete cervical SCIs over a total treatment period of 16 weeks. Clients were randomly assigned to one of four groups receiving physical exercise, NMES, or EMGBF. Group 1 received EMGBF followed by physical exercise, group 2 received EMGBF followed by NMES, group 3 received NMES followed by physical exercise, and group 4 received 16 weeks of exercise only. Measurements of muscle strength, self-care ratings, mobility scores, and voluntary EMG were conducted at baseline, treatment midpoint, and conclusion of all interventions. All groups demonstrated improvement across the treatment period on all measures except voluntary EMG; however, no significant differences were seen among the four groups.166 At least one other study compared conventional intervention, EMGBF, electrical stimulation, and combined stimulation with biofeedback over a 6-week period in individuals with quadriplegia. An examiner who was blinded to the intervention protocol evaluated 45 subjects in the four treatment groups. All groups improved in the parameters evaluated, and no significant difference among groups was noted.167 These results again emphasize the need to consider carefully cost as well as time and effort for setup and equipment operation in intervention planning.
A study by Carvalho and colleagues168 studied the use of treadmill gait training with NMES to improve bone mass in people with SCI. Their research study included 21 males with chronic quadriplegia between C4 to C8, randomly assigned to a control and treatment groups. Those in the treatment group received treadmill training with NMES, unweighting the body of 30% to 50% of body weight. The training was done for 20 minutes, twice a week for 6 months. Results showed that gait training with NMES, even with 30% to 50% unweighting, resulted in the improvement of bone mass in the subjects with chronic quadriplegia. In another study also by Carvalho,169 NMES with partial body weight support resulted in hypertrophy of the quadriceps femoris muscle also in a population of subjects with quadriplegia. In both studies, NMES was helpful in producing stimulation that allowed for the subjects to advance their lower extremities while walking with body weight supported on the treadmill.
In a few studies, the application of NMES appears to be beneficial in improving impairments associated with SCI. Bittar and Cliquet170 found that the use of NMES on the quadriceps and anterior tibialis of individuals with SCI was beneficial in that it allowed the feet and ankles to be placed in a better biomechanical position for ambulation. In addition, a study by de Abreu and colleagues171 found improvements in the cross-sectional area of the quadriceps after NMES to these muscles.
In a study by De Biase172 EMGBF training was noted to have resulted in increased EMG response of the rectus femoris muscle in 20 subjects with chronic SCI.
Upper-extremity management
The use of electrically stimulated hand orthotic systems for patients with C6 or higher level SCI have been refined to allow greater functional independence for a select group of patients.87,96,97,115,173–175 Because hand function does not occur in a cyclical pattern, the onset and termination of stimulation must be controlled by the patient in some manner, with a myoelectric or contact closing switch.96 Multichannel stimulation is then applied with intramuscular electrodes for the flexors and extensors of the fingers and thumb, with computer-configured interplay between the different muscles to achieve a functional grasp. A chest-mounted position transducer (operated by shoulder elevation or depression and protraction or retraction) allows the user to initiate stimulation and lock the stimulation to maintain a grasp as well as unlock it for release. A toggle switch mounted on the chest allows a choice between electronically stimulated lateral or palmar grasp patterns.87,97 Some investigators have used contralateral shoulder slings as well as elbow accelerometers to trigger the needed stimulation.176,177 Multiple authors have described successful implantation and use, with a few drawbacks, of upper-extremity FES prostheses.178–181 Use of this type of system may allow patients with SCI at the C5 level to operate at the same level of independence (or even a higher level) as those with C6 quadriplegia with tenodesis, gaining the ability to perform more activities of daily living (ADLs) without an attendant. Patients with SCI at the C6 level may be able to manipulate a greater variety of objects without special adaptations.
Lower-extremity management
Standing.
In an excellent review of the use of FES for the purpose of standing patients with SCI, Gardner and Baker182 described the easiest approach to stimulating the quadriceps femoris to allow paraplegic clients to stand. More complex systems may incorporate stimulation of the gluteus maximus, gluteus medius, hamstring, adductor magnus, gastrocnemius, and soleus muscles for longer-duration and better-quality standing performance. Multichannel surface and implantable systems have been and continue to be developed for assistance in sit-stand and transfer activities.183–185 Surgical procedures, client selection, and technology are also factors cited in successful interventions. Despite these efforts, the duration of standing with electrically stimulated systems ranges from a few minutes to several hours. The client with SCI may be able to use this technology to perform functional activities that require standing. The use of these systems depends on the functions unavailable to a client without use of the technology, and the ease with which a system can be used and maintained. Peripheral to, but no less important, are the reactions of joints to these interventions. Two studies186,187 have reported positive benefits to the structure and functions of lower-extremity joints of adolescents with SCI after participating in FES programs. A recent study has also identified that the physiological responses to standing in SCI may provide a cardiorespiratory stress sufficient to meet minimal requirements for exercise conditioning.100
Cycling.
The use of systems to stimulate reciprocal lower limb motions electrically has increased for stationary cycling. The benefits of these interventions for the client with SCI may relate to prevention of cardiovascular disease in the wheelchair-dependent client. Physiological changes noted with electrically stimulated cycling include improvement of peripheral muscular and cardiovascular fitness, as demonstrated by increased power output after training with leg cycle ergometry.85,94,95,101,102 Combining FES and lower-extremity cycling with upper-extremity ergometry induced a higher level of cardiovascular fitness than lower-extremity ergometry alone.188 Exercise session frequency as little as two times a week induced positive changes in cardiovascular fitness.189 When testing of clients with paraplegia or quadriplegia is conducted with arm crank ergometry after a training program with electrically stimulated leg cycle ergometry, clients do not demonstrate differences in pretest and posttest measures of hemodynamic and pulmonary responses. These findings may relate to the specificity of the leg exercise training or the presence of a peripheral rather than a central circulatory response to the training procedure.95 As previously noted, many cardiovascular factors can be improved and the improvements retained for at least 8 weeks after a program of FES ergometry.
Ambulation.
As technology continues to progress, the use of electrically stimulated systems for ambulation may become more practical and useful for the patient with SCI.98,190,191 Acceptance and use of the systems by clients outside the clinic have been mixed, but the systems have been shown to have positive effects on characteristics of ambulation.149,192 Improvements in functional applications and use will also take place as the ability to select appropriate candidates improves.193,194 Benefits of these systems may include increased muscle bulk, a reduced risk of pressure sores and osteoporosis, and psychological benefit. Generally, improvements in functional ability are expected to produce positive psychological factors. Addressing these factors directly, Bradley,195 in a study measuring the effects of participation in an FES program on the affect of 37 individuals with SCI, demonstrated that positive affect was not significantly altered. Significant changes in negative affect occurred, however, with particular items of hostility and depression evident in those individuals in the treatment group who had unrealistic expectations. The author noted that these individuals need to be identified and monitored through the course of rehabilitation. Other drawbacks relate to the expense of the equipment and personnel and the lack of long-term efficacy studies. The speed with which a client with a complete SCI is able to walk with electrically stimulated systems remains relatively low (2 to 54 m/min) compared with normal rates of 78 to 90 m/min.196,197 Many of the published reports do not provide information on the maximal distance patients are able to walk with these systems, but reported distances range from 100 to 400 m.
Some clients may perceive the technology of electrically stimulated standing and walking as moving them toward a cure for their paralysis. With a complete injury, however, the stimulation occurs passively, without expectation that voluntary control will return.195 In cases of incomplete injury, electrically stimulated ambulation may assist the client in using and bolstering active control so that movement without the stimulation is more feasible. In considering use of electrically stimulated cycling and ambulation, discussion of the goals of treatment and the costs of the procedure must be conducted openly with the client to allow an educated choice to be made about the use of this expensive technology.
Traumatic brain injury (see chapter 24)
NMES may be a useful tool with clients having sustained brain injury, with potential benefits of managing contractures by increasing ROM, facilitating active control, and reducing spasticity by strengthening the antagonist of a spastic muscle.198 In cases in which an understanding of the purpose and principles of NMES is not feasible for a client, the comfort of the stimulation may be critical in ensuring its continued use. Comfort may be enhanced by increasing the ramp-on time and selecting waveforms that allow stimulation at lower amplitudes yet still obtain the desired contraction.36 Use of NMES with a client at Rancho Level IV and below is not appropriate because the client may not be able to understand the purpose and meaning of the stimulus and thereby may perceive the stimulus as noxious.
EMGBF applications for clients with brain injury can be similar to those used with stroke, given similar motor presentations.199 Therapists must consider residual cognitive deficits after brain injury in determining the appropriateness of EMGBF.
Guillain-barré syndrome (see chapter 17)
EMGBF in clients with Guillain-Barré syndrome demonstrated improvements in muscle strength in upper and lower extremities, although inconsistent improvement in functional use of the upper extremities was noted.200,201 Treatment regimens consisted of EMGBF for 10 trials per muscle conducted in 45-minute treatment sessions twice a week, in one case for 78 weeks and in the other case for 46 weeks.201
Multiple sclerosis (see chapter 19)
In a case series, Wahls and colleagues202 found improvements in ambulation of patients with primary or secondary progressive multiple sclerosis. The authors cited the possible positive effects of NMES application on muscle spasm, muscle pain, and disuse atrophy as possible reasons behind the improvements in ambulatory function in their subjects.
Pediatric applications
Cerebral palsy (see chapter 12)
The use of NMES with children with cerebral palsy has been addressed to some degree, with several case study reports.203–205 Carmick203,204 described a variety of applications with children at 1.6, 6.7, and 10 years of age, integrating NMES into a treatment regimen that focused on a “task-oriented model of motor learning.” Improvements were noted in upper- and lower-extremity movement and functional use across a variety of tasks appropriate to the age and the movement dysfunction each child demonstrated. In a study by Nunes and colleagues,206 the once-a-week application of NMES on the tibialis anterior muscle of 10 children with spastic hemiplegia resulted in improvements in gross motor function, passive ROM of ankle dorsiflexion, and muscle strength.
Ozer and collegues207 studied the effect of using NMES combined with dynamic bracing and found the intervention to be more effective than either alone in reducing upper-extremity spasticity in their sample of children with spastic hemiplegic cerebral palsy. A similar study by Postans and colleagues208 found that NMES combined with dynamic splinting reduced upper-limb contractures in children with cerebral palsy. In another study,209 the application of NMES to the gluteus medius improved gait parameters in children with spastic diplegia.
Advancements in technology have allowed the use of EMGBF in increasing contexts, such as the computer-assisted feedback (CAF) system, which can be used to provide feedback about muscle activity during ambulation.210 Data examining use of this system to provide feedback about the level of triceps surae activity during gait of children with cerebral palsy suggest potential improvements in gait symmetry, velocity, and appropriate muscle activation patterns as a result of this intervention. Use of this modality as an adjunct to physical therapy may prove beneficial.
Spina bifida (see chapter 15)
Five subjects with spina bifida (aged 5 to 21 years) were treated with daily NMES over an 8-week period to strengthen the quadriceps femoris muscles. Increases in maximal quadriceps torque production were observed in two of the five subjects in the treated limb. Improvements in functional activity speeds were noted for all of the subjects. Lack of improvement in torque production by three subjects was speculated to be related to lack of adherence to the exercise regimen and the heterogeneity of the subject sample.211
Spinal muscular atrophy (see chapter 13)
One study has reported the use of low-intensity electrical stimulation in children with types II and III spinal muscular atrophy in an attempt to determine any effect on arm strength and function. After 6 to 12 months of stimulation no statistically significant differences were noted between experimental and placebo-control arms in strength, muscle mass, or function.212
Scoliosis
Axelgaard and Brown84 demonstrated in the 1970s that surface NMES could reduce idiopathic scoliosis. Criteria for this treatment required curves measuring 20 to 45 degrees by the Cobb method, at least 1 year of growth remaining, idiopathic and progressive nature of the curve, cooperative and psychologically stable and compliant patient, and tolerance of the stimulation. Electrodes were placed laterally over the midaxillary line on the convex side. A paraspinous location on the convex side was sometimes used. Settings included a pulse duration of 220 μs, frequency of 25 pps, and an on-off ratio of 6 seconds on and 6 seconds off. The treatment time was gradually increased to 8 hours of stimulation per day (the stimulation was typically done at night if tolerated). High success and low dropout rates were reported.213 Others, however, reported much lower success rates.214 Intolerance of the treatment resulting in low compliance may be the cause of these differences. This disorder is much more common in adolescent girls. When NMES first was introduced for scoliosis, the uncomfortable and cosmetically undesirable Milwaukee brace was still the norm for treatment. With more advanced materials and orthotic management that is much more acceptable cosmetically, the use of NMES in scoliosis management has significantly declined.
Contraindications and precautions
Any electrical stimulation application is contraindicated for clients who have epilepsy or demand-type pacemakers. In addition, contraindications exist to applications over the transthoracic area or the uterus in pregnancy as well as in a cancerous area and the carotid sinus. Other factors require precaution but are not strict contraindications, such as sensory deficits, skin problems (sensitivity to stimulation, electrodes, or gel; edema; open wounds), tolerance of stimulation intensity sufficient to elicit muscle contraction, client’s capability to participate in the training process, and financial considerations.56–58,215
Matthews and colleagues216 reported changes in blood pressure and heart rate suggestive of autonomic hyperreflexia when electrical stimulation was applied in seven subjects with SCI above the T6 level. FES to the quadriceps produced the noted changes as stimulation intensity was increased. The mechanism for this reaction is unclear. Clinicians should monitor vital signs in clients with SCI (and possibly all clients), at least during the initial application of electrical stimulation.