Chapter 58 Electronically Enhanced Endoscopic Imaging
Introduction
Although ex vivo histology remains the current standard of confirming all lesions, the concept of real-time in vivo histology of lesions or optical biopsies during ongoing endoscopy has been introduced.1 In vivo histology could play an important role in a busy GI endoscopy practice not only in diagnosing small lesions of otherwise unknown significance before making a decision regarding their necessary resection but also in targeting biopsies and directing treatment decisions during surveillance endoscopic evaluation, such as in inflammatory bowel disease or Barrett’s esophagus.
Electronically enhanced endoscopy is also expected to improve lesion detection rates. Conventional white light videoendoscopy was found to be associated with alarming miss rates for subtle lesions (e.g., flat and depressed adenomas).2 Even experienced gastroenterologists can miss 6% of advanced adenomas and 30% of all adenomas.3,4 Some studies confirmed the development of colorectal cancer, especially in the right site of the colon, despite a surveillance colonoscopy program.5,6 Because subtle dysplastic and early neoplastic lesions are often not easily recognized during regular standard white light endoscopy, electronically enhanced endoscopic technologies are intended to improve their detection. These electronically enhanced imaging technologies including broad field and point fields (Table 58.1) combined together are expected to improve diagnostic yield and to allow in vivo histology diagnosis, minimize the burden of unnecessary biopsies, and lead to immediate decisions for definitive management.
| Methods | Technology | Aims |
|---|---|---|
| Broad field electronic enhancement technologies | Virtual chromoendoscopy: NBI, i-Scan, AFI | Increased mucosal contrast and detection of flat and depressed lesions |
| Point field electronic enhancement technologies | Endoscope-based CLE, probe-based CLE | In vivo histology |
AFI, autofluorescence imaging; CLE, confocal laser endomicroscopy; NBI, narrow band imaging.
Broad Field Image Enhancement Techniques: High-Resolution Endoscopy, Virtual Chromoendoscopy, and Other Optical Techniques
Broad image enhanced endoscopy (IEE) technologies include traditional dye-based IEE with chromoendoscopy and optical methods including equipment-based IEE with narrow band imaging (NBI), electronically based IEE with spectral estimation technologies such as Fuji Intelligent Chromo Endoscopy (FICE) and i-Scan, and autofluorescence imaging (AFI).7 These technologies are used with more recently developed high-resolution endoscopes.
High-Resolution and High-Definition Endoscopy
High-definition (HD) systems can display 1080 scanning lines on a screen compared with standard definition analog systems, which can generate 576 scanning lines. These HD endoscopes with high-density couple charge devices, combined with HD 1080-line television monitors, produce images with increased spatial resolution. A high-definition imaging system was used in more recent trials and showed very high adenoma detection rates greater than 50% in screening patients with average colorectal cancer risk.8–11
The use of HD colonoscopy was associated with a higher adenoma detection rate compared with SD colonoscopy in a large cohort study of 2430 patients.12 This higher rate was most apparent for smaller adenomas (<10 mm) and adenomas in the left colon. Other colonoscopic studies comparing standard definition white light endoscopy with HD white light endoscopy have revealed mixed results.11,13–15 In a study by East and colleagues11 of 132 patients undergoing routine screening colonoscopies by one dedicated and highly experienced endoscopist, adenoma detection rate was higher with HD colonoscopy (71% vs. 60%), but this trend did not reach statistical significance in the group of 58 HD patients and 72 standard definition patients. Pellise and coworkers13 showed no statistically significant advantage in detection of adenomas (HD, 26%; standard definition, 25%) and all polyps (HD, 43%; standard definition, 38%) using a HD colonoscope versus a standard definition colonoscope in 639 patients undergoing routine screening colonoscopy. However, this study was underpowered to detect small differences between groups in a population with a prevalence of adenomas of less than 30%, which is typical of average-risk individuals.
Narrow Band Imaging
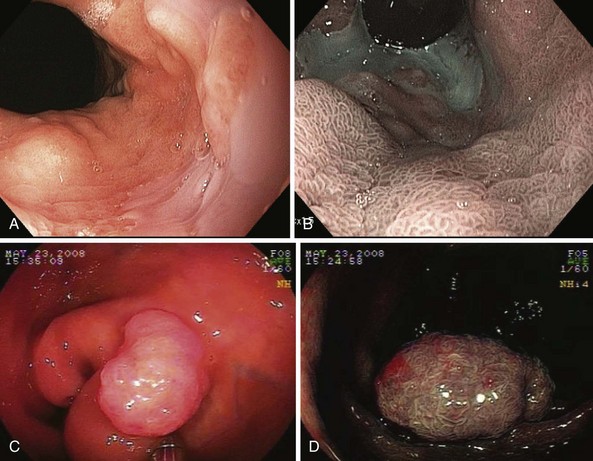
NBI (Olympus Corp, Tokyo, Japan) is a method of virtual chromoendoscopy that has a potential to improve detection of mucosal abnormalities without time-consuming and impractical application of chromic agents. NBI is currently the most investigated advanced endoscopic imaging technique for the detection of Barrett’s dysplasia and colorectal polyps (Fig. 58.1). Conventional white light endoscopy uses the full visible wavelength range (400 to 700 nm) to produce a red/green/blue image, whereas NBI combined with magnification endoscopy illuminates the tissue surface using special filters that narrow the red/green/blue bands and simultaneously increases the relative intensity of the blue band.
The technology was developed by Gono and associates16 in 1999 as a joint project of the Japanese National Cancer Center Hospital East and Olympus Corporation. The investigators studied variations of conventional endoscopy that potentially could visualize early changes of angiogenesis associated with the development of dysplasia and superficial neoplasia. Using light filters, the contribution of blue light is increased by narrowing the band widths of the red, green, and blue components of the excitation light and reducing the amount of green light and eliminating the red light. The resulting “narrow band” blue/green light improves imaging of mucosal patterns because of the limited optical scattering and shallow penetration depth. This blue light is also absorbed by hemoglobin (because the hemoglobin absorption band [Soret band] lies at 415 nm) for optimal detection of mucosal glandular and vascular patterns and the presence of abnormal blood vessels that are associated with the development of dysplasia.
Numerous studies have shown the value of this technology in the evaluation of patients with upper GI lesions including Barrett’s esophagus dysplasia and gastric lesions.17,18 The systematic review by Curvers and associates18 summarized performance and clinical utility of NBI in upper GI endoscopy with a focus on the primary detection of premalignant lesions and the differentiation between neoplastic and nonneoplastic lesions.
A prospective, blinded, tandem study in 65 patients referred for evaluation of Barrett’s dysplasia by Wolfsen and colleagues17 showed that in patients evaluated for Barrett’s esophagus with dysplasia, NBI detected significantly more patients with dysplasia and higher grades of dysplasia with fewer biopsy samples compared with standard resolution endoscopy. Specifically, NBI-targeted biopsies found dysplasia in more patients (37 patients [57%]) compared with standard resolution endoscopy with targeted plus random biopsies (28 patients [43%]; P < .001). NBI also found higher grades of dysplasia in 12 patients (18%) compared with no cases in which standard resolution endoscopy with targeted and random biopsies detected a high grade of histology (0%; P < .001). In addition, more biopsy samples were taken using standard resolution endoscopy with targeted plus random biopsies (mean 8.5 biopsy samples per case) compared with NBI-directed biopsies (mean 4.7 biopsy samples per case; P < .001). This ability of high-resolution endoscopy combined with NBI to detect dysplasia in significantly more patients with Barrett’s esophagus using fewer biopsy specimens supports the role of this technology in surveillance evaluation.
NBI technology was also evaluated for the detection of colorectal lesions.19,20 More recent studies revealed conflicting results with overall no improvement of adenoma detection rates.9,21,22 In a study by Adler and coworkers23 of 401 patients randomly assigned to undergo either standard definition colonoscopy or NBI colonoscopy, a higher adenoma detection rate in the NBI group compared with the standard group (23% vs. 17%) was shown in the initial phase of the study, suggesting a learning effect with the introduction of a new method influencing the conventional technology as well.
The use of NBI for the detection of dysplasia in long-standing ulcerative colitis has not led to improvement of neoplasia detection.24 East and colleagues25 showed that NBI improved adenoma detection in high-risk patients with hereditary nonpolyposis colorectal cancer syndrome, in whom a second additional colonoscopic examination with NBI doubled the total number of adenomas detected. A systematic review by van den Broek and coworkers22 summarized data on the performance and clinical utility of NBI during colonoscopy. Their review did not show a significant improvement in adenoma detection using NBI, but it confirmed the value of NBI for differentiation of neoplastic from nonneoplastic colonic polyps when used by experts.
The main advantage of NBI seems to be not detection but rather characterization because NBI has a high sensitivity of 90% to 95% and a specificity of 80% to 85% for differentiation of neoplastic from nonneoplastic lesions.26 Based on available studies, this level of accuracy is comparable to that of expert chromoendoscopists.27,28 It is anticipated that with appropriate training, NBI will be used routinely for lesion discrimination and management.
More recent trials confirmed very high accuracy of NBI for the diagnosis of small colorectal lesions (<10 mm) in selected cases of high-quality and high-confidence stored images interpreted offline.29,30 Rex29 introduced the concept of confidence levels to the endoscopic interpretation of colorectal polyp histology. This concept allowed sufficient accuracy (>91%) for the use of NBI to identify distal hyperplastic polyps that do not need resection and to plan postpolypectomy surveillance without pathologic evaluation of polyps 5 mm or smaller.29
Ignjatovic and colleagues30 showed that for polyps less than 10 mm, in vivo optical diagnosis using NBI as virtual chromoendoscopy or in a few cases dye-based chromoendoscopy can be an acceptable approach for polyp characterization. Wada and associates31 showed that both NBI and chromoendoscopy can be useful in distinguishing between neoplastic and nonneoplastic colorectal lesions based on analysis of pit pattern and vascular patterns.
Rastogi and coworkers32 also showed that using a simple surface mucosal and vascular pattern classification, NBI without magnification was highly accurate and significantly superior to high-definition white light imaging for prediction of adenomas. It is anticipated that with appropriate training, NBI will be used routinely for better lesion discrimination and characterization.
Fluorescence and Trimodal Imaging
In a prospective randomized study, AFI endoscopy significantly increased the diagnostic yield of surveillance endoscopy to detect intraepithelial neoplasia in high-risk patients with ulcerative colitis.33 However, AFI has not been shown to enhance the diagnostic yield of screening colonoscopy in a tandem colonoscopy trial by the same group.34
Curvers and colleagues35 published the initial results of four expert endoscopy centers using trimodal imaging for the evaluation of 84 patients with Barrett’s dysplasia in their uncontrolled trial. The use of AFI increased the number of patients found to have high-grade dysplasia from 53% to 90%. The use of NBI reduced the false-positive rate of AFI from 81% to 26%. The same group more recently published their subsequent randomized crossover trial evaluating endoscopic trimodal imaging (ETMI), which combines high-resolution endoscopy, NBI, and AFI for the detection of high-grade dysplasia and early cancer of Barrett’s esophagus.36 The ETMI system improved the targeted detection of high-grade dysplasia and early cancer compared with standard white light endoscopy, although NBI was found to have a limited value in characterizing the mucosal changes. Based on these results, ETMI cannot replace random biopsies for the detection of lesions and targeted biopsies for characterization of the lesions.
A subsequent study by the same group37 compared the role of ETMI with standard definition endoscopy for the detection of neoplasia in 99 patients with Barrett’s esophagus in their community practice. ETMI had a significantly higher targeted histologic yield because of additional detection of 22 lesions with low-grade intraepithelial neoplasia, high-grade intraepithelial neoplasia (HGIN), or carcinoma by AFI. There was no significant difference in the overall histologic yield (targeted plus random) between ETMI and standard definition endoscopy. HGIN or carcinoma was diagnosed only by random biopsies in 6 of 24 patients and 7 of 24 patients with ETMI and standard definition endoscopy. ETMI performed in a community-based setting did not improve the overall detection of dysplasia compared with standard definition endoscopy. The diagnosis of dysplasia still required identification of a significant number of patients with dysplasia by random biopsies.
Overall studies on AFI for colorectal adenoma have shown very mixed results. Matsuda and colleagues38 showed that AFI detected more polyps in the right-sided colon compared with white light colonoscopy. van den Broek and coworkers39 evaluated interobserver agreement and accuracy for differentiation of polyps among experienced and nonexperienced gastroenterologists and confirmed that AFI had higher interobserver agreement and accuracy for diagnosing polyps than NBI. In a randomized prospective study, Takeuchi40 showed that AFI colonoscopy with a transparent hood detected significantly more colorectal neoplasms than conventional colonoscopy without a hood. Overall, studies on AFI have shown very mixed results, and the future application of AFI is still to be determined.
FICE and i-Scan
The FICE system was used to evaluate esophageal neoplasia and showed improvement in detection of early neoplasia.41 In this prospective crossover randomized pilot study, the detection of HGIN or early cancer in 57 patients with Barrett’s esophagus was compared using conventional chromoendoscopy with acetic acid with the FICE system. The sensitivity of targeted biopsies for HGIN or early cancer on a “per lesion” basis was 87% (26 of 30) for chromoendoscopy and FICE. Computed virtual chromoendoscopy is a helpful adjunct for surveillance of Barrett’s esophagus and seems to be as accurate as conventional chromoendoscopy in the detection of HGIN or early cancer.
The FICE system can be useful in evaluation of colorectal neoplasia. However, in the prospective randomized trial of tandem colonoscopy by Chung and associates,42 there was also no objective advantage of the FICE technique over conventional high-resolution endoscopy in terms of improved adenoma detection rate. The adenoma miss rate with FICE showed no significant difference compared with white light colonoscopy (6.6% vs. 8.3%; P = .59).
In a prospective randomized trial, Pohl and associates43 compared a computed virtual chromoendoscopy system (FICE) with other modalities such as standard colonoscopy and conventional chromoendoscopy with indigo carmine in low-magnification and high-magnification modes for determination of colonic lesion histology. Based on this study, the FICE system was able to identify morphologic details that efficiently predict adenomatous histology and was superior to standard colonoscopy and equivalent to conventional chromoendoscopy. The same investigators were also able to show that computed virtual chromoendoscopy is a helpful adjunct for surveillance of Barrett’s esophagus, and it seems to be as accurate as traditional chromoendoscopy in the detection of HGIN or early cancer.41
Hoffman and coworkers44 showed that high-definition endoscopy combined with i-Scan is significantly superior in detecting colorectal neoplasia compared with standard videocolonoscopy, and it allows prediction of histology of the identified lesions and surface enhancement. In their study, high-definition endoscopy plus colonoscopy with i-Scan identified significantly more patients with at least one neoplasm compared with standard resolution endoscopy (38% vs. 13%; P < .001). The histology of polyps was predicted accurately with i-Scan with a sensitivity of 98% and a specificity of 100% (95% confidence interval sensitivity 90.6 to 99.6, specificity 92.8 to 100.) Although the techniques of NBI, FICE, and i-Scan are very promising, further studies are needed to evaluate their final application not only in tertiary referral centers but also in routine endoscopy practices in terms of efficiency and cost-effectiveness in both detection and characterization of malignant GI lesions.
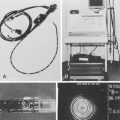
Confocal Laser Endomicroscopy
Intravenous fluorescein (1.0 to 5.0 mL of 10% solution) distributes throughout the capillary network and connective matrix, whereas acriflavine accumulates in superficial epithelial cells and nuclei, which raised a concern for a potential mutagenic risk and limited its broader use. In a cross-sectional survey of 16 international medical centers with active research protocols involving 2272 patients, no serious adverse events were reported with the use of intravenous fluorescein.45
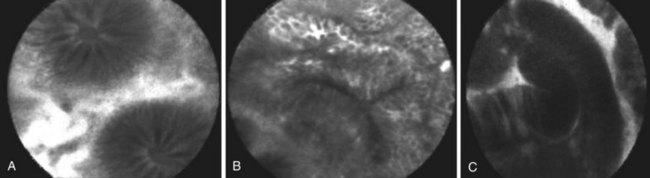
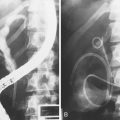
Numerous studies have supported the application of both CLE systems, endoscope-based and probe-based, as novel tools for in vivo histopathology in various clinical settings, including colorectal neoplasia (Fig. 58.2), Barrett’s esophagus, and ulcerative colitis.46–49 The CLE systems have been shown to distinguish accurately dysplastic Barrett’s esophagus from nondysplastic Barrett’s esophagus.46,50 In the initial pilot study, Kiesslich and colleagues46 developed criteria for gastric mucosa, intestinal metaplasia, and neoplastic epithelium based on the confocal vascular and cellular features. It enabled prediction of Barrett’s esophagus dysplasia versus nondysplasia with a sensitivity of 93% and specificity of 98%.
These initial results have been validated further in a prospective randomized crossover study comparing standard endoscopy with random biopsy and endomicroscopy (endoscope-based CLE) with targeted biopsies.51 CLE with targeted biopsy almost doubled the diagnostic yield for endoscopically inapparent Barrett’s esophagus neoplasia (from 17% to 33%) in 16 patients. Two-thirds of 23 patients in the surveillance group did not need any mucosal biopsies at all because no neoplasia was present during endomicroscopic imaging. CLE with targeted biopsy also greatly reduces the number of biopsies needed per patient and allows some patients without neoplasia to forgo mucosal biopsy.15
The other confocal system—probe-based CLE—has been also tested in Barrett’s esophagus. Pohl and colleagues43 developed probe-based CLE criteria for neoplastic Barrett’s esophagus that included irregular epithelial lining, variable width of epithelial lining, fusion of glands, presence of dark areas, and an irregular vascular pattern. These criteria were established based on 95 biopsy specimens from 15 patients and were validated prospectively in 23 patients with assessment of not only accuracy of probe-based CLE but also interobserver agreement. The overall sensitivity, specificity, positive predictive value, and negative predictive value were 80%, 94%, 44%, and 99%, with good interobserver agreement (κ = 0.6). Subsequently, Wallace and colleagues50 evaluated the accuracy of probe-based CLE for prediction of dysplasia and dysplastic Barrett’s esophagus among endoscopists and estimated overall sensitivity of 88% and specificity of 96% with good interobserver agreement (κ = 0.72).
The probe-based CLE criteria also have been validated more recently in a large multicenter randomized controlled trial (DON’T BIOPCE trial) that used blinded endoscopists to perform tandem endoscopic procedures to evaluate the sensitivity and specificity of probe-based CLE, in addition to white light endoscopy, for the detection of high-grade dysplasia and early adenocarcinoma in Barrett’s esophagus.52 Preliminary data on 39 patients with Barrett’s esophagus and 51 suspicious lesions and 245 random locations showed sensitivity of white light endoscopy, white light endoscopy plus NBI, and probe-based CLE to be 0.32, 0.46, and 0.59 and specificity to be 0.92, 0.91, and 0.89.
Both systems have been shown to improve accuracy in diagnosing colorectal neoplasia.47,49 Kiesslich and colleagues47 published the first report of the use of endoscope-based CLE in 42 patients during ongoing colonoscopy in diagnosing intraepithelial neoplasia and colorectal cancer. After staining with methylene blue, 134 small lesions (mean size 4 mm) were identified during colonoscopy. With the help of the confocal endoscope, intraepithelial neoplasia could be predicted with a sensitivity of 97% and a specificity of 99% (accuracy 99%). Buchner and associates49 compared virtual chromoendoscopy systems (NBI, FICE) with probe-based CLE for classification of colorectal polyps and confirmed higher sensitivity of probe-based CLE with similar specificity in accurate differentiation between neoplastic and nonneoplastic lesions. Sanduleanu and colleagues53 showed that endoscope-based CLE can reliably distinguish low-grade dysplasia from high-grade dysplasia in colorectal lesions with very good interobserver and intraobserver agreement.
The chromoendoscopy-guided endomicroscopy technique also has been evaluated in patients with long-term ulcerative colitis.48 The results confirmed that the presence of dysplastic changes could be predicted by endomicroscopy with high accuracy (sensitivity 94.7%, specificity 98.3%, accuracy 97.8%). Endomicroscopy has the potential to determine if ulcerative colitis lesions identified by chromoscopy should undergo biopsy examination and increase the diagnostic yield and reduce the need for biopsy examinations. Chromoscopy-guided endomicroscopy may lead to significant improvement in the long-term with clinical management of ulcerative colitis. Despite these promising results, CLE is used mainly as a research tool in academic centers.
1 Kiesslich R, Galle PR, Neurath MF. Endoscopic surveillance in ulcerative colitis: Smart biopsies do it better. Gastroenterology. 2007;133:742-745.
2 van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: A systematic review. Am J Gastroenterol. 2006;101:343-350.
3 Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24-28.
4 Postic G, Lewin D, Bickerstaff C, et al. Colonoscopic miss rates determined by direct comparison of colonoscopy with colon resection specimens. Am J Gastroenterol. 2002;97:3182-3185.
5 Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981.
6 Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: A multicentre randomised controlled trial. Lancet. 2010;375:1624-1633.
7 Kaltenbach T, Sano Y, Friedland S, et al. American Gastroenterological Association (AGA) Institute technology assessment on image-enhanced endoscopy. Gastroenterology. 2008;134:327-340.
8 Paggi S, Radaelli F, Amato A, et al. The impact of narrow band imaging in screening colonoscopy: A randomized controlled trial. Clin Gastroenterol Hepatol. 2009;7:1049-1054.
9 Rex DK, Helbig CC. High yields of small and flat adenomas with high-definition colonoscopes using either white light or narrow band imaging. Gastroenterology. 2007;133:42-47.
10 Kahi CJ, Anderson JC, Waxman I, et al. High-definition chromocolonoscopy vs. high-definition white light colonoscopy for average-risk colorectal cancer screening. Am J Gastroenterol. 2010;105:1301-1307.
11 East JE, Stavrindis M, Thomas-Gibson S, et al. A comparative study of standard vs. high definition colonoscopy for adenoma and hyperplastic polyp detection with optimized withdrawal technique. Aliment Pharmacol Ther. 2008;28:768-776.
12 Buchner AM, Shahid MW, Heckman MG, et al. High-definition colonoscopy detects colorectal polyps at a higher rate than standard white-light colonoscopy. Clin Gastroenterol Hepatol. 2010;8:364-370.
13 Pellise M, Fernandez-Esparrach G, Cardenas A, et al. Impact of wide-angle, high-definition endoscopy in the diagnosis of colorectal neoplasia: A randomized controlled trial. Gastroenterology. 2008;135:1062-1068.
14 Burke CA, Burke CA, Choure AG, Sanaka MR, et al. A comparison of high-definition versus conventional colonoscopes for polyp detection. Dig Dis Sci. 2010;55:1716-1720.
15 Tribonias G, Theodoropoulou A, Konstantinidis K, et al. Comparison of standard versus high-definition, wide-angle colonoscopy for polyp detection: A randomized controlled trial. Colorectal Dis. 2009;12:e260-e266.
16 Gono K, Obi T, Yamaguchi M, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568-577.
17 Wolfsen HC, Crook JE, Krishna M, et al. Prospective, controlled tandem endoscopy study of narrow band imaging for dysplasia detection in Barrett’s esophagus. Gastroenterology. 2008;135:24-31.
18 Curvers WL, van den Broek FJ, Reitsma JB, et al. Systematic review of narrow-band imaging for the detection and differentiation of abnormalities in the esophagus and stomach (with video). Gastrointest Endosc. 2009;69:307-317.
19 Su MY, Hsu CM, Ho YP, et al. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol. 2006;101:2711-2716.
20 Hirata M, Tanaka S, Oka S, et al. Magnifying endoscopy with narrow band imaging for diagnosis of colorectal tumors. Gastrointest Endosc. 2007;65:988-995.
21 Kaltenbach T, Friedland S, Soetikno R. A randomised tandem colonoscopy trial of narrow band imaging versus white light examination to compare neoplasia miss rates. Gut. 2008;57:1406-1412.
22 van den Broek FJ, Reitsma JB, Curvers WL, et al. Systematic review of narrow-band imaging for the detection and differentiation of neoplastic and nonneoplastic lesions in the colon (with videos). Gastrointest Endosc. 2009;69:124-135.
23 Adler A, Pohl H, Papanikolaou IS, et al. A prospective randomised study on narrow-band imaging versus conventional colonoscopy for adenoma detection: Does narrow-band imaging induce a learning effect? Gut. 2008;57:59-64.
24 Dekker E, van den Broek FJ, Reitsma JB, et al. Narrow-band imaging compared with conventional colonoscopy for the detection of dysplasia in patients with longstanding ulcerative colitis. Endoscopy. 2007;39:216-221.
25 East JE, Suzuki N, Stavrinidis M, et al. Narrow band imaging for colonoscopic surveillance in hereditary non-polyposis colorectal cancer. Gut. 2008;57:65-70.
26 East JE, Saunders BP. Narrow band imaging at colonoscopy: Seeing through a glass darkly or the light of a new dawn? Exp Rev. Gastroenterol Hepatol. 2008;2:1-4.
27 East JE, Suzuki N, Saunders BP. Comparison of magnified pit pattern interpretation with narrow band imaging versus chromoendoscopy for diminutive colonic polyps: A pilot study. Gastrointest Endosc. 2007;66:310-316.
28 Machida H, Sano Y, Hamamoto Y, et al. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: A pilot study. Endoscopy. 2004;36:1094-1098.
29 Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology. 2009;136:1174-1181.
30 Ignjatovic A, East JE, Suzuki N, et al. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): A prospective cohort study. Lancet Oncol. 2009;10:1171-1178.
31 Wada Y, Kashida H, Kudo SE, et al. Diagnostic accuracy of pit pattern and vascular pattern analyses in colorectal lesions. Dig Endosc. 2010;22:192-199.
32 Rastogi A, Keighley J, Singh V, et al. High accuracy of narrow band imaging without magnification for the real-time characterization of polyp histology and its comparison with high-definition white light colonoscopy: A prospective study. Am J Gastroenterol. 2009;104:2422-2430.
33 van den Broek FJ, Fockens P, van Eeden S, et al. Endoscopic tri-modal imaging for surveillance in ulcerative colitis: Randomised comparison of high-resolution endoscopy and autofluorescence imaging for neoplasia detection; and evaluation of narrow-band imaging for classification of lesions. Gut. 2008;57:1083-1089.
34 van den Broek FJ, Fockens P, Van Eeden S, et al. Clinical evaluation of endoscopic trimodal imaging for the detection and differentiation of colonic polyps. Clin Gastroenterol Hepatol. 2009;7:288-295.
35 Curvers W, Baak L, Kiesslich R, et al. Chromoendoscopy and narrow-band imaging compared with high-resolution magnification endoscopy in Barrett’s esophagus. Gastroenterology. 2008;134:670-679.
36 Curvers WL, Herrero LA, Wallace MB, et al. Endoscopic tri-modal imaging is more effective than standard endoscopy in identifying early-stage neoplasia in Barrett’s esophagus. Gastroenterology. 2010;139:1106-1114.
37 Curvers WL, van Vilsteren FG, Baak LC, et al. Endoscopic trimodal imaging versus standard video endoscopy for detection of early Barrett’s neoplasia: A multicenter, randomized, crossover study in general practice. Gastrointest Endosc. 2011;73:195-203.
38 Matsuda T, Saito Y, Fu KI, et al. Does autofluorescence imaging videoendoscopy system improve the colonoscopic polyp detection rate? A pilot study. Am J Gastroenterol. 2008;103:1926-1932.
39 van den Broek FJ, van Soest EJ, Naber AH, et al. Combining autofluorescence imaging and narrow-band imaging for the differentiation of adenomas from non-neoplastic colonic polyps among experienced and non-experienced endoscopists. Am J Gastroenterol. 2009;104:1498-1507.
40 Takeuchi Y. Autofluorescence imaging with a transparent hood for detection of colorectal neoplasms: A prospective, randomized trial. Gastrointest Endosc. 2010;72:1006-1013.
41 Pohl J, et al. Comparison of computed virtual chromoendoscopy and conventional chromoendoscopy with acetic acid for detection of neoplasia in Barrett’s esophagus. Endoscopy. 2007;39:594-598.
42 Chung SJ, et al. Efficacy of computed virtual chromoendoscopy on colorectal cancer screening: A prospective, randomized, back-to-back trial of Fuji Intelligent Color Enhancement versus conventional colonoscopy to compare adenoma miss rates. Gastrointest Endosc. 2010;72:136-142.
43 Pohl J, May A, Rabenstein T, et al. Computed virtual chromoendoscopy for classification of small colorectal lesions: A prospective comparative study. Am J Gastroenterol. 2008;103:562-569.
44 Hoffman A, Kagel C, Goetz M, et al. Recognition and characterization of small colonic neoplasia with high-definition colonoscopy using i-Scan is as precise as chromoendoscopy. Dig Liver Dis. 2010;42:45-50.
45 Wallace MB, Meining A, Canto MI, et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 2010;31:548-552.
46 Kiesslich R, Gossner L, Goetz M, et al. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4:979-987.
47 Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706-713.
48 Kiesslich R, Goetz M, Lammersdorf K, et al. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874-882.
49 Buchner AM, Shahid MW, Heckman MG, et al. Comparison of probe-based confocal laser endomicroscopy with virtual chromoendoscopy for classification of colon polyps. Gastroenterology. 2010;138:834-842.
50 Wallace MB, Sharma P, Lightdale C, et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett’s esophagus with probe-based confocal laser endomicroscopy. Gastrointest Endosc. 2010;72:19-24.
51 Dunbar KB, Okolo P3rd, Montgomery E, et al. Confocal laser endomicroscopy in Barrett’s esophagus and endoscopically inapparent Barrett’s neoplasia: A prospective, randomized, double-blind, controlled, crossover trial. Gastrointest Endosc. 2009;70:645-654.
52 Sharma P, Meining A, Coron E, et al. Detection of neoplastic tissue in patients with Barrett’s esophagus using probe based confocal endomicroscopy: Interim results from the “DONT BIOPCE” trial. Gastroenterology. 2010;138:S-155.
53 Sanduleanu S, Driessen A, Gomez-Garcia E, et al. In vivo diagnosis and classification of colorectal neoplasia by chromoendoscopy-guided confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2010;8:371-378.